Abstract
It is becoming increasingly clear that demanding cognitive tasks rely on an extended network engaging task‐relevant areas and, importantly, disengaging task‐irrelevant areas. Given that alpha activity (8–12 Hz) has been shown to reflect the disengagement of task‐irrelevant regions in attention and working memory tasks, we here ask if alpha activity plays a related role for long‐term memory formation. Subjects were instructed to encode and maintain the order of word sequences while the ongoing brain activity was recorded using magnetoencephalography (MEG). In each trial, three words were presented followed by a 3.4 s rehearsal interval. Considering the good temporal resolution of MEG this allowed us to investigate the word presentation and rehearsal interval separately. The sequences were grouped in trials where word order either could be tested immediately (working memory trials; WM) or later (LTM trials) according to instructions. Subjects were tested on their ability to retrieve the order of the three words. The data revealed that alpha power in parieto‐occipital regions was lower during word presentation compared to rehearsal. Our key finding was that parieto‐occipital alpha power during the rehearsal period was markedly stronger for successfully than unsuccessfully encoded LTM sequences. This subsequent memory effect demonstrates that high posterior alpha activity creates an optimal brain state for successful LTM formation possibly by actively reducing parieto‐occipital activity that might interfere with sequence encoding. Hum Brain Mapp, 2011. © 2010 Wiley Periodicals, Inc.
Keywords: MEG (magnetoencephalography), oscillations, synchronization, EEG (electroencephalography), association, inhibition
INTRODUCTION
Higher level cognition relies on the interplay between various brain regions. Long‐term memory (LTM) processing is an example of a cognitive task engaging an extended network (for a review [Paller and Wagner,2002]). Given the high cognitive demands of LTM formation it is important that relevant memory structures are engaged, but memory formation may also require the disengagement of potentially interfering regions not involved in the task [Daselaar et al.,2004; Otten and Rugg,2001; Uncapher and Wagner,2009].
Numerous studies have focused on identifying the regions and temporal dynamics important for long‐term memory formation. Typically subsequent memory paradigms are used to study declarative memory formation. In these paradigms, a set of items is presented to the subjects while brain activity is recorded. Later, memory for this material is tested and brain activity associated with successful versus unsuccessful memory encoding is characterized. Investigations using fMRI (functional Magnetic Resonance Imaging) and PET (Positron Emission Tomography) have revealed that in particular the medial temporal lobe, inferior prefrontal cortex, and parietal regions support declarative memory formation [Brewer et al.,1998; Davachi et al.,2001; Murray and Ranganath,2007; Qin et al.,2009; Staresina and Davachi,2009; Wagner et al.,1998]. Long‐term memory formation has also been studied by electrophysiological means. Studies on evoked responses have identified a distributed network activating ∼400 ms after stimulus presentation [Fernandez et al.,1999; Khader et al.,2007; Sanquist et al.,1980; Takashima et al.,2006]. In the frequency domain subsequent memory effects have been found in various frequency bands. Increases theta power [Hanslmayr et al.,2009; Klimesch et al.,1996a; Osipova et al.,2006; Sederberg et al.,2003; Summerfield and Mangels,2005] and gamma power [Fell et al.,2001,2003; Gruber et al.,2004; Osipova et al.,2006] have been associated with successful memory formation. In contrast decreases in alpha [Hanslmayr et al.,2009; Klimesch et al.,1996b; Sederberg et al.,2007; Weiss and Rappelsberger,2000] and beta power [Hanslmayr et al.,2009; Sederberg et al.,2007] support memory formation. In these studies the brain activity is analyzed when stimuli are presented. In the current study, we have separated the presentation and rehearsal interval. This allows us to focus on brain activity associated with rehearsal only.
While previous studies have concentrated on brain activity and regions contributing to long‐term memory formation, less is known about the modulation of activity in regions not directly important for memory formation. Recent electroencephalography (EEG) and MEG findings support the notion that oscillatory activity in the alpha band (∼10 Hz) plays an important functional role by disengaging task‐irrelevant regions [Jokisch and Jensen,2007; Kelly et al.,2006; Klimesch et al.,2007; Sauseng et al.,2009; Thut and Miniussi,2009; Van Der Werf et al.,2008] but see [Palva and Palva,2007]. In particular, increased alpha activity in task‐irrelevant regions was related to optimal performance in a somatosensory working memory task [Haegens et al.,2009]. A recent EEG study reported stronger alpha activity associated with LTM encoding of objects and letter strings [Khader et al.,2010].
We set out to investigate the functional role of alpha activity for long‐term memory encoding and working memory maintenance during stimulus presentation and rehearsal, respectively. Therefore, we used a paradigm that allowed us to investigate LTM encoding and working memory maintenance of word sequences while we recorded the MEG activity. Our main aim was to quantify how posterior alpha activity reflected successful memory formation during encoding and rehearsal of the word sequences both in terms of time course and neuronal sources.
METHODS
Participants
Twenty‐five participants (14 females, 11 males) gave written informed consent to participate in this study. All participants reported to be right handed, native Dutch speakers and had no history of neurological or psychiatric disorders including dyslexia.
Stimuli
We obtained 2,119 high frequency (>90 occurrences per million) concrete nouns with a word length of 2–13 letters from the Celex database (http://celex.mpi.nl/) and the Dutch spoken word corpus (http://lands.let.kun.nl/cgn/). The collected set of words was divided in three categories with the lowest (average frequency: 157), intermediate (average frequency: 346), and highest word frequencies (average frequency: 2,507). All trials contained three words, one randomly picked from each category to match the sequences for word frequency. Words from each category were equally distributed over first, second, and last position in the sequence. Each word in the set was shown only once to each subject. To minimize repetition of word combinations which are very easy to learn (like “steak” “pepper” “plate”) over subjects, eight lists with stimuli were made. Because of differences in individual performance, blocks with 9 (complete task contained 35 blocks) and 11 (complete task contained 29 blocks) sequences were designed. Six participants did the 11‐sequences version of the task and the remaining 19 subjects did the 9‐sequences version. This ensured an appropriate balance between the number of later remembered and forgotten trials.
Experimental Design
The task contained 35 (or 29) blocks and each block had three parts. In the first part, subjects were instructed to encode the order of three words in each of the 9 (or 11) sequences in LTM (“LTM trials”). The three words were presented sequentially (0.6 s/word) followed by a rehearsal interval of 3.4 s (Fig. 1A). Subjects were instructed to encode the order of the three words in long term memory. We suggested them to make sentences of the three words to make it easier to remember the order. The next trial started immediately after the rehearsal interval.
Figure 1.
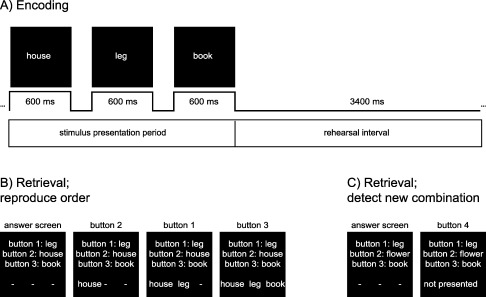
The paradigm consisted of 35 (or 29) blocks. A: During memory encoding three words were presented followed by a rehearsal interval. Immediately after the rehearsal interval the next word list followed. A block consisted of three parts. For the first part (9 or 11 trials; “LTM trials”), subjects were informed that they later would be tested on the order of the word lists. In the second part followed 6 (or 7) trials (“WM trials”) where subjects could be tested on word order immediately after the rehearsal interval. B: In the third part, participants had to reproduce the order of the words encoded in LTM earlier in the block. Three words were shown each represented by a button. Subjects had to reconstruct the sequence by pressing the buttons in the correct order. C: Extra sequences, not presented in the LTM encoding part, were added as catch trials in the retrieval part. When participants detected a new word combination, they were instructed to press button 4.
In the second part of a block, subjects were instructed to maintain the order of the words in WM (“WM trials”). Either 6 or 7 sequences were presented. This part contained two types of trials: trials with three different words (Load 3, “house” “leg” “book”) and trials with a word repeated three times (Load 1, “flower” “flower” “flower”). Subjects maintained these sequences during the 3.4 s rehearsal interval and were not explicitly tested. Additional probe WM trials (20% of the total number) were included randomly to test whether the subjects maintained the words. In these trials subjects had to reconstruct the sequence immediately after the rehearsal interval. For Load 3 trials, testing was the same as for the LTM retrieval described below. For Load 1 trials, the same or a different word was shown (3 times) and subjects were asked to give an “old/new” response.
To keep the stimulus characteristics for the WM trials the same as during the LTM trials, 20% additional test trials where randomly included amongst the LTM encoding trials in the first part of each block.
In the third part of each block, participants were asked to reconstruct the sequences they learned in the encoding part; for example [“house,” “leg,” “book”] (Fig. 1B). Every word was represented by a button. In the example “leg” is represented by button 1, “house” by button 2, and “book” by button 3. To give the correct response button 2 should be pressed first, then button 1 and finally button 3. In addition 20% catch trials were added. Here, one of the words was replaced by a word from another sequence (Fig. 1C). Participants had to indicate the catch trials with new word combinations by pressing button 4. The catch trials were added to enforce that subjects encoded the full word sequence.
Procedure
Participants came to the laboratory twice at two successive days. On the first day, the task was explained to them and they practiced the task for about half an hour. To reduce variance caused by usage of different strategies, we suggested subjects to subvocally construct sentences of the three words in each trial to remember the order later on. Depending on performance during the practice session, participants got the easy or difficult version of the task (9 versus 11 trials per block). We aimed at about 70% correctly recalled sequences for each participant.
On the second day, the full experiment was performed while brain activity was recorded using MEG [Hansen et al.,2010]. Word lists were counter‐balanced over subjects and days. Brain activity was recorded by a 275 axial gradiometer MEG system (VSM/CTF systems, Port Coquitlam, Canada) while the subjects were in supine position. The data were low pass filtered at ∼250 Hz and sampled at 1,200 Hz. Bipolar electrodes were attached to record the electrocardiogram (ECG) and electrooculogram (EOG). Three coils were placed respectively at the nasion and in both ear canals to determine the head position. The recording sessions lasted about 2 h with a 15 min break half way. After the experiment participants were asked to fill in an evaluation form to check which strategies they applied to encode the sequences.
Finally, an anatomical MRI scan was acquired using a 1.5 T (Siemens, Magnetom Avanto) or a 3T (Siemens, Magnetom Trio) MRI scanner. To realign the MEG source reconstructions and the structural MRI data, ear plugs containing oil with vitamin E were placed in the ear canal during MRI acquisition.
Data Analysis
The MEG data were analyzed using Fieldtrip; a Matlab toolbox developed at the Donders Institute for Brain, Cognition and Behaviour (website: http://www.ru.nl/neuroimaging/fieldtrip). First, trials contaminated with muscle or SQUID artifacts were rejected. After artifact rejection, there were at least 50 trials per condition per dataset left to be used for further analysis. Eye and heart beat artifacts were removed from the data using independent component analysis (ICA).
Spectral Analysis
Time‐frequency representations of power (TFRs; 4–32 Hz) based on a sliding time window (steps of 50 ms) were computed from data segments recorded during the presentation of the words (2.2 s) and the rehearsal interval (3.4 s). The length of the time window was adapted to the frequency and contained four cycles (i.e. 400 ms for 10 Hz; 200 ms for 20 Hz). Prior to Fourier analysis, a Hanning taper with the same length as the time window was multiplied to each data segment. Absolute differences between spectra were reported.
Source Analysis
To identify the sources of the oscillatory activity, we applied a beamforming approach using an adaptive filtering technique (Dynamic Imaging of Coherent Sources, DICS) [Gross et al.,2001]. First, cross‐spectral density matrices were obtained from the Fourier transformed data for both conditions. Then, for each subject a realistically shaped single‐shell description of the brain was constructed based on the anatomical MRI [Nolte,2003]. Each participant's brain volume and a brain volume based on a template MRI in MNI (Montreal Neurological Institute) coordinates were divided into a regular 1 cm three‐dimensional grid. Subsequently, each individual's MRI was warped to the template MRI using SPM2 (http://www.fil.ion.ucl.ac.uk/spm), and the inverse of that warp was applied to the individual's dipole grids. Because of this warping, a specific grid point is at the same location in the MRIs of all participants and in the template MRI. Next, per participant the lead field was calculated for each grid point. Using the cross‐spectral density matrix and the lead field matrix, a spatial filter was calculated for each grid point. By applying the filter to the Fourier transformed data, the power at each grid point was estimated. The estimated power was averaged per condition for each participant and the relative difference between the averages of the respective conditions was overlaid on the participants MRI. This analysis was applied to the data recorded during the rehearsal interval in the LTM part of the task where significant differences in alpha power were identified in the sensor level analysis; i.e., 0.5–3.0 s at 10 ± 2 Hz.
Statistical Analysis
To compare conditions, we applied statistical tests to the interval −1.7–0.0 s during word presentation and the interval 0.5–3.0 s during rehearsal of the words (t = 0 s is start of the rehearsal interval). A nonparametric cluster‐based randomization test was applied to the sensor and source level data [Maris and Oostenveld,2007]. This test controls for Type 1 errors in situations involving multiple comparisons by clustering neighboring channels or grid points which show the same effect. Sensors/grid points became part of a cluster when the t‐value of the difference between the conditions exceeded a threshold (P < 0.05). When the clusters were formed, the cluster‐level statistic was defined as the sum of the t‐values of the sensors/grid points in the cluster. The cluster with the maximum sum (summed t‐values in a cluster) was used as a test statistic. To make the randomization null‐distribution, the following step was repeated 1,000 times: per participant the averages for both conditions were randomly divided over two groups and the maximum cluster level statistic was calculated. Last, the test statistic was compared to the randomization null‐distribution.
RESULTS
Subjects were instructed to encode and maintain the order of three words per sequence. Each block consisted of LTM memory encoding trials (9 or 11) where subjects knew that they later would be probed for word order. Then followed WM trials (6 or 7) requiring the subjects to be ready to reproduce the order information at the end of each trial. Finally during LTM retrieval subjects were asked to reconstruct the word sequences from the encoding part (see Fig. 1). Each subject performed 35 (or 29) of these blocks containing respectively 315 (or 319) LTM trials and 210 (or 203) WM trials.
Behavioral Results
Participants remembered the order of the words‐triplets successfully in 64.6% ± 10.2% of the trials. This is clearly above chance level which is 16.6%. In the LTM and WM parts of each block, catch trials were added immediately after the rehearsal interval to check if the word order was maintained correctly. Respectively 96.0% ± 3.6% and 94.4% ± 6.4% of these trials were retrieved correctly for the LTM and WM part.
Alpha Band Activity was Suppressed During Word Presentation Compared With the Rehearsal Interval
Time‐frequency representations (TFRs) of power were calculated and averaged for the respective conditions: LTM remembered, LTM forgotten, WM Load 3, and WM Load 1. The average power in the alpha band over all trials and conditions revealed relatively little alpha activity during stimulus presentation followed by a significant increase of alpha activity during the rehearsal interval over posterior sensors (P < 0.0001, Fig. 2A). The alpha activity was significantly more suppressed for LTM compared to WM trials during the presentation interval (−1.7–0.0 s; P < 0.0001). During the rehearsal interval there was no significant difference in alpha power between LTM and WM trials.
Figure 2.
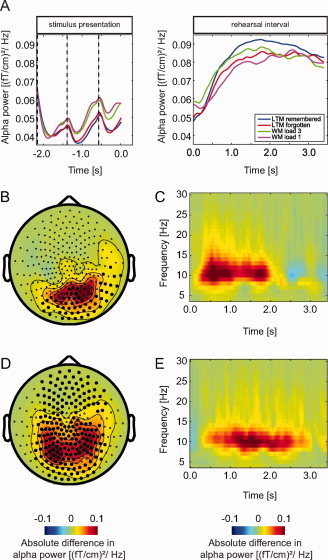
Sensor analysis during the rehearsal interval. A: Average alpha power (8–12 Hz) in occipital and parietal sensors for all conditions (LTM, later remembered; LTM, later forgotten; WM Load 3 and WM Load 1) during presentation of the words and the rehearsal interval. B: The topography of the difference in alpha power when comparing WM Load 3 and WM Load 1 during the rehearsal interval. Marked sensors are part of the significant cluster (P < 0.001) in this contrast. C: Time‐frequency representation of the difference in oscillatory activity (4–32 Hz) between WM Load 3 and WM Load 1 trials during the rehearsal interval (t = 0–3.4 s) averaged over parietal and occipital sensors is shown. D: Topography of the alpha power when comparing LTM later remembered and LTM later forgotten trials during the rehearsal interval. This comparison revealed strong alpha power (8–12 Hz) between 0.5 and 3.0 s associated with successful memory formation. Marked sensors are part of the significant cluster (P < 0.001) when comparing later remembered and forgotten trials. E: A time‐frequency representation of the average alpha power increase over parietal and occipital sensors.
Alpha Power was Stronger for WM Load 3 Compared With WM Load 1 Trials During the Rehearsal Interval
We then compared the TFRs of WM Load 3 and Load 1 trials during the word presentation and rehearsal interval. During presentation of the words there was no significant difference in alpha power between the two conditions; however, in the rehearsal interval we observed an increase in alpha power with increasing memory load. The alpha power increase appeared strongest between 0.5 and 2.0 s (Fig. 2C). The cluster randomization procedure showed a significant effect (0.5–3.0 s; P = 0.01) over parietal and occipital sensors (Fig. 2B).
Alpha Power was Stronger for Later Remembered Compared With Later Forgotten Sequences During the Rehearsal Interval
Next, we compared the TFRs of power for the word presentation and rehearsal interval with respect to later remembered and later forgotten LTM trials. During stimulus presentation, we did not find a significant difference between these two conditions. The same comparison revealed significantly stronger alpha power during the rehearsal interval between 0.5 and 3.0 s for the remembered compared to forgotten trials (0.5–3.0 s; P < 0.0001) (Fig. 2E). The topography displayed the strongest effect at sensors over occipital and parietal regions (Fig. 2D). To identify the dominant source of this subsequent memory effect we applied a beamforming approach. The relative difference in source level power was then subjected to a cluster‐randomization procedure. Figure 3 shows the relative difference in power between later remembered and later forgotten trials for the significant cluster (P = 0.006). The source is consistent with the topography of the effect measured at the sensor level. It included mainly grid points in the occipital cortex (>60% of the grid points in BA 17, 18, and 19) but also in parietal areas (50–60% of the grid points in BA 7 and 39). Note, however, that the spatial extend of the source cannot be estimated reliably. This is an inherent limitation of source modeling caused by the inverse problem [Hansen et al.,2010].
Figure 3.
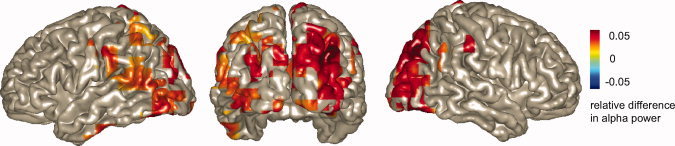
Source analysis during the rehearsal interval. Source reconstruction of the subsequent memory effect in the alpha band (8–12 Hz, 0.5–3.0 s) is shown. Later remembered and forgotten trials are compared. A highly significant cluster (P = 0.006; corrected) was identified in occipital and parietal areas.
Theta Power was Stronger for Later Remembered Compared With Later Forgotten Sequences During Stimulus Presentation
Next to modulations of alpha activity, the time‐frequency representation of power demonstrated a modulation in theta band activity (4–8 Hz) during presentation of the words. Interestingly the theta activity peaked at the time of word presentation rather than after (Fig. 4A). When comparing later remembered and later forgotten trials, we identified a subsequent memory effect in the theta band (Fig. 4B). Theta power was significantly increased over frontal and temporal sensors during presentation of the words (−1.7–0.0 s, P = 0.002) (Fig. 4C). No significant subsequent memory effect was found during the rehearsal interval in the theta band. We also tested for differences in the alpha and beta band during stimulus presentation; however, the task dependent effects were constrained to the theta band.
Figure 4.
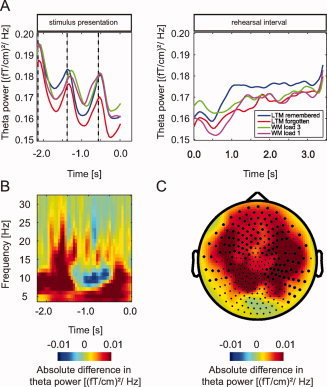
Analysis of theta band activity. A: Average theta power (4–8 Hz) over significant sensors, per condition during presentation of the three words and the rehearsal interval. B: Average time‐frequency representation over sensors in the significant cluster (P = 0.002) when comparing oscillatory activity between later remembered and forgotten trials during presentation of the words. An increase in theta band activity can be observed. C: The topography of the increase in theta power. Marked sensors indicate the significant cluster.
DISCUSSION
In this study, we have investigated oscillatory activity during LTM encoding of word‐sequences using MEG. The alpha activity was relatively low during the 2.2 s word presentation interval but increased during the 3.4 s rehearsal interval. Our key finding was stronger alpha activity during the rehearsal interval for later remembered compared to later forgotten word‐sequences. We identified the sources of the alpha activity in occipital areas extending into superior and ventral parietal regions. During word presentation, activity in the theta band was larger for remembered compared with forgotten trials over frontal and temporal areas.
The finding that an alpha power increase reflects subsequent memory could be a surprise to many, since alpha often is expected to decrease with cognitive efforts. Consistent with previous studies on working memory and attention we propose that the alpha power increase reflects an active disengagement of posterior regions not required for the task [Fu et al.,2001; Jensen et al.,2002; Tuladhar et al.,2007; Worden et al.,2000]. This disengagement could serve to reduce the processing of visual input [Mathewson et al.,2009; van Dijk et al.,2008]. It could also reflect active suppression of top‐down generated activity in visual regions. The consequence of inhibiting the posterior task‐irrelevant regions might be to allocate resources to memory regions involved in rehearsal. This interpretation is consistent with a recent fMRI study demonstrating that a decrease in BOLD activity in the occipital cortex has positive effects on memory performance under acute psychological stress [Henckens et al.,2009]. Stress led to a hypervigilant state with more occipital cortex processing. This result supports the notion that memory formation is impaired when the visual system is “over‐engaged,” e.g., in a hypervigilant state. To what extend this effect is reflected in alpha activity is not yet known but could be investigated by combined EEG and fMRI recordings.
In the WM part of the task, we found stronger alpha activity during the rehearsal period for Load 3 compared with Load 1. This replicates earlier studies reporting an increase in posterior alpha activity with memory load [Jensen et al.,2002; Sauseng et al.,2005; Scheeringa et al.,2009; Tuladhar et al.,2007]. In addition, we found lower alpha activity for LTM trials compared with WM trials during the word‐list presentation. This suggests that the visual system is more engaged for the more demanding LTM task during the actual word presentation. In sum, these effects support the notion that demanding tasks not requiring visual processing result in an increase in posterior alpha; however, during periods of stimulus presentation alpha activity is suppressed to allow optimal flow of visual information.
The notion that increased alpha power reflects disengagement originates from several MEG and EEG findings on attention and working memory [Klimesch et al.,2007]. Studies on covert attention have found that alpha power decreases in engaged areas, while alpha power increases are found in nonengaged areas [Fu et al.,2001; Kelly et al.,2006; Rihs et al.,2007; Worden et al.,2000]. Similar findings have been reported in WM studies [Haegens et al.,2009; Jokisch and Jensen,2007; Sauseng et al.,2009]. For instance in studies described above, increased alpha power with WM Load is explained as increased disengagement of irrelevant areas [Jensen et al.,2002; Scheeringa et al.,2009; Tuladhar et al.,2007]. A recent study on somatosensory delayed‐match‐to‐sample WM revealed a positive effect of alpha activity on performance [Haegens et al.,2009]. Increased alpha activity ipsilateral to the engaged hand during maintenance reflected successful performance. All these results are in line with the functional inhibition hypothesis in which alpha synchronization reflects top‐down inhibitory control [Klimesch et al.,2007; Thut and Miniussi,2009]. Alternatively one might suggest that the alpha power increase is a direct correlate of the neuronal mechanism responsible for the task at hand; in this study LTM encoding and WM maintenance (see e.g. [Kolev et al.,2001; Maltseva et al.,2000; Palva and Palva,2007; Sewards and Sewards,1999]). We find such an interpretation less likely since it cannot account for the findings demonstrating an increase in alpha power in task‐irrelevant regions.
This report is one of the first human electrophysiological studies suggesting that the disengagement of occipital‐parietal areas during rehearsal is important for optimal LTM encoding. Related results have been found in an EEG study using letter strings and line drawings as memory items [Khader et al.,2010]. Other studies found a decrease in alpha activity during presentation of the stimuli to correlate with successful encoding in LTM [Hanslmayr et al.,2009; Klimesch et al.,1996b; Sederberg et al.,2007; Weiss and Rappelsberger,2000]. In our study, we did not find a significant difference in alpha activity during presentation of the words. This might be explained by the fact that the current study relies on creating associations between the presented words rather than remembering the individual items. This implies that memory encoding during the rehearsal period is more important than during the presentation interval for the task we have applied.
Studies applying fMRI have identified both BOLD increases and decreases in parietal and occipital areas associated with subsequent memory [Daselaar et al.,2004; Davachi et al.,2001; Henckens et al.,2009; Kim et al.,2010; Otten and Rugg,2001; Wagner and Davachi,2001]. In particular, the picture has emerged that BOLD activity in superior parietal cortex reflects memory formation whereas activity in ventral parietal areas is detrimental for memory formation [Uncapher and Wagner,2009]. However, the alpha source we identified reflecting subsequent memory formation included the occipital lobe and extended into both posterior superior and ventral parietal regions. We attribute the difference between the fMRI studies and our MEG findings to the fact that the time resolution of MEG allows us to distinguish the stimulus presentation and rehearsal intervals. Because of the slow time resolution of the BOLD signal, stimulus presentation and rehearsal intervals are typically not separated in fMRI studies. Thus, we believe that our findings are not in contradiction to the existing literature, but rather they contribute new complementary insight.
Last we identified a subsequent memory effect in the theta band during the word presentation interval. We identified the subsequent theta band effect in frontal and temporal areas. This finding is consistent with previous reports [Hanslmayr et al.,2009; Osipova et al.,2006; Sederberg et al.,2003; Summerfield and Mangels,2005]. It should be mentioned that the theta effect is present during the visual presentation of the words only. Given that the presentation of words also is associated with evoked responses which might have frequency content in the theta band, the effect might be partly explained by ERFs reflecting subsequent memory [Paller and Wagner,2002; Rugg and Allan,2000].
CONCLUSION
Our key finding is that strong parieto‐occipital alpha activity predicts successful long‐term memory encoding. This suggests that active inhibition of parieto‐occipital regions is a necessity for optimal task performance. We posit that this inhibition serves to reduce potential interfering visual input and allocate processing resources to brain areas responsible for LTM encoding.
Acknowledgements
The authors thank Marcel van Gerven, Barbara Haendel, and Jurrian van der Werf for fruitful discussions about the analysis of this dataset.
REFERENCES
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD ( 1998): Making memories: Brain activity that predicts how well visual experience will be remembered. Science 281: 1185–1187. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R ( 2004): When less means more: Deactivations during encoding that predict subsequent memory. Neuroimage 23: 921–927. [DOI] [PubMed] [Google Scholar]
- Davachi L, Maril A, Wagner AD ( 2001): When keeping in mind supports later bringing to mind: Neural markers of phonological rehearsal predict subsequent remembering. J Cogn Neurosci 13: 1059–1070. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CE, Fernandez G ( 2001): Human memory formation is accompanied by rhinal‐hippocampal coupling and decoupling. Nat Neurosci 4: 1259–1264. [DOI] [PubMed] [Google Scholar]
- Fell J, Fernandez G, Klaver P, Elger CE, Fries P ( 2003): Is synchronized neuronal gamma activity relevant for selective attention? Brain Res Brain Res Rev 42: 265–272. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Effern A, Grunwald T, Pezer N, Lehnertz K, Dumpelmann M, Van Roost D, Elger CE ( 1999): Real‐time tracking of memory formation in the human rhinal cortex and hippocampus. Science 285: 1582–1585. [DOI] [PubMed] [Google Scholar]
- Fu KM, Foxe JJ, Murray MM, Higgins BA, Javitt DC, Schroeder CE ( 2001): Attention‐dependent suppression of distracter visual input can be cross‐modally cued as indexed by anticipatory parieto‐occipital alpha‐band oscillations. Brain Res Cogn Brain Res 12: 145–152. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R ( 2001): Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA 98: 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber T, Tsivilis D, Montaldi D, Muller MM ( 2004): Induced gamma band responses: An early marker of memory encoding and retrieval. Neuroreport 15: 1837–1841. [DOI] [PubMed] [Google Scholar]
- Haegens S, Osipova D, Oostenveld R, Jensen O ( 2009): Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Hum Brain Mapp 31: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen P, Kringelbach M, Salmelin R, editors ( 2010): MEG: An Introduction to Methods. New York: Oxford University Press. [Google Scholar]
- Hanslmayr S, Spitzer B, Bauml KH ( 2009): Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cereb Cortex 19: 1631–1640. [DOI] [PubMed] [Google Scholar]
- Henckens MJ, Hermans EJ, Pu Z, Joels M, Fernandez G ( 2009): Stressed memories: How acute stress affects memory formation in humans. J Neurosci 29: 10111–10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE ( 2002): Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short‐term memory task. Cereb Cortex 12: 877–882. [DOI] [PubMed] [Google Scholar]
- Jokisch D, Jensen O ( 2007): Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci 27: 3244–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ ( 2006): Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol 95: 3844–3851. [DOI] [PubMed] [Google Scholar]
- Khader P, Ranganath C, Seemuller A, Rosler F ( 2007): Working memory maintenance contributes to long‐term memory formation: Evidence from slow event‐related brain potentials. Cogn Affect Behav Neurosci 7: 212–224. [DOI] [PubMed] [Google Scholar]
- Khader PH, Jost K, Ranganath C, Rosler F ( 2010): Theta and alpha oscillations during working‐memory maintenance predict successful long‐term memory encoding. Neurosci Lett 468: 339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Daselaar SM, Cabeza R ( 2010): Overlapping brain activity between episodic memory encoding and retrieval: Roles of the task‐positive and task‐negative networks. Neuroimage 49: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T ( 1996a) : Theta band power in the human scalp EEG and the encoding of new information. Neuroreport 7: 1235–1240. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Doppelmayr M, Ripper B, Schwaiger J, Pfurtscheller G ( 1996b): Event‐related desynchronization (ERD) and the Dm effect: Does alpha desynchronization during encoding predict later recall performance? Int J Psychophysiol 24: 47–60. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S ( 2007): EEG alpha oscillations: The inhibition‐timing hypothesis. Brain Res Rev 53: 63–88. [DOI] [PubMed] [Google Scholar]
- Kolev V, Yordanova J, Schurmann M, Basar E ( 2001): Increased frontal phase‐locking of event‐related alpha oscillations during task processing. Int J Psychophysiol 39: 159–165. [DOI] [PubMed] [Google Scholar]
- Maltseva I, Geissler HG, Basar E ( 2000): Alpha oscillations as an indicator of dynamic memory operations—Anticipation of omitted stimuli. Int J Psychophysiol 36: 185–197. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R ( 2007): Nonparametric statistical testing of EEG‐ and MEG‐data. J Neurosci Methods 164: 177–190. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T ( 2009): To see or not to see: prestimulus alpha phase predicts visual awareness. J Neurosci 29: 2725–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray LJ, Ranganath C ( 2007): The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J Neurosci 27: 5515–5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte G ( 2003): The magnetic lead field theorem in the quasi‐static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys Med Biol 48: 3637–3652. [DOI] [PubMed] [Google Scholar]
- Osipova D, Takashima A, Oostenveld R, Fernandez G, Maris E, Jensen O ( 2006): Theta and gamma oscillations predict encoding and retrieval of declarative memory. J Neurosci 26: 7523–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD ( 2001): When more means less: Neural activity related to unsuccessful memory encoding. Curr Biol 11: 1528–1530. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD ( 2002): Observing the transformation of experience into memory. Trends Cogn Sci 6: 93–102. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva JM ( 2007): New vistas for alpha‐frequency band oscillations. Trends Neurosci 30: 150–158. [DOI] [PubMed] [Google Scholar]
- Qin S, Rijpkema M, Tendolkar I, Piekema C, Hermans EJ, Binder M, Petersson KM, Luo J, Fernandez G ( 2009): Dissecting medial temporal lobe contributions to item and associative memory formation. Neuroimage 46: 874–881. [DOI] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G ( 2007): Mechanisms of selective inhibition in visual spatial attention are indexed by alpha‐band EEG synchronization. Eur J Neurosci 25: 603–610. [DOI] [PubMed] [Google Scholar]
- Rugg M, Allan K ( 2000): Event‐related potential studies of memory In: Tulving E, Craik F, editors. The Oxford Handbook of Memory. Oxford: Oxford UP; pp 521–537. [Google Scholar]
- Sanquist TF, Rohrbaugh JW, Syndulko K, Lindsley DB ( 1980): Electrocortical signs of levels of processing: Perceptual analysis and recognition memory. Psychophysiology 17: 568–576. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Doppelmayr M, Pecherstorfer T, Freunberger R, Hanslmayr S ( 2005): EEG alpha synchronization and functional coupling during top‐down processing in a working memory task. Hum Brain Mapp 26: 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Glennon M, Gerloff C, Birbaumer N, Hummel FC ( 2009): Brain oscillatory substrates of visual short‐term memory capacity. Curr Biol 19: 1846–1852. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Petersson KM, Oostenveld R, Norris DG, Hagoort P, Bastiaansen MC ( 2009): Trial‐by‐trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage 44: 1224–1238. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR ( 2003): Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci 23: 10809–10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Schulze‐Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, Tully MS, Kahana MJ ( 2007): Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex 17: 1190–1196. [DOI] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA ( 1999): Alpha‐band oscillations in visual cortex: Part of the neural correlate of visual awareness? Int J Psychophysiol 32: 35–45. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L ( 2009): Mind the gap: Binding experiences across space and time in the human hippocampus. Neuron 63: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Mangels JA ( 2005): Coherent theta‐band EEG activity predicts item‐context binding during encoding. Neuroimage 24: 692–703. [DOI] [PubMed] [Google Scholar]
- Takashima A, Jensen O, Oostenveld R, Maris E, van de Coevering M, Fernandez G ( 2006): Successful declarative memory formation is associated with ongoing activity during encoding in a distributed neocortical network related to working memory: A magnetoencephalography study. Neuroscience 139: 291–297. [DOI] [PubMed] [Google Scholar]
- Thut G, Miniussi C ( 2009): New insights into rhythmic brain activity from TMS‐EEG studies. Trends Cogn Sci 13: 182–189. [DOI] [PubMed] [Google Scholar]
- Tuladhar AM, ter Huurne N, Schoffelen JM, Maris E, Oostenveld R, Jensen O ( 2007): Parieto‐occipital sources account for the increase in alpha activity with working memory load. Hum Brain Mapp 28: 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD ( 2009): Posterior parietal cortex and episodic encoding: Insights from fMRI subsequent memory effects and dual‐attention theory. Neurobiol Learn Mem 91: 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf J, Jensen O, Fries P, Medendorp WP ( 2008): Gamma‐band activity in human posterior parietal cortex encodes the motor goal during delayed prosaccades and antisaccades. J Neurosci 28: 8397–8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O ( 2008): Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci 28: 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Davachi L ( 2001): Cognitive neuroscience: Forgetting of things past. Curr Biol 11: R964–R967. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL ( 1998): Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science 281: 1188–1191. [DOI] [PubMed] [Google Scholar]
- Weiss S, Rappelsberger P ( 2000): Long‐range EEG synchronization during word encoding correlates with successful memory performance. Brain Res Cogn Brain Res 9: 299–312. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV ( 2000): Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha‐band electroencephalography increases over occipital cortex. J Neurosci 20: RC63. [DOI] [PMC free article] [PubMed] [Google Scholar]


