Abstract
Although elevated serum high‐sensitivity C‐reactive protein (hsCRP) is related to atherosclerosis, brain infarction, and cognitive decline, it has not been clarified whether increased hsCRP is associated with the decline in brain gray matter volume. Therefore, the purpose of this study was to determine the relationship between hsCRP levels and brain regional gray matter volume using brain magnetic resonance imaging (MRI) data from 109 community‐dwelling healthy elderly subjects. Brain MRIs were processed with voxel‐based morphometry using a custom template by applying diffeomorphic anatomical registration using the exponentiated lie algebra (DARTEL) procedure. We found a significant negative correlation between regional gray matter volume of the posterior and lateral aspects of the left temporal cortex and hsCRP level after adjusting for age, gender, and intracranial volume. Our results suggest that subjects who have mild inflammation related to arteriosclerosis have decreased regional gray matter volume in the posterior and lateral aspects of the left temporal cortex. Thus, preventing the progression of arteriosclerosis may be important for preventing a decrease in gray matter volume in healthy elderly subjects. Hum Brain Mapp 34:2418–2424, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: gray matter, high‐sensitivity C‐reactive protein, magnetic resonance imaging, elderly, voxel‐based morphometry, DARTEL
INTRODUCTION
Recent studies have shown that chronic inflammation plays a crucial role in the development of atherosclerosis [Ross, 1999]. High‐sensitivity C‐reactive protein (hsCRP) is a sensitive marker of systemic low‐grade inflammation [Pearson et al., 2003] and is associated with plaque progression and instability in large arteries [Hashimoto et al., 2001; Yamagami et al., 2004]. Additionally, hsCRP is useful to predict several diseases such as stroke and cardiovascular diseases [Pearson et al., 2003; Teunissen et al., 2003; Yaffe et al., 2003]. Increased serum concentrations of hsCRP have been associated with poor memory [Teunissen et al., 2003], poor global cognitive performance [Pearson et al., 2003], and vascular dementia [Ravaglia et al., 2007]. Several higher cognitive functions have been shown to be associated with regional gray matter volume, for example, episodic memory with hippocampus gray matter [Kramer et al., 2007], working memory with dorsolateral prefrontal cortex gray matter [Takeuchi et al., 2010], semantic memory with temporal pole gray matter [Taki et al., 2011], and executive function with lateral prefrontal cortex gray matter [Kramer et al., 2007; Zimmerman et al., 2006]. Therefore, it is plausible that there is a correlation between hsCRP level and regional gray matter volume. Investigating the correlation between hsCRP level and regional gray matter volume is important because a significant correlation would indicate that cognitive decline owing to atherosclerosis is derived from a reduction in gray matter volume, and that medical treatment for atherosclerosis is important not only to prevent the decrease in cognitive function but also to prevent a decrease in gray matter volume.
However, few studies have tested the correlation between hsCRP level and brain structure. A recent study focused on the correlation between hsCRP level and white matter microstructure in healthy adults, and showed that a higher hsCPR level was related with reduced fractional anisotropy of the frontal lobe, the corona radiata, and the corpus callosum [Wersching et al., 2010]. Another study showed that hsCRP level was related with hippocampal volume in patients with type 2 diabetes mellitus [Anan et al., 2011]; nondemented patients with type 2 diabetes mellitus and a higher hsCRP level and showed more hippocampal atrophy compared with those with a normal hsCRP level. Although these findings suggest a significant correlation between hsCRP and regional gray matter volume of the hippocampus, that study focused on patients with type 2 diabetes mellitus, who show atrophy of several gray matter regions including the hippocampus [Korf et al., 2007]. That study also focused only on hippocampal volume, not regional gray matter volume. Therefore, it has not yet been clarified whether there is a significant correlation between hsCRP and regional gray matter volume in healthy subjects using a whole‐brain analysis.
The purpose of this study was to investigate the correlation between hsCRP level and regional gray matter volume using brain magnetic resonance imaging (MRI) of 109 community‐dwelling healthy elderly subjects within a narrow age window. We applied the brain‐image analysis technique of voxel‐based morphometry (VBM) [Ashburner and Friston, 2000]. VBM analysis enables a global analysis of brain structures without a priori identification of a region of interest. This approach permits the identification of unsuspected potential brain structural abnormalities. We hypothesized that the gray matter volume of regions fed by the middle cerebral artery would be negatively correlated with hsCRP level because symptomatic atherosclerotic disease is mostly observed in the middle cerebral artery [Kim et al., 2005]; therefore, it was thought that the middle cerebral artery would be more vulnerable to atherosclerosis compared with other intracranial arteries such as the anterior cerebral artery.
METHODS
Study Population
The subjects were selected from participants in the Tsurugaya Project, a comprehensive geriatric assessment (CGA) of the elderly population, which includes assessments of medical status, depressive symptoms, and physical and cognitive functions. Recruitment of subjects was described previously [Taki et al., 2011]. Briefly, the project enrolled 2,730 subjects aged 69 years or older living in Tsurugaya district, Sendai, Japan. The subjects responded to interviews by psychologists and geriatrists based on questionnaires, including the Rome II Modular Questionnaire 8, Geriatric Depression Scale (GDS; http://www.stanford.edu/~yesavage/GDS.html) [Brink et al., 1982; Yesavage et al., 1982], and the Mini‐Mental State Examination (MMSE) [Folstein et al., 1975]as part of the CGA. Next, we asked the subjects whether they were willing to undergo an MRI of their brains. We defined healthy subjects as those who showed GDS scores <10 and MMSE scores of 28 or higher. Additionally, we excluded subjects with a history of brain tumors, cerebrovascular diseases, head trauma, or any neuropsychiatric disease. In total, 79 men and 96 women fulfilled the criteria of “healthy” and gave their consent to undergo brain MRI. From these subjects, we selected healthy male and female subjects separately by random sampling and obtained their brain MRIs. We also obtained hsCRP levels from the blood in each subject. As a result, the study subjects consisted of 55 men and 54 women. The characteristics of the subjects were reported previously [Taki et al., 2011].
Written informed consent was obtained from each subject after a full explanation of the purpose and procedures of the study prior to brain MRI according to the declaration of Helsinki (1991). Approval for these experiments was obtained from the institutional review board of Tohoku University.
Measurement of Serum hsCRP
Blood was extracted from the antecubital vein after an overnight fast. All subjects underwent routine laboratory tests, including assays for serum total cholesterol, serum triglycerides, serum high‐density lipoprotein cholesterol, and serum low‐density lipoprotein cholesterol. hsCRP concentrations were determined using an immunotechnique on a Behring BN II analyzer (Dade Behring, Tokyo, Japan). The BN II high sensitivity assay utilizes a monoclonal antibody coated on polystyrene particles and fixed‐time kinetic nephelometric measurements [Ledue et al., 1998]. The detection limit of this assay is 0.02 mg/L. Clinical characteristics of the subjects are summarized in Table 1.
Table 1.
Subject clinical characteristics
| Men | Women | ||
|---|---|---|---|
| (Range, mean ± SD) | (Range, mean ± SD) | P | |
| Age (years old) | 70–75, 72.4 ± 1.3 | 69–75, 72.0 ± 1.9 | 0.187 |
| MMSEa (max. 30) | 28–30, 29.2 ± 0.8 | 28–30, 29.0 ± 0.9 | 0.321 |
| GDSb (max. 30) | 0–9, 5.2 ± 2.4 | 1–9, 5.4 ± 2.5 | 0.632 |
| Duration of education (year) | 8–25, 14.4 ± 3.7 | 8–18, 11.6 ± 2.3 | <0.001 |
| Systolic blood pressure (mm Hg) | 81–167, 132.6 ± 16.7 | 105–203, 137.8 ± 20.3 | 0.163 |
| Diastolic blood pressure (mm Hg) | 50–104, 77.1 ± 10.8 | 63–104, 78.4 ± 10.2 | 0.544 |
| hsCRPc (mg/dL) | 0.05–21.7, 1.46 ± 3.1 | 0.09–34.3, 2.00 ± 4.7 | 0.491 |
| Total cholesterol (mg/dL) | 113–259, 191.8 ± 32.1 | 146–311, 191.8 ± 32.1 | <0.001 |
| Triglyceride (mg/dL) | 62–495, 179.8 ± 100.0 | 58–272, 148.6 ± 59.8 | 0.050 |
| HDL‐Cd (mg/dL) | 32–104, 50.1 ± 13.7 | 29–113, 59.6 ± 14.8 | <0.001 |
Mini‐Mental State Examination.
Geriatric Depression Scale.
High‐sensitivity C‐reactive protein.
High‐density lipoprotein cholesterol.
Image Acquisition
Brain MRI was acquired from each subject using two 0.5‐T MRI scanners of the same model (Signa Contour, GE‐Yokogawa Medical Systems, Tokyo, Japan). The scanner was routinely calibrated using the same standard GE phantom. No major hardware upgrades occurred during the period. All subjects were scanned with identical pulse sequences: 124 contiguous, 1.5‐mm‐thick axial planes of three‐dimensional T1‐weighted images (spoiled gradient recalled acquisition in steady state: repetition time, 40 ms; echo time, 7 ms; flip angle, 30; voxel size, 1.02 × 1.02 × 1.5 mm).
Image Analysis
We applied VBM to conduct the image analyses. Specifically, we used the Statistical Parametric Mapping 8 software (SPM8, Wellcome Department of Cognitive Neurology, London, United Kingdom) for the structural segmentation, longitudinal registration, and group statistics, and the “Diffeomorphic Anatomical Registration using Exponentiated Lie algebra (DARTEL)” [Ashburner, 2007] deformation framework for intersubject spatial normalization. As DARTEL produces more accurate registration [Klein et al., 2009], it allows improved sensitivity such as that needed to assess the correlation between regional gray matter volume and hsCRP. Practically, all of the T1‐weighted images were first segmented using the New Segmentation algorithm in SPM8 [Ashburner and Friston, 2005] and their resulting gray matter maps were rigidly registered onto their common mean image. Then, the DARTEL toolbox was used to estimate a best set of smooth, pure nonlinear and reversible deformation sets from each subject's tissue map to a common, custom template. To achieve this goal, DARTEL uses an iterative process: by computing constrained warping fields from all subject data to the current average at each step, successive and increasingly sharp average templates are generated, as well as corresponding warping fields for each subject. Finally, our resulting custom template was itself matched to the Montreal Neurological Institute (MNI) space using an affine‐only registration to map our custom coordinate space to the more standard MNI space [Bergouignan et al., 2009]. We applied modulation, which compensated for the volume change induced by warping [Good et al., 2001], to preserve the amount of gray matter in the process. Finally, the warped gray matter images were smoothened by convolving an 8‐mm full‐width at half‐maximum isotropic Gaussian kernel.
Statistical Analysis
A multiple regression analysis was performed to investigate the correlation between hsCRP level and regional gray matter volume after adjusting for age, gender, and intracranial volume. The intracranial volume was calculated by summing the gray matter volume, white matter volume, and CSF space volume derived in the abovementioned image preprocess step. Intracranial volume adjustment was performed using intracranial volume as an independent variable as described below. As the distribution of hsCRP levels was highly deviated from a normal distribution, the hsCRP data were log transformed to achieve a normal distribution. Age, gender, intracranial volume, and log‐transformed hsCRP levels were used as independent variables, and regional gray matter volume was used as the dependent variable. We set the significance level at P <0.05 by controlling false‐discovery rate when performing multiple tests of topological features (i.e., clusters of voxels) and with random field theory when performing multiple tests [Chumbley et al., 2010; Morrell et al., 2010].
RESULTS
The distribution of log‐transformed hsCRP levels is shown in Figure 1. A significant negative correlation was observed between regional gray matter volume of the posterior and lateral aspects of the left temporal cortex and hsCRP levels after adjusting for age, gender, and intracranial volume (x, y, z = −60, −33, −2; t = 4.46, r = ‐0.345, P = 0.014; Fig. 2). This result indicates that individuals with mild inflammation, which is related with arteriosclerosis, show a decrease in regional gray matter volume in the posterior and lateral aspects of the left temporal cortex. If the statistical threshold was set to a more liberal condition (P < 0.001, uncorrected), a correlation between regional gray matter volume and hsCRP level was observed in the bilateral posterior and lateral aspect of the temporal cortex and temporo–parieto–occipital regions after adjusting for age, gender, and intracranial volume. In no region did gray matter show a significant positive correlation with hsCRP level.
Figure 1.
The distribution of log‐transformed hsCRP P‐levels.
Figure 2.
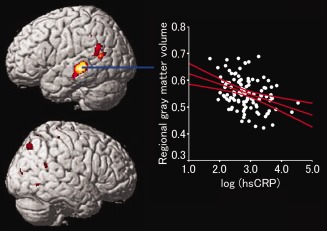
Gray matter regions that had a significant negative correlation with the log‐transformed hsCRP P‐levels and regional gray matter volume after adjusting for age, gender, and intracranial volume. Upper: Correlation results were superimposed onto right and left lateral‐view structural magnetic resonance images. Lower: Relationship between high‐sensitivity C‐reactive protein P‐levels and regional gray matter volume (Talairach coordinates, x = −60, y = −33, z = −2). Color scales indicate the t‐scores. We show the multiple comparisons data (P < 0.001, uncorrected) to clarify the extent of the regions. The 95% confidence interval of the regression line is also shown.
DISCUSSION
This is the first study to show a significant negative correlation between regional gray matter volume and hsCRP level in healthy elderly subjects. A significant negative correlation was observed in the posterior and lateral aspects of the left temporal cortex. Although the mechanism for the correlation between regional gray matter volume and hsCRP is not well clarified, it is thought that atherosclerosis is associated with gray matter volume loss because hsCRP is a sensitive marker of systemic low‐grade inflammation such as that seen in atherosclerosis [Pearson et al., 2003]. Atherosclerosis may be related to a variety of vascular pathologies, including carotid artery wall thickening [De Michele et al., 2002], vascular and coronary endothelial dysfunction [Brook et al., 2001; Sorisky, 2002; Williams et al., 2002], peripheral resistance, and arterial stiffness [Yki‐Jarvinen and Westerbacka, 2000]. Therefore, it was thought that atherosclerosis may predispose a patient to a progressive decrease in cerebral blood flow [Nobili et al., 1993; Rodriguez et al., 1987] and alter the supply of oxygen and other nutrients to neurons, leading to chronic ischemia and tissue loss. Additionally, the lower limit of cerebral blood flow autoregulation is shifted to the right on the blood pressure axis to maintain a constant cerebral blood flow in the hypertensive state [Strandgaard, 1976]. This state is vulnerable to minor hypotensive episodes and easily leads to ischemia. From these phenomena, atherosclerosis may result in neuronal loss and gray matter volume decline.
We showed that the volume of the posterior and lateral aspects of the left temporal cortex was significantly and negatively correlated with hsCRP level. As hypothesized, this region is fed by the middle cerebral artery [Martin and Neuroanatomy, 2003]. As symptomatic atherosclerotic disease is mostly observed in the middle cerebral artery [Kim et al., 2005], it is thought that the middle cerebral artery is more vulnerable to atherosclerosis compared with other intracranial arteries such as the anterior cerebral artery. Additonally, the left lateral temporal lobe is significantly and negatively correlated with the rate of brain perfusion and age [Van Laere et al., 2001]; therefore, it may be particularly vulnerable to aging by the atherosclerotic interaction in the left lateral temporal lobe. Furthermore, we showed that the gray matter regions of the rather posterior aspect of the brain showed a tendency toward correlation with regional gray matter volume and hsCRP level in the liberal threshold analysis. Posterior circulation fed by the bilateral vertebral arteries shows vulnerability to atherosclerosis [Baker and Iannone, 1959; Strassburger et al., 1997]. Thus, it is thought that the gray matter regions of the posterior aspect of the brain had a tendency toward correlation with regoinal gray matter volume and hsCRP level.
Although a recent study suggested a significant correlation between hsCRP level and regional gray matter volume of the hippocampus [Anan et al., 2011], we did not find this correlation. This inconsistency may be derived from differences in subject characteristics, as that study focused not on healthy subjects but on patients with type 2 diabetes mellitus, who show atrophy in several gray matter regions such as the hippocampus [Korf et al., 2007]. Therefore, it is possible that diabetes mellitus itself may have affected the results of the correlation between hippocampal volume and hsCRP level. Additionally, although we applied VBM, which enables a global analysis of brain structures without a priori identification of a region of interest, Anan and colleagues focused only on the hippocampus. Thus, the inconsistency in the results was thought to be derived from both the subject characteristics and the methodology applied. In addition, although another study showed that higher hsCPR levels were related to reduced fractional anisotropy of the frontal lobe [Wersching et al., 2010], we did not find a significant correlation between regional gray matter volume in the frontal lobe and hsCRP level. Although there is also a negative correlation between gray matter volume in several regions in the dorsal frontal lobe and hsCRP level if a more liberal threshold such as P < 0.01, uncorrected for multiple comparisons is used (data not shown), we cannot deny the possibility that mechanism(s) of the correlation between hsCRP level and gray matter, and white matter structure, may be different. Further studies may help elucidate correlations between brain structure and hsCRP levels.
The present study had several limitations. First, it was a cross‐sectional study. Thus, although we have shown a relationship between hsCRP level and gray matter volume, we cannot clarify a causal relationship between hsCRP level and gray matter volume. Longitudinal studies are needed to clarify this issue. Second, we cannot exclude the possibility that our sample may have included subjects with mild cognitive deficits or even early‐stage dementia because we used only the MMSE and GDS to screen for what our study defined as “healthy” subjects. To reduce this possibility, we set strict inclusion criteria for healthy elderly subjects by requiring that the MMSE score be 28 or higher. Third, we used two 0.5‐T MRI scanners, and although we adjusted the pulse sequences to collect optimized images, the sensitivity of the results may be lower than could be obtained using a 1.5‐T MRI scanner, which is more commonly used. Fourth, we did not collect other measures of atherosclerosis, such as plaque thickening or calcification of the internal carotid or middle cerebral artery. Therefore, further studies may be needed to show the correlation between the extent of atherosclerosis and gray matter volume decline.
In summary, we analyzed the correlation between hsCRP level and brain regional gray matter volume in 109 community‐dwelling healthy elderly subjects. We found a significant negative correlation between regional gray matter volume of the posterior and lateral aspects of the left temporal cortex and hsCRP level after adjusting for age, gender, and intracranial volume. Our results suggest that individuals with mild inflammation, which is related with arteriosclerosis, show a decrease in regional gray matter volume in the posterior and lateral aspects of the left temporal cortex. Therefore, preventing the progress of arteriosclerosis may help to prevent decreases of gray matter volume in healthy elderly subjects.
ACKNOWLEDGMENTS
The authors thank Prof. Hiroyuki Arai for organizing the Tsurugaya Project.
REFERENCES
- Anan F, Masaki T, Shimomura T, Fujiki M, Umeno Y, Eshima N, Saikawa T, Yoshimatsu H (2011): High‐sensitivity C‐reactive protein is associated with hippocampus volume in nondementia patients with type 2 diabetes mellitus. Metab Clin Exp 60:460–466. [DOI] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2000): Voxel‐based morphometry—The methods. Neuroimage 11:805–821. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Baker AB, Iannone A (1959): Cerebrovascular disease. I. The large arteries of the circle of Willis. Neurology 9:321–332. [DOI] [PubMed] [Google Scholar]
- Bergouignan L, Chupin M, Czechowska Y, Kinkingnehun S, Lemogne C, Le Bastard G, Lepage M, Garnero L, Colliot O, Fossati P (2009): Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression? Neuroimage 45:29–37. [DOI] [PubMed] [Google Scholar]
- Brink T, Yesavage J, Lum B, Heersma P, Adey M, Rose TL (1982): Screening tests for geriatric depression. Clin Gerontol 1:37–43. [DOI] [PubMed] [Google Scholar]
- Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S (2001): Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol 88:1264–1269. [DOI] [PubMed] [Google Scholar]
- Chumbley J, Worsley K, Flandin G, Friston K (2010): Topological FDR for neuroimaging. Neuroimage 49:3057–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Michele M, Panico S, Iannuzzi A, Celentano E, Ciardullo AV, Galasso R, Sacchetti L, Zarrilli F, Bond MG, Rubba P (2002): Association of obesity and central fat distribution with carotid artery wall thickening in middle‐aged women. Stroke 33:2923–2928. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975): “Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ (2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14:21–36. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Kitagawa K, Hougaku H, Shimizu Y, Sakaguchi M, Nagai Y, Iyama S, Yamanishi H, Matsumoto M, Hori M (2001): C‐reactive protein is an independent predictor of the rate of increase in early carotid atherosclerosis. Circulation 104:63–67. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kang DW, Kwon SU (2005): Intracranial atherosclerosis: incidence, diagnosis and treatment. J Clin Neurol 1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV (2009): Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 46:786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf ES, van Straaten EC, de Leeuw FE, van der Flier WM, Barkhof F, Pantoni L, Basile AM, Inzitari D, Erkinjuntti T, Wahlund LO, Rostrup E, Schmidt R, Fazekas F, Scheltens P, LADIS Study G (2007): Diabetes mellitus, hypertension and medial temporal lobe atrophy: The LADIS study. Diabet Med 24:166–171. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Weiner MW, Chui HC (2007): Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology 21:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledue TB, Weiner DL, Sipe JD, Poulin SE, Collins MF, Rifai N (1998): Analytical evaluation of particle‐enhanced immunonephelometric assays for C‐reactive protein, serum amyloid A and mannose‐binding protein in human serum. Ann Clin Biochem 35:745–753. [DOI] [PubMed] [Google Scholar]
- Martin J. Neuroanatomy (2003):Text and Atlas, 3rd edNew York:McGraw‐Hill Professional. [Google Scholar]
- Morrell MJ, Jackson ML, Twigg GL, Ghiassi R, McRobbie DW, Quest RA, Pardoe H, Pell GS, Abbott DF, Rochford PD, Jackson GD, Pierce RJ, O'Donoghue FJ, Corfield DR (2010): Changes in brain morphology in patients with obstructive sleep apnoea. Thorax 65:908–914. [DOI] [PubMed] [Google Scholar]
- Nobili F, Rodriguez G, Marenco S, De Carli F, Gambaro M, Castello C, Pontremoli R, Rosadini G (1993): Regional cerebral blood flow in chronic hypertension. A correlative study. Stroke 24:1148–1153. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon3rdRO , Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith JrSC , Taubert K, Tracy RP, Vinicor F, Centers for Disease Control and, P, American Heart, A (2003): Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107:499–511. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Chiappelli M, Montesi F, Tumini E, Mariani E, Licastro F, Patterson C (2007): Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiol Aging 28:1810–1820. [DOI] [PubMed] [Google Scholar]
- Rodriguez G, Arvigo F, Marenco S, Nobili F, Romano P, Sandini G, Rosadini G (1987): Regional cerebral blood flow in essential hypertension: Data evaluation by a mapping system. Stroke 18:13–20. [DOI] [PubMed] [Google Scholar]
- Ross R (1999): Atherosclerosis—An inflammatory disease. N Engl J Med 340:115–126. [DOI] [PubMed] [Google Scholar]
- Sorisky A (2002): Molecular links between obesity and cardiovascular disease. Am J Ther 9:516–521. [DOI] [PubMed] [Google Scholar]
- Strandgaard S (1976): Autoregulation of cerebral blood flow in hypertensive patients. The modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug‐induced hypotension. Circulation 53:720–727. [DOI] [PubMed] [Google Scholar]
- Strassburger TL, Lee HC, Daly EM, Szczepanik J, Krasuski JS, Mentis MJ, Salerno JA, DeCarli C, Schapiro MB, Alexander GE (1997): Interactive effects of age and hypertension on volumes of brain structures. Stroke 28:1410–1417. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Kawashima R (2010): Effects of working memory training on cognitive functions and neural systems. Rev Neurosci 21:427–449. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Goto R, Wu K, Kawashima R, Fukuda H (2011): Correlation between gray/white matter volume and cognition in healthy elderly people. Brain Cogn 75:170–176. [DOI] [PubMed] [Google Scholar]
- Teunissen CE, van Boxtel MP, Bosma H, Bosmans E, Delanghe J, De Bruijn C, Wauters A, Maes M, Jolles J, Steinbusch HW, de Vente J (2003): Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol 134:142–150. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Versijpt J, Audenaert K, Koole M, Goethals I, Achten E, Dierckx R (2001): 99mTc‐ECD brain perfusion SPET: variability, asymmetry and effects of age and gender in healthy adults. Eur J Nucl Med 28:873–881. [DOI] [PubMed] [Google Scholar]
- Wersching H, Duning T, Lohmann H, Mohammadi S, Stehling C, Fobker M, Conty M, Minnerup J, Ringelstein EB, Berger K, Deppe M, Knecht S (2010): Serum C‐reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 74:1022–1029. [DOI] [PubMed] [Google Scholar]
- Williams IL, Wheatcroft SB, Shah AM, Kearney MT (2002): Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes Relat Metab Disord 26:754–764. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T (2003): Inflammatory markers and cognition in well‐functioning African‐American and white elders. Neurology 61:76–80. [DOI] [PubMed] [Google Scholar]
- Yamagami H, Kitagawa K, Nagai Y, Hougaku H, Sakaguchi M, Kuwabara K, Kondo K, Masuyama T, Matsumoto M, Hori M (2004): Higher levels of interleukin‐6 are associated with lower echogenicity of carotid artery plaques. Stroke 35:677–681. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982): Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17:37–49. [DOI] [PubMed] [Google Scholar]
- Yki‐Jarvinen H, Westerbacka J (2000): Vascular actions of insulin in obesity. Int J Obes Relat Metab Disord 24:S25–S28. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, Cohen RA, Aloia MS, Williams LM, Clark CR, Whitford TJ, Gordon E (2006): The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am J Geriatr Psychiatry 14:823–833. [DOI] [PubMed] [Google Scholar]


