Abstract
Major depression has been repeatedly associated with amygdala hyper‐responsiveness to negative (but not positive) facial expressions at early, automatic stages of emotion processing using subliminally presented stimuli. However, it is not clear whether this “limbic bias” is a correlate of depression or represents a vulnerability marker preceding the onset of the disease. Because childhood maltreatment is a potent risk factor for the development of major depression in later life, we explored whether childhood maltreatment is associated with amygdalar emotion processing bias in maltreated but healthy subjects. Amygdala responsiveness to subliminally presented sad and happy faces was measured by means of fMRI at 3 T in N = 150 healthy subjects carefully screened for psychiatric disorders. Childhood maltreatment was assessed by the 25‐item childhood trauma questionnaire (CTQ). A strong association of CTQ‐scores with amygdala responsiveness to sad, but not happy facial expressions emerged. This result was further qualified by an interaction of emotional valence and CTQ‐scores and was not confounded by trait anxiety, current depression level, age, gender, intelligence, education level, and more recent stressful life‐events. Childhood maltreatment is apparently associated with detectable changes in amygdala function during early stages of emotion processing which resemble findings described in major depression. Limbic hyper‐responsiveness to negative facial cues could be a consequence of the experience of maltreatment during childhood increasing the risk of depression in later life. Limitation: the present association of limbic bias and maltreatment was demonstrated in the absence of psychopathological abnormalities, thereby limiting strong conclusions. Hum Brain Mapp 34:2899–2909, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: childhood maltreatment, amygdala, stress, fMRI, depression, anxiety
INTRODUCTION
Depression ranks among the most debilitating diseases with a life‐time prevalence of 16% and occupies a top rank among the major causes of disability adjusted life years worldwide (World Health Organization, 2001). Understanding neurobiological vulnerability markers involved in the onset and maintenance of depression is a major research goal. The acute state of depression has been extensively investigated by human neuroimaging studies. It has been repeatedly reported that acutely depressed patients show increased amygdala responsiveness to various emotionally negative stimuli (Abler et al., 2007; Fu et al., 2004; Peluso et al., 2009; Sheline et al., 2001; Siegle et al., 2002, 2007). Using subliminally presented facial expressions, two independent studies recently demonstrated that acute depression is associated with increased amygdala responsiveness to negative but not positive faces already on automatic stages of emotion processing (Suslow et al., 2010a; Victor et al., 2010). However, to date it is not known whether amygdala hyper‐responsiveness represents a state marker of acute depression or trait characteristic of depression vulnerability.
Among the strongest risk factors for developing major depression are experiences of childhood maltreatment (Gilbert et al., 2009), with up to 30% of all maltreated children fulfilling DSM‐IV criteria for major depression in their late 20s (Widom et al., 2007). It was estimated that up to 30–40% of the adult population have experienced at least some form of maltreatment during childhood (Scher et al., 2004). Furthermore, childhood maltreatment was already repeatedly associated with reduced hippocampal volumes (Bremner et al., 1997; Edmiston et al., 2011; Frodl et al., 2010; Teicher et al., 2012; Vythilingam et al., 2002), which is also a frequent finding in major depression (MacQueen and Frodl, 2010).
Given the highly increased risk for adults having experienced maltreatment as children, it could be speculated that these subjects could already demonstrate an automatic limbic bias. In line with this notion, it was demonstrated that childhood maltreatment is associated with increased amygdala responsiveness to sad faces in depressed patients (Grant et al., 2011), to fearful/angry faces in a large sample of healthy subjects (Dannlowski et al., 2012), and to different emotion categories in a mixed patient and healthy control sample (van Harmelen et al., 2012). However, all three studies the stimuli were presented overtly, and therefore, the tasks did not tap the rapid, automatic stages of emotion processing. It seems to be of particular importance to examine automatic responding to emotional stimuli because in general emotions are involuntarily elicited and emerge without conscious effort in everyday life (Bargh and Chartrand, 1999; Scherer, 1993).
Therefore, in this study we sought to clarify the impact of childhood maltreatment on automatic amygdala responsiveness to negative and positive facial cues in a large sample of healthy adults. We used a subliminal priming paradigm specifically designed to target the automatic stages of emotion processing (particularly in the amygdala), which has already been used in several previous imaging studies of our group. We hypothesized that healthy adults having experienced maltreatment as children would show amygdala hyper‐responsiveness to negative but not positive facial expressions also in a subliminal presentation condition.
METHODS
Subjects
A total of N = 150 right‐handed subjects (71 male, 79 female) were thoroughly investigated by experienced psychologists and were free from any life‐time history of psychiatric disorders according to DSM‐IV criteria (American Psychiatric Association, 1994), as diagnosed with the SCID interview (Wittchen et al., 1997), see (Dannlowski et al., 2012) for details. Exclusion criteria were scores ≥ 10 on the Beck Depression Inventory (BDI), any neurological abnormalities, any history of psychiatric disorders, history of seizures, head trauma or unconsciousness, intake of any psychotropic medication, and the usual MRI‐contraindications. The Childhood Trauma Questionnaire (CTQ) was administered to assess maltreatment during childhood. The CTQ is a 25‐item retrospective self‐report questionnaire designed to assess five types of negative childhood experiences (Bernstein et al., 1994). The reliability was high in this sample (internal consistency; Cronbach's α = 0.95; please see Supporting Information Figure 1 for a histogram regarding CTQ score distribution in the sample). Furthermore, the perceived stress scale (PSS) and the List of Threatening Experiences Questionnaire (LTE‐Q) were administered as measures of more recent stressful life‐events. The PSS assesses the degree to which situations in the subjects' life are experienced as stressful and how unpredictable, uncontrollable, and overloaded respondents find their lives during the past month (Cohen et al., 1983). The LTE‐Q assesses 12 stressful life‐events during the last 12 months (Brugha and Cragg, 1990). Trait anxiety was measured with the State‐Trait Anxiety Inventory (STAI, trait version) (Laux et al., 1981). Verbal intelligence was estimated by the Mehrfachwahl‐Wortschatz‐Intelligenztest (multiple choice vocabulary intelligence test; MWT‐B) (Lehrl, 1995). We further assessed harm avoidance as measured with the Tridimensional Personality Questionnaire (Weyers et al., 1995). Table 1 lists sociodemographic, questionnaire, and behavioral data of the final study sample. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the University of Münster. After complete description of the study to the participants, written informed consent was obtained. Participants received a financial compensation.
Figure 1.
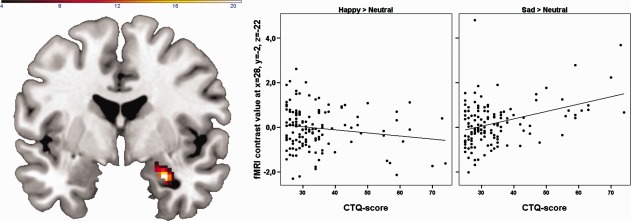
Childhood maltreatment (CTQ‐scores) is positively associated with right amygdala responsiveness to negative but not positive facial expressions. Left panel: Coronal view (y = −2) depicting the interaction of emotional valence (sad vs. happy) and childhood maltreatment (CTQ‐scores). Color bar, F‐value (df = 1, 264). Middle and right panel: Scatter plot depicting amygdala responsiveness to masked happy (middle panel) and sad (right panel) facial expressions dependent on self‐reported childhood maltreatment. There was no significant association of CTQ‐scores and amygdala responsiveness to happy faces (r = −0.16, P = 0.064) but a strong positive association of CTQ‐scores and amygdala responsiveness to negative faces (r = 0.37, P < 0.001). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 1.
Sociodemographic, questionnaire, and behavioral data of all study participants (N = 134) of the final fMRI sample; mean ± SE (range)
| Age | 34.5 ± 10.6 (20–57) |
| Education years | 15.6 ± 2.2 (10–18) |
| Sex (m/f) | 63/71 |
| Verbal intelligence (IQ)a | 117.5 ± 11.6 (94–145) |
| BDI | 1.5 ± 1.8 (0–8) |
| STAI‐T | 31.8 ± 6.8 (20–53) |
| Harm avoidance | 9.6 ± 4.5 (0–20) |
| CTQ‐score | 34.2 ± 10.7 (25–74) |
| CTQ emotional neglect | 9.1 ± 4.3 (5–25) |
| CTQ emotional abuse | 7.5 ± 3.8 (5–23) |
| CTQ physical abuse | 5.7 ± 2.1 (5–20) |
| CTQ physical neglect | 6.5 ± 2.0 (5–13) |
| CTQ sexual abuse | 5.2 ± 0.9 (5–14) |
| PSS | 18.5 ± 6.5 (5–35) |
| LTE‐Q | 1.0 ± 1.3 (0–7) |
| Mean evaluation sad prime condition | 0.022 ± 0.27 |
| Mean evaluation happy prime condition | 0.021 ± 0.26 |
| Mean evaluation neutral prime condition | 0.019 ± 0.27 |
| Mean evaluation no‐face prime condition | 0.014 ± 0.27 |
| RT sad prime condition | 1469.7 ± 355.7 |
| RT happy prime condition | 1440.1 ± 342.1 |
| RT neutral prime condition | 1408.3 ± 363.0 |
| RT no face prime condition | 1469.7 ± 355.7 |
Assessed with the Mehrfachwahl‐Wortschatz‐Intelligenztest, MWT‐B (Lehrl, 1995).
Subliminal Affective Priming Paradigm
This paradigm is based on previous behavioral studies in major depression (Dannlowski et al., 2006) and has been widely used in imaging studies for investigating the early, automatic stages of emotion processing in patients and healthy subjects as described previously (Dannlowski et al., 2010b; Kugel et al., 2008; Rauch et al., 2010; Reker et al., 2010; Suslow et al., 2009, 2010a, 2010b). Briefly, facial stimuli consisted of grey‐scale normalized sad, happy, and neutral expressions of 10 individuals from the Ekman & Friesen stimulus set (Ekman and Friesen, 1976). Emotional and neutral faces were presented for 33 ms and masked by neutral faces of the same individuals. To avoid identity of prime and mask in the neutral face condition, vertically mirrored faces were used as neutral primes. A total of 80 trials were shown: 20 with sad, 20 with happy, and 20 with neutral prime faces. In 20 trials, no‐face primes were presented. The no‐face prime condition consisted of neutral faces in which central facial features (i.e., eyes, nose, and mouth) had been replaced by a surface without contours. Faces were shown in two fixed pseudo‐random sequences with the restriction of no repetition of an individual and no more than one repetition of a prime condition on consecutive trials. Each trial lasted 9 s. A fixation cross presented for 800 ms preceded a prime face shown for 33 ms which was followed by the corresponding neutral face mask, presented for 467 ms. A blank screen followed for 7,700 ms. During this time‐period, subjects had to evaluate whether the neutral (mask) face expressed rather negative or positive feelings, by pressing one of four buttons (−1.5, −0.5, +0.5, and +1.5). In each hand, participants held a fiber‐optic response pad with two buttons (the positive or the negative response keys). One half of the sample gave positive responses with the left hand; the other with the right hand. Judgments and reaction times were registered.
Prime Detection Task
After the fMRI experiment, all subjects answered a fixed set of questions whether they had noticed any features of the subliminally presented faces in the affective priming task. Then, the subjects were informed about the presence of the emotional prime faces and took part in a forced‐choice prime detection task outside the scanner to assess potential objective awareness. The prime detection task consisted of 40 trials of the same stimulus presentation conditions and the same stimuli as in the fMRI experiment (33 ms prime presentation, followed by a neutral face mask of the same actor). However, in the prime detection task, the subjects were asked to indicate the prime condition that was presented before the neutral mask via button press. The hitrate was calculated for each subject. A binominal distribution test indicated that >15/40 hits were significantly above chance level (P < 0.05, two‐tailed).
fMRI Methods
Images were projected to the rear end of the scanner (Sharp XG‐PC10XE with additional HF shielding). T2* functional data were acquired at a 3 T scanner (Gyroscan Intera 3T, Philips Medical Systems, Best, NL), using a single shot echoplanar sequence with parameters selected to minimize distortion in the region of central interest, while retaining adequate signal to noise ratio (S/N) and T2* sensitivity. Volumes consisting of 34 slices were acquired (matrix 64 × 64, resolution 3.6 × 3.6 × 3.6 mm3; TR = 2.1 s, TE = 30 ms, FA = 90°). The slices were tilted 25° from the AC/PC line to minimize drop out artifacts in the orbitofrontal and mediotemporal region.
Functional imaging data were realigned and unwarped, spatially normalized to standard MNI space (Montreal Neurological Institute) and smoothed (Gaussian kernel, 6 mm FWHM) using Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm). Three subjects had to be excluded for anatomical abnormalities discovered in the structural MRI‐Images (abnormally enlarged ventricles). Furthermore, 13 subjects were excluded due to excessive head movement (exclusion criterion > 2 mm and/or 2°), leaving N = 134 complete datasets for fMRI analyses. These 16 excluded subjects did not differ from the included 134 subjects regarding CTQ‐scores or any sociodemographic or questionnaire data (all P > 0.25).
fMRI Data Analysis
We tested our main hypothesis of amygdala responsiveness modulation by childhood maltreatment via regressing CTQ scores on amygdala responsiveness to sad‐neutral and happy‐neutral facial expressions separately. The amygdala was defined according to the AAL‐atlas (Tzourio‐Mazoyer et al., 2002), and the amygdala mask was created by means of the WFU pickatlas (http://fmri.wfubmc.edu/software/PickAtlas). To control for multiple statistical testing, we maintained a cluster‐level false‐positive detection rate at P < 0.05 using a voxel threshold of P < 0.05 with a cluster (k) extent empirically determined by Monte Carlo simulations (n = 1000 iterations), by means of the AlphaSim procedure (Forman et al., 1995), implemented in the REST toolbox (http://restfmri.net/forum/index.php). The empirically determined cluster threshold was k = 33 voxels for the bilateral amygdala mask.
In a second step, the mean contrast values of significant clusters from these analysis were extracted for each participant and further analyzed with PASW Statistics 18: We conducted a multiple regression model predicting amygdala responsiveness with CTQ‐scores, age, total education time (years), verbal intelligence, harm avoidance, trait anxiety and depression level, as well as PSS‐scores and LTE‐Q‐scores. Furthermore, each of the 5 CTQ subscales was separately correlated with amygdala responsiveness to explore which maltreatment type was the strongest predictor. Additionally, we conducted nonparametric correlations of CTQ and amygdala responsiveness.
For exploratory reasons, a whole‐brain analysis of emotional valence × childhood trauma interaction was conducted at an uncorrected threshold (P < 0.001, k = 10), by means of a multiple regression analysis predicting brain activation by sad > happy faces by CTQ‐scores, again regressing out age, gender, total education time (years), detection task performance, verbal intelligence, harm avoidance, trait anxiety and depression level, as well as PSS‐scores and LTE‐Q‐scores.
RESULTS
Detection Task
The N = 134 subjects had a mean hit rate of 12.8 corrects hits/40 trials (range 4‐22). Although about half of all subjects reported being able to see “a brief flash of light” in the detection task (after being informed about the presence of backward‐masked faces), none of the subjects reported being able to consciously detect the emotional prime faces, neither in the detection task nor during the fMRI experiment. However, N = 31 subjects (23.1%) showed a hit rate above chance level (>15 hits) and were, therefore, considered as “detecting.” The remaining N = 103 subjects scored within the range of chance level and were, therefore, considered “nondetecting.” To account for differences in detection task performance, we conducted two additional analysis strategies: First, in addition to our main analysis in the entire sample, we analyzed the data of “detecting” and “nondetecting” subjects separately and second, within the entire sample, we entered detection task performance as nuisance regressor.
Behavioral Results
Table 1 lists mean reaction times and evaluative responses for the experimental conditions. There were no significant associations of amygdala responsiveness to sad or happy faces with any behavioral measure (reaction times and evaluative responses).
fMRI Results
As in previous studies, the paradigm significantly activated the bilateral amygdala in response to subliminally sad faces (opposed to the neutral face baseline; right: x = 26, y = 2, z = −14; Z = 3.02, P uncorrected < 0.001; cluster size k = 146; left: x = −24, y = 0, z = −28; Z = 3.34, P uncorrected < 0.001; cluster size k = 86) and happy faces (right: x = 26, y = 2, z = −14; Z = 3.26, P uncorrected < 0.001; cluster size k = 108; left: x = −22, y = 0, z = −26; Z = 3.55, P uncorrected < 0.001; cluster size k = 83).
The regression analysis conducted with SPM8 yielded a strong positive association of CTQ‐scores and amygdala responsiveness to sad‐neutral faces (right: x = 26, y = −2, z = −22; t = 4.2, df = 134, P uncorrected < 0.0001; P FWE‐corrected = 0.005; r = 0.37, cluster size k = 183; left: x = −24, y = 0, z = −20; t = 4.0, df = 134, P uncorrected < 0.0001; P FWE‐corrected = 0.01; r = 0.34, cluster size k = 142). In contrast, the regression of CTQ‐scores on amygdala responsiveness to happy‐neutral facial expressions produced no significant cluster.
To explicitly test an emotion by CTQ interaction, a full factorial model was conducted including the within‐subjects factor emotion (happy vs. sad) and CTQ as covariate, modeled as interaction term with the emotion factor. The interaction was highly significant in the right amygdala (x = 28, y = −2, z = −22; F(1,264) = 20.6, P uncorrected < 0.0001; P FWE‐corrected = 0.002, cluster size k = 115), whereas a cluster in the left amygdala failed to survive the cluster correction (x = −24, y = 2, z = −20; F(1,264) = 8.78, P uncorrected = 0.003; P FWE‐corrected = 0.28; r = 0.38, cluster size k=20). Thus, in the right amygdala, a significantly stronger correlation of CTQ‐scores and neural activity to sad faces compared with happy faces emerged (see Fig. 1). To determine the amygdalar subregion where differential processing of emotion faces occurred, the SPM Anatomy toolbox Version 1.5 was administered (Eickhoff et al., 2005). The emotion × group interaction was located in the lateral and basal nuclei of the right amygdala.
Subsequently, we extracted the activity at these coordinates in response to sad and happy facial expressions (contrast values) for each subject separately for further processing with PASW Statistics 18. To assess potential effects of detection task performance, we conducted the correlations of CTQ and amygdala responsiveness additionally for “detecters” and “nondetecters,” separately. The correlation of CTQ‐scores and amygdala responsiveness to sad faces was highly significant among the nondetecters (N = 103, r = 0.35, P = 0.0002) and the detecters (N = 31, r = 0.54, P = 0.0014). No association of CTQ‐scores and amygdala responsiveness to happy faces could be discerned in the whole sample or in either subgroup.
In a multiple regression analysis conducted in the entire sample predicting, the mean activation of the right amygdala responsiveness to sad faces by CTQ‐score, detection task performance, BDI, harm avoidance, STAI trait, PSS, LTE‐Q, age, verbal intelligence, and total education time, the strong effect of CTQ remained unchanged (β = 0.35, t(122) = 4.04, P < 0.0001). No other predictor had any significant effect (all P > 0.25). Thus, the association of childhood maltreatment and limbic hyper‐responsiveness was apparently unconfounded by detection task performance, recent stressful life events, current levels of subclinical depression and anxiety symptoms, verbal intelligence, or sociodemographic factors. Also, a nonparametric correlation of CTQ‐scores and amygdala responsiveness (Spearman's rho) was highly significant r s = 0.25, P = 0.004.
From all five subscales, emotional neglect (r = 0.37, P < 0.0001) and emotional abuse (r = 0.33, P = 0.0001) were the strongest predictors for amygdala responsiveness to sad faces, followed by physical neglect (r = 0.28, P = 0.001) and physical abuse (r = 0.23, P = 0.008). The correlation of CTQ‐scores and sexual abuse reached a trend level of significance (r = 0.16, P = 0.068). However, the results from different subscales should be treated with care because the two “emotional” scales showing the highest correlations were also the subscales with the largest variance, and furthermore, there were no significant differences regarding the highest correlation (emotional neglect, r = 0.37) and the lowest correlation (sexual abuse, r = 0.16), P = 0.08.
Conducting the same multiple regression model as mentioned above, predicting amygdala responsiveness to happy faces yielded no significant effect of CTQ‐scores (β = −0.15, t(122) = −1.6, P = 0.11). However, there was a significant association of LTE‐Q scores (β = −0.29, t(122) = −3.34, P = 0.001, indicating that a larger number of stressful life‐events during the year before study participation predicted a weaker amygdala responsiveness to happy facial expressions. No other regressor yielded any significant result (all P > 0.25).
Regarding our whole‐brain regression analysis, in addition to the right amygdala, also other clusters show a similar emotion × childhood trauma interaction pattern, including ventromedial PFC, the anterior cingulate gyrus, and the insula (see Table 2 for details). All areas showing a significant interaction revealed the same pattern as in the amygdala‐a stronger positive association of CTQ scores and neural responsiveness to sad faces, opposed to the association of CTQ scores and neural responsiveness to happy faces (which was either less positive or even nominally negative).
Table 2.
Results of a multiple regression analysis predicting brain activation by sad > happy faces by CTQ‐scores, regressing out age, gender, total education time (years), detection task performance, verbal intelligence, harm avoidance, trait anxiety and depression level, as well as PSS‐scores and LTE‐Q‐scores (conducted at P < 0.001, uncorrected, k = 10 voxels)
| Anatomical region | BA | Side | Cluster size | X | Y | z | Z‐score | P‐value (uncorr.) |
|---|---|---|---|---|---|---|---|---|
| Angular gyrus, MOG | 19 | L | 30 | −38 | −62 | 24 | 4.53 | <0.0001 |
| SFG, precentral gyrus | 6 | R | 58 | 24 | −16 | 54 | 4.34 | <0.0001 |
| SPG | 7 | L | 24 | −20 | −76 | 48 | 4.15 | 0.00002 |
| Amygdala | — | R | 15 | 26 | −2 | −22 | 4.10 | 0.00002 |
| Insula | — | R | 25 | 32 | 34 | 8 | 4.00 | 0.00003 |
| Putamen | — | L | 12 | −16 | 12 | −10 | 3.69 | 0.00011 |
| ACC, SFG (medial part) | 32 | L | 19 | −12 | 44 | 2 | 3.64 | 0.00014 |
| Fusiform gyrus | 19 | R | 11 | 26 | −82 | −12 | 3.55 | 0.00019 |
| MFG | 8/9 | L | 11 | −36 | 20 | 48 | 3.49 | 0.00024 |
Coordinates are given in MNI space. MFG, Middle frontal gyrus; SFG, superior frontal gyrus; IFG, inferior frontal gyrus; ACC, anterior cingulate cortex; SPG, superior parietal gyrus; MOG, middle occipital gyrus.
DISCUSSION
This data suggest a strong effect of childhood maltreatment on automatic amygdala excitability to negative but not positive facial expressions that seems to persist in later life. A significant difference between the negative and positive experimental condition regarding maltreatment effects on amygdala responsiveness was qualified by an emotion (happy, sad) × maltreatment (CTQ‐scores) interaction. There was no evidence for confounding effects of age, verbal intelligence, education, current depression, harm avoidance and trait anxiety levels, level of perceived stress during the past month or stressful life‐events during the year before participation. This association was discerned in the absence of any current or life‐time history of psychiatric disorders.
The amygdala plays a core role in a neural circuit processing emotional valence and generating rapid affective responses (Davis and Whalen, 2001; Domschke and Dannlowski, 2010; Phillips et al., 2003). In addition to a slower, cortical route, the amygdala receives direct projections from thalamic nuclei, allowing a rapid response to emotionally salient stimuli, even before conscious cortical representations emerge (Ledoux, 2000). These notions were widely confirmed in human neuroimaging studies, showing that amygdala responses can be discerned particularly to negative facial expressions even if the stimuli were presented subliminally (Killgore and Yurgelun‐Todd, 2004; Morris et al., 1996, 1999; Nomura et al., 2004; Whalen et al., 1998), or under binocular suppression (Williams et al., 2004) and thus were “unseen,” and processed without explicit knowledge. Our present results indicate that maltreatment experiences during childhood affect the “low route,” biasing emotion processing on an automatic level.
Our results indicate that the emotion × maltreatment interaction was located in the basolateral parts of the right amygdala, albeit due to the limited spatial resolution of our fMRI sequence, these results should be taken with care. The basolateral amygdala represents the central input structure of the amygdaloid complex and is critically involved in the generation of affect (Davis and Whalen, 2001). Also in humans, neuroimaging studies have identified particularly the right basolateral amygdala as being the main subarea associated with hyperactivity in major depression or anxiety (Etkin et al., 2004; Suslow et al., 2010a). A lateralization of our findings to the right amygdala is in line with previous findings of right lateralized stress responses in the brain (Sullivan and Gratton, 2002), a particular role of the right amygdala in automatic stress responses (Gläscher and Adolphs, 2003), and stronger right amygdala responsiveness to subliminally presented stimuli (Costafreda et al., 2008). However, this laterality finding should be treated with care, because the left amygdala showed a similar but nonsignificant pattern in our sample.
Our results are, furthermore, in line with recent data obtained in rodent models via in vivo single cell recordings (Rosenkranz et al., 2010). Paralleling our imaging results, the authors reported that chronic stress induces amygdala hyper‐excitability in the lateral nuclei of the amygdaloid complex.
It is known that the amygdala modulates vigilance and attention to enhance subsequent information processing throughout the brain (Ledoux, 2000). A low automatic reactivity of the amygdala to positive facial expressions could result in less engagement in the encoding of such positively valenced stimuli, or reduced recruitment of attention resources bringing emotional stimuli to conscious awareness. In contrast, higher amygdala responsiveness to negatively valenced stimuli could bias attention and other cognitive processes negatively. High amygdala responsiveness to negative stimuli was shown to be associated with trait anxiety (Etkin et al., 2004; Sehlmeyer et al., 2010), harm avoidance (Dannlowski et al., 2011), depression level (Lee et al., 2007), and cognitive biases favoring the processing of negative stimuli (Dannlowski et al., 2007a, 2007b; Hamilton and Gotlib, 2008). Furthermore, several studies have already demonstrated that patients suffering from clinical major depression show amygdala hyper‐responsiveness to different kinds of negative stimuli (Abler et al., 2007; Fitzgerald et al., 2008; Fu et al., 2004; Kessler et al., 2011; Peluso et al., 2009; Sheline et al., 2001; Siegle et al., 2002, 2007) but rather reduced responsiveness to positive cues (Fu et al., 2007; Lawrence et al., 2004). This limbic emotion processing bias was demonstrated also for subliminally presented faces in two independent studies (Suslow et al., 2010a; Victor et al., 2010), suggesting that early, automatic stages of emotion processing are biased in depression.
However, it has not been clear so far whether such limbic biases represent a feature of acute depression or a risk factor preceding depression onset. Only few studies are available investigating subjects at risk for depression regarding amygdala responsiveness to emotional stimuli. On the one hand, automatic amygdala hyper‐responsiveness was shown to resolve (at least partly) under antidepressant treatment (Sheline et al., 2001; Victor et al., 2010). On the other hand, elevated amygdala responsiveness to aversive stimuli was also demonstrated in remitted patients without antidepressant medication (Victor et al., 2010) and in healthy subjects at high risk for depression (Joormann et al., 2011; Wolfensberger et al., 2008; Zhong et al., 2011), indicating that such a “limbic bias” could represent a vulnerability marker and not a symptom of depression. Pharmaco‐fMRI studies in healthy subjects suggest that the reduction of amygdala responsiveness is rather an effect of medication than an effect of remission, because it can occur only after a single dose (Murphy et al., 2009). Furthermore, there are several studies demonstrating that amygdala responsiveness to negative stimuli is strongly influenced by genotype (Baune et al., 2010; Brown et al., 2005; Canli et al., 2008; Dannlowski et al., 2005, 2007c, 2010a, 2012; Domschke et al., 2010; Hariri et al., 2002; Heinz et al., 2005; Munafò et al., 2008). Using the very same paradigm as in this study, it was shown that healthy risk allele carrier in a frequently studied serotonin transporter polymorphism (5‐HTTLPR) show stronger automatic amygdala responsiveness to negative but not positive facial expressions compared to the nonrisk variant (Dannlowski et al., 2010b). Because childhood maltreatment is a strong risk factor for developing depression, our present data further suggest that increased amygdala responsiveness particularly to negative stimuli could be a risk factor rather than a state marker of acute illness.
Other brain areas revealing similar associations between maltreatment experiences and neuronal processing of subliminal sad but not happy faces included the anterior insula, rostral ACC, and medial prefrontal areas. Interestingly, these areas have strong connections to the amygdala and belong to a para‐limbic anterior emotion processing system (Phillips et al., 2003, 2008) involved in the initial generation and experience of affective states.
Our findings indicating a strong effect of childhood maltreatment on amygdala excitability to negative facial expression are in line with results from a recent fMRI study examining medial temporal lobe functioning in youths as a function of a history of emotional neglect and caregiver deprivation (Maheu et al., 2010). In this study, exaggerated activation of the amygdala in response to threatening faces was observed in emotionally neglected and caregiver‐deprived youths relative to a comparison group regardless of attentional instructions during the perception of faces. A similar study reported amygdala hyperresponsiveness to fearful faces in children with a history of early deprivation (Tottenham et al., 2011). A heightened sensitivity to threatening facial expression might serve to protect neglected and abused children against additional aversive situations by rapidly identifying negative cues and preparing defensive responses. It is known from behavioral studies that youths who were neglected or abused are faster than comparison youths in recognizing threat‐related faces (Masten et al., 2008). A further recent study using not only negative but also happy facial expressions compared adults reporting childhood maltreatment with nonmaltreated adults (van Harmelen et al., 2012). The authors reported increased amygdala responsiveness to all facial expressions in maltreated subjects without a maltreatment × emotional valence interaction. However, the authors used overtly presented faces, and furthermore, the effects size of maltreatment on amygdala responsiveness was stronger in the angry, fearful, and sad condition compared to the happy condition.
Interestingly, in addition to childhood traumatic experiences, we further found a significant negative effect of the number of stressful life‐events during the past year on amygdala responsiveness to happy faces. This could reflect a blunted emotional reactivity to pleasant stimuli in subjects having experienced also more recent negative experiences in their lives.
It should be noted that while the present findings were discussed in the context of depression vulnerability, childhood maltreatment also increases the risk for several other psychiatric disorders, including substance abuse, borderline personality disorders or anxiety disorders. Furthermore, amygdala hyper‐responsiveness to negative facial expressions has also been demonstrated in anxiety disorders or borderline patients. Therefore, the relevance of the present findings might not be specific for the context of depression vulnerability but could refer to a more general mechanism for developing psychiatric disorders.
Some limitations must be acknowledged. The assessment of traumatic experiences during childhood was performed retrospectively by means of a self‐report measure. Therefore, we cannot completely rule out that subjects with high amygdala responsiveness to negative faces were able to recall negative events during their childhood more sufficiently. Although we could regress out the effects of more recent life stress as well as current depression‐ and anxiety levels, only prospective studies could definitely rule out this possibility. Our sample consisted of carefully screened healthy subjects without any current psychopathology, which limits our conclusions. Future studies should include subjects with a wider range of affective symptoms, e.g., patient samples or subjects explicitly selected for a history of maltreatment. Furthermore, future studies should carefully assess family history of all psychiatric disorders, and depression in particular, preferably including the investigation of the affected relatives. In our sample, more than 20% of all subjects scored above chance level in the detection task and, therefore, could have gained some “awareness” of the subliminally presented prime faces. However, none of the subjects reported subjectively having “seen” the prime faces in the fMRI experiment after being informed about their presence. Furthermore, all results reported here were also found in the subsample of “nondetecting” subjects alone and adding detection task performance as regressor of no interest did not change the pattern of results.
In sum, we provide further evidence that childhood maltreatment is associated with amygdala hyper‐responsiveness specifically to negative stimuli on automatic stages of emotion processing that persists into adulthood.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
This work was supported by grants of Innovative Medizinische Forschung (IMF) of the Medical Faculty of Münster. This funding source had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The authors thank Mrs. Nina Nagelmann for her skillful technical support during the fMRI sessions. All authors have no conflicts of interest to declare, financial or otherwise.
Udo Dannlowski and Harald Kugel contributed equally to this work.
REFERENCES
- Abler B, Erk S, Herwig U, Walter H (2007): Anticipation of aversive stimuli activates extended amygdala in unipolar depression. J Psychiatr Res 41:511–522. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1994): Diagnostic and Statistical Manual of Mental Disorders, 4th ed Washington, DC:American Psychiatric Association. [Google Scholar]
- Bargh JA, Chartrand TL (1999): The unbearable automaticity of being. Am Psychol 54:462–479. [Google Scholar]
- Baune BT, Dannlowski U, Domschke K, Janssen DGA, Jordan MA, Ohrmann P, Bauer J, Biros E, Arolt V, Kugel H, Baxter AG, Suslow T (2010): The interleukin 1 beta (IL1B) gene is associated with failure to achieve remission and impaired emotion processing in major depression. Biol Psychiatr 67:543–549. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J (1994): Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatr 151:1132–1136. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen R, Mazure CM, Capelli S, McCarthy G, Innis RB, Charney DS (1997): Magnetic resonance imaging‐based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—A preliminary report. Biol Psychiatr 41:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Peet E, Manuck SB, Williamson DE, Dahl RE, Ferrell RE, Hariri AR (2005): A regulatory variant of the human tryptophan hydroxylase‐2 gene biases amygdala reactivity. Mol Psychiatr 10:884–888. [DOI] [PubMed] [Google Scholar]
- Brugha TS, Cragg D (1990): The list of threatening experiences: The reliability and validity of a brief life events questionnaire. Acta Psychiatr Scand 82:77–81. [DOI] [PubMed] [Google Scholar]
- Canli T, Congdon E, Lesch K‐P, Todd Constable R (2008): Additive effects of serotonin transporter and tryptophan hydroxylase‐2 gene variation on neural correlates of affective processing. Biol Psychol 79:118–125. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R (1983): A global measure of perceived stress. J Health Soc Behav 24:385–396. [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CHY (2008): Predictors of amygdala activation during the processing of emotional stimuli: A meta‐analysis of 385 PET and fMRI studies. Brain Res Rev 58:57–70. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Kersting A, Donges U‐S, Lalee‐Mentzel J, Arolt V, Suslow T (2006): Masked facial affect priming is associated with therapy response in clinical depression. Eur Arch Psychiatr Clin Neurosci 256:215–221. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, Kersting A, Baune BT, Suslow T (2007a): Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: A 3 T fMRI study. J Psychiatr Neurosci 32:423–429. [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, Suslow T (2007b): Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatr Res 154:13–20. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Baune BT, Hohoff C, Kersting A, Arolt V, Heindel W, Deckert J, Suslow T (2007c): Serotonergic genes modulate amygdala activity in major depression. Genes Brain Behav 6:672–676. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Deckert J, Hohoff C, Kugel H, Arolt V, Heindel W, Kersting A, Baune BT, Suslow T (2008): 5‐HTTLPR biases amygdala activity in response to masked facial expressions in major depression. Neuropsychopharmacology 33:418–424. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Konrad C, Arolt V, Suslow T (2010a): Neurogenetics of emotional processes. Neuroimaging findings as endophenotypes for depression. Der Nervenarzt 81:24–31. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Konrad C, Kugel H, Zwitserlood P, Domschke K, Schöning S, Ohrmann P, Bauer J, Pyka M, Hohoff C, Zhang W, Baune BT, Heindel W, Arolt V, Suslow T (2010b): Emotion specific modulation of automatic amygdala responses by 5‐HTTLPR genotype. NeuroImage 53:893–898. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Franke F, Stuhrmann A, Hohoff C, Zwanzger P, Lenzen T, Grotegerd D, Suslow T, Arolt V, Heindel W, Domschke K (2011): Neuropeptide‐S (NPS) receptor genotype modulates basolateral amygdala responsiveness to aversive stimuli. Neuropsychopharmacology 36:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H, Lenzen T (2012): Limbic scars: Long‐term consequences of childhood maltreatment revealed by functional and structural MRI. Biol Psychiatr 71:286–293. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ (2001): The amygdala: Vigilance and emotion. Mol Psychiatr 6:13–34. [DOI] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U (2010): Imaging genetics of anxiety disorders. NeuroImage 53:822–831. [DOI] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U, Hohoff C, Ohrmann P, Bauer J, Kugel H, Zwanzger P, Heindel W, Deckert J, Arolt V, Suslow T, Baune BT (2010): Neuropeptide Y (NPY) gene: Impact on emotional processing and treatment response in anxious depression. Eur Neuropsychopharmacol 20:301–309. [DOI] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, Blumberg HP (2011): Corticostriatal‐limbic gray matter morphology in adolescents with self‐reported exposure to childhood maltreatment. Arch Pediatr Adolesc Med 165:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV (1976): Pictures of Facial Affect. Palo Alto:Consulting Psychologists Press. [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J (2004): Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron 44:1043–1055. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller JJ, Daskalakis ZJ (2008): A meta‐analytic study of changes in brain activation in depression. Hum Brain Mapp 29:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33:636–647. [DOI] [PubMed] [Google Scholar]
- Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM (2010): Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res 44:799–807. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET (2004): Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event‐related functional magnetic resonance imaging study. Arch Gen Psychiatr 61:877–889. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Brammer MJ, Suckling J, Kim J, Cleare AJ, Walsh ND, Mitterschiffthaler MT, Andrew CM, Pich EM, Bullmore ET (2007): Neural responses to happy facial expressions in major depression following antidepressant treatment. Am J Psychiatr 164:599–607. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S (2009): Burden and consequences of child maltreatment in high‐income countries. Lancet 373:68–81. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R (2003): Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci 23:10274–10282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, Cannistraci C, Hollon SD, Gore JC, Shelton RC (2011): Childhood trauma history differentiates amygdala response to sad faces within MDD. J Psychiatr Res 45:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Gotlib IH (2008): Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatr 63:1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Kolachana BS, Tessitore A, Fera F, Goldman D, Egan MF, Weinberger DR (2002): Serotonin transporter genetic variation and the response of the human amygdala. Science 297:400–403. [DOI] [PubMed] [Google Scholar]
- Harmelen A‐L van, Tol M‐J van, Demenescu LR, Wee NJA van der, Veltman DJ, Aleman A, Buchem MA van, Spinhoven P, Penninx BWJH, Elzinga BM (2012): Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc Cogn Affect Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grüsser SM, Flor H, Schumann G (2005): Amygdala‐prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci 8:20–21. [DOI] [PubMed] [Google Scholar]
- Joormann J, Cooney RE, Henry ML, Gotlib IH (2011): Neural correlates of automatic mood regulation in girls at high risk for depression. J Abnorm Psychol 121:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler H, Taubner S, Buchheim A, Münte TF, Stasch M, Kächele H, Roth G, Heinecke A, Erhard P, Cierpka M, Wiswede D (2011): Individualized and clinically derived stimuli activate limbic structures in depression: An fMRI study. PLoS ONE 6:e15712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun‐Todd DA (2004): Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. NeuroImage 21:1215–1223. [DOI] [PubMed] [Google Scholar]
- Kugel H, Eichmann M, Dannlowski U, Ohrmann P, Bauer J, Arolt V, Heindel W, Suslow T (2008): Alexithymic features and automatic amygdala reactivity to facial emotion. Neurosci Lett 435:40–44. [DOI] [PubMed] [Google Scholar]
- Laux L, Glanzmann P, Schaffner P, Spielberger CD (1981): Das State‐Trait Angstinventar.Weinheim, Germany:Beltz. [Google Scholar]
- Lawrence NS, Williams AM, Surguladze SA, Giampietro VP, Brammer MJ, Andrew CM (2004): Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatr 55:578–587. [DOI] [PubMed] [Google Scholar]
- Ledoux JE (2000): Emotion circuits in the brain. Annu Rev Neurosci 23:155–184. [DOI] [PubMed] [Google Scholar]
- Lee B‐T, Seong Whi Cho, Hyung Soo Khang, Lee B‐C, Choi I‐G, Lyoo IK, Ham B‐J (2007): The neural substrates of affective processing toward positive and negative affective pictures in patients with major depressive disorder. Prog Neuro‐psychopharmacol Biol Psychiatr 31:1487–1492. [DOI] [PubMed] [Google Scholar]
- Lehrl S (1995): Mehrfachwahl‐Wortschatz‐Intelligenztest MWT‐B.Göttingen:Hogrefe. [Google Scholar]
- MacQueen GM, Frodl T (2010): The hippocampus in major depression: Evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatr 16:252–264. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Jenness J, Lau JYF, Ackerman JP, Pine DS, Ernst M (2010): A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci 10:34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Guyer AE, Hodgdon HB, McClure EB, Charney DS, Ernst M, Kaufman J, Pine DS, Monk CS (2008): Recognition of facial emotions among maltreated children with high rates of post‐traumatic stress disorder. Child Abuse Negl 32:139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ (1996): A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383:812–815. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ (1999): A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci USA 96:1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR (2008): Serotonin transporter (5‐HTTLPR) genotype and amygdala activation: A meta‐analysis. Biol Psychiatr 63:852–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, O'sullivan U, Cowen PJ, Harmer CJ (2009): Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatr 194:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Ohira H, Haneda K, Iidaka T, Sadato N, Okada T, Yonekura Y (2004): Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: An event‐related fMRI study. NeuroImage 21:352–363. [DOI] [PubMed] [Google Scholar]
- Peluso MAM, Glahn DC, Matsuo K, Monkul ES, Najt P, Zamarripa F, Li J, Lancaster JL, Fox PT, Gao J‐H, Soares JC (2009): Amygdala hyperactivation in untreated depressed individuals. Psychiatr Res 173:158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R (2003): Neurobiology of emotion perception. I. The neural basis of normal emotion perception. Biol Psychiatr 54:504–514. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC (2008): A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatr 13: 829, 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch AV, Reker M, Ohrmann P, Pedersen A, Bauer J, Dannlowski U, Harding L, Koelkebeck K, Konrad C, Kugel H, Arolt V, Heindel W, Suslow T (2010): Increased amygdala activation during automatic processing of facial emotion in schizophrenia. Psychiatr Res 182:200–206. [DOI] [PubMed] [Google Scholar]
- Reker M, Ohrmann P, Rauch AV, Kugel H, Bauer J, Dannlowski U, Arolt V, Heindel W, Suslow T (2010): Individual differences in alexithymia and brain response to masked emotion faces. Cortex 46:658–667. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M (2010): Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatr 67:1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher CD, Forde DR, McQuaid JR, Stein MB (2004): Prevalence and demographic correlates of childhood maltreatment in an adult community sample. Child Abuse Negl 28:167–180. [DOI] [PubMed] [Google Scholar]
- Scherer KR (1993): Neuroscience projections to current debates in emotion psychology. Cogn Emot 7:1–41. [Google Scholar]
- Sehlmeyer C, Dannlowski U, Schöning S, Kugel H, Pyka M, Pfleiderer B, Zwitserlood P, Schiffbauer H, Heindel W, Arolt V, Konrad C (2010): Neural correlates of trait anxiety in fear extinction. Psychol Med 41:789–798. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun M a (2001): Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatr 50:651–658. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS (2002): Can't shake that feeling: event‐related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatr 51:693–707. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson WK, Carter CS, Steinhauer SR, Thase ME (2007): Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol Psychiatr 61:198–209. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A (2002): Prefrontal cortical regulation of hypothalamic‐pituitary‐adrenal function in the rat and implications for psychopathology: Side matters. Psychoneuroendocrinology 27:99–114. [DOI] [PubMed] [Google Scholar]
- Suslow T, Kugel H, Rauch AV, Dannlowski U, Bauer J, Konrad C, Arolt V, Heindel W, Ohrmann P (2009): Attachment avoidance modulates neural response to masked facial emotion. Hum Brain Mapp 30:3553–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schöning S, Ohrmann P, Bauer J, Pyka M, Kersting A, Arolt V, Heindel W, Dannlowski U (2010a)Automatic mood‐congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatr 67:155–160. [DOI] [PubMed] [Google Scholar]
- Suslow T, Kugel H, Reber H, Bauer J, Dannlowski U, Kersting A, Arolt V, Heindel W, Ohrmann P, Egloff B (2010b)Automatic brain response to facial emotion as a function of implicitly and explicitly measured extraversion. Neuroscience 167:111–123. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A (2012): PNAS Plus: Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA 109(9):E563–E572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin J, Casey BJ (2011): Elevated amygdala response to faces following early deprivation. Dev Sci 14:190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Öhman A, Drevets WC (2010): Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatr 67:1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Heim CM, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD (2002): Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatr 159:2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyers P, Krebs H, Janke W (1995): Reliability and construct validity of the German version of Cloninger's tridimensional personality questionnaire. Pers Individ Differ 19:853–861. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA (1998): Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ (2007): A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatr 64:49–56. [DOI] [PubMed] [Google Scholar]
- Williams MA, Morris AP, McGlone F, Abbott DF, Mattingley JB (2004): Amygdala responses to fearful and happy facial expressions under conditions of binocular suppression. J Neurosci 24:2898–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H‐U, Wunderlich U, Gruschwitz S, Zaudig M (1997): SKID‐I. Strukturiertes Klinisches Interview für DSM‐IV.Göttingen:Hogrefe. [Google Scholar]
- Wolfensberger SPA, Veltman DJ, Hoogendijk WJG, Boomsma DI, Geus EJC de (2008): Amygdala responses to emotional faces in twins discordant or concordant for the risk for anxiety and depression. NeuroImage 41:544–552. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2001): Mental Health: New Understanding, New Hope. Geneva, Switzerland:World Health Organization. [Google Scholar]
- Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, Wang W, Yao S (2011): Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol Psychol 88:233–242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


