Abstract
Structural and functional studies have shown that schizophrenia is often associated with frontolimbic abnormalities in the prefrontal and mediotemporal regions. It is still unclear, however, if such dysfunctional interaction extends as well to relay regions such as the thalamus and the anterior insula. Here, we measured gray matter volumes of five right‐hemisphere regions in 68 patients with schizophrenia and 77 matched healthy subjects. The regions were amygdala, thalamus, and entorhinal cortex (identified as anomalous by prior studies on the same population) and dorsolateral prefrontal cortex and anterior insula (isolated by voxel‐based morphometry analysis). We used structural equation modeling and found altered path coefficients connecting the thalamus to the anterior insula, the amygdala to the DLPFC, and the entorhinal cortex to the DLPFC. In particular, patients exhibited a stronger thalamus‐insular connection than healthy controls. Instead, controls showed positive entorhinal‐DLPFC and negative amygdalar‐DLPFC connections, both of which were absent in the clinical population. Our data provide evidence that schizophrenia is characterized by an impaired right‐hemisphere network, in which intrahemispheric communication involving relay structures may play a major role in sustaining the pathophysiology of the disease. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: dorsolateral prefrontal cortex, amygdala, entorhinal cortex, structural equation modeling, voxel‐based morphometry
INTRODUCTION
Schizophrenia is a psychiatric disorder characterized by impaired high cognitive functions, such as perception, memory, or decision making [Bellani et al., 2009a, b, c;]. A wealth of studies in recent decades have found this syndrome to be associated with extensive brain structural abnormalities, including decreased gray matter volume in cortical regions (e.g., frontal, mediotemporal, and insular cortex) as well as within‐specific subcortical regions, such as the thalamus [see Chan et al., 2011; Ellison‐Wright and Bullmore, 2010, as meta‐analyses of voxel‐based morphometry (VBM) studies]. Both these functional and structural abnormalities have often been interpreted in terms of dysconnectivity; anatomically, dysconnectivity has been posited as the result of “miswirings” of association fibers, whereas at the functional level, it has been described as the result of impairments of synaptic transmission and plasticity [Bullmore et al., 1997; Friston, 1998; Stephan et al., 2006]. Evidence for dysconnectivity in schizophrenia arises by electrophysiological studies, which reported reduced interregional gamma‐band synchrony during sensory processing in patients [Spencer et al., 2004; Symond et al., 2005], and by functional neuroimaging studies, which showed reduced coupling between the signal from frontal and temporal cortex during execution of linguistic/memory tasks [Friston and Frith, 1995; Lawrie et al., 2002; Meyer‐Lindenberg et al., 2005]. Further evidence comes from the study of the correlation between brain regional volumetric measures; high correlation between the volume of two brain structures is held to reflect the presence of mutual trophic effects and, therefore, the presence of an anatomical connection [Colibazzi et al., 2008; Lerch et al., 2006; Mitelman et al., 2005; Yeh et al., 2010] and a strong functional interaction [Mitelman et al., 2005; Schlaepfer et al., 1994]. In particular, studies compared the volumetric correlations among brain regions in both patients with schizophrenia and healthy controls and found that the frontal cortex correlated with portions of both medial and lateral temporal cortex differently in the two groups [Mitelman et al., 2005; Wible et al., 1995, 2001; Woodruff et al., 1997].
Although the idea of frontotemporal dysconnectivity in schizophrenia is well established in the scientific community [Andreone et al., 2007], little is still known about to which extent such dysfunctional interaction involves as well other regions and, in particular, those neural structures playing a key role in monitoring/integrating frontotemporal communications. Of particular interest for our purpose is the role played by the thalamus and anterior insula. Both regions have been described as important interface centers, which are highly interconnected to each other and to both frontal and temporal cortex, with which they play a key role in processes such as awareness, attention, decision making, perception, and emotion regulation [Craig, 2009; Lambe et al., 2007; Sutcliffe and de Lecea, 2002]. Crucially, both regions have been described structurally and functionally impaired in subjects suffering from schizophrenia. Previous magnetic resonance imaging (MRI) studies have identified structural thalamic abnormalities at both the volume [Agarwal et al., 2008; Andreasen et al., 1994; Dasari et al., 1999; Ettinger et al., 2007; Flaum et al., 1995; Gilbert et al., 2001; Gur et al., 1998; Staal et al., 1998] and microstructure levels [Agarwal et al., 2008]. Postmortem studies have reported reduced neuronal [Byne et al., 2006; Pakkenberg, 1990; Popken et al., 2000; Young et al., 2000] and oligodendrocyte [Byne et al., 2006] populations in the thalamus of patients when compared with those of controls. Furthermore, functional neuroimaging studies have reported reduced thalamic neural activity in patients with schizophrenia during linguistic and memory tasks [Andreasen et al., 1996; Andrews et al., 2006; Camchong et al., 2006; Fox et al., 2005; Schneider et al., 2007]. Likewise, volumetric insular reduction (prevalently in the right hemisphere) [Chan et al., 2011; Crespo‐Facorro et al., 2000; Jang et al., 2006; Kim et al., 2003; Wright et al., 1999] and altered insular activity during verbal processing (in both hemispheres) [Curtis et al., 1998] or primary experience of auditory hallucinations (in the right hemisphere) [Sommer et al., 2008] have been reported in schizophrenia.
This study aims at extending the literature's results by investigating altered volumetric correlation patterns in a large sample of patients with schizophrenia in a network comprehending not only frontal and mediotemporal regions but also thalamus and insula. Gray matter volumes of five regions of interests (ROIs) were considered. Two of them were the dorsolateral prefrontal cortex (DLPFC) and the anterior insula, which VBM analysis on our 68 patients reveals as showing decreased gray matter volumes (see Results section). The remaining three were the amygdala, the thalamus, and the entorhinal cortex, which previous (non‐VBM) studies on the same population of patients described as exhibiting structural and microstructural anomalies [Agarwal et al., 2008; Baiano et al., 2008; Tomasino et al., 2011]. We then used structural equation modeling [SEM—Joreskog and Wold, 1982; Wold, 1982] to identify the anatomical model that best explained the putative relations between gray matter volume of these regions for the control population. Finally, we compared the results from the best model in both populations to assess discrepancies in the estimated interregional coefficients between the patient and the control group. Of specific interest was the assessment of intergroup differences not only in the connections between frontal and mediotemporal regions (which would converge with neuroimaging studies reviewed above) but also in the connections pertaining thalamus and anterior insula.
MATERIALS AND METHODS
Participants
We tested 68 patients diagnosed with schizophrenia (43 males, average age 40.15 ± 12.06 years) and 77 healthy individuals (40 males, average age = 39.63 ± 10.73 years—see Table I for more details). Patients met the Diagnostic and Statistical Manual of Mental Disorders criteria [American Psychiatric Association, 2000] for schizophrenia and were recruited from the South Verona Psychiatric Case Register [Tansella and Burti, 2003]. Control subjects had no DSM‐IV axis I disorders, as determined by a brief modified version of the SCID‐NP (nonpatient version), no history of psychiatric disorders among first‐degree relatives, no history of alcohol or substance abuse, and no current major medical illness. They were recruited from the same catchment area. All participants gave signed informed consent, having been informed of all potential risks of research participation, as approved by the biomedical Ethics Committee of the Azienda Ospedaliera of Verona. Of note, all 68 patients and 72 of 77 healthy controls were also tested in our previous studies reporting schizophrenia‐related amygdalar, thalamic, and entorhinal structural anomalies [Agarwal et al., 2008; Baiano et al., 2008; Tomasino et al., 2011].
Table I.
Demographic and clinical characteristics of the sample
| Patients (n = 68) | Controls (n = 77) | |
|---|---|---|
| Age (years) | 40.15 ± 12.06 | 39.63 ± 10.73 |
| Males | 43 (63.23%) | 40 (51.95%) |
| Age of onset (years) | 26.41 ± 9.46 | — |
| Illness duration (years) | 9.66 ± 11.17 | — |
| Hospital addmissions | 14.38 ± 15.05 | — |
| BPRS total score | 75.52 ± 15.05 | — |
| Medication use | ||
| Patients on antipsychotics (AP) | 68 (100%) | |
| Patients on atypical AP | 45 (66%)a | |
| Patients on typical AP | 24 (35%)a |
BPRS, Brief Psychiatric Rating Scale.
One patient is on both typical and atypical.
MRI Data Acquisition
MRI scans were acquired using a 1.5‐T Siemens Magnetom Symphony Maestro Class, Syngo MR 2002B. All participants were provided with earplugs to reduce acoustic noise, and their head was comfortably placed in a head holder and held stable to minimize movement artifact. Initially, exploratory T1‐weighted images (TR = 450 ms, TE = 14 ms, flip angle = 90°, FOV = 230 × 230, slice thickness = 5 mm, and matrix size = 384 × 512) were obtained to verify the subject's head position and the quality of the image. A sequence of DP/T2‐weighted images were then obtained (TR = 2,500 ms, TE = 24/121 ms, flip angle = 180°, FOV = 230 × 230, slice thickness = 5 mm, and matrix size = 410 × 512) according to an axial plane parallel to the anterior–posterior commissures (AC‐PC) to exclude focal lesions. Subsequently, a coronal 3D MPR sequence was acquired (TR = 2,060 ms, TE = 3.9 ms, flip angle = 15°, FOV = 176 × 235, slice thickness = 1.25 mm, matrix size = 270 × 512, and TI = 1,100) to obtain images covering the entire brain.
MRI Data Processing
Preprocessing
Statistical analysis was carried out using the SPM5 software package (http://www.fil.ion.ucl.ac.uk/spm/). Unless stated otherwise, software default settings were applied. For each subject, an image describing gray matter volume was computed and spatially normalized to the Montreal Neurological Institute (MNI) single‐subject template [Collins et al., 1994; Evans et al., 1992; Holmes et al., 1998] using the “unified segmentation” function in SPM5. This algorithm is based on a probabilistic framework that enables image registration, tissue classification, and bias correction to be combined within the same generative model. The resulting parameters of a discrete cosine transform, which define the deformation field necessary to move the subjects' data into the space of the MNI tissue probability maps [Evans et al., 1994], were then combined with the deformation field transforming between “MNI tissue probability maps” and the MNI single‐subject template. The ensuing deformation was subsequently applied to the estimated gray matter volume image, which was thereby transformed into standard stereotaxic space and resampled at 2 × 2 × 2 mm3 voxel size. The quality of the resulting normalized, segmented images was checked visually to detect gross misregistrations. The intensity value of each gray matter voxel was then modulated (i.e., multiplied) by the Jacobian determinant from spatial normalization, thus yielding voxel intensity values that reflected gray matter volume rather than concentration. Finally, the normalized and modulated images were spatially smoothed using a 12‐mm FWHM Gaussian kernel.
Voxel‐based morphometry
Volumetric differences in smoothed gray matter volume were tested for on a voxel‐by‐voxel basis using the general linear model approach implemented on SPM. We excluded from the analysis those voxels that did not exceed an absolute threshold of 0.05. To avoid confounds due to global differences, for each subject, voxel intensities were scaled to the subject's total intracranial volume (ICV), which in turn was calculated as the sum of the segmented gray matter, white matter, and cerebrospinal fluid volumes [Mechelli et al., 2005a; Pell et al., 2008]. An independent sample t‐test on scaled, smoothed, modulated, gray matter images was carried out, including age and gender as covariates of no interest. Furthermore, and specifically for the case of patients, we included as well as covariates the length of illness (in years), the number of hospitalizations, the daily chlorpromazine consumption (mg), and the Brief Psychiatric Rating Scale (BPRS) score [Ventura et al., 1993]. We investigated putative changes in gray matter volume, throughout the whole brain, using two one‐sided comparisons (patients > controls and patients < controls). Areas were identified as significant if they exceeded a height‐level threshold of t (135) > 4.62, corresponding to P < 0.05 family‐wise error (FWE) corrected for multiple comparisons for the whole brain.
Path Modeling
We examined the interactions between key regions exhibiting gray matter volume anomalies in schizophrenic patients using the partial least squares approach to SEM, also known as partial‐least‐squares path modeling (PLS‐PM) [Tenenhaus et al., 2005; Wold, 1982]. In contrast to the traditional maximum‐likelihood approach [Joreskog and Wold, 1982], which requires large sample sizes (≈200 or larger), observed variables to be normally distributed and models that should not excessively exceed in complexity to avoid misconvergences, PLS‐PM does not impose any assumption in the distribution of the observed variables, can tolerate smaller sample sizes and allows the number of observations to be limited with respect to the number of variables [Joreskog and Wold, 1982]. For our purposes, PLS‐PM analysis involved four steps: identification of the network of interest, identification of the anatomical model of the network, model fitting, and comparison of the estimated path coefficients between the two groups. PLS‐PM analysis was carried out using the plspm package implemented in R 2.9.1 (http://cran.r-project.org/) open source software.
Network of interest
We built a network involving five regions identified both through the current VBM analysis and following previous studies on the same population [Agarwal et al., 2008; Baiano et al., 2008; Tomasino et al., 2011]. The regions were the DLPFC, the anterior insula (see VBM analysis results), the amygdala, the thalamus, and the entorhinal cortex (implicated in previous studies). As the current VBM analysis outlined exclusively right‐hemisphere regions (see Results section), our analysis focused exclusively on the right hemisphere. We extracted the signal from each voxel subtending each region. At variance from our previous studies in which each region was localized through visual inspection of its main anatomical landmarks on subjects' native (unnormalized) brain, to take advantage of our morphometric results, we localized each region in the MNI space. For the DLPFC and the anterior insula, we considered those voxels that exhibited a significant decrease of gray matter volume in schizophrenic patients associated with a P < 0.05 (FWE corrected for multiple comparisons for the whole brain). For amygdala, thalamus, and entorhinal cortex, we considered those voxels within a priori defined ROIs. ROIs were defined according to an anatomically constrained morphometric principle. We first localized those voxels most likely corresponding to thalamic, amygdalar, and entorhinal structures in standard MNI space; subsequently, we selected, among the anatomically defined voxels, those showing significant (uncorrected) effects on the current VBM analysis. Anatomical localization of amygdala and entorhinal cortex was based on the database of the SPM Anatomy toolbox (http://www.fz-juelich.de/inm/inm-1/spm_anatomy_toolbox) [Amunts et al., 2005; Eickhoff et al., 2005], which aims at MNI template parcellation on cytoarchitectonical bases. Such database was built on postmortem brains, which were analyzed cytoarchitectonically and then MNI normalized. Probabilistic maps were then built describing, for each voxel in the space, its likelihood to be identified as a given region. Following Eickhoff et al. [ 2005], we included only those voxels that were identified as the entorhinal/amygdalar nuclei in at least 40% of the tested postmortem brains. Unfortunately, the anatomy toolbox does not report, at the present stage, probabilistic maps of thalamic nuclei. Thus, anatomical localization of the thalamus was based on the Automatic Anatomical Labeling database [Tzourio‐Mazoyer et al., 2002], which provides MNI template parcellation through the identification of main anatomical landmarks of a single MNI‐normalized brain. Subsequently, we selected, for each anatomically defined ROI, those voxels that exhibited in the previous VBM analysis significant effects in the contrast “controls > patients” of at least t (136) > 1.66 (P < 0.05 uncorrected). Please note that no amygdalar voxel survived such threshold. Thus, exclusively for the case of the amygdala, we considered only region's local maxima, which was associated with t (136) = 1.54 (P = 0.063—see Table II). Our approach insured that the data we fed in our SEM analysis reflected only those portions of thalamic, amygdalar, and entorhinal structures in which degenerative effects of Schizophrenia were maximized. Finally, putative linear effects of age, gender, group, ICV and, for patients only, years from the pathology onset, hospitalizations, chlorpromazine, and BPRS were removed from the signals of each voxel, and the resulting detrended signals were z‐transformed.
Table II.
Network of interest
| Approach | Voxels | Height threshold | MNI coordinates | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| DLPFC | VBM | 18 | FWE‐corr. | 36 | 40 | 46 |
| Ins | VBM | 106 | FWE‐corr. | 46 | 16 | 4 |
| Tha | ROIa | 74 | Uncorrected | 2 | −20 | 4 |
| EC | ROIb | 18 | Uncorrected | 32 | −10 | −40 |
| Am | ROIb | 1 | Local maxima | 16 | −8 | −18 |
Each of the regions included in the network is described in terms of approach (DLPFC and anterior insula were isolated by VBM analysis, whereas the remaining three regions were isolated through anatomically constrained morphometric ROIs), number of voxel included, height threshold for voxel inclusion, and maximally activated foci MNI coordinates.
DLPFC, dorsolateral prefrontal cortex; Ins, anterior insula; Tha, thalamus; EC, entorhinal cortex; Am, amygdale; FWE‐corr, family‐wise error corrected for multiple comparisons for the whole brain. x, distance (mm) to the right (+) or the left (−) of the midsagittal line; y, distance anterior (+) or posterior (−) to the vertical plane through the anterior commissure (AC); z, distance above (+) or below (−) the intercommissural (AC‐PC) line.
ROI built from AAL atlas [Tzourio‐Mazoyer et al., 2002].
ROI built from cytoarchitectonic maps [Amunts et al., 2005].
Model selection
Path modeling analysis usually involves two types of variables: latent and manifest. Latent variables are those among which causal relations are being assessed in the selected model; in our case, the gray matter volume in the five regions of our network. These are not measured directly, but can be estimated using manifest variables; in our case, these are the gray matter volumes in each of the voxels included in the network. PLS‐PM is an iterative process in which two approximations for the latent variables are alternated until convergence: (a) an outer approximation, in which latent variables are estimated linearly through their corresponding manifest variables (reflective mode) and (b) an inner approximation, which reflects the putative relations between the latent variables in the selected model (latent variables are weighted such that they could be predicted by their antecedents and be good predictors of their subsequents—path weighting scheme). Although PLS‐PM does not optimize any global scalar index (as it is the case of maximum likelihood), Tenenhaus et al. [ 2005] recently introduced a goodness of fit (GoF) as a global index for model validation/comparison:
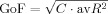 , where C reflects the average of the squared correlation coefficients between each manifest variable and its corresponding latent variable, and avR
2 is the average of the variance of each endogenous latent variable explained (R
2) by other putatively connected latent variables.
, where C reflects the average of the squared correlation coefficients between each manifest variable and its corresponding latent variable, and avR
2 is the average of the variance of each endogenous latent variable explained (R
2) by other putatively connected latent variables.
Rather than focusing on an a priori model (as is the usual case in path analysis), we performed an exploratory analysis in other to identify the anatomical model associated with the greatest GoF and that, therefore, best explains the putative relations between the voxels of our network in the control population. However, as GoF may privilege complex models (R
2 of each endogenous variable increases the more predictors are modeled), we used an adjusted GoF (aGoF),
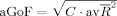 , where
, where
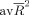 is the average of the coefficient R
2 associated with each endogenous variable and adjusted for the number of predictors. All possible interactions between the five regions of our network were considered, yielding a web of 10 connections each of which could point in either direction. Only nonrecursive models were considered (i.e., no loops in the structural relations), where all 10 connections are unidirectional. This leads to 120 possible models, each of which was optimized by assessing whether the aGoF improved by setting one or more of the 10 connections to 0. Specifically, optimization was done as follows. First, we calculated the aGoFs associated with alternative models, in which each of the 10 connections was set to 0. We then assessed whether the largest of the resulting 10 aGoFs was larger than the aGoF of the original model. If this were not the case, the optimization procedure stopped; otherwise, the alternative model associated with the largest aGoF replaced the original model and was itself subjected to the same optimization procedure. Finally, we choose, from the 120 optimized models, the one associated with the largest aGoF.
is the average of the coefficient R
2 associated with each endogenous variable and adjusted for the number of predictors. All possible interactions between the five regions of our network were considered, yielding a web of 10 connections each of which could point in either direction. Only nonrecursive models were considered (i.e., no loops in the structural relations), where all 10 connections are unidirectional. This leads to 120 possible models, each of which was optimized by assessing whether the aGoF improved by setting one or more of the 10 connections to 0. Specifically, optimization was done as follows. First, we calculated the aGoFs associated with alternative models, in which each of the 10 connections was set to 0. We then assessed whether the largest of the resulting 10 aGoFs was larger than the aGoF of the original model. If this were not the case, the optimization procedure stopped; otherwise, the alternative model associated with the largest aGoF replaced the original model and was itself subjected to the same optimization procedure. Finally, we choose, from the 120 optimized models, the one associated with the largest aGoF.
Model fitting and groups comparison
We tested the chosen model on the overall population (both patients and controls individuals). Statistical validation of the differences, in the estimated path coefficients, between the two groups, was assessed using a t‐test based on bootstrap standard errors, which were extrapolated by testing 5,000 resamples of the original dataset.
RESULTS
Voxel‐Based Morphometry
The only two regions in the whole brain volume exhibiting suprathreshold gray matter decreases in patients when compared with controls were the right anterior insula (MNI coordinates: x = 46, y = 16, z = −4, t (136) = 5.40, P‐FWE < 0.01, cluster size = 106 voxels) and the right DLPFC (x = 36, y = 40, z = 46, t (136) = 5.00, P‐FWE < 0.05, cluster size = 18 voxels—see Fig. 1). We found no instances of suprathreshold gray matter volume increase in patients when compared with healthy controls.
Figure 1.
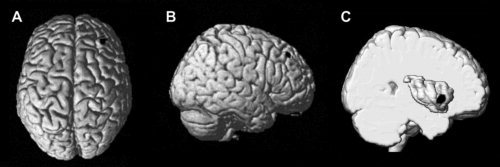
Surface renderings of the contrasts testing significant decrease of gray matter volume in patients as opposed to controls. (A, B) Significant decrease of gray matter volume at the level of the prefrontal cortex. (C) Significant decrease of gray matter volume at the level of the insula. The surface rendering was obtained from an MNI‐normalized single‐subject brain in which the most lateral regions were removed, thus allowing free vision of the surface of the insular cortex.
Path Modeling
We used path modeling to examine the interactions among the DLPFC, the anterior insula, thalamus, amygdala, and entorhinal cortex. We first selected the best functional‐anatomical model, which explained the largest amount of variance in the gray matter volume of the control population (see Materials and Methods section). This yielded one model, in which amygdala and thalamus are defined as exogenous and the other three regions as endogenous; specifically, the entorhinal cortex was assumed to be influenced by the amygdala, the insula by thalamus and entorhinal cortex, and the DLPFC by all other four regions.
The selected model was tested on the overall population to assess differential effects on one brain region over another in the two groups (see Table III). We found significant group variation in both the path connecting the thalamus to the anterior insula (t (143) = 2.62, P < 0.01) and the path connecting the amygdala to the DLPFC (t (143) = 2.49, P < 0.05); for both, the coefficients were larger in the clinical when compared with the healthy population. We also found a significant group difference with regards to the path connecting the entorhinal cortex to the DLPFC (t (143) = 2.88, P < 0.01—see Figs. 2 and 3). Here, the path coefficient was larger in the healthy, as opposed to clinical, population.
Table III.
Path Modeling
| Path | Global | Controls | Patients | T‐diff(143) |
|---|---|---|---|---|
| Am → EC | 0.85 | 0.85 | 0.85 | 0.16 |
| Am → DLPFC | −0.18 | −0.47 | 0.13 | 2.49 a |
| Tha → DLPFC | 0.09 | 0.16 | −0.04 | 1.35 |
| Tha → Ins | 0.35 | 0.22 | 0.55 | 2.62 b |
| EC → DLPFC | 0.22 | 0.59 | −0.15 | 2.88 b |
| EC → Ins | 0.36 | 0.45 | 0.23 | 1.71 |
| Ins→ DLPFC | 0.52 | 0.40 | 0.65 | 1.55 |
Path coefficients of the model for both the overall population and each group. T‐values associated with each of the paths are also displayed. Bold values refer to significant t‐tests.
Am, amygdale; Tha, thalamus; Ins, anterior insula; EC, entorhinal cortex; DLPFC, dorsolateral prefrontal cortex.
P < 0.05.
P < 0.01.
Figure 2.
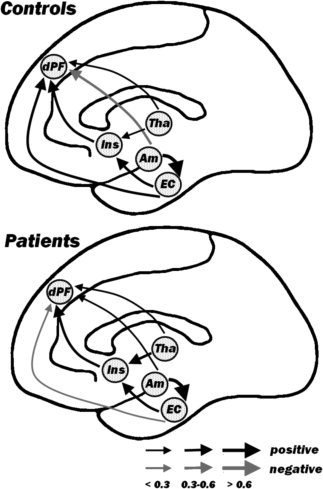
Path modeling. Line drawing of a sagittal brain section displaying, for each group, the five regions subtending the network used in the present analysis and the strength of their connection. Black arrows refer to positive path coefficients, whereas gray arrows refer to negative path coefficients. Thickness of each arrow is proportional with the absolute magnitude of the coefficient. Am, amygdala; Tha, thalamus; Ins, anterior insula; EC, entorhinal cortex; dPF, dorsolateral prefrontal cortex.
Figure 3.
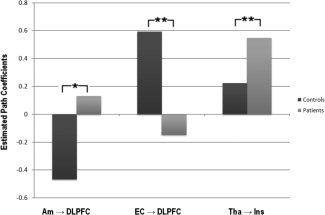
Barplot displaying path coefficients connecting the amygdala to the dorsolateral prefrontal cortex, the entorhinal cortex to the dorsolateral prefrontal cortex, and the anterior insula to the thalamus. Light gray bars refer to path coefficients estimated in the patient population, whereas dark gray bars refer to path coefficients estimated in the healthy/control population. Significances associated with t‐test based on bootstrap standard errors are also reported. “*” refers to t (143) > 1.98 (corresponding to P < 0.05), whereas “**” refers to t (143) > 2.61 (corresponding to P < 0.01).
DISCUSSION
In this study, we compared correlation patterns of healthy controls and patients with schizophrenia in a network comprehending five right‐hemisphere regions: DLPFC, amygdala, thalamus, entorhinal cortex, and the insula. We utilized SEM to (a) identify the anatomical model that best explained the relationships between the gray matter volumes of these five regions in the control population and to (b) assess, using this model, putative discrepancies in path coefficients between the patient and the control group. We found that three path coefficients were significantly different across groups: the path connecting the thalamus to the insula, the path connecting the amygdala to the DLPFC, and the path connecting the entorhinal cortex to the DLPFC (see Figs. 2 and 3). Our data converge, but also extend, findings from previous studies by documenting dysfunctional connectivity in patients with schizophrenia not only at the frontotemporal but also at the thalamic‐insular level.
Volumetric Correlation as a Connectivity Measure
Our interpretation of correlation patterns between regional morphometric parameters as connectivity patterns is not new in current neuroscience research, and it is strengthened by two classes of evidence. First, brain morphometric properties were found not depending exclusively on global volumetric features (e.g., ICV) or other genetically related factors (e.g., gender and age) but also on environmental and experience‐dependent features such as the acquisition of new skills. For example, using VBM, Draganski et al. [ 2004] found increased gray matter volume in mediotemporal regions as a result of 3‐month juggling training. Likewise, gray matter volume in a network comprehending auditory, motor, cingulate, and frontal regions was documented to increase as a result of musical training [Hyde et al., 2009]. Second, the volumetric and morphometric properties of different brain areas correlate with one another in a pattern that is reminiscent of (although not identical to) known anatomical connectivity. For instance, Mechelli et al. [ 2005b] argued that the degree of morphometric covariation between homologous regions in the two hemispheres reflected the amount of interhemispheric connection between these regions. Lerch et al. [ 2006] measured the cortical thickness of the inferior frontal gyrus (over and around Broca's area) on almost 300 adolescents and found it to be correlated with the thickness of other posterior temporal regions, consistent with diffusion tensor maps of the arcuate fasciculus [Parker et al., 2005]. Furthermore, Colibazzi et al. [ 2008] used SEM and found that a model based on the anatomical pathways within cortico‐striatal‐thalamic‐cortical circuits could account for volumetric covariances in group of almost 100 subjects.
Changes to brain regions' structural properties have been often interpreted as the result of trophic influences on anatomically and functionally connected structures, which promote the formation of new connections from dendritic/axonal growth and arborization [Draganski et al., 2004; Lerch et al., 2006; Mechelli et al., 2005b]. Such growth has been documented also in the animal model: Hihara et al. [ 2006], for example, reported the emergence of new corticocortical connections in the macaque brain, between the intraparietal sulcus and premotor/temporoparietal neurons, following 3 weeks of demanding tool‐use training.
In this perspective, correlation patterns of the volume of different brain regions can be considered as an indirect measure of their interconnectivity, which is particularly relevant in the case of investigations of those psychiatric syndromes, such as schizophrenia, thought to involve impairment of synaptic transmission and plasticity [Bullmore et al., 1997; Friston, 1998; Stephan et al., 2006].
Thalamus‐Insula Pathway
The path connecting the right thalamus with the anterior portion of the right insula was found to be significantly stronger in patients with schizophrenia than in healthy controls. Abnormal positive coefficients in patients when compared with healthy subjects could reflect (a) a neural network with stronger than normal interregional associations or (b) a causal role played by gray matter degeneration in one region toward the other, due to spared interregional trophic effects [Mitelman et al., 2005]. As in this study ROIs were selected according to the presence of gray matter degeneration, we believe the latter interpretation to be more suitable for our thalamic‐insular connection.
Although the right insula and thalamus have previously been documented to exhibit structural and functional abnormalities in patients affected by schizophrenia (see introduction), to the best of our knowledge, this is the first study documenting also their interaction to be dysfunctional. Direct anatomical connections between the thalamus and the anterior insula in the right hemisphere have been extensively mapped in previous studies, utilizing anatomical tracing methods on species such as macaques [Burton and Jones, 1976; Friedman and Murray, 1986; Jones and Burton, 1976; Mufson and Mesulam, 1984], cats [Clascá et al., 1997; Minciacchi et al., 1986], or rats [Allen et al., 1991; Groenewegen, 1988; Guldin and Markowitsch, 1983; Ray and Price, 1992; Saper, 1982], and diffusion tensor imaging (DTI) on the human brain [Moisset et al., 2010].
Furthermore, a wealth of neuroimaging and neuropsychological studies have implicated networks that comprise both the (right hemisphere) thalamus and insula; in various cognitive and affective processes, the dysfunction of which might result in symptoms similar to those characterizing schizophrenia. For example, thalamus and insula are both part of the pain matrix [Apkarian et al., 2005; Derbyshire, 2000; Rainville, 2002]: both regions were found active during direct nociceptive sensation [Geuze et al., 2007; Roy et al., 2009], as in the case of the DTI study described above [Moisset et al., 2010] in which the thalamic‐insular portions connected by the reconstructed fibers were localized functionally through visceral pain stimulation. Dysfunctional properties in schizophrenia on the pain matrix converge with the long‐lasting evidence of decreased pain sensitivity in patients affected by schizophrenia [Bonnot et al., 2009; Potvin and Marchand, 2008] including those not under pharmacological treatment, thus ruling out a potential role played by analgesic properties of antipsychotic drugs [Potvin and Marchand, 2008]. Furthermore, thalamus and insula have also been implicated in motor awareness and control, as shown by motor agency tasks, in which participants feel an event as the sensory consequence of one's own movement [e.g., Blakemore et al., 1998; Corradi‐Dell'Acqua et al., 2008; Farrer and Frith, 2002]. The inability to distinguish between the sensory consequences of self‐produced and other‐produced movements is commonly observed in schizophrenia [Blakemore et al., 2000; Shergill et al., 2005] and classified as one of its first‐rank symptoms [Stephan et al., 2009]. Stephan et al. [ 2009] argued that these symptoms might occur by a failure to distinguish between self‐ and other‐generated events and, specifically, by a dysconnection between motor acts and sensory consequences thereof. However, neural structures whose dysconnection underlies altered motor awareness and control are, to date, still to be elucidated. We believe the thalamic‐insular network described in this study to be a strong candidate for such role, consistently with Farrer et al. [ 2004], who report activation in the insular cortex during motor agency tasks in healthy participants, but not in patients affected by schizophrenia.
Mediotemporo‐Frontal Pathways
The analysis of the control population revealed a positive path coefficient connecting the right entorhinal cortex with the DLPFC and a negative path coefficient connecting the amygdala with the DLPFC. Both coefficients were found to be significantly closer to 0 in patients with schizophrenia. Abnormally small coefficients (positive or negative) in patients, as opposed to controls, can be interpreted as reflections of a weakened interregional trophic influence (either excitatory or inhibitory), which itself might be the result of from developmental dissociation between the two areas that define the path [Bullmore et al., 1997; Mitelman et al., 2005]. At variance with the case of the thalamic‐insular connection, in which two regions exhibited common susceptibility to gray matter degeneration presumably due to spared connectivity, the analysis of mediotemporo‐frontal connections is instead suggestive of a degeneration, which extends to the connections themselves.
Our results converge with a large number of studies reporting altered connectivity patterns between mediotemporal regions and the prefrontal cortex in patients with schizophrenia. This has been documented both at the functional level, through altered covariation between the neural activity of the prefrontal cortex and hippocampal‐amygdalar complex [Hoptman et al., 2010; Meyer‐Lindenberg et al., 2005; Wolf et al., 2009], and at the structural level, with the identification of anisotropy abnormalities in the uncinate and cingulate fasciculus [Burns et al., 2003; Kubicki et al., 2002, 2003; Mandl et al., 2010; Voineskos et al., 2010], as well as altered covariation between prefrontal and hippocampal‐amygdalar volumes [Mitelman et al., 2005; Wible et al., 1995, 2001]. In contrast to previous studies, our data have the important merit of distinguishing between two cytoarchitectonically distinct portions of the medial temporal cortex, which, although highly positively correlated with one another in terms of gray matter volume (see Table I), exhibit opposite effects in their relation with the DLPFC.
Although our evidence of right amygdala degeneration in schizophrenia is faint (P ≈ 0.06, uncorrected—see Materials and Methods sections), several studies have not only reported reduced amygdalar volumes in patients affected by this syndrome [Chan et al., in press; Exner et al., 2004; Gur et al., 1998; Joyal et al., 2002] but also associated such a reduction with impaired emotional learning (but not with learning per se) (Exner et al., 2004]. Furthermore, functional neuroimaging studies have documented abnormal amygdalar activation in schizophrenia associated with the processing of emotional stimuli [Gur et al., 2002; Kosaka et al., 2002; Paradiso et al., 2003; Phillips et al., 1999; Schneider et al., 1998]. It is conceivable that emotional impairments exhibited by patients affected by schizophrenia are not exclusively reflective of degraded amygdalar function, but rather of a more extended damage that involves also those structures, such as the lateral prefrontal cortex and the anterior cingulate cortex, who receive information from (and exert control over) neurons in the amygdala [Pessoa, 2008]. Our data are consistent with this latter account.
Mediotemporo‐frontal dysconnectivity in schizophrenia may also be interpreted in terms of memory (and, in particular, episodic memory) impairments [Brebion et al., 1997; Huron et al., 1995; Kazes et al., 1999; Weiss et al., 2008]. Weiss et al. [ 2008] described patients with impairments specifically in familiarity‐based memory, a fast process which informs whether the item has been previously experienced in the absence of contextual details, but not in recollection‐based memory, a slow process which refers to the recovery of specific contextual information associated with a previously experienced item [see Yonelinas, 2002, as review]. Recently, Daselaar et al. [ 2006] tested young (≈22 years old) and older (≈70 years old) healthy volunteers and found that recollection‐based activity in the hippocampus, and the strength of its functional connection with temporoparietal and retrosplenial regions, decreased with age. On the other hand, familiarity‐based activity in the rihinal cortex, and the strength of its functional connection with prefrontal regions, increased with age. The evidence that the rihinal‐prefrontal network is implicated in familiarity‐based memory [Daselaar et al., 2006], together with reports of selective impairment of familiarity‐based memory in schizophrenia [Weiss et al., 2008], is suggestive of a rihinal‐prefrontal dysconnectivity in patients affected by this psychiatric syndrome. Our data support this evidence. Furthermore, not only our previous study reported volume reduction of the entorhinal cortex (but not in the hippocampus) in patients affected by schizophrenia [Baiano et al., 2008], but we also report affected correlation between the entorhinal cortex of these same patients and the DLPFC.
Limitations of the Study and Future Directions
Although correlation/covariation patterns within regional volumetric measures appear to be consistent with known anatomical connectivity data [Colibazzi et al., 2008; Lerch et al., 2006; Mitelman et al., 2005], studies report absent relation between brain structures, which are known to be structurally interconnected based on the primate anatomical literature [Mitelman et al., 2005]. Thus, anatomical connection between two regions is not sufficient for their volume to be significantly related. It has been suggested that what volumetric measures correlations instead more directly reflect primary connections between regions that exert strong, mutual trophic effects [Bullmore et al., 1997]. Furthermore, a correlation between structural properties of two regions could also be resultant from something distinct from connectivity, such as shared vulnerability to an insult in a clinical population [Mitelman et al., 2005]. In our case, the exclusion of alternative reasons is strengthened by the fact that the correlated regions: (a) are anatomically/functionally connected, as revealed by DTI and functional neuroimaging evidence and (b) are engaged in cognitive/emotional processes, which, if dysfunctional, might lead to known schizophrenia symptoms.
Second, caution should also be taken in comparing data from the current VBM study to that from previous studies using different volumetric measures [e.g., Agarwal et al., 2008; Baiano et al., 2008; Mitelman et al., 2005; Tomasino et al., 2011]. In particular, it has been argued that although VBM has the methodological advantage of semiautomatic and operator‐independent processing and seems more sensitive in capturing focal changes, it might not be as sensitive as standard manual volumetry in terms of detecting changes in brain areas with large anatomical variability [Tisserand et al., 2002]. From this perspective, the anatomically constrained morphometric principle adopted for our ROIs (see Materials and Methods section) is significant, allowing us to base our SEM analysis only on those voxels in which the differences between the two groups are captured by VBM.
Third, our data are based on a model that oversimplifies the dysconnectivity pattern which might be found in schizophrenia. The first simplification pertains to the number of regions included in our network, which is (a) a subportion of the regions previously described as exhibiting structural and functional abnormalities in schizophrenia and (b) limited to the right hemisphere. This choice was justified by the fact that our five regions were only identified as exhibiting structural anomalies in the population of patients included in our analysis, as revealed by both the VBM analysis described here, and by previous volumetric measures on the same dataset [Agarwal et al., 2008; Baiano et al., 2008; Tomasino et al., 2011]. In all but one case, the reduction was the largest in the right hemisphere. Therefore, it should be stressed that our results do not exclude the presence of additional dysconnectivity patterns pertaining regions which either were found dysfunctional by other research groups or might not be dysfunctional per se but simply in their interactions with other neural structures. Further studies will address this issue.
The second simplification pertains the nature of the connections that, due to mathematical constraints of the modeling procedure, were restricted to be unidirectional and to nonrecursive structures. Both of these constraints have weak biological justifications, making more pertinent the argument that the dysconnectivity pattern presented here might not match in complexity what that which is the real pattern. Further caution should be taken in interpreting the direction of the paths in the estimated models; in contrast to functional connectivity measures, which are endowed with temporal information about neural signal change, volumetric correlation between brain regions is the result of mutual trophic exchanges during lifetime, thus yielding no reliable information about the main direction of such connection. In SEM, direction is usually selected on the basis of a priori knowledge about the network of interest or like in the case of the present and previous studies [e.g., Stein et al., 2007; Zhuang et al., 2005], by the identification of one, among many competing models, which maximizes a global scalar index. This strategy, however, cannot be considered as a direct measure of connection directionality. These limitations might be overcome by further research with functional neuroimaging techniques, which will not only allow the implementation of more complex networks but also allow to link putative dysconnectivity patterns with on‐line patients' performance in cognitive/affective tasks.
Acknowledgements
The authors thank Elizabeth Clark‐Polner for proofreading the manuscript.
REFERENCES
- Agarwal N, Rambaldelli G, Perlini C, Dusi N, Kitis O, Bellani M, Cerini R, Isola M, Versace A, Balestrieri M, Gasparini A, Mucelli RP, Tansella M, Brambilla P ( 2008): Microstructural thalamic changes in schizophrenia: A combined anatomic and diffusion weighted magnetic resonance imaging study. J Psychiatry Neurosci 33: 440–448. [PMC free article] [PubMed] [Google Scholar]
- Allen GV, Saper CB, Hurley KM, Cechetto DF ( 1991): Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol 311: 1–16. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association ( 2000): Diagnostic and Statistical Manual of Mental Disorders DSM‐IV‐TR, 4th ed Washington DC: American Psychiatric Publishing, Inc; Joreskog and Wold, 1982. [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K ( 2005): Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol 210: 343–352. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Arndt S, Swayze V, Cizadlo T, Flaum M, O'Leary D, Ehrhardt JC, Yuh WT ( 1994): Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science 266: 294–298. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD ( 1996): Schizophrenia and cognitive dysmetria: A positron‐emission tomography study of dysfunctional prefrontal‐thalamic‐cerebellar circuitry. Proc Natl Acad Sci USA 93: 9985–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreone N, Tansella M, Cerini R, Versace A, Rambaldelli G, Perlini C, Dusi N, Pelizza L, Balestrieri M, Barbui C, Nosè M, Gasparini A, Brambilla P ( 2007): Cortical white‐matter microstructure in schizophrenia. Diffusion imaging study. Br J Psychiatry 191: 113–119. [DOI] [PubMed] [Google Scholar]
- Andrews J, Wang L, Csernansky JG, Gado MH, Barch DM ( 2006): Abnormalities of thalamic activation and cognition in schizophrenia. Am J Psychiatry 163: 463–469. [DOI] [PubMed] [Google Scholar]
- Apkarian A, Bushnell M, Treede R, Zubieta J ( 2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484. [DOI] [PubMed] [Google Scholar]
- Baiano M, Perlini C, Rambaldelli G, Cerini R, Dusi N, Bellani M, Spezzapria G, Versace A, Balestrieri M, Mucelli RP, Tansella M, Brambilla P ( 2008): Decreased entorhinal cortex volumes in schizophrenia. Schizophr Res 102: 171–180. [DOI] [PubMed] [Google Scholar]
- Bellani M, Perlini C, Brambilla P, ( 2009c): Language disturbances in schizophrenia. Epidemiol Psichiatr Soc. 18: 314–317. [PubMed] [Google Scholar]
- Bellani M, Fagnani C, Brambilla P ( 2009a): Twin studies in psychotic disorders. Epidemiol Psichiatr Soc 18: 195–199. [PubMed] [Google Scholar]
- Bellani M, Tomelleri L, Brambilla P ( 2009b): Emotion‐based decision making in schizophrenia: Evidence from the Iowa Gambling Task. Epidemiol Psichiatr Soc 18: 104–106. [PubMed] [Google Scholar]
- Blakemore S, Rees G, Frith CD ( 1998): How do we predict the consequences of our actions? A functional imaging study. Neuropsychologia 36: 521–529. [DOI] [PubMed] [Google Scholar]
- Blakemore S, Smith J, Steel R, Johnstone CE, Frith CD ( 2000): The perception of self‐produced sensory stimuli in patients with auditory hallucinations and passivity experiences: Evidence for a breakdown in self‐monitoring. Psychol Med 30: 1131–1139. [DOI] [PubMed] [Google Scholar]
- Bonnot O, Anderson GM, Cohen D, Willer JC, Tordjman S ( 2009): Are patients with schizophrenia insensitive to pain? A reconsideration of the question. Clin J Pain 25: 244–252. [DOI] [PubMed] [Google Scholar]
- Brebion G, Smith MJ, Amador X, Malaspina D, Gorman JM ( 1997): Clinical correlates of memory in schizophrenia: Differential links between depression, positive and negative symptoms, and two types of memory impairment. Am J Psychiatry 154: 1538–1543. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Frangou S, Murray RM ( 1997): The dysplastic net hypothesis: An integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res 28: 143–156. [DOI] [PubMed] [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, Lawrie SM ( 2003): Structural disconnectivity in schizophrenia: A diffusion tensor magnetic resonance imaging study. Br J Psychiatry 182: 439–443. [PubMed] [Google Scholar]
- Burton H, Jones EG ( 1976): The posterior thalamic region and its cortical projection in new world and old world monkeys. J Comp Neurol 168: 249–301. [DOI] [PubMed] [Google Scholar]
- Byne W, Kidkardnee S, Tatusov A, Yiannoulos G, Buchsbaum MS, Haroutunian V ( 2006): Schizophrenia‐associated reduction of neuronal and oligodendrocyte numbers in the anterior principal thalamic nucleus. Schizophr Res 85: 245–253. [DOI] [PubMed] [Google Scholar]
- Camchong J, Dyckman KA, Chapman CE, Yanasak NE, McDowell JE ( 2006): Basal ganglia‐thalamocortical circuitry disruptions in schizophrenia during delayed response tasks. Biol Psychiatry 60: 235–241. [DOI] [PubMed] [Google Scholar]
- Chan RCK, Di X, McAlonan GM, Gong Q ( 2011): Brain anatomical abnormalities in high‐risk individuals, first‐episode, and chronic schizophrenia: An activation likelihood estimation meta‐analysis of illness progression. Schizophr Bull (in press). 27: 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clascá F, Llamas A, Reinoso‐Suárez F ( 1997): Insular cortex and neighboring fields in the cat: A redefinition based on cortical microarchitecture and connections with the thalamus. J Comp Neurol 384: 456–482. [DOI] [PubMed] [Google Scholar]
- Colibazzi T, Zhu H, Bansal R, Schultz RT, Wang Z, Peterson BS ( 2008): Latent volumetric structure of the human brain: Exploratory factor analysis and structural equation modeling of gray matter volumes in healthy children and adults. Hum Brain Mapp 29: 1302–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC ( 1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Corradi‐Dell'Acqua C, Ueno K, Ogawa A, Cheng K, Rumiati RI, Iriki A ( 2008): Effects of shifting perspective of the self: An fMRI study. Neuroimage 40: 1902–1911. [DOI] [PubMed] [Google Scholar]
- Craig ADB ( 2009): How do you feel—Now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70. [DOI] [PubMed] [Google Scholar]
- Crespo‐Facorro B, Kim J, Andreasen NC, O'Leary DS, Bockholt HJ, Magnotta V ( 2000): Insular cortex abnormalities in schizophrenia: A structural magnetic resonance imaging study of first‐episode patients. Schizophr Res 46: 35–43. [DOI] [PubMed] [Google Scholar]
- Curtis VA, Bullmore E, Brammer MJ, Wright IC, Williams SC, Morris RG, Sharma TS, Murray RM, McGuire PK ( 1998): Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry 155: 1056–1063. [DOI] [PubMed] [Google Scholar]
- Dasari M, Friedman L, Jesberger J, Stuve TA, Findling RL, Swales TP, Schulz SC ( 1999): A magnetic resonance imaging study of thalamic area in adolescent patients with either schizophrenia or bipolar disorder as compared to healthy controls. Psychiatry Res 91: 155–162. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R ( 2006): Effects of healthy aging on hippocampal and rhinal memory functions: An event‐related fMRI study. Cereb Cortex 16: 1771–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SW ( 2000): Exploring the pain “neuromatrix”. Curr Pain Headache Rep 4: 467–477. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A ( 2004): Neuroplasticity: Changes in grey matter induced by training. Nature 427: 311–312. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Ellison‐Wright I, Bullmore E ( 2010): Anatomy of bipolar disorder and schizophrenia: A meta‐analysis. Schizophr Res 117: 1–12. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Picchioni M, Landau S, Matsumoto K, van Haren NE, Marshall N, Hall M, Schulze K, Toulopoulou T, Davies N, Ribchester T, McGuire PK, Murray RM ( 2007): Magnetic resonance imaging of the thalamus and adhesio interthalamica in twins with schizophrenia. Arch Gen Psychiatry 64: 401–409. [DOI] [PubMed] [Google Scholar]
- Evans AC, Marrett S, Neelin P, Collins DL, Worsley K, Dai W, Milot S, Meyer E, Bub D ( 1992): Anatomical mapping of functional activation in stereotactic coordinate space. Neuroimage 1: 43–53. [DOI] [PubMed] [Google Scholar]
- Evans AC, Kamber M, Collins DL, MacDonald D ( 1994): An MRI based probabilistic atlas of neuroanatomy In Shorvon S, Fish D, Andermann F, Byder GM, editors. Magnetic resonance scanning and epilepsy. New York: Plenum; pp 263–274. [Google Scholar]
- Exner C, Boucsein K, Degner D, Irle E, Weniger G ( 2004): Impaired emotional learning and reduced amygdala size in schizophrenia: A 3‐month follow‐up. Schizophr Res 71: 493–503. [DOI] [PubMed] [Google Scholar]
- Farrer C, Frith CD ( 2002): Experiencing oneself vs another person as being the cause of an action: The neural correlates of the experience of agency. Neuroimage 15: 596–603. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Frith CD, Decety J, Georgieff N, d'Amato T, Jeannerod M ( 2004): Neural correlates of action attribution in schizophrenia. Psychiatry Res 131: 31–44. [DOI] [PubMed] [Google Scholar]
- Flaum M, Swayze VW, O'Leary DS, Yuh WT, Ehrhardt JC, Arndt SV, Andreasen NC ( 1995): Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry 152: 704–714. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, McAvoy MP, Barch DM, Raichle ME ( 2005): The BOLD onset transient: Identification of novel functional differences in schizophrenia. Neuroimage 25: 771–782. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Murray EA ( 1986): Thalamic connectivity of the second somatosensory area and neighboring somatosensory fields of the lateral sulcus of the macaque. J Comp Neurol 252: 348–373. [DOI] [PubMed] [Google Scholar]
- Friston KJ ( 1998): The disconnection hypothesis. Schizophr Res 30: 115–125. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD ( 1995): Schizophrenia: A disconnection syndrome? Clin Neurosci 3: 89–97. [PubMed] [Google Scholar]
- Geuze E, Westenberg HGM, Jochims A, de Kloet CS, Bohus M, Vermetten E, Schmahl C ( 2007): Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry 64: 76–85. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS ( 2001): Thalamic volumes in patients with first‐episode schizophrenia. Am J Psychiatry 158: 618–624. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ ( 1988): Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal‐prefrontal topography. Neuroscience 24: 379–431. [DOI] [PubMed] [Google Scholar]
- Guldin WO, Markowitsch HJ ( 1983): Cortical and thalamic afferent connections of the insular and adjacent cortex of the rat. J Comp Neurol 215: 135–153. [DOI] [PubMed] [Google Scholar]
- Gur RC, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC ( 1998): Subcortical MRI volumes in neuroleptic‐naive and treated patients with schizophrenia. Am J Psychiatry 155: 1711–1717. [DOI] [PubMed] [Google Scholar]
- Gur RC, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, Kohler C, Alsop D, Maldjian J, Ragland JD, Gur RC ( 2002): An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry 159: 1992–1999. [DOI] [PubMed] [Google Scholar]
- Hihara S, Notoya T, Tanaka M, Ichinose S, Ojima H, Obayashi S, Fujii N, Iriki A ( 2006): Extension of corticocortical afferents into the anterior bank of the intraparietal sulcus by tool‐use training in adult monkeys. Neuropsychologia 44: 2636–2646. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins DL, Woods R, Toga AW, Evans AC ( 1998): Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22: 324–333. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, D'Angelo D, Catalano D, Mauro CJ, Shehzad ZE, Kelly AMC, Castellanos FX, Javitt DC, Milham MP ( 2010): Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophr Bull 36: 1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huron C, Danion JM, Giacomoni F, Grangé D, Robert P, Rizzo L ( 1995): Impairment of recognition memory with, but not without, conscious recollection in schizophrenia. Am J Psychiatry 152: 1737–1742. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Lerch J, Norton A, Forgeard M, Winner E, Evans AC, Schlaug G ( 2009): Musical training shapes structural brain development. J Neurosci 29: 3019–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang D, Kim J, Chung T, An SK, Jung YC, Lee J, Lee J, Kim I, Kim SI ( 2006): Shape deformation of the insula in schizophrenia. Neuroimage 32: 220–227. [DOI] [PubMed] [Google Scholar]
- Jones EG, Burton H ( 1976): Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal and temporal regions of primates. J Comp Neurol 168: 197–247. [DOI] [PubMed] [Google Scholar]
- Joreskog KG, Wold H ( 1982): The ML and PLS techniques for modeling with latent variables: Historical and comparative aspects In Joreskog KG, Wold H, editors. Systems Under Indirect Observation. Amsterdam: Elsevier Science Ltd; pp 263–270. [Google Scholar]
- Joyal CC, Laakso MP, Tiihonen J, Syvälahti E, Vilkman H, Laakso A, Alakare B, Räkköläinen V, Salokangas RKR, Hietala J ( 2002): A volumetric MRI study of the entorhinal cortex in first episode neuroleptic‐naive schizophrenia. Biol Psychiatry 51: 1005–1007. [DOI] [PubMed] [Google Scholar]
- Kazes M, Berthet L, Danion J, Amado I, Willard D, Robert P, Poirier M ( 1999): Impairment of consciously controlled use of memory in schizophrenia. Neuropsychology 13: 54–61. [DOI] [PubMed] [Google Scholar]
- Kim J, Youn T, Lee JM, Kim IY, Kim SI, Kwon JS ( 2003): Morphometric abnormality of the insula in schizophrenia: A comparison with obsessive‐compulsive disorder and normal control using MRI. Schizophr Res 60: 191–198. [DOI] [PubMed] [Google Scholar]
- Kosaka H, Omori M, Murata T, Iidaka T, Yamada H, Okada T, Takahashi T, Sadato N, Itoh H, Yonekura Y, Wada Y ( 2002): Differential amygdala response during facial recognition in patients with schizophrenia: An fMRI study. Schizophr Res 57: 87–95. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Westin C, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME ( 2002): Uncinate fasciculus findings in schizophrenia: A magnetic resonance diffusion tensor imaging study. Am J Psychiatry 159: 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin C, Nestor PG, Wible CG, Frumin M, Maier SE, Kikinis R, Jolesz FA, McCarley RW, Shenton ME ( 2003): Cingulate fasciculus integrity disruption in schizophrenia: A magnetic resonance diffusion tensor imaging study. Biol Psychiatry 54: 1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Liu R, Aghajanian GK ( 2007): Schizophrenia, hypocretin (orexin), and the thalamocortical activating system. Schizophr Bull 33: 1284–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC ( 2002): Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry 51: 1008–1011. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC ( 2006): Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage 31: 993–1003. [DOI] [PubMed] [Google Scholar]
- Mandl RCW, Schnack HG, Luigjes J, van den Heuvel MP, Cahn W, Kahn RS, Hulshoff Pol HE ( 2010): Tract‐based analysis of magnetization transfer ratio and diffusion tensor imaging of the frontal and frontotemporal connections in schizophrenia. Schizophr Bull 36: 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J ( 2005a): Voxel‐based morphometry of the human brain: Methods and applications. Curr Med Imaging Rev 1: 105–113. [Google Scholar]
- Mechelli A, Friston KJ, Frackowiak RS, Price CJ ( 2005b): Structural covariance in the human cortex. J Neurosci 25: 8303–8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer‐Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF ( 2005): Regionally specific disturbance of dorsolateral prefrontal‐hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 62: 379–386. [DOI] [PubMed] [Google Scholar]
- Minciacchi D, Bentivoglio M, Molinari M, Kultas‐Ilinsky K, Ilinsky IA, Macchi G ( 1986): Multiple cortical targets of one thalamic nucleus: The projections of the ventral medial nucleus in the cat studied with retrograde tracers. J Comp Neurol 252: 106–129. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Buchsbaum MS, Brickman AM, Shihabuddin L ( 2005): Cortical intercorrelations of frontal area volumes in schizophrenia. Neuroimage 27: 753–770. [DOI] [PubMed] [Google Scholar]
- Moisset X, Bouhassira D, Ducreux D, Glutron D, Coffin B, Sabaté J ( 2010): Anatomical connections between brain areas activated during rectal distension in healthy volunteers: A visceral pain network. Eur J Pain 14: 142–148. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM ( 1984): Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol 227: 109–120. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B ( 1990): Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry 47: 1023–1028. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Andreasen NC, Crespo‐Facorro B, O'Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD ( 2003): Emotions in unmedicated patients with schizophrenia during evaluation with positron emission tomography. Am J Psychiatry 160: 1775–1783. [DOI] [PubMed] [Google Scholar]
- Parker GJM, Luzzi S, Alexander DC, Wheeler‐Kingshott CAM, Ciccarelli O, Lambon Ralph MA ( 2005): Lateralization of ventral and dorsal auditory‐language pathways in the human brain. Neuroimage 24: 656–666. [DOI] [PubMed] [Google Scholar]
- Pell GS, Briellmann RS, Chan CH, Pardoe H, Abbott DF, Jackson GD ( 2008): Selection of the control group for VBM analysis: Influence of covariates, matching and sample size. Neuroimage 41: 1324–1335. [DOI] [PubMed] [Google Scholar]
- Pessoa L ( 2008): On the relationship between emotion and cognition. Nat Rev Neurosci 9: 148–158. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams L, Senior C, Bullmore E, Brammer MJ, Andrew C, Williams SCR, David AS ( 1999): A differential neural response to threatening and non‐threatening negative facial expressions in paranoid and non‐paranoid schizophrenics. Psychiatry Res: Neuroimaging 92: 11–31. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Bunney WE, Potkin SG, Jones EG ( 2000): Subnucleus‐specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci USA 97: 9276–9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Marchand S ( 2008): Hypoalgesia in schizophrenia is independent of antipsychotic drugs: A systematic quantitative review of experimental studies. Pain 138: 70–78. [DOI] [PubMed] [Google Scholar]
- Rainville P ( 2002): Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol 12: 195–204. [DOI] [PubMed] [Google Scholar]
- Ray JP, Price JL ( 1992): The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain‐prefrontal cortex topography. J Comp Neurol 323: 167–197. [DOI] [PubMed] [Google Scholar]
- Roy M, Piché M, Chen J, Peretz I, Rainville P ( 2009): Cerebral and spinal modulation of pain by emotions. Proc Natl Acad Sci USA 106: 20900–20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB ( 1982): Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol 210: 163–173. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Harris GJ, Tien AY, Peng LW, Lee S, Federman EB, Chase GA, Barta PE, Pearlson GD ( 1994): Decreased regional cortical gray matter volume in schizophrenia. Am J Psychiatry 151: 842–848. [DOI] [PubMed] [Google Scholar]
- Schneider F, Weiss U, Kessler C, Salloum JB, Posse S, Grodd W, Müller‐Gärtner HW ( 1998): Differential amygdala activation in schizophrenia during sadness. Schizophr Res 34: 133–142. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Reske M, Kellermann T, Stöcker T, Shah NJ, Zilles K, Braus DF, Schmitt A, Schlösser R, Wagner M, Frommann I, Kircher T, Rapp A, Meisenzahl E, Ufer S, Ruhrmann S, Thienel R, Sauer H, Henn FA, Gaebel W ( 2007): Neural correlates of working memory dysfunction in first‐episode schizophrenia patients: An fMRI multi‐center study. Schizophr Res 89: 198–210. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM ( 2005): Evidence for sensory prediction deficits in schizophrenia. Am J Psychiatry 162: 2384–2386. [DOI] [PubMed] [Google Scholar]
- Sommer IEC, Diederen KMJ, Blom J, Willems A, Kushan L, Slotema K, Boks MPM, Daalman K, Hoek HW, Neggers SFW, Kahn RS ( 2008): Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain 131: 3169–3177. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW ( 2004): Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA 101: 17288–17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal WG, Hulshoff Pol HE, Schnack H, van der Schot AC, Kahn RS ( 1998): Partial volume decrease of the thalamus in relatives of patients with schizophrenia. Am J Psychiatry 155: 1784–1786. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer‐Lindenberg A ( 2007): A validated network of effective amygdala connectivity. Neuroimage 36: 736–745. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ ( 2006): Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry 59: 929–939. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD ( 2009): Dysconnection in schizophrenia: From abnormal synaptic plasticity to failures of self‐monitoring. Schizophr Bull 35: 509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L ( 2002): The hypocretins: Setting the arousal threshold. Nat Rev Neurosci 3: 339–349. [DOI] [PubMed] [Google Scholar]
- Symond MP, Symond MB, Harris AWF, Gordon E, Williams LM ( 2005): “Gamma synchrony” in first‐episode schizophrenia: A disorder of temporal connectivity? Am J Psychiatry 162: 459–465. [DOI] [PubMed] [Google Scholar]
- Tansella M, Burti L ( 2003): Integrating evaluative research and community‐based mental health care in Verona, Italy. Br J Psychiatry 183: 167–169. [DOI] [PubMed] [Google Scholar]
- Tenenhaus M, Vinzi VE, Chatelin Y, Lauro C ( 2005): PLS path modeling. Comput Stat Data Anal 48: 159–205. [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB ( 2002): Regional frontal cortical volumes decrease differentially in aging: An MRI study to compare volumetric approaches and voxel‐based morphometry. Neuroimage 17: 657–669. [PubMed] [Google Scholar]
- Tomasino B, Bellani M, Perlini C, Rambaldelli G, Cerini R, Isola M, Balestrieri M, Calì S, Versace A, Pozzi Mucelli R, Gasparini A, Tansella M, Brambilla P ( 2011): Altered microstructure integrity of the amygdala in schizophrenia: A bimodal MRI and DWI study. Psychol Med 41: 301–311. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP ( 1993): Training and quality assurance with the Brief Psychiatric Rating Scale: “The drift busters.” Int J Methods Psychiatr Res 3: 221–244. [Google Scholar]
- Voineskos AN, Lobaugh NJ, Bouix S, Rajji TK, Miranda D, Kennedy JL, Mulsant BH, Pollock BG, Shenton ME ( 2010): Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain 133( Part 5): 1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AP, Goff DC, Duff M, Roffman JL, Schacter DL ( 2008): Distinguishing familiarity‐based from source‐based memory performance in patients with schizophrenia. Schizophr Res 99: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible CG, Shenton ME, Hokama H, Kikinis R, Jolesz FA, Metcalf D, McCarley RW ( 1995): Prefrontal cortex and schizophrenia. A quantitative magnetic resonance imaging study. Arch Gen Psychiatry 52: 279–288. [DOI] [PubMed] [Google Scholar]
- Wible CG, Anderson J, Shenton ME, Kricun A, Hirayasu Y, Tanaka S, Levitt JJ, O'Donnell BF, Kikinis R, Jolesz FA, McCarley RW ( 2001): Prefrontal cortex, negative symptoms, and schizophrenia: An MRI study. Psychiatry Res: Neuroimaging 108: 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold H ( 1982): Soft modeling: The basic design and some extensions In Joreskog KG, Wold H, editors. Systems Under Indirect Observation. Amsterdam: Elsevier Science Ltd; pp 1–54. [Google Scholar]
- Wolf RC, Vasic N, Sambataro F, Höse A, Frasch K, Schmid M, Walter H ( 2009): Temporally anticorrelated brain networks during working memory performance reveal aberrant prefrontal and hippocampal connectivity in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 33: 1464–1473. [DOI] [PubMed] [Google Scholar]
- Woodruff PW, Wright IC, Shuriquie N, Russouw H, Rushe T, Howard RJ, Graves M, Bullmore E, Murray RM ( 1997): Structural brain abnormalities in male schizophrenics reflect fronto‐temporal dissociation. Psychol Med 27: 1257–1266. [DOI] [PubMed] [Google Scholar]
- Wright IC, Ellison ZR, Sharma T, Friston KJ, Murray RM, McGuire PK ( 1999): Mapping of grey matter changes in schizophrenia. Schizophr Res 35: 1–14. [DOI] [PubMed] [Google Scholar]
- Yeh P, Zhu H, Nicoletti MA, Hatch JP, Brambilla P, Soares JC ( 2010): Structural equation modeling and principal component analysis of gray matter volumes in major depressive and bipolar disorders. Psychiatry Res 184: 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP ( 2002): The nature of recollection and familiarity: A review of 30 years of research. J Mem Lang 46: 441–517. [Google Scholar]
- Young KA, Manaye KF, Liang C, Hicks PB, German DC ( 2000): Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psychiatry 47: 944–953. [DOI] [PubMed] [Google Scholar]
- Zhuang J, LaConte S, Peltier S, Zhang K, Hu X ( 2005): Connectivity exploration with structural equation modeling: An fMRI study of bimanual motor coordination. Neuroimage 25: 462–470. [DOI] [PubMed] [Google Scholar]


