Abstract
Simple writer's cramp (WC) is a task‐specific form of dystonia, characterized by abnormal movements and postures of the hand during writing. It is extremely task‐specific, since dystonic symptoms can occur when a patient uses a pencil for writing, but not when it is used for sharpening. Maladaptive plasticity, loss of inhibition, and abnormal sensory processing are important pathophysiological elements of WC. However, it remains unclear how those elements can account for its task‐specificity. We used fMRI to isolate cerebral alterations associated with the task‐specificity of simple WC. Subjects (13 simple WC patients, 20 matched controls) imagined grasping a pencil to either write with it or sharpen it. On each trial, we manipulated the pencil's position and the number of imagined movements, while monitoring variations in motor output with electromyography. We show that simple WC is characterized by abnormally increased activity in the dorsal premotor cortex (PMd) when imagined actions are specifically related to writing. This cerebral effect was independent from the known deficits in dystonia in generating focal motor output and in processing somatosensory feedback. This abnormal activity of the PMd suggests that the task‐specific element of simple WC is primarily due to alterations at the planning level, in the computations that transform a desired action outcome into the motor commands leading to that action. These findings open the way for testing the therapeutic value of interventions that take into account the computational substrate of task‐specificity in simple WC, e.g. modulations of PMd activity during the planning phase of writing. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: task‐specificity, dystonia, dorsal premotor cortex, action planning, functional MRI
INTRODUCTION
Writer's cramp (WC) is a form of primary focal hand dystonia [Sheehy and Marsden,1982], a movement disorder thought to develop in individuals with a genetic susceptibility following repeated performance of skilled movements [Byl et al.,1996; Hallett,2006]. Patients with focal hand dystonia have clear motor planning alterations, as shown by the loss of surround inhibition, leading to unnecessary contractions of more muscles than required in a certain motor task [Berardelli et al.,1998; Hallett,2004; Hallett,2006]. Other evidence suggests that somatosensory processing and sensorimotor integration are altered in these patients [Braun et al.,2003; Byl et al.,1996; Candia et al.,2003; Rosenkranz et al.,2008; Tempel and Perlmutter,1993], possibly as a consequence of underlying maladaptive synaptic plasticity [Byl et al.,1996; Quartarone et al.,2003, 2005]. These three mechanisms are fundamental contributors to the pathophysiology of dystonia. Yet, in some forms of dystonia like simple WC, these neurobiological alterations appear to occur within a scarcely considered psychological context, e.g., only for actions having the goal of writing. For instance, a patient could develop dystonic symptoms when writing, but not when sharpening a pencil [Sheehy and Marsden CD,1982]. In some cases, dystonia occurs even when just approaching a pencil for writing. Task‐specificity is thus an intrinsic element of simple WC, such that the alterations in motor excitability and somatosensory processing generally observed in dystonia emerge only when triggered by a motor plan selected to achieve the goal of writing [Braun et al.,2003; Butz et al.,2006; Ceballos‐Baumann et al.,1995; Hallett,2000; Hamano et al.,1999; Lerner et al.,2004; Odergren et al.,1996;1998; Pujol et al.,2000; Quartarone et al.,2005; Tempel and Perlmutter JS,1993]. Here we used fMRI to study cerebral alterations evoked by selecting a motor plan for writing in WC patients. The rationale is to examine the stage where goal‐related alterations influence the sensorimotor machinery, rather than the consequences of those alterations. Accordingly, we focus on action selection, i.e. a stage where desired action outcome is used to specify a series of motor control parameters [Shadmehr and Krakauer,2008; Wolpert and Ghahramani,2000], rather than action execution. Previous studies on dystonia have not directly touched on this issue, as these were mainly focused on the cerebral effects of the production of dystonic movements [Braun et al.,2003; Butz et al.,2006; Ibanez et al.,1999; Lerner et al.,2004; Odergren et al.,1998; Pujol et al.,2000]. However, cerebral activity related to overt motor responses could reflect altered somatosensory activity due to the dystonic movements; altered premotor activity due to alternative movement strategies; primary abnormalities in the motor plans supporting the writing movements; or a combination of these factors.
Motor imagery offers a way to distinguish these factors from alterations related to the specification of the motor parameters leading to a desired action outcome. Although motor imagery is likely to engage only parts of the cerebral circuits controlling our actions, it has proven to be an effective and relevant tool for studying alterations of the motor system [de Lange et al.,2004; Fiorio et al.,2006; Helmich et al.,2009; Snijders et al.,2011]) being sensitive to motor control variables like movement speed, force, and current state of the body [de Lange et al.,2006; Gentili et al.,2004; Nico et al.,2004]. Building on the observation that motor imagery relies on neural processes similar to those evoked during performance and planning of the same movements [Cisek and Kalaska,2004; Jeannerod,1995; la Fougere et al.,2010; Miller and Wingfield,2010; Stephan et al.,1995], we combine this tool with fMRI to isolate cerebral responses driven by tool‐effector interactions functionally associated with simple WC. We asked patients with simple WC and age‐matched healthy controls to imagine grasping a pencil for either writing with it, or sharpening it. We verified whether imagery performance was sensitive to motor parameters by manipulating pencil position and number of imagined movements, while we monitored variations in motor output with electromyography.
MATERIALS AND METHODS
Subjects
Thirteen patients with simple writer's cramp (5 men, 8 women) with a mean age of 53 years (range, 36–63) and 20 healthy controls (10 men, 10 women) with a mean age of 55 years (range, 32–69) were included after giving written informed consent according to the Declaration of Helsinki. Age and gender were matched (i.e., not significantly different) between the two groups (age P = 0.41; gender P = 0.57). The study was approved by the local ethics committee. All participants were consistent right‐handers (mean latency quotient 96%) [Oldfield,1971]. They all had a full range of motion of the right arm and shoulder. All patients were affected at the right hand. During clinical testing, all patients developed dystonic symptoms during writing, but not during other movements (such as sharpening). Four patients receiving Botulinum injections were investigated three months after the last injection. No other neurotropic medication was used by the participants. The primary complaint of the patients was cramping (11) or loss of control (8) during writing. Altered flexion of the thumb or index finger were the most common disturbances during writing. Two patients also had some writing tremor. In addition to a neurological examination, the Writer's cramp rating scale (WCRS) and the Burke‐Fahn‐Marsden rating scale (BFM) were used to rate the severity of the dystonia [Burke et al.,1985; Wissel et al.,1996] (Table I).
Table I.
Patient details
| Patient No. | Sex | Age (years) | Age at disease onset | BFM | WCRS | Rx |
|---|---|---|---|---|---|---|
| 1 | F | 58 | 42 | 2 | 1 | BTX |
| 2 | F | 45 | 25 | 1 | 7 | none |
| 3 | F | 63 | 42 | 2 | 3 | none |
| 4 | M | 53 | 37 | 3 | 2 | none |
| 5 | F | 61 | 41 | 3 | 6 | none |
| 6 | F | 57 | 38 | 2 | 6 | none |
| 7 | M | 68 | 44 | 2 | 2 | none |
| 8 | F | 36 | 25 | 2 | 4 | none |
| 9 | M | 63 | 43 | 2 | 2 | BTX |
| 10 | F | 46 | 44 | 2 | 12 | BTX |
| 11 | M | 50 | 47 | 1 | 1 | none |
| 12 | F | 39 | 29 | 2 | 2 | none |
| 13 | M | 53 | 43 | 3 | 3 | BTX |
BTX, botulinum toxin; BFM, Burke Fahn Marden dystonia rating scale; F, female; M, male; No., number; Rx, treatment; WC, Writer's Cramp; WCRS, Writer's Cramp rating scale.
Experimental Design
The experimental design consisted of three factors: task (two levels), movement repetitions (four levels), and biomechanical difficulty (two levels), fully randomized within each of the two experimental groups (patients, controls). This yielded a total of 16 different stimuli, repeated 15 times, for a total of 240 trials during the whole fMRI experiment. In the following sections we describe these factors of the experimental design in more detail.
Task
Because we were interested in task‐specific activity during motor planning, we contrasted a writing task with a control task that involved pencil manipulation without writing movements (pencil sharpening). During the writing task, subjects were presented with pictures showing a pencil and a blank sheet of paper. They were asked to imagine themselves grasping the pencil and write the letter “e,” using small distal joints movements—similar to writing on a paper as opposed to writing on a blackboard. During the sharpening task, subjects were presented with pictures showing a pencil and a sharpener. They were asked to imagine themselves grasping the pencil with the right hand and sharpen it by rotating it inside the sharpener (Fig. 1). The prediction is that there should be differences in cerebral activity between patients and controls in the writing task, but not in the sharpening task (Group × Task interaction).
Figure 1.
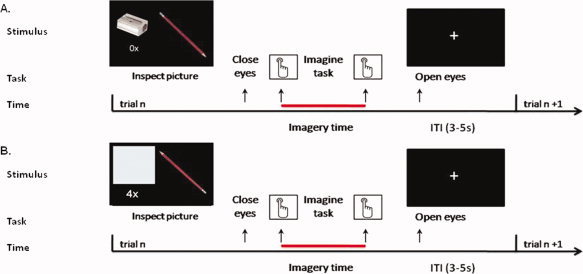
Time course of motor imagery trials. During each trial, after inspection of the picture on display, the subjects closed their eyes and pressed a button with the index finger of their left hand to signal they had started imagining to grasp the pencil for writing or sharpening it. During the sharpening task, a pencil and a handheld sharpener were shown (A). During the writing task, a pencil and a blank piece of paper were shown (B). The pencil was shown in two orientations that afforded either a biomechanically difficult (A) or easy (B) movement. The subjects pressed the button again when they finished imagining writing or sharpening the pencil according to the number of movement repetitions (0×, 2×, 4×, 6×) stated in the visual instructions. Following the second button press, a fixation cross was presented on the screen and the subjects could open their eyes. After a variable intertrial interval (ITI, intertrial interval, 3–5 s) a new picture was shown. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Movement Repetitions
To monitor the subjects' ability to perform motor imagery, we asked them to perform the task (imagine either to write or to sharpen the pencil) for a given number of times (repetitions) on each trial. More precisely, subjects were asked to imagine writing zero, two, four, or six times the letter “e” on the piece of paper. Similarly, they were asked to imagine rotating the pencil zero, two, four, or six times inside the sharpener. For the zero repetition the subjects were asked to imagine grasping and moving the pencil to the piece of paper or sharpener without subsequent writing or sharpening. The prediction is that there should be clear behavioural differences in imagery times as a function of the number of repetitions.
Biomechanical Difficulty
In principle, finding a relationship between duration of the imagined movements and number of movement repetitions could be attributable to knowledge about how long it would take to actually execute that movement [Pylyshyn,2002]. Therefore, we introduced a second manipulation to test whether subjects' motor imagery performance was influenced by biomechanical constraints dependent on the current posture of their body, a strong test for the presence of first‐person kinaesthetic imagery independent from propositional representations [de Lange et al.,2005, 2006]. Stimuli were shown in two orientations, chosen on the basis of a behavioral pilot study involving actual movements. Biomechanically easy stimuli consisted of pictures with the pencil tip pointing at a 135° angle from the horizontal plane. Biomechanically difficult stimuli consisted of pictures with the pencil tip pointing at a 315° angle from the horizontal plane (Fig. 1).
Experimental Procedures
Training session
Before scanning, subjects performed actual writing and sharpening movements in a set‐up consisting of the elements presented in the pictures, namely the pencil, an empty sheet of paper, and a sharpener, with the same spatial configuration as in the pictures. For instance, on each trial the pencil was positioned at either of the two orientations used in the imagery experiment, and subjects were asked to grasp the pencil and write on a piece of paper, first while lying outside the MRI scanner (duration: 10 min; see Supporting Information Fig. 1) and then while lying in a dummy‐scanner exactly reproducing the MR scanner setting (duration: 10 min). The piece of paper and the sharpener were held in place with appropriate support, allowing the subjects to perform the task with their right hand only. Before starting the motor imagery task, subjects were instructed to imagine the tasks as vividly as possible, in a first‐person perspective, as if their right arm and hand were actually moving, but without making any actual movements.
fMRI session—Set‐up
The two motor imagery tasks (writing, sharpening) were performed in two sessions of 20 minutes each (with the order of experimental factors pseudo‐randomized within each session), separated by a break outside the scanner. During the experiment, subjects were lying supine in the MR‐scanner. Head movements were minimized by an adjustable padded head holder. Visual stimuli (pictures) were projected onto a screen at the back of the scanner and were seen through a mirror above the subjects' heads. The stimuli subtended a visual angle of ∼ 10°. Stimuli presentation was controlled through a PC running Presentation software (Neurobehavioural systems, Albany, USA). Motor responses, i.e. left index finger flexions resulting in button presses, were recorded via an MR‐compatible keypad (Current Designs, Philadelphia, USA) positioned on the left side of the subject's abdomen.
fMRI session—Trial time course
Both tasks started with the presentation of a picture (Fig. 1). After a short inspection of the photograph on display, the subjects closed their eyes and pressed a button with their left index finger. The button‐press was used to mark the onset of the imagery episode. They were instructed not to imagine reaching for the pencil until knowing how to grasp the pencil. Subjects opened their eyes and pressed the button again after they imagined writing or sharpening. Following the second button press, a fixation cross was presented on the screen during a randomly jittered intertrial interval (ITI) between 3,000 ms and 5,000 ms (Fig. 1).
fMRI Data Collection and Preprocessing
MR images were acquired on a 3T Trio MRI system (Siemens, Erlangen, Germany), using a standard circular polarized head coil for radio‐frequency transmission and signal reception. Blood oxygenation level‐dependent (BOLD) sensitive functional images were acquired using a single shot gradient EPI sequence (TR/TE = 2,380 ms/30 ms; 50 ms gap between successive volumes; 35 transversal slices; ascending acquisition; voxel size 3.5 × 3.5 × 3.0 mm3; FOV = 224 mm2). High‐resolution anatomical images were acquired using an MP‐RAGE sequence (TE/TR 3.03/2,300 ms, 192 sagittal slices, voxel size 1.0 × 1.0 × 1.0 mm3, FOV 256 mm2).
Functional data were preprocessed and analyzed with SPM5 (Statistical Parametric Mapping, http://www.fil.ion.ucl.ac.uk/spm). First, the functional images were spatially realigned using a sinc interpolation algorithm that estimates rigid body transformations (translations, rotations) by minimizing head‐movements between each image and the reference image [Friston,1995]. Subsequently, the time‐series for each voxel was temporally realigned to the acquisition of the first slice. Images were normalized to a standard EPI template centered in MNI (Montreal Neurological Institute) space [Ashburner and Friston, 1997] by using linear transformations and resampled at an isotropic voxel size of 2 mm. The normalized images were smoothed with an isotropic 10 mm full‐width‐at‐half‐maximum Gaussian kernel. Anatomical images were spatially coregistered to the mean of the functional images [Ashburner and Friston, 1997] and spatially normalized by using the same transformation matrix applied to the functional images.
EMG Data Collection and Analysis
A concern in the comparison of patients and controls is that differences in actual movements (related to dystonia or overt hand movements during motor imagery of writing) might result in changes in cerebral activity. To control for these factors, muscle activity from the right hand was measured during the fMRI experiment in patients and controls using MR‐compatible surface EMG‐electrodes (BrainAmp, Brain Products, Munich, Germany) on the flexors and extensors of the right hand. A neutral electrode was placed on the head of the ulna. Following amplification and A/D conversion (Brain Products GmBH, Gilching, Germany), an optical cable fed the EMG signal to a dedicated PC outside the MR room for further off‐line analysis (sampling rate: 5000 Hz). Because of technical problems no muscle activity was measured in one patient and one control (one session).
MR artifact correction followed the method described by Allen and Van Duinen, including low‐pass filtering (400 Hz), and down‐sampling (1000 Hz). [van Duinen et al., 2005] Finally, we applied high‐pass filtering (10 Hz, to remove possible movement artifacts), and rectification. For each trial, we considered the root mean square (RMS) of the EMG signals measured during the imagery task and the intertrial interval, excluding the EMG signal measured during the button presses (500 ms). We tested the effects of GROUP (patients, controls) and TASK (writing, sharpening) using mixed‐effects repeated measures ANOVA.
Behavioural Analysis
We monitored task performance by testing whether the imagery times were modulated by the angle of the pencil (factor Biomechanical Difficulty) and by the number of times the writing/sharpening actions were repeated (factor Movement Repetitions). Imagery time was measured as the interval between the two button presses within a trial that marked the beginning and the end of the imagery period (Fig. 1). We analyzed the influence of the factors Group (patients, controls), Task (writing, sharpening), Biomechanical Difficulty (difficult, easy) and Movement Repetitions (0, 2, 4, 6 times) on imagery time by means of a 2 × 2 × 2 × 4 mixed‐effects repeated‐measures ANOVA. The alpha‐level was set a P < 0.05. The Greenhouse‐Geisser method was used to correct for nonsphericity.
fMRI Analysis (First Level)
The preprocessed fMRI time series were analyzed on a subject‐by‐subject basis using an event‐related approach in the context of the General Linear Model [Friston,1995]. For each subject, regressors of interest were defined to characterize the cerebral response evoked during the performance of the imagery task in each of the sixteen different conditions of the 2 × 2 × 4 design [i.e., TASK (writing, sharpening), Biomechanical Difficulty (difficult, easy) and Movement Repetitions (0, 2, 4, 6 times)]. These effects were modelled as square waves time‐locked to picture presentation, extending in time during each trial over the imagery time averaged across all imagery times of the subject. We also considered and modelled several confounds: 12 head‐motion regressors (describing translation and rotation in each of the three dimensions, and their temporal derivatives), as derived from the spatial realignment procedure in the statistical model; two regressors for signals arising from segmented white matter and cerebral spinal fluid [Verhagen et al.,2008] and one regressor describing the EMG‐activity measured during the experiment [Bakker et al.,2008; Helmich et al.,2010]. Data were high‐pass filtered (cutoff, 128 s) to remove low‐frequency confounds such as scanner drifts. The statistical significance of the estimated haemodynamic responses was assessed using Z‐statistics. We calculated the parameter estimates for the effects of Task, Biomechanical Difficulty, and Movement Repetitions for each subject, bringing 16 contrast images to the second level for each subject.
fMRI Analysis—Second Level
We report the results of a random effects analysis. For clarity, we indicate the number of movement repetitions or biomechanical difficulty considered in the contrast with a superscript, and the group with a subscript. We tested for the following effects.
First, we tested for a linear increase of cerebral activity as a function of movement repetitions (Movement Repetitions: writing0 < writing2 < writing4 < writing6 ∩ sharpening0 < sharpening2 < sharpening4 < sharpening6) and for increased cerebral activity during biomechanically difficult imagined movements (Biomechanical Difficulty: writingeasy < writingdifficult ∩ sharpeningeasy sharpeningdifficult), across both groups. These analyses were meant to isolate imagery‐related activity shared across the two groups (see Table I; Supporting Information).
Second, we considered the effects of TASK. This analysis was meant to isolate writing‐related effects, both in patients and controls. We tested for writing‐related activity shared across the two groups [(writing2,4,6)patients ∩ (writing2,4,6)controls], and for differential writing‐related activity shared across the two groups [(writing2,4,6 > sharpening2,4,6)patients ∩ (writing2,4,6 > sharpening2,4,6)controls], with two conjunction analyses [Nichols et al.,2005]. Also, we tested for generic effects of performing motor imagery tasks shared across the two groups [(writing0,2,4,6)patientsn (sharpening0,2,4,6)patients ∩ (writing0,2,4,6)controls ∩ (sharpening0,2,4,6)controls]. In order to limit the effects to those areas showing clear motor imagery effects, we masked this contrast with the effects of Movement Repetitions and Biomechanical Difficulty.
Third, we considered the Group × Task interaction, i.e., differential cerebral activity between patients and controls evoked during imagery of writing compared to sharpening [(writing2,4,6 >sharpening2,4,6)patients > (writing2,4,6 > sharpening2,4,6)controls]. In order to focus the interaction effects to those areas showing clear motor imagery effects, we masked the interaction contrast with the effects of Movement Repetitions and Biomechanical Difficulty.
Fourth, we tested whether there was a significant Group × Task interaction when limiting the analysis to those trials involving imagery of reaching‐grasping movements of the pencil, without any subsequent writing or sharpening [(writing0 > sharpening0)patients > (writing0 > sharpening0)controls]. To limit the analysis to those voxels showing clear motor imagery‐related effects, we limited the search to those voxels showing an effect of Biomechanical Difficulty.
Statistical Inference
Statistical inference on common task‐related and differential task‐related cerebral effects used a false‐discovery rate (FDR) corrected for multiple comparisons over the whole brain and over the selected search volume (P < 0.05), respectively [Genovese et al.,2002]. The search volume consisted of ROIs centered on cerebral areas previously shown to be involved in writing in healthy controls. Spherical volumes (radius: 6 mm) were centered at the reported coordinates of left dorsal premotor cortex, PMd [−28 −1 65] and left ventral premotor cortex, PMv [−53 11 29] [Longcamp et al.,2003]. In addition, as previous studies on dystonia emphasized the role of primary sensory cortex (S1) and basal ganglia, we performed an exploratory analysis on an additional set of ROIs in both left and right hemisphere: primary sensory cortex, caudate putamen, and pallidum (as defined with the WFU Pick Atlas, [Maldjian et al.,2003]. FWHM was 10.3 mm × 10.4 mm × 9.8 mm.
fMRI Analysis—Anatomical Inference
Anatomical details of significant signal changes were obtained by superimposing the SPMs on the structural images of the subjects. The atlas of [Duvernoy et al., 1991] was used to identify relevant anatomical landmarks. When applicable, the anatomical position of our significant clusters and local maxima was formally tested against published (three‐dimensional) probabilistic cytoarchitectonic maps (SPM Anatomy Toolbox, [Eickhoff et al.,2005; Mayka et al.,2006].
RESULTS
Patient Characteristics
Patient characteristics are shown in Table I. The median BFM score for patients was 2, range, 1–3. The median WCRS score was 3, range, 1–14. The scores for controls were 0.
Behavioral Results
Overall task performance was well matched between the two experimental groups. There was no effect of Group, Task, or Group × Task interaction on imagery times (main effect Group: F(1, 31) = 0.89 P = 0.35; main effect Task: F(1,31) = 0.07, P = 0.79; Task × Group interaction: F(1,31) = 3.14, P = 0.09). Both groups performed the imagery task by taking into account motor components: imagery times increased with increasing number of movements (main effect of Movement Repetitions: F(1.11, 34.34) = 247.97, P < 0.001), and as a function of biomechanical difficulty (main effect Biomechanical Difficulty: F(1, 31) = 12.56, P = 0.001) across both tasks. Importantly, these effects did not differ between groups (Movement Repetitions × Group interaction: F(1,31) = 0.35, P = 0.58; Biomechanical Difficulty × Group interaction: F(1,31) = 0.27, P = 0.61) or between tasks (Task × Movement Repetitions interaction: F(2.05,63.56) =1.73, P = 0.19; Task × Biomechanical Difficulty interaction (F(1, 31) = 1.01, P = 0.32) (Fig. 2).
Figure 2.
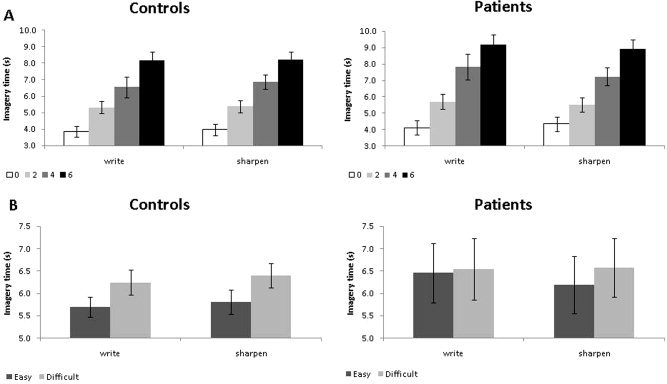
Behavioural performance during scanning. This figure illustrates the imagery times (IT) measured in trials involving (A) different movement repetitions (0, 2, 4, and 6) and (B) different biomechanical constraints (easy, difficult) separately for each task (write, sharpen) and group (controls, patients).
Muscular Activity
There were no differences in EMG activity measured during performance of the motor imagery tasks and during the intertrial intervals (main effect Epoch F(1, 31) = 1.88, P = 0.18). The two groups did not significantly differ in overall EMG activity (main effect Group F(1,30) = 3.00 P = 0.09). No group‐differences were found across tasks (Group × Task interaction (F(1, 30) = 1.20, P = 0.28; Group x Epoch x Task (F(1, 30) = 0.14, P = 0.71). Finally, there were no significant between‐groups differences in EMG‐related cerebral activity.
Common Task‐Related Cerebral Effects Across Groups
Motor imagery of writing evoked shared patterns of cerebral activity across the two groups in parts of the motor system. A conjunction analysis across patients and control groups [(writing2,4,6)patients ∩ (writing2,4,6)controls] revealed significant effects in the precentral gyrus (0 −2 60, Z = 8). This cluster fell in Brodmann Area 6 (BA 6) [60%, (Eickhoff et al.,2005)], and it was functionally defined as supplementary motor area [SMA; >95% (Mayka et al.,2006)]. There were also effects in the left superior frontal gyrus (–22–4 70, Z = 5.43). This cluster also fell in BA 6 [50%, (Eickhoff et al.,2005)], and it was functionally defined as dorsal premotor cortex [PMd; 50% (Mayka et al.,2006)]. This conjunction analysis revealed effects also in the left inferior frontal gyrus;–56 6 22, Z = 5.48—corresponding to BA 44 [40%, [Eickhoff et al.,2005] /ventral premotor cortex (PMv) >95%, [Mayka et al.,2006]]; in the right cerebellum [48–58–30, Z = 5.67; lobule 7a [100%, (Eickhoff et al.,2005)]; in the left cerebellum [–36–52–32, Z = 6.04; lobule 6 (80%, Eickhoff et al.,2005)]; anterior parietal cortex [–40–42 52, Z = 6.00—corresponding to BA 2 [40%, (Eickhoff et al.,2005)]; and in the superior parietal lobule [–12–70 62, Z = 6.34– corresponding to BA7a (60%, Eickhoff et al.,2005)]—see Table II, Supporting Information data.
Table II.
Stereotactic coordinates of the local maxima showing differential cerebral activity during motor imagery of writing across groups
| Contrast | Functional mask (inclusive) | Anatomical label | Functional label | Cluster size | Hemi‐ sphere | Z‐value | P‐value (corrected for search volume) | x | y | z |
|---|---|---|---|---|---|---|---|---|---|---|
| (writing2,4,6 > sharpening2,4,6)patients | Biomechanical difficulty and Movement repetitions | Superior frontal gyrus | PMd | 59 | L | 2.89 | 0.048 | −24 | −6 | 66 |
| > | ||||||||||
| (writing2,4,6 > sharpening2,4,6)controls | ||||||||||
| (writing0 > sharpening0)patients | Biomechanical difficulty | Superior frontal gyrus | PMd | 71 | L | 3.14 | 0.047 | −28 | 2 | 62 |
| > | Inferior frontal gyrus | PMv | 6 | L | 2.13 | 0.066 | −52 | 8 | 34 | |
| (writing0 > sharpening0)controls |
Brain areas with a significant Group × Task interaction, i.e., a larger increase in activity for motor imagery of writing versus motor imagery of sharpening in patients than in controls. The first row describes effects obtained when considering trials involving imagery of 2, 4, or 6 repetitions of either writing or sharpening movements (as indicated by the 2, 4, 6 superscript). The second row describe effects obtained when considering trials involving imagery of grasping movements only (as indicated by the 0 superscript), but occurring in the context of periods involving writing or sharpening movements. In order to isolate cerebral responses sensitive to motor processes, we searched for GROUP × TASK interactions occurring within those region showing increased activity for imagined movements of larger biomechanical difficulty and duration [effects of angle (difficult > easy) and movement repetitions (linear increase as a function of number of movement repetitions), see Methods]. Results are based on the ROI coordinates centered on cerebral areas previously shown to be involved in writing in healthy controls, using spherical volumes (radius: 6 mm) centered at these coordinates: left dorsal premotor cortex, PMd [−28 −1 65] and left ventral premotor cortex, PMv [−53 11 29] (Longcamp 2003) using small volume correction on the selected VOIs (FDR < 0.05). Stereotactic coordinates are reported in Montreal Neurological Institute (MNI) space. Details on the anatomical and functional labelling can be found in the Methods and Results sections. L, left; PMd, dorsal premotor cortex; PMv, ventral premotor cortex; R, right.
There were no suprathreshold effects shared across groups when considering differential responses between writing and sharpening [(writing2,4,6 > sharpening2,4,6)patients ∩ (writing2,4,6 > sharpening2,4,6)controls]. There were additional effects in the left putamen [–24 16 4; Z = 3.33; 100%, (Eickhoff et al.,2005)], and a trend in the left pallidum [–18 6 0; Z = 2.20; 100%; (Eickhoff et al.,2005)] when considering shared effects across groups and tasks [(writing0,2,4,6)patients n (sharpening0,2,4,6)patients ∩ (writing0,2,4,6)controls ∩ (sharpening0,2,4,6)controls]—see Table III, Supporting Information data).
Differential Task‐Related Cerebral Effects Between Groups (Task × Group Interaction)
Motor imagery of writing evoked differential patterns of cerebral activity (as compared to imagery of pencil sharpening) between the dystonic and the control groups in portions of the precentral gyrus contralateral to hand used during the imagery of writing [–24–6 66, Z = 2.89 P = 0.048, 60% probability in BA6 [Eickhoff et al.,2005]; 75% probability in PMd (Mayka et al.,2006)]. This effect was driven by a relative increase in activity during motor imagery of writing in the patients group (Table II, Fig. 3). This effect was significant even when analysis of the Task × Group interaction was limited to those trials requiring no writing or sharpening movements, but just a reaching‐grasping movement towards the pencil in the context of trials involving writing or sharpening (i.e., the zero‐repetition trials, see Experimental design). This effect was localized to the same portion of the left dorsal premotor cortex [–28 2 62, Z = 3.14, P = 0.047; 20% probability in BA 6 [Eickhoff et al.,2005]; 90% probability in PMd [Mayka et al.,2006]; Table II, Fig. 3].
Figure 3.
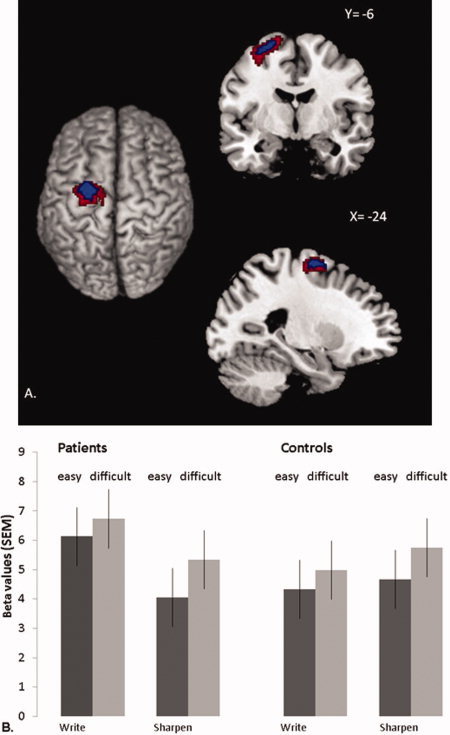
Differential cerebral effects of imagined writing in patients and controls. Panel A shows cerebral activity that increased during motor imagery of writing vs. motor imagery of sharpening, and more in patients than in controls. This contrast is shown at an uncorrected threshold of P < 0.05 and P < 0.01 (for graphical purposes), overlaid onto the structural scan of a representative subject from the MNI series. To restrict these effects to areas specifically involved in motor imagery, we included only voxels where activity increased linearly with repetition (2, 4, or 6 imagined movements) and where activity increased more for biomechanically difficult than easy trials (using inclusive masking at an uncorrected threshold of P < 0.05). The left side of the figure is the left side of the brain. Panel B shows cerebral responses over the left dorsal premotor cortex (PMd; −24 −6 +66]. This region showed a significant Group × Task interaction (P = 0.048, FDR‐corrected). For each group, the histograms show parameter estimates (in S.E.M. units) evoked by writing (the left two bars for each group) or sharpening (the right two bars for each group), separately for trials involving biomechanically easy movements (dark gray bars) or biomechanically difficult movements (light gray bars). Effects are averaged over the different repetitions (2, 4, or 6 imagined movements). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Further analysis of the Task × Group interaction (across the different repetition levels) revealed a statistical trend in the left PMv [–52 8 34, Z = 2.13; P = 0.066; 10% probability in BA6 [Eickhoff et al.,2005]; 90% probability in PMv (Mayka et al.,2006)]. There were no significant Task × Group interaction effects in the right PMd, PMv, S1, or basal ganglia, nor differential effects when comparing controls versus patients and sharpening versus writing.
DISCUSSION
We used motor imagery as a tool to investigate alterations in neural activity related to planning of writing in dystonic patients with simple writer's cramp (WC). These patients showed an increase of activity in the contralateral dorsal premotor (PMd) cortex when imagining grasping a pencil and writing with it using their dystonic hand, as compared with imagining grasping the same pencil with the same hand and sharpening it. Imagining grasping the pencil, without any subsequent movement, was sufficient to evoke altered responses in the same PMd region, provided that the imagined grasping movement occurred in the context of trials involving writing movements. This cerebral finding matches and qualifies the clinical observation that some WC patients show dystonic symptoms already while reaching for a pen to write.
The cerebral effect was not confounded by between‐groups differences in task‐related motor performance, muscular activity, or somatosensory processing. The effect was embedded within portions of the motor system supporting imagery of writing across both experimental groups (i.e., left frontal cortex, left superior parietal lobule, left postcentral gyrus, and the right cerebellum). These results show that simple WC is primarily characterized by altered action plans, over and above the known deficits of these patients in generating focal motor output and in processing somatosensory feedback [Braun et al.,2003; Butz et al.,2006; Ceballos‐Baumann et al.,1995; Hu et al.,2006; Lerner et al.,2004; Odergren et al.,1998; Pujol et al.,2000; Quartarone et al.,2003; Tempel and Perlmutter,1993]. The present findings provide a cerebral and computational substrate for the known task‐specificity of dystonic symptoms in simple WC. Namely, this disorder appears linked to alterations in the computations that transform a desired action outcome into a series of motor parameters leading to that action, i.e., writing.
ALTERED MOVEMENT REPRESENTATIONS IN WC PATIENTS
There was a relative increase in cerebral activity in the PMd of patients with simple WC during motor imagery of writing, as compared with sharpening. This effect was not present in healthy controls, and it was sensitive to motor constraints, as indicated by the significant modulations of activity induced by biomechanical difficulty and duration of the imagined movements. This result confirms and extends the findings of previous studies that showed (and used) altered electrophysiological responses in the PMd of patients with dystonia: alteration of the contingent negative variation (CNV) in WC [Hamano et al.,1999; Ikeda et al.,1996; Kaji et al.,1995]; alteration of the functional interhemispheric pathway (Koch et al., 2008) and the intrahemispheric pathway [Huang et al.,2010] between the PMd and motor cortex; and improvement of WC after 1Hz repetitive transcranial magnetic stimulation [Murase et al.,2005] and after theta burst stimulation [Huang et al.,2010] of the PMd.
These altered PMd responses could reflect the increased computational load imposed on this structure by the need to control altered sensorimotor representations in primary motor and somatosensory cortices in patients with dystonia [Beck et al.,2009; Braun et al.,2003; Candia et al.,2003]. However, this account would not explain why the altered PMd responses observed in this study is linked to a prediction of the specific functional outcome of the movement (i.e., writing), over and above its effector‐specific characteristics. Namely, we show that the same hand‐object interaction (grasping) leads to altered premotor responses when preceded by imagined writing actions, but not when preceded by imagined sharpening actions, irrespective of whether writing actions are actually part of the imagined movements. The present findings therefore indicate that the PMd alterations observed in dystonia are more likely related to the known role of this region in processing the outcome of a goal‐directed action according to learned stimulus‐response mappings [Hoshi and Tanji,2006; Majdandzic et al.,2009; Toni et al.,2001b]. More precisely, a desired end‐state involves a learned association between sensory cues and motor responses (i.e., the spatial relation between the pencil and the fingers when holding it for writing). The PMd appears to be preferentially and necessarily involved in deriving the motor parameters that lead to that end‐state [Hoshi and Tanji,2006; Passingham,1986; Toni et al.,2001b; Toni et al.,2001a; Toni et al.,2002]. The present findings suggest that patients with simple WC have a problem in dealing with this type of sensorimotor computations.
INTERPRETATIONAL ISSUES
It might be argued that the current findings either simply replicate or fail to replicate changes in PMd activity previously observed in patients with dystonia [Ceballos‐Baumann et al.,1995; Ibanez et al.,1999; Kadota et al.,2010; Odergren et al.,1998; Preibisch et al.,2001; Pujol et al.,2000]. In fact, here we report a novel writing‐specific planning impairment that was not addressed in previous reports [Ceballos‐Baumann et al.,1995; Ibanez et al.,1999; Lerner et al.,2004; Odergren et al.,1996; Preibisch et al.,2001; Pujol et al.,2000]. Both groups performed the tasks adequately, without overall differences in imagery times between groups or tasks, an indication that the two tasks evoked imagined actions of comparable length across subjects. Similarly, there were no between‐groups differences in EMG activity specifically related to writing (as compared to sharpening). These results exclude task difficulty, imagined cramping movements, or actual dystonic symptoms as an explanation for between‐group cerebral differences during motor imagery.
The imagery times of both groups were equally sensitive to the biomechanical constraint associated with the pencil positions and with the duration of the movements. This result indicates that patients and controls were equally capable of performing the imagery task, and that the task was solved by using motor processes. The cerebral location and lateralization of the writing‐related effects provide further independent support for the notion that subjects used their motor system when imagining writing with their right hand.
In contrast to the motor imagery study in WC patients by Fiorio and colleagues, our patients do not show longer imagery times in comparison to controls. There are, however, four clear differences between the current study and the report by Fiorio et al. First, the patients selected in the study by Fiorio et al. are more severely affected than those recruited in this study (mean BFM: Fiorio 6.0; Delnooz 3.0, P = 0.004; {BFM = Burke Fahn and Marsden rating scale}). Second, we only included patients with simple writer's cramp, whereas both simple writer's cramp patients and dystonic writer's cramp patients were included by Fiorio et al. Taken together, these clinical differences suggest that the patients studied by Fiorio et al. might have had a more widespread impairment of hand motor control, influencing more severely their performance during motor imagery. Third, the current study uses a different motor imagery task than Fiorio et al. (2006). Rather than focusing on motor imagery of a body part (as in Fiorio et al.), here we ask patients to imagine a goal‐oriented interaction between their hand and a tool. Fourth, the patients tested in the current study were instructed to engage in motor imagery, whereas the hand‐laterality judgement task used in Fiorio et al. implicitly relies on motor imagery. Taken together, these task‐related differences suggest that Fiorio et al. might have been particularly sensitive to alterations in multimodal hand representations used to solve the hand laterality judgment task, whereas the current task might be more appropriate to study the goal‐related specificity of simple WC.
In addition to the primary role of the dorsal premotor cortex in dystonia one might hypothesize that the changes described above are secondary to long‐term dystonia. As fMRI is a correlative measure, it does not allow causal links between activity patterns, behavioural responses and neurological symptoms. Therefore it is possible that the hyperactivity found in the PMd is due to a secondary plasticity change in the circuits involved in motor planning. Further longitudinal studies will need to address this possibility.
Finding altered PMd activity when WC patients imagined grasping a pencil might appear at odds with the dominant role of the ventral premotor cortex (PMv) in controlling reaching‐grasping movements [Davare et al.,2010; Fogassi et al.,2001]. In fact, it has been repeatedly shown that the PMv is crucial when hand movements are selected for grasping an object according to its visuospatial properties [Hoshi and Tanji,2006], rather than according to arbitrarily instructed action outcomes (as in this study; [Kurata and Hoffman,1994; Majdandzic et al.,2009; Toni et al.,2001a]. In other words, learned action‐outcome associations (processed in PMd) are necessary to uniquely specify which of the different grasping actions afforded by the visuospatial properties of a pencil (processed in PMv) should be selected. Accordingly, we found a statistically weaker effect in PMv when patients imagined grasping a pencil, in the context of trials involving writing (Table II). Furthermore, given the importance of the PMv region in the online control of grasping movements [Fogassi et al.,2001; Verhagen et al.,2008], and given that the present set‐up does not involve such control, it appears plausible that the PMv might contribute to simple WC during the actual performance of visually guided grasping and writing movements.
In contrast to previous studies that examined actual writing movements in WC and other types of dystonia, we found no significant between‐groups differences in the somatosensory cortex during imagery of writing relative to sharpening. However, there were robust imagery‐related somatosensory responses in both groups, in line with the notion that the generation of motor plans relies on an estimation of the current state of the system [Shadmehr et al.,2010], and that motor plans involve a prediction of the somatosensory consequences of those plans even in the absence of sensory feedback [Blankenburg et al.,2006; Christensen et al.,2007; Tanne‐Gariepy et al.,2002]. Accordingly, we suggest that somatosensory alterations are unlikely to embody the task‐specific features of simple WC. Rather, those somatosensory alterations could reflect more general abnormalities found in WC and other types of dystonia during actual motor performance.
Finally, we found no significant task‐specific differences in the basal ganglia between patients and controls. Given the important role of the basal ganglia in the storage and retrieval of learned sensorimotor associations [Nixon et al.,2004], and their involvement in dystonia [Berardelli et al.,1998; Hallett,2004], this result could be a false negative reflecting the selectivity of the differential effects assessed in this study. Alternatively, it is possible that the basal ganglia contributions to dystonia emerge only once altered goal‐specific motor plans are implemented and executed, such as in the loss of the surround inhibition that is (partly) driven by the PMd [Beck et al.,2009].
Follow‐up studies with a larger sample size are required to address this issue and to confirm whether the present findings are statistically robust, beyond the increased power but limited scope afforded by the current region of interest analysis. However, given the relative rarity of simple WC in the task‐specific form studied here, it might prove difficult to assemble a considerably larger sample of patients.
CONCLUSION
We have tested whether simple writer's cramp is characterized by altered task‐specific motor plans. We show that the dorsal premotor cortex of WC patients is disproportionally active when patients imagine grasping a pencil to write with it. We suggest that this dorsal premotor alteration reflects a computational impairment in deriving the motor parameters adequate to achieve an outcome arbitrarily linked to sensory cues, e.g., the learned spatial configuration that the fingers need to take around a pencil when writing. Given that maladaptive cortical plasticity within the motor system represents a fundamental trait of dystonia [Quartarone et al.,2003, 2005, 2008], it is conceivable that simple WC might emerge when synaptic plasticity in PMd remains up‐regulated despite the highly overtrained stage of the arbitrary sensorimotor associations supporting writing. In this perspective, the present findings suggest that down‐regulating synaptic plasticity in the dorsal premotor cortex during the planning phase of a writing movement, as can be realized with some transcranial magnetic stimulation procedures [Huang et al.,2010; Murase et al.,2005] may alleviate and perhaps treat writer's cramp.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
The authors thank J. Meulstee, MD, Canisius Wilhelmina Hospital Nijmegen, for his contribution to the recruitment of patients.
REFERENCES
- Bakker M, de Lange FP, Helmich RC, Scheeringa R, Bloem BR, Toni I ( 2008): Cerebral correlates of motor imagery of normal and precision gait. Neuroimage 41: 998–1010. [DOI] [PubMed] [Google Scholar]
- Beck S, Houdayer E, Richardson SP, Hallett M ( 2009): The role of inhibition from the left dorsal premotor cortex in right‐sided focal hand dystonia. Brain Stimul 2: 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD ( 1998): The pathophysiology of primary dystonia. Brain 121 (Pt 7): 1195–1212. [DOI] [PubMed] [Google Scholar]
- Blankenburg F, Ruff CC, Deichmann R, Rees G, Driver J ( 2006): The cutaneous rabbit illusion affects human primary sensory cortex somatotopically. PLoS Biol 4: e69–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C, Schweizer R, Heinz U, Wiech K, Birbaumer N, Topka H ( 2003): Task‐specific plasticity of somatosensory cortex in patients with writer's cramp. Neuroimage 20: 1329–1338. [DOI] [PubMed] [Google Scholar]
- Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J ( 1985): Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 35: 73–77. [DOI] [PubMed] [Google Scholar]
- Butz M, Timmermann L, Gross J, Pollok B, Dirks M, Hefter H, Schnitzler A ( 2006): Oscillatory coupling in writing and writer's cramp. J Physiol Paris 99: 14–20. [DOI] [PubMed] [Google Scholar]
- Byl NN, Merzenich MM, Jenkins WM ( 1996): A primate genesis model of focal dystonia and repetitive strain injury: I. Learning‐induced dedifferentiation of the representation of the hand in the primary somatosensory cortex in adult monkeys. Neurology 47: 508–520. [DOI] [PubMed] [Google Scholar]
- Candia V, Wienbruch C, Elbert T, Rockstroh B, Ray W ( 2003): Effective behavioral treatment of focal hand dystonia in musicians alters somatosensory cortical organization. Proc Natl Acad Sci USA 100: 7942–7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos‐Baumann AO, Passingham RE, Warner T, Playford ED, Marsden CD, Brooks DJ ( 1995): Overactive prefrontal and underactive motor cortical areas in idiopathic dystonia. Ann Neurol 37: 363–372. [DOI] [PubMed] [Google Scholar]
- Christensen MS, Lundbye‐Jensen J, Geertsen SS, Petersen TH, Paulson OB, Nielsen JB ( 2007): Premotor cortex modulates somatosensory cortex during voluntary movements without proprioceptive feedback. Nat Neurosci 10: 417–419. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF ( 2004): Neural correlates of mental rehearsal in dorsal premotor cortex. Nature 431: 993–996. [DOI] [PubMed] [Google Scholar]
- Davare M, Rothwell JC, Lemon RN ( 2010): Causal connectivity between the human anterior intraparietal area and premotor cortex during grasp. Curr Biol 20: 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange FP, Hagoort P, Toni I ( 2005): Neural topography and content of movement representations. J Cogn Neurosci 17: 97–112. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Helmich RC, Toni I ( 2006): Posture influences motor imagery: an fMRI study. Neuroimage 33: 609–617. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Kalkman JS, Bleijenberg G, Hagoort P, van der Werf SP, van der Meer JW, Toni I ( 2004): Neural correlates of the chronic fatigue syndrome–an fMRI study. Brain 127: 1948–1957. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, Cabanis EA, Vannson JL ( 1991): The human brain: surface, and three‐dimensional sectional anatomy and MRI. New York: Springer‐Verlag. [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Fiorio M, Tinazzi M, Aglioti SM ( 2006): Selective impairment of hand mental rotation in patients with focal hand dystonia. Brain 129: 47–54. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G ( 2001): Cortical mechanism for the visual guidance of hand grasping movements in the monkey: A reversible inactivation study. Brain 124: 571–586. [DOI] [PubMed] [Google Scholar]
- Friston KJ ( 1995): Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp 2: 56–78. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Gentili R, Cahouet V, Ballay Y, Papaxanthis C ( 2004): Inertial properties of the arm are accurately predicted during motor imagery. Behav Brain Res 155: 231–239. [DOI] [PubMed] [Google Scholar]
- Hallett M ( 2000): Disorder of movement preparation in dystonia. Brain 123 (Part 9): 1765–1766. [DOI] [PubMed] [Google Scholar]
- Hallett M ( 2004): Dystonia: abnormal movements result from loss of inhibition. Adv Neurol 94: 1–9. [PubMed] [Google Scholar]
- Hallett M ( 2006): Pathophysiology of writer's cramp. Hum Mov Sci 25: 454–463. [DOI] [PubMed] [Google Scholar]
- Hamano T, Kaji R, Katayama M, Kubori T, Ikeda A, Shibasaki H, Kimura J ( 1999): Abnormal contingent negative variation in writer's cramp. Clin Neurophysiol 110: 508–515. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Aarts E, de Lange FP, Bloem BR, Toni I ( 2009): Increased dependence of action selection on recent motor history in Parkinson's disease. J Neurosci 29: 6105–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I ( 2010): Spatial remapping of cortico‐striatal connectivity in Parkinson's disease. Cereb Cortex 20: 1175–1186. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J ( 2006): Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J Neurophysiol 95: 3596–3616. [DOI] [PubMed] [Google Scholar]
- Hu XY, Wang L, Liu H, Zhang SZ ( 2006): Functional magnetic resonance imaging study of writer's cramp. Chin Med J (Engl) 119: 1263–1271. [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Lu CS, Wang J, Chen RS ( 2010): Restoration of motor inhibition through an abnormal premotor‐motor connection in dystonia. Mov Disord 25: 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez V, Sadato N, Karp B, Deiber MP, Hallett M ( 1999): Deficient activation of the motor cortical network in patients with writer's cramp. Neurology 53: 96–105. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Shibasaki H, Kaji R, Terada K, Nagamine T, Honda M, Hamano T, Kimura J ( 1996): Abnormal sensorimotor integration in writer's cramp: study of contingent negative variation. Mov Disord 11: 683–690. [DOI] [PubMed] [Google Scholar]
- Jeannerod M ( 1995): Mental imagery in the motor context. Neuropsychologia 33: 1419–1432. [DOI] [PubMed] [Google Scholar]
- Kadota H, Nakajima Y, Miyazaki M, Sekiguchi H, Kohno Y, Amako M, Arino H, Nemoto K, Sakai N ( 2010): An fMRI study of musicians with focal dystonia during tapping tasks. J Neurol 257: 1092–1098. [DOI] [PubMed] [Google Scholar]
- Kaji R, Ikeda A, Ikeda T, Kubori T, Mezaki T, Kohara N, Kanda M, Nagamine T, Honda M, Rothwell JC, et al. ( 1995): Physiological study of cervical dystonia. Task‐specific abnormality in contingent negative variation. Brain 118 (Part 2): 511–522. [DOI] [PubMed] [Google Scholar]
- Kurata K, Hoffman DS ( 1994): Differential effects of muscimol microinjection into dorsal and ventral aspects of the premotor cortex of monkeys. J Neurophysiol 71: 1151–1164. [DOI] [PubMed] [Google Scholar]
- la Fougere C, Zwergal A, Rominger A, Forster S, Fesl G, Dieterich M, Brandt T, Strupp M, Bartenstein P, Jahn K ( 2010): Real versus imagined locomotion: a [18F]‐FDG PET‐fMRI comparison. Neuroimage 50: 1589–1598. [DOI] [PubMed] [Google Scholar]
- Lerner A, Shill H, Hanakawa T, Bushara K, Goldfine A, Hallett M ( 2004): Regional cerebral blood flow correlates of the severity of writer's cramp symptoms. NeuroImage 21: 904–913. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Anton JL, Roth M, Velay JL ( 2003): Visual presentation of single letters activates a premotor area involved in writing. Neuroimage 19: 1492–1500. [DOI] [PubMed] [Google Scholar]
- Majdandzic J, Bekkering H, van Schie HT, Toni I ( 2009): Movement‐specific repetition suppression in ventral and dorsal premotor cortex during action observation. Cereb Cortex 19: 2736–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE ( 2006): Three‐dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta‐analysis. Neuroimage 31: 1453–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P, Wingfield A ( 2010): Distinct effects of perceptual quality on auditory word recognition, memory formation and recall in a neural model of sequential memory. Front Syst Neurosci 4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N, Rothwell JC, Kaji R, Urushihara R, Nakamura K, Murayama N, Igasaki T, Sakata‐Igasaki M, Mima T, Ikeda A, Shibasaki H ( 2005): Subthreshold low‐frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer's cramp. Brain 128: 104–115. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB ( 2005): Valid conjunction inference with the minimum statistic. Neuroimage 25: 653–660. [DOI] [PubMed] [Google Scholar]
- Nico D, Daprati E, Rigal F, Parsons L, Sirigu A ( 2004): Left and right hand recognition in upper limb amputees. Brain 127: 120–132. [DOI] [PubMed] [Google Scholar]
- Nixon PD, McDonald KR, Gough PM, Alexander IH, Passingham RE ( 2004): Cortico‐basal ganglia pathways are essential for the recall of well‐established visuomotor associations. Eur J Neurosci 20: 3165–3178. [DOI] [PubMed] [Google Scholar]
- Odergren T, Iwasaki N, Borg J, Forssberg H ( 1996): Impaired sensory‐motor integration during grasping in writer's cramp. Brain 119 (Pt 2): 569–583. [DOI] [PubMed] [Google Scholar]
- Odergren T, Stone‐Elander S, Ingvar M ( 1998): Cerebral and cerebellar activation in correlation to the action‐induced dystonia in writer's cramp. Mov Disord 13: 497–508. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Passingham RE ( 1986): Cues for movement in monkeys (Macaca mulatta) with lesions in premotor cortex. Behav Neurosci 100: 695–703. [DOI] [PubMed] [Google Scholar]
- Preibisch C, Berg D, Hofmann E, Solymosi L, Naumann M ( 2001): Cerebral activation patterns in patients with writer's cramp: a functional magnetic resonance imaging study. J Neurol 248: 10–17. [DOI] [PubMed] [Google Scholar]
- Pujol J, Roset‐Llobet J, Rosines‐Cubells D, Deus J, Narberhaus B, Valls‐Sole J, Capdevila A, Pascual‐Leone A ( 2000): Brain cortical activation during guitar‐induced hand dystonia studied by functional MRI. NeuroImage 12: 257–267. [DOI] [PubMed] [Google Scholar]
- Pylyshyn ZW ( 2002): Mental imagery: in search of a theory. Behav Brain Sci 25: 157–182. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Morgante F, Sant'angelo A, Crupi D, Romano M, Messina C, Berardelli A, Girlanda P ( 2005): Corticospinal excitability during motor imagery of a simple tonic finger movement in patients with writer's cramp. Mov Disord 20: 1488–1495. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Siebner HR, Dattola V, Scalfari A, Morgante F, Battaglia F, Romano M, Girlanda P ( 2003): Abnormal associative plasticity of the human motor cortex in writer's cramp. Brain 126: 2586–2596. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Morgante F, Sant'angelo A, Rizzo V, Bagnato S, Terranova C, Siebner HR, Berardelli A, Girlanda P ( 2008): Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry 79: 985–990. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Butler K, Williamon A, Cordivari C, Lees AJ, Rothwell JC ( 2008): Sensorimotor reorganization by proprioceptive training in musician's dystonia and writer's cramp. Neurology 70: 304–315. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW ( 2008): A computational neuroanatomy for motor control. Exp Brain Res 185: 359–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW ( 2010): Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33: 89–108. [DOI] [PubMed] [Google Scholar]
- Sheehy MP, Marsden CD ( 1982): Writers' cramp‐a focal dystonia. Brain 105 (Pt 3): 461–480. [DOI] [PubMed] [Google Scholar]
- Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, Toni I ( 2011): Gait‐related cerebral alterations in patients with Parkinson's disease with freezing of gait. Brain 134: 59–72. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos‐Baumann AO, Frith CD, Frackowiak RS ( 1995): Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol 73: 373–386. [DOI] [PubMed] [Google Scholar]
- Tanne‐Gariepy J, Rouiller EM, Boussaoud D ( 2002): Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res 145: 91–103. [DOI] [PubMed] [Google Scholar]
- Tempel LW, Perlmutter JS ( 1993): Abnormal cortical responses in patients with writer's cramp. Neurology 43: 2252–2257. [DOI] [PubMed] [Google Scholar]
- Toni I, Rushworth MF, Passingham RE ( 2001a): Neural correlates of visuomotor associations. Spatial rules compared with arbitrary rules. Exp Brain Res 141: 359–369. [DOI] [PubMed] [Google Scholar]
- Toni I, Shah NJ, Fink GR, Thoenissen D, Passingham RE, Zilles K ( 2002): Multiple movement representations in the human brain: an event‐related fMRI study. J Cogn Neurosci 14: 769–784. [DOI] [PubMed] [Google Scholar]
- Toni I, Thoenissen D, Zilles K ( 2001b): Movement preparation and motor intention. Neuroimage 14: S110–S117. [DOI] [PubMed] [Google Scholar]
- Verhagen L, Dijkerman HC, Grol MJ, Toni I ( 2008): Perceptuo‐motor interactions during prehension movements. J Neurosci 28: 4726–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissel J, Kabus C, Wenzel R, Klepsch S, Schwarz U, Nebe A, Schelosky L, Scholz U, Poewe W ( 1996): Botulinum toxin in writer's cramp: objective response evaluation in 31 patients. J Neurol Neurosurg Psychiatry 61: 172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z ( 2000): Computational principles of movement neuroscience. Nat Neurosci 3 Suppl: 1212–1217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


