Abstract
Relatively discrete experimental literatures have grown to support the insula's role in the domains of interoception, focal exteroceptive attention and cognitive control, and the experience of anxiety, even as theoretical accounts have asserted that the insula is a critical zone for integrating across these domains. Here we provide the first experimental demonstration that there exists a functional topography across the insula, with distinct regions in the same participants responding in a highly selective fashion for interoceptive, exteroceptive, and affective processing. Although each insular region is associated with areas of differential resting state functional connectivity relative to the other regions, overall their functional connectivity profiles are quite similar, thereby providing a map of how interoceptive, exteroceptive, and emotional awareness are integrated within the insular cortex. Hum Brain Mapp 34:2944–2958, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: insula, interoception, exteroception, anxiety
INTRODUCTION
Located within the Sylvian Fissure, the insula is situated at the intersection of the frontal, temporal, and parietal lobes. Neuroanatomical tract‐tracing studies demonstrate that the posterior and middle aspects of the insula receive substantial, reciprocal projections with the surrounding somatosensory cortex, and also receive nociceptive, thermal, and visceral afferent projections that convey interoceptive information about bodily states important for homeostatic regulation [Craig, 2002]. The anterior insula, in contrast, is heavily interconnected with prefrontal cortical and limbic structures, including the anterior cingulate cortex, orbitofrontal cortex, amygdala, and ventral striatum [Mesulam and Mufson, 1982; Ongur and Price, 2000]. Perhaps because of its position at the nexus of multiple neural systems underlying sensation, emotion, and cognition, the insular cortex is one of the most frequently reported areas of activation in the functional neuroimaging literature [Kurth et al., 2010; Nelson et al., 2010]. Insula involvement has been observed during the performance of a wide array of experimental tasks in healthy humans, and abnormalities of insular function have been reported in populations manifesting a variety of psychiatric or neurological disorders [Craig, 2009]. The insula has been implicated in cognitive and emotional processes as diverse as maternal love [Bartels and Zeki, 2004], disgust [Jabbi et al., 2008], sexual arousal [Kuhn and Gallinat, 2011], pain [Brooks et al., 2002], empathy [Fan et al., 2011], drug craving [Naqvi et al., 2007], and bowel distension [Eickhoff et al., 2006].
Drawing on influential theories of the relationship between emotion and bodily states (e.g., James‐Lange Theory of Emotion and Damasio's Somatic Marker Hypothesis), Craig 2009 proposed that the insula's role in these diverse phenomena may be accounted for by its involvement in interoception, and in particular, successively higher order re‐representations along the insula's caudal to rostral axis of interoceptive information and its associated homeostatic significance. This axis may provide a medium for integrating interoceptive experience, represented in its most embodied form in mid‐insula and posterior insula, and in a more abstracted format in the anterior insula, with information about emotional salience and hedonic potential represented in brain regions to which the anterior insula is strongly connected, including the amygdala, orbitofrontal cortex, and striatum [Mufson and Mesulam, 1982]. This theoretical account is well supported by experiments demonstrating insula activation when participants are made aware of interoceptive sensations, including thirst, air hunger, heartbeat detection, and distension of the esophagus, stomach, bladder, or rectum [for reviews see (Craig, 2002; Craig, 2009)], as well as evidence that insula damage impairs interoceptive awareness [Khalsa et al., 2009].
Interoception generally activates posterior and mid‐insular regions [Kurth et al., 2010], with some noteworthy exceptions finding the anterior insula active during interoceptive awareness [Critchley et al., 2004]. In contrast, processing emotional stimuli, particularly negatively valenced stimuli, activates the anterior insula [Kurth et al., 2010]. For example, disgust, guilt, perception of emotional and fear‐related pictures, and retrieval of sad autobiographical memories are all associated with anterior insula activation [Jabbi et al., 2008; Liotti et al., 2000; Mathews et al., 2004; Shin et al., 2000; Wicker et al., 2003]. Insula involvement in anxiety is particularly well supported. The anterior insula is activated during risk and the anticipation of punishment, and anxiety‐prone individuals exhibit greater activity in the anterior insula during emotion processing [Paulus et al., 2003; Simmons et al., 2006]. Similarly, worrying about self‐relevant aversive events (e.g., losing a job) is associated with anterior insula activation [Hoehn‐Saric et al., 2004]. Evidence additionally suggests that insular involvement in anxiety may partially underlie some clinical anxiety disorders, as symptom provocation in patients with specific phobias, post‐traumatic stress disorder, and obsessive–compulsive disorder is associated with anterior insula activation [Rauch et al., 1997]. Interestingly, Caseras et al. 2011 used conjunction analyses of fMRI data and showed that overlapping regions of the anterior and mid‐insula respond both to interoception and a phobic symptom provocation paradigm. In light of this, the findings described above can certainly be accommodated by the aforementioned theories asserting a hierarchical organization in the insula, with anterior regions supporting the integration of interoceptive and emotional awareness [Paulus and Stein, 2010]. They could also, however, indicate that the insula plays a primary role in emotional awareness independent of its involvement in the representation of interoceptive information.
Finally, evidence has accrued that the anterior insula may be part of a domain‐general, “multiple demand” network of brain regions that support task‐level control and focal attention to salient stimuli [Dosenbach et al., 2007; Duncan and Owen, 2000; Menon and Uddin, 2010; Nelson et al., 2010; Seeley et al., 2007; Sterzer and Kleinschmidt, 2010]. For example, in “extended reveal” paradigms that progressively reveal a stimulus out of visual noise, the anterior insula responds strongly at the moment of recognition when the participant perceives the stimulus as a salient object [Ploran et al., 2007; Wheeler et al., 2008]. Similarly, using simple experimental paradigms that employ relatively impoverished nonemotional stimuli, several groups have demonstrated robust anterior insula responses to deviant stimuli embedded amid continuous stimulus streams [Crottaz‐Herbette and Menon, 2006; Downar et al., 2002]. One interpretation of these findings is that the anterior insula may be engaged during focal attention to the detection of salient stimuli [Menon and Uddin, 2010; Nelson et al., 2010]. If so, this characterization would suggest that the anterior insula plays a more general role in cognition beyond interoception and emotional awareness per se.
Amid the wealth of neuropsychological, electrophysiological, and functional neuroimaging insula findings, there are unfortunately no studies that directly compare insula activity and functional organization within the same experimental paradigm and with the same participants for these separable sensory, emotional, and cognitive processes. For example, although studies have directly compared interoception and exteroception [Farb et al., 2012], or examined the conjunction across different tasks of interoception and the processing of emotional stimuli [Caseras et al., 2011], no studies have directly compared activity within the insula to all three sensory, emotional, and cognitive processes within the same scanning task context. Because this gap exists in the experimental database regarding the insula's functional organization, researchers across diverse literatures have been left to speculate about which sensory, emotional, or cognitive process might correspond to the particular area of the insula activated in their respective task. For example, changes in insula activity reported during tasks that evoke acute anxiety states, such as perceiving threatening stimuli, conceivably may reflect an epiphenomenal consequence of participants' interoceptive awareness of autonomic changes associated with anxiety, the heightened deployment of focal attention associated with processing emotionally evocative stimuli, or the emotional experience of fear. A study that directly compares and localizes these sub‐processes within the insula is needed.
To address this need, we asked participants to undergo BOLD fMRI while alternating among tasks requiring interoceptive attention to visceral sensations, exteroceptive attention to salient visually presented targets, and anxious attention to apprehensive life events, with similar stimulus properties among experimental conditions. Here we show that there exists a heterogeneous functional topography across the insula, with distinct regions responding in a highly selective fashion for interoceptive, exteroceptive, and emotional processing. In addition, we used these functionally selective insula regions as seeds in resting‐state functional connectivity analyses, and show that each seed region exhibits selective resting‐state functional connectivity to brain regions associated with interoceptive, exteroceptive, or anxious‐attention in the independent task data. Importantly, however, although the insula seed regions exhibited distinct functional selectivities in the task data, they shared commonalities in their overall resting‐state functional connectivity. Collectively, these findings elucidate the insula's functional topographic organization and provide a map of how interoceptive, exteroceptive, and emotional attention are integrated within the insula's functional topography.
METHODS
Participants
Fourteen, right‐handed native English‐speaking volunteers (8 female; mean age = 29 years; range 21–43 years) participated in the study. All participants were paid for their participation and provided written informed consent as approved by The University of Oklahoma Institutional Review Board. All participants completed detailed physical health screens and mental health evaluations using both structured and unstructured diagnostic interviews. Exclusion criteria included a prior history of major medical or psychiatric disorders, head injury or neurological disorders, current pregnancy, lifetime history of substance dependence, substance abuse within 1 year, exposure within 3 weeks to psychotropic or other medications expected to influence cerebral blood flow or function, or general MRI exclusions.
Experimental Design
Stimuli were back‐projected on to a screen located at the foot of the scanner bore, and viewed through a mirror system mounted on the head‐coil. Stimulus presentation and response collection were controlled using Eprime2 software (http://www.pstnet.com).
Upon entering the scanner, participants first underwent a resting‐state fMRI scan, during which they viewed a black fixation‐cross presented against a white background. Participants were asked to clear their mind and not think of anything in particular for the duration of the 7 min 30‐second scan. Because of participant head motion or technical difficulties during image acquisition, only 12 of the 14 participants' resting‐state scans were available for analysis of the resting BOLD data.
Upon completing the resting‐state scan, participants received verbal instructions about the experimental task, and underwent a short training run during which they practiced alternating among the three different task conditions: interoceptive attention to visceral sensations, exteroceptive attention to visually presented targets, and attention to apprehensive life events. Immediately following the training the participants performed the experimental task during four fMRI scanning runs, each lasting 7 min 30 s.
During the interoception condition, participants viewed the words “HEART” and “STOMACH” presented individually four times in each scanning run in black font against a white background for 15 s. Participants were instructed that for the entire time the word remained on the screen they should focus their attention on any sensations they felt in the part of the body to which the word referred. Immediately following one‐half of the interoception blocks, participants were asked to rate how full their stomach felt or how quickly their heart was beating during the preceding 15‐s period. Participants provided their ratings via a magnetic resonance‐compatible handheld scroll‐wheel that moved a cursor along a visual analog scale numbered from 1 to 7, with 1 indicating no sensation, and 7 indicating either an extremely full sensation, or extremely fast heartbeat. Participants had 5 s to provide their intensity ratings. The rating periods were included in the task to help ensure participants remained attentive to the task.
During the “anxiety” condition, participants saw either the word “RELATIONSHIP,” “MONEY,” “CLIMATE,” or “TERRORISM” presented four times in each run in black font against a white background for 15 s. Participants were instructed that for the entire time the word remained on the screen they should focus on anxieties and concerns they experience related to each of these four topics. Immediately following one‐half of the “worry” blocks, participants were asked to rate how intensely they experienced anxieties regarding the word during the preceding 15‐s period. Again, participants provided ratings via a handheld scroll‐wheel that moved a cursor along a visual analog scale numbered from 1 to 7, with 1 indicating no anxiety and 7 indicating extremely intense anxieties. Participants had 5 s to provide their ratings.
During the exteroception condition, participants fixated on an “O” presented four times in each run in black font against a white background for 15 s. At random intervals during the 15‐s block, the “O” was replaced with a target character (an “X”) for 500 ms. The exteroception targets appeared between 1 and 7 times during each 15‐s exteroception block. Participants were instructed to attend to the exteroception task for the entire 15‐s period and to keep track of how many targets were presented. Immediately following one‐half of the exteroception blocks, participants were asked to indicate the number of targets they detected during the preceding 15‐s period. Participants provided responses via a handheld scroll‐wheel that moved a cursor along a visual analog scale numbered from 1 to 7. Participants had 5 seconds to provide their ratings.
In each of the four fMRI task scanning runs, the three conditions were presented in a pseudo‐random order optimized for fMRI analysis by Optseq2 (http://surfer.nmr.mgh.harvard.edu/optseq/). Each condition block was separated by a variable‐duration interstimulus interval lasting between 2.5 and 15 s (mean interval = 6.14 s), during which time participants saw only a black fixation mark against a white background.
Data Acquisition
All functional and structural MR images were collected using a General Electric Discovery MR750 whole‐body 3 Tesla MRI scanner. The scanner is equipped with a scalable 32‐channel digital MRI receiver capable of performing massively parallel fMRI. A brain dedicated and optimized for parallel‐imaging, receive‐only 32‐element surface coils array (Nova Medical) was used for MRI signal reception. A single‐shot gradient‐recalled EPI sequence with Sensitivity Encoding (SENSE) depicting blood oxygenation level depended (BOLD) contrast, was used for functional scans. The following EPI imaging parameters were used: FOV/slice/gap = 240/2.9/0 mm, 46 axial slices per volume, acquisition matrix = 96 × 96, repetition/echo time TR/TE = 2,000/30 ms, SENSE acceleration factor R = 2 in the phase encoding (anterior–posterior) direction, flip angle = 90°, sampling bandwidth = 250 kHz, number of volumes 180, scan time 7 min 30 s. The EPI images were reconstructed into a 128 × 128 matrix, in which the resulting fMRI voxel volume was 1.875 × 1.875 × 2.9 mm3. Additionally, simultaneous physiological pulse oximetry and respiration waveform recordings were conducted (with 50 Hz sampling) for each fMRI run. A photoplethysmograph with an infra‐red emitter placed under the pad of the participant's left index finger was used for pulse oximetry, and a pneumatic respiration belt was used for respiration measurements. A T1‐weighted magnetization‐prepared rapid gradient‐echo (MPRAGE) sequence with SENSE was used to provide an anatomical reference for the fMRI analysis. The anatomical scan had the following parameters: FOV = 240 mm, axial slices per slab = 128, slice thickness = 1.2 mm, image matrix = 256 × 256, voxel volume 0.9 × 0.9 × 1.2 mm3, TR/TE = 5/1.9 ms, acceleration factor R = 2, flip angle = 80, delay time TD = 1,400 ms, inversion time TI = 725 ms, sampling bandwidth = 31.2 kHz, scan time = 4 min 58 s.
Data Preprocessing and Analyses
Prior to statistical analyses, imaging preprocessing was performed using AFNI (http://afni.nimh.nih.gov/afni). The anatomical scan was registered to the first run of the EPI data using AFNI's anatomical‐to‐epi alignment procedure. The anatomical scan was then spatially transformed to the stereotaxic array of Talairach and Tournoux [1988] using AFNIs automated algorithm and the transformation parameters were saved for use later in the pre‐processing. The first four volumes of each gradient‐echo EPI time‐course were excluded from data analysis to allow the fMRI signal to reach steady state, and a slice time correction was applied to all EPI volumes. Estimates of the transformations necessary to register all EPI volumes to the first volume of the first EPI time‐course (i.e., the resting‐state scan, to which the anatomical scan was registered) were then saved both for the next step in the preprocessing, and also for use in the statistical analyses. Motion correction and spatial transformation of the EPI data were then implemented in a single image transformation, and the data were subsequently resampled to a 2 mm × 2 mm × 2 mm grid. The EPI data were then smoothed with a 6 mm full‐width at half‐maximum Gaussian kernel, and the signal value for each EPI volume was normalized to the percent signal change from the voxel's mean signal across the time‐course.
Preprocessing of the resting‐state scans employed a modified version of the ANATICOR method [Jo et al., 2010]. The first four volumes of the resting state‐scan were excluded to remove T1 effects in the data. A despiking interpolation algorithm (AFNI's 3dDespike) was then used to remove any transient signal spikes from the data that might artificially inflate estimates of the correlation among voxels' time‐series, followed by slice time correction. Each volume in the resting state EPI time‐course was then registered to the first volume (which was registered to the anatomical scan). Masks of the participant's ventricles and white matter were constructed using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/), and each was eroded slightly to prevent partial volume effects that might include signal from gray matter voxels in the mask. First we calculated the average time course during the resting‐state run within the ventricle mask. Next, to produce estimates of the local physiological noise, we calculated for each gray matter voxel the average signal time‐course for all white matter voxels within a 1.5 cm radius. We also used the respiration and cardiac traces collected during the resting‐state scan to calculate Respiration Volume per Time (RVT) [Birn et al., 2008] parameters using the RetroTS.m plugin for Matlab. Mean, linear, quadratic, and cubic trends were removed from the all the regressors of non‐interest described above. In total, the estimates of physiological and nonphysiological noise included the 6 motion parameters (3 translations, 3 rotations), the average ventricle signal, the average local white matter signal, and 9 respiration regressors from Retroicor and RVT. The predicted time‐course for these nuisance variables was constructed using AFNI's 3dTfitter program, and then subtracted from each resting‐state voxel time‐course, yielding a residual time‐course for each voxel. This residual resting‐state time‐course was then smoothed with a 6 mm FWHM Gaussian kernel, resampled to a 2 mm × 2 mm × 2 mm grid, and spatially transformed for all subsequent analyses.
Statistical Analyses
Multiple regression was used to analyze the task data. The regression model included regressors for the interoception, exteroception, and anxiety conditions. To adjust the model for the shape and delay of the BOLD function, the three task regressors were constructed by convolution of a gamma‐variate function and a box‐car function having a 15‐second width beginning at the onset of each occurrence of the condition. Additionally, the regression model included regressors of non‐interest to account for each run's signal mean, linear, quadratic, and cubic signal trends, as well as 6 motion parameters (3 translations, 3 rotations) computed during the image registration preprocessing. The individual participants' beta weights from the regression analysis were then combined in a group random effects ANOVA to evaluate condition effects at the population‐level.
We used conjunction analyses to identify regions of the insula that responded selectively to a particular condition [Nichols et al., 2005]. For example, interoception‐selective voxels were defined by a conjunction of t‐tests with interoception > exteroception AND interoception > anxiety, with each of the individual tests separately FDR‐corrected for multiple comparisons (pFDR < 0.05). To reduce the chance that the identified voxels responded to the other (noninteroception) conditions, we additionally masked out all voxels exhibiting a reliable difference in activity between the exteroception and anxiety conditions (pFDR < 0.05). Finally, to rule out the possibility that small areas of intersection among the t‐maps could be induced by spatial smoothing and resampling, we applied a modest cluster‐size threshold of 10 voxels to the conjunction maps of interoception‐selective voxels. Exteroception‐selective, and anxiety‐selective voxels were identified in a like manner, with exteroceptive‐selective voxels defined as exteroception > interoception AND exteroception > anxiety and no reliable differences observed between interoception and anxiety, and anxiety‐selective voxels defined by anxiety > interoception AND anxiety > exteroception and no reliable differences observed between interoception and exteroception. All t‐maps were separately corrected for multiple comparisons using the false discovery rate (pFDR < .05). Because spatial smoothing and resampling could induce small areas of intersection among the t‐maps, we then also applied a cluster‐size threshold of 10 voxels to the conjunction maps of condition‐selective voxels. This step was taken to guarantee that any clusters we observed could not simply be due to the smoothing together of boundaries between functionally heterogeneous regions. Thus, not only are the maps for each condition corrected for multiple comparisons, we then applied an additional (cluster) threshold. The rationale for doing the analyses on group data in this manner is that the random effects analysis takes into account the variability across the participants, thus both guaranteeing that the preponderance of subjects show a given effect at a voxel, and that the observed effect can be inferred to be a property of the population, rather than the sample. Both of these inferences become stronger the higher the p‐value threshold applied to the data. We used pFDR < 0.05 as our threshold as this both controls the Type I error by correcting for comparisons, while also minimizing the Type‐II false‐negative rate. Taken together, we believe that our use of conjunctions of individually corrected random effects statistical maps (which take into account inter‐individual variability and balance Types I and II error), plus the application of an additional cluster size threshold on the conjunction map, is highly conservative.
Finally, to localize condition‐selective clusters within the insula, we inclusively masked both insular lobes using an anatomical mask drawn on the TT‐N27 atlas brain within AFNI.
Functional connectivity analyses on resting‐state scan data were conducted as follows. At the participant‐level, the seed time‐series for each of the condition‐selective clusters was constructed by calculating the average time series during the resting‐state scan within all voxels in each condition‐selective cluster identified in the conjunction analyses of the task data. Using multiple regression analysis we produced maps of the time‐course correlations (r‐values) between each of the seed regions and all voxels in the brain. These r‐values were then transformed to Z‐scores using Fisher's r‐to‐Z transformation. To identify voxels exhibiting greater correlations with one seed voxel than all other seeds (i.e., selective functional connectivity), we implemented a series of group‐level random effects paired‐sample t‐tests comparing the Z‐scores associated with each seed against each of the other seeds' Z‐scores. We then used conjunction analyses on the t‐maps to identify those voxels where functional connectivity with a particular seed was greater than each of the other seeds at P < 0.005. For example, voxels that were identified as being selectively functionally connected to the ventral interoceptive‐selective cluster were those voxels where Zventral_Interoception > Zdorsal_Interoception AND Zventral_Interoception > ZExteroception AND Zventral_Interoception > Anxiety, with each t‐map separately thresholded at p < .005. An additional cluster size threshold of at least 10 voxels was applied to the conjunction maps. The resulting conjunction maps show voxels, derived from an independent resting‐state dataset, that were selectively functionally connected to the condition‐selective clusters identified in the task data.
To identify regions of shared functional connectivity among the seed regions, correlation maps with each seed region's average time series were produced in the manner described above, and converted to Z‐maps. At the group‐level, all participants' Z‐maps were combined in a random effects one‐sample t‐test to identify voxels with mean scores across participants that differed from zero with P < 0.005. We then took the union of these maps to identify voxels with shared functional connectivity to all of the condition‐selective clusters identified in the task data. Additionally, the condition‐selective clusters were examined for similar whole‐brain resting‐state functional connectivity using the eta2 analysis method described by Cohen et al. 2008. In the context of fMRI, eta2 measures the overall similarity of two functional connectivity maps, with a value of 1 indicating perfect similarity, and 0 indicating no similarity. Eta2 values for each participant were calculated between each pair of Z‐score maps for each of the task‐selective clusters. The group eta2 values were then averaged to obtain a mean eta2 matrix (presented in Fig. 3c).
Figure 3.
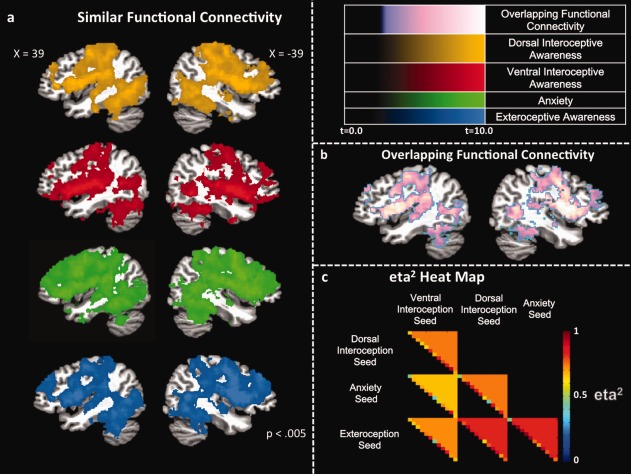
Random effects group conjunction analyses demonstrating regions of overlapping resting‐state functional connectivity to the interoception, exteroception, or anxiety insular seed regions. (a) Sagittal images show regions exhibiting resting‐state functional connectivity (P < 0.005) to the dorsal mid‐insula interoception seed region (gold), ventral mid‐insula interoception seed (red), ventral anterior insula anxiety seed (green), and dorsal anterior insula exteroception seed region. (b) Sagittal images showing regions of overlapping resting‐state functional connectivity (i.e., the conjunction of all four maps in Fig. 3a). The color intensity gradations in this figure represent the average t‐statistic at a voxel across all t‐maps included in the union of the t‐images. (c) eta2 heat map demonstrating high overall similarity in the whole‐brain resting‐state functional connectivity maps for the interoception, exteroception, or anxiety insular seed regions. The color of each triangle indicates the average eta2 value across all participants, with the individual participants' values indicated by the colors along the hypotenuse of each triangle.
RESULTS
Behavioral Responses During the Scanner Task
Subjects' behavioral responses indicated that they performed the tasks as instructed. Responses to exteroceptive target detection trials were highly accurate (Mean = 88.5%, SD = 8.2%). Ratings following interoceptive attention trials indicated that participants were clearly able to attend to their interoceptive experience, and reported moderately strong interoceptive sensations, with mean ratings near the middle of the 7‐point scale (Mean = 3.2, SD = 1.2). Similarly, intensity ratings following the anxiety trials indicated that participants experienced moderately high levels of anxiety, with mean ratings near the middle of the 7‐point scale. (Mean = 4.1, SD = 1.7).
Functional Selectivity within the Insular Cortex
As described in the Methods, functional selectivity for a task was herein defined in a statistically conservative manner, requiring reliably greater activation for one condition than each of the other conditions, with each contrast separately corrected for multiple comparisons, and with the additional constraint that we excluded those voxels exhibiting a reliable difference in the amount of activation between the other two tasks. This definition insures that any region identified as “functionally selective” exhibits reliably greater responses to a particular condition than to all other conditions, and the region's activity does not also reliably discriminate between the other two conditions.
Four regions of the insula exhibited activation profiles across conditions that satisfied this definition of “functional selectivity” (Fig. 1, Table 1). Two mid‐insula regions exhibited functionally selective responses during interoception. One region was located in the dorsal mid‐insula near the fundus of the circular insular sulcus. The other interoception‐selective region was located more ventrally and rostrally along the anterior short and middle short insular gyri. In contrast, exteroception selectively activated a region in the right dorsal anterior insula and frontal operculum in the fundus of the circular insular sulcus. Finally, and in contrast to the other two conditions, a region in the left ventral, anterior insula, near the intersection of the anterior short and accessory insular gyri, was selectively activated during the anxiety condition.
Figure 1.
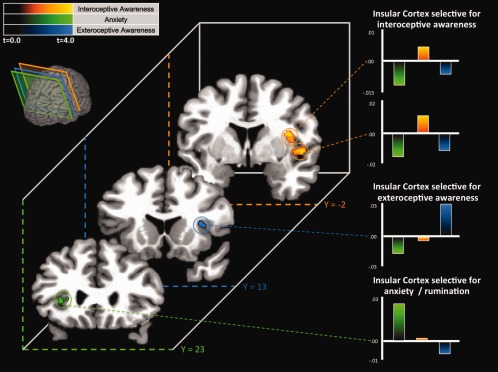
Random effects group conjunction analyses reveals insular regions responding selectively during interoception (orange), exteroception (blue), and anxiety (green). The coronal slices show the four regions of the insula exhibiting functionally selective responses as defined in the random effects conjunction analyses. For example, the two orange clusters at Y = −2 show regions in the right mid‐insula where activity during the interoception condition was greater than during the exteroception condition (P < 0.05 corrected) AND activity during interoception condition was greater than during the anxiety condition (P < 0.05 corrected) AND no reliable differences were observed in the activity during the exteroception and anxiety conditions. The bar graphs show the average percent signal change across participants in the four functionally selective clusters relative to the signal baseline. The colored planes running through the rendered brain in the top left corner of the figure show the locations of the three coronal slices. The color intensity gradations in each cluster represent the average t‐statistic at that voxel across all t‐maps included in the conjunction analysis.
Table 1.
Insular regions responding selectively under the interoception, exteroception, or anxiety conditions
| Side/location | Coordinates | Peak T | Volume (mm3) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Interoception | |||||
| Right mid‐insula | +37 | −3 | +16 | 4.18 | 232 |
| Right mid‐insula | +45 | +1 | 0 | 4.40 | 376 |
| Anxiety | |||||
| Left anterior insula | −31 | +23 | +4 | 4.21 | 160 |
| Exteroception | |||||
| Right dorsal anterior insula | +33 | +15 | +10 | 3.87 | 472 |
Outside the insula, functionally selective regions were observed for all three tasks, particularly in brain areas associated in earlier research with focal attention, emotion, and interoception (Table 2).
Table 2.
Regions outside the insula that responded selectively for either the interoception, exteroception, or anxiety conditions
| Side/location | Coordinates | Peak T | Volume (mm3) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Interoception | |||||
| L postcentral gyrus | −51 | −21 | +32 | 5.14 | 1,704 |
| R insula and precentral gyrus | +53 | −5 | +8 | 6.04 | 1,352 |
| R postcentral gyrus | +51 | −15 | +18 | 3.72 | 1,272 |
| L precuneus | −11 | −63 | +46 | 4.46 | 1,032 |
| L middle frontal gyrus | −41 | +31 | +28 | 4.02 | 464 |
| R dorsal insula | +37 | −3 | +16 | 4.18 | 232 |
| R cuneus | +13 | −77 | +24 | 4.02 | 208 |
| R inferior parietal lobule | +41 | −27 | +26 | 4.13 | 192 |
| R inferior frontal gyrus | +21 | +25 | −6 | 3.88 | 128 |
| L precentral gyrus | −55 | +5 | +32 | 3.93 | 88 |
| L postcentral gyrus | −55 | −21 | +16 | 3.28 | 80 |
| Anxiety | |||||
| L medial frontal gyrus | −5− | +45 | +28 | 8.39 | 15,152 |
| L superior temporal gyrus | −43 | +5 | −22 | 8.75 | 7,640 |
| R cerebellum | +31 | −71 | −34 | 8.36 | 4,832 |
| R posterior cingulate | +1 | −51 | +24 | 6.23 | 2,408 |
| L precuneus | −43 | −71 | +34 | 6.98 | 2,352 |
| L caudate | −5 | +15 | +12 | 5.24 | 2,072 |
| L parahippocampal gyrus | −19 | −25 | −12 | 5.64 | 1,800 |
| L posterior cingulate | −13 | −47 | +22 | 4.94 | 1,632 |
| L subgenual cingulate | −1 | +9 | −4 | 4.93 | 1,160 |
| L medial frontal gyrus | −9 | +13 | +44 | 6.22 | 776 |
| R superior temporal gyrus | +43 | +5 | −16 | 5.62 | 536 |
| L insula | −31 | +23 | +4 | 4.21 | 496 |
| R cerebellar tonsil | +7 | −51 | −34 | 5.37 | 400 |
| L middle frontal gyrus | −31 | +39 | −4 | 4.31 | 336 |
| R hippocampus | +29 | −21 | −8 | 3.54 | 208 |
| L anterior thalamus | −3 | −3 | +10 | 3.44 | 200 |
| R inferior frontal gyrus | +53 | +19 | +14 | 3.84 | 192 |
| L culmen | −13 | −53 | −18 | 3.29 | 168 |
| L middle temporal gyrus | −57 | −33 | −4 | 3.64 | 168 |
| L caudate tail | −17 | −31 | +22 | 3.48 | 136 |
| L cingulate gyrus | −1 | −17 | +38 | 4.72 | 120 |
| R inferior temporal gyrus | +45 | −7 | −32 | 3.59 | 112 |
| R culmen | +25 | −35 | −22 | 3.78 | 80 |
| L middle frontal gyrus | −25 | +17 | +56 | 4.68 | 80 |
| Exteroception | |||||
| R inferior parietal lobule | +51 | −55 | +38 | 8.81 | 23,328 |
| R precentral gyrus | +39 | +1 | +36 | 7.75 | 19,872 |
| R thalamus | +9 | −25 | +12 | 7.64 | 7,640 |
| L cerebellar tonsil | −31 | −45 | −30 | 5.75 | 5,200 |
| R middle frontal gyrus | +41 | +39 | −2 | 7.44 | 3,200 |
| R medial frontal gyrus | +5 | +33 | +38 | 6.18 | 1,952 |
| L middle occipital gyrus | −39 | −63 | −2 | 5.34 | 1,368 |
| R medial frontal gyrus | +3 | −1 | +52 | 4.37 | 800 |
| L precuneus | −23 | −65 | +24 | 4.57 | 496 |
| R lentiform nucleus | +17 | +7 | −8 | 3.94 | 400 |
| R superior frontal gyrus | +21 | +39 | −6 | 4.75 | 304 |
| R caudate | +7 | +11 | +6 | 5.27 | 232 |
| R cerebellar tonsil | +11 | −31 | −34 | 3.09 | 160 |
| R lentiform nucleus | +29 | −17 | +2 | 3.70 | 128 |
| R culmen | +9 | −35 | −24 | 3.04 | 112 |
| R cingulate gyrus | +5 | −9 | +32 | 3.75 | 112 |
| L precentral gyrus | −41 | −11 | +44 | 3.13 | 112 |
| L cerebellum | −15 | −27 | −34 | 3.95 | 88 |
| R parahippocampal gyrus | +19 | −13 | −24 | 3.91 | 88 |
| R cerebellar tonsil | +19 | −37 | −38 | 3.41 | 80 |
Resting‐State Functional Connectivity to Insular Cortex Regions Selective for Interoception, Anxiety, or Exteroception
For each of the functionally selective insular clusters described above, we sought to identify brain regions exhibiting reliably greater covariation in the spontaneous resting‐state BOLD fluctuations relative to the other functionally selective clusters. As described in the Methods, we did so by defining as seed regions the four condition‐selective insula clusters, and then performing conjunction analyses to identify brain regions that exhibited reliably greater correlations with the signal variation in one of those seed regions than to each of the other seeds. We refer to these as regions exhibiting “selective functional connectivity”.
Although the two insular regions selective for interoception exhibited highly similar response patterns across tasks (see bar graphs Fig. 1), and both regions were located in the mid‐insular region of the same hemisphere, these regions exhibited marked differences in their selective functional connectivity (Fig. 2 and Table 3). The dorsal mid‐insula seed region exhibited selective functional connectivity to regions in the somatosensory network, including the post‐central gyrus bilaterally, the right posterior insula, and the left mid‐insula. In contrast, the ventral mid‐insular interoception seed‐region exhibited selective functional connectivity with the entire ventral insula bilaterally, including regions immediately adjacent to the two anxiety‐ and exteroception‐selective insula regions. Importantly, the ventral interoception seed region was additionally selectively functionally connected to the left temporoparietal junction (TPJ) and subgenual anterior cingulate cortex (ACC), two regions well known for their involvement respectively in the conscious experience of physical embodiment and autonomic interactions with emotion [De Ridder et al., 2007; Drevets et al., 2008].
Figure 2.
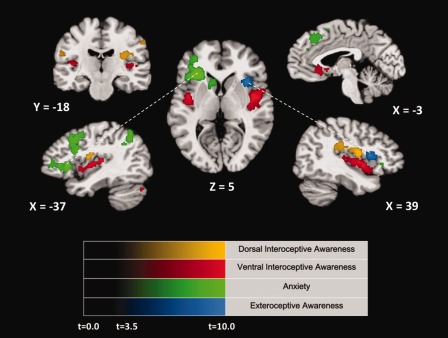
Random effects group conjunction analyses demonstrating regions of selective resting‐state functional connectivity to either the interoception, exteroception, or anxiety insular seed regions. Regions in the mid‐ and posterior‐insula and somatosensory cortex highlighted in gold exhibited greater resting‐state functional connectivity to the dorsal mid‐insula cluster that responded selectively during the interoception condition in the task data, than to each of the other three seed regions defined in the task data (with each statistical contrast separately thresholded at P < 0.005). Regions in the ventral insula and subgenual anterior cingulate highlighted in red exhibited greater resting state functional connectivity to the ventral mid‐insula cluster that responded selectively during the interoception condition in the task data, relative to each of the other seed regions (again, with each statistical test separately thresholded at P < 0.005). Likewise, green and blue highlights show regions of selective functional connectivity to the anxiety‐ and exteroception‐selective clusters defined in the task data. The color intensity gradations in each cluster represent the average t‐statistic at that voxel across all t‐maps included in the conjunction analysis.
Table 3.
Regions exhibiting selective functional connectivity to one of the interoception, exteroception, or anxiety seed regions
| Side/location | Coordinates | Peak T | Volume (mm3) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Ventral interoception cluster seed region | |||||
| R insula | +43 | −5 | +4 | 27.92 | 3504 |
| L insula | −41 | −13 | +8 | 17.17 | 1624 |
| L subgenual anterior cingulate | +1 | +23 | −8 | 6.08 | 720 |
| L cerebellum | −39 | −71 | −22 | 5.57 | 256 |
| L declive | −15 | −85 | −20 | 6.68 | 128 |
| R superior temporal gyrus/ temporoparietal junction | +67 | −35 | +6 | 4.69 | 88 |
| Dorsal interoception cluster seed region | |||||
| R insula | +35 | +1 | +14 | 28.14 | 5080 |
| L insula | −33 | −3 | +12 | 11.18 | 832 |
| L postcentral gyrus | −47 | −19 | +20 | 13.51 | 544 |
| R precentral gyrus | +53 | −23 | +40 | 13.35 | 224 |
| R paracentral lobule | +31 | −41 | +48 | 13.41 | 192 |
| R postcentral gyrus | +43 | −27 | +38 | 9.80 | 104 |
| L middle cingulate gyrus | −15 | −9 | +40 | 6.76 | 96 |
| R paracentral lobule, R SMA | +15 | −19 | +58 | 5.49 | 96 |
| Anxiety/rumination cluster seed region | |||||
| L insula | −25 | +23 | +6 | 25.80 | 5224 |
| L inferior frontal gyrus | −35 | +9 | +28 | 12.42 | 4920 |
| L caudate | −13 | +7 | +10 | 22.51 | 2392 |
| L angular gyrus, L precuneus, L inferior parietal lobe | −27 | −51 | +36 | 6.38 | 2040 |
| L medial frontal gyrus | −7 | +19 | +44 | 9.61 | 1464 |
| L superior frontal/L cingulate gyrus | −13 | +27 | +24 | 11.14 | 1328 |
| L middle frontal gyrus | −3 | +11 | +54 | 14.96 | 448 |
| R caudate | +13 | +9 | +14 | 21.04 | 320 |
| L medial dorsal thalamus | −7 | −17 | +14 | 11.74 | 152 |
| R inferior frontal gyrus | +35 | +29 | +2 | 9.62 | 144 |
| R superior frontal gyrus | +17 | +49 | +18 | 6.72 | 144 |
| L medial frontal gyrus | −21 | +51 | +20 | 6.98 | 112 |
| R superior frontal gyrus | +13 | +45 | +34 | 5.32 | 104 |
| L superior frontal gyrus, L superior orbital gyrus | −13 | +53 | −2 | 6.65 | 80 |
| R caudate | +11 | +11 | +8 | 15.58 | 80 |
| Exteroception cluster seed region | |||||
| R insula | +33 | +17 | +8 | 31.38 | 2416 |
| R middle frontal gyrus | +37 | +37 | +34 | 11.35 | 240 |
The ventral anterior insula anxiety seed‐region exhibited selective functional connectivity to a network of regions that included the left dorsolateral, medial‐orbitofrontal, and inferior prefrontal cortex, the midline supplementary motor area (SMA), the dorsal parietal cortex, and the left caudate (Table 3). The dorsal anterior insula exteroception seed‐region exhibited selective functional connectivity to a region of the right dorsal anterior prefrontal cortex previously implicated in the cingulo‐opercular attention control network [Dosenbach et al., 2007].
Many anatomical and functional connectivity studies have demonstrated that the insula and anterior cingulate cortex (ACC) form the core of an insula‐ACC salience and control network [Dosenbach et al., 2007]. The lack of significant insula‐ACC functional connectivity findings may be accounted for, however, by the fact that these analyses identify regions that are reliably more functionally connected to one of the seed regions than to any of the other seed regions. In contrast to mapping the regions exhibiting selective functional connectivity to any one of the seed regions relative to the other seeds, we also identified regions exhibiting shared functional connectivity to all of the seed regions. Importantly, the four regions of the insula exhibiting distinct (functionally selective) responses in the task data also exhibited similar patterns of functional connectivity with the wider brain (Fig. 3a,b). In fact, large portions of the frontal lobes, including the ACC, the parietal, posterior temporal, and occipital lobes, and the striatum, thalamus, and amygdala exhibited spontaneous resting‐state BOLD fluctuations that were reliably correlated with all four functionally selective regions of the insula. This finding highlights the insula as a region capable of supporting the integration of information across neural systems. In fact, the overall similarity of the four seed regions' whole‐brain functional connectivity profiles is demonstrated by their eta2 similarity matrices [Cohen et al., 2008] (Fig. 3c), with all pairs of seed regions exhibiting similarity values in excess of 0.66.
DISCUSSION
The findings reported here demonstrate a heterogeneous functional topography along the insula with distinct regions responding during interoceptive‐, exteroceptive‐, and emotional‐attention. These functionally selective insula regions exhibit both distinct and overlapping patterns of resting‐state functional connectivity across the brain; a finding that supports both the selectivity of the responses observed in the task data, and a role for the insula as an important region for integrating across neural systems.
Interoceptive Attention in the Insula
It is well established that focal attention on a perceptual modality amplifies activity in brain regions underlying that modality [Jancke et al., 1999; Johansen‐Berg et al., 2000; Somers et al., 1999]. By instructing participants to focus on their naturally occurring interoceptive sensations, we used this attentional spotlight effect to identify two insular regions selectively activated during interoception of visceral sensations.
One of the interoceptive‐selective regions in the present study was located in an area of the dorsal mid‐insula characterized by granular cortex [Mesulam and Mufson, 1982]. This insular region is near the terminus of the ascending neural pathway that carries information from the viscera through the nucleus of the solitary tract, and on to the ventral medial nucleus of the thalamus, before arriving in the mid‐dorsal insula [Craig, 2002]. The other interoception‐selective region was located ventral and anterior to the dorsal insula cluster, in the dysgranular insula. Both areas of activation localize to insular regions implicated in interoception [Farb et al., 2012; Kurth et al., 2010; Pollatos et al., 2007], and both exhibited nearly identical degrees of selectivity for interoception relative to the other conditions. As a result, on the bases of their functional selectivity alone, it would be difficult to infer whether they make distinct contributions to interoceptive attention. Importantly, however, previous studies have demonstrated differences in both structural and functional connectivity across the insula [Cauda et al., 2011; Cerliani et al., 2011; Cloutman et al., 2012; Jakab et al., 2011; Nelson et al., 2010]. The findings from the present study agree with this earlier work by finding distinctive patterns of selective resting‐state functional connectivity for the two different interoceptive‐selective regions within the insula, a finding that suggests markedly different roles for these two regions. Relative to other seed regions, spontaneous resting‐state BOLD fluctuations in the dorsal mid‐insula interoceptive cluster were significantly more correlated with activity in sensorimotor cortex, including the post‐central gyrus and the posterior insula. Activity in the dorsal insula thus may be related to the representation of visceral somatosensation. In contrast, spontaneous resting‐state BOLD fluctuations in the ventral mid‐insular interoception region were significantly more correlated with activity in the anterior insula adjacent to the regions that responded selectively during the exteroception and emotion conditions, as well as to the subgenual ACC and the temporoparietal cortex. The ventral interoception region thus may play a role in the integration of interoceptive signals with information in dorsal anterior insular regions subserving salience and focal attention. Additionally, because its activity appears yoked to ventral anterior insula regions adjacent to the cortex that was functionally selective for emotion processing, as well as to the subgenual ACC, this ventral mid‐insula interoception region may be an important mediator in the emotional modulation of autonomic activity. The strong relationship between the ventral interoception‐selective cluster and the subgenual ACC is particularly interesting as dysfunction within this region is thought to underlie disruptions of autonomic regulation with emotion that are observed in clinical depression [Drevets et al., 2008].
To summarize, attention to interoceptive states selectively activates multiple insula regions, one closely related to the somatosensory system, the other slightly more anterior that is tightly coupled to a region of ventromedial prefrontal cortex implicated in emotional regulation of autonomic responses, and a region of temporoparietal cortex previously implicated in body schemata and ‘out of body’ experiences [De Ridder et al., 2007]. These findings appear to support theories [Craig, 2009; Damasio, 1993; Singer et al., 2009] positing the existence of multiple hierarchically organized insula regions underlying “meta‐representations” of the body's homeostatic state. For both Damasio and Craig [Craig, 2009; Damasio, 1993], these so‐called “somatic markers” give rise to the emotionally textured sentience colloquially described as the “self”. If so, aberrations within this circuit should lead to disruptions of one's sense of self. It is thus significant that the insular region associated here with interoception, and the subgenual ACC and temporoparietal regions to which it is strongly functionally connected, have all been repeatedly implicated in depersonalization disorder, a psychiatric condition characterized by feelings of detachment from one's own body and mental processes [Sierra and David, 2011].
Insula Activity During Anxious Rumination
In this study, which is the first to directly compare emotional and interoceptive processing in the same participants, anxious rumination selectively activated a region of the left ventral anterior insula. As evident in Figure 1, not only was this region reliably more active during anxious rumination than in all other conditions, interoceptive attention did not activate the region over the signal baseline (e.g., fixation cross). This finding is significant because it demonstrates that interoception and anxiety are at least dissociable within the insula. For example, previous studies have demonstrated increased anterior insula activity during anxious anticipation of aversive stimuli [Nitschke et al., 2006]. The findings reported here appear to exclude the possibility that the anterior insula's role in these responses is strictly an epiphenomenal consequence of participants' interoceptive attention to the autonomic changes they manifest while anticipating those stimuli.
At least one of two alternative explanations thus appears more likely. One possibility derives from the aforementioned theories of Craig and Damasio positing hierarchically organized regions within the insula representing information about the body's homeostatic state [Craig, 2002, 2009; Damasio, 1993]. Whereas the interoceptive‐selective regions we observed in the mid‐insula may represent relatively lower‐level physical sensations experienced in the body, the anxiety‐selective region we observed in the anterior insula may represent a type of high‐level homeostatic information, perhaps about the overall state of the body, which is an important component of emotional experience and a sense of well‐being. Paulus and Stein 2010 in particular have noted the importance of the anterior insula for alliesthesia, a process by which subjective evaluations of homeostatic signals are linked to external stimuli. By this account, high‐level representations of the body's current homeostatic state in the anterior insula support beliefs about the significance for one's well‐being of external stimuli or internal thoughts. The ventral anterior insula emotion‐selective region observed in this study may be the site of these high‐level homeostatic representations, thereby linking interoceptive signals represented in more posterior insular cortex with perceptual information about the outside world and high‐level reasoning about its contents represented in other brain regions to which the anterior insula is connected. This process of linking homeostatic information with perceptual and high‐level cognitive representations may allow assessments of the self‐relevance of events both outside and inside the mind, which in turn should be critical for producing appropriate affective responses. A second possible account of the findings in the present study, however, is that this ventral anterior insular region may represent an emotion‐processing system within the insula that is completely distinct from interoceptive information. We find this account unlikely given the theoretical reasons for grounding at least part of emotional experience in interoceptive awareness, as well the wealth of data indicating a relationship between interoceptive and emotional acuity [Barrett et al., 2004; Dunn et al., 2010]. The main goal in directly comparing interoception and anxious rumination in the present study was simply to identify whether the two conditions are even dissociable within the insula. Ultimately, the definitive interpretation of the roles played by the emotion‐selective cluster in the present study must await future experiments designed to more directly assess the information content of this region.
Exteroceptive Attention in the Insula
Exteroceptive attention selectively activated the right dorsal anterior insula. The exteroception‐selective region of the dorsal anterior insular cortex exhibited differential functional connectivity with a region of the right anterior prefrontal cortex implicated in the ventral attention network [Fox et al., 2006] and cingulo‐opercular control network [Dosenbach et al., 2007]. This finding agrees very well with recent findings demonstrating functional connectivity between the dorsal anterior insula and the anterior prefrontal region implicated in attention and cognitive control [Deen et al., 2011; Touroutoglou et al., 2012].
Given the strong connectivity with the anterior prefrontal portion of the ventral attention/cingulo‐opercular control networks, it may be initially surprising to see that the exteroceptive‐selective insula region was not also selectively functionally connected with another important component of this network, the dorsal ACC [Taylor et al., 2009]. In fact, rather than exhibiting selective‐functional connectivity with only the exteroceptive‐selective insula region, the dorsal ACC exhibited spontaneous resting‐state BOLD fluctuations that were correlated with each of the condition‐selective clusters in the insula. This highlights an important point about the functional connectivity findings in this study. Although there were many regions where spontaneous resting‐state activity was more highly correlated with one of the four condition‐selective insula clusters than all the others, the four interoception‐, exteroception‐, or anxiety‐selective clusters shared many regions of functional connectivity in common. For example, as with the cingulate gyrus, the amygdala also exhibited correlated spontaneous resting‐state BOLD fluctuations with all four condition‐selective insula clusters. This finding makes sense in light of anatomical tracings demonstrating reciprocal connections between the amygdala and the posterior, mid, and anterior insular cortex [Mesulam and Mufson, 1982]. The gross similarity in the functional connectivity profiles for the four interoceptive‐, exteroceptive‐, and emotion‐selective clusters is demonstrated by the high eta2 values shared among the functional connectivity maps (Fig. 3c).
Laterality, Limitations, and Future Directions
In this study there was evidence of laterality effects, particularly in the affective and exteroceptive attention conditions. The exteroceptive attention task selectively activated a region of the right anterior insula previously implicated in focal attention and “moment of recognition” [Dosenbach et al., 2007; Nelson et al., 2010; Touroutoglou et al., 2012; Wheeler et al., 2008]. In contrast, the anxiety condition activated the left anterior insula. Left anterior insula involvement has been reported previously in neuroimaging studies of worry [Shin et al., 2000], and multiple neuroimaging studies suggest preferential left > right anterior insula activation during tasks of emotional processing and awareness [Campbell‐Sills et al., 2011; Craig, 2009]. Though in separate studies, tasks involving either emotional processing or focal attention have shown bilateral anterior insula activation. This study, however, directly compared the two conditions and found evidence supporting the account of preferential left anterior insula activation for emotional processing. After FDR correction, conjunction analysis, and application of a cluster threshold, our interoceptive‐selective insular regions were confined to the right insula. This observation could lend support to current theories of sympathetic/parasympathetic hemisphere lateralization [Craig, 2009]. It is worth noting however, that at the same statistical threshold, but a lower clustering threshold, we observed selective mid‐insula activation for interoceptive attention in the left hemisphere as well. Future studies should be undertaken to replicate the laterality effects observed in this study, and in particular to explore the relative selectivity for interoception in the two hemispheres.
Additionally, the paradigm used in this study required subjective ratings for the interoceptive and anxious rumination conditions, but an objective response (i.e., number of targets detected) for the exteroceptive condition. As a result, accuracy scores cannot be compared between the conditions, which makes it hard to ensure that the tasks are well matched for difficulty. We believe it is highly unlikely, however, that differences in task‐difficulty account for the findings. Participants reported after the scan session that the conditions were approximately equally difficult, and both the task and functional connectivity findings agree well with previous reports in the literature about the neuroanatomical and functional bases of interoception, exteroception, and emotional awareness. In future studies it would be good to measure activity within the interoceptive‐selective regions reported here while objectively measuring the acuity of participants' interoceptive awareness. It would be ideal, however, if this hypothetical experimental paradigm does not also impose a co‐occurring exteroceptive task demand, such as in paradigms requiring participants to compare their heartbeats to a series of co‐occurring (exteroceptive) auditory beats.
CONCLUSIONS
The insula is one of the most commonly activated brain regions across sensory, emotional, and cognitive tasks. Large, relatively discrete experimental literatures have grown up to support its role in particular domains, even as influential theoretical accounts have asserted that the insula is a critical zone for integrating across these domains. It has thus been surprising that heretofore no studies have directly compared insula activity and functional organization within the same participants and experimental context for the key sensory, emotional, and cognitive functions so often ascribed to the insula. This study is the first experimental demonstration that a heterogenous functional topography exists across the insular cortex, with different regions exhibiting remarkably distinct functional selectivity during interoceptive, exteroceptive, and affective processing, as well as differential functional connectivity with regions outside the insula. Nevertheless, the close proximity of interoceptive, exteroceptive, and affective regions within the insula, as well as their grossly similar functional connectivity with the wider brain, reflect the insula's role as a region for relaying and integrating information across neural systems underlying diverse cognitive, sensory, and emotional processes.
ACKNOWLEDGMENTS
The authors thank Alex Martin for insightful feedback regarding the manuscript.
REFERENCES
- Barrett LF, Quigley KS, Bliss‐Moreau E, Aronson KR (2004): Interoceptive sensitivity and self‐reports of emotional experience. J Pers Soc Psychol 87:684–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S (2004): The neural correlates of maternal and romantic love. Neuroimage 21:1155–1166. [DOI] [PubMed] [Google Scholar]
- Birn RM, Murphy K, Bandettini PA (2008): The effect of respiration variations on independent component analysis results of resting state functional connectivity. Hum Brain Mapp 29:740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N (2002): fMRI of thermal pain: Effects of stimulus laterality and attention. Neuroimage 15:293–301. [DOI] [PubMed] [Google Scholar]
- Campbell‐Sills L, Simmons AN, Lovero KL, Rochlin AA, Paulus MP, Stein MB (2011): Functioning of neural systems supporting emotion regulation in anxiety‐prone individuals. Neuroimage 54:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X, Murphy K, Mataix‐Cols D, Lopez‐Sola M, Soriano‐Mas C, Ortriz H, Pujol J, Torrubia R (2011): Anatomical and functional overlap within the insula and anterior cingulate cortex during interoception and phobic symptom provocation. Hum Brain Mapp. DOI: 10.1002/hbm.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A (2011): Functional connectivity of the insula in the resting brain. Neuroimage 55:8–23. [DOI] [PubMed] [Google Scholar]
- Cerliani L, Thomas RM, Jbabdi S, Siero JC, Nanetti L, Crippa A, Gazzola V, D'Arceuil H, Keysers C (2011): Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Hum Brain Mapp. DOI: 10.1002/hbm.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman LL, Binney RJ, Drakesmith M, Parker GJ, Lambon Ralph MA (2012): The variation of function across the human insula mirrors its patterns of structural connectivity: Evidence from in vivo probabilistic tractography. Neuroimage 59:3514–3521. [DOI] [PubMed] [Google Scholar]
- Cohen AL, Fair DA, Dosenbach NU, Miezin FM, Dierker D, Van Essen DC, Schlaggar BL, Petersen SE (2008): Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage 41:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666. [DOI] [PubMed] [Google Scholar]
- Craig AD (2009): How do you feel‐‐now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ (2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–195. [DOI] [PubMed] [Google Scholar]
- Crottaz‐Herbette S, Menon V (2006): Where and when the anterior cingulate cortex modulates attentional response: Combined fMRI and ERP evidence. J Cogn Neurosci 18:766–780. [DOI] [PubMed] [Google Scholar]
- Damasio AR (1993): Descartes' Error: Emotion, Reason, and the Human Brain. New York:Putnam. [Google Scholar]
- De Ridder D, Van Laere K, Dupont P, Menovsky T, Van de Heyning P (2007): Visualizing out‐of‐body experience in the brain. N Engl J Med 357:1829–1833. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA (2011): Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex 21:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE (2007): Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD (2002): A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol 87:615–620. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M (2008): The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 13:663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM (2000): Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23:475–483. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Galton HC, Morgan R, Evans D, Oliver C, Meyer M, Cusack R, Lawrence AD, Dalgleish T (2010): Listening to your heart. How interoception shapes emotion experience and intuitive decision making. Psychol Sci 21:1835–1844. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Lotze M, Wietek B, Amunts K, Enck P, Zilles K (2006): Segregation of visceral and somatosensory afferents: An fMRI and cytoarchitectonic mapping study. Neuroimage 31:1004–1014. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G (2011): Is there a core neural network in empathy? An fMRI based quantitative meta‐analysis. Neurosci Biobehav Rev 35:903–911. [DOI] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Anderson AK (2012): Attentional modulation of primary interoceptive and exteroceptive cortices. Cerebral Cortex. DOI: 10.1093/cercor/bhr385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME (2006): Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn‐Saric R, Schlund MW, Wong SH (2004): Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Res 131:11–21. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Bastiaansen J, Keysers C (2008): A common anterior insula representation of disgust observation, experience and imagination shows divergent functional connectivity pathways. PLoS ONE 3:e2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab A, Molnar PP, Bogner P, Beres M, Berenyi EL (2011): Connectivity‐based parcellation reveals interhemispheric differences in the insula. Brain Topogr. DOI: 10.1007/s10548‐011‐0205‐y. [DOI] [PubMed] [Google Scholar]
- Jancke L, Mirzazade S, Shah NJ (1999): Attention modulates activity in the primary and the secondary auditory cortex: A functional magnetic resonance imaging study in human subjects. Neurosci Lett 266:125–128. [DOI] [PubMed] [Google Scholar]
- Jo H, Saad Z, Simmons W, Milbury L, Cox R (2010): Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage 52:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen‐Berg H, Christensen V, Woolrich M, Matthews PM (2000): Attention to touch modulates activity in both primary and secondary somatosensory areas. Neuroreport 11:1237–1241. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Feinstein JS, Tranel D (2009): The pathways of interoceptive awareness. Nat Neurosci 12:1494–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J (2011): A quantitative meta‐analysis on cue‐induced male sexual arousal. J Sex Med 8:2269–2275. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB (2010): A link between the systems: Functional differentiation and integration within the human insula revealed by meta‐analysis. Brain Struct Funct 214( 5–6):519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT (2000): Differential limbic–cortical correlates of sadness and anxiety in healthy subjects: Implications for affective disorders. Biol Psychiatry 48:30–42. [DOI] [PubMed] [Google Scholar]
- Mathews A, Yiend J, Lawrence AD (2004): Individual differences in the modulation of fear‐related brain activation by attentional control. J Cogn Neurosci 16:1683–1694. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214( 5–6):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ (1982): Insula of the old world monkey. III. Efferent cortical output and comments on function. J Comp Neurol 212:38–52. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM (1982): Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol 212:23–37. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007): Damage to the insula disrupts addiction to cigarette smoking. Science 315:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE (2010): Role of the anterior insula in task‐level control and focal attention. Brain Struct Funct 214( 5–6):669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB (2005): Valid conjunction inference with the minimum statistic. Neuroimage 25:653–660. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ (2006): Functional neuroanatomy of aversion and its anticipation. Neuroimage 29:106–116. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL (2000): The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10:206–219. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB (2003): Increased activation in the right insula during risk‐taking decision making is related to harm avoidance and neuroticism. Neuroimage 19:1439–1448. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB (2010): Interoception in anxiety and depression. Brain Struct Funct 214( 5–6):451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploran EJ, Nelson SM, Velanova K, Donaldson DI, Petersen SE, Wheeler ME (2007): Evidence accumulation and the moment of recognition: dissociating perceptual recognition processes using fMRI. J Neurosci 27:11912–11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C (2007): Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res 1141:178–187. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Alpert NM, Fischman AJ, Jenike MA (1997): The functional neuroanatomy of anxiety: A study of three disorders using positron emission tomography and symptom provocation. Biol Psychiatry 42:446–452. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Dougherty DD, Orr SP, Pitman RK, Lasko M, Macklin ML, Alpert NM, Fischman AJ, Rauch SL (2000): Activation of anterior paralimbic structures during guilt‐related script‐driven imagery. Biol Psychiatry 48:43–50. [DOI] [PubMed] [Google Scholar]
- Sierra M, David AS (2011): Depersonalization: A selective impairment of self‐awareness. Conscious Cogn 20:99–108. [DOI] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB (2006): Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety‐prone subjects. Biol Psychiatry 60:402–409. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K (2009): A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci 13:334–340. [DOI] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RB (1999): Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci USA 96:1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P, Kleinschmidt A (2010): Anterior insula activations in perceptual paradigms: Often observed but barely understood. Brain Struct Funct 214( 5–6):611–622. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD (2009): Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 30:2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Barrett LF (2012): Dissociable large‐scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage 60:1947–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Nelson SM, Ploran EJ, Velanova K (2008): Dissociating early and late error signals in perceptual recognition. J Cogn Neurosci 20:2211–2225. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G (2003): Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron 40:655–664. [DOI] [PubMed] [Google Scholar]


