Abstract
A growing number of studies suggest that early visual processing is not only affected by low‐level perceptual attributes but also by higher order cognitive factors such as attention or emotion. Using high‐density electroencephalography, we recently demonstrated that attentional load of a task at fixation reduces the response of primary visual cortex to irrelevant peripheral stimuli, as indexed by the C1 component. In the latter study, peripheral stimuli were always presented during intervals without task‐relevant stimuli. Here, we use a similar paradigm but present central task stimuli and irrelevant peripheral stimuli simultaneously while keeping all other stimulus characteristics constant. Results show that rather than to suppress responses to peripheral stimulation, high attentional load elicits higher C1 amplitudes under these conditions. These findings suggest that stimulus timing can profoundly alter the effects of attentional load on the earliest stages of processing in human visual cortex. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: C1, early vision, EEG, V1
INTRODUCTION
A growing body of evidence indicates that even the earliest stages of cortical visual processing in humans may be modified by higher level factors such as emotion [Pourtois et al.,2004; Stolarova et al.,2006], learning [Pourtois et al.,2008; Schwartz et al.,2002], or attention [Kelly et al.,2008; Rauss et al.,2009; Schwartz et al.,2005]. Although early attentional effects are in accordance with results from animal experiments [Crist et al.,2001; Gilbert and Sigman,2007; Gilbert et al.,2000], previous electroencephalography (EEG) studies in humans found effects of attention in primary visual cortex (V1) only at a later latencies, suggesting delayed feedback influences [Di Russo et al.,2003; Heinze et al.,1994; Hillyard et al.,1998; Martinez et al.,1999; Noesselt et al.,2002]. Reasons for these inconsistent findings may partly lie in the large variability of human visual cortex anatomy [Amunts et al.,2000; Dougherty et al.,2003; Hasnain et al.,1998] that potentially precludes adequate overlap of individual EEG topographies [Kelly et al.,2008] as well as stimulation parameters not optimally adapted to V1 characteristics [for detailed discussions, see Pourtois et al.,2008; Rauss et al.,2009].
In a previous visual‐evoked potential (VEP) experiment [Rauss et al.,2009], we manipulated the attentional load [Lavie,1995,2005; Lavie et al.,2004] of a central task while presenting occasional irrelevant stimuli in the periphery in either the upper or the lower visual field. Using high‐density EEG, we observed that the C1 component elicited by such peripheral probes in the upper visual field was reduced under conditions of high attentional load in the central task. This result suggests a very early reduction of neural activity elicited by peripheral stimulation as a function of the attentional demands of a task at fixation.
In this study, we further explored the boundary conditions of such early load effects. We asked whether neural activity elicited by peripheral stimuli would still be reduced under high attentional load when peripheral probes are presented simultaneously with central task stimuli. As foveally presented stimuli do not elicit a reliable C1 component in the majority of subjects [Clark et al.,1995; Jeffreys and Axford,1972], simultaneous vs. nonsimultaneous presentation of central and peripheral stimuli should not per se affect this first component of the VEP. This allowed us to test the effects of stimulus timing on early visual processing while leaving other low‐level stimulus characteristics as well as higher‐level task demands constant with respect to our earlier experiment [Rauss et al.,2009].
Several theoretical accounts suggest that such a change in stimulus timing could disrupt the load‐induced reduction in C1 amplitudes we observed previously [Rauss et al.,2009]. For example, a simultaneously presented, task‐irrelevant stimulus could interfere more strongly with the processing of task‐relevant stimuli than a nonsimultaneously presented one because of automatic capture of attention [Kahneman et al.,1983; Khoe et al.,2005; Olivers and Nieuwenhuis,2005; Santangelo and Spence,2007; Van der Burg et al.,2008a]. Alternatively, the visual system may use the temporal structure of a given task to gate the processing of incoming stimuli [Correa et al.,2006; Nobre et al.,2007], such that increased attentional demands (e.g., high load) lead to increased focusing on the expected onset time of task‐relevant stimuli. This ramping‐up of attentional resources could in turn modulate the processing of simultaneously presented irrelevant stimuli. Independent of the underlying mechanisms, if attentional effects on early visual processing were modulated by stimulus timing, this could explain some of the inconsistencies in the literature noted above. It would also entail that future studies should take into account not just the spatial but also the temporal structure of their stimulation protocols to adequately assess the characteristics of early visual processing.
METHODS
Subjects
Nineteen healthy, right‐handed participants without neurological or psychiatric history gave written informed consent to participate in the study, which was conducted in accordance with the Declaration of Helsinki and approved by the local ethics committee. Data from five subjects had to be excluded because of poor behavioral performance (i.e., error rates were two standard deviations above the mean of all participants) and/or excessive artifacts on the EEG recordings. The remaining 14 participants (11 females) were aged between 21 and 31 (median: 26).
Stimuli
Visual stimuli were presented using Cogent (http://www.vislab.ucl.ac.uk/Cogent2000), a MATLAB toolbox allowing precise timing and synchronization with the EEG system, and shown on a 17″ CRT screen (viewing distance 40 cm, refresh cycle 60 Hz). T‐shapes of six different colors (blue, green, red, rose, violet, and yellow) and two orientations (upright vs. upside‐down) were rapidly presented at the bottom of the screen (1.35° × 1.90° of visual angle; Fig. 1A). Task‐irrelevant arrays of white horizontal line elements were flashed in the periphery (∼41° × 18°, distance from upper edge of task stimuli ∼3.8°) on 22% of central events. Peripheral probe stimuli were presented simultaneously with the T‐shaped central task stimuli, unlike in our previous study where they were interleaved with central task stimuli [Rauss et al.,2009]. Stimuli were presented for 500 ms, followed by a blank screen of variable duration (250–393 ms). The screen background remained black throughout the experiment. Figure 1 shows a single‐trial time course.
Figure 1.
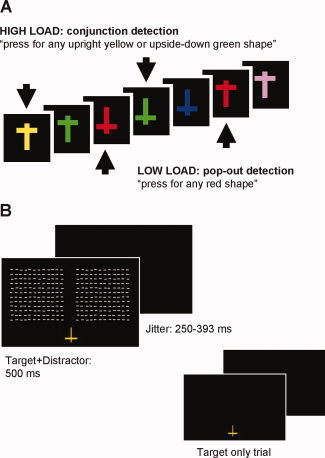
Experimental paradigm. A: During EEG recording, subjects performed either an easy (color pop‐out detection) or a more demanding (color/orientation conjunction detection) task on the same stream of centrally presented stimuli. B: Irrelevant probes were presented simultaneously with the central stimulus on 22% of trials, with approximately equal proportions of target and nontarget central stimuli accompanied by peripheral probes (see Methods). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
As poorer performance was expected for synchronous than asynchronous task‐probe presentation [Kahneman et al.,1983], we adapted stimulus duration so as to equate difficulty with our previous study [Rauss et al.,2009]: central task stimuli (and thus peripheral probes) were presented for 500 ms in this study, whereas they were presented for 250 ms each in our previous experiment. In addition, we used peripheral stimuli covering the whole vertical extent of the screen, whereas different eccentricities were tested with smaller probes in our previous study.
Procedure
Participants sat in a quiet, dimly lit and electrically shielded recording booth. Four blocks of 410 trials were presented yielding 60 probe trials of interest per load condition. At the beginning of each block, participants were instructed to press the space bar of a standard computer keyboard either (i) if they saw an upright or upside‐down red T‐shape (pop‐out detection, low attentional load) or (ii) if they saw an upright yellow or an upside‐down green T‐shape (conjunction discrimination, high attentional load). The two tasks were alternated across blocks, with the starting condition randomized across subjects. Pseudo‐random trains of stimuli were created for each block, with an equal number of imperative central task stimuli requiring motor responses (32) in the low‐ and high‐load conditions. Stimulus sequences were constructed such that they could be used in either load condition, effectively equating stimulus characteristics between conditions. Instructions stressed that randomly occurring stimuli in the periphery were task‐irrelevant and to be ignored. Each block lasted ∼6 min, including a short break after half of the trials had been completed.
Data Recording and Analysis
Scalp‐EEG was recorded from 62 Ag/AgCl electrodes (Neuroscan, Synamps, El Paso, TX) positioned according to the extended international 10‐20 EEG system and referenced to the tip of the nose. Signals were amplified at 30 K and bandpass‐filtered between 0.01 and 100 Hz; a 50‐Hz notch filter was applied to filter line noise. Horizontal and vertical electrooculograms (EOGs) were monitored using four bipolar electrodes. Both EEG and EOG were acquired continuously at 500 Hz.
Using Brain Vision Analyzer 1.05 (Brain Products, Munich, Germany), eye‐blink artifacts were semiautomatically corrected using the procedure described by Gratton et al. [1983]. Continuous data were then high‐pass filtered at 0.5 Hz to remove slow drifts (filter roll‐off: 12 dB/oct), and epochs from −100 to +600 ms around stimulus onset were extracted for task‐only as well as task‐plus‐probe trials. Epochs were baseline‐corrected for the 100 ms preceding stimulus‐onset and semiautomatically inspected for artifacts, using a rejection criterion of ±80 μV. Single trials were averaged for each condition, and the C1 (only on task‐plus‐probe trials), P1, and N1 components were semiautomatically identified based on their distinctive polarities, latencies, and topographic properties. Their peak amplitudes and latencies were measured in each participant for electrode sites and time windows determined from the grand averages (for details, see Results). Repeated‐measures analyses of variance (ANOVAs) were conducted on peak amplitude and latency values, with load condition, hemisphere (if applicable), and electrode locations as within‐subjects factors. Greenhouse–Geisser correction of degrees of freedom was applied where appropriate.
A local autoregressive average (LAURA) procedure [Grave de Peralta Menendez et al.,2004] was used to estimate electrical sources in the brain volume during selected periods of interest. This distributed source localization analysis does not require any a priori assumptions about the number and position of neural generators but determines the most likely configuration of activity simultaneously in a large number of solution points (4,024 in this study) evenly distributed throughout the gray matter of an individual or template brain (the MNI template in our case). Effects of attentional load were assessed via paired t‐tests conducted on the activity of each solution point in each subject across experimental conditions.
RESULTS
Behavior
Nonparametric Friedman tests were used to assess the influence of attentional load and experiment block on accuracy measures, i.e., the number of misses and false alarms. Overall error rates were low, with no subject committing more than three misses per block and only one subject committing more than 10 false alarms in one block. As expected, accuracy dropped in the high‐ compared with the low‐load condition (misses: P < 0.05; false alarms, P < 0.001, one‐tailed tests).
An ANOVA was conducted on the mean reaction‐time (RT) data, with attentional load (low vs. high) and block (first vs. second) as within‐subjects factors. As expected, the effect of attentional load was highly significant (F[1, 13] = 229.75, P < 0.001, partial η2 = 0.95), as indicated by slower detection of imperative central task stimuli in the high‐load (mean ± SE, 613 ± 9 ms) relative to the low‐load condition (481 ± 7 ms). The main effect of block and the load × block interaction were not significant (both P > 0.19), suggesting that effects of attentional load were stable during the course of the experiment.
These results are comparable with our previous study using a similar paradigm with nonsimultaneous task‐probe presentations [Rauss et al.,2009]. They confirm clear effects of the load manipulation and indicate that task difficulty was successfully equated between the two stimulation protocols (see Methods). We also examined behavioral effects of the presence of peripheral stimuli by comparing performance on target trials with and without simultaneous probe presentation. Because of the relatively low number of such events, a maximum of 24 target‐plus‐probe trials were compared to 40 target‐only trials per load condition. An ANOVA on RTs across load conditions and probe presence/absence did not indicate any effects of the latter factor (P = 0.27); nonparametric tests of the number of misses per load × probe condition remained similarly nonsignificant (P = 0.68). This indicates that the presence of peripheral probes did not impair performance on the central task.1
Task‐Only VEPs
We first analyzed VEPs elicited by nontarget stimuli appearing in the central RSVP stream. This excluded all trials that required a motor response and any false alarms. As shown in Figure 2, these non‐imperative central task stimuli elicited a conspicuous occipital P1 component [Hillyard and Anllo‐Vento,1998], followed by an occipital N1 component [Vogel and Luck,2000]. The inset shows that no clear C1 component was evident at the level of the grand averages for these foveally presented stimuli, in accordance with the known characteristics of the C1 [Clark et al.,1995; Jeffreys and Axford,1972].2
Figure 2.
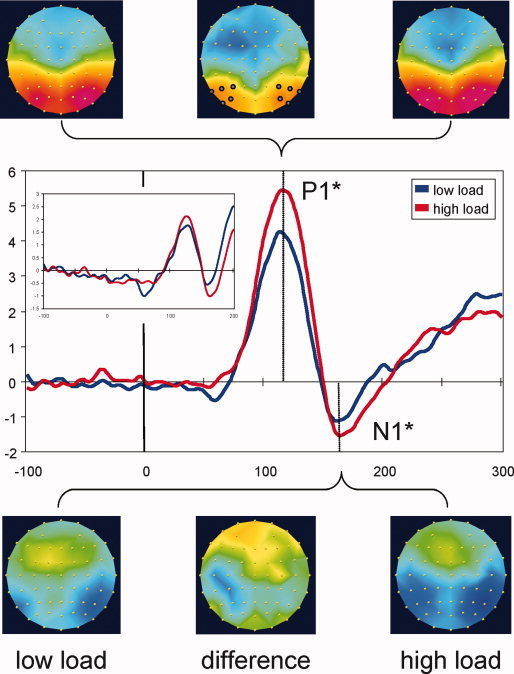
Task‐only VEPs. Waveforms show VEPs elicited by nonimperative central task stimuli in the absence of peripheral probes and represent grand averages across 10 lateral posterior electrodes highlighted on the top‐central map. Topographic maps are shown for the P1 (115 ms) and N1 (165 ms) peak latencies, for low‐load and high‐load conditions (scaled to ±5 μV) as well as their difference (high load minus low load, scaled to ±2.5 μV). N1 differences were significant when measured peak‐to‐peak (N1 minus P1). The inset shows the average across six central posterior electrodes used for C1 analyses (see below) and indicates that a C1 component was absent following foveal stimulation. *P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Following standard practice, the P1 component was measured at lateral parieto‐occipital electrodes P7, P5, P3, P4, P6, P8, PO7, PO5, PO6, and PO8. Peak amplitudes were entered into a repeated‐measures ANOVA with factors attentional load (low/high), hemisphere (left/right), and electrode (see above). Results indicated a highly significant effect of load (F[1, 13] = 1874.74, P = 0.001, partial η2 = 0.59), with high load leading to substantially higher P1 amplitudes than low load (mean ± SE = 6.17 ± 0.81 vs. 4.83 ± 0.75 μV), consistent with an early gain control mechanism acting on attended visual stimuli [Hillyard et al.,1998; Luck,1995]. Analyses of peak latencies showed no significant effect of load, and this was the case for all analyses reported below, unless noted otherwise.
Amplitude values for the N1 component, measured at the same electrodes as the P1 and analyzed using the same factors, did not show a significant effect of attentional load (P = 0.23). Considering the effect of attentional load on the preceding P1 component, we repeated N1 analyses using peak‐to‐peak measurements [Picton et al.,2000]. This complementary analysis did indicate a significant effect of attentional load (F[1, 13] = 8.41, P = 0.012, partial η2 = 0.39), suggesting that P1 differences may be responsible for the nonsignificant N1 differences at the level of absolute voltages.3
Task‐Plus‐Probe VEPs
We then analyzed trials in which the central stimulus did not require a motor response but was accompanied by an irrelevant and nonpredictive peripheral probe (see Methods). On the basis of the grand averages (Fig. 3), we selected central‐parietal and parietal electrodes CP1, CPz, CP2, P1, Pz, and P2 for analyses of the C1 component [see also Rauss et al.,2009]. This is in keeping with previous studies showing that this VEP component reaches its maximum amplitude over posterior leads along the midline, consistent with generators localized along the calcarine sulcus [Clark et al.,1995; Di Russo and Spinelli,1999; Jeffreys and Axford,1972]. Peak voltages were analyzed with attentional load (low/high) and electrode as within‐subjects factors. Results demonstrated a significant effect of load (F[1, 13] = 5.88, P = 0.031, partial η2 = 0.311), with higher C1 amplitudes under high than low load (−6.59 ± 0.76 vs. −5.07 ± 0.75 μV). Although the electrode factor was significant, it did not interact with load (F < 1) and was therefore not followed up.
We further investigated subsequent VEP components elicited by the combination of the central task and peripheral probe stimuli. Analysis of P1 amplitudes followed the same procedure as for task‐only trials, i.e., using electrodes P7, P5, P3, P4, P6, P8, PO7, PO5, PO6, and PO8 with attentional load, hemisphere, and electrode as within‐subjects factors. The effect of attentional load was nonsignificant (F < 1). There was a tendency toward higher P1 amplitudes over the right hemisphere (P = 0.064), and the electrode factor was significant, but with no interaction effect with attentional load (P > 0.1). As a C1 was evident at some of the lateral posterior electrodes used to measure the P1 component, we repeated these analyses using peak‐to‐peak measurements (P1 relative to C1). This analysis revealed that the effect of attentional load was significant (F[1,13] = 4.76, P = 0.048, partial η2 = 0.27), suggesting that differences at the level of the C1 component may have partly masked the load‐related effects on the P1 for absolute voltage values. Analyses of N1 peak amplitudes, conducted on the same set of electrodes and using the same factors as for the P1 component, did not indicate any significant main or interaction effects.4
Additional C1 Analyses
The results obtained for the C1 component are striking in that they demonstrate a reversal of the effect of attentional load on early visual processing observed in our earlier study [Rauss et al.,2009]. That is, although we previously reported reduced C1 amplitudes following peripheral stimulation under increased attentional load, the present results indicate increased C1 amplitudes to task‐irrelevant information under high attentional load if peripheral probes appear simultaneously with central task stimuli. To corroborate these findings, we performed several control analyses.
First, to test the basic assumption that C1 amplitudes were not affected by the simultaneous presentation of central task stimuli per se, we conducted analyses on the difference waveforms between task‐plus‐probe minus task‐only VEPs. Results indicated that the effect of attentional load on C1 amplitudes was still significant (F[1, 13] = 7.26, P = 0.018, partial η2 = 0.358) after controlling for activity elicited by the central task stimuli in this way.
Second, the grand averages displayed in Figure 3 suggested differences between load conditions during the prestimulus interval. We systematically explored these early differences to test whether they could have affected the early poststimulus effects described above. Running t‐tests were applied to compare load conditions across all subjects and electrodes for each time point from 100 ms prestimulus onset to 300 ms poststimulus, with a liberal significance criterion of P < 0.05 (uncorrected for multiple comparisons) and an equally liberal temporal stability criterion (≥10 ms). Results indicated significant differences at posterior electrodes throughout the 25 ms preceding stimulus onset. The next period of significant differences was the C1 interval between 45 and 70 ms poststimulus. The fact that these two intervals of significant differences were clearly separated by a period without any obvious attentional effects rules out a simple baseline shift in one of the two conditions as a likely explanation of the observed C1 effect. In addition, baseline differences were not correlated across subjects with C1 differences, either when the latter were measured across the interval of significant differences as defined by the point‐wise t‐tests (P = 0.20) or when C1 peak differences were used (P = 0.18).
Figure 3.
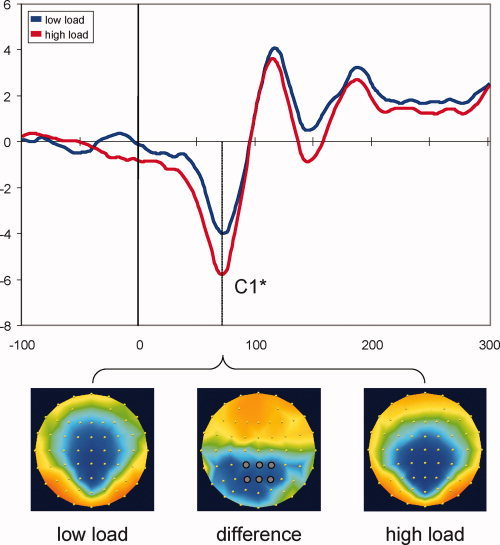
Task‐plus‐probe VEPs. Waveforms show VEPs elicited by non‐imperative task stimuli accompanied by peripheral probes, averaged across six central posterior electrodes highlighted on the central map. C1 activity is largely determined by the peripheral stimulation (compare with Fig. 2). C1 peak amplitudes differed significantly between load conditions, with higher probe‐related activity in the high‐load condition. Topographic maps are shown for the C1 peak latency (75 ms), separately for low‐ and high‐load conditions (scaled to ±5 μV), as well as their difference (high load minus low load, scaled to ±2.5 μV). *P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Third, to exclude that these correlation analyses as well as the C1 results themselves might be affected by the baseline correction procedure, we recalculated VEPs without baseline correction, using a more liberal amplitude rejection criterion of ±100 μV instead of the original ±80 μV. The C1 effect remained significant (F[1,13] = 6.58, P = 0.024, partial η2 = 0.34) in these analyses. Apart from a general shift in the baseline in both load conditions, grand averages were equivalent to those obtained in the original analyses (compare Fig. 3 with Supporting Information Fig. 1). These results establish that the load‐dependent modulation of the C1 reported above was not confounded by obvious changes during the prestimulus baseline interval across the two load conditions.
Finally, we tested whether the load‐related modulation of C1 peak amplitudes (reflecting differential processing of peripheral probes) correlated across subjects with the attentional effects observed in task‐only trials (reflecting differential processing of central stimuli). No significant correlation was observed between task‐plus‐probe C1 differences and attentional modulations of the task‐only P1 (P = 0.334). However, C1 modulations correlated positively with the effect of attentional load on task‐only N1 amplitudes, both for raw N1 voltages (r = 0.65, P = 0.011) and peak‐to‐peak measurements (see above; r = 0.58, P = 0.029). This result suggests a linear relationship between the extent of attentional focusing on the central task, as indexed by more negative N1 amplitudes, and increased processing of peripheral probes in early visual cortex under high attentional load, as indexed by increased C1 amplitudes. Interestingly, there was also a marginally significant correlation between task‐only P1 and N1 modulations (r = −0.47, P = 0.087; note that the negative coefficient is due to the components' opposite polarities). In combination with the absence of a significant correlation between C1 and task‐only P1 modulations, this finding suggests that partly distinct aspects of task‐only N1 variance relate to C1 and task‐only P1 variability, respectively.
In addition to these control analyses, we calculated inverse solutions to delimit the neural sources of the observed C1 effect. Distributed source localization analyses were calculated for each subject's task‐plus‐distractor VEPs using LAURA [Grave de Peralta Menendez et al.,2004] and subsequently averaged across conditions and subjects. Results indicated maximal activity in early visual cortex as well as anterior temporal areas during the C1 interval (45–70 ms; Fig. 4A). The latter finding is in accordance with a recent report by Plomp et al. [2010], indicating that temporal cortices may be activated very early following the onset of a visual stimulus. We then compared activity at each of the 4,024 solution points between conditions and across subjects for each time point using a running t‐test. Finally, we averaged t‐values across the time interval of interest (i.e., 45–70 ms) and applied a liberal significance criterion of P < 0.10. As shown in Figure 4B, maximal differences were observed in bilateral posterior cingulate cortex (PCC) stretching into the precuneus, with higher activity seen under low attentional load. Marginally significant differences were found in lateral prefrontal and temporoparietal areas of the right hemisphere, potentially corresponding to the frontal eye field (FEF; cf. Plomp et al.,2010] and temporoparietal junction (TPJ), respectively. Marginally significant differences were also seen in the cuneus, where activity appeared higher under high attentional load, in accordance with the VEP results reported above.
Figure 4.
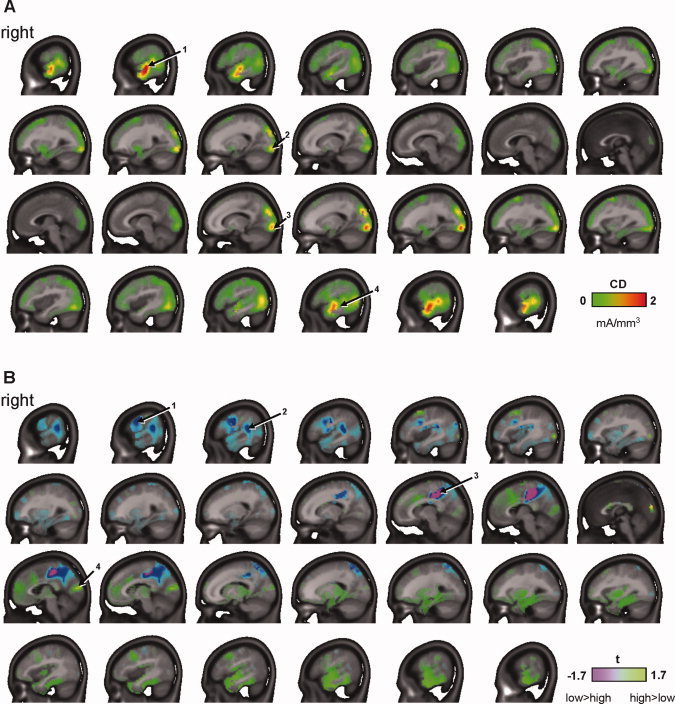
Results of distributed inverse solutions using LAURA. Sagittal slices are shown from right (top) to left (bottom). A: Inverse solutions over the C1 interval (45–70 ms), averaged across subjects and load conditions. Principal activation foci were located bilaterally in lower visual cortex (2, 3) and middle temporal cortex (1, 4). Data shown are current densities (CDs) in mA/mm3. B: Inverse solutions were compared between load conditions for each subject using paired point‐by‐point t‐tests (high minus low load) and subsequently averaged over the C1 interval and across subjects. Maps of t‐values shown are scaled to P = 0.10 for df = 13. Results indicated higher activity under low load in regions including right FEF (1), right TPJ (2), and bilateral PCC (3). In accordance with VEP results, a marginally significant increase in activity under high load was observed in the cuneus bilaterally (4). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Direct Comparisons Between Simultaneous and Nonsimultaneous Presentation Conditions
To directly assess similarities and differences between simultaneous (present experiment) and nonsimultaneous conditions [Rauss et al.,2009], we conducted additional analyses including VEP data from both studies (N = 14 each), using experiment as a between‐subjects factor.
At the behavioral level, mixed‐model ANOVAs across both experiments indicated significant main effects of attentional load on RT and performance variables, but no main or interaction effects involving the experiment factor. This result shows that task difficulty and participants' speed were successfully matched between the two studies.
We then examined the possible role of stimulus differences between the two experiments: here, full‐screen probes were employed, whereas in our previous experiment, we manipulated the eccentricity of task‐irrelevant stimulation as an additional factor, such that peripheral stimuli covered only half of the vertical extent of the screen. Considering the functional anatomy of human visual cortex and the cortical magnification of locations closer to the fovea [Slotnick et al.,2001], more eccentric stimulation should lead to activations that are weaker and less comparable across subjects than those following parafoveal stimulation [Amunts et al.,2000; Dougherty et al.,2003; Hasnain et al.,1998]. Thus, the larger vertical extent of probes used in this study should not have led to a systematic increase of C1 amplitudes. To formally examine this, we compared C1 amplitudes across all subjects from both experiments. Although the load × experiment interaction was highly significant (F[1, 26] = 11.31, P = 0.002, partial η2 = 0.30), in accordance with the reversed effect of attention on C1 amplitudes, no overall differences between C1 amplitudes in the two experiments were observed (F < 1). Accordingly, the inverted effect of attentional load observed in this study is unlikely to be due to low‐level stimulus differences or idiosyncratic differences between participants.
Finally, regarding the significant correlation between C1 and task‐only N1 modulations by attentional load, we found a similar pattern of results for our previous experiment: although there was no correlation between C1 and task‐only P1 amplitudes (r = 0.39, P = 0.17), a significantly negative relationship was observed between modulations of C1 and task‐only N1 (r = −0.55, P = 0.043). Thus, reductions in C1 amplitudes following peripheral stimulation under high attentional load were linearly related across participants to attentional focusing, as indexed by increased N1 amplitudes.
DISCUSSION
This study demonstrates that C1 amplitudes increase under high attentional load when central task stimuli and peripheral probes are presented simultaneously. These new data extend our previous work [Rauss et al.,2009] by demonstrating for the first time that the attentional load of a task at fixation may either increase or decrease C1 responses to peripheral, task‐irrelevant stimuli, depending on the relative timing of task and probe streams. More generally, our study adds to increasing evidence for attentional [Fu et al.,2009,2010; Karns and Knight,2009; Kelly et al.,2008; Poghosyan and Ioannides,2008; Rauss et al.,2009] and other top‐down influences [Pourtois et al.,2004,2008; Stolarova et al.,2006] on the earliest sweep of cortical visual‐evoked activity.
We did not observe clear load‐induced differences at the level of the P1 and N1 components following simultaneous central and peripheral stimulation. However, after removing overlap from the C1 component via peak‐to‐peak measurements, a significant difference did emerge for the task‐plus‐probe P1. As activity at extrastriate levels necessarily reflects compound processing of central task and peripheral probe stimuli, these results may be explained in terms of saturation effects. That is, attentional modulations of task processing could be masked by activity elicited by the large peripheral probes, whereas attentional modulations in probe processing may quickly dissipate beyond the level of the C1. Additional analyses on peak amplitudes from both task‐only and task‐plus‐probe VEPs (with probe presence as an additional within‐subjects factor) showed that this explanation may apply to the task‐plus‐probe P1: both the effect of probe presence (P = 0.003) and the interaction between load and probe presence (P = 0.037) were significant and together suggested a ceiling effect in the presence of peripheral probes. However, the same pattern did not emerge for the task‐plus‐probe N1 component.
Looking at VEPs elicited by the central task stimuli only, we observed a significant increase in P1 amplitudes and some evidence for differences at the level of the N1, although the latter effect was significant only when the component was measured relative to the preceding P1. Interestingly, we did not observe any P1 modulations in our previous study, although the central task stimuli used were exactly the same. Two factors could explain this discrepancy between the two studies: either the slight changes in stimulus timing (longer stimulus duration and shorter ITIs in this study, see Methods) modified the response of extrastriate visual areas to the RSVP task or the potential co‐occurrence of central and peripheral stimuli in this study led to adaptive changes in attentional gain control, e.g., to reduce interference. Considering the well‐known electrophysiological properties of the P1 [Handy et al.,2001; Hillyard and Anllo‐Vento,1998; Hopfinger and Handy,1998; Luck,1995], we are inclined to favor the latter explanation, although this issue cannot be resolved based on the present data alone.
Importantly, attentional modulations of the (task‐plus‐probe) C1 component correlated with task‐only N1 modulations across subjects. The N1 component is known to reflect target discrimination processes [Luck,1995], which presumably constitute one of the main differences between the low‐ and high‐load conditions. The fact that its modulation was linearly related to even earlier neural activity elicited by peripheral stimuli in a different class of trials suggests a functional relationship between the attentional resources deployed at fixation and the processing of irrelevant information in the periphery. The finding of a similarly significant correlation of opposite sign in our previously published experiment [Rauss et al.,2009] indicates that the nature of this link is strongly dependent on stimulus timing.
Finally, auxiliary source analyses indicated higher activity in PCC and precuneus under low attentional load as well as marginally higher neural activity in early visual cortex. Within the limits of spatial resolution offered by distributed source localization procedures, we propose that the first result may be linked to reduced activity in the so‐called default network [Gusnard and Raichle,2001] under increasing task demands. Differences at the level of early visual cortex under high load are consistent with the C1 results, but the marginal significance of this effect may be partly due to the limited sensitivity when using a template brain in combination with high anatomical variability in these regions [Amunts et al.,2000; Dougherty et al.,2003]. More advanced methods based on individually tailored inverse solutions [Ales et al.,2010; Hagler et al.,2009] may prove useful to corroborate and extend these findings in the future.
As noted in the Introduction, several models may explain the present results. Our original assumption was that simultaneous presentation of irrelevant stimuli in the periphery should increase their salience and thereby disrupt the attentional modulation of C1 amplitudes observed under nonsimultaneous presentation conditions. More specifically, attentional capture by simultaneously onsetting stimuli has been demonstrated both in the visual domain [Fournier,1994; Kahneman et al.,1983; Kritikos et al.,2008; Wilson and Singer,1981] and across different modalities [Olivers and Nieuwenhuis,2005; Santangelo and Spence,2007; Van der Burg et al.,2008a,b). Because the C1 reduction observed in our previous study relates to endogenous attentional processes (with instructions determining attentional load, while stimuli were the same in both conditions), the present results might suggest that attentional capture by salient (though completely task irrelevant) stimuli can override endogenously driven suppression of early visual cortex activity. However, this interpretation rests on the comparison of two studies in different subjects and would therefore need to be followed up with additional, within‐subjects experiments. Also, such an interpretation would only explain a reduction of the C1 effect observed in our previous study [Rauss et al.,2009], not its reversal. One would thus have to assume an additional mechanism that “hijacks” increased recruitment of attentional resources under high load, such that peripheral probes may paradoxically elicit more neural activity. One such mechanism could be temporal grouping [Blake and Lee,2005], an extended version of the Gestalt law of common fate assuming that stimuli with simultaneous onset and offset will preferentially be processed together.
However, a more parsimonious interpretation may be found in the temporal structure of the task: if attentional resources were recruited periodically around the time of expected stimulus onset, this temporally focused recruitment could be increased under high attentional load, and it could boost neural activity elicited by all stimuli occurring around the expected onset time. (Note that this account and the aforementioned interpretation in terms of temporal grouping are not mutually exclusive, as temporally focused recruitment of attention might lead to temporal grouping between central targets and peripheral probes.) The significant C1–N1 correlations observed in the present and our previous experiment [Rauss et al.,2009] are in accordance with this interpretation: periodical recruitment of attentional resources, reflected in increased task‐only N1 amplitudes, could explain both increased C1 amplitudes elicited by concurrently presented probe stimuli here, as well as C1 reductions when probes are presented during putative attentional troughs between task stimuli as in our previous study. The pervasive effects of temporal attention have been extensively investigated in recent years [for a review, see Nobre et al.,2007], but further studies would be required to determine whether the jittered stimulus onsets in this study would disrupt the effects observed with fixed SOAs in previous reports. In addition, it is unclear whether temporal attention can affect early VEP components, which have traditionally been found to be modulated by spatial attention only [Hillyard and Anllo‐Vento,1998]. However, recent evidence suggests that this may be the case [Correa et al.,2006; Doherty et al.,2005].
To further refine these interpretations, future studies should manipulate SOAs parametrically in the same subjects [cf. Kahneman et al.,1983]. Independent of their underlying mechanisms, the effects reported here and their comparison with our previously published results indicate that the temporal structure of a given task may profoundly change the effects of attention on the earliest stages of processing in the visual cortex, as reflected by scalp VEPs. Thus, temporal task structure should be carefully considered both in designing and interpreting experiments that probe top‐down effects at the initial levels of visual perception.
It could be argued that the effects observed in this study are the consequence of a spillover of spatial attention onto the peripheral probes. Thus, if part of the peripheral stimulus fell into the task‐centered attentional spotlight [Brefczynski and DeYoe,1999], probe‐elicited C1 amplitudes might be enhanced as a result of increased attentional gain under high attentional load. We believe that this alternative interpretation is unlikely because previous studies have overwhelmingly reported a decrease of the size of an attentional spotlight with increasing task difficulty [Bahcall and Kowler,1999; McMains and Somers,2004; Schwartz et al.,2005; Williams,1984,1985], which should reduce spillover effects under high attentional load.
One might also interpret the differences between the present and our previous study in terms of an attentional blink‐like phenomenon [Raymond et al.,1992] in the case of nonsimultaneous probe presentation. However, this explanation seems unlikely because the timing of our stimuli was clearly different from the values reported in the attentional blink literature. In particular, SOAs in attentional blink paradigms are usually smaller than 500 ms [Hommel et al.,2006], and stimuli are presented during very short periods [e.g., 15 ms in the original study of Raymond et al.,1992]. Both stimulus durations and SOAs were considerably longer in our experiments.
Finally, it could be that peripheral probes, consisting of arrays of horizontal line elements, were processed differentially because their task relevance differed between conditions. That is, although in the low‐load condition, detection of the color red was sufficient to elicit a correct response, participants additionally had to distinguish object orientations under high attentional load. As the orientation of the task stimuli was defined by the location of a horizontal line either at the top or bottom of a vertical line, the peripheral arrays of horizontal lines might have gained task relevance under high load and could thus have attracted more processing resources [Folk et al.,1992]. We cannot exclude such an effect based on the present dataset, but note that it would not by itself explain the effects of stimulus timing that we observed across our two studies. Moreover, such an interpretation rests on the assumption that feature‐based attentional mechanisms can modulate neural processing at the level of the C1, something which to the best of our knowledge remains to be shown [for evidence of early feature‐based attentional modulations at the level of the P1 component, see Zhang and Luck,2009].
In summary, our results demonstrate that relatively small changes in stimulation parameters can have a profound influence on top‐down effects measured at the earliest cortical stages of visual processing in humans. As the number of studies demonstrating early attentional effects on visual processing increases, an important aim is to better characterize the conditions under which these effects may be replicated. Recent work from our [Pourtois et al.,2004,2008; Rauss et al.,2009] and other groups [Karns and Knight,2009; Kelly et al.,2008; Khoe et al.,2005; Poghosyan and Ioannides,2008; Stolarova et al.,2006] shows that attentional suppression or enhancement may be observed very early following stimulus onset at the level of V1, as a function of stimulus relevance and task demands. This study adds important information to these reports by demonstrating that stimulus timing may critically affect modulations of early visual cortex activity by attentional load [Rauss et al.,2009; Schwartz et al.,2005]. Temporal and spatial factors may thus combine to shape the effects of attention on the earliest stimulus‐evoked responses of the visual system.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1
Acknowledgements
Topographic maps and inverse solution images were produced using Cartool, programed by Denis Brunet of the Functional Brain Mapping Lab at the University of Geneva and supported by the Centre d'Imagerie Biomédical (CIBM) at the Universities of Geneva and Lausanne and the Ecole Polytechnique Fédérale de Lausanne. Rolando Grave de Peralta and Sara Gonzalez Andino programmed the realistic head model and inverse solutions used in this work. The authors thank Mélanie Genetti, Christoph Michel, Tonia Rihs, and Gregor Thut for helpful discussions, and the anonymous reviewers for their thoughtful suggestions on earlier versions of the manuscript.
Footnotes
It is mainly for this reason that we refer to the peripheral stimuli as “probes,” whereas we described them as “distractors” in our previous study. Note, however, that an absence of behavioral effects does not mean that these stimuli cannot exert distracting effects on low‐level visual processing.
The small deviation seen in the low‐load condition led us to measure and analyze this possible component using the same settings as those employed for the C1 analyses described below, i.e., we checked for the most negative amplitudes over electrodes CP1, CPz, CP2, P1, Pz, and P2 between 40 and 90 ms poststimulus. Repeated‐measures ANOVA with factors attentional load and electrode indicated no effect of load (F < 1) and no interaction between the two factors (P = 0.16). A similar null result was obtained for analyses based on mean amplitudes measured over the same interval (P = 0.47).
There is a possibility that low‐level stimulus differences could have affected these results because red crosses were included only in the high‐load VEPs, being imperative targets in the low‐load condition. We therefore recalculated these analyses using VEPs based on both nontarget and target stimuli. Results were equivalent to those reported above, arguing against low‐level stimulus differences as an explanation for the observed effects of attentional load.
As for task‐only VEPs, there is a possibility that low‐level stimulus differences may have contributed to the effects observed (see footnote 3). We, therefore, calculated VEPs including imperative target stimuli and repeated all analyses. Results were largely equivalent to those reported above, with the effect of attentional load on C1 amplitudes significant at P = 0.048. However, the effect of load on P1 amplitudes was nonsignificant both for raw voltages and peak‐to‐peak measurements. In addition, P1 and N1 peaked slightly earlier under high load (both P < 0.05), with mean differences of 3 and 6 ms, respectively. In light of our 2‐ms sampling interval, we regard these differences as unimportant.
REFERENCES
- Ales J, Carney T, Klein SA ( 2010): The folding fingerprint of visual cortex reveals the timing of human V1 and V2. Neuroimage 49: 2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K ( 2000): Brodmann's areas 17 and 18 brought into stereotaxic space—Where and how variable? Neuroimage 11: 66–84. [DOI] [PubMed] [Google Scholar]
- Bahcall DO, Kowler E ( 1999): Attentional interference at small spatial separations. Vis Res 39: 71–86. [DOI] [PubMed] [Google Scholar]
- Blake R, Lee S‐H ( 2005): The role of temporal structure in human vision. Behav Cogn Neurosci Rev 4: 21–42. [DOI] [PubMed] [Google Scholar]
- Brefczynski JA, DeYoe EA ( 1999): A physiological correlate of the ‘spotlight’ of visual attention. Nat Neurosci 2: 370–374. [DOI] [PubMed] [Google Scholar]
- Clark VP, Fan S, Hillyard SA ( 1995): Identification of early visual evoked potential generators by retinotopic and topographic analyses. Hum Brain Mapp 2: 170–187. [Google Scholar]
- Correa A, Lupianez J, Madrid E, Tudela P ( 2006): Temporal attention enhances early visual processing: A review and new evidence from event‐related potentials. Brain Res 1076: 116–128. [DOI] [PubMed] [Google Scholar]
- Crist RE, Li W, Gilbert CD ( 2001): Learning to see: Experience and attention in primary visual cortex. Nat Neurosci 4: 519–525. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Spinelli D ( 1999): Electrophysiological evidence for an early attentional mechanism in visual processing in humans. Vis Res 39: 2975–2985. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Hillyard SA ( 2003): Source analysis of event‐related cortical activity during visuo‐spatial attention. Cereb Cortex 13: 486–499. [DOI] [PubMed] [Google Scholar]
- Doherty JR, Rao A, Mesulam MM, Nobre AC ( 2005): Synergistic effect of combined temporal and spatial expectations on visual attention. J Neurosci 25: 8259–8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA ( 2003): Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J Vis 3: 586–598. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW, Johnston JC ( 1992): Involuntary covert orienting is contingent on attentional control settings. J Exp Psychol Hum Percept Perform 18: 1030–1044. [PubMed] [Google Scholar]
- Fournier LR ( 1994): Selective attentional delays and attentional capture among simultaneous visual onset elements. Percept Psychophys 56: 536–550. [DOI] [PubMed] [Google Scholar]
- Fu S, Huang Y, Luo Y, Wang Y, Fedota J, Greenwood PM, Parasuraman R ( 2009): Perceptual load interacts with involuntary attention at early processing stages: Event‐related potential studies. Neuroimage 48: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Fedota J, Greenwood PM, Parasuraman R ( 2010): Early interaction between perceptual load and involuntary attention: An event‐related potential study. Neurosci Lett 468: 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M ( 2007): Brain states: Top‐down influences in sensory processing. Neuron 54: 677–696. [DOI] [PubMed] [Google Scholar]
- Gilbert C, Ito M, Kapadia M, Westheimer G ( 2000): Interactions between attention, context and learning in primary visual cortex. Vis Res 40: 1217–1226. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E ( 1983): A new method for off‐line removal of ocular artifact. Electroencephalogr Clin Neurophysiol 55: 468–484. [DOI] [PubMed] [Google Scholar]
- Grave de Peralta Menendez R, Murray MM, Michel CM, Martuzzi R, Gonzalez Andino SL ( 2004): Electrical neuroimaging based on biophysical constraints. Neuroimage 21: 527–539. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME ( 2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Halgren E, Martinez A, Huang M, Hillyard SA, Dale AM ( 2009): Source estimates for MEG/EEG visual evoked responses constrained by multiple, retinotopically‐mapped stimulus locations. Hum Brain Mapp 30: 1290–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handy TC, Soltani M, Mangun GR ( 2001): Perceptual load and visuocortical processing: Event‐related potentials reveal sensory‐level selection. Psychol Sci 12: 213–218. [DOI] [PubMed] [Google Scholar]
- Hasnain MK, Fox PT, Woldorff MG ( 1998): Intersubject variability of functional areas in the human visual cortex. Hum Brain Mapp 6: 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze HJ, Mangun GR, Burchert W, Hinrichs H, Scholz M, Münte TF, Gös A, Scherg M, Johannes S, Hundeshagen H, Gazzaniga MS, Hillyard SA ( 1994): Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature 372: 543–546. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo‐Vento L ( 1998): Event‐related brain potentials in the study of visual selective attention. Proc Natl Acad Sci USA 95: 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ ( 1998): Sensory gain control (amplification) as a mechanism of selective attention: Electrophysiological and neuroimaging evidence. Philos Trans R Soc Lond B Biol Sci 353: 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B, Kessler K, Schmitz F, Gross J, Akyürek E, Shapiro K, Schnitzler A ( 2006): How the brain blinks: Towards a neurocognitive model of the attentional blink. Psychol Res 70: 425–435. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Handy TC ( 1998): Reflexive attention modulates processing of visual stimuli in human extrastriate cortex. Psychol Sci 9: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys DA, Axford JG ( 1972): Source locations of pattern‐specific components of human visual evoked potentials. I. Component of striate cortical origin. Exp Brain Res 16: 1–21. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Treisman A, Burkell J ( 1983): The cost of visual filtering. J Exp Psychol Hum Percept Perform 9: 510–522. [DOI] [PubMed] [Google Scholar]
- Karns CM, Knight RT ( 2009): Intermodal auditory, visual, and tactile attention modulates early stages of neural processing. J Cogn Neurosci 21: 669–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP, Gomez‐Ramirez M, Foxe JJ ( 2008): Spatial attention modulates initial afferent activity in human primary visual cortex. Cereb Cortex 18: 2629–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoe W, Mitchell JF, Reynolds JH, Hillyard SA ( 2005): Exogenous attentional selection of transparent superimposed surfaces modulates early event‐related potentials. Vis Res 45: 3004–3014. [DOI] [PubMed] [Google Scholar]
- Kritikos A, McNeill J, Pavlis A ( 2008): Temporal dissociation between distractors and targets: The impact of residual distractor processing on target responses. J Mot Behav 40: 29–42. [DOI] [PubMed] [Google Scholar]
- Lavie N ( 1995): Perceptual load as a necessary condition for selective attention. J Exp Psychol Hum Percept Perform 21: 451–468. [DOI] [PubMed] [Google Scholar]
- Lavie N ( 2005): Distracted and confused? Selective attention under load. Trends Cogn Sci 9: 75–82. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E ( 2004): Load theory of selective attention and cognitive control. J Exp Psychol Gen 133: 339–354. [DOI] [PubMed] [Google Scholar]
- Luck SJ ( 1995): Multiple mechanisms of visual‐spatial attention: Recent evidence from human electrophysiology. Behav Brain Res 71: 113–123. [DOI] [PubMed] [Google Scholar]
- Martinez A, Anllo‐Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA ( 1999): Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci 2: 364–369. [DOI] [PubMed] [Google Scholar]
- McMains SA, Somers DC ( 2004): Multiple spotlights of attentional selection in human visual cortex. Neuron 42: 677–686. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Correa A, Coull JT ( 2007): The hazards of time. Curr Opin Neurobiol 17: 465–470. [DOI] [PubMed] [Google Scholar]
- Noesselt T, Hillyard SA, Woldorff MG, Schoenfeld A, Hagner T, Jancke L, Tempelmann C, Hinrichs H, Heinze HJ ( 2002): Delayed striate cortical activation during spatial attention. Neuron 35: 575–587. [DOI] [PubMed] [Google Scholar]
- Olivers CN, Nieuwenhuis S ( 2005): The beneficial effect of concurrent task‐irrelevant mental activity on temporal attention. Psychol Sci 16: 265–269. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ ( 2000): Guidelines for using human event‐related potentials to study cognition: Recording standards and publication criteria. Psychophysiology 37: 127–152. [PubMed] [Google Scholar]
- Plomp G, Michel CM, Herzog MH ( 2010): Electrical source dynamics in three functional localizer paradigms. Neuroimage 53: 257–267. [DOI] [PubMed] [Google Scholar]
- Poghosyan V, Ioannides AA ( 2008): Attention modulates earliest responses in the primary auditory and visual cortices. Neuron 58: 802–813. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vuilleumier P ( 2004): Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cereb Cortex 14: 619–633. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Rauss KS, Vuilleumier P, Schwartz S ( 2008): Effects of perceptual learning on primary visual cortex activity in humans. Vis Res 48: 55–62. [DOI] [PubMed] [Google Scholar]
- Rauss KS, Pourtois G, Vuilleumier P, Schwartz S ( 2009): Attentional load modifies early activity in human primary visual cortex. Hum Brain Mapp 30: 1723–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM ( 1992): Temporary suppression of visual processing in an RSVP task: An attentional blink? J Exp Psychol Hum Percept Perform 18: 849–860. [DOI] [PubMed] [Google Scholar]
- Santangelo V, Spence C ( 2007): Multisensory cues capture spatial attention regardless of perceptual load. J Exp Psychol Hum Percept Perform 33: 1311–1321. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Maquet P, Frith C ( 2002): Neural correlates of perceptual learning: A functional MRI study of visual texture discrimination. Proc Natl Acad Sci USA 99: 17137–17142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Vuilleumier P, Hutton C, Maravita A, Dolan RJ, Driver J ( 2005): Attentional load and sensory competition in human vision: Modulation of fMRI responses by load at fixation during task‐irrelevant stimulation in the peripheral visual field. Cereb Cortex 15: 770–786. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Klein SA, Carney T, Sutter EE ( 2001): Electrophysiological estimate of human cortical magnification. Clin Neurophysiol 112: 1349–1356. [DOI] [PubMed] [Google Scholar]
- Stolarova M, Keil A, Moratti S ( 2006): Modulation of the C1 visual event‐related component by conditioned stimuli: Evidence for sensory plasticity in early affective perception. Cereb Cortex 16: 876–887. [DOI] [PubMed] [Google Scholar]
- Van der Burg E, Olivers CN, Bronkhorst AW, Theeuwes J ( 2008a): Audiovisual events capture attention: Evidence from temporal order judgments. J Vis 8: 1–10. [DOI] [PubMed] [Google Scholar]
- Van der Burg E, Olivers CN, Bronkhorst AW, Theeuwes J ( 2008b): Pip and pop: Nonspatial auditory signals improve spatial visual search. J Exp Psychol Hum Percept Perform 34: 1053–1065. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ ( 2000): The visual N1 component as an index of a discrimination process. Psychophysiology 37: 190–203. [PubMed] [Google Scholar]
- Williams LJ ( 1984): Information processing in near peripheral vision. J Gen Psychol 111: 201–207. [DOI] [PubMed] [Google Scholar]
- Williams LJ ( 1985): Tunnel vision induced by a foveal load manipulation. Hum Factors 27: 221–227. [DOI] [PubMed] [Google Scholar]
- Wilson JT, Singer W ( 1981): Simultaneous visual events show a long‐range spatial interaction. Percept Psychophys 30: 107–113. [DOI] [PubMed] [Google Scholar]
- Zhang W, Luck SJ ( 2009): Feature‐based attention modulates feedforward visual processing. Nat Neurosci 12: 24–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1


