Abstract
Placebo analgesia (PA) is one of the most studied placebo effects. Brain imaging studies published over the last decade, using either positron emission tomography (PET) or functional magnetic resonance imaging (fMRI), suggest that multiple brain regions may play a pivotal role in this process. However, there continues to be much debate as to which areas consistently contribute to placebo analgesia‐related networks. In the present study, we used activation likelihood estimation (ALE) meta‐analysis, a state‐of‐the‐art approach, to search for the cortical areas involved in PA in human experimental pain models. Nine fMRI studies and two PET studies investigating cerebral hemodynamic changes were included in the analysis. During expectation of analgesia, activated foci were found in the left anterior cingulate, right precentral, and lateral prefrontal cortex and in the left periaqueductal gray (PAG). During noxious stimulation, placebo‐related activations were detected in the anterior cingulate and medial and lateral prefrontal cortices, in the left inferior parietal lobule and postcentral gyrus, anterior insula, thalamus, hypothalamus, PAG, and pons; deactivations were found in the left mid‐ and posterior cingulate cortex, superior temporal and precentral gyri, in the left anterior and right posterior insula, in the claustrum and putamen, and in the right thalamus and caudate body. Our results suggest on one hand that the modulatory cortical networks involved in PA largely overlap those involved in the regulation of emotional processes, on the other that brain nociceptive networks are downregulated in parallel with behavioral analgesia. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: placebo, analgesia, humans, experimental pain, regional cerebral blood flow, PET, fMRI
INTRODUCTION
Placebo analgesia (PA) is one of the best‐studied placebo effects. The neurobiology of PA was born with the discovery that the opioid receptor antagonist naloxone can reverse PA in clinical and experimental pain [Benedetti, 1996; Levine et al., 1978]. Other studies suggest that endogenous opioids are not the only neurochemicals involved in PA. Indeed, nonopioid mechanisms have also been described, particularly in the context of previous preconditioning with nonopioid agents [Amanzio and Benedetti, 1999]. Many brain systems besides the endogenous opioid system may contribute to the placebo effect, such as the dopamine reward system [Scott et al., 2007, 2008].
The advent of brain imaging tools provided neuroscientists with a window into the brain activity orchestrating PA. Specifically, functional magnetic resonance imaging (fMRI) studies of blood oxygen level‐dependent (BOLD) signals or positron emission tomography (PET) studies of regional cerebral blood flow (rCBF) or neurochemical changes have enabled researchers to study changes in neural activity in experimental placebo paradigms. Placebo effects can be mediated by increased descending modulation via brainstem inhibitory systems and activation of endogenous opioid systems, which can lead to changes in the affective/‐motivational dimension of pain processing through the activation of cortical regions such as the anterior cingulate cortex (ACC) and the dorsolateral and orbitofrontal/‐ventrolateral prefrontal cortices (DLPFC, OFC, and VLPFC respectively; for review, see Benedetti et al. [2005]). In particular, the DLPFC cortex has been proved to be consistently associated with cognitive control of representation and maintenance of information, consistent with a role in expectation [Miller and Cohen, 2001]. Because the activation of both the DLPFC and OFC is correlated with brainstem activation in placebo expectation [Wager et al., 2004], the PFC may trigger PA responses through activation of other pain modulatory regions besides the ACC, including the thalamus and brainstem areas such as the periaqueductal gray (PAG) and rostral ventromedial medulla.
Importantly, Kong et al. [2006] underline that multiple pathways and mechanisms may explain the apparent inconsistencies in placebo imaging studies. One reason for the diverging evidence on the involvement of different brain regions in placebo‐related networks is represented by the heterogeneity of the experimental designs, such as pain paradigms and the degree of certainty associated with pain relief expectations [Ploghaus et al., 2003].
To identify human brain regions that are consistently implicated in placebo analgesia, we adopted a coordinate‐based meta‐analysis approach (activation likelihood estimation; ALE), which is considered to be the best method to search for the cortical areas implicated in the phenomenon of interest [Eickhoff et al., 2009; Salimi‐Khorshidi et al., 2009].
The aim of this study is to provide a quantitative meta‐analysis of the neuroimaging literature—using PET and fMRI to investigate cerebral hemodynamic changes—in order to delineate consistent activation of brain regions associated with PA in human experimental pain. Deactivation foci related to PA are also considered here in a separate analysis from that used to study activation sites. The neural correlates of PA were investigated through two different analyses, aimed at assessing changes occurring either during expectations of analgesia or during noxious stimulation.
MATERIALS AND METHODS
Literature Search, Selection, and Methodological Challenges
We followed the guidelines for meta‐analyses and systematic reviews of observational studies, MOOSE [Stroup et al., 2000].
A systematic search strategy was used to identify relevant studies, published on or before November 1, 2010, across the online database most frequently used in the international literature (Medline database with Pubmed literature search: http://www.pubmed.org; see also the PubAtlas search included in the Supporting Information).
All studies that included one of the following terms were considered: “Pain”; “Placebo effect”; “fMRI”; “PET.”
We also searched published meta‐analyses and reviews on PA to identify additional studies using the above‐mentioned neuroimaging techniques, which were not included in the Pubmed literature search database. The references included in the reviews identified in these searches were used as an additional source to identify other studies.
All articles were reviewed to establish that (1) the experimental pain paradigms included a baseline condition, in order to study functional activity during PA minus resting state conditions; (2) the results were reported in Talairach/Tournoux or in Montreal Neurological Institute (MNI) coordinates; (3) the field of view was not confined to a restricted region of the cortex [Radua and Mataix‐Cols, 2009; Radua et al., 2010] (4) the studies reported cerebral hemodynamic changes, as assessed by BOLD‐fMRI or rCBF‐PET.
We also tried to identify any instances of multiple reports of single data sets across articles to ensure that only one report of a study contributed to the coordinates for the present meta‐analysis. This assertion is true for all the selected studies, except for those by Price et al. [2007] and Craggs et al. [2008]; although both refer to the same experimental design and the same group of patients, the former reports deactivation foci, the latter the activation foci related to placebo analgesia.
The focus of our study was on PA in human experimental pain paradigms. However, two studies (described in three of the selected papers: Craggs et al., 2008; Price et al., 2007; Lu et al., 2010) considered patients, who were suffering either from irritable bowel syndrome (IBS; n = 9) or from functional gastrointestinal disorder (FGID; n = 14). Although IBS and FGID can cause discomfort, they do not harm the intestines or lead to any serious disease (see the Digestive Diseases Dictionary at http://www.digestive.niddk.nih.gov/index.html). Besides, as stated by Lu et al. (2010), none of the 14 FGID patients were taking drugs affecting gastrointestinal motor functions or pain perception. Finally, in all studies considered for the present meta‐analysis, PA did modulate experimentally induced pain, rather than spontaneous ongoing pain. On these grounds, we believe that it is appropriate to include the results from the three above‐mentioned papers in our meta‐analysis.
Studies were independently ascertained and checked by two of the authors for any discrepancies, which were resolved by a third author. In four cases (Craggs, Kong, Nemoto, and Petrovic), we contacted authors to avoid any errors in our interpretation of the experimental conditions and/or of the functional significance of the foci identified in the studies (all the authors kindly replied and resolved our doubts).
Following the suggestions of Rainville and Duncan [2006] in terms of methodological challenges and recommendations in experimental studies on PA, we carefully checked the conditions and experiments, placebo induction, pain assessment, possible selection of placebo responders, and the brain mechanisms related to PA. Descriptive information was extracted from each article including imaging and experimental modality, sample size, and pain stimulus attributes, such as modality, location (site, side), and duration. Tables I and II provide a detailed description of the methods used in the selected studies. A fixed‐intensity paradigm was defined as an experiment that used the same level of stimulation for all participants, whereas a subject‐dependent paradigm was defined as an experiment that used different stimulus intensities among subjects. In the latter paradigm, the intensity of stimuli used was individually determined by a prescan scaling procedure [Farrel et al., 2005].
Table I.
Experimental procedures and correspondent numbers of activation and deactivation foci (with reference to all studies included in the meta‐analysis)
| First author | Technique | Pain stagea | Stimulus | Site | Side | Duration | Intensity of stimulib | Stage 1 | Stage 2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Activation foci | Deactivation foci | Activation foci | Deactivation foci | ||||||||
| Bingel, 2006 | fMRI | 2 | Laser heat | Hand | Right/Left | 1 ms | Fixed | 0 | 0 | 2 | 0 |
| Craggs, 2008 Price, 2007 | fMRI | 2 | Rectal baloon distension | Rectum | 20 s | Subject dependent | 0 | 0 | 10 | 9 | |
| Eippert, 2009a | fMRI | 1, 2, 3 | Contact heat | Forearm | Right | 20 s | Subject dependent | 0 | 2 | 11 | 16 |
| Kong, 2006 | fMRI | 2 | Contact heat | Forearm | Right | 10 s | Subject dependent | 0 | 0 | 16 | 0 |
| Lu, 2010 | fMRI | 1, 2 | Esophageal baloon distension | Esophagus | 30 s | Subject dependent | 1 | 0 | 27 | 0 | |
| Lui, 2010 | fMRI | 1, 2 | Laser heat | Foot dorsum | Right/Left | 1–10 ms | Subject dependent | 1 | 0 | 3 | 7 |
| Nemoto, 2007 | PET | 3 | Laser heat | Volar forearm | Right | 6s | Fixed | 0 | 0 | 4 | 4 |
| Petrovic, 2002 | PET | 2 | Contact heat | Dorsum hand | Left | 70 s | Fixed | 0 | 0 | 4 | 0 |
| Wager [S1], 2004 | fMRI | 1, 2 | Electrical | Wrist | Right | 30 s | Subject dependent | 14 | 0 | 26 | 1 |
| Wager [S2], 2004 | fMRI | 1, 2 | Contact heat | Forearm | Left | 20 s | Subject dependent | 5 | 0 | 26 | 7 |
| Watson, 2009 | fMRI | 1, 2 | Laser heat | Dorsal surface of forearm | Right | 4 ms | Subject dependent | 6 | 0 | 3 | 0 |
| Experimental paradigm (%) | Subjects | Stage 1 | Stage 2 | ||||||||
| Activation foci | Deactivation foci | Activation foci | Deactivation foci | ||||||||
| Rectal baloon distension (9.09%) | 18 | 0 | 0 | 10 | 9 | ||||||
| Contact heat (36.36%) | 80 | 5 | 2 | 57 | 23 | ||||||
| Esophageal baloon distension (9.09%) | 14 | 1 | 0 | 27 | 0 | ||||||
| Laser heat (36.36%) | 63 | 7 | 0 | 12 | 11 | ||||||
| Electrical (9.09%) | 24 | 14 | 0 | 26 | 1 | ||||||
| Total | 199 | 27 | 2 | 132 | 44 | ||||||
Considered with reference to stage 1 (prestimulation pain expectation), stage 2 (start and end of the noxious stimuli administration).
A fixed‐intensity paradigm was defined as an experiment that used the same level of stimulation for all participants, a subject‐dependent paradigm was defined as an experiment that used dissimilar stimuli for each subject.
Table II.
Composition of samples in each individual study and of the actual overall sample
| First author | ♂ | ♀ | Menstrual cycle phase | Average age | Manual dominance | Partecipants |
|---|---|---|---|---|---|---|
| Bingel, 2006 | 12 | 4 | Unknown | 24 | Right | Healthy |
| Craggs, 2008; Price, 2007 | 0 | 9 | Unknown or PMPa | 27.7 | Unknown | IBSb |
| Eippert, 2009a | 19 | 0 | 26.1 | Unknown | Healthy | |
| Kong, 2006 | 9 | 7 | Unknown | 28.4 | Right | Healthy |
| Lu, 2010 | 5 | 9 | Mid‐Proliferative | 23.9 | Right | FGIDc |
| Lui, 2010 | 13 | 18 | Unknown | 23.5 | Right (26), left (1) | Healthy |
| Nemoto, 2007 | Unknown | Unknown | 23 | Right | Healthy | |
| Petrovic, 2002 | 9 | 0 | Range 20–70 | Unknown | Healthy | |
| Wager [S1], 2004 | Unknown | Unknown | Unknown | Healthy | ||
| Wager [S2], 2004 | Unknown | Unknown | Right | Healthy | ||
| Watson, 2009 | 5 | 6 | 27.5 | Right | Healthy | |
| Subjects (N) | Gender | Menstrual cycle phase (N) | Average age | Manual dominance (N) | Health status (N) | |
| 199 | ♂ (72) | MPPd (9) | 28.18 (± 7.93) | Right (148); Left (1) | Healthy subjects (172) | |
| ♀ (62) | PMP (9) | Left‐Right (4) | FGID (9) | |||
| Unknown (65) | Unknown (44) | Unknown (46) | IBS (18) | |||
Premenopausal phase.
Irritable bowel syndrome.
Functional gastrointestinal disorders.
The number of activation and deactivation foci was also established for each study.
We also checked the phase in which the placebo‐related brain activity had been studied, considering the three temporal stages of PA as defined by Kong et al. [2007] (Fig. 1):
Stage 1: Expectation/anticipation: Before the pain starts, expectation or anticipation of pain relief could modulate perception of the subsequent pain stimuli. In an fMRI paradigm, this corresponds to the period of “expected analgesia” [period of time between the beginning of the scan (or of each trial, in event‐related fMRI paradigms), and the beginning of the stimulus].
Stage 2: Noxious stimuli administration: During administration of painful stimuli, placebo treatment linked with previous knowledge about analgesic effects could inhibit the incoming signals of noxious stimuli. In particular, this component is related to brain activity between the beginning of the stimulus and its termination.
Stage 3: Subjective pain intensity rating (appraisal of pain). After the pain stimulus has ended, previous knowledge of treatment effect may unconsciously distort subjective pain rating.
Figure 1.
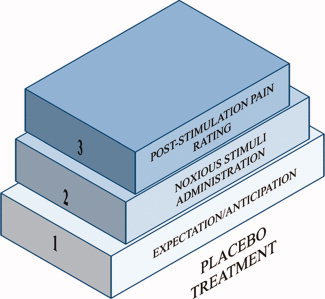
Placebo treatment can target each of the three stages described in the figure and the final outcome of pain. The treatment is considered as a function of time. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table I also represents studies in terms of placebo‐related activity during stages 1, 2, and 3 of analgesia (see Fig. 1). Six of eleven studies assessed alterations in stage 1 [Eippert et al., 2009a; Lu et al., 2010; Lui et al., 2010; Study 1 and 2 by Wager et al., 2004; Watson et al., 2009]. Ten of eleven studies assessed PA in the second stage condition [Bingel et al., 2006; Craggs et al., 2008; Eippert et al., 2009a; Kong et al., 2006; Lu et al., 2010; Lui et al., 2010; Petrovic et al., 2002; Price et al., 2007; Study 1 and 2 by Wager et al., 2004; Watson et al., 2009]. Two of eleven studies assessed PA also in the third stage condition [Eippert et al., 2009a; Nemoto et al., 2007].
In particular, Wager et al. [2004] sought to discriminate the activity induced by PA in the anticipation of pain from changes registered during pain experience. In the study by Kong et al. [2006], placebo acupuncture treatment was accompanied by analgesic expectations that were manipulated by lowering the temperature in the placebo side of the arm but not in the control side, causing the participants to experience analgesia. The study by Nemoto et al. [2007] investigated the last stage of PA in which participants were classified as responders or nonresponders based on subjective reports of reactions to painful stimuli after 7 days of pill placebo administration. The study by Eippert et al. [2009a] analyzed PA considering each of the three stages. Last, the study by Lui et al. [2010] investigated placebo‐related brain activity during stage 1 of analgesia (as compared to anticipation of pain) and during stage 2, in a model of conditioned placebo analgesia. All the other studies reported data in the second stage condition; on the other hand, Lu et al. [2010] also considered placebo‐related brain activity during stage 1 of analgesia.
We performed here two different analyses. In the first, we examined the neural changes related to prestimulation pain expectation of analgesia (stage 1 of the PA components in the model represented in Fig. 1), whereas in the second, we analyzed the changes in brain activity occurring during noxious stimulation (stage 2 in the model represented in Fig. 1).
Because of the paucity of data in the literature, we did not examine neural changes occurring during stage 3 or during placebo conditioning. In particular, the only paper where stages 2 and 3 were somehow intermingled is that by Nemoto et al. [2007]. However, because stimulation was continued throughout the acquisition scans, we believe that their results can be attributed to the stage 2. The foci of the study by Eippert et al. [2009a] referring to stage 3 were instead not considered in our analysis.
We used automated GingerALE 2.0 routines [Eickhoff et al., 2009; Laird, 2009] to convert MNI coordinates into Talairach/Tournoux space using the icbm2tal transform [Lancaster et al., 2007]. Importantly, the findings of Lancaster et al. [2007] show that MNI/Talairach coordinate bias associated with reference frame (position and orientation) and scale (brain size) can be substantially reduced using the best‐fit icbm2tal transform.
Coordinate‐Based Meta‐Analysis
Quantitative meta‐analysis techniques, such as ALE [Turkeltaub et al., 2002], provide information about the anatomical reliability of the results reported in a collection of related studies within the existing literature. ALE meta‐analysis is a quantitative voxel‐based meta‐analysis method that can be used to estimate consistent activation across different imaging studies [Laird et al., 2005]. This method does not rely upon author‐assigned anatomical labels; rather, it requires only that activation foci be reported in standard stereotactic space [Laird et al., 2005]. During an ALE analysis, each activation focus from each article is modeled as the center of a Gaussian probability distribution. These 3D Gaussian distributions are subsequently summed to create a statistical map that estimates the likelihood of activation for each voxel as determined by the entire set of studies. This map is then thresholded using permutation testing as proposed by the authors of the ALE methodology (Laird et al., 2005; Lancaster et al., 2000, 2007) and with reference to the literature on this matter (see http://www.brainmap.org).
ALE activation maps are usually derived on the basis of foci of interest, where multiple studies have reported statistically significant peak activation locations. To limit the intersubject and interlaboratory variability typical of neuroimaging studies, we used an algorithm that estimates the spatial uncertainty of each focus taking into account the possible differences among studies related to sample size [Eickhoff et al., 2009]. This algorithm was preferred to a prespecified FWHM as in the original ALE approach. The advantage of such an algorithm is that it comprises a method to calculate the above‐chance clustering between experiments (i.e., random effects analysis, RFX), rather than between foci (fixed effects analysis, FFX) [Eickhoff et al., 2009]. Accordingly, to improve the output, we performed an accurate coordinate transformation using the most recent and unbiased method [Eickhoff et al., 2009].
ALE maps were computed using a Java‐based version of ALE software named GingerALE (version 2.0.4) at an FDR‐corrected threshold of P < 0.05 and a minimum cluster size of K > 50 mm3. This software requires the coordinates to be provided in Talairach space and produces its output in the same standard. ALE maps were then visualized using Mricron (http://www.cabiatl.com/mricro/mricron/index.html) and BrainVoyager QX 2.2 [Goebel et al., 2006].
The location of clusters in the Talairach space was assigned by identifying the location of the coordinates of the maximum ALE value using the Talairach Daemon (http://www.talairach.org/) [Lancaster et al., 2000]. Each label was provided automatically by the program. Because important areas related to pain and placebo may fall within areas where the Talairach Daemon has insufficient coverage (e.g., pons and brain stem), we identified these locations separately using the “Duvernoy's Atlas of the Human Brain Stem and cerebellum” [Naidich et al., 2009].
Jackknife Analysis
To rule out the possibility of some activations being driven by the contribution of a small subset of studies, we performed a jackknife analysis. The jackknife is a nonparametric method for estimating the sampling distribution of a statistic [Fan and Wang, 1996; Radua and Mataix‐Cols, 2009; Radua et al., 2010; Wu, 1986; Shao and Tu, 1995]. Given a sample data set and a desired statistic (e.g., the mean), the jackknife works by computing the desired statistic with an element (or a group of elements) deleted. This is done for each element of the data set. The collection of these statistics is used as an estimate of the sampling distribution. A radar plot of the sampling distribution is usually performed on the computed values of the statistic [Fan and Wang, 1996; Wu, 1986].
RESULTS
The studies that were excluded are listed in the appendix in the Supporting Information along with the reason for their exclusion. In particular, we excluded 43 fMRI studies and 21 PET studies (see the trial flow represented in the Graph 1).
Illustration .
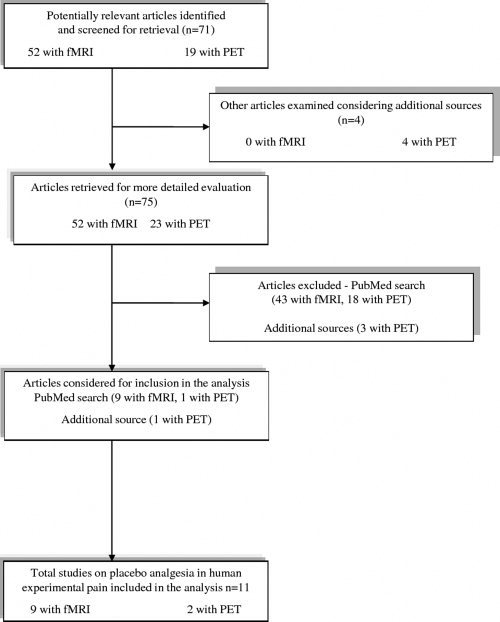
Graph 1: Trial flow: Selection of study reports (see Appendix I for more details).
The characteristics of the 11 studies (reporting nine experiments with BOLD‐fMRI and two experiments with rCBF‐PET), designated as suitable for meta‐analysis, are reported in Table I. They included the studies described in the work by Wager et al. [2004], which analyzed two experimental sessions and were consequently coded as two reports, and the works by Price et al. [2007] and Craggs et al. [2008], which are based upon the same experiments (see Methods section). Together, these studies include data from 199 subjects (154 for fMRI and 45 for PET paradigms) and report 159 activation and 46 deactivation foci. Most studies explored pain‐related brain activity using fMRI (81.81%) in healthy subjects (86.43%) and used contact noxious heat stimuli (36.36%) or laser noxious heat stimuli (36.36%). The remaining 18.18% of the studies used rectal balloon or esophageal balloon distension.
The 11 experiments yielded tabulated coordinates for 36 contrasts involving left‐side stimulation and 98 contrasts for right‐side stimulation. The ALE analysis of the left side incorporated 118 foci and the right analysis 87 foci. The labels ascribed to the activation sites by the authors of the studies are summarized in the Supporting Information Table I.
The average age of the samples (calculated from 9 studies) was 28.18 years (see Table II). Manual dominance was only specified for 76.88% of cases; most subjects were right‐handed (96.73%). There was a lack of information about gender for 32.66% of our sample. The remaining 36.18% were men, while 31.15% were women. About 86.43% of the samples were healthy.
Clusters of Neural Activity Changes
The brain regions identified in the meta‐analysis are presented in Tables III–V.
Table III.
Areas of increased activity associated with placebo analgesia during stage 1a
| Cluster no. | Volume (mm3) | X | Y | Z | Hemisphere | Label | BA |
|---|---|---|---|---|---|---|---|
| 1 | 520 | 38 | 0 | 32 | R | Precentral gyrus | 6 |
| 2 | 328 | −8 | 38 | −2 | L | rACC | |
| 3 | 248 | −6 | 22 | 30 | L | Anterior mid‐cingulate | 32 |
| 4 | 80 | 26 | 44 | 0 | R | Lateral prefrontal cortex | 10 |
| 5 | 64 | −2 | −26 | −10 | L | PAG |
aStage 1 (prestimulation pain expectation).
L, left; R, right; BA, Brodmann area; PAG, periaqueductal gray; rACC, rostral anterior cingulate.
PA‐related changes during prestimulation pain expectation (stage 1)
Expectation component cluster analysis for stage 1 of PA identified three clusters with a volume of more than 200 mm3 where there was increased placebo‐related activity compared to baseline values. These included the right precentral gyrus and the left ACC.
Two clusters with volumes exceeding 50 mm3 were located in the right rostral prefrontal cortex and the left periacqueductal gray (Table III and Figs. 2 and 3).
Figure 2.
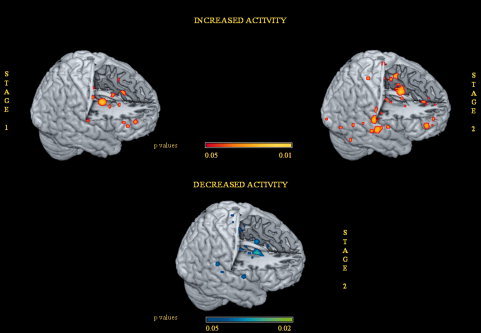
Areas of increased activity associated with placebo analgesia in the expectation stage 1 and during noxious stimulation stage 2. Areas of decreased activity associated with placebo analgesia in stage 2 are also shown. ALE maps were computed using GingerALE 2.0.4 at an FDR‐corrected threshold of P < 0.05, with a minimum cluster size of K > 50 mm3 and visualized using MRIcron. They were projected onto a 3D rendering model of the brain.
PA‐related changes during noxious stimulation (stage 2)
The noxious stimulation cluster analysis identified five clusters with a volume of more than 300 mm3 showing increased PA activity compared to baseline values. These included the left medial dorsal nucleus of the thalamus, the bilateral rostral ACC (rACC), and the pons. Five other clusters with a volume of more than 200 mm3 exhibited a network of co‐activated regions including the anterior insula, the periacqueductal gray, the parahippocampal gyrus, the inferior parietal lobule (all of which were more left lateralized), and the orbito‐frontal area with a right lateralization. Another cluster with a volume of more than 100 mm3 was the left postcentral gyrus (Table IV).
Table IV.
Areas of increased activity associated with placebo analgesia during stage 2a
| Cluster no. | Volume (mm3) | X | Y | Z | Hemisphere | Label | BA |
|---|---|---|---|---|---|---|---|
| 1 | 640 | −4 | −14 | 12 | L | Medial dorsal nucleus | |
| 2 | 512 | 18 | 8 | 40 | R | rACC | 32 |
| 3 | 328 | 0 | 36 | 16 | L | rACC | 32 |
| 4 | 312 | −6 | −30 | −32 | L | Pons | |
| 5 | 288 | −50 | −10 | 12 | L | Anterior insula | 13 |
| 6 | 280 | −4 | −34 | −16 | L | PAG | |
| 7 | 272 | −12 | −30 | −4 | L | Parahippocampal gyrus | 30 |
| 8 | 232 | −58 | −26 | 24 | L | Inferior parietal lobule | 40 |
| 9 | 208 | 0 | 40 | −18 | R | Orbito‐frontal area | 11 |
| 10 | 176 | −66 | −14 | 24 | L | Postcentral gyrus | 1 |
| 11 | 64 | −14 | −38 | −38 | L | Pons | |
| 12 | 64 | −22 | 38 | 34 | L | DLPFC | 9 |
| 13 | 64 | −40 | −26 | 52 | L | Postcentral gyrus | 3 |
| 14 | 56 | −6 | −2 | −12 | L | Hypothalamus | |
| 15 | 56 | −2 | −10 | 48 | L | Medial frontal gyrus | 6 |
Stage 2 (start and end of noxious stimuli administration).
L, left; R, right; BA, Brodmann area; DLPFC, dorsolateral prefrontal cortex; PAG, periaqueductal gray; rACC, rostral anterior cingulate.
Two clusters of decreased PA activity with a volume of more than 600 mm3 were found in the claustrum, in the putamen, and in the posterior cingulate, all of which were more left lateralized. Two other clusters of deactivation with a volume more than 300 mm3 were seen in the left superior temporal gyrus. Other clusters with a volume more than 100 mm3 were seen in the left mid‐cingulate cortex, in the right caudate body, and putamen. Smaller clusters were found in the left anterior insula and in the right posterior insula (Table V and Figs. 4 and 5).
Table V.
Areas of decreased activity associated with placebo analgesia during stage 2a
| Cluster no. | Volume (mm3) | X | Y | Z | Hemisphere | Label | BA |
|---|---|---|---|---|---|---|---|
| 1 | 1256 | −34 | 0 | −2 | L | Claustrum | |
| −26 | 10 | −2 | L | Putamen | |||
| 2 | 608 | −6 | −18 | 46 | L | Posterior cingulate | 31 |
| 3 | 328 | −40 | −20 | 2 | L | Superior temporal gyrus | |
| 4 | 320 | −52 | −30 | 20 | L | Superior temporal gyrus | 41 |
| 5 | 272 | −2 | 6 | 32 | L | Mid‐cingulate | 24 |
| 6 | 128 | 16 | 4 | 12 | R | Caudate body | |
| 22 | 4 | 18 | R | Putamen | |||
| 7 | 80 | −38 | −10 | −6 | L | Subgyral | 21 |
| 8 | 80 | 56 | −38 | 20 | R | Posterior insula | 13 |
| 9 | 64 | 2 | −16 | 12 | R | Medial dorsal nucleus | |
| 10 | 64 | −56 | −6 | 12 | L | Precentral gyrus | 43 |
| 11 | 56 | 36 | −6 | −6 | R | Claustrum | |
| 12 | 56 | −42 | −4 | 8 | L | Anterior insula | 13 |
Stage 2 (start and end of noxious stimuli administration).
L, left; R, right; BA, Brodmann area.
Figure 4.
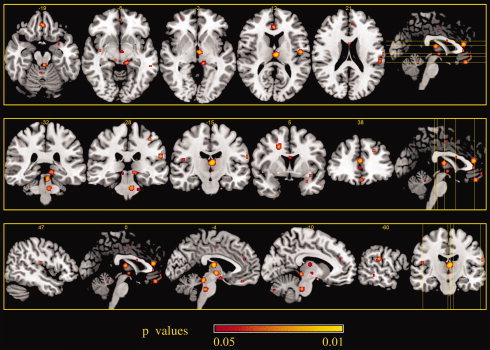
Areas of increased activity associated with placebo analgesia during noxious stimulation stage 2. ALE maps were computed using GingerALE 2.0.4 at an FDR‐corrected threshold of P < 0.05, with a minimum cluster size of K > 50 mm3 and visualized using MRIcron.
Figure 5.
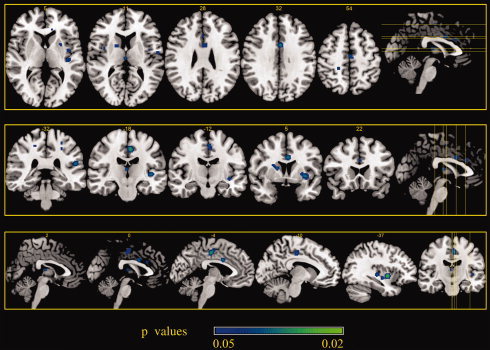
Areas of decreased activity associated with placebo analgesia during noxious stimulation stage 2. ALE maps were computed using GingerALE 2.0.4 at an FDR‐corrected threshold of P < 0.05, with a minimum cluster size of K > 50 mm3 and visualized using MRIcron.
Reliability of the Identified Clusters: Jackknife Analysis
The jackknife analysis described in the Methods section allowed us to affirm the validity of the results obtained through the ALE methodology. Indeed, no identified blob was driven by a single paper, and reliability was ≥ 50%, for all clusters (see Fig. 6 and Figs. A and B of the Supporting Information).2, 3, 4, 5
Figure 6.
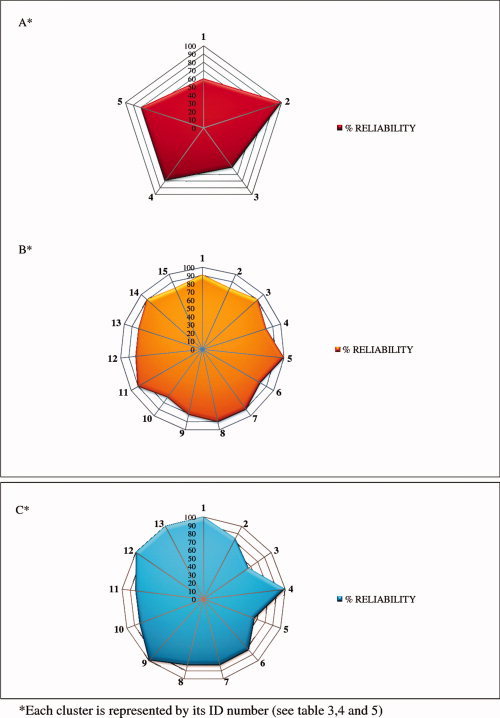
Reliability of increased activity associated with placebo analgesia in stage 1 (A) and in stage 2 (B). In (C), reliability of decreased activity associated with placebo analgesia in stage 2. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
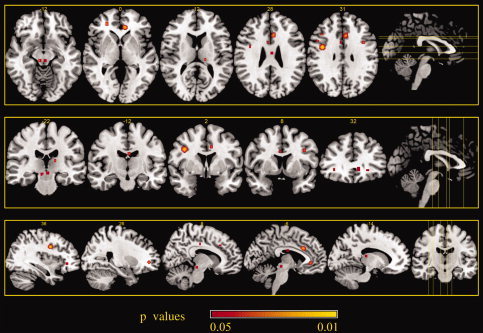
Areas of increased activity associated with placebo analgesia in the expectation stage 1. ALE maps were computed using GingerALE 2.0.4 at an FDR‐corrected threshold of P < 0.05, with a minimum cluster size of K > 50 mm3 and visualized using MRIcron.
DISCUSSION
The goal of this coordinate‐based ALE meta‐analysis was to quantitatively analyze the results of neuroimaging studies investigating cerebral hemodynamic changes, aiming to investigate the neural correlates of placebo analgesia. Our study represents the first attempt to consider all the data from the literature in a single analysis, in order to provide a more objective overall perspective. Meta‐analyses are prone to selection biases, although we tried to control as many factors as possible [Rainville and Duncan, 2006]. Importantly, by performing different analyses, we were able to consider the neural changes related to expectations of analgesia separately from those concerning PA‐related changes during the noxious stimulation (see Methods section).
Notably, our study represents the first attempt to summarize the brain areas involved in PA in human experimental pain. Despite the small number of studies suitable for inclusion, our analyses identified significant ALE clusters, which were broadly consistent with the proposed regions involved in PA in human experimental pain. Indeed, altogether, these studies included data from 199 subjects (154 for fMRI and 45 for PET paradigms) and reported 162 activation and 46 deactivation foci. These numbers were sufficient for us to objectively proceed with the ALE analysis (as stated by Laird in his “Users' Manual for BrainMap GingerALE 2.0”). Moreover, as seen in Figure 6, none of the “blobs” that we identified through our analyses could be deemed to have been guided by a single work. On the basis of the Jackknife analysis findings, we can state that even the use of a small number of works does not lead to spotty results.
From this evidence, we can now draw a possible theoretical explanation of placebo analgesia.
During noxious stimulation, we observed an increased activity in the ACC, the insula, and the diencephalon (the thalamus and the hypothalamus) as well as in brainstem regions such as the PAG and pons, whose involvement in descending and specifically the opioid analgesia is well established [Yaksh, 1997]. Descending influences from the ACC, insular and prefrontal cortex and diencephalon that elicit inhibition or facilitation of nociceptive transmission via brainstem structures are now thought to occur during PA (see the review of Tracey and Mantyh [2007]).
Conversely, we demonstrate that PA is accompanied by decreased activity in some portions of the pain matrix [Melzack, 1999; Tracey and Mantyh, 2007], such as the mid‐cingulate cortex and the anterior and posterior insula, as well as in the basal ganglia. Because placebo‐induced decreases were detectable during noxious stimulation, they are likely to reflect actual reduction of nociceptive processing, rather than modulation of the cognitive evaluation of pain intensity. Indeed, there is preliminary evidence that placebo‐induced antinociceptive effects can be identified in the spinal cord [Eippert et al., 2009b], although confirmatory data are required in this regard. The lack of consistent deactivation in the somatosensory cortices is conceivably related to the different body sites and stimuli employed in the different studies.
Altogether, these results lend further support to the hypothesis that PA is based at least in part upon changes in actual processing of nociceptive information and, therefore, in pain perception [Lui et al., 2010; Price et al., 2007], although additional components cannot be ruled out.
Other interesting insights about the mechanisms of PA can be gained by focusing on changes in brain activity that take place during the expectation phase (stage 1). In fact, expectation of benefit can induce a placebo effect even without the physical administration of a placebo. Because no placebo is actually given, these effects may be more appropriately called “placebo‐like” effects. Thus, activity in pain areas following an identical painful stimulus can be modulated just by varying the subject's expectation of the level of stimulation: the higher the expected level of the stimulus, the stronger the activity, that is, in the ACC and the PAG and other areas implicated in the activation of the descending inhibitory pathway [Koyama et al., 2005]. Indeed, it has been shown that the activation of the same neurotransmitter systems in the brain can be obtained by a pharmacological (drug) or a psychological (placebo) means [Zubieta and Stohler, 2009]. As far as the expectation of analgesia network was concerned, we observed an increased activity associated with PA in areas such as the ACC and the PAG. Interestingly, an area selectively activated in the PA expectation component and not present when we analyzed the PA during noxious stimulation is BA10, demonstrating that anticipatory responses may well be part of a more general mechanism that is not confined to pain. This area is considered as a cognitive control region and one of the most fascinating puzzles in cognitive neuroscience [Burgess et al., 2005]. It is consistently implicated in tasks where one has to “bear something in mind” whilst doing something else, for example, voluntary task switching after a delay [e.g., Koechlin et al., 1999], prospective memory [Burgess et al., 2001, 2003; Okuda et al., 1998], and “monitoring” type tasks [e.g., MacLeod et al., 1998]. Thus, the characterization of the rostral processing system as cross‐domain [Burgess et al., 2003] and serving the purpose of guiding behavior in situations where the optimal course of action is not obvious or established seems secure [Burgess et al., 2000; Goel and Grafman, 2000; Pollman, 2004]. In particular, an analysis of context, in terms of cognitive flexibility, prospective memory, monitoring, and executive attention abilities, appears to indicate different responses in the working memory counterpart [Damasio, 1996; Wager et al., 2004], a particular condition impaired in AD patients [Amanzio et al., 2011], in which loss of expectancy mechanisms disrupts PA and thus makes analgesic therapies less effective [Benedetti et al., 2006].
The results of both our analyses demonstrate how the areas engaged by PA in human experimental pain may be part of a general circuit underlying the voluntary regulation of affective responses [Bechara et al., 1997; Damasio, 1996; Petrovic et al., 2005; Wager et al., 2011].
Apart from the involvement of similar regions in the processing of pain and emotion, both PA [Lieberman et al., 2004; Petrovic and Ingvar, 2002; Wager et al., 2004] and emotional regulation [Bishop et al., 2004; Ochsner et al., 2002] are associated with increased activation in a modulatory network that includes the rACC and prefrontal cortices. This suggests a functional–anatomical relationship between PA and emotional regulation in which top–down modulation of the pain or emotional network is implemented. We suggest that the placebo phenomenon may be applied to any emotional experience, in terms of a reduction in negative emotions; indeed, our data demonstrate the involvement of a modulatory array of brain regions very near to those observed by Petrovic et al. [2005] in the emotional placebo.
CONCLUSIONS
The present data suggest that pain perception may be reduced through a placebo treatment by networks similar to those described for the modulation of emotional experience and the anticipation of therapeutic benefit [Petrovic et al., 2005].
Indeed, both PA [Lieberman et al., 2004; Petrovic et al., 2002; Wager et al., 2004] and emotional regulation [Bishop et al., 2004; Ochsner et al., 2002] are associated with increased activation in the ACC, the lateral prefrontal and orbitofrontal cortices, the periaqueductal gray, and with decreased activation of the regions involved in mood changes, thus suggesting a role of anxiety reduction through the placebo treatment. This points to a functional–anatomical relationship between PA and emotional regulation in which a top–down modulation of the pain or emotional network is implemented.
Specifically, PA analgesia is accompanied by decreased activation in some regions of the pain matrix during noxious stimulation. These findings point to a true antinociceptive effect associated with placebo analgesia, in addition to its potential modulation of cognitive evaluation of pain intensity.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
The authors thank the anonymous reviewers for their contribution to the improvement of the article.
REFERENCES (Included studies are emphasized by an asterisk)
- Amanzio M, Benedetti F ( 1999): Neuropharmacological dissection of placebo analgesia: Expectation‐activated opioid systems versus conditioning‐activated specific subsystems. J Neurosci 19: 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanzio M, Torta DME, Sacco K, Cauda F, D'Agata F, Duca S, Leotta D, Palermo S, Geminiani G ( 2011): Unawareness of deficits in Alzheimer's disease: Role of the cingulate cortex. Brain 134: 1061–1076. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR ( 1997): Deciding advantageously before knowing the advantageous strategy. Science 275: 1293–1295. [DOI] [PubMed] [Google Scholar]
- Benedetti F ( 1996): The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. Pain 64: 535–543. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK ( 2005): Neurobiological mechanisms of the placebo effect. J Neurosci 25: 10390–10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Arduino C, Costa S, Vighetti S, Tarenzi L, Rainero I, Asteggiano G ( 2006): Loss of expectation‐related mechanisms in Alzheimer's disease makes analgesic therapies less effective. Pain 121: 133–144. [DOI] [PubMed] [Google Scholar]
- *Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C ( 2006): Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain 120: 8–15. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD ( 2004): Prefrontal cortical function and anxiety: Controlling attention to threat‐related stimuli. Nat Neurosci 7: 184–188. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T ( 2000): The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia 38: 848–863. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD ( 2001): Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia 39: 545–555. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Scott SK, Frith CD ( 2003): The role of the rostral frontal cortex (area 10) in prospective memory: A lateral versus medial dissociation. Neuropsychologia 41: 906–918. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Simons JS, Dumontheil I, Gilbert SJ ( 2005): The gateway hypothesis of rostral PFC functioning In: Duncan, Phillips JL, McLeod P, editors. Measuring the Mind: Speed, Control and Age. Oxford: Oxford University Press; pp 215–246. [Google Scholar]
- *Craggs JG, Price DD, Perlstein WM, Verne GN, Robinson ME ( 2008): The dynamic mechanisms of placebo induced analgesia: Evidence of sustained and transient regional involvement. Pain 139: 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR ( 1996): The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 351: 1413–1420. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT ( 2009): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30: 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C ( 2009a): Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63: 533–543. [DOI] [PubMed] [Google Scholar]
- Eippert F, Finsterbusch J, Bingel U, Buchel C ( 2009b): Direct evidence for spinal cord involvement in placebo analgesia. Science 326: 404. [DOI] [PubMed] [Google Scholar]
- Fan X, Wang L ( 1996): Comparability of Jackknife and Bootstrap results: An investigation for a case of canonical correlation analysis. J Exp Educ 64: 173–189. [Google Scholar]
- Farrell MJ, Laird AR, Egan GF ( 2005): Brain activity associated with painfully hot stimuli applied to the upper limb: A meta‐analysis. Human Brain Mapp 25: 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E ( 2006): Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single‐subject to cortically aligned group general linear model analysis and self‐organizing group independent component analysis. Hum Brain Mapp 27: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel V, Grafman J ( 2000): Role of the right prefrontal cortex in ill‐structured planning. Cogn Neuropsychol 17: 415–436. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J ( 1999): The role of the anterior prefrontal cortex in human cognition. Nature 399: 148–151. [DOI] [PubMed] [Google Scholar]
- *Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ ( 2006): Brain activity associated with expectancy‐enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci 26: 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Kaptchuk TJ, Polich G, Kirsch I, Gollub I ( 2007): Placebo analgesia: Findings from brain imaging studies and emerging hypotheses. Rev Neurosci 18: 173–190. [DOI] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC ( 2005): The subjective experience of pain: Where expectations become reality. Proc Natl Acad Sci USA 102: 12950–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR ( 2009): Users' Manual for BrainMap GingerALE 2.0. Copyright 2003–2009, Research Imaging Center, UTHSCSA. [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT ( 2005): ALE meta‐analysis: Controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT ( 2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT ( 2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28: 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Fields HL ( 1978): The mechanism of placebo analgesia. Lancet 2: 654–657. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Jarcho JM, Berman S, Naliboff BD, Suyenobu BY, Mandelkern M, Mayer EA ( 2004): The neural correlates of placebo effects: A disruption account. Neuroimage 22: 447–455. [DOI] [PubMed] [Google Scholar]
- *Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, Porro CA ( 2010): Neural bases of conditioned placebo analgesia. Pain 151: 815–823. [DOI] [PubMed] [Google Scholar]
- *Lu HC, Hsieh JC, Lu CL, Niddam DM, Wu YT, Yeh TC, Cheng CM, Chang FY, Lee SD ( 2010): Neuronal correlates in the modulation of placebo analgesia in experimentally‐induced esophageal pain: A 3T‐fMRI study. Pain 148: 75–83. [DOI] [PubMed] [Google Scholar]
- MacLeod AK, Buckner RL, Miezin FM, Petersen SE, Raichle ME ( 1998): Right anterior prefrontal cortex activation during semantic monitoring and working memory. Neuroimage 7: 41–48. [DOI] [PubMed] [Google Scholar]
- Melzack R ( 1999): From the gate to the neuromatrix. Pain Suppl 6: S121–S126. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD ( 2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Naidich TP, Duvernoy HM, Delman BN, Sorensen AG, Kollias SS, Haacke EM ( 2009): Duvernoy's Atlas of the Human Brain Stem and Cerebellum. New York: SpringerWien. [Google Scholar]
- *Nemoto H, Nemoto Y, Toda H, Mikuni M, Fukuyama H ( 2007): Placebo analgesia: A PET study. Exp Brain Res 179: 655–664. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD ( 2002): Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14: 1215–1229. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Yamadori A, Kawashima R, Tsukiura T, Fukatsu R, Suzuki K, Ito M, Fukuda H ( 1998): Participation of the prefrontal cortices in prospective memory: Evidence from a PET study in humans. Neurosci Lett 253: 127–130. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M ( 2005): Placebo in emotional processing‐induced expectations of anxiety relief activate a generalized modulatory network. Neuron 46: 957–969. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M ( 2002): Imaging cognitive modulation of pain processing. Pain 95: 1–5. [DOI] [PubMed] [Google Scholar]
- *Petrovic P, Kalso E, Petersson KM, Ingvar M ( 2002): Placebo and opioid analgesia—Imaging a shared neuronal network. Science 295: 1737–1740. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Becerra L, Borras C, Borsook D ( 2003): Neural circuitry underlying pain modulation: Expectation, hypnosis, placebo. Trends Cogn Sci 7: 197–200. [DOI] [PubMed] [Google Scholar]
- Pollmann S ( 2004): Anterior prefrontal cortex contributions to attention control. Exp Psychol 51: 270–278. [DOI] [PubMed] [Google Scholar]
- *Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME ( 2007): Placebo analgesia is accompanied by large reductions in pain‐related brain activity in irritable bowel syndrome patients. Pain 127: 63–72. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix‐Cols D ( 2009): Voxel‐wise meta‐analysis of grey matter changes in obsessive‐compulsive disorder. Br J Psychiatry 195: 393–402. [DOI] [PubMed] [Google Scholar]
- Radua J, Via E, Catani M, Mataix‐Cols D ( 2010): Voxel‐based meta‐analysis of regional white‐matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med 16: 1–12. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH ( 2006): Functional brain imaging of placebo analgesia: Methodological challenges and recommendations. Pain 121: 177–180. [DOI] [PubMed] [Google Scholar]
- Salimi‐Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE ( 2009): Meta‐analysis of neuroimaging data: A comparison of image‐based and coordinate‐based pooling of studies. Neuroimage 45: 810–823. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK ( 2007): Individual differences in reward responding explain placebo‐induced expectations and effects. Neuron 55: 325–536. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK ( 2008): Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry 65: 220–231. [DOI] [PubMed] [Google Scholar]
- Shao J, Tu D ( 1995): The Jackknife and Bootstrap. New York: Springer‐Verlag. [Google Scholar]
- Stroup DF, Berlin JA, Morton CS, Olkim I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB ( 2000): Meta‐analysis of observational studies in epidemiology: A proposal for reporting. JAMA 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW ( 2007): The cerebral signature for pain perception and its modulation. Neuron 55: 377–391. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA ( 2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. Neuroimage 16: 765–780. [DOI] [PubMed] [Google Scholar]
- *Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD ( 2004): Placebo‐induced changes in FMRI in the anticipation and experience of pain. Science 303: 1162–1167. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK ( 2011): Predicting individual differences in placebo analgesia: Contributions of brain activity during anticipation and pain experience. J Neurosci 31: 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A, El‐Deredy W, Iannetti GD, Lloyd D, Tracey I, Vogt BA, Nadeau V, Jones AK ( 2009): Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain 145: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CFJ ( 1986): Jackknife, bootstrap and other resampling methods in regression analysis. Ann Stat 14: 1261–1295. [Google Scholar]
- Yaksh TL ( 1997): Pharmacology and mechanisms of opioid analgesic activity. Acta Anaesthesiol Scand 41: 94–111. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Stohler CS ( 2009): Neurobiological mechanisms of placebo responses. Ann NY Acad Sci 1156: 198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


