Abstract
Compared with complex coordinated orofacial actions, few neuroimaging studies have attempted to determine the shared and distinct neural substrates of supralaryngeal and laryngeal articulatory movements when performed independently. To determine cortical and subcortical regions associated with supralaryngeal motor control, participants produced lip, tongue and jaw movements while undergoing functional magnetic resonance imaging (fMRI). For laryngeal motor activity, participants produced the steady‐state/i/vowel. A sparse temporal sampling acquisition method was used to minimize movement‐related artifacts. Three main findings were observed. First, the four tasks activated a set of largely overlapping, common brain areas: the sensorimotor and premotor cortices, the right inferior frontal gyrus, the supplementary motor area, the left parietal operculum and the adjacent inferior parietal lobule, the basal ganglia and the cerebellum. Second, differences between tasks were restricted to the bilateral auditory cortices and to the left ventrolateral sensorimotor cortex, with greater signal intensity for vowel vocalization. Finally, a dorso‐ventral somatotopic organization of lip, jaw, vocalic/laryngeal, and tongue movements was observed within the primary motor and somatosensory cortices using individual region‐of‐interest (ROI) analyses. These results provide evidence for a core neural network involved in laryngeal and supralaryngeal motor control and further refine the sensorimotor somatotopic organization of orofacial articulators. Hum Brain Mapp 33:2306–2321, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: fMRI, sparse sampling, motor control, orofacial articulators, somatotopy, speech production
INTRODUCTION
The neural correlates of orofacial motor control have been extensively studied in the context of coordinated actions such as mastication (Lund and Kolat, 2006; Nakamura and Katakura, 1995; Onozuka et al., 2007), swallowing (Hamdy et al., 1999; Humbert and Robbins, 2007; Leopold and Daniels, 2009; Martin et al., 2001, 2004, 2007; Peeva et al., 2009; Sawczuk and Mosier, 2005), whistling (Dresel et al., 2005), phonation and vocalization (Brown et al., 2008; Jürgens, 2002, 2009; Schulz et al., 2005; Simonyan et al., 2009; Smotherman, 2007) as well as speech production (Bohland and Guenther, 2006; Brown et al., 2005; Brown, Ngan and Liotti, 2008; Chang et al., 2009; Guenther et al., 2006; Lotze et al., 2000a; Murphy et al., 1997; Özdemir et al., 2006; Riecker et al., 2000a, b, 2005, 2008; Sörös et al., 2006; Terumitsu et al., 2006; Wise et al., 1999).
However, compared with complex coordinated orofacial actions, less is known about the shared and distinct neural substrates of supralaryngeal and laryngeal articulatory movements when they are performed independently (Brown et al., 2008; Dhanjal et al., 2008; Hesselmann et al., 2004; Lotze et al., 2000a; Pulvermüller et al., 2006; Terumitsu et al., 2006). Previous neuroimaging studies of simple orofacial movements have primarily focused on the somatotopic organization of orofacial articulators within the primary sensorimotor cortex (Brown et al., 2008; Hesselmann et al., 2004; Lotze et al., 2000a; Pulvermüller et al., 2006; Terumitsu et al., 2006). Since the early electrocortical mapping studies during awake neurosurgical operations (Penfield and Boldrey, 1937; Penfield and Rasmussen, 1950), it is well known that the motor and somatosensory cortices are broadly arranged somatotopically, with an orderly organization of sensorimotor areas responsible for motor control and sensory integration of different parts of the human body. Despite large activation overlap, Penfield and Rasmussen (1950) observed a dorso‐ventral somatotopic organization for the lips, jaw, tongue, and pharynx, respectively. Since then, these results have been partly reproduced using functional magnetic resonance imaging (fMRI), with recent studies demonstrating topographically dorso‐ventral ordered positions within the primary sensorimotor cortex for lip and tongue movements (Hesselmann et al., 2004; Lotze et al., 2000a; Pulvermüller et al., 2006). The location of a larynx‐specific area in the primary motor cortex appears less clear with two recent studies reporting different positions for laryngeal movements among lips and/or tongue motor areas and varying across hemispheres (Brown et al., 2008; Terumitsu et al., 2006). Finally, in our best knowledge, no brain‐imaging study attempted to localize an area controlling jaw movements and to place this area in a somatotopic context.
While the existence of an orofacial sensorimotor somatotopy per se makes no doubt in light of previous studies, only few of them explored and directly compared within the same participants the neural correlates of simple supralaryngeal and laryngeal movements (Brown et al., 2008; Dhanjal et al., 2008). It appears, however, important to further precise how the basic orofacial articulators are mutually organized in the human brain. Notably, in the context of speech studies, speech production models often involve detailed sensorimotor maps in which each articulator has a specific role and a specific representation (Guenther et al., 2006; Guenther and Vladusich, in press). In this framework, while lips and tongue are often considered as the two basic supralaryngeal articulators for speech production, jaw is also considered as playing a crucial role in the development of speech motor control particularly in relation with the acquisition of syllables (MacNeilage, 1998). The coordination of laryngeal and supralaryngeal articulators during speech production is also essential at both the segmental and suprasegmental levels. From this view, previous neuroimaging studies on orofacial motor control provide “an overall picture of somatotopy with overlap” (Takai et al., 2010) and suggest a highly integrated system for laryngeal and supralaryngeal functions.
In this study, we used fMRI with sparse temporal image acquisition to further clarify the shared and distinct neural substrates of simple lip, tongue, jaw and vocalic/laryngeal movements. For supralaryngeal articulators, participants performed independent lip protrusion, jaw lowering, and tongue retraction movements. To determine laryngeal motor activity, participants produced the steady‐state/i/vowel. Although vowel vocalization involves activity of other orofacial articulators, a larynx‐specific area in the primary motor cortex has been shown to be activated comparably by vocalic and nonvocalic laryngeal tasks (i.e., production of the schwa vowel vs. forced adduction of the vocal folds in the absence of any voicing; Brown et al., 2008). Furthermore, vocalization of the unrounded front high/i/vowel likely involved lower activity of the lip and jaw muscles than for other rounded and/or low vowels. In summary, our design improves upon previous studies by precising at once (1) the shared and distinct neural substrates of supralaryngeal and laryngeal movements in relation to speech and non‐speech orofacial gestures and (2) the sensorimotor somatotopic organization of lip, jaw, vocalic/laryngeal, and tongue movements, using the sparse temporal sampling method to minimize articulatory‐related movement image artifacts.
Abbreviations.
- ANOVA
analysis of variance
- BOLD
blood‐oxygen‐level dependence
- COG
centre of gravity
- EPI
echoplanar imaging
- FIR
finite impulse response
- fMRI
functional magnetic resonance imaging
- GLM
general linear model
- MNI
Montreal neurological institute
- ROI
region‐of‐interest
- TR
repetition time
METHODS
Participants
Thirteen healthy adults (eleven males and two females with a mean age of 29 years, ranging in age from 21 to 44 years), native French speakers, participated in the study after giving their informed consent. All were right‐handed according to a standard handedness inventory (Oldfield, 1971), had normal or corrected‐to‐normal vision and reported no history of motor, speaking or hearing disorders. Participants were screened for neurological, psychiatric, other possible medical problems and contraindications to MRI. The protocol was approved by the Grenoble University Ethical Committee and was carried out in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Tasks
The experimental paradigm of the present study is depicted in Fig. 1. All participants first performed a vowel vocalization task in three functional runs, as part of a larger speech production study (including other speech stimuli to be produced; data not reported here). Participants were instructed to overtly produce the steady‐state/i/vowel, which requires laryngeal activation for phonation. A resting condition, without any movement, served as baseline. Importantly, although the vowel vocalization task was performed to determine laryngeal motor activity, it also involved activity of tongue and lip muscles. However, the rationale to include this condition was based on the following points. First, because some previous studies dealing with laryngeal activity also used a vowel vocalization task (Brown et al., 2008; Terumitsu et al., 2006), this condition allowed to compare and to discuss the observed activity in this condition. Second, compared with other vowels, the steady‐state vocalization of the unrounded/front/high/i/vowel likely involved lower activity of the lip and jaw muscles than for rounded/low vowels. Third, compared with a pure motor laryngeal task (as vocal‐fold adduction movements performed in Brown et al.'s study, 2008), the/i/vowel was hypothesized to require minimal involvement of the pharyngeal part of the tongue and to be easier and more natural to produce. Finally, it is also worthwhile noting that a larynx‐specific area in the primary motor cortex has been shown to be activated comparably by nonvocal and vocal laryngeal movements (Brown et al., 2008) and that vowel vocalization and non‐speech orofacial movements have been shown to involve very similar activation of the sensorimotor system, besides specific auditory and phonological activations in the bilateral temporal cortices for vowel vocalization (Sörös et al., 2006).
Figure 1.
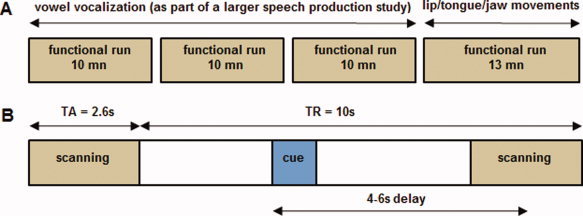
(A) Experimental design. The vowel vocalization task was performed in three functional runs (as part of a larger speech production study) preceding a functional run related to the supralaryngeal motor tasks. Each motor or resting condition occurred 18 times in a pseudorandomized order. (B) Timeline of a single trial. For each trial, the time interval between the visual instruction onset and the midpoint of the following functional scan acquisition was randomly varied between 4 s, 5 s, or 6 s. In each trial, a 1,000 ms visual instruction informed the participants about the motor condition or the resting baseline (blue rectangle). TR: repetition time; TA = acquisition time. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In a subsequent functional run, done within the same imaging session and using exactly the same acquisition parameters, three orofacial motor tasks were performed independently and without phonation: a lip protrusion movement, a tongue retraction movement (the tongue turned in the back of the mouth) and a jaw lowering movement. As previously, a resting condition was also added.
For all motor conditions, participants were instructed to initiate and end each movement from a resting state position, with the mouth closed and the tongue and jaw relaxed. In each trial, a 1,000 ms visual instruction informed the participants about the motor condition (“i,” “tongue,” “lip,” “jaw”) or the resting baseline (“pause”) and indicated the onset and offset of the movement or the vowel vocalization for the motor conditions. Participants were instructed to initiate each motor task as soon as they perceived the visual instruction and to maintain the production/movement until the visual cue disappeared.
Apart from articulatory movements, participants were instructed not to move during the whole experimental session to avoid head‐movement artifacts. They were trained a few days before the scanning session and all the motor tasks were practiced again just before entering into the scanner. No participant reported any difficulty performing the tasks.
Data Acquisition
Magnetic resonance images were acquired with a 3T whole‐body MRI scanner (Bruker Medspec S300). Participants laid supine in the scanner with head movements minimized with a standard birdcage head coil and foam cushions. Visual instructions were presented using Presentation software (Neurobehavioral Systems, Albany) and displayed on a screen situated behind the scanner and viewed on a mirror fixed above the subject's eyes.
A high‐resolution T1‐weighted whole‐brain structural image was first acquired for each participant (MP‐RAGE, sagittal volume of 256 × 224 × 176 mm3 with a 1 mm isotropic resolution, inversion time = 900 ms, two segments, segment repetition time = 2,500 ms, segment duration = 1,795 ms, TR/TE = 16/5 in ms with 35% partial echo, flip angle = 30°). Functional images were then obtained using a T2*‐weighted, echoplanar imaging (EPI) sequence with whole‐brain coverage (TR = 10 s, acquisition time = 2,600 ms, TE = 30 ms, flip angle = 90°). Each functional scan comprised forty axial slices parallel to the anteroposterior commisural plane acquired in interleaved order (72 × 72 matrix; field of view: 216 mm; 3 × 3 mm2 in plane resolution with a slice thickness of 3 mm without gap).
To avoid movement artifacts due to vowel vocalization and articulatory movements (Birn et al., 1999; Bohland and Guenther, 2006; Gracco et al., 2005; Hall et al., 1999; Sörös et al., 2006), and to minimize scanner noise, a “sparse sampling” acquisition paradigm was used (see Fig. 1). This acquisition technique is based on neurophysiological properties of the slowly rising hemodynamic response, which is estimated to occur with a 4–6 s delay in case of overt speech sequences or articulatory movements (Bohland and Guenther 2006; Gracco et al., 2005; Sörös et al., 2006). In this study, functional scanning therefore occurs only during a fraction of the TR, alternating with silent interscanning periods, where participants produced oral movements. For each TR, the time interval between the visual instruction onset and the midpoint of the following functional scan acquisition was varied between 4 s, 5 s or 6 s. The order of delay times was pseudorandomly counterbalanced within both runs and conditions (the same delay of acquisition never occurred twice in successive scans). In addition, each motor or resting condition occurred 18 times in a pseudorandomized order (the same condition never occurring twice in succession). Altogether, 108 functional scans were therefore acquired (4 + 2 conditions × 18 trials). Three “dummy” scans at the beginning of each run were added to allow for equilibration of the MRI signal and were removed from the analyses.
Data Analysis
Data were analyzed using the SPM5 software package (Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK) running on Matlab 7.1 (Mathworks, Natick, MA). Brain activated regions were labeled using the SPM Anatomy toolbox (Eickhoff et al., 2005) and, when necessary, using the Talairach Daemon software (Lancaster et al., 2000). Region‐of‐interest (ROI) analyses were performed using the SPM PickAtlas Toolbox (Maldjian et al., 2003). For visualization, activation maps were superimposed on a standard brain template using the MRICRON software (http://www.sph.sc.edu/comd/rorden/mricron/).
Data preprocessing
Before statistical analyses, the first three volumes of each run (‘dummy’ scans) were discarded and 108 volumes were used for the analyses. For each participant, the functional series were first realigned by estimating the six movement parameters of a rigid‐body transformation to control for head movements between scans. After segmentation of the T1 structural image and coregistration to the mean functional image, all functional images were spatially normalized into standard stereotaxic space of the Montreal neurological institute (MNI) using segmentation parameters of the T1 structural image. All functional images were then smoothed using a 6 mm full‐width at half maximum Gaussian kernel, to improve the signal‐to‐noise ratio and to compensate for the anatomical variability among individual brains.
Group analysis
For each participant, the neural correlates related to the motor tasks were analyzed using the general linear model (GLM; Friston et al., 1995). The GLM included regressors of interest related to the four conditions (vowel, lip, tongue, and jaw conditions) and realignment parameters, with the silent trials forming an implicit baseline. Each condition (regressors of interest and baseline) included 18 functional images. The blood‐oxygen‐level dependence (BOLD) response for each event was modeled using a single‐bin finite impulse response (FIR) basis function spanning the time of acquisition (2.6 s). Before estimation, a high‐pass filtering with a cutoff period of 128 s was applied. Beta weights associated with the modeled FIR responses were then computed to fit the observed BOLD signal time course in each voxel for each condition. Individual statistical maps were calculated for each condition contrasted with the baseline and subsequently used for group statistics. To draw population‐based inferences (Friston et al., 1999), a second‐level random effect group analysis was carried‐out. A one‐way repeated measures analysis of variance (ANOVA) was performed, with the “motor” condition as within‐subject factor with four levels (lips, tongue, jaw, vowel) while “subjects” variable was considered as a random factor. Due to the separation of the motor tasks into different runs, the level measurements were defined as having unequal variance. Covariance components were therefore estimated using restricted maximum likelihood and used to adjust the statistics and degrees of freedom during inferences.
Four t‐contrasts were calculated to determine brain regions specifically activated for each of the orofacial motor tasks (versus baseline). To identify overlapping activation for all motor conditions, a conjunction analysis (Friston et al., 1999; Nichols et al., 2005) was subsequently conducted. Finally, an F‐contrast was calculated to determine the main effect of the motor factor and to determine brain regions showing significant variation of the MR signal between the four motor tasks. All activations for the group analysis are reported at a family‐wise (FWE; Nichols and Hayasaka, 2003) corrected level of P < 0.05 and a cluster extent of at least 10 voxels (T > 5.91 for the t‐contrasts and F > 17.71 for the F‐contrast). The activation peaks were first determined in each cluster and then labeled according to probabilistic cytoarchitectonic maps (Eickhoff et al., 2005) as implemented in the SPM Anatomy toolbox. If a brain region was assigned with a probability lower than 50% or if it was not specified in the SPM Anatomy toolbox, the coordinates of the activation peak was converted from MNI space to the standard stereotactic space of Talairach and Tournoux (1988) and the related brain region determined using the Talairach Daemon software (Lancaster et al., 2000).
In addition, a second analysis was performed to determine motor activations related to the three delays of image acquisition and irrespective of the articulator (see Supporting Information).
Individual sensorimotor somatotopy analysis
Individual ROI analyses were carried out to test for somatotopic organization of motor activations in the primary motor and somatosensory cortices for each participant individually. Three specific cytoarchitectonic ROIs related to the primary motor and somatosensory cortices were first created using the SPM anatomy toolbox (Eickhoff et al., 2005; see Fig. 4a). The first ROI was related to the primary motor cortex (combined cytoarchitectonic maps of areas 4a and 4p; see Gayer et al., 1996). Given the anatomical locations of BA3 and BA1 (in the fundus of the central sulcus and on the crown of the postcentral gyrus, respectively; see Geyer et al., 1999), two distinct ROIs related to the primary somatosensory cortex were created (combined cytoarchitectonic maps of areas BA3a and BA3b and cytoarchitectonic map of area BA1). ROI analyses were then performed using the SPM Anatomy toolbox for each participant and each task, with small volume correction applied on each ROI at a threshold of P = 0.0001 uncorrected. MNI coordinates of the maximum activation peak and the centre of gravity (COG) within the primary motor and somatosensory cortices, (areas BA4, BA3 and BA1) cortex were determined for both hemispheres. Two subjects were removed from these analyses because no activations were observed in the three ROIs and in both hemispheres at a threshold of P = 0.0001 uncorrected. For each ROI, two‐way repeated measures ANOVAs with the hemisphere (left, right) and the articulator (lips, jaw, larynx, tongue) as within‐subjects factors were performed on x, y, and z MNI coordinates of activation peaks (for x coordinates, absolute values were used). For all analyses, the significance level was set at P = 0.05 and Greenhouse‐Geisser corrected when appropriate. When required, post‐hoc analyses were conducted with Fisher's protected LSD tests.
Figure 4.
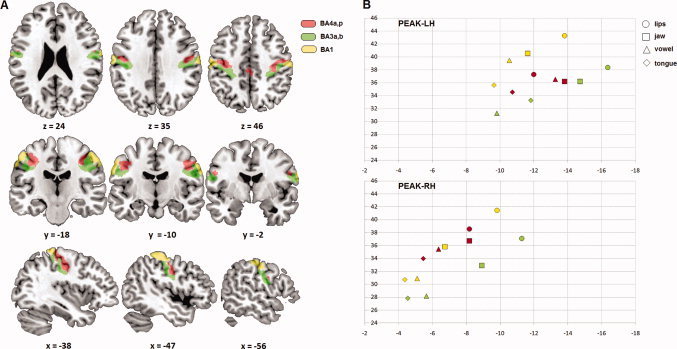
Individual sensorimotor somatotopy (ROI analysis). (A) ROIs related to BA4a,p (red), BA3a,b (green), and BA1 (yellow). (B) Mean y‐ and z‐axis values of activation peaks in MNI coordinates and their projections for the four articulators. Regarding a somatotopic organization, a more posterior position emerged in the caudo‐rostral (y) dimension for lips compared with tongue as well as a more dorsal position in the dorso‐ventral (z) dimension for lips compared with tongue and vowel and for jaw compared with tongue. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
RESULTS
Basic Articulatory Network
Results from the group analysis showed regions that were largely overlapping across the four motor conditions. The conjunction analysis revealed a bilateral set of common brain areas classically involved in orofacial motor control. Table I summarizes activations for each of the four motor conditions (compared with baseline), as well as activations provided by the conjunction analysis. Activation maps are illustrated in Figure 2.
Table I.
Activation peak summary observed in each motor task (versus baseline) and in the conjunction analysis (random‐effect group analysis, P < .05, FWE corrected, cluster extent threshold of 10 voxels, T > 5.91)
| Tongue | Vowel | Jaw | Lips | Conjunction | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | H | BA | x | y | z | T | BA | x | y | z | T | BA | x | y | z | T | BA | x | y | z | T | BA | x | y | z | T |
| Primary motor cortex | ||||||||||||||||||||||||||
| L | 4 | −20 | −28 | 58 | 7.48 | 4 | −60 | −4 | 18 | 15.49 | 4 | −48 | −12 | 34 | 10.53 | 4 | −48 | −12 | 36 | 8.57 | 4 | −48 | −12 | 36 | 8.57 | |
| R | 4 | 56 | −4 | 30 | 19.73 | 4 | 52 | −6 | 32 | 14.67 | 4 | 54 | −6 | 32 | 11.59 | 4 | 40 | −12 | 46 | 7.95 | ||||||
| Primary somatosensory cortex | ||||||||||||||||||||||||||
| L | 1,2,3 | −52 | −8 | 32 | 21.95 | 1,2,3 | −52 | −8 | 32 | 15.70 | 1,2,3 | −52 | −8 | 32 | 11.33 | 1,2,3 | −54 | −16 | 42 | 9.97 | 1,2,3 | −50 | −16 | 40 | 9.31 | |
| R | 1,2,3 | 44 | −8 | 30 | 13.31 | 1,2,3 | 60 | −10 | 34 | 8.30 | ||||||||||||||||
| Frontal regions | ||||||||||||||||||||||||||
| Superior frontal gyrus | L | 6 | −18 | −4 | 68 | 6.02 | 6 | −22 | −2 | 66 | 7.23 | |||||||||||||||
| Supplementary motor area | L | 6 | 0 | −6 | 58 | 20.58 | 6 | −4 | −4 | 62 | 8.93 | 6 | −10 | −2 | 70 | 6.60 | 6 | −4 | −4 | 62 | 8.93 | |||||
| R | 6 | 8 | −4 | 60 | 8.18 | 6 | 2 | −6 | 58 | 13.78 | 6 | 2 | −6 | 58 | 11.81 | |||||||||||
| Premotor cortex | L | 6 | −62 | 0 | 26 | 15.50 | 6 | −60 | −4 | 36 | 11.21 | 6 | −44 | −12 | 60 | 8.97 | 6 | −44 | −12 | 60 | 9.04 | 6 | −56 | −4 | 40 | 7.56 |
| R | 6 | 54 | −6 | 38 | 15.55 | 6 | 54 | −6 | 38 | 11.99 | 6 | 54 | −6 | 40 | 12.23 | 6 | 54 | −6 | 40 | 11.98 | ||||||
| Inferior frontal gyrus/ | L | 44/9 | −58 | 8 | 30 | 14.76 | 44/9 | −58 | 8 | 30 | 8.22 | |||||||||||||||
| Prefrontal gyrus | R | 44/9 | 58 | 8 | 28 | 16.33 | 44 | 58 | 8 | 28 | 8.07 | 44/9 | 58 | 8 | 30 | 8.20 | 44/9 | 58 | 6 | 30 | 7.36 | |||||
| Temporal regions | ||||||||||||||||||||||||||
| Transverse temporal gyrus | L | 41/42 | −42 | −24 | 8 | 13.43 | 41/42 | −40 | −26 | 12 | 6.71 | |||||||||||||||
| R | 41/42 | 50 | −16 | 6 | 12.30 | |||||||||||||||||||||
| Superior temporal gyrus | R | 22 | 52 | −20 | 0 | 10.71 | ||||||||||||||||||||
| Parietal regions | ||||||||||||||||||||||||||
| Supramarginal gyrus | L | 40 | −38 | −44 | 54 | 9.24 | 40 | −62 | −30 | 34 | 7.52 | 40 | −48 | −26 | 38 | 9.17 | ||||||||||
| R | 40 | 58 | −26 | 24 | 8.18 | 40 | 64 | −26 | 22 | 8.50 | ||||||||||||||||
| Parietal operculum/ | L | 43/40 | −46 | −28 | 20 | 10.24 | 43/40 | −48 | −28 | 20 | 8.80 | 43/40 | −48 | −28 | 20 | 8.80 | ||||||||||
| Supramarginal gyrus | R | 43/40 | 52 | −28 | 20 | 7.31 | ||||||||||||||||||||
| Parietal operculum | L | 43 | −62 | −6 | 18 | 16.84 | 43 | −58 | −6 | 10 | 13.98 | 43 | −62 | −6 | 18 | 9.33 | ||||||||||
| R | 43 | 60 | −4 | 6 | 10.36 | 43 | 60 | −16 | 22 | 7.84 | ||||||||||||||||
| Superior parietal lobule | L | 7 | −20 | −52 | 64 | 7.50 | 7 | −20 | −52 | 64 | 6.73 | |||||||||||||||
| R | 7 | 14 | −64 | 56 | 7.84 | |||||||||||||||||||||
| Subcortical regions | ||||||||||||||||||||||||||
| Insula/parietal operculum | R | 13 | 36 | 16 | 8 | 7.01 | 13 | 46 | 8 | 2 | 7.06 | 43/13 | −38 | −12 | 18 | 6.96 | 43/13 | −38 | −14 | 20 | 6.86 | |||||
| Cingulate cortex | L | 32 | 10 | 14 | 34 | 7.14 | 24 | −6 | 8 | 38 | 7.72 | 24 | −6 | 8 | 38 | 8.23 | ||||||||||
| Amygdala | R | 26 | −2 | −8 | 8.37 | |||||||||||||||||||||
| Putamen | L | −26 | −2 | −4 | 13.69 | −26 | −8 | −6 | 10.60 | −26 | −2 | −4 | 7.28 | −26 | −2 | −4 | 7.28 | |||||||||
| R | 24 | 8 | 6 | 13.56 | 24 | −4 | −2 | 6.84 | 26 | 6 | −6 | 8.00 | ||||||||||||||
| Pallidum | L | −24 | 0 | −4 | 8.69 | |||||||||||||||||||||
| R | 18 | −8 | −4 | 9.04 | 22 | −10 | −4 | 8.05 | ||||||||||||||||||
| Caudate nucleus | R | 18 | 4 | 20 | 7.41 | |||||||||||||||||||||
| Claustrum | R | 32 | −8 | 12 | 7.81 | |||||||||||||||||||||
| Thalamus | L | −14 | −20 | 0 | 13.37 | −14 | −20 | 2 | 9.35 | |||||||||||||||||
| R | 16 | −20 | −2 | 8.21 | 14 | −18 | 4 | 9.51 | 16 | −20 | −2 | 6.97 | ||||||||||||||
| Cerebellum | ||||||||||||||||||||||||||
| Cerebellum (declive) | L | −16 | −62 | −20 | 14.76 | −12 | −64 | −16 | 12.95 | −16 | −62 | −18 | 8.56 | −16 | −62 | −18 | 6.93 | −16 | −62 | −18 | 6.93 | |||||
| R | 18 | −60 | −20 | 13.75 | 20 | −60 | −20 | 12.28 | 20 | −60 | −20 | 7.96 | 18 | −58 | −20 | 7.13 | 18 | −58 | −20 | 7.13 | ||||||
| Cerebellum (culmen) | R | 10 | −58 | −14 | 8.66 | |||||||||||||||||||||
| Occipital regions | ||||||||||||||||||||||||||
| Calcarine gyrus | 18 | −62 | 10 | 8.66 | ||||||||||||||||||||||
Figure 2.
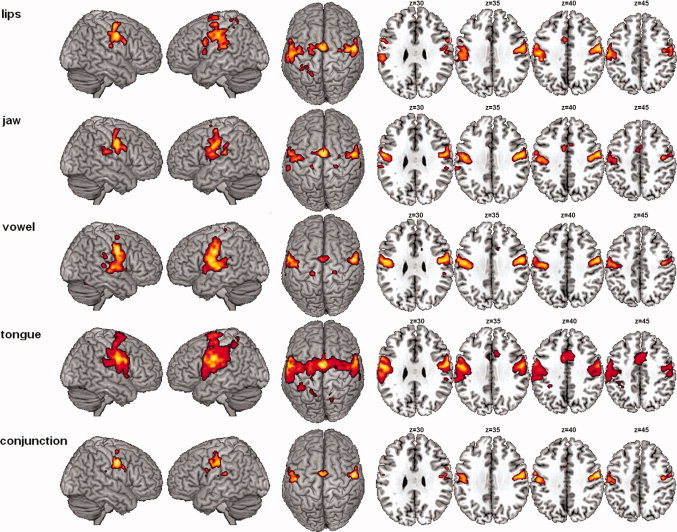
Cerebral networks of lip, jaw, vocalic/laryngeal and tongue movements, and conjunction map (random‐effect group analysis, t‐contrasts, P < 0.05, FWE corrected, cluster extent threshold of 10 voxels, T > 5.91). Significant activations are rendered on cortical surfaces (left) and on axial slices covering the orofacial motor cortex (right—z values refer to planes in MNI space). The conjunction analysis revealed a bilateral set of largely overlapping brain areas classically involved in motor control: the sensorimotor and premotor cortices, bilaterally, the right inferior frontal gyrus, the supplementary motor area, the left parietal operculum and the adjacent inferior parietal lobule, the basal ganglia and the cerebellum. Due to spatial overlapping, no clear sensorimotor somatotopy of the four articulators was observed at the group level. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The minimal network for supralaryngeal and laryngeal movements (conjunction analysis; Table I and Fig. 2) concerned, bilaterally, the activation of the central sulcus extending rostrally onto the precentral gyrus and caudally onto the postcentral gyrus. Two large bilateral clusters of activations enclosed the superior portion of the ventral premotor, motor, and somatosensory cortices. In addition, two smaller bilateral clusters of activations of the precentral gyrus were also observed in the dorsal premotor cortex. Moreover, bilateral activations were found in the supplementary motor area and the superior cerebellar hemispheres (declive region of neocerebellum). Additional frontal activation was observed at the border of the right inferior frontal and middle frontal gyri. Left activations were also observed around the ventral part of the postcentral sulcus, extending rostrally onto the parietal operculum, as well as in the supramarginal gyrus, and in the dorsal striatum of basal ganglia (putamen).
Main Effect of Motor Tasks
The main effect of motor tasks (Table II and Fig. 3) revealed significant activity differences in the ventral precentral and postcentral gyri (with two activation peaks located in the ventrolateral primary motor and somatosensory cortices) and in the bilateral primary auditory cortex (the anterior and posterior parts of the transverse temporal gyrus) bilaterally. All these regions showed stronger activation for vowel vocalization compared with the other tasks. As previously mentioned, due to the separation of the motor tasks into different runs, these results have to be interpreted with caution. Nevertheless, our results are quite coherent with those reported in a previous study (Sörös et al., 2006), with stronger activity observed in the auditory cortex for the vowel condition compared with orofacial movements.
Table II.
Activation peak summary observed in the main effect analysis (random‐effect group analysis, P < .05, FWE corrected, cluster extent threshold of 10 voxels, F > 17.71). Apart from the bilateral superior temporal gyri for vowel production, the only significant difference between the four motor tasks was observed in the left ventro‐lateral sensorimotor cortex, with stronger activity for vowel vocalization than for the other tasks
| Region | H | Main effect | ||||
|---|---|---|---|---|---|---|
| BA | x | y | z | F | ||
| Primary motor cortex | L | 4 | −60 | −4 | 18 | 26.44 |
| Primary somatosensory cortex | L | 3 | −52 | −8 | 32 | 25.66 |
| Transverse temporal gyrus | L | 41/42 | −52 | −16 | 4 | 24.55 |
| R | 41/42 | 44 | −22 | 4 | 20.72 | |
Figure 3.
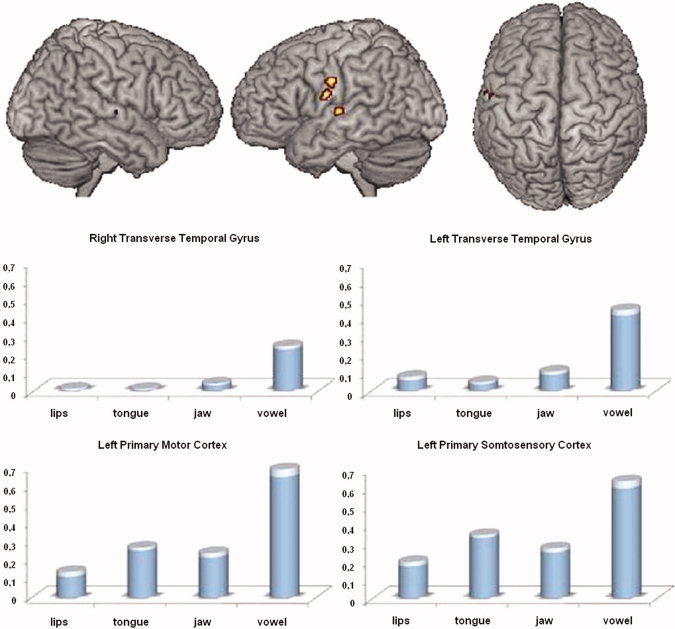
Main effect of motor task (random‐effect group analysis, F‐contrast, P < 0.05, FWE corrected, cluster extent threshold of 10 voxels, F > 17.71): brain regions showing activity differences between the four motor tasks (top) and contrast estimates reflecting percentage BOLD signal change from baseline for the observed activation peaks and the four motor tasks (bottom). Compared with the other tasks, stronger activations were observed in the left ventrolateral sensorimotor cortex and the bilateral primary auditory cortex for vowel vocalization. SEM are indicated in transparency. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Individual Articulatory Somatotopy
As no clear sensorimotor somatotopy for the four articulators emerged from the group analysis, individual ROI analyses within the primary motor and somatosensory cortices (areas 4a/p, 3a/b, 1; Geyer et al., 1996, 1999) were carried out to further test at the individual level a sensorimotor somatotopic organization of supralaryngeal and laryngeal activations. To this aim, MNI coordinates in the medio‐lateral (x), caudo‐rostral (y), and dorso‐ventral (z) dimensions of the maximum activation peaks within areas BA4, BA3, and BA1 were determined in both hemispheres for each condition (see Tables III and IV and Fig. 4).
Table III.
Mean y‐ and z‐axis values of COGs and activation peaks in MNI coordinates observed in the ROI analyses
| Sensorimotor ROIs | Left hemisphere | Right hemisphere | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lips | Jaw | Vowel | Tongue | Lips | Jaw | Vowel | Tongue | |||||||||||||||||
| x | y | z | x | y | z | x | y | z | x | y | z | x | y | z | x | y | z | x | y | z | x | y | z | |
| Activation Peak | ||||||||||||||||||||||||
| BA 4a,p | −46 | −12 | 37 | −43 | −14 | 36 | −43 | −13 | 37 | −47 | −11 | 35 | 51 | −8 | 39 | 48 | −8 | 37 | 51 | −6 | 35 | 51 | −5 | 34 |
| BA 3a,b | −46 | −16 | 38 | −46 | −15 | 36 | −50 | −10 | 31 | −50 | −12 | 33 | 48 | −11 | 37 | 51 | −9 | 33 | 55 | −6 | 28 | 56 | −5 | 28 |
| BA 1 | −52 | −14 | 43 | −52 | −12 | 41 | −53 | −11 | 39 | −57 | −9 | 38 | 54 | −10 | 41 | 57 | −7 | 36 | 59 | −5 | 31 | 60 | −4 | 31 |
| Mean coordinates | −48 | −14 | 40 | −47 | −13 | 38 | −49 | −11 | 36 | −51 | −11 | 34 | 51 | −10 | 39 | 52 | −8 | 35 | 55 | −6 | 32 | 55 | −5 | 31 |
| Center of gravity (COG) | ||||||||||||||||||||||||
| BA 4a,p | −44 | −11 | 39 | −43 | −12 | 39 | −42 | −12 | 39 | −45 | −10 | 36 | 47 | −8 | 38 | 47 | −8 | 37 | 49 | −7 | 36 | 49 | −6 | 34 |
| BA 3a,b | −45 | −16 | 39 | −47 | −14 | 36 | −50 | −10 | 31 | −49 | −11 | 33 | 48 | −12 | 36 | 51 | −9 | 33 | 55 | −6 | 28 | 54 | −7 | 28 |
| BA 1 | −54 | −13 | 44 | −54 | −11 | 42 | −55 | −11 | 40 | −57 | −9 | 38 | 55 | −11 | 41 | 58 | −7 | 35 | 60 | −6 | 31 | 60 | −7 | 33 |
| Mean coordinates | −48 | −14 | 40 | −48 | −12 | 39 | −49 | −11 | 37 | −50 | −10 | 36 | 50 | −10 | 38 | 52 | −8 | 35 | 55 | −6 | 32 | 54 | −7 | 32 |
Table IV.
Review of main activation peaks observed in the sensorimotor motor cortex during simple articulatory gestures in previous fMRI studies. x,y,z coordinates are reported in MNI space
| > | Left hemisphere | Right hemisphere | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lips | Jaw | Larynx | Tongue | Lips | Jaw | Larynx | Tongue | |||||||||||||||||
| Reference | x | y | z | x | y | z | x | y | z | x | y | z | x | y | z | x | y | z | x | y | z | x | y | z |
| Corfield et al. (1999)*—horizontal tongue movement | −63 | −7 | 24 | 64 | −5 | 19 | ||||||||||||||||||
| Lotze et al. (2000a)*—lip pursing, vertical tongue movement | −53 | −18 | 40 | −53 | 0 | 35 | 55 | −8 | 41 | 67 | −3 | 26 | ||||||||||||
| Lotze et al. (2000b)*—lip pursing | −59 | −16 | 43 | 53 | −10 | 39 | ||||||||||||||||||
| Riecker et al. (2000)—horizontal tongue movement | −63 | −3 | 30 | 57 | −3 | 24 | ||||||||||||||||||
| Stippich et al. (2002)*—vertical tongue movement | −54 | −12 | 34 | 53 | −8 | 34 | ||||||||||||||||||
| Fesl et al. (2003)*—horizontal tongue movement | −63 | −8 | 37 | 67 | −8 | 32 | ||||||||||||||||||
| Gerardin et al. (2003)*—lip pursing | −58 | −15 | 45 | 61 | −9 | 52 | ||||||||||||||||||
| Shinagawa et al. (2003)*—tongue protrusion | −48 | −14 | 34 | 51 | −8 | 37 | ||||||||||||||||||
| Shinagawa et al. (2003)*—right tongue movement | −48 | −14 | 36 | 51 | −8 | 37 | ||||||||||||||||||
| Shinagawa et al. (2003)*—left tongue movement | −48 | −14 | 38 | 59 | −1 | 24 | ||||||||||||||||||
| Hesselmann et al. (2004)*—lip pursing, horizontal tongue movement | −41 | −21 | 44 | −55 | −12 | 27 | 53 | −21 | 49 | 58 | −13 | 30 | ||||||||||||
| Hanakawa et al. (2005)*—unilateral lip stretching | −54 | −8 | 41 | 57 | −3 | 40 | ||||||||||||||||||
| Pulvermüller et al. (2006)—up/down tongue or lip movement | −48 | −10 | 36 | −56 | −8 | 28 | ||||||||||||||||||
| Terumitsu et al. (2006)—tongue movements and vowel phonation | −40 | −19 | 42 | −55 | −8 | 32 | 51 | −11 | 38 | 59 | −6 | 30 | ||||||||||||
| −56 | −3 | 21 | ||||||||||||||||||||||
| Vincent et al. (2006)*—horizontal/vertical tongue movement | 61 | −1 | 26 | |||||||||||||||||||||
| Brown et al. (2008)*—lip protrusion and vertical tongue movement | −52 | −14 | 36 | −64 | −12 | 30 | 58 | −12 | 34 | 58 | −14 | 34 | ||||||||||||
| Brown et al. (2008)*—glottal stops | −38 | −16 | 34 | 44 | −12 | 36 | ||||||||||||||||||
| Brown et al. (2008)*—vowel phonation | −40 | −12 | 32 | 44 | −10 | 36 | ||||||||||||||||||
| Stippich et al. (2008)*—vertical tongue movement | −54 | −16 | 41 | 54 | −11 | 39 | ||||||||||||||||||
| Mean coordinates | −52 | −15 | 41 | −44 | −13 | 32 | −55 | −10 | 34 | 56 | −11 | 43 | 46 | −11 | 37 | 58 | −7 | 31 | ||||||
(*)If originally reported in Talairach space, coordinates were converted in MNI space using the Talairach Daemon software (Lancaster et al., 2000) and a nonlinear transformation originally described by Matthew Brett (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html).
For all sensorimotor ROIs, almost all analyses showed a significant effect of the hemisphere, with a more medial, posterior and dorsal position in the left hemisphere compared with the right hemisphere. Regarding a somatotopic organization, a more posterior position significantly emerged in the caudo‐rostral (y) dimension for lips compared with tongue as well as a more dorsal position in the dorso‐ventral (z) dimension for lips compared with tongue and vowel and for jaw compared with tongue.
Medio‐Lateral (x) dimension
BA4: Significant effects of the hemisphere and the articulator were observed [F(1,10) = 24.7; P < 0.001; F(3,30) = 5.1; P < 0.006], with a more medial position in the left than in the right hemisphere (45 vs. 50 mm) and a more lateral position for lips and tongue compared with vowel and jaw (48, 49, 46, 47 mm, respectively). The interaction was not significant [F(3,30) = 2.1].
BA3: Significant effects of the hemisphere and the articulator were observed [F(1,10) = 20.1; P < 0.001; F(3,30) = 8.7; P < 0.001], with a more medial position in the left than in the right hemisphere (48 vs. 53 mm) and a more lateral position for vowel and tongue compared with lips and jaw (53, 53, 48, 48 mm, respectively). The interaction was not significant (F(3,30) = 0.6).
BA1: Significant effects of the hemisphere and the articulator were observed [F(1,10) = 21.9; P < 0.001; F(3,30) = 3.8; P < 0.02], with a more medial position in the left than in the right hemisphere (53 vs. 57 mm) and a more lateral position for tongue compared with lips and jaw (58, 53, 54 mm, respectively). The interaction was not significant [F(3,30) = 1.2].
Caudo‐rostral (y) dimension
BA4: Significant effects of the hemisphere and the articulator were observed [F(1,10) = 31.4; P < 0.001; F(3,30) = 6.6; P < 0.001] with a more posterior position in the left than in the right hemisphere (−12 vs. −7 mm) and a more anterior position for tongue compared with lips, jaw and vowel (−8, −10, −11, −10 mm, respectively). The interaction was not significant [F(3,30) = 1.9].
BA3: Significant effects of the hemisphere and the articulator were observed [F(1,10) = 45.9; P < 0.001; F(3,30) = 12.9; P < 0.001], with a more posterior position in the left than in the right hemisphere (−13 vs. −8 mm) and a more anterior position for vowel and tongue compared with lips and jaw (−8, −8, −14, −12 mm, respectively). The interaction was not significant [F(3,30) = 0.9].
BA1: Significant effects of the hemisphere and the articulator were observed [F(1,10) = 19.4; P < 0.001; F(3,30) = 4.7; P < 0.008], with a more posterior position in the left than in the right hemisphere (−11 vs. −6 mm) and a more anterior position for tongue and vowel compared with lips (−8, −7, −12 mm, respectively). The interaction was not significant [F(3,30) = 0.2].
Dorso‐ventral (z) dimension
BA4: A significant effect of the articulator was observed [F(3,30) = 7.0; P < 0.001], with a more dorsal position for lips, jaw, and vowel compared with tongue (38, 36, 36, 34 mm, respectively) as well as a more dorsal position for lips compared with vowel. The interaction was not significant [F(3,30) = 1.2]. The effect of the hemisphere [F(1,10) = 0.1] and the interaction [F(3,30) = 0.8] were not significant.
BA3: Significant effects of the hemisphere and the articulator were observed [F(1,10) = 5.3; P < 0.05; y: F(3,30) = 11.1; P < 0.001], with a more dorsal position in the left than in the right hemisphere (35 vs.31 mm) and a more dorsal position for lips and jaw compared with vowel and tongue (38, 35, 30, 31 mm, respectively). The interaction was not significant [F(3,30) = 0.5].
BA1: Significant effects of the hemisphere and the articulator were observed [F(1,10) = 10.2; P < 0.009; y: F(3,30) = 7.5; P < 0.001], with a more dorsal position for lips compared with vowel and tongue (42, 35, 33 mm, respectively) as well as a more dorsal position for jaw compared with tongue (35, 33 mm, respectively). The interaction was not significant [F(3,30) = 1.1].
DISCUSSION
Using sparse temporal acquisition, the goal of this fMRI study was to clarify the neural networks underlying motor control of simple supralaryngeal and laryngeal movements and to refine the sensorimotor somatotopic organization of lip, jaw, vocalic/laryngeal, and tongue motor representations. Three main findings were observed. First, orofacial movements activated a set of largely overlapping, common brain areas forming a core neural network involved in orofacial motor control. Second, apart from the auditory temporal cortex for vowel production, differences between the motor tasks were restricted to ventrolateral regions of the primary motor and somatosensory cortices, with greater signal intensity for vowel vocalization likely reflecting more complex neuromuscular coordination and sensory feedback processing. Finally, using individual ROI analyses, a sequential dorso‐ventral somatotopic organization of lip, jaw, vocalic/laryngeal, and tongue movements was observed in the primary motor and somatosensory cortices.
Before discussing these results, it is important to highlight an important limitation of this study. As previously noted, although the vowel vocalization task was performed to determine laryngeal motor activity, it also requires muscle activity of the tongue and lips. In addition, jaw movements likely induce tongue movements as well given the anatomical connection of the jaw and the tongue. Hence, although this study confirms “an overall picture of somatotopy with overlap” (Takai et al., 2010), a mutual contribution of orofacial articulators during vowel productions and jaw movements cannot be ruled out.
Basic Articulatory Network
The basic neural network for supralaryngeal movements and vowel vocalization (see Table I and Fig. 2) revealed strong bilateral activations within the sensorimotor cortex (including the primary motor, ventral and dorsal premotor, and somatosensory cortices), the supplementary motor area and the superior cerebellar hemispheres. Activations were also found in the right posterior inferior frontal gyrus (pars opercularis), the left parietal operculum and the adjacent inferior parietal lobule, and the left dorsal striatum of basal ganglia. The observed basic neural network for orofacial motor actions is fully consistent with previous fMRI studies on orofacial motor control. Notably, common neural activity for jaw and tongue movements (Dhanjal et al., 2008) were observed in the medial and lateral prefrontal cortex including the ventral lateral premotor cortex, the frontal operculum ventral to the central sulcus and the adjacent insular cortex, the primary motor and somatosensory cortices, the supplementary motor area and the cerebellum (lobule VI). Lotze et al. (2000a) have previously reported these findings for simple tongue and lip movements (although, surprisingly, without significant activation observed in the supplementary motor area).
Focusing on orofacial coordinated actions, our results also fit well with studies dealing with swallowing and breathing (Loucks et al., 2007; Sawczuk and Mosier, 2001), vocalization (Jürgens 2002, 2009) and overt speech production (Bohland and Guenther, 2006; Brown et al., 2005; Guenther, Ghosh and Tourville 2006; Riecker et al., 2000, 2005, 2008; Sörös et al., 2006). First, the neural control of swallowing and breathing has been shown to partly rely on an overlapping neural network, including bilateral activation of the primary sensorimotor cortices, the supplementary motor area, the thalamus, the cerebellum, the caudate nucleus, the globus pallidum, and the medulla (Sawczuk and Mosier, 2001; see also McKay et al., 2003). In line with our results, a number of speech production studies have led to the suggestion of a “minimal network for overt speech production” (Bohland and Guenther, 2006), including mesiofrontal structures (supplementary motor area and anterior cingulate gyrus), bilateral pre‐ and postcentral convolutions, extending rostrally into posterior parts of the inferior frontal gyrus, the left anterior insula as well as bilateral components of the basal ganglia (notably the putamen and the globus pallidus), the cerebellum (notably the lobule VI, including the declive), the thalamus and the superior temporal gyrus (Bohland and Guenther, 2006; Brown et al., 2005; Guenther et al., 2006; Riecker et al., 2008; Sörös et al., 2006). Finally, Chang et al. (2009) showed common activations during speech and non‐speech vocal tract gestures in the posterior inferior frontal gyrus, the ventral premotor cortex, the supplementary motor area, the superior temporal gyrus, the insula, the supramarginal gyrus, the cerebellum and the basal ganglia, a result suggesting a more general role for these regions than just speech production. Finally, this basic neural network is also consistent with that observed during wordless singing (for a review, Brown et al., 2005) and whistling (Dresel et al., 2005), despite differences in the amygdala, not reported for singing, and in the insula, not reported for whistling.
As the basic neural network for supralaryngeal movements and vowel vocalization comprises a similar set of brain areas, our results refine the core neural network involved in speech and non‐speech orofacial motor control. This network encompasses the ventral sensorimotor and ventral/dorsal premotor cortices, bilaterally, the right posterior inferior frontal gyrus (pars opercularis), the supplementary motor area, the left parietal operculum and the adjacent inferior parietal lobule, the basal ganglia and the cerebellum. Although specific functions in the motor‐coordinating neural network of these cortical and subcortical brain regions remain largely discussed, they can be broadly assigned to motor preparation, execution and regulation loops (for reviews, Jürgens, 2002; Riecker et al., 2008). Initiation and suppression of voluntary movements is traditionally assigned to the supplementary motor area (Pickard and Strick, 2001) while fine‐grained motor control of orofacial gestures is carried out by the primary motor cortex and basal ganglia via the pyramidal and extrapyramidal pathways (Jürgens, 2002; Riecker et al.; 2005; Wise et al., 1999). The motor cortex receives motor plans from the premotor cortex (Pickard and Strick, 2001), and the adjacent posterior inferior frontal gyrus as well as proprioceptive inputs from primary and associative somatosensory areas and the inferior parietal cortex (Riecker et al., 2005; Smith, 1998). Importantly, although some previous studies on speech and lip, tongue and or jaw motor control failed to observe activation in the inferior frontal gyrus and/or the inferior parietal cortex (Corfield et al., 1997; Dhanjal et al., 2008; Lotze et al., 2000a; Murphy et al., 1997; Nota and Honda, 2004; Wise et al., 1999), the present results and that of Chang et al. (2009) support these regions as part of the core neural network involved in orofacial motor control. Finally, the basal ganglia play an important role not only in the selection but also in the regulation of motor commands via thalamo‐motor projections (Houk et al., 2007). In parallel, the cerebellum receives motor and sensory information and is involved in fine muscular coordination of the intended movement (Houk et al., 2007; Thach, 1998).
Main Effect of Motor Tasks
In this study, the neural networks involved in simple orofacial movements strongly overlap and share similar cortical and subcortical brain areas. Apart from the bilateral superior temporal gyri for vowel production, the only significant difference between the four motor tasks was observed in the left ventrolateral sensorimotor cortex, with stronger activity for vowel vocalization than for the other tasks (see Table II and Fig. 3).
Due to the separation of the supralaryngeal and vocalic/laryngeal motor tasks into different runs, this result has to be interpreted with caution. It is tempting to hypothesize that these regions might be related specifically to laryngeal movements during vowel production. In that case, the closely located activation peaks observed in the individual ROI analyses for vowel vocalization and tongue movement (see below, section “articulatory somatotopy”), slightly dorsal than the present activations, would likely reflect the contribution of tongue muscles during vocalization, rather than pure laryngeal activity. On the other hand, these two activation peaks in the left ventrolateral primary motor and somatosensory cortices were also found to be activated during jaw and tongue movement (see Fig. 2), albeit at a lower extent. Although tongue retraction movements also induce prominent sensory feedback, an alternative interpretation is that this stronger sensorimotor activity would be due to more complex sensorimotor interactions required for steady‐state vowel vocalization. To accurately reach and maintain the precise target speech sound, the left ventrolateral primary motor and somatosensory cortices would be strongly engaged in online motor control and registration of orosensory and auditory feedback (Guenther, 2006; Guenther and Bohland, 2006; Guenther and Vladusich, in press).
Articulatory Somatotopy
Due to significant activation overlap between the four articulators, no clear sensorimotor somatotopy was observed at the group level. To better take into account possible individual anatomical and functional differences across participants, individual ROI analyses on the bilateral primary motor and somatosensory cortices were carried out to evaluate individual somatotopic organization of supralaryngeal and laryngeal articulators.
Overall, the observed coordinates for lip, jaw, tongue, and vocalic/laryngeal representations in both hemispheres and the three sensorimotor ROIs (averaged across participants) fit very well with those observed in previous fMRI studies of simple articulatory gestures (see mean coordinates in Tables III and IV). For all sensorimotor ROIs, a more posterior position emerged in the caudo‐rostral (y) dimension for lips compared with tongue as well as a more dorsal position in the dorso‐ventral (z) dimension for lips compared with tongue and vowel and for jaw compared with tongue. The main finding from these ROI analyses is therefore a dorso‐ventral somatotopic organization of lips, vowel, and tongue, respectively, with the sensorimotor representations of lip movements occupying the most dorso‐caudal position and that of vocalic/laryngeal and tongue movements the most ventrorostral positions, the jaw position being dorsal to the tongue position. These results appear consistent with the well‐established more dorsal position for the lip area than for the tongue area in the human sensory and motor homunculi, as shown by previous electrocortical stimulation and fMRI studies (Hesselmann et al., 2004; Lotze et al., 2000a; Penfield and Rasmussen, 1950; Pulvermüller et al., 2006). In agreement with earlier electrocortical mapping by Penfield and Rasmussen (1950), we also observed the jaw area located a little more ventral than the lip area and more dorsal than the larynx and tongue areas (although no significant differences were observed between lips and jaw positions and the only significant differences between jaw and vowel was observed in BA3). A previous fMRI study also showed a little more medial and dorsal position in the sensorimotor cortex for jaw (opening/closing movement) compared with that for the tongue (vertical movement), but unfortunately did not report precise activation peaks separately for the two articulators (Dhanjal et al., 2008). Finally, the motor location of the vocalic/laryngeal movement was found to be located more dorsally than the tongue position. Brown et al. (2008) reported similar activation peaks within the left motor cortex for both nonvocal and vocal laryngeal movements (vocal‐fold abduction and vowel vocalization) and located between those observed for lip and tongue movements. However, they report a more dorsal position for the larynx area than for both tongue and lip areas in the right hemisphere. We note however that in their study the right lip and tongue activation peak in the primary cortex are quite exactly the same (see Tables III and IV), a result which does not correspond to previous studies showing the lip motor area above the tongue area in both hemispheres (Hesselmann et al., 2004; Lotze et al., 2000a, see Tables III and IV). Another study using both vowel vocalization and tongue movement also observed a more dorsal position in both hemispheres for the larynx area than for the tongue area (Terumitsu et al., 2006), although another activation peak was found in the left primary motor cortex more ventral than the tongue area. Altogether, the somatotopy of lip, jaw, vocalic/laryngeal, and tongue movements displayed in this study could be of importance for both better specification of speech production models and their brain correlates (Guenther et al., 2006) and for future brain‐imaging studies investigating speech motor control and disorders as well as cortical reorganization following injury.
Finally, irrespective of the articulators, results also showed a significant hemispheric asymmetry of sensorimotor representations, with activation sites globally more medial, posterior and dorsal in the left than in the right hemisphere. A review of fMRI studies of simple lip, tongue, and larynx movements show a similar hemispheric sensorimotor asymmetry for all articulators, with a more medial and posterior position of the activation site in the left hemisphere (see mean coordinates in Tables III and IV). This result might therefore suggest some anatomical and/or functional asymmetry of the primary sensorimotor cortex (Sowman et al., 2009) irrespective of participants' handedness or gender (Amunts et al., 2000).
CONCLUSION
At the group level, a core functional motor network controlling laryngeal and supralaryngeal movements was observed, including the sensorimotor and premotor cortices, bilaterally, the right inferior frontal gyrus, the supplementary motor area, the left parietal operculum and the adjacent inferior parietal lobule, the basal ganglia, and the cerebellum. Apart from the auditory temporal cortex for vowel production, activity differences across motor tasks were found in the left ventrolateral sensorimotor cortex with greater signal intensity for vowel vocalization. Finally, although no clear sensorimotor somatotopy of the four articulators emerged at the group level due to large spatial overlapping, a sequential dorso‐ventral somatotopic organization of lips, jaw, vocalic/laryngeal, and tongue movements was observed using individual ROI analyses within the primary sensory and motor cortices. The observed core neural network of orofacial movements and their sequential dorso‐ventral somatotopic organization may be of particular interest for future brain‐imaging studies investigating speech motor control and disorders as well as cortical reorganization following injury.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Supporting Information Figure 1
Supporting Information Table 1
Acknowledgements
The authors thank Vincent Gracco and two Reviewers for their insightful and constructive comments as well as all participants of the study.
Contributor Information
Krystyna Grabski, Email: krystyna.grabski@gipsa-lab.inpg.fr or marc.sato@gipsa-lab.inpg.fr.
Marc Sato, Email: marc.sato@gipsa-lab.inpg.fr.
REFERENCES
- Ackermann H, Riecker A ( 2004): The contribution of the insula to motor aspects of speech production: A review and a hypothesis. Brain Lang 89: 320–328. [DOI] [PubMed] [Google Scholar]
- Alkadhi H, Crelier GR, Boendermaker SH, Golay X, Hepp‐Reymond MC, Kollias SS ( 2002): Reproducibility of primary motor cortex somatotopy under controlled conditions. Am J Neuroradiol 23: 1524–1532. [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Jäncke L, Mohlberg H, Steinmetz H, Zilles K ( 2000): Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia 38: 304–312. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH ( 2006): An fMRI investigation of syllable sequence production. NeuroImage 32: 821–841. [DOI] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Shaker R ( 1999): Event‐related fMRI of tasks involving brief motion, Human Brain Mapp 7: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT ( 2005): Stuttered and fluent speech production: An ALE meta‐analysis of functional neuroimaging studies. Hum Brain Mapp 25: 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Ngan E, Liotti M ( 2008): A larynx area in the human motor cortex. Cerebral Cortex 18: 837–845. [DOI] [PubMed] [Google Scholar]
- Brown S, Laird A, Pfordresher PQ, Thelen SM, Turkeltaub P, Liotti M ( 2009): The somatotopy of speech. Phonation and articulation in the human motor cortex. Brain Cognition 70: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunton K ( 2008): Speech versus nonspeech: Different tasks, different neural organization. Semin Speech Lang 29: 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Kenney MK, Loucks TM, Poletto CJ, Ludlow CL ( 2009): Common neural substrates support speech and non‐speech vocal tract gestures. Neuroimage 47: 314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield DR, Murphy K, Josephs O, Fink GR, Frackowiak RS, Guz A, Adams L, Turner Desmuget, Grethe R ( 1999): Cortical and subcortical control of tongue movement in humans: A functional neuroimaging study using fMRI. J Appl Physiol 86: 1468–1477. [DOI] [PubMed] [Google Scholar]
- Dhanjal NS, Handunnetthi L, Patel MC, Wise RJ ( 2008): Perceptual systems controlling speech production. J Neurosci 28: 9969–9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresel C, Castrop F, Haslinger B, Wohlschlaeger AM, Hennenlotter A, Ceballos‐Baumann AO ( 2005): The functional neuroanatomy of coordinated orofacial movements: Sparse sampling fMRI of whistling. Neuroimage 28: 588–597. [DOI] [PubMed] [Google Scholar]
- Dronkers NF ( 1996): A new brain region for coordinating speech articulation. Nature 384: 159–161. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Geyer S, Naito E ( 2003): Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body‐part‐specific motor representations. J Neurophysiol 90: 3304–3316. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Fesl G, Moriggl B, Schmid UD, Naidich TP, Herholz K, Yousry TA ( 2003): Inferior central sulcus: Variations of anatomy and function on the example of the motor tongue area. NeuroImage 20: 601–610. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner Desmuget, Grethe R ( 1995): Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ ( 1999): How many subjects constitute a study? NeuroImage 10: 1–5. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ ( 1999): Multisubject fMRI studies and conjunction analyses. NeuroImage 10: 385–396. [DOI] [PubMed] [Google Scholar]
- Gaab N, Gaser C, Zaehle T, Jancke L, Schlaug G ( 2003): Functional anatomy of pitch memory: An fMRI study with sparse temporal sampling. Neuroimage 19: 1417–1426. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP ( 1995): Current issues in directional motor control. Trends Neurosci 18: 506–510. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Gerardin E, Lehéricy S, Pochon JB, Tézenas du Montcel S, Mangin JF, Poupon F, Agid Y, Le Bihan D, Marsault C ( 2003): Foot, hand, face and eye representation in the human striatum. Cerebral Cortex 13: 162–169. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Bürgel U, Klingberg T, Larsson J, Zilles K, Roland PE ( 1996): Two different areas within the primary motor cortex of man. Nature 382: 805–807. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K ( 1999): Areas 3a, 3b, and 1 of human primary somatosensory cortex: 1. Microstructural organization and interindividual variability. NeuroImage 10: 63–83. [DOI] [PubMed] [Google Scholar]
- Gracco VL, Tremblay P, Pike GB ( 2005): Imaging speech production using fMRI. NeuroImage 26: 294–301. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Woods RP, Mazziotta JC, Phelps ME ( 1991): Somatotopic mapping of the primary motor cortex in humans: Activation studies with cerebral blood flow and positron emission tomography. J Neurophysiol 66: 735–743. [DOI] [PubMed] [Google Scholar]
- Guenther FH, Vladusich T: A neural theory of speech acquisition and production (in press). [DOI] [PMC free article] [PubMed]
- Guenther FH, Ghosh SS, Tourville JA ( 2006): Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang 96: 280–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliott MR, Gurney EM, Bowtell RW ( 1999): “Sparse” temporal sampling in auditory fMRI. Hum Brain Mapp 7: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE ( 1999): Cortical activation during human volitional swallowing: An event‐related fMRI study. Am J Physiol 277: G219–G225. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Parikh S, Bruno MK, Hallett M ( 2005): Finger and face representations in the ipsilateral precentral motor areas in humans. J Neurophysiol 93: 2950–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann V, Sorger B, Lasek K, Guntinas‐Lichius O, Krug B, Sturm V, Goebel R, Lackner K ( 2004): Discriminating the cortical representation sites of tongue and lip movement by functional MRI. Brain Topography 16: 159–167. [DOI] [PubMed] [Google Scholar]
- Houk JC, Bastianen C, Fansler D, Fishbach A, Fraser D, Reber PJ, Roy SA, Simo LS ( 2007): Action selection and refinement in subcortical loops through basal ganglia and cerebellum. Philos Trans R Soc Lond B Biol Sci 362: 1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert IA, Robbins J ( 2007): Normal swallowing and functional magnetic resonance imaging: A systematic review. Dysphagia 22: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens U ( 2002): Neural pathways underlying vocal control. Neurosci Biobehav Rev 26: 235–258. [DOI] [PubMed] [Google Scholar]
- Jürgens U ( 2009): The neural control of vocalization in mammals: A review. J Voice 23: 1–10. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey‐Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG ( 1998): The acquisition of skilled motor performance: Fast and slow experience‐driven changes in primary motor cortex. Proc Natl Acad Sci U S A 95: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT ( 2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold NA, Daniels SK. 2010. Supranuclear control of swallowing. Dysphagia 25: 250–257. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A ( 2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157. [DOI] [PubMed] [Google Scholar]
- Lotze M, Seggewies G, Erb M, Grodd W, Birbaumer N ( 2000a): The representation of articulation in the primary sensorimotor cortex. Neuroreport 11: 2985–2989. [DOI] [PubMed] [Google Scholar]
- Lotze M, Erb M, Flor H, Huelsmann E, Godde B, Grodd W ( 2000b): fMRI evaluation of somatotopic representation in human primary motor cortex. Neuroimage 11: 473–481. [DOI] [PubMed] [Google Scholar]
- Loucks TMJ, Poletto CJ, Simonyan K, Reynolds CL, Ludlow CL ( 2007): Human brain activation during phonation and exhalation: Common volitional Control for two upper airway functions. Neuroimage 36: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JP, Kolta A ( 2006): Brainstem circuits that control mastication: Do they have anything to say during speech? J Commun Disord 39( 5), 381–390. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. NeuroImage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Martin RE, Goodyear BG, Gati JS, Menon RS ( 2001): Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 85: 938–950. [DOI] [PubMed] [Google Scholar]
- Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, Menon RS ( 2004): Cerebral areas processing swallowing and tongue movement are overlapping but distinct: A functional magnetic resonance imaging study. J Neurophysiol 92: 2428–2443. [DOI] [PubMed] [Google Scholar]
- Martin R, Barr A, MacIntosh B, Smith R, Stevens T, Taves D, Gati J, Menon R, Hachinski V ( 2007): Cerebral cortical processing of swallowing in older adults. Exp Brain Res 176: 12–22. [DOI] [PubMed] [Google Scholar]
- McKay LC, Evans KC, Frackowiak RS, Corfield DR ( 2003): Neural correlates of voluntary breathing in humans. J Appl Physiol 95: 1170–1178. [DOI] [PubMed] [Google Scholar]
- MacNeilage PF ( 1998): The frame/content theory of evolution of speech production. Behav Brain Sci 21: 499–546. [DOI] [PubMed] [Google Scholar]
- Murphy K, Corfield DR, Guz A, Fink GR, Wise RJ, Harrison J, Adams L ( 1997): Cerebral areas associated with motor control of speech in humans. J Appl Physiol 83: 1438–1447. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Katakura N ( 1995): Generation of masticatory rhythm in the brainstem. Neurosci Res 23: 1–19. [PubMed] [Google Scholar]
- Nota Y, Honda K ( 2004): Brain regions involved in motor control of speech. Acoust Sci Technol 25: 286–289. [Google Scholar]
- Nichols T, Hayasaka S ( 2003): Controlling the familywise error rate in functional neuroimaging: A comparative review. Stat Methods Med Res 12: 419–446. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–114. [DOI] [PubMed] [Google Scholar]
- Onozuka M, Hirano Y, Tachibana A, Kim W, Ono Y, Sasaguri K, Kubo K, Niwa M, Kanematsu K, Watanabe K ( 2007): Interactions between chewing and brain activity in humans In Onozuka M, Yen CT, editors. Novel Trends in Brain Science, Springer: pp 99–113. [Google Scholar]
- Özdemir E, Norton A, Schlaug G ( 2006): Shared and distinct neural correlates of singing and speaking. NeuroImage 33: 628–635. [DOI] [PubMed] [Google Scholar]
- Peeva MG, Guenther FH, Tourville JA, Nieto‐Castanon A, Anton JL, Nazarian B, Alario FX ( 2009): Distinct representations of phonemes, syllables, and supra‐syllabic sequences in the speech production network. Neuroimage 50: 626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Boldrey E ( 1937): Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60: 389–443. [Google Scholar]
- Penfield W, Rasmussen T. 1950. The Cerebral Cortex of Man. New York: Macmillan. [Google Scholar]
- Pickard N, Strick P ( 2001): Imaging the premotor areas. Curr Opin Neurobiol 11: 663–672. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Huss M, Kherif F, Moscoso del Prado Martin F, Hauk O, Shtyrov Y ( 2006): Motor cortex maps articulatory features of speech sounds. PNAS 103: 7865–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Hammeke TA, Bandettini PA, Bobholz JA, Frost JA, Myklebust BM, Jacobson RD, Hyde JS ( 1995): Somatotopic mapping of the human primary motor cortex with functional magnetic resonance imaging. Neurology 45: 919–924. [DOI] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Meyer J, Dogil G, Haider H, Grodd W ( 2000a): Articulatory/phonetic sequencing at the level of the anterior perisylvian cortex: A functional magnetic resonance imaging (fMRI) study. Brain Lang 75: 259–276. [DOI] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W ( 2000b): Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport 11: 1997–2000. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, Ackermann H ( 2005): fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology 64: 700–706. [DOI] [PubMed] [Google Scholar]
- Riecker A, Brendel B, Ziegler W, Erb M, Ackermann H ( 2008): The influence of syllable onset complexity and syllable frequency on speech motor control. Brain Lang 107: 102–113. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Mosier KM ( 2001): Neural control of tongue movement with respect to respiration and swallowing. Crit Rev Oral Biol Med 12: 18–37. [DOI] [PubMed] [Google Scholar]
- Schulz GM, Varga M, Jeffires K, Ludlow CL, Braun AR ( 2005): Functional neuroanatomy of human vocalization: An H215O PET study. Cereb Cortex 15: 1835–1847. [DOI] [PubMed] [Google Scholar]
- Shinagawa H, Ono T, Ishiwata Y, Honda E, Sasaki T, Taira M, Iriki A, Kuroda T ( 2003): Hemispheric dominance of tongue control depends on the chewing‐side preference. J Dental Res 82: 278–283. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Ostuni J, Ludlow CL, Horwitz B ( 2009): Functional but not structural networks of the human laryngeal motor cortex show left hemispheric lateralization during syllable but not breathing production. J Neurosci 29: 14912–14923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A ( 1998): The control of orofacial movements in speech. Crit Rev Oral Biol Med 3: 233–267. [DOI] [PubMed] [Google Scholar]
- Smotherman MS ( 2007): Sensory feedback control of mammalian vocalizations. Behav Brain Res 182: 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowman PF, Flavel SC, McShane CL, Sakuma S, Miles TS, Nordstrom MA ( 2009): Asymmetric activation of motor cortex controlling human anterior digastric muscles during speech and target‐directed jaw movements. J Neurophysiol 102: 159–166. [DOI] [PubMed] [Google Scholar]
- Sörös P, Sokoloff LG, Bose A, McIntosh AR, Graham SJ, Stuss DT ( 2006): Clustered functional MRI of overt speech production. NeuroImage 32: 376–387. [DOI] [PubMed] [Google Scholar]
- Stippich C, Ochmann H, Sartor K ( 2002): Somatotopic mapping of the human primary sensorimotor cortex during motor imagery and motor execution by functional magnetic resonance imaging. Neurosci Lett 331: 50–54. [DOI] [PubMed] [Google Scholar]
- Stippich C, Blatow M, Durst A, Dreyhaupt J, Sartor K ( 2007): Global activation of primary motor cortex during voluntary movements in man. Neuroimage 34: 1227–1237. [DOI] [PubMed] [Google Scholar]
- Takai, O , Brown, S , Liotti, M ( 2010): Representation of the speech effectors in the human motor cortex: Somatotopy or overlap? Brain Lang 113: 39–44. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 1988. Co‐planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers. [Google Scholar]
- Terumitsu M, Fujii Y, Suzuki K, Kwee IL, Nakada T ( 2006): Human primary motor cortex shows hemispheric specialization for speech. Neuroreport 17: 1091–1095. [DOI] [PubMed] [Google Scholar]
- Thach WT ( 1998): A role for the cerebellum in learning movement coordination. Neurobiol Learn Mem 70: 177–188. [DOI] [PubMed] [Google Scholar]
- Vincent DJ, Bloomer CJ, Hinson VK, Bergmann KJ ( 2006): The range of motor activation in the normal human cortex using bold FMRI. Brain Topogr 18: 273–280. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Miura N, Watanabe Y, Maeda Y, Matsue Y, Kawashima R ( 2004): The human parietal cortex is involved in spatial processing of tongue movement: An fMRI study. NeuroImage 21: 1289–1299. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Ackermann H, Klose U, Kardatzki B, Grodd W ( 1996): Functional lateralization of speech production at primary motor cortex: A fMRI study. Neuroreport 7: 2791–2795. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Greene J, Büchel C, Scott SK ( 1999): Brain regions involved in articulation. Lancet 353: 1057–1061. [DOI] [PubMed] [Google Scholar]
- Yetkin FZ, Haughton VM, Cox RW, Hyde J, Birn RM, Wong EC, Prost R ( 1996): Effect of motion outside the field of view on functional MR. Am J Neuroradiol 17: 1005–1009. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Supporting Information Figure 1
Supporting Information Table 1


