Abstract
Behavioral studies indicate that directional gaze and hand pointing are fundamental social signals that may capture spatial attention more powerfully than directional arrows. By using fMRI, we explored whether reflexive shifts of attention triggered by different distracters were influenced by the motor effector used for performing an overt response. In separate blocks, healthy participants performed a directional saccadic or a hand pointing movement. Color changes of a central black fixation point constituted the imperative instruction signal to make a leftward (red color) or a rightward (blue color) movement while ignoring distracting leftward or rightward oriented gaze, hand pointing, or arrow. Distracters that were directionally incongruent with the instruction cue impaired the saccadic and pointing‐release RTs. The comparison of incongruent vs. congruent conditions showed an increase of BOLD signal in the frontal eye field (FEF), the intraparietal sulcus (IPS), and the posterior parietal cortex (PPC) bilaterally. Importantly, a specific relationship between distracter and effector used for the response was found in these frontal and parietal regions. In particular, higher activity in the FEF, for distracting gaze was found mainly during the saccadic response task. In the same vein, higher activity in the left and right IPS regions was found for the distracting hand mainly in the hand pointing task. The results suggest that reflexive shifts of attention triggered by social signals are coded in the fronto‐parietal cortex according to effector‐specific mapping rules. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: reflexive attention, gaze, hand, mirroring, coordinate systems, pointing, saccadic eye movements
INTRODUCTION
Allocation of attention to a specific point in space may be automatically triggered by biological (e.g., averted gaze or pointing hands) as well as non‐biological directional signals (e.g., regulatory or warning road arrows) [Frischen et al.,2007; Itier and Batty,2009; Langton et al.,2000]. Whether biological cues are pre‐eminent in determining attentional shifts with respect to non‐biological cues is hotly debated [Bonato et al.,2009; Eimer,1997; Friesen and Kingstone,1998; Friesen et al.,2004,2005; Hietanen,1999; Jonides,1981; Ristic et al.,2002,2007; Stevens et al.,2008; Tipples,2002], mainly because laboratory based paradigms use impoverished tasks that hardly reproduce the situational complexity of real life human interactions [Birmingham and Kingstone,2009; Kingstone,2009]. Indeed, under daily life condition, fundamental socio‐cognitive operations like intention and mind reading, are inherently linked to the power of gaze in capturing the attention of an observer and in triggering reflexive joint attention [Kuhn and Land,2006; Smilek et al.,2006].
Possibly, because eye contact is a hallmark of interpersonal interactions, a considerable number of behavioral studies focused on the role of gaze perception in modulating social attention [Kingstone,2009; Klein et al.,2009; Nummenmaa and Calder,2009]. Importantly, unlike non‐social orienting cues such as arrows, gaze cues not only signal a seen agent's direction of attention but are also used to infer current goals and intentions of other individuals. This difference raises the important issue, explored by recent functional neuroanatomy and electrophysiological studies, of whether orienting attention to biological, socially relevant cues, such as gaze, may engage neural mechanisms distinct from those engaged by orienting to non‐social cues. Hietanen et al. [2006], for example, explored at behavioral and neural levels the effect of responding to left or right visual targets preceded by central non‐predictive gaze or arrow cues pointing to same or opposite direction. Although the interference effect (IE) of cue‐target directional incongruence was found for both gaze and arrows, changes of BOLD signal revealed that while gaze‐cued orienting recruits occipital regions, arrow‐cued orienting also recruits parietal and frontal regions. That arrow‐cues related orienting activates a larger network with respect to gaze‐cue related orienting is also suggested by an event‐related study showing that changes of parietal and frontal attention‐directed neuroelectric signatures are found for arrow—but not for gaze‐cues [Hietanen et al.,2008]. However, using an ingenious event‐related fMRI design in which the central cue was an ambiguous stimulus that could appear as an eye in profile or an arrow, Tipper and colleagues [2008] demonstrated that attention to social and non‐social cues activates a largely overlapping neural network centered upon ventral and dorsal fronto‐parietal and lateral occipital regions. Since activation in two regions of this network, namely the ventral frontal and the lateral occipital cortex, was higher for gaze‐ than arrow‐cues, the authors suggested that quantitative more than qualitative differences underlie the social vs. non‐social mapping of attentional shifts [Tipper et al.,2008].
Although most of the original studies focused on the importance of gaze in social attention, body parts other than the eyes play a fundamental role in triggering joint attention. Studies demonstrate, for example, that full body/head orientation as well as hand orientation of a model modulates attentional shifts of an observer [Langton and Bruce,2000; Langton,2000; Pierno et al.,2008]. Much less is known on whether shifts of attention are similarly triggered by different person‐related cues. Information on whether reflexive social attention triggered by different person‐related cues is mapped according to the social valence of the cue or in body‐centered coordinates is very scanty. Studies indicate that social attention may recruit a more extensive neural network with respect to non‐social spatial attention. Indeed, areas involved in face, gaze, hand, and even full body perception may be called into play specifically in social attention tasks [Nummenmaa and Calder,2009]. This raises the question of whether social spatial attention may be coded according to body‐centered coordinate systems.
In a recent behavioral study, we explored whether the IE of person‐related cues (averted gaze and pointing hands) and of non‐social stimuli (arrow) was specifically influenced by the type of effector used for responding namely, saccadic movements and hand pointing [Crostella et al.,2009]. We expected that a non‐specific spatial interference of social stimuli would produce higher interference of gaze and pointing hands than arrows, regardless of the body part performing the action. By contrast, we hypothesized that finding a relation between the type of distracting stimulus and the type of response would suggest that additional reference frames are called into action in the task. The results showed that distracting gaze stimuli interfere specifically with saccadic performance and distracting hand stimuli with pointing performance. Relevant to this issue is the fMRI study showing that mere observation of directional and non‐directional eyes, hands, and arrows in the absence of any motor response, activated overlapping neural regions that included the posterior superior temporal sulcus (STS), the inferior parietal lobule (IPL), the inferior frontal gyrus (IFG), and the occipital cortices in the right hemisphere [Sato et al.,2009].
Capitalizing on such behavioral and neuroimaging evidence we sought to determine whether the neural activity in the network underpinning the observation of person‐ and non‐person‐related signals was modulated by the relationship between type of distracter and type of effector used for the response. We recorded changes of BOLD fMRI signal associated to conditions where three different distracters (gaze, hand, or arrows) influenced overt directional saccadic or hand responses triggered by central instruction signals. This design allowed us to highlight: (i) the neural network activated during reflexive shifts of attention triggered by social and non‐social distracters; (ii) the possible modulatory role of gaze and hand distracters on saccadic and hand pointing responses, respectively.
We predicted a specific involvement of dorsal fronto‐parietal structures in modulating attentional shifts triggered by directional, socially relevant stimuli (i.e., eyes and hand vs. arrow). The fronto‐parietal attention system, which includes portions of the intraparietal cortex (e.g., the intraparietal sulcus, IPS) and of the superior frontal cortex (e.g., frontal eye field, FEF) [Corbetta et al., 2002], is involved in the selection of stimuli and goal‐directed responses for goal‐directed actions. Importantly, specific sections of this system (FEF and some parts of IPS) may be differentially active when subjects plan and perform visually guided hand movements, instead than eye movements [Astafiev et al.,2003; Corbetta and Shulman,2002; Corbetta et al.,2008]. It is also relevant that clinical and brain imaging studies suggest the presence in humans of a segregated pattern of effector representations in the parietal lobe [De Renzi,1982; Jeannerod,1986; Seitz et al.,1991].
On the basis of this evidence, we investigated whether the tendency of an onlooker to imitate the actions of the observed model reflects the activity of a resonant system that works according to body‐part specific reference frames.
MATERIALS AND METHODS
Participants
Eighteen right‐handed volunteers (10 males and 8 females, mean age = 28 years, range: 23–36 years) took part in the study. All subjects had normal or contact‐corrected‐to‐normal visual acuity. All were in good health, free of psychotropic or vasoactive medication, with no history of psychiatric or neurological disease. After having received an explanation of the procedures, participants gave their written consent. The study was approved by the independent Ethics Committee of the Santa Lucia Foundation (Scientific Institute for Research Hospitalization and Health Care). Behavioral and imaging data were analyzed for subjects who showed reliable IE (slower responses for incongruent vs. congruent condition both for saccade and pointing task). Five subjects did not meet this criterion and therefore were not included in the analyses that were performed on 13 subjects (8 males, mean age: 27.5 years; 5 female, mean age: 27 years; range: 23–32 years).
Stimuli and Procedure
Participants were positioned in the scanner, in a dimly lit environment. The experimental visual stimuli were presented via a mirror mounted on the MRI headcoil (total display size 19.5° × 14.6° degrees of visual angle, 1.024 × 768 screen resolution, 60 Hz refresh rate). The visual stimuli were back‐projected on a screen behind the magnet. Stimulus presentation was controlled with Cogent2000 (http://www.vislab.ucl.ac.uk/Cogent/).
Each trial started with the appearance of a black central fixation mark (0.5° × 0.5° in size), presented centrally against a gray background, and of two black squares (1.4° × 1.4° in size), presented for 500 ms at 7.5° of eccentricity in the left and the right visual field. The distracting stimuli consisted of digital Photoshop 8.0.1 (Adobe, CA) modified photographs of gaze, hand, or arrow. The three distracters were created by using colored photographs of: (i) an emotional neutral‐expression, full‐face of a young woman looking to the right; (ii) a man hand pointing to the right; (iii) an arrow pointing to the right obtained by digitally scrambling the hand distracter. The mirror images of these pictures were created to produce leftward directed stimuli. To make the attention‐capture effect conspicuous and the scenario reminiscent of what can be encountered under daily life conditions, the stimuli were animated by presenting two frames in rapid sequence. The first frame depicted a straight gaze, an upward pointing fist or a T‐like shape. The second frame, which depicted a leftward or rightward oriented gaze, extended finger or arrow, replaced the first frame. The direction of the distracter and the one indicated by the instruction cue could be 50% of the time congruent or incongruent. Before starting the fMRI acquisition, each participant was asked to perform outside the scanner a training task in which they had to learn with 100% accuracy on 30 consecutive trials per task, the association between instruction signal (red or blue) with leftward or rightward saccadic or pointing movements.
In the scanner, each trial started with the presentation behind the black fixation mark of a straight gaze, an upward pointing fist or a T‐like shape which lasted 500 ms. At 500 ms, a second frame, that depicted leftward or rightward oriented gaze, extended finger or an arrow, replaced the first frame and created a strong animation effect. The directional distracters remained on until the end of the trial and 75 ms after the oriented distracter presentation, the black central fixation mark (instruction cue) changed to either blue or red color. This was the instruction signal for the subjects to make, in separate runs, a saccade or a right index pointing movement towards the left (change into red) or the right (change into blue) target square (for saccades) and the left or right button of a home‐made keypad (for pointing). Thus, the direction of the distracter and that indicated by the instruction cue could be congruent (left‐red or right‐blue) or incongruent (left‐blue or right‐red). The colored cue remained visible until the end of the trial (See Fig. 1).
Figure 1.
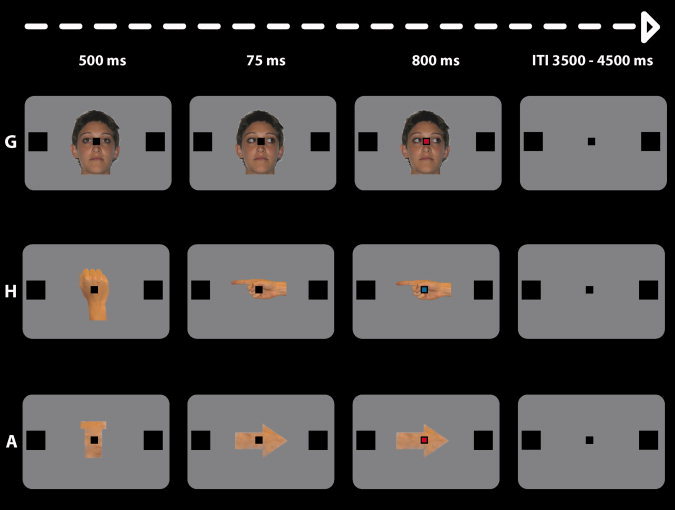
Schematic depiction of the events occurring during a representative trial. The three possible distracting stimuli namely: G, gaze; H, pointing hand; A, arrow; are reported. At the beginning of the trial, a straight gaze, an upward pointing fist, or a T‐like shape was presented behind a black fixation mark (500 ms). Turning the black fixation point into red was the imperative instruction signal for leftward saccades or hand pointing movements. Only incongruent conditions are represented for the sake of simplicity.
To engage automatic processes and minimize expectations, the directional cues were equiprobable (50% congruent) and non‐predictive. It is worth noting that the subjects were instructed to ignore the distracters and to focus on the central mark color change. Moreover, they were explicitly informed that the instruction cue was not informative about the direction of the distracters. In the hand pointing task, subjects were also instructed to fixate the central cross for the entire trial. This allowed us to measure attentional shifts independent of eye movements. To avoid subjects anticipating stimuli, a random inter‐trial interval ranging from 3.5 to 4.5 s was used.
Twelve event types were organized in a 3 × 2 × 2 factorial design. One factor was the Distracter: gaze, hand (both biological distracters with social valence), and arrow (non biological and non social distracter). The second factor was the type of Effector: saccadic vs. pointing movements. To minimize any task‐switching requirements, each participant performed three fMRI runs of saccadic movement and three fMRI runs of pointing movement. The order of the effectors was counterbalanced across participants. On each run, participants were verbally instructed about the motor response to be performed (saccadic or pointing task). The third factor was the Condition: congruent vs. incongruent direction between instruction signal and distracter. Congruent and incongruent directional combinations of instruction cues and distracters were presented in unpredictable and randomized order. Thus, fMRI data were acquired via a mixed, blocked (Distracter, Effector)/event‐related (Condition) protocol. All participants underwent six fMRI runs. Each participant completed a total of 720 trials (360 for each effector), therefore each imaging session consisted of 40 repetitions for each of the three distracters (Gaze/Hand/Arrow), respectively, 20 for congruent and 20 for incongruent conditions (balanced for left/right direction and red‐blue imperative‐cues). Each scanning session lasted ∼8 min for a total experiment duration of about 50 min.
Eye Movements Recording
In the training session outside the scanner, subjects sat in front of a computer screen. In all subjects, eye position and saccadic movements were monocularly monitored using an infrared video camera (Sony EVI D31, color video camera, Sony JP). Participants were instructed to look at the location indicated by the instruction cue and then to quickly look back at the fixation point. During the scanning session, again the participants' saccadic movements were monocularly monitored in real‐time by means of an ASL eye‐tracking system that was adapted for use in the scanner (Applied Science Laboratories, Bedford, MA; Model 504, sampling rate: 60 Hz). For each subject, the eye‐tracking system was calibrated before fMRI scanning. The calibration was repeated during the experiment whenever necessary. Eye‐position traces were examined in a 1,175 ms time window, beginning with the imperative cue onset until the end of the trial. In the sessions requiring pointing movements, the maintenance of central fixation was monitored throughout the trial. We defined losses of fixation as changes in horizontal eye‐position >±2° of visual angle with durations at least 100 ms. For trials requiring saccadic responses (Saccadic Task), the saccadic RTs were calculated from the target onset time to when an horizontal eye position exceeded 2°. Moreover, we did not compute RTs for the trials in which subjects made a saccade to the wrong side (e.g., saccade to the left target after the central cue turned into blue) or did not perform any saccade at all.
Hand Movement Recording
In the training session outside the scanner, participants sat in front of a computer screen by keeping their right index finger on a central response key until the occurrence of the instruction signal. Then, based on the directional instruction cue, subjects pointed towards a left or a right key located 2 cm laterally with respect to the central position. In the scanner, the right hand was positioned in correspondence of the low abdomen in a relaxed posture with the right index finger extended and all other fingers flexed. This position allowed participants to perform central‐cue instructed index finger movements toward the right or the left button key. The right shoulder and arm were supported and immobilized with cushioning wedged between the scanner bed and the coil surface. When the central mark changed color, subjects pointed as quickly as possible in the direction of the target location (lateral key presses) and then returned to the resting position. Pointing involved a minimal rotation of the wrist with extending index without movements of the shoulder or the arm [see Astafiev et al.,2003]. For pointing data, we computed a Release RTs measure and we only analyzed trials in which subjects maintained fixation on the central fixation mark.
Magnetic Resonance Imaging
A Siemens Allegra (Siemens Medical Systems, Erlangen, Germany) operating at 3T and equipped for echo‐planar imaging (EPI) acquired functional magnetic resonance (MR) images. A quadrature volume head coil was used for radio frequency transmission and reception. Head movements were minimized by mild restraint and cushioning. Thirty‐six slices of functional MR images were acquired using blood oxygenation level‐dependent imaging (3.0 × 3.0 × 2.5 mm thick, 50% distance factor, TR = 2.34 s, TE = 30 ms), covering the entire cortex.
Data Analysis
We used the statistical parametric mapping package SPM5 (http://www.fil.ion.ucl.ac.uk) implemented in MATLAB (v 7.1, The MathWorks, Natick, MA) for data pre‐processing and statistical analyses. For all participants, we acquired 1.290 fMRI volumes, 215 for each run. The first four image volumes of each run were used for stabilizing longitudinal magnetization and were discarded from the analysis. Pre‐processing included rigid‐body transformation (realignment) and slice timing to correct for head movement and slice acquisition delay. Residual effects of head motion were corrected including the six estimated motion parameters for each subject as regressors of no interest. Slice‐acquisition delays were corrected using the middle slice as a reference. All images were normalized to the standard SPM5 EPI template, resampled to 2 mm isotropic voxel size, and spatially smoothed using an isotropic Gaussian kernel of 8 mm FWHM. Statistical inference was based on a random effects approach [Penny and Holmes,2004]. First, for each participant, the data were best‐fitted at every voxel using a combination of effects of interest. These were delta functions representing the onsets of the 12 conditions given by the crossing of our 3 × 2 × 2 factorial design: Distracter (gaze/hand/arrow) × Condition (congruent/incongruent) × Effector (saccadic movement/pointing movement) convolved with the SPM5 hemodynamic response function. The onset of the hemodynamic response function was aligned with the onset of the imperative cue with duration = 0. Onsets of trials in which an erroneous response or an eye movement toward the wrong side occurred were included in the design matrix as covariates of no interest, but excluded from any further analysis. Linear contrasts were used to determine differential activation for incongruence minus congruence conditions separately for 3 × 2 (Distracter × Effector) (e.g., [Gaze (Incong) > Gaze (Cong)] for saccadic movement) factors, averaging the three fMRI runs (three for the saccade and three for the pointing task). These six contrasts images were entered in a 3 × 2 factorial ANOVA with Distracter (gaze, hand, and arrow) and Effector (saccadic movement, pointing movement). Finally, linear compounds (contrasts) were used to compare the Incongruency effect using between‐participants variance (rather than between scans). Correction for nonsphericity [Friston et al.,2002] was used to account for possible differences in error variance across conditions and non‐independent error terms for the repeated measures.
The analyses aimed at determining: (i) the brain regions called into action when directional cue and distracters provided conflicting directional information (incongruent condition); (ii) whether any modulation exerted by the biological distracters (gaze and hand) was specifically linked to the effector the onlookers used for responding; (iii) whether reflexive joint attention was differentially modulated by the biological (gaze and hand) vs. non‐biological distracters (arrow), irrespective of motor effector.
We first sought to determine any specific cortical attentional network associated with the directional incongruence conditions (comparing incongruent vs. congruent condition, irrespective of distracter and effector). Thus, the main effect of Incongruence allowed us to identify the network activated by the directional conflict between task‐irrelevant distracters and instruction signals. For this comparison, the SPM threshold was set to P corr. = <0.05 at cluster level (cluster extent estimated a P uncorr. = 0.001), considering the whole brain as the volume of interest. To test for the interaction between the IE with Motor‐Effector and Distracter, we created regions of interest (ROIs) extracting average BOLD signals (MarsBar 0.41, “MARSeille Boîte À Région d'Intérêt” SPM toolbox) from the peak activity of the voxels that showed a main effect of Incongruence. Each ROI was defined as a 10 mm radius sphere centered on the corresponding maxima of the whole‐brain analysis (see Table II), and P values were Bonferroni‐corrected. We expected that our manipulations of IE would affect activity within the dorsal fronto‐parietal attentional systems depending on specific relationships with Distracter and Effector [Corbetta and Shulman,2002; Crostella et al.,2009; Ricciardelli et al.,2002]. Accordingly, we used a combination of anatomical and functional criteria to identify six ROIs in the dorsal attentional system: the FEF, the posterior parietal cortex (PPC), the IPS bilaterally.
Table II.
Mean MNI coordinates of activation foci associated with incongruence effect
| Anatomical area | Cluster size | P corr. | x | y | z | z scores |
|---|---|---|---|---|---|---|
| Parietal lobe | ||||||
| R PPC | 7,804 | <0.001 | 14 | −66 | 58 | 6.09* |
| L PPC | −22 | −70 | 46 | 5.67* | ||
| R IPS | 42 | −56 | 58 | 3.97* | ||
| L IPS | −32 | −44 | 40 | 4.98* | ||
| Frontal lobe | ||||||
| R FEF | 930 | <0.001 | 36 | 0 | 56 | 5.81* |
| L FEF | 507 | <0.001 | −28 | 0 | 54 | 5.54* |
| R Cingulum Mid | 397 | <0.001 | 8 | 14 | 46 | 4.16 |
| L Precentral G | 376 | 0.002 | −52 | 2 | 38 | 4.90 |
| R Insula | 1,346 | <0.001 | 34 | 24 | 8 | 5.21 |
| L Insula | 564 | <0.001 | −30 | 20 | 6 | 4.89 |
Anatomical locations, peak coordinates in MNI space (Montreal Neurological Institute), and statistical values for the main effect of incongruence (incongruent > congruent trials, irrespective of distracter and effector). P values are corrected for multiple comparisons at the cluster level, considering the whole brain as the volume of interest. R/L PPC = Right/Left Posterior Parietal Cortex; R/L IPS = Right/Left Intraparietal Sulcus; R/L FEF = Right/Left Frontal Eye Field; R/L Insula = Right/Left Insula; R Cingulum Mid = Right Middle Cingulum; L Precentral G = left Precentral Gyrus. With the asterisk (*) we indicated the ROIs within the dorsal fronto‐parietal attentional network. ROIs were extracted averaging BOLD signals (see Methods) from a 10 mm sphere centered on the cluster peak.
The bilateral frontal ROIs included a portion of middle frontal gyrus (FEF) located laterally within the superior frontal sulcus [Paus,1996]. Because of the large extension of parietal cortex clusters, we decided to distinguish between posterior and anterior anatomical regions, bilateral PPC and IPS, respectively. Bilateral PPC included a portion of superior parietal lobule close to superior parietal gyrus and precuneus. Bilateral IPS ROIs were instead located anteriorly and close to the inferior parietal lobule, the angular gyrus and the IPS.
For each ROI, we tested the three‐way interaction IE × Effector × Distracter to provide information on whether the cost of directional incongruence was mapped on different brain regions depending on specific relationships with distracter and motor effector. For example, this interaction allowed exploring whether observation of incongruent saccades performed by the distracting gaze induced differential brain responses in the onlookers' when performing the saccadic with respect to the hand‐pointing movement. It should be noted that main effect and interactions are orthogonal and, therefore, our ROI selection procedure was unbiased. Moreover, based on the prediction that IEs are stronger when elicited by social (gaze and hand) than by non‐social (arrow) distracters, we tested the interaction between the IE and the biological vs. non‐biological distracters, irrespective of motor effector.
RESULTS
Behavioral Performance
Both saccadic and release mean RTs were calculated collapsing left and right target trials. Incorrect responses (movements performed following distracters instead than instruction cues), misses (no response), anticipations (RTs < 100 ms), and retards (RTs > 1.500 ms) were not included in the analysis. Overall, we discarded 12.7% of trials for saccadic sessions and 7.5% of trials for pointing sessions. Following previous studies [Kitagawa and Spence,2005; Murphy and Klein,1998; Spence et al.,2001a,b], we computed an inverse efficiency score by dividing, for each condition and in each subject, the mean correct RTs by the percentage of directionally correct responses. The inverse efficiency score provides a way to combine RT and accuracy measures of performance into a single measure [Townsend and Ashby,1983] and allows controlling for any speed‐accuracy trade‐off effects. As for RT and error measures, higher inverse efficiency scores indicate worse performance. Table I reports inverse efficiency scores in the saccadic and hand pointing tasks, for each distracter type and incongruent and congruent conditions, acquired during fMRI scanning.
Table I.
Behavioural performance for Saccadic and Hand‐pointing tasks
| Distracter | ||||
|---|---|---|---|---|
| G | H | A | ||
| Saccade | Congruent | 512 (33) | 511 (44) | 494 (30) |
| Incongruent | 608 (56) | 634 (41) | 648 (55) | |
| Hand pointing | Congruent | 528 (30) | 511 (24) | 499 (21) |
| Incongruent | 552 (30) | 521 (25) | 534 (24) | |
Inverse efficiency scores (mean RT/percentage of correct responses, S.E.M. in brackets) are represented separately for saccadic and release RTs as a function of Distracter (G = Gaze/H = Hand/A = Arrow) and Condition (Congruent/Incongruent).
The inverse efficiency scores were entered in two separate 3 × 2 repeated‐measures ANOVAs (one for saccadic and one for pointing task) with Distracter (gaze, hand, and arrow), and Condition (congruent and incongruent) as within‐subjects effects. In the saccadic task, the main effect of Condition [F (1, 12) = 22.297, P = 0.001] was explained by the worse performance in the incongruent than congruent trials (629 vs. 505 ms/percentage of correct trials). No other effects or interactions were significant. Also in the pointing task, the main effect of Condition was significant [F (1, 12) = 8.521, P = 0.01] because of the worse performance in the incongruent than congruent trials (535 vs. 510 ms/percentage of correct responses). Again, no other effects or interactions were significant.
To sum up, saccadic and release RTs/percentage of correct trials scores during MR scanning show that the incongruent cues worsened both saccadic and pointing performances. However, this effect was independent from the type of Distracter (as indicated by the non‐significant Distracter × Effector interaction).
fMRI Data
Main effect of incongruence
To highlight the neural underpinnings of the IE triggered by incongruent distracters, we tested for the main effect of incongruence irrespective of Distracter and Effector (See Table II).
This contrast revealed the expected activation of the ventral and dorsal frontal and parietal regions. The parietal region consisted of a large cluster including the right superior and inferior parietal cortex bilaterally. The frontal region included the left precentral gyrus, the right middle frontal cortex bilaterally, the right supplementary motor area, the most posterior portion of the inferior frontal gyrus, the operculum, bilaterally, and the pars triangularis extending into the insula and the middle portion of the right cingulate cortex (See Fig. 2).
Figure 2.
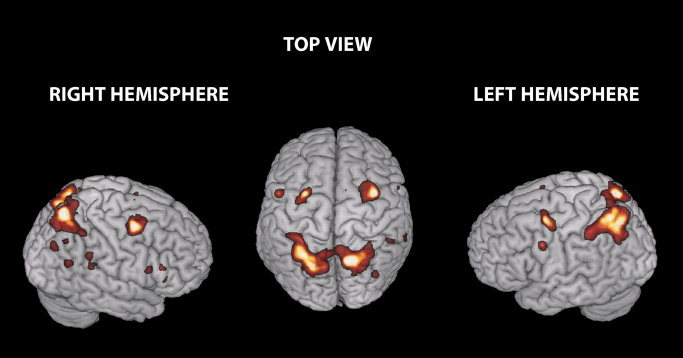
Brain regions activated by incongruence (Incongruent > Congruent trials). Clusters showing higher activity in the incongruent than congruent condition irrespective of distracter and effector are rendered on three‐dimensional (3D) views of the SPM template. This contrast revealed the activation of frontal and parietal regions. The frontal region included the left Precentral Gyrus (L Precentral G), the right Middle Frontal (L/R FEF) cortex bilaterally, the right Supplementary Motor Area (R SMA), the most posterior portion of the Inferior Frontal Gyrus, the Operculum, bilaterally, and the Pars Triangularis (IFG) extending into the Insula and the middle portion of the right Cingulate Cortex. The parietal region included the right superior and inferior Parietal Cortex bilaterally. These regions were used as ROI to assess any differential influence of distracter/instruction signal incongruence on brain responses (SPM thresholds are set to P corr. = 0.05 at cluster level).
The main effect of Incongruence considering the three distracters (G = Gaze/H = Hand/A = Arrow, averaging across saccadic/hand‐pointing motor effector) was used to define the center of each ROI in the two hemispheres. Within each ROI, we tested for: (i) the critical interaction: IE of Distracter (Gaze and Hand) on the paired saccadic and hand pointing task; (ii) the IE of biological (Gaze and Hand) vs. non‐biological distracters (Arrow), irrespective to effector.
IE of distracters in the saccadic and hand pointing tasks
We investigated the possible influence on BOLD signal of the pairing between body‐part related (gaze or pointing hand) distracter of motor‐effector used for the response (eyes or hand) within each frontal and parietal ROI. The mean BOLD activation for each Distracter and Effector in the frontal and parietal ROIs is shown in Figure 3. Statistics, for the interaction effect and additional t test in each ROI are reported in Table III.
Figure 3.
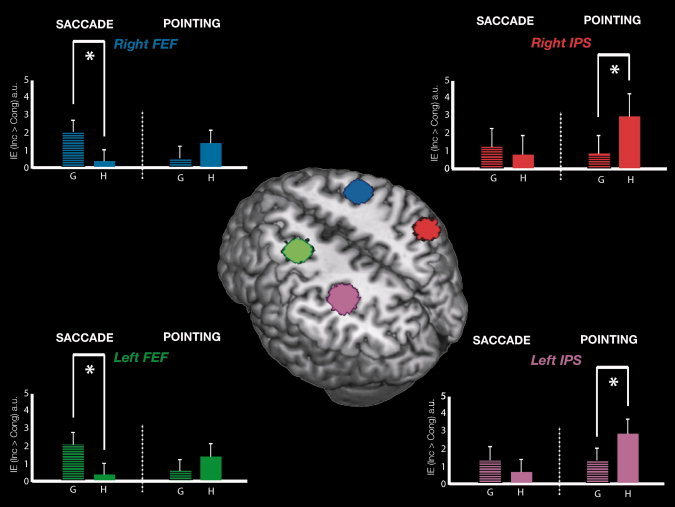
Activity in the bilateral FEF and IPS regions elicited by the IE of the two social distracters during Saccadic and Pointing movements. Central panel: 3D rendering of the canonical MNI template showing the localization of four ROI corresponding to the left (green) and right (blue) FEF and to the left (pink) and right (red) IPS is reported in the axial section. Left panel: signal plots for the IE [IE (inc > cong)] in the right FEF (up) and the left FEF (down) as a function of the two biological distracters (G = Gaze/H = Hand) and effectors (Saccade/Pointing). Right panel: signal plots for the IE [IE (inc > cong)] in the right IPS (up) and the left IPS (down) for each biological distracter (G = Gaze/H = Hand) during saccadic and hand‐pointing task. In each plot, the level of activity for the four conditions represents the average amplitude of the hemodynamic response for the [IE (inc > cong)] belonging to the corresponding condition (e.g., Gaze or Hand trials, for Saccade) and expressed in arbitrary units (a.u., ±90% confidence interval). The asterisks indicate significant (G vs. H) difference for left/right FEF and (H vs. G) difference for left/right IPS.
Table III.
IE of distracters in Saccadic and Hand pointing tasks
| Side | Anatomical area | IE for distracter by effector | IE for Saccade G > H | IE for Hand pointing H > G | |||
|---|---|---|---|---|---|---|---|
| t test values | P corr. | t test values | P uncorr. | t test values | P uncorr. | ||
| L | FEF | 3.06 | <0.01 | 3.04 | <0.01 | 1.82 | n.s. |
| IPS | 2.47 | <0.05 | 1.32 | n.s. | 2.52 | <0.01 | |
| PPC | 1.87 | n.s. | 1.43 | n.s. | 1.36 | n.s. | |
| R | FEF | 2.33 | 0.068 | 2.36 | <0.05 | 1.41 | n.s. |
| IPS | 2.32 | 0.069 | 0.71 | n.s. | 2.79 | <0.01 | |
| PPC | 2.33 | 0.068 | 2.57 | <0.01 | 1.22 | n.s. | |
Anatomical locations of ROIs, t test, and P values (Bonferroni‐corr) for the IE of social distracter (Gaze = G/Hand = H) by effector (Saccade/Pointing), in the Left/Right (L/R) hemispheres. A significant interaction was found for L FEF and L IPS, whereas a trend toward significance was found for R FEF and R IPS. Additional t test (P uncorr) confirmed a significant larger differential effect for (G > H) distracter in the L and R FEF, whereas bilateral IPS showed a larger IE for (H > G; see Results section for more details), indicating a selective correspondence between G/H body‐part and saccadic/hand‐pointing effector.
Left FEF was specifically modulated by the interaction IE × Distracter × Effector, while right FEF showed a trend toward significance. To further confirm the specificity of these effects, we compared the IE of gaze vs. hand distracter for saccadic motor effector. This revealed that left and right FEF were modulated by the selective correspondence between “Gaze” body‐part and “Saccadic” effector (See bars Fig. 3, left panel: G > H). The opposite pattern was found in left IPS region; as for left FEF, this region resulted specifically influenced by the interaction IE × Distracter × Effector, whereas right IPS showed a trend toward significance. Additional t‐tests confirmed that this effect was due to a larger IE for “Hand pointing” than gaze‐distracter during “Hand pointing” movements (See bars Fig. 3, right panel: H > G). This demonstrates that activity in these regions is specially influenced by the motor effectors used to perform the task. This effect was stronger in the left than in the right hemisphere. Finally, left PPC was not sensitive to this interaction given that results were not replicated (albeit a significant IE for Gaze more than Arrow was found for right PPC).
fMRI Activations Associated to the IE of Biological vs. Non‐Biological Distracters
To explore whether reflexive joint attention was differentially modulated by the different categories of distracters (e.g., biological and social vs. non‐biological non‐social cues) independently from motor‐effector, we compared the IE for biological (gaze and hand) vs. non‐biological distracters (arrow).
Statistics for the interaction effect and additional t‐tests in each ROI are reported in Table IV.
Table IV.
IE of biological vs. non‐biological distracters
| Side | Anatomical area | IE for Bio vs. Non‐Bio | IE for G > A | IE for H > A | |||
|---|---|---|---|---|---|---|---|
| t test values | P corr. | t test values | P uncorr. | t test values | P uncorr. | ||
| L | FEF | 1.74 | n.s. | 2.03 | <0.05 | 1.09 | n.s. |
| IPS | 3.00 | <0.05 | 2.18 | <0.05 | 2.24 | <0.001 | |
| PPC | 2.52 | <0.05 | 2.26 | <0.05 | 2.24 | <0.05 | |
| R | FEF | 1.38 | n.s. | 1.52 | 0.066 | 1.00 | n.s. |
| IPS | 1.41 | n.s. | 0.71 | n.s. | 2.13 | <0.05 | |
| PPC | 3.07 | <0.01 | 2.82 | <0.01 | 2.74 | <0.01 | |
Anatomical locations of ROIs, t test, and P values (Bonferroni‐corr) for the IE of biological (Gaze = G/Hand = H) vs. non‐biological distracter (Arrow = A), in the Left/Right (L/R) hemispheres. A significant interaction was found for L/R PPC and L IPS. Additional t test (P uncorr) confirmed significant larger differential effect for biological vs. non‐biological (G > A) and (H > A) in bilateral PPC and L IPS, irrespective to Effector (see Results section for more details; albeit some trends were found for bilateral FEF and R IPS). These results confirm a larger IE driven by the biological distracters with social valence (Gaze and Hand) respect to Arrow.
Significant interactions, mainly in the left hemisphere were found within the parietal ROIs. In particular, in the left and the right PPC, the activation for the IE triggered by biological distracters (gaze and hand) was larger than the activation for the IE triggered by non‐biological distracter (arrow; See Bars Fig. 4: G > A). In other words, the BOLD signal in these regions was higher when the directional conflict between distracter‐instruction signals involved biological (gaze and hand) distracters than when the conflict involved the non‐biological (arrow) distracter. Confirmatory t tests demonstrated that this effect was due to both a significant IE for gaze vs. arrow distracters and to a significant IE for hand vs. arrow distracters in left IPS and bilateral PPC. Finally, these analyses did not reveal any significant interaction for right and left FEF or right IPS, with the exception of a larger IE for G > A in bilateral FEF and a larger IE for H > A in right IPS.
Figure 4.
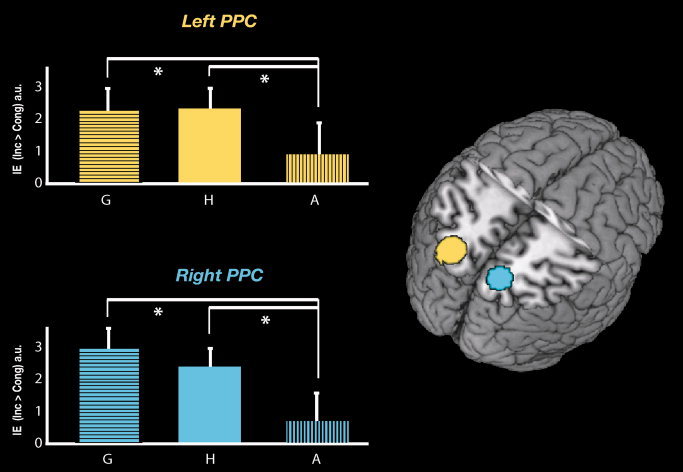
Activity in the bilateral PPC regions elicited by the IE of the two biological distracters respect to non‐biological distracter. Right panel: 3D rendering of the canonical MNI template showing the localization of two ROI corresponding to the left (yellow) and right (light blue) PPC is reported in the axial section. Left panel: the relative plots show the mean IE [IE (inc < cong)] of the three distracters (averaged across the two effector, respectively). A significant interaction was observed in these ROIs: biological distracter (G = Gaze/H = Hand) interfered on shifts of attention more than the non‐biological (A = Arrow) distracter. The asterisks indicate significant higher IE for (G than A), and higher IE for (H than A) in both regions. The level of activation is expressed in arbitrary units (a.u., ±90% confidence interval).
DISCUSSION
The present study had two main aims: (i) to ascertain whether the possible differential attention orienting‐power of directional gaze, hand, and arrow distracters relies upon commons neural substrates; (ii) to explore whether the relationship between gaze and hand distracters and the motor effector used in the experimental task (Saccadic or Hand pointing response) was reflected in a specific modulation of the activity in the dorsal fronto‐parietal nodes of the reflexive attention network. Finally, the study explored the architecture of the reflexive orienting triggered by biological (Gaze and Hand) and non‐biological (Arrow) distracters, irrespective of motor effector.
Behavioral and Neural Correlates of Reflexive Attention
A cost of directional incongruence between distracters and instructions signals was found. All distracters in the behavioral performance showed a congruency effect both for saccadic and pointing task. This is in keeping with studies showing that attention is captured by gaze and arrows to a similar extent [Kuhn and Benson,2007; Kuhn and Kingstone,2009; Sato et al.,2009] and at variance from studies showing that social distracters like averted gaze or pointing hands induce stronger attentional capture more than symbolic arrow [Langton and Bruce,2000; Ricciardelli et al.,2002]. It is worth noting that in many complex daily life interactions, the tendency to follow others seems to be very strong. Thus, the lack of predominance of gaze‐ over arrow‐distracters in triggering reflexive attention of arrows in some studies may be due to a floor effect induced by the extremely simplified reality of laboratory conditions [Birmingham and Kingstone,2009; Kingstone,2009]. However, one may observe that the present behavioral results differed also from our previous study where gaze and hand distracters interfered more with eye and hand pointing movements respectively [Crostella et al.,2009]. It should be noted however, that, different from Crostella et al. [2009] in the current study the pointing movement was defined as a index finger extension toward the right or the left button key with the shoulder and the arm immobilized instead of a free hand arm movement in the space, accounting substantial difference in motor programming and executing.
At any rate, the present study demonstrated that, despite the instruction to focus on the imperative signal, subjects could not ignore the distracters. Importantly, the behavioral interference of directional incongruence between instruction signal and distracters was reflected in an increase of the BOLD signal. Such increase occurred in a fronto‐parietal network that included the left precentral gyrus and the right middle frontal gyrus bilaterally, the right supplementary motor area, the most posterior portion of the inferior frontal gyrus, the operculum, bilaterally, and the pars triangularis extending into the insula and the middle portion of the right cingulate cortex as well as posterior regions of the superior and inferior parietal cortex bilaterally. Previous studies highlighted the importance of fronto‐parietal networks in a variety of attentional tasks, including covert and overt reorienting of attention to non biological stimuli [Corbetta and Shulman,2002; Corbetta et al.,2008; Szczepanski et al.,2010], as well as to the direction of others' gaze [Grosbras et al.,2005]. Although studies indicate that gaze and arrows may modulate attention‐shifts related activity in different brain regions [Hietanen et al.,2006] even in the absence of differences in behavioral tasks [Engell et al., 2010], only one study has thus far explored the neural network activated by mere observation of directional vs. non‐directional eye gaze, hand‐pointing gestures and arrows [Sato et al.,2009]. This study showed activation in inferior frontal and inferior parietal areas as well as in the superior temporal sulcus common to the three distracters, even if an increase of activity in temporo‐parietal clusters and in the amygdala was found for directional arrows and directional eyes respectively.
Body‐Part Specific Reference Frames for Mapping Reflexive Social Attention in the Fronto‐Parietal Cortex
In keeping with previous neuroimaging studies [Grosbras et al.,2005; Hietanen et al.,2006; Sato et al.,2009; Tipper et al.,2008], our results highlight the fundamental role of fronto‐parietal structures in mediating gaze and hand related shifts of attention. However, our study expands significantly previous knowledge by combining, for the first time, two main issues, namely the possible specificity of the neural representation of different effectors used for response and the influence of social and non social distracters in modulating reflexive attention. It is widely held that movements performed with different effectors are coded in different cortical regions. Distinct posterior parietal modules, for example, may preferentially code for saccades and reaches, respectively [Colby and Goldberg,1999; Glimcher,2003]. More recent studies indicate that far from being a strict principle, effector‐selectivity implies a gradual transition of preference from one effector to another, with areas of balanced activation to saccades and reaches and areas with significant preference for reaches [Levy et al.,2007]. Similarly, effector preference was found in parieto‐frontal areas during eye or hand movement planning but no region responded exclusively to either effector [Beurze et al.,2009]. A predominance of left lateralized maps for coding the preparation of pointing movements in the presence of equivalent coding of saccadic and reaches preparation in frontal areas has also been reported [Astafiev et al.,2003]. Testing the hypothesis of a difference in the visuospatial maps recruited by pointing and saccades, Hagler and colleagues [2007] identified multiple maps in both PPC and superior frontal cortex recruited for eye and hand movements, including maps not observed in previous studies. Although their analysis revealed subtle differences between pointing and saccades, including hemispheric asymmetries, no evidence of pointing‐specific maps of visual space was found.
In the present study, we explored whether biological directional distracters such as directional gaze and pointing gestures, influenced the neural underpinnings of reflexive shifts of attention in relation to the motor effectors used for the response, namely eyes or hands. To this aim, we compared the BOLD signal in the fronto‐parietal ROIs that turned out to be involved in reflexive attention. We found a functional dissociation in the frontal and parietal nodes of the reflexive joint attention network, hinting at a specific influence of gaze and hand distracters in the saccadic and hand pointing tasks, respectively. Overall, the fMRI data indicated that the observed interference with voluntary orienting varied as a function of central distracter‐type and motor‐effector. In particular, we observed greater IE‐related activation in the frontal ROIs for shifts of spatial attention triggered by gaze in the saccadic task and in the parietal ROIs, specifically bilateral IPS, for shifts of attention triggered by hand in the pointing task. This result is in keeping with previous studies indicating the importance of parietal regions in mediating interference of hand movements incongruous with planning of a different hand movement [Grefkes et al.,2004] or of hand‐related attention switching tasks [Rushworth et al.,2001].
Tellingly, a main point of novelty of the present study is that the fronto‐parietal network subserving reflexive shifts of social attention is specially sensitive to the relationship between specific body‐related distracters and the responding body parts. Importantly, an effector‐specific activation of fronto‐parietal networks in humans has also been found in a recent study on cortical temporal dynamics of visually guided behavior [Hinkley et al., 2010]. In this study, high‐gamma activity was observed in SEF and subsequently in visual cortex and FEF bilaterally, followed by a low‐beta power decrease over caudal PPC during saccade execution. Thus, hand or saccadic movements implied a different functional connectivity between frontal and parietal areas.
Mirroring of Attention in the Fronto‐Parietal System
In our experimental paradigm, participants were specifically instructed to ignore the visual distracting stimuli (gaze, hand, and arrow), to focus on the central imperative go signal and to maintain the fixation on the central point. Given that the distracter was presented before the unpredictable central cue, the cost of re‐orienting to fully irrelevant‐task distracters is likely due to interference with ongoing action programs. This may be in keeping with pre‐motor theories of attention [Rizzolatti et al.,1987] and with the notion of mirroring others' actions [Rizzolatti and Sinigaglia,2010]. Behavioral studies indicate that priming a given motor response is more effective if the visual prime shares specific properties with the requested response suggesting that perceptual codes and action plans may share a common representational medium [Craighero et al.,2002]. Neuroimaging studies indicate that viewing hand, mouth and foot actions may induce a specific increase of the BOLD signal in the frontal and parietal representations of the acting body parts [Buccino et al.,2001]. A clear link between action mirroring and sharing of attention between individuals has been established in a single cell recording study from the monkey parietal lobe [Shepherd et al.,2009]. This study demonstrates an increase of activity of parietal neurons not only when the monkey oriented his attention towards their receptive field, but also during observation of another monkey orienting in the same direction. It is also relevant that overlapping fronto‐parietal cortical representations are called into play during executed, observed, and imagined reaching in humans [Filimon et al.,2007]. That reflexive shifts of social attention may be coded in body‐part specific coordinates and may reflect a specific tendency to imitate other movements, is indirectly suggested by a behavioral study showing that distracting gaze and hand pointing distracters impaired saccadic and pointing performance, respectively [Crostella et al.,2009]. The pattern of activation found in the present study likely represents neural evidence that mirroring of attention may be coded according to body‐part specific reference frames.
Influence of Social vs. Non‐Social Distracters on Changes of BOLD Signal in the Fronto‐Parietal Network Underlying Reflexive Attention
As reported in the results section, the performance to incongruent trials was impaired with respect to congruent trials irrespectively of the distracter (gaze, pointing hand, or arrow). Importantly, however, despite the equivalent IE of the three distracters at the behavioral level, higher changes of BOLD signal for biological (gaze and hand‐pointing) than non biological distracters were found in the bilateral PPC and left IPS regions. This suggests that hemodynamic brain responses may be more sensitive than behavioral responses in signaling selective influences on attentional shifts and thus in highlighting the special contribution of the parietal‐frontal network to reflexive social attention [Deaner and Platt,2003]. Thus biological stimuli, possibly because of their social relevance, may have an inherently higher power in catching attention than non‐biological stimuli even when this is not elected in the behavioural performance. This result is in keeping with a recent fMRI study showing that even though the interference of gaze and arrows was comparable at the behavioral level, only the latter distracter modulated neural activity in the temporo‐parietal attention network, thus indicating that different neural substrates underpin reflexive attention mediated by biological and non‐biological cues [Engell et al., 2010].
CONCLUSION
Our study indicates that frontal and parietal cortical regions map the conflict between a central cue instructing leftward or rightward saccadic or hand pointing movements directions and to‐be‐ignored distracters (gaze, hand, and arrow) pointing in opposite direction. Crucially, however, the detrimental effect of the directional conflict induced by gaze and hand distracters brought about differential activation in parietal and frontal structures depending on whether subjects performed a saccadic or hand‐pointing task. In particular, the distracting effect of pointing gestures is associated with higher parietal activity when the motor task is performed with the hand. By contrast, the distracting effect of averted gaze is associated with high frontal activity when the motor task is performed with the eyes. It is worth noting that the distracting effect of arrows induced increased responses in the fronto‐parietal network independently from the effector used for the response but overall to lesser degree than biological distracter. This pattern of results indicates, for the first time, that reflexive social attention is coded in the fronto‐parietal cortex according to body‐part centered coordinate systems.
Acknowledgements
The authors thank Dr. Valerio Santangelo for providing helpful comments and Dr. Paolo Alessandrini for his technical assistance. The Neuroimaging Laboratory of the Fondazione Santa Lucia is supported by The Italian Ministry of Health. The financial contribution from MIUR (Ministero Italiano Università e Ricerca, PRIN 2009) and IIT (Italian Institute of Technology, SEED, Prot. Num. 21538) to S.M.A. is gratefully acknowledged.
Contributor Information
Valentina Cazzato, Email: valentina.cazzato@uniroma1.it.
Salvatore Maria Aglioti, Email: salvatoremaria.aglioti@uniroma1.it.
REFERENCES
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M ( 2003): Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci 23: 4689–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurze SM, de Lange FP, Toni I, Medendorp WP ( 2009): Spatial and effector processing in the human parietofrontal network for reaches and saccades. J Neurophysiol 101: 3053–3062. [DOI] [PubMed] [Google Scholar]
- Birmingham E, Kingstone A ( 2009): Human social attention. Prog Brain Res 176: 309–320. [DOI] [PubMed] [Google Scholar]
- Bonato M, Priftis K, Marenzi R, Zorzi M ( 2009): Normal and impaired reflexive orienting of attention after central nonpredictive cues. J Cogn Neurosci 21: 745–759. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund H‐J ( 2001): Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. Eur J Neurosci 13: 400–404. [PubMed] [Google Scholar]
- Colby CL, Goldberg ME ( 1999): Space and attention in parietal cortex. Annu Rev Neurosci 22: 319–349. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL ( 2008): The reorienting system of the human brain: From environment to theory of mind. Neuron 58: 306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Craighero L, Bello A, Fadiga L, Rizzolatti G ( 2002): Hand action preparation influences the response to hand pictures. Neuropsychol 40: 492–502. [DOI] [PubMed] [Google Scholar]
- Crostella F, Carducci F, Aglioti SM ( 2009): Reflexive social attention is mapped according to effector‐specific reference systems. Exp Brain Res 197: 143–151. [DOI] [PubMed] [Google Scholar]
- Deaner RO, Platt ML ( 2003): Reflexive social attention in monkeys and humans. Curr Biol 13: 1609–1613. [DOI] [PubMed] [Google Scholar]
- De Renzi E ( 1982): Disorders of Space Exploration and Cognition. New York: John Wiley & Sons, Inc. [Google Scholar]
- Eimer M ( 1997): Uninformative symbolic cues may bias visual‐spatial attention: Behavioral and electrophysiological evidence. Biol Psychol 46: 67–71. [DOI] [PubMed] [Google Scholar]
- Engell AD, Nummenmaa L, Oosterhof NN, Henson RN, Haxby JV, Calder AJ: Differential activation of fronto‐parietal attention networks by social and symbolic spatial cues. Soc Cogn Affect Neurosci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filimon F, Nelson JD, Hagler DJ, Sereno MI ( 2007): Human cortical representations for reaching: Mirror neurons for execution, observation, and imagery. Neuroimage 37: 1315–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen CK, Kingstone A ( 1998): The eyes have it: Reflexive orienting is triggered by nonpredictive gaze. Psychon Bull Rev 5: 490–495. [Google Scholar]
- Friesen CK, Ristic J, Kingstone A ( 2004): Attentional effects of counterpredictive gaze and arrow cues. J Exp Psychol Hum Percept Perform 30: 319–329. [DOI] [PubMed] [Google Scholar]
- Friesen CK, Moore C, Kingstone A ( 2005): Does gaze direction really trigger a reflexive shift of spatial attention? Brain Cogn 57: 66–69. [DOI] [PubMed] [Google Scholar]
- Frischen A, Bayliss AP, Tipper SP ( 2007): Gaze cueing of attention: Visual attention, social cognition, and individual differences. Psychol Bull 133: 694–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J ( 2002): Classical and Bayesian inference in neuroimaging: Applications. NeuroImage 16: 484–512. [DOI] [PubMed] [Google Scholar]
- Glimcher PW ( 2003): The neurobiology of visual‐saccadic decision making. Annu Rev Neurosci 26: 133–179. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Ritzl A, Zilles K, Fink GR ( 2004): Human medial intraparietal cortex subserves visuomotor coordinate transformation. Neuroimage 23: 1494–1506. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Laird AR, Paus T ( 2005): Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum Brain Map 25: 140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ Jr, Riecke L, Sereno MI ( 2007): Parietal and superior frontal visuospatial maps activated by pointing and saccades. Neuroimage 35: 1562–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietanen JK ( 1999): Does your gaze direction and head orientation shift my visual attention? Neuroreport 10: 3443–3447. [DOI] [PubMed] [Google Scholar]
- Hietanen JK, Nummenmaa L, Nyman MJ, Parkkola R, Hämäläinen H ( 2006): Automatic attention orienting by social and symbolic cues activates different neural networks: An fMRI study. NeuroImage 33: 406–413. [DOI] [PubMed] [Google Scholar]
- Hietanen JK, Leppänen JM, Nummenmaa L, Astikainen P ( 2008): Visuospatial attention shifts by gaze and arrow cues: An ERP study. Brain Res 1215: 123–136. [DOI] [PubMed] [Google Scholar]
- Hinkley LB, Nagarajan SS, Dalal SS, Guggisberg AG, Disbrow EA (2010): Cortical temporal dynamics of visually guided behavior. Cereb Cortex [Epub ahead of print] doi: 10.1093/cercor/bhq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier RJ, Batty M ( 2009): Neural bases of eye and gaze processing: The core of social cognition. Neurosci Biobehav Rev 33: 843–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M ( 1986): The Neural and Behavioural Organisation of Goal‐Directed Movements. Oxford: Oxford University Press. [Google Scholar]
- Jonides J ( 1981): Voluntary vs. automatic control over the mind's eye's movement In: Long JB, Baddeley AD, editors. Attention and Performance IX. Hillsdale, NJ: Erlbaum; pp 187–203. [Google Scholar]
- Kingstone A ( 2009): Taking a real look at social attention. Curr Opin Neurobiol 19: 52–56. [DOI] [PubMed] [Google Scholar]
- Kitagawa N, Spence C ( 2005): Investigating the effect of a transparent barrier on the crossmodal congruency effect. Exp Brain Res 161: 62–71. [DOI] [PubMed] [Google Scholar]
- Klein JT, Shepherd SV, Platt ML ( 2009): Social attention and the brain. Curr Biol 19: 958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn G, Benson V ( 2007): The influence of eye‐gaze and arrow pointing distractor cues on voluntary eye movements. Percept Psychophys 69: 966–971. [DOI] [PubMed] [Google Scholar]
- Kuhn G, Kingstone A ( 2009): Look away! Eyes and arrows engage oculomotor responses automatically. Atten Percept Psychophys 71: 314–327. [DOI] [PubMed] [Google Scholar]
- Kuhn G, Land MF ( 2006): There's more to magic than meets the eye. Curr Biol 16: 950–951. [DOI] [PubMed] [Google Scholar]
- Langton SR, Watt RJ, Bruce II ( 2000): Do the eyes have it? Cues to the direction of social attention. Trends Cogn Sci 4: 50–59. [DOI] [PubMed] [Google Scholar]
- Langton SR ( 2000): The mutual influence of gaze and head orientation in the analysis of social attention direction. Q J Exp Psychol A 53: 825–845. [DOI] [PubMed] [Google Scholar]
- Langton SR, Bruce V ( 2000): You must see the point: Automatic processing of cues to the direction of social attention. J Exp Psychol Hum Percept Perform 26: 747–757. [DOI] [PubMed] [Google Scholar]
- Levy I, Schluppeck D, Heeger DJ, Glimcher PW ( 2007): Specificity of human cortical areas for reaches and saccades. J Neurosci 27: 4687–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Klein RM ( 1998): The effects of nicotine on spatial and non‐spatial expectancies in a covert orienting task. Neuropsychologia 36: 1103–1114. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Calder AJ ( 2009): Neural mechanism of social attention. Trends Cogn Sci 13: 135–143. [DOI] [PubMed] [Google Scholar]
- Paus T ( 1996): Location and function of the human frontal eye‐field: A selective review. Neuropsychologia 34: 475–483. [DOI] [PubMed] [Google Scholar]
- Penny W, Holmes AP ( 2004): Random‐effects analysis In: Frackowiak RSJ, Ashburner JT, Penny WD, Zeki S, Friston KJ, Frith CD, Dolan RJ, Price CJ, editors. Human Brain Function. San Diego: Elsevier; pp 843–850. [Google Scholar]
- Pierno AC, Becchio C, Tubaldi F, Turella L, Castiello U ( 2008): Motor ontology in representing gaze‐object relations. Neurosci Lett 430: 246–251. [DOI] [PubMed] [Google Scholar]
- Ricciardelli P, Bricolo E, Aglioti SM, Chelazzi L ( 2002): My eyes want to look where your eyes are looking: Exploring the tendency to imitate another individual's gaze. Neuroreport 13: 2259–2264. [DOI] [PubMed] [Google Scholar]
- Ristic J, Friesen CK, Kingstone A ( 2002): Are eyes special? It depends on how you look at it. Psychon Bull Rev 9: 507–513. [DOI] [PubMed] [Google Scholar]
- Ristic J, Wright A, Kingstone A ( 2007): Attentional control and reflexive orienting to gaze and arrow cues. Psychon Bull Rev 14: 964–969. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umiltá C ( 1987): Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia 25: 31–40. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C ( 2010): The functional role of the parieto‐frontal mirror circuit: Interpretations and misinterpretations. Nat Rev Neurosci 11: 264–274. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Paus T, Sipila PK ( 2001): Attention systems and the organization of the human parietal cortex. J Neurosci 21: 5262–5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Yoshikawa S ( 2009): Commonalities in the neural mechanisms underlying automatic attentional shifts by gaze, gestures, and symbols. NeuroImage 45: 984–992. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Roland PE, Bohm C, Greitz T, Stone‐Elander S ( 1991): Somatosensory discrimination of shape: Tactile exploration and cerebral activation. Eur J Neurosci 3: 481–492. [DOI] [PubMed] [Google Scholar]
- Shepherd SV, Klein JT, Deaner RO, Platt ML ( 2009): Mirroring of attention by neurons in macaque parietal cortex. Proc Natl Acad Sci USA 106: 9489–9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilek D, Birmingham E, Cameron D, Bischof W, Kingstone A ( 2006): Cognitive ethology and exploring attention in real‐world scenes. Brain Res 1080: 101–119. [DOI] [PubMed] [Google Scholar]
- Spence C, Kingstone A, Shore DI, Gazzaniga MS ( 2001a) Representation of visuotactile space in the split brain. Psychol Sci 12: 90–93. [DOI] [PubMed] [Google Scholar]
- Spence C, Nicholls ME, Driver J ( 2001b) The cost of expecting events in the wrong sensory modality. Percept Psychophys 63: 330–336. [DOI] [PubMed] [Google Scholar]
- Stevens SA, West GL, Al‐Aidroos N, Weger UW, Pratt J ( 2008): Testing whether gaze cues and arrow cues produce reflexive or volitional shifts of attention. Psychon Bull Rev 15: 1148–1153. [DOI] [PubMed] [Google Scholar]
- Szczepanski SM, Konen CS, Kastner S ( 2010): Mechanisms of spatial attention control in frontal and parietal cortex. J Neurosci 30: 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper CM, Handy TC, Giesbrecht B, Kingstone AF ( 2008): Brain responses to biological relevance. J Cogn Neurosci 20: 879–891. [DOI] [PubMed] [Google Scholar]
- Tipples J ( 2002): Eye gaze is not unique: Automatic orienting in response to uninformative arrows. Psychon Bull Rev 9: 314–318. [DOI] [PubMed] [Google Scholar]
- Townsend JT, Ashby FG ( 1983): The Stochastic Modelling of Elementary Psychological Processes. Cambridge: Cambridge University Press. [Google Scholar]


