Abstract
The feeling of guilt is a complex mental state underlying several human behaviors in both private and social life. From a psychological and evolutionary viewpoint, guilt is an emotional and cognitive function, characterized by prosocial sentiments, entailing specific moral believes, which can be predominantly driven by inner values (deontological guilt) or by more interpersonal situations (altruistic guilt). The aim of this study was to investigate whether there is a distinct neurobiological substrate for these two expressions of guilt in healthy individuals. We first run two behavioral studies, recruiting a sample of 72 healthy volunteers, to validate a set of stimuli selectively evoking deontological and altruistic guilt, or basic control emotions (i.e., anger and sadness). Similar stimuli were reproduced in a event‐related functional magnetic resonance imaging (fMRI) paradigm, to investigate the neural correlates of the same emotions, in a new sample of 22 healthy volunteers. We show that guilty emotions, compared to anger and sadness, activate specific brain areas (i.e., cingulate gyrus and medial frontal cortex) and that different neuronal networks are involved in each specific kind of guilt, with the insula selectively responding to deontological guilt stimuli. This study provides evidence for the existence of distinct neural circuits involved in different guilty feelings. This complex emotion might account for normal individual attitudes and deviant social behaviors. Moreover, an abnormal processing of specific guilt feelings might account for some psychopathological manifestation, such as obsessive‐compulsive disorder and depression. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: guilt, emotion, fMRI, deontological, altruistic, anterior cingulate cortex
INTRODUCTION
Guilt is a complex mental state, involving both emotional and cognitive aspects, that humans spontaneously experience in their everyday life. From an evolutionary viewpoint, guilt has been identified as a prosocial sentiment entailing specific moral believes [Moll et al., 2008]. Within such a context, guilt can be predominantly driven by inner values (deontological guilt) or by more interpersonal situations (altruistic guilt). Deontological guilt is usually characterized by a sense of responsibility leading to punishment seeking and sin expiation. Indeed, we can feel guilty after the violation of inner moral rules, in the absence of any direct damage or victim. For example, breaking a religious rule, as it may happen to a Roman Catholic who has sexual intercourse before marriage, might evoke a sense of deontological guilt [Haidt and Hersh, 2001]. Conversely, guilt may arise simply by observing one who is luckier than someone else, who has been unjustly penalized by chance (altruistic guilt). A traditional example of altruistic guilt is represented by air crash survivors, where empathy and feelings of sorrow might even lead to sacrifice oneself in the attempt to mitigate victim's suffering, even knowing others bad luck has occurred by chance (Baumeister, 2005; Hoffman, 1981; O'Connor et al., 2000; Weiss et al., 1986]. Altruistic guilt clearly differs from both “kin selected” and “reciprocal” altruistic behaviors, where individuals intentionally enter into a pact to exchange favours [Humphrey, 1997].
Only few neuroimaging studies have investigated the neural correlates involved in experiences of guilt, although none of them have made any distinction between different kinds of guilt that may arise in response to different circumstances. Shin et al. [2000], using positron emission tomography (PET), investigated regional changes of cerebral blood flow (rCBF) in individuals while recalling personal guilt experiences. Guilt against neutral conditions showed a significant increase of rCBF in the anterior cingulate gyrus, in the left insular cortex and inferior frontal gyrus, and in the anterior temporal pole bilaterally. Takahashi et al. [2004] used block‐design functional magnetic resonance imaging (fMRI) to measure regional activation associated with judgments of guilty, embarrassment, and neutral statements. Guilt relative to neutral condition produced greater activity in the medial prefrontal cortex and in the left posterior superior temporal sulcus. Another study investigated brain activation while subjects were reading sentences inducing guilt‐, other‐anger‐, self‐anger‐, and compassion [Kedia et al., 2008]. The association of guilt and other‐anger sentences contrasted with the remaining conditions revealed significantly increased activity in the anterior cingulate cortex, extending to the medial prefrontal cortex, the caudate, the precuneus, and the temporal pole. Taken altogether, these studies show a significant involvement of the anterior cingulate cortex, extending to more prefrontal areas, and of the temporal poles in the experience of guilt. These brain areas are traditionally involved in information integration, in one's own and others' mental state monitoring and in emotional processing [Bush et al., 2000; Lane et al., 1997; Shallice, 2001]. Nevertheless, these previous studies have some methodological limitations, such as the use of block design and the absence of control in subjective guilt experiences. More recently, Zahn et al. [2009], using event‐related fMRI, has reported a direct association between empathic concern and activation in the subgenual cingulate cortex of subjects undergoing stimuli evoking guilty feelings.
To investigate the neuronal substrate of both deontological and altruistic guilt, we employed an event‐related fMRI paradigm based on the presentation of emotional facial expressions [Ekman and Friesen, 1976] followed by contextual sentences, aiming to generate two different types of guilt, namely deontological and altruistic (Fig. 1). Randomly intermixed events, including sentences evoking anger and sadness, were also introduced to control for guilt‐emotion processing.
Figure 1.
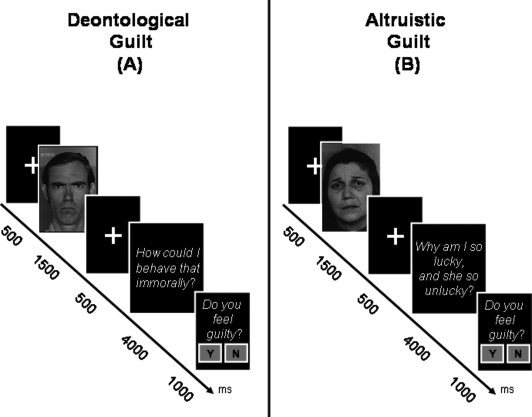
fMRI paradigm. The timing of each event in each trial is illustrated schematically. Each trial included the presentation of an emotional or neutral face, followed by a contextual sentence. The content of the sentence leads to two types of guilt (deontological and altruistic) and two control conditions (anger and sadness). At the end of each trial, subjects were asked to indicate, in a forced yes/no choice, whether they experienced guilt. Two examples are illustrated showing a trial with emotional face plus sentence inducing deontological guilt (A), and a trial with emotional face plus sentence inducing altruistic guilt (B).
MATERIALS AND METHODS
Subjects
The present event‐related fMRI study involved a group of 22 right‐handed, native Italian‐speaking healthy volunteers (13 women and 9 men; mean [SD] age = 26.8 [3.0] years; range = 21–38) with no history of medical or psychiatric disorders. All were recruited from a pool of psychology students. Ethical approval from the Ethics Committee of the Santa Lucia Foundation and written informed consent from each participant were obtained before study initiation.
Stimuli Validation
Stimuli used during fMRI were preliminarily validated in two behavioural studies. In the first study, we tested specific association between angry and sad faces and both kinds of guilt statements, while in the second one, we investigated whether all four emotional stimuli (deontological and altruistic guilt, anger, and sadness) were effectively evoking expected emotional responses.
Ekman's “Pictures of Facial Affect” [Ekman and Friesen, 1976] depicting specific emotional facial expressions were used in both behavioral experiments. Each face was followed by a short sentence to evoke deontological or altruistic guilt or other basic emotions (anger, sadness). Subjects were instructed to observe each face and to imagine that an external person was experiencing that specific emotion directed toward themselves. Then, a short sentence representing an inner dialogue in response to the specific facial expression was shown. Subjects were asked to rate the intensity of each of the randomly occurring stimuli, rating each of nine emotions (deontological guilt, altruistic guilt, shame, sadness, anger, compassion, fear, disgust, absence of emotion) on an eighteen‐point visual analogue scale (VAS: 0 = not present, 18 = very intense). Each VAS answer was defined by the emotion's name, its distinctive action tendency, and a short description. Typical target trials evoking deontological guilt were elicited by associating an angry face with sentences like: “Oh my God! How could I do such a thing!?” or “How could I behave that immorally!” Conversely, altruistic guilt was elicited by the association of a sad face and a sentence such as: “Why am I so lucky, and why is he/she so unlucky?” or “How unfair! I am doing so well, while she/he is so unlucky!”
In the first study, we recruited 32 healthy volunteers (mean [SD] age = 29.9 [5.22] years; range = 26–53). To test for the specific association between angry and sad faces with, respectively, deontological and altruistic guilt statements we confronted subjects with angry and sad faces, combined with both kinds of guilt statements (2 × 2 design).
Once guilt specific face/sentence coupling was established, we run a second behavioral experiment, involving 40 new healthy volunteers (mean [SD] age = 30.23 [4.38] years; range = 25–43), adding two control emotional sentences (anger and sadness). Anger statements, preceded by the same angry faces used in deontological guilt stimuli, included sentences as: “How dare she? Staring at me in such a way!” or “Who does he think he is?! Looking at me in such a way!”. Sad sentences, associated with previously used sad facial expressions, included statements like: “Oh my God! What has happened to her, she looks so sad!” or “He must be really desperate! Crying in such a way!” Four trials for each condition were used in both experiments, and sentences were matched for length and number of syllables. All statistical analyses were performed using the SPSS 13.0 statistical package.
fMRI Image Acquisition and Analysis
During our event‐related fMRI paradigm, we used 30 trials for each of the four early mentioned conditions (that is: deontological and altruistic guilt, and anger and sadness as correspondent controls), randomly intermixed (inter‐trail‐interval was jittered between 1,350 and 1,650 ms). Differently from previous behavioral studies, four additional neutral‐face conditions were introduced, to control for emotional facial expression effect on the succeeding emotional sentence (Fig. 1). Finally, the fMRI experiment resulted in eight equally balanced experimental conditions: (1) neutral face + deontological guilt sentence; (2) anger face + deontological guilt sentence; (3) neutral face + anger sentence; (4) anger face + angry sentence; (5) neutral face + altruistic guilt sentence; (6) sad face + altruistic guilt sentence; (7) neutral face + sad sentence; (8) sad face + sad sentence. The eight experimental conditions were presented in four fMRI sessions (duration for each session = 11 min and 26 s; total experimental duration = 45.04 min). Two sessions included randomly occurring trials evoking deontological guilt and anger (conditions 1–4, above), while the other two included trials evoking altruistic guilt and sadness (conditions 5–8, above). During MR scanning, subjects were instructed to observe the face and to image that an external person was experiencing that specific emotion. After viewing the face, participants were asked to read and empathize with the content of the presented short sentence, as representing an inner dialog in response to the previously shown face. Finally, subjects were confronted with two alternative forced choice task (“Do you feel guilty”: YES/NO), which allowed us to assess whether guilt was elicited on a trial‐by‐trial basis.
Data Acquisition
MRI data were acquired from a 3‐Tesla Allegra system (Siemens, Erlangen, Germany) equipped with a circularly polarized transmit‐receive coil. The maximum gradient strength is 40 mT/m, with a maximum slew rate of 400 mT/m/ms.
Functional images were collected by echo‐planar (EPI) T2* sequence using blood‐oxygenation‐level‐dependent (BOLD) contrast. Each acquired volume consisted of 32 axial slices with a 3‐mm thickness and a 1.3‐mm distance factor to cover the entire brain, with an effective repetition time of 2.08 s. The scanner was synchronized with the presentation of each session, and the ratio of interscan to interstimulus interval ensured that voxels were sampled at different phases relative to stimulus onset.
Data Analysis
Data were processed using MATLAB 7.0 (MathWork, Natick, MA) and SPM5 (http://www.fil.ion.ucl.ac.uk/spm/), and analyzed with the general linear model (GLM) for event‐related designs, using a random‐effects analysis. For each fMRI session, the first four volumes were discarded to allow for T1 equilibration effects. All the acquired EPI images were then realigned to the first image of the first session using the “Realign” routine in SPM5, normalized to a standard echo‐planar image template, and smoothed with a Gaussian kernel of 8 mm full‐width half maximum. Data were globally scaled and high‐passed‐filtered to 1/128 Hz to remove low‐frequency noise. For each subject, the following conditions were modeled (independently of guilt‐evoking ratings) using the time of sentence disappearance as onset (with a duration of 4,000 ms): (1) neutral face + deontological guilt sentence; (2) anger face + deontological guilt sentence; (3) neutral face + anger sentence; (4) anger face + angry sentence; (5) neutral face + altruistic guilt sentence; (6) sad face + altruistic guilt sentence; (7) neutral face + sad sentence; (8) sad face + sad sentence.
The resulting contrast‐images representing the amplitude of BOLD response for each subject and each condition were included in the random‐effects analysis using a one‐way analysis of variance (ANOVA) design. Correction for nonsphericity was used to account for possible differences in error variance across conditions and any non‐independent error terms for the repeated measures analysis.
Within this ANOVA, we tested for the following effects: (1) emotional versus neutral faces stimuli, to highlight the overall network responsive to our emotional stimuli; (2) the main effect of both types of guilt (deontological and altruistic) against basic emotions (anger and sadness), to reveal guilt‐related brain activation; and (3) interactions between guilt‐type (deontological/altruistic) and the corresponding basic emotion (anger/sadness), to reveal brain activation specific for one or the other type of guilt. For the first two comparisons, we assigned corrected P‐value at the cluster‐level (P corr. < 0.05; cluster size estimated at P unc. = 0.005, voxel‐level), considering the whole brain as the volume of interest. For the interaction effects, corrected P‐values (P corr. < 0.05, voxel‐level) were assigned using a small volume procedure (SVC) [Worsley et al., 1996]. The volume of interest (VOI) included all voxels in the medial frontal cortex and the insula bilaterally that showed an overall effect of our emotional stimuli (Fig. 3). Additional guilt‐specific VOIs, which had been identified in previous studies [i.e. temporal poles, including posterior superior temporal sulcus/temporo‐parietal junction, precuneus; Kedia et al., 2008; Shin et al., 2000; Takahashi et al., 2004], were used for further SVC analyses.
Figure 3.
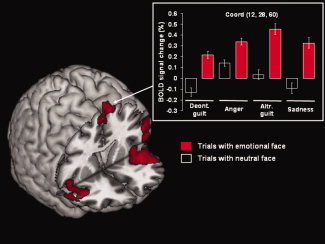
Effect of emotional faces. Brain activation for the comparison of all trials containing an emotional face (columns filled in red) versus trials with neutral faces (columns filled in black). Significant activation was observed in right superior frontal gyrus (BA6), in the inferior frontal gyrus (BA45), extending to the middle frontal gyrus (BA9) and the right orbitofrontal cortex (BA 47, not visible in this projection), and the insula bilaterally. The plot shows the BOLD signal changes in the right superior frontal gyrus for all experimental conditions. The activation map is rendered at a threshold of P corr. = 0.05, at the cluster level. Deont., deontological; Altr., altruistic.
RESULTS
Stimuli Validation
In the first behavioral study, specific association of angry faces with deontological guilt sentences evoked significantly more intense deontological guilt VAS answers, compared to altruistic guilt (mean [SD] = 8 [4.7], mean [SD] = 5.1 [3.7]; t(31) = 4.84, two‐tailed P < 0.000). On the other hand, sad facial expressions associated with altruistic guilt elicited significantly more intense altruistic guilt answers, when compared to stimuli showing angry faces followed by altruistic guilt sentences (mean [SD] = 4.4 [3], mean [SD] = 1,7 [2.1]; t(31) = 3.37 two‐tailed P < 0.001).
In the second preliminary study, we tested all four emotional conditions together (deontological guilt, anger, altruistic guilt, and sadness). When subjects were asked to rate deontological guilt stimuli, VAS answers on the corresponding deontological guilt scale (mean [SD] = 8.80[5.3]) were significantly greater than for altruistic guilt VAS responses (mean [SD] = 2.48 [2.7]) (t(39) = 8.25, two‐tailed P < 0.001). Within altruistic guilt trials, the expected altruistic guilt VAS answers (mean [SD] = 8.88[5.7]) were statistically greater than for deontological guilt responses (mean [SD] = 2.44 [2.06]) (t(39) = 7.47, two‐tailed P < 0.001). These results confirm that the selected stimuli evoked the expected feelings of guilt. Similarly, anger (mean [SD] = 9.7 [4.3]) (t(39) = 11.89, two‐tailed P < 0.000) and sad stimuli (mean [SD] = 10 [4.2]) (t(39) = −12.21, two‐tailed P < 0.000) as well were found to evoke significantly more intense anger and sad, respectively, VAS responses. Concerning sad stimuli, it should be noted that VAS responses revealed a constant association between sadness and compassion (means [SD] = 10 [4.21] and 9.47 [4.34], respectively) (t(39) = –0.827 two‐tailed P = 0.413).
fMRI
Accuracy and reaction time data
Behavioral responses confirmed that the two critical guilt conditions (emotional faces + deontological or altruistic guilt sentences) evoked a significantly higher rate of guilty responses compared, respectively, to each of their control conditions (emotional faces followed by anger or sad sentences) (t(18) = 3.25, two‐tailed P < 0.002, t‐test) (Fig. 2). A within‐subject ANOVA of reaction times (RT) was performed on subjects button‐pressing. Both deontological guilt (F(1,18) = 7.46, P < 0.01) and altruistic guilt (F(1,18) = 8, P < 0.01) required longer RTs in answering, when compared to their control conditions.
Figure 2.
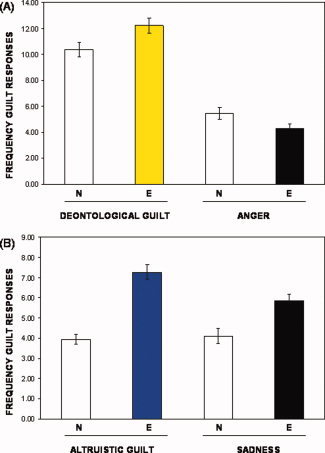
fMRI behavioral responses. Behavioral responses showing frequency of target‐guilt answers across all eight conditions. (A) Neutral (N) and emotional (E) faces plus sentence inducing deontological guilt and anger, respectively. (B) Neutral (N) and emotional (E) faces followed by statements inducing altruistic guilt and sadness.
Imaging Findings
Effect of emotional faces
Significantly higher activation was observed in three clusters. The first cluster included the left inferior frontal gyrus, extending to the middle frontal gyrus and the left insula. The second cluster included the right inferior frontal gyrus, extending to the right orbitofrontal cortex and the right insula. The third cluster was centered on the right superior frontal gyrus. These results are summarized in Table I and illustrated in Figure 3.
Table I.
Effect of emotional faces
| Brain area | BA | Coordinates | Z‐val | Size | P‐corr | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Inf frontal gyrus | 45 | −48 | 32 | 0 | 4.39 | 2,141 | <0.001 |
| Mid frontal gyrus | 9 | −58 | 18 | 26 | 2.95 | ||
| Insula | — | −48 | 18 | −14 | 3.41 | ||
| Inf frontal gyrus | 45 | 46 | 24 | −14 | 3.73 | 922 | <0.001 |
| Orbitofrontal cortex | 47 | 36 | 28 | −4 | 3.71 | ||
| Insula | — | 46 | 14 | 0 | 3.47 | ||
| Sup frontal gyrus | 6 | 12 | 28 | 60 | 3.59 | 710 | <0.001 |
Inf, inferior; Mid, middle; Sup, superior; BA, Brodmann area.
Maxima of regions showing significant BOLD signal changes in a direct comparison between trials with emotional faces versus trials with neutral faces. The extension of each area (size) is expressed in number of voxels. Stereotaxic coordinates are reported in Talairach space. See text for further detail.
Effect of guilty feeling
Next, we directly compared trials including guilt sentences, both deontological and altruistic, versus trials that included only the basic control emotions (i.e. anger and sadness). This contrast revealed greater activation in two clusters. The first one included the ventral and dorsal parts of the anterior cingulate and the medial frontal gyrus. The second cluster included the posterior cingulate bilaterally. These results are summarized in Table II and illustrated in Figure 4 (regions filled in red). For completeness, we also compared each single guilt‐type (i.e. deontological or altruistic) versus the corresponding emotional control condition (anger or sadness). This revealed some dissociation of regional brain activity within the anterior cluster, in the medial frontal cortex. Deontological guilt induced grater activation in the anterior cingulate bilaterally, extending posteriorly to the cingulate gyrus (Table II and Fig. 4, regions in yellow). Conversely, altruistic guilt elicited greater activation in more anterior regions of the medial prefrontal cortex including the left medial frontal gyrus (Table II and Fig. 4, regions in blue).
Table II.
Effect of guilty feeling
| Main effect of guilt | Simple main effects in the frontal cluster | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain area | BA | Coordinates | Z‐val | Size | P‐corr | Guilt type | Coordinates | Z‐val | Size | P‐corr | ||||
| x | y | z | x | y | z | |||||||||
| Ant cingulate | 32/24 | 0 | 50 | −2 | 3.46 | 1,717 | <0.001 | Deontological | −4 | 30 | 24 | 3.63 | 793 | 0.018 |
| Med frontal gyrus | 9/10 | −12 | 40 | 12 | 3.05 | <0.001 | Altruistic | −4 | 52 | 20 | 3.14 | 458 | 0.144 | |
| Post cingulate | 23 | −4 | −52 | 40 | 3.19 | 453 | 0.149 | |||||||
Ant, anterior; Med, medial; Post, posterior; BA, Brodmann area.
Maxima of regions showing significant BOLD signal changes in comparison of guilt (deontological and altruistic) as compared to control conditions (anger and sadness) (left side). There are also reported here the results obtained by comparing each single guilt‐type (i.e. deontological or altruistic) versus the corresponding emotional control condition (anger or sadness) (right side).
Stereotaxic coordinates are reported in Talairach space. See text for further details.
Figure 4.
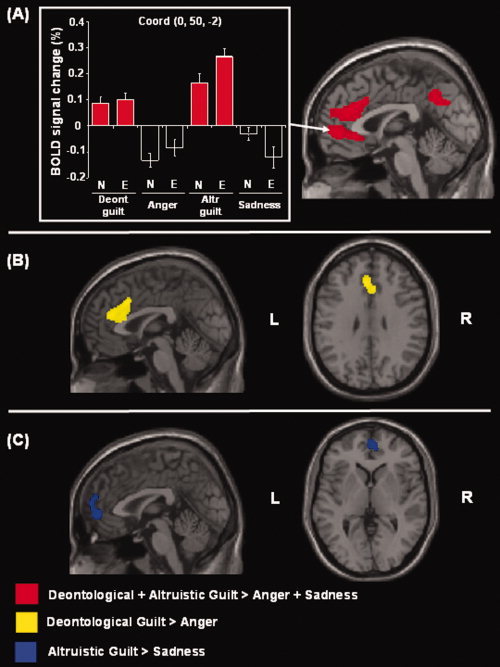
Effects of deontological and altruistic guilty feelings. Average activity for deontological and altruistic guilt as compared with the control conditions (anger and sadness) revealed greater activation in the anterior cingulate extending to the medial frontal gyrus, plus a cluster in the posterior cingulate. The localization of these clusters is highlighted on the sagittal anatomical section (red coloured; display threshold: P corr. = 0.05, at the cluster level). The signal plot shows the level of activation at the peak of the anterior cluster, with the critical four guilt conditions highlighted in red. When each type of guilt (deontological or altruistic) was compared separately to the corresponding basic emotion (anger or sadness respectively), two different patterns of brain activation were identified. (A) Deontological guilt showed greater activation in the anterior cingulate bilaterally (in yellow) (B), while altruistic guilt induced greater activation in the left medial frontal gyrus (in blue) (C). For these additional comparisons, the two activation maps are displayed at a voxel‐level threshold of P unc. = 0.005. Deont., deontological; Altr., altruistic.
Interactions Between Deontological and Altruistic Guilt
To formally asses brain activation associated specifically with one or the other type of guilt, we tested the interaction between guilt‐type (deontological/altruistic) and the corresponding basic emotion (anger/sadness). This revealed a significant modulation of activity in the left insula (x, y, z = −44, 10, −10; Z‐score = 3.32; P corr < 0.05) where the interaction was driven by deontological guilt stimuli [(deontological guilt vs. anger) > (altruistic guilt vs. sadness)]. An analogous pattern of activation was present also in the right insula, although it did not reach full statistical significance after correction for multiple comparisons (x, y, z = 48, 10, −8; Z‐score = 2.85; P unc < 0.002). These results are illustrated in Figure 5. The reverse interaction [(altruistic guilt vs. sadness) > (deontological guilt vs. anger)] did not reveal any significant effect after correction for multiple comparisons. Because of study design, we could not perform a direct contrast between “anger” and “sad” conditions, as these stimuli were presented in distinct sessions, to respectively control for deontological and altruistic guilt.
Figure 5.
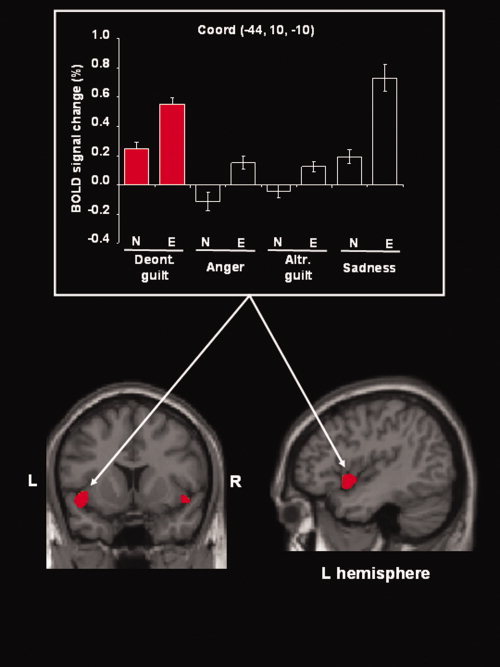
Selective activation of the insula for deontological guilt. Anatomical localisation and signal plot of the left insula that showed a selective effect for deontological guilt (columns filled in red), i.e. the interaction: (deontological guilt vs. anger) > (altruistic guilt vs. sadness). A similar pattern of activation was found in the right insula (also visible in the coronal section), where this effect only approached statistical significance (see main text). The activation map is rendered at a voxel‐level threshold of P unc. = 0.002, to reveal also the activation of the right insula. Deont., deontological; Altr., altruistic; L, left; R, right.
No additional activation was found using VOIs identified in previous guilt studies, nor between any guilt condition against its control.
DISCUSSION
In this study, we designed an event‐related fMRI paradigm to investigate the neural substrate of deontological and altruistic guilt. To control for guilt‐emotions, additional conditions including basic emotions, such as anger and sadness, were introduced.
As expected from previous perceptual‐emotional studies [Bechara et al., 2000; Blair, 2004; Steele and Lawrie, 2004], the overall effect of emotional versus neutral faces revealed activation of prefrontal regions. These areas play a role in recognizing and processing emotional cues [for a review see Murphy and Lawrence, 2003; Phan et al., 2002] that are impaired in patients with frontal lesions [Damasio, 1994; Hornak et al., 2003; Rolls et al., 1999]. The activation we observed here in the orbitofrontal cortex seems to be particularly relevant in highlighting the emotional aspects of our current fMRI paradigm. This brain region is involved in the identification of facial expressions and has been previously reported to be implicated in social feedback and in behaviour regulation [Rolls, 2005]. The additional modulation of activity in the insula, which is connected to the limbic system, may correspond to the engagement of general emotional processes [Murphy and Lawrence, 2003; Phan et al., 2002], and more specifically to the generation of feelings of disgust [Blair, 2004; Phillips et al., 1998; Rozin et al., 2000; Wicker et al., 2003]. We might speculate that, in evolutionary terms, the insula may have developed widespread connections with higher cognitive brain structures (i.e., anterior cingulate), thus accounting for complex mental states such as guilt.
Both deontological and altruistic guilt compared to their control conditions (anger and sadness) showed significantly greater activity in the anterior and posterior cingulate, and in the left medial frontal gyrus. These findings are consistent with previous functional neuroimaging studies (Kédia et al., 2008; Moll et al., 2007; Shin et al., 2000; Takahashi et al., 2004] reporting a direct involvement of the anterior cingulate in the experience of guilt. Here, we found increased activity in the most ventral and dorsal portions of the cingulate cortex, which, respectively, represent the affective and cognitive divisions of this brain structure [Bush et al., 2000]. The engagement of the anterior cingulate is consistent with the notion that guilt reflects a complex mental state, in which both emotional [Devinsky et al., 1995; Drevets and Raichle, 1998] and cognitive processes [for a review see, Drevets and Raichle, 1998; Posner and DiGirolamo, 1998] are implicated. Longer RTs observed in both guilt conditions, compared to control emotions, might reflect the difference between more cognitively demanding information processes, and more basic emotions, such as anger and sadness, which are more automatically and directly processed. As suggested by several authors [Kedia et al., 2008; Moll et al., 2007; Zahn et al., 2009], the anterior cingulate might be directly implicated in empathic moral feelings, entailing both attachment towards another person (altruistic guilt) or abstract moral rules (deontological guilt). Similarly to Zahn et al. [2009], who reported a direct association between activation in the subgenual cingulate and empathic concern under guilt‐evoking stimuli, we found a broad increase of activation in the same brain area (extending also to the anterior cingulate) when looking at both kinds of guilt together. Admittedly, within the subgenual part of the cingulate, we were unable to identify a specific response to altruistic or deontological guilt in isolation. Nevertheless, in view of previous findings [Zahn et al., 2009], we might hypothesize that the subgenual cingulate is more likely related with altruistic than deontological guilt processing. In fact, this specific brain area has also been shown to be specifically involved in charitable altruistic behaviour [Moll et al., 2006]. Finally, Singer et al. [2006] found significantly higher activation in females, compared against males, within anterior cingulate cortices, when feeling empathic toward a cooperative person who is suffering. According with these findings, we can hypothesize that gender might also play a role in the processing of altruistic and deontological guilt. Future studies including larger populations of subjects are needed to address this issue.
Further, the increased activation of the posterior cingulate corroborates previous findings suggesting the role of this brain structure in the evaluation of emotional and social information [Maddock, 1999; Vogt et al., 2000]. The anterior and posterior cingulate are strongly interconnected and both receive afferent inputs from limbic areas, including the insula, the amygdala, the nucleus accumbens [Devinsky et al., 1995], and the prefrontal and orbital cortices. All these considerations reinforce our hypothesis that an increase in connectivity between affective and cognitive brain regions may represent the evolutionary development of more structured emotions, such as guilty feelings.
Finally, the experience of deontological guilt induced a selective activation of the insula, which is a well‐known structure implicated in emotional processing, and more specifically in feelings of disgust [Lane et al., 1997; Phillips et al., 1997, 1998; Rozin et al., 2000]. Some authors define disgust as a complex emotion which is classified, despite its predominant physical manifestations [Rozin et al., 2000; Phillips et al., 2003], as a “moral emotion” [Miller, 1997]. Consistently, our results support this view indicating a functional‐anatomical correspondence between deontological guilt and moral‐disgust feelings, which might be oriented toward oneself. Furthermore, since the insula seems to be involved in aversive emotions processing, we might suggest that deontological guilt entails more negative characteristics, compared to altruistic guilt.
In addition, when we examined the pattern of activation for the two types of guilt separately (compared with the corresponding controls) we found that the anterior cingulate activated primarily for the deontological guilt conditions, while processing of altruistic guilt appeared to involve primarily a more anterior region in the medial prefrontal cortex. The effect in the anterior cingulate for deontological guilt is consistent with the hypothesis that this structure plays a relevant role in processing both emotional and cognitive aspects of stimuli [Bush et al., 2000], and fits well with the idea that guilt is a complex human experience based on basic emotion and cognitive processes.
On the other hand, the activation of medial prefrontal brain areas for altruistic guilt can be related with previous studies on Theory of Mind (Blair, 1995; Shallice, 2001]. For instance, “mind‐reading” tasks typically entail experiencing social and interpersonal emotions, compared to inner and more private emotional experiences, such as sadness. Similar activation was also found in a recent study by Moll et al. [2007], where the medial prefrontal cortex seemed to be involved in empathic guilt and compassion feelings. Further, activation in the medial prefrontal cortex was observed in subjects experiencing moral sentiments, and also when viewing other people sad face [for reviews, see Moll et al., 2005a, and Moll et al., 2005b]. This suggests that altruistic guilt, falling within sadness and compassion emotional domain, might share with these emotions a partially common neural substrate. Finally, implication of prefrontal areas in guilt processing may contribute to understand why patients with lesions in this anatomical locations are described as more insensitive to guilt, when compared to healthy controls [Koenigs et al., 2007; Krajbich et al., 2009].
CONCLUSIONS
Overall, our study supports Sinnott‐Armstrong's [1996] and Haidt's [Haidt and Hersh, 2001] efforts to identify multiple domains associated with morality, by suggesting that different varieties of morally reprehensible acts are based on similar but differential psychological and neurobiological mechanisms. Our results provide evidence for the existence of distinct brain areas involved in different kinds of guilt processing. Beside previous findings investigating guilt as a single component emotion [i.e. Kédia et al., 2008; Moll et al., 2007; Shin et al., 2000], here we show that our brain is differentially specialised in terms of neural substrates for situations implicating suffering in others (altruistic guilt), and those violating inner moral rules (deontological guilt). This formulation has clinical implications for abnormal experiences of guilt in relation to certain neurological disorders [Mendez, 2006], antisocial behavior [Link et al., 1977; Pardini et al., 2003], and obsessive (Rachman, 1993; Salkovskis, 1989; Shafran et al., 1996] or depressive symptoms [Gilbert, 1992; O'Connor et al., 1999, 2002].
Specifically, depression may be associated with an abnormal processing of altruistic guilt, as suggested by previous clinical studies [Gilbert, 1992; O'Connor et al., 1999, 2002]. Moreover, some authors have shown an increased metabolic activity in the ventral cingulate cortex of patients with depression [Mayberg et al., 1999], which could be reduced, concomitantly with a clinical improvement, following deep brain stimulation [Mayberg et al., 2005]. In contrast, abnormal processing of deontological guilt might characterize aspects of the obsessive compulsive disorder, as argued by others [Rachman, 1993; Salkovskis, 1989; Shafran et al., 1996]. Thus, there might be a connection between deontological and altruistic guilt and prosocial behavior promotion, norm compliance, and self‐blaming when exacerbated.
The dissociation of altruistic from deontological guilt may also help to clarify other complex human behaviors such as cooperation or its lack. In this perspective, individual variability in guilt processing may explain why individuals from similar sociocultural backgrounds react differently to the same environmental stimuli in similar situations. A better characterization of the physiological basis of guilt should increase our understanding of individual behaviors that constitute a continuous spectrum from ‘normality’ to frank psychopathology, with possible attendant clinical and forensic implications.
Acknowledgements
The Neuroimaging Laboratory of the Santa Lucia Foundation is supported by the Italian Ministry of Health. RSJF was funded by a Welcome Trust Programme grant.
REFERENCES
- Baumeister RF ( 2005): The Cultural Animal: Human Nature, Meaning, and Social Life. New York: Oxford University Press. [Google Scholar]
- Bechara A, Damasio H, Damasio AR ( 2000): Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 10: 295–307. [DOI] [PubMed] [Google Scholar]
- Blair RJ ( 1995): A cognitive developmental approach to morality: Investigating the psychopath. Cognition 57: 1–29. [DOI] [PubMed] [Google Scholar]
- Blair RJ ( 2004): The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn 55: 198–208. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Damasio AR ( 1994): Descartes' Error: Emotion, Reason, and the Human Brain. New York: Grosset/Putnam. [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA ( 1995): Contributions of anterior cingulate cortex to behaviour. Brain 118: 279–306. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME ( 1998): Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition Emotion 12: 353–385. [Google Scholar]
- Ekman P, Friesen WV ( 1976): Pictures of Facial Affect. Palo Alto: Consulting Psychologists. [Google Scholar]
- Gilbert P ( 1992): Depression: The Evolution of Powerlessness. Hove: Lawrence Erlbaum Associates. [Google Scholar]
- Haidt J, Hersh MA ( 2001): Sexual morality: The cultures and reasons of liberals and conservatives. J Appl Soc Psychol 31: 191–221. [Google Scholar]
- Hoffman M ( 1981): Is altruism a part of human nature? J Pers Soc Psychol 40: 121–137. [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O'Doherty J, Bullock PR ( 2003): Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain 126: 1691–1712. [DOI] [PubMed] [Google Scholar]
- Humphrey N ( 1997): Varieties of altruism—And the common ground between them. Social Res 64: 199–209. [Google Scholar]
- Kédia G, Berthoz S, Wessa M, Hilton D, Martinot JL ( 2008): An agent harms a victim: A functional magnetic resonance imaging study on specific moral emotions. J Cogn Neurosci 20: 1788–1798. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A ( 2007): Damage to the prefrontal cortex increases utilitarian moral judgments. Nature 19: 908–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajbich I, Adolphs R, Tranel D, Denburg NL, Camerer CF ( 2009): Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. J Neurosci 29: 2188–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ ( 1997): Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry 154: 929–933. [DOI] [PubMed] [Google Scholar]
- Link NF, Scherer SE, Byrne PN ( 1977): Moral judgement and moral conduct in the psychopath. Can Psychiatr Assoc J 22: 341–346. [DOI] [PubMed] [Google Scholar]
- Maddock RJ ( 1999): The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends Neurosci 22: 310–316. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. ( 1999): Reciprocal limbic‐cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry 156: 675–682. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH ( 2005): Deep brain stimulation for treatment‐resistant depression. Neuron 3: 651–660. [DOI] [PubMed] [Google Scholar]
- Mendez MF ( 2006): What frontotemporal dementia reveals about the neurobiological basis of morality. Med Hypotheses 67: 411–418. [DOI] [PubMed] [Google Scholar]
- Miller WI ( 1997): The Anatomy of Disgust. Cambridge: Harvard University Press. [Google Scholar]
- Moll J, de Oliveira‐Souza R, Moll FT, Ignácio FA, Bramati IE, Caparelli‐Dáquer EM, Eslinger PJ ( 2005a): The moral affiliations of disgust: A functional MRI study. Cogn Behav Neurol 18: 68–78. [DOI] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira‐Souza R, Krueger F, Grafman J ( 2005b): Opinion: The neural basis of human moral cognition. Nat Rev Neurosci 6: 799–809. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira‐Souza R, Grafman J ( 2006): Human fronto‐mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci USA 103: 15623–15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira‐Souza R, Garrido GJ, Bramati IE, Caparelli‐Daquer EM, Paiva ML, Zahn R, Grafman J ( 2007): The self as a moral agent: Linking the neural bases of social agency and moral sensitivity. Soc Neurosci 2: 336–352. [DOI] [PubMed] [Google Scholar]
- Moll J, De Oliveira‐Souza R, Zahn R ( 2008): The neural basis of moral cognition: Sentiments, concepts, and values. Ann N Y Acad Sci 1124: 161–180. [DOI] [PubMed] [Google Scholar]
- Murphy NI, Lawrence AD ( 2003): Functional neuroanatomy of emotion: A meta‐analysis. Cogn Affect Behav Neurosci 3: 207–233. [DOI] [PubMed] [Google Scholar]
- O'Connor LE, Berry JW, Weiss J ( 1999): Interpersonal guilt, shame and psychological problems. J Social Clin Psychol 18: 356–361. [Google Scholar]
- O'Connor LE, Berry JW, Weiss J, Schweitzer D, Sevier M ( 2000): Survivor guilt, submissive behaviour and evolutionary theory: The down‐side of winning in social comparison. Br J Med Psychol 73: 519–530. [DOI] [PubMed] [Google Scholar]
- O'Connor LE, Berry JW, Weiss J, Gilbert P ( 2002): Guilt, fear, submission, and empathy in depression. J Affec Disord 71: 19–27. [DOI] [PubMed] [Google Scholar]
- Pardini DA, Lochman JE, Frick PJ ( 2003): Callous/unemotional traits and social‐cognitive processes in adjudicated youths. J Am Acad Child Adolesc Psychiatry 42: 364–371. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I ( 2002): Fuctional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS ( 1997): A specific neural substrate for perceiving facial expressions of disgust. Nature 389: 495–498. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, Williams SC, Bullmore ET, Brammer M, Gray JA ( 1998): Neural responses to facial and vocal expressions of fear and disgust. Biol Sci 265: 1809–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R ( 2003): Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry 54: 504–514. [DOI] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ ( 1998): Executive attention: Conflict, target detection and cognitive control In: Parasuraman R. (Ed.), The Attentive Brain. Cambridge, MA: MIT Press; pp 401–423. [Google Scholar]
- Rachman S ( 1993): Obsessions, responsibility and guilt. Beh Res Ther 31: 149–154. [DOI] [PubMed] [Google Scholar]
- Rolls ET ( 2005): Emotion Explained. Oxford: Oxford University Press. [Google Scholar]
- Rolls ET, Tovee MJ, Panzeri S ( 1999): The neurophysiology of backward visual masking: Information analysis. J Cogn Neurosci 11: 335–346. [DOI] [PubMed] [Google Scholar]
- Rozin P, Haidt J, McCauley CR ( 2000): Disgust In: Lewis M, Haviland‐Jones J, editors. Handbook of Emotions, 2nd ed. New York: Guilford Press; pp 673–653. [Google Scholar]
- Salkovskis P ( 1989): Cognitive‐behavioral factors and the persistence of intrusive thought in obsessional problems. Behav Res Ther 27: 677–682. [DOI] [PubMed] [Google Scholar]
- Shafran R, Watkins E, Charman T ( 1996): Guilt in obsessive‐compulsive disorder. J Anxiety Disord 10: 509–516. [Google Scholar]
- Shallice T ( 2001): 'Theory of mind' and the prefrontal cortex. Brain 124: 247–248. [DOI] [PubMed] [Google Scholar]
- Shin LM, Dougherty DD, Orr SP, Pitman RK, Lasko M, Macklin ML, Alpert NM, Fischman AJ, Rauch SL ( 2000): Activation of anterior paralimbic structures during guilt‐related script‐driven imagery. Biol Psychiatry 48: 43–50. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD ( 2006): Empathic neural responses are modulated by the perceived fairness of others. Nature 26: 466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott‐Armstrong W ( 1996): Moral Knowledge? New York: Oxford University Press. [PubMed] [Google Scholar]
- Steele JD, Lawrie SM ( 2004): Segregation of cognitive and emotional function in the prefrontal cortex: A stereotactic meta‐analysis. Neuroimage 21: 868–875. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y ( 2004): Brain activation associated with evaluative processes of guilt and embarrassment: An fMRI study. Neuroimage 23: 967–974. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Abscher JR, Bush G ( 2000): Human retrosplenial cortex: Where is it and is it involved in emotion? Trends Neurosci 23: 195–196. [DOI] [PubMed] [Google Scholar]
- Weiss E, O'Connell AN, Siiter R ( 1986): Comparisons of second‐generation holocaust survivors, immigrants, and non‐immigrants on measures of mental health. J Pers Soc Psychol 50: 828–531. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G ( 2003): Both of us disgusted in my insula: The common neural basis of seeing and feeling disgust. Neuron 40: 655–664. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC ( 1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Zahn R, de Olivera‐Souza, Bramati I, Garrido G, Moll J ( 2009): Subgenual cingulated activity reflects individual differences in empathic concern. Neurosci Lett 457: 107–110. [DOI] [PubMed] [Google Scholar]


