Abstract
Functional imaging is increasingly being used to provide a noninvasive alternative to intracarotid sodium amobarbitol testing (i.e., the Wada test). Although magnetoencephalography (MEG) has shown significant potential in this regard, the resultant output is often reduced to a simplified estimate of laterality. Such estimates belie the richness of functional imaging data and consequently limit the potential value. We present a novel approach that utilizes MEG data to compute “complex laterality vectors” and consequently “laterality maps” for a given function. Language function was examined in healthy controls and in people with epilepsy. When compared with traditional laterality index (LI) approaches, the resultant maps provided critical information about the magnitude and spatial characteristics of lateralized function. Specifically, it was possible to more clearly define low LI scores resulting from strong bilateral activation, high LI scores resulting from weak unilateral activation, and most importantly, the spatial distribution of lateralized activation. We argue that the laterality concept is better presented with the inherent spatial sensitivity of activation maps, rather than being collapsed into a one‐dimensional index. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: magnetoencephalography, Wada test, presurgical functional mapping, neurological planning, language, memory, cognition
INTRODUCTION
Magnetoencephalography (MEG) provides a noninvasive, patient friendly approach to study brain function with relatively high spatial resolution (millimeters) and real‐time temporal resolution (milliseconds). Common clinical applications of MEG have included seizure localization in epilepsy and preoperative functional mapping for brain tumor resection (Funke et al.,2009; Stufflebeam et al.,2009].
One emerging epilepsy application for MEG is preoperative evaluation of critical cognitive functions like language and memory. Historically, hemispheric dominance, or laterality, has been determined through the invasive Wada test [Wada,1949]. In the Wada test, a barbiturate is injected into one internal carotid artery of an awake patient to disrupt cognitive function within the injected hemisphere. Neuropsychological testing is done concurrently to evaluate the likelihood of language and memory impairments. The Wada test is being phased out in many centers, in large part, due to invasiveness and the availability of amobarbital [Baxendale,2009; Van der Haegen et al.,2011]. Consequently, the critical need for a noninvasive alternative from functional imaging has become increasingly clear. Advances in MEG and functional magnetic resonance imaging (fMRI) represent the leading solutions in this realm [Baxendale,2009; Binder,2011; Papanicolaou et al.,2004].
Functional Imaging, Language, and the Wada Test
Significant effort has gone into validating MEG and fMRI laterality testing for language. Often, the functional imaging results are converted into laterality index (LI) scores, which represent hemispheric dominance in terms of a numeric index that often ranges from 1 (left) to −1 (right).
For example, Papanicolaou et al. [2004] used MEG with auditory word presentation to calculate LI scores for language laterality. The results were concordant with those of the Wada test in 74 of 85 patients (87%). In terms of sensitivity and specificity, MEG LI scores were greater than 90 and 80%, respectively, when compared with the Wada test. More recently, McDonald et al. [2009] compared MEG activation between visually presented words and false fonts. LI scores based on a temporoparietal region of interest (ROI) corresponded with Wada testing in 75% of cases, whereas LI scores from a frontal ROI matched in all cases. The pattern of closer frontal, than temporal, correspondence with Wada testing has been seen with other studies involving verb generation and picture naming [Bowyer et al.,2005].
In fMRI, LI scores are also strongly consistent with results of the Wada test. Using a semantic decision task, Binder et al. [1996] showed that LI scores based on the number of significantly activated voxels in each hemisphere were in agreement with Wada results in all 22 patients, including typical and atypical language distributions [Binder et al.,1996]. Woermann et al. [2003] measured fMRI activation in a large sample of patients performing a covert naming task (n = 100). The results, based on blinded visual inspection, matched the Wada results in 91% of completed cases. Interestingly, fMRI activation was bilateral in 82% of cases, suggesting that involvement of both hemispheres in language processes, rather than unilateral processing, is the norm in patients with epilepsy.
The concordance of MEG/fMRI LI scores with Wada test results remains high despite the different tasks used across studies. However, recent work suggests that clinical outcome may be a more appropriate “gold standard” comparator than the Wada test [Baxendale,2009; Binder,2011]. Binder et al. [2008] found that including fMRI laterality scores in presurgical assessment improved the predictive accuracy after surgery. In contrast, the Wada test results made no appreciable difference. In a study of patients with epilepsy involving the left anterior temporal lobe, Sabsevitz et al. [2003] compared the accuracy of fMRI and Wada language tests in predicting postsurgical language deficits. They too reported better predictive performance for fMRI based on a temporal lobe ROI as compared to the Wada test.
Revisiting Laterality Scores
Questions have also been raised about reliance about over reliance on the LI [Jones et al.,2011; Sharan et al.,2011]. In particular, MEG and fMRI studies have remained largely wedded to traditional LI calculations. LI scores have been defined based on the well‐known basic formula:
| (1) |
where Q represents a measure of activation in the appropriate hemisphere. Thus, LI ranges from −1 to 1 to indicate right to left hemispheric dominance. The measure of activation, Q, may be (1) the number of active sources in a hemisphere or ROI [Binder et al.,1996; Binder et al.,2008; Kamada et al.,2007; Papanicolau et al.,2004; Sabsevitz,2003]; (2) the magnitude of activation in a ROI [Hirata et al.,2010; Kim and Chung,2008; McDonald et al.,2009]; or (3) the length of time for which activation occurs in a ROI [Bowyer et al.,2005].
While useful, the LI approach has two main disadvantages. First, the LI scores discard information about activation strength, such that scores near 0 are ambiguous—they may represent either weak or strong bilateral activation. Second, the LI measure suffers from the coarse spatial sensitivity, as it is dependent on the selection of specific ROIs or is summed over the entire hemisphere. Consequently, LI scores, in effect, are under representative of the valuable patient‐specific, high‐resolution neuroanatomical data provided by functional imaging.
Objectives and Hypotheses
This study was designed to redefine the LI score by taking advantage of the rich data available in functional imaging. This new approach extracts information about the degree of laterality, magnitude, and location of neural activity by scoring laterality as a complex number, and subsequently remapping this information on a patient‐specific cortical surface. Laterality maps were generated based on MEG scans during a verbal memory task. The results for both healthy controls and people with epilepsy were compared against standard LI scores derived from traditional ROIs.
There were two main hypotheses: (1) the language‐related laterality maps would match closely with the known functional anatomy that supports language; and (2) the laterality map would provide more specific and clinically relevant information about activation magnitude and location, when compared with typical LI scores for both healthy controls and patients.
METHODS
Participants
Twenty adult participants were included across two groups. The control group included 10 healthy controls (mean age = 28.2 years, standard deviation (SD) = 9.4 years; six females). All subjects were right handed and fluent in English. The patient group included 10 people with epilepsy (mean age = 32.4 years, SD = 10.5 years; six females). The patient sample was recruited from the Epilepsy Monitoring Unit at the Halifax Infirmary, Halifax, Nova Scotia. For all patients, the lateralization of language function was in question. Data from one patient could not be included due to an inability to perform the task (reducing the patient sample size to n = 9). A summary of the patient group characteristics is provided in Table I. Seven of nine remaining patients were right handed, and all were fluent in English. No patient had a Wada test as part of the investigation. The study had research ethics board approval, and all participants provided informed consent.
Table I.
Patient characteristics
| Patients | Sex | Age | Hand | Onset | Education/occupation | Clinical diagnosis | EEG | MRI | Medication at time of study | Surgery/outcome (as of July 12, 2011) |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | F | 30 | R | 5 | Unemployed; BA | Symptomatic cortical dysplasia | Scalp: F3‐FZ spikes; FZ onset seizures; Stereotactic depth EEG: Seizure onset within the cortical dysplasia | Left frontal cortical dysplasia | Levetiracetam | Resection cortical dysplasia |
| Focal onset; Secondary generalized | Lamotrigine | Seizure free; off all antiepileptic drugs (AEDs) | ||||||||
| P2 | F | 39 | R | 25 | Housewife; Grade 12 | Symptomatic malformation of cortical development | Scalp EEG: Right frontal spikes, seizures | Right orbital frontal malformation of cortical development | Levetiracetam | Awaiting resective surgery |
| Subdural EEG: seizure onset right orbital frontal | Lamotrigine | |||||||||
| P3 | F | 49 | R | 39 | Salesperson | Idiopathic epilepsy | Scalp EEG: Left temporal spikes, seizures | Normal | Divalproex Lacosamide | No surgery |
| Nearly seizure free on Lacosamide | ||||||||||
| P4 | M | 21 | R | 10 | Student; Grade 12 plus University | Idiopathic epilepsy | Scalp EEG: Generalized polyspike and wave | Not done | Phenytoin | Seizure with Divalproex |
| Divalproex | ||||||||||
| P5 | M | 28 | L | 17 | Unemployed; Grade 12 | Cryptogenic epilepsy | Scalp EEG: Multifocal spikes and generalized spike waves | Normal | Divalproex Lamotrigine | No invasive electrodes; No surgery planned |
| P6 | M | 45 | R | 27 | Student; Grade 12, plus University | Cryptogenic epilepsy | Scalp EEG: Right temporal spikes, seizures | Normal | Awaiting bitemporal subdural electrode implantation | |
| P7 | F | 41 | R | 5 | Grade 12 | Cryptogenic epilepsy | Scalp EEG: Bifrontal spike waves, maximum right frontal seizures | Normal | Lamotrigine Divalproex | Awaiting stereotaxic EEG evaluation |
| P8 | M | 19 | R | 12 | Grade 12; plus University | Idiopathic epilepsy | Scalp EEG: Bifrontal spike waves, max right frontal | Normal | Topirimate | Seizure free with Topirimate and Lacosamide |
| Lamotrigine | ||||||||||
| Lacosamide | ||||||||||
| P9 | F | 30 | L | 13 | Grade 12 | Symptomatic epilepsy | Scalp EEG: Right mid‐temporal, posterior temporal, right central spikes | Right cingulate lesion; dysembryoplastic neuroepithelial tumor; also right temporal periventricular heterotopia | Lamotrigine Divalproex | Awaiting stereotactic EEG study |
| Seizures: right hemisphere, not localized |
Experimental Paradigm
Figure 1a provides an overview of the experimental paradigm. Participants sat upright in the MEG and completed a simple verbal working memory task [adapted from D'Arcy et al.,2011]. Each trial started with an encoding phase, wherein three consecutive pictures were presented visually (1 s duration per picture, no interstimulus interval). Each trial used stimuli that were either intact colored line drawings of common objects [Rossion and Pourtois,2004] or the same line drawings scrambled on a 9 × 14 grid. Subjects were instructed to covertly name each intact object and to try to remember the appearance of each scrambled picture. Encoding of the three pictures was followed by a variable 3–4‐s retention period, during which participants were instructed to remember the pictures they had just seen (covert repetition of names or visually remembering the “unnamable” scrambled pictures). Following this was a verification phase with the presentation of a probe picture, in which there was either one of the three pictures presented in the encoding phase (old) or a new one (2 s duration). Novel pictures were used for each trial of the task. Participants were instructed to indicate, using a button press response, whether the probe picture was old or new (50:50). There was a variable 4–5 s delay between trials. Every eighth trial was followed by a short animation (5 s) to break up the task. In total, 128 trials were presented over four blocks to each participant.
Figure 1.
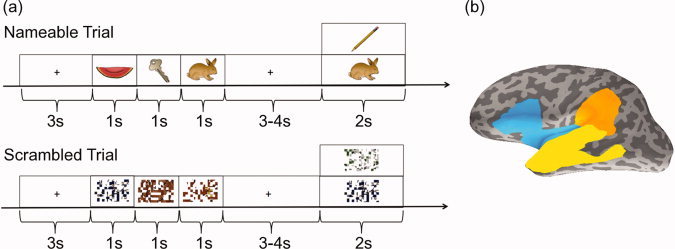
Task design (a) and regions of interest (ROIs; b) are shown. In each trial, participants performed a verbal working memory task. The contrast between nameable and scrambled pictures revealed language processing. ROIs defined in the inferior frontal lobe (blue), superior/middle temporal lobe (yellow), and supramarginal gyrus (orange) are depicted on the left hemisphere. These ROIs were used for calculating downsampled LI/CLV scores.
Stimuli presentation and response monitoring were achieved using Presentation software (Neurobehavioral Systems, Albany, CA). Stimuli were back‐projected on a translucent screen positioned 1 m away. The visual angle for stimuli was 5.7°. A practice run with visual feedback was used before scanning to ensure task proficiency. No image was repeated within the experiment. Response hand and the order of image presentation were counterbalanced across subjects.
Acquisition
Magnetic fields were recorded using an Elekta Neuromag® whole head 306‐channel MEG system located within a magnetically shielded room, equipped with 102 magnetometers and 204 gradiometers (Elekta Neuromag®, Helsinki, Finland).1 A separate MEG scan was performed for each block of the experimental paradigm. MEG data were band‐pass filtered at 0.1–330 Hz and sampled at 1,000 Hz. Electromagnetic head position indicator (HPI) coils were used to track head motion during the scan. The three‐dimensional (3D) positions of these coils with respect to the nasion and the left and right preauricular points were obtained using a 3D position monitoring system (Polhemus, Colchester, VT). In addition, 100–150 points along the head were digitized to coregister MEG data with the anatomical magnetic resonance imaging (MRI).
Anatomical MRI data were acquired using a 1.5T GE scanner (GE Medical Systems, Waukesha, WI). A 3D T1‐weighted anatomical image was acquired using an spoiled gradient recalled (SPGR) sequence (inversion time (TI) = 400 ms, recovery time (TR) = echo time (TE) = min, Flip angle = 12°, field of view (FOV) 25.6 cm, 256 × 256, 2 mm slices). Functional MRI data were acquired in the same session, but the results were analyzed separately.
Behavioral Data Analysis
Behavioral analysis included 128 retrieval trials divided by four conditions (nameable‐old, nameable‐new, scrambled‐old, and scrambled‐new). Reaction time (RT) and accuracy data for the verification probe phase were analyzed using a repeated measures analysis of variance (ANOVA), with conservative degrees of freedom [Greenhouse and Geisser,1959]. Posthoc t‐tests were conducted on significant interactions. An alpha level of P < 0.05 was used to establish statistical significance.
MEG Data Analysis
Temporal signal‐space separation [Taulu et al.,2004] was applied to the raw data for environmental noise reduction using standard Elekta Neuromag® software. MEG scans were excluded if intrascan movement of any HPI coil exceeded 1 cm. MEG data were segmented with respect to the onset of each picture in the encoding phase (−100 to 1,000 ms), baseline corrected, low‐pass filtered (40 Hz), and down‐sampled (250 Hz). Principle components of MEG epochs that exceeded the threshold of 1.0 pT (magnetometers) and 150 fT/cm2 (gradiometers) were removed as artifacts [Kobayashi and Kuriki,1999; Lagerlund et al.,1997]. For patient data, segments containing interictal activity were rejected based on visual inspection.
For signal averaging to compute evoked data, trials were categorized into two types: nameable and scrambled. The contrast between these two trial types in the encoding phase was used to isolate language processing. Nameable and scrambled conditions were presented in four separate scans with 48 segments for each of the signal‐averaged conditions (192 epochs in total per condition, less errors).
Spatiotemporal activation maps for nameable and scrambled evoked field data were generated using dynamic statistical parametric mapping (dSPM) of the change in activation from the prestimulus interval as a pseudo‐Z statistic [Dale et al.,2000]. The maps were averaged across the four separate scans for each condition. Examination of the differences between the nameable and scrambled spatiotemporal dSPM activation maps for healthy control participants' using the partial‐least squares method applied to event‐related MEG source data [Moses et al.,2009] revealed predominant differences between 200 and 600 ms poststimulus. For each subject, the difference between nameable and scrambled activation maps was used to generate language‐related spatiotemporal maps. A dSPM threshold for significant language‐related activation was set to the 99.5% largest value in the null‐hypothesis distribution using the 100 ms baseline. In the 200–600‐ms poststimulus period, only vertices (∼5,000 elements per hemisphere) above the dSPM threshold for more than 100 ms were included for further analysis. Time‐collapsed language‐related maps were plotted on the anatomical MRI by taking the maximum suprathreshold dSPM activation occurring 200–600‐ms poststimulus for each included location and were used in the laterality calculations below.
Complex Laterality Vector Calculation
For each location on the spatially normalized cortex of the left hemisphere, a two‐dimensional (2D) construct called a complex laterality vector (CLV) was calculated that included the magnitude of activation at the left hemisphere location of interest and the mirror location in the right hemisphere. Mathematically, this 2D information was represented as a complex number, as in Formula 2, which contains both a “real” and “imaginary” component. The imaginary component was generated by multiplying the magnitude of the second dimension by the mathematical constant i. The “real” component was defined as the magnitude of left hemisphere (LH), dSPM activation at the location of interest (Q LH). The “imaginary” component was defined as the magnitude of dSPM activation at the mirror location in the right hemisphere (Q RH). The same calculation was also made for each location on the right hemisphere.
| (2) |
For visualization, laterality was overlaid on the left hemisphere of the individual's cortical surface, showing left and bilateral activation only. Laterality was also overlaid on the right hemisphere of the individual's cortical surface, showing right and bilateral activation only. On both hemispheres, the real (left) component of CLV was plotted on a red‐to‐yellow color scale, and the imaginary (right) component was plotted on a blue‐to‐light blue color scale. Bilateral activation was represented as the sum of both color scales.
ROI Analysis
To compare laterality maps to standard LI scores, ROIs were defined in the inferior frontal lobe/insula, supramarginal gyrus, and superior/middle temporal region. ROIs were selected using the FreeSurfer cortical surface reconstruction [Desikan et al.,2006; Fischl et al.,2004]. The ROIs are shown in Figure 1b. Within each ROI, mean activation and LI scores were calculated based on the language activation maps. For comparison, CLV scores were calculated using the same mean activation.
RESULTS
Behavioral Performance Verification
Overall task performance was high for both controls (mean accuracy = 90.8%, standard error = 1.2%) and patients (mean accuracy = 87.2%, standard error = 1.2%). Accuracy and RT data were analyzed using a repeated measures ANOVA with between‐subjects (nine patients and 10 controls) and within‐subjects factors (old/new and nameable/scrambled). The performance results are presented in Table II. In brief, the results showed a trend towards a group difference in performance (controls better than patients; P = 0.051), stimulus type differences (nameable better than scrambled; P < 0.001), and memory differences (new better than old; P < 0.05). There was also a stimulus type by memory interaction (P < 0.05), reflecting the worst performance for the recognition of scrambled objects that were previously seen.
Table II.
Behavioral results
| Factors | degrees of freedom (DOF) | Mean (%) | Standard error (%) | F | P |
|---|---|---|---|---|---|
| Accuracy | |||||
| Group | 1,17 | Controls: 90.8 | Controls: 1.17 | 4.4 | 0.051 |
| Patients: 87.2 | Patients: 1.23 | ||||
| Type | 1,17 | Nameable: 97.9 | Nameable: 0.45 | 135.6 | <0.001 |
| Scrambled: 80.1 | Scrambled: 1.55 | ||||
| Memory | 1,17 | New: 91.9 | New: 1.09 | 8.5 | 0.01 |
| Old: 86.0 | Old: 1.52 | ||||
| Type × memory | 1,17 | 6.6 | 0.02 | ||
| Factors | DOF | Mean (ms) | Standard error (ms) | F | P |
| Reaction time | |||||
| Group | 1,17 | Controls: 856 | Controls: 34.7 | 1.6 | 0.23 |
| Patients: 918 | Patients: 36.6 | ||||
| Type | 1,17 | Nameable: 804 | Nameable: 24.2 | 114 | <0.001 |
| Scrambled: 971 | Scrambled: 28.3 | ||||
| Memory | 1,17 | New: 869 | New: 25.5 | 4.5 | 0.048 |
| Old: 906 | Old: 27.7 | ||||
| Type × memory | 1,17 | 6.1 | 0.024 | ||
Healthy Control Laterality Maps
Language laterality maps for the control group are presented in Figure 2. Laterality data were overlaid on the left (in red) and right (in blue) hemispheres. Bilateral activation (in purple) is shown on both hemispheres. These maps convert activation intensity into the relative strength of lateralization.2 Across the group, the results show the expected trend of left hemisphere asymmetry in the inferior frontal, temporal, and inferior parietal regions, which is highly consistent with the functional neuroanatomy of language.
Figure 2.
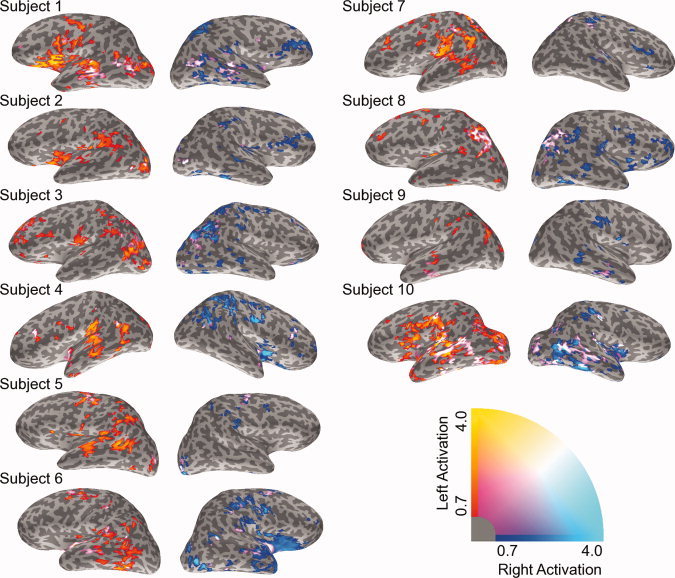
Language‐related laterality maps are shown for all healthy control subjects. All subjects were right handed. Left and bilateral activation are shown on the left hemisphere. Right and bilateral activation are shown on the right hemisphere. Left lateralized activation is depicted as red‐to‐yellow and right lateralized activation is depicted as blue‐to‐cyan. Bilateral activation is shown as the combination of both color scales (purple). Significant activation only is shown (P < 0.01).
Figure 3 shows language laterality calculated as both (a) LI and (b) CLV scores for each ROI and for all control subjects. Using a standard cutoff (LI score = ±0.1), 8/10 controls were left dominant for language based on the combined ROIs. The angle of the CLV closely corresponds to a measure of laterality. The length of the x (right) and y (left) components of the CLV represent the magnitude of activation in each hemisphere. In the CLV plots (Fig. 3b), the line x = y is drawn which would correspond to an LI of 0. Vectors to the left of that line indicate leftward laterality, and vectors right of that line, rightward laterality. It can be seen, however, that this measure provides richer information than the simple LIs plotted in the top of Figure 3, as the length of the vector varies with activation strength. Thus, a vector along the x = y line will differentiate no or weak activation from strong bilateral activation. In the present data, the CLV scores were consistent with the LI measures in terms of the overall proportion of controls that were left lateralized for language, based on the combined ROI.
Figure 3.
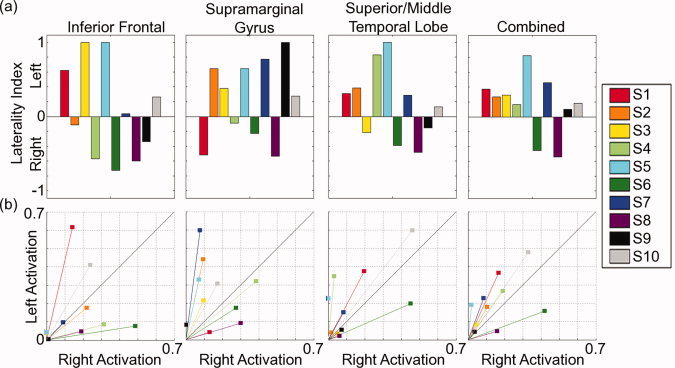
Laterality scores for each ROI and for all healthy control subjects are shown as (a) laterality indices (LIs) and (b) complex laterality vectors (CLVs). LIs are plotted as bars between −1 and 1, indicating right‐to‐left hemisphere dominance. CLVs are plotted as 2D vectors, where angle indicates laterality and length indicates activation magnitude.
Importantly, there were several cases where the LI and CLV data did not agree. These are quite instructive. For example, compare Subjects 6 and 8 for the temporal lobe ROI in Figure 3. The LI indicates strong rightward lateralization for both these subjects. However, in the CLV plots, it can be seen that Subject 6 is truly right lateralized (a long vector right of the x = y line), whereas Subject 8 shows a vector with the same slope as Subject 6, but a much shorter length. In other words, while both of these people demonstrate a similar ratio of left–right activation, only one (Subject 6) actually shows robust levels of activation in the right hemisphere. A second situation can be seen, also for the temporal lobe ROI, in comparing Subjects 9 and 10. These two people showed similar, weak LI strengths, though in opposite directions (i.e., slightly right lateralized for Subject 9, slightly left lateralized for Subject 10). However, the CLV plots reveal a very different story for the two subjects. The CLV score of Subject 9 is quite short, indicating low levels of activation in this region. The CLV score of Subject 10, however, is the longest of any subject for this ROI. In other words, a similar magnitude LI represented weak overall activation in Subject 9, but strong activation in both hemispheres of Subject 10. In both of these cases, the LI scores did not accurately represent the underlying dSPM activation, as shown in the corresponding dSPM activation maps (Supporting Information Fig. 1).
Patient Laterality Maps
Language laterality maps for the patient group are presented in Figure 4 using the same plotting criteria as in Figure 2. LI and CLV scores based on down‐sampled patient laterality maps are shown in Figure 5. Patient laterality maps show a trend similar to healthy controls of dominant activation in the inferior frontal, temporal, and inferior parietal regions. However, examination of the laterality maps and CLV scores revealed a general trend of reduced activation intensity, as compared to the control group.3
Figure 4.
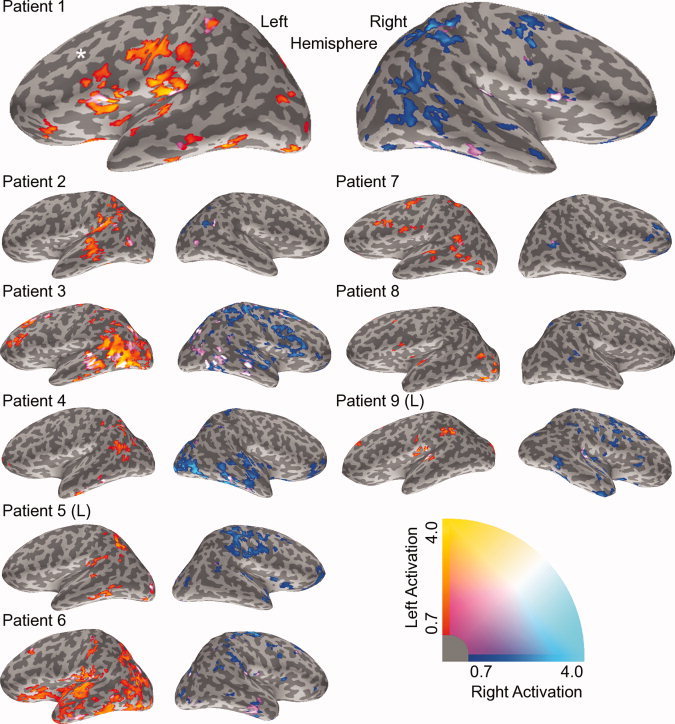
Language‐related laterality maps are shown for all patients (all details as in Fig. 2). Note the spatial sensitivity for bilateral activation in Patient 1. The white asterisk indicates the dipole location for epileptogenic activity, which was colocalized with the cortical dysplasia in the left frontal lobe.
Figure 5.
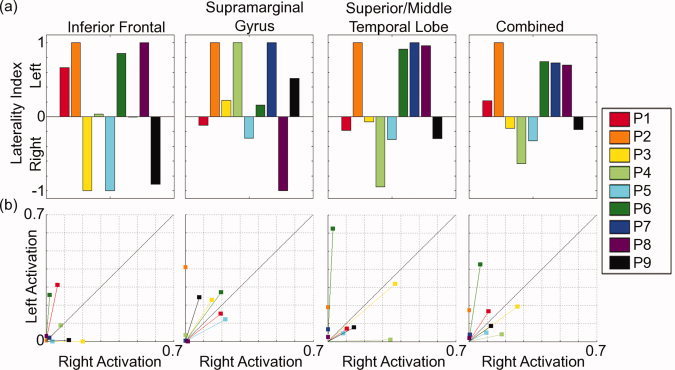
Laterality for each ROI and for all patients is shown as (a) LI scores and (b) CLV scores. All other details as in Figure 3.
Using a standard cutoff (LI score = ±0.1), 5/9 patients were left dominant for language based on the combined ROIs. Again, the CLV scores were consistent with the LI in terms of the overall assessment of laterality summed across ROIs. However, as with the healthy control results, the CLV scores identified instances of strong bilateral activation resulting in low LI scores, and weak unilateral activation resulting in strong LI scores.
For example, a patient case demonstrates the potential value of the spatial data from the laterality maps. Patient 1 (right‐handed female) had a medically refractory seizure disorder caused by a left frontal cortical dysplasia. Previous scalp electroencephalography (EEG) revealed F3 spikes with seizure onset at F3‐Fz. The seizures were proven, by stereotactic EEG depth electrodes, to arise from the depth of the sulcus involved by the cortical dysplasia. MEG spike dipole localization during interictal recording was highly consistent with both the EEG localization and the structural magnetic resonance (MR) findings (Fig. 4, white asterisk). Surgical resection of the cortical dysplasia (Type IIb) resulted in seizure freedom and no observable postoperative cognitive impairment.
For this patient, both LI and CLV scores indicated left lateralization of language processing in the inferior frontal ROI. Examination of the spatial activation data may reduce concern preoperatively given that the lesion was not in the immediate vicinity of the left frontal activation. Preoperative concern would be further reduced through examination of the laterality maps, which showed bilateral contributions from Broca's area and the right hemisphere homolog. Importantly, the standard activation maps would have revealed this spatial distinction, but not the bilateral cluster. Thus, the clinical value of the laterality map is the efficient and effective representation of all data necessary for clinical evaluation in a single image.
DISCUSSION
Overview
The CLV laterality map results were highly consistent with the functional anatomy involved in language (Hypothesis 1). The overall pattern for right‐handed individuals showed a left‐greater‐than‐right asymmetry, predominately involving lateral/inferior frontal, superior/posterior temporal, and inferior parietal lobes (Fig. 2). This result is highly consistent with prior MEG/EEG studies of language [Breier and Papanicolaou,2008; D'Arcy et al.,2005; Kujala et al.,2004] as well as fMRI studies aimed at localizing language‐related MEG/EEG components [D'Arcy et al.,2004; Newman et al.,2001]. Furthermore, examination of the healthy control LI and CLV scores showed that 8/10 subjects were left lateralized (combined ROI), consistent with the high proportion of left dominance in right handed persons that is typically reported [Knecht et al.,2000]. The overall pattern of results provides validation of the method and sample characteristics.
Importantly, the CLV and laterality maps contributed critical information when compared to the LI scores (Hypothesis 2). CLV scores identified instances where low LI scores resulted from strong bilateral activation and strong LI scores resulted from weak unilateral activation. In these instances, the LI scores incorrectly represented the underlying activation. Resolving these ambiguities would require referring to the original activation data, which has inherent complications discussed below.
The challenge with the original activation data relates to spatial sensitivity for laterality. That is, the activation data contain no laterality metric. Plotting CLVs as a laterality map on the individual's MRI increases the clinical utility by improving spatial sensitivity when identifying vital regions of hemispheric dominance and/or bilateral activation. Evidence for this was seen in our first single patient example. The first patient case had undergone surgical resection of a left frontal cortical dysplasia (Fig. 4; right‐handed female). Surgery resulted in a seizure‐free outcome and no postoperative cognitive impairment. In this instance, both LI/CLV scores and the activation data indicated left lateralization for the inferior frontal ROI. Examination of the laterality maps showed bilateral activation in Broca's area (and the right homolog), which was not located in the planned resection zone. Only the spatial sensitivity of laterality maps could demonstrate this important bilateral cluster.
LIs calculated from brain imaging data have typically collapsed activation across one or more specific ROIs [Kim and Chung,2008; Papanicolaou et al.,2004; Woermann et al.,2003]. These approaches compress clinically valuable information into a single numerical index, which is taken as representative of a highly complex situation. However, the intended application of identifying laterality is to spare specific brain areas involved in critical cognitive functions. During surgical assessment, it is arguably more beneficial to fully express the laterality distribution. One can posit then that ROIs can be refined for this purpose. Extended to the spatial limit though, this argument leads to the laterality maps presented here. The laterality map effectively achieves spatial sensitivity by taking better advantage of the high localization accuracy of neuroimaging data. When computed from MEG data, it is further possible to utilize temporal resolution by examining the maximal time points for language‐related activation. This, combined with the spatial information, allows for improved specificity and sensitivity.
Future Directions
This study proposes that the concept of laterality be represented in a more complex framework than that of one‐dimensional “left/right dominant” dichotomy. With respect to language function, this is consistent with the general understanding that bilateral language processing is common [Woermann et al,2003]. It is important to note that CLV scores and the laterality mapping method we have presented can be applied to other cognitive functions (e.g., memory) and other imaging modalities (e.g., fMRI). Indeed, the laterality map provides a modality independent framework in which MEG and fMRI results can be compared directly. Future work in this area will examine multimodal differences between fMRI and MEG in mapping language laterality.
LI scores provided the immediate comparison for CLV score and laterality maps. Follow‐up comparisons against the Wada test and outcome are necessary and can be implemented with existing datasets related to presurgical noninvasive functional mapping for language and, by extension, memory. In this respect, it is noteworthy that previous studies report differences in the predictive power between different anatomical regions/ROIs [e.g., Bowyer et al.,2005; Sabsevitz et al.,2003]. However, the addition of magnitude and spatial information clearly complicates any evaluation of regional differences in LI scores (Figs. 3 and 5).
According to the recent Clinical Practice Guidelines for Presurgical Functional Brain Mapping using Magnetic Evoked Fields [Burgess et al.,2011], reporting MEG laterality results require the following:
“When calculated, the LI should be stated, along with a clear statement of which hemisphere is language dominant (left dominant, right dominant, bilateral, and inconclusive).”
“Plotting of language‐related data on spatially aligned MRI is at the discretion of each site and should be based on their experience concerning the reliability of localization information. Such plots may give the impression to neurosurgeons that areas without plotted activity are safe to resect. This type of error (false‐negative) cannot be excluded systematically, so qualifying statements may be appropriate.” (pp. 361)
The first point underscores the importance of the CLV scores disambiguating laterality in terms of relative differences in magnitude. The current results demonstrate that LI scores alone cannot appropriately demonstrate left dominant, right dominant, bilateral, and inconclusive situations. The second point underscores the importance of the spatial sensitivity in mapping laterality rather than activation. This approach provides improved sensitivity, as lateral and bilateral activations become an emergent property of the map. In addition, the patient example demonstrates improved specificity, as important clusters are better detected. Gains in sensitivity and specificity should translate into improved capabilities for evaluating potential clinical outcome.
CONCLUSIONS
Spatiotemporal laterality mapping with MEG provides a noninvasive alternative to riskier hospital procedures such as the Wada test. Importantly, the results of the current study demonstrate that CLV and laterality maps take better advantage of the rich information within MEG for the clinical implementation of presurgical functional mapping.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1
Supporting Information Figure 2
Acknowledgements
The authors thank Wendy Smith‐D'Arcy and Patrick McGrath for their assistance with this study. The authors also thank Elekta NeuroMag® and Elekta Atlantic for invaluable contributions to the work.
Footnotes
10–20 electroencephalography (EEG) data were also recorded using a MEG compatible electrode cap (Elekta Neuromag®, Helsinki, Finland). The EEG data were compared to the MEG data for verification, but did not otherwise add a significant contribution to the results.
Healthy control dSPM activation maps used to derive the laterality maps are provided in Supporting Information Figure 1.
Patient dSPM activation maps used to derive the laterality maps are provided in Supporting Information Figure 2.
REFERENCES
- Baxendale S. ( 2009): The Wada test. Curr Opin Neurol 22: 185–189. [DOI] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, Benbadis S, Frost JA, Rao SM, Haughton VM. ( 1996): Determination of language dominance using functional MRI: A comparison with the Wada test. Neurology 46: 978–984. [DOI] [PubMed] [Google Scholar]
- Binder JR, Sabsevitz DS, Swanson SJ, Hammeke TA, Raghavan M, Mueller WM. ( 2008): Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia 49: 1377–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR. ( 2011): Functional MRI is a valid noninvasive alternative to Wada testing. Epilepsy Behav 20: 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer SM, Moran JE, Weiland BJ, Mason KM, Greenwald ML, Smith BJ, Barkley GL, Tepley N. ( 2005): Language laterality determined by MEG mapping with MR‐FOCUSS. Epilepsy Behav 6: 235–241. [DOI] [PubMed] [Google Scholar]
- Breier JI, Papanicolaou AC. ( 2008): Spatiotemporal patterns of brain activation during an action naming task using magnetoencephalography. J Clin Neurophysiol 25: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess RC, Barkley GL, Bagic AI. ( 2011): Turning a new page in clinical magnetoencephalography: Practicing according to the first clinical practice guidelines. J Clin Neurophysiol 28: 339–340. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. ( 2000): Dynamic statistical parametric mapping: Combining fMRI and MEG for high‐resolution imaging of cortical activity. Neuron 26: 55–67. [DOI] [PubMed] [Google Scholar]
- D'Arcy RC, Ryner L, Richter W, Service E, Connolly JF. ( 2004): The fan effect in fMRI: Left hemisphere specialization in verbal working memory. Neuroreport 15: 1851–1855. [DOI] [PubMed] [Google Scholar]
- D'Arcy RC, Service E, Connolly JF, Hawco CS. ( 2005): The influence of increased working memory load on semantic neural systems: A high‐resolution event‐related brain potential study. Brain Res Cogn Brain Res 22: 177–191. [DOI] [PubMed] [Google Scholar]
- D'Arcy RC, Gawryluk JR, Beyea SD, Hajra SG, Feindel KW, Clarke DB. ( 2011): Tracking cognitive changes in new‐onset epilepsy: Functional imaging challenges. Epilepsia 52( Suppl 4): 43–46. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. ( 2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31: 968–980. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. ( 2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14: 11–22. [DOI] [PubMed] [Google Scholar]
- Funke M, Constantino T, Van Orman C, Rodin E. ( 2009): Magnetoencephalography and magnetic source imaging in epilepsy. Clin EEG Neurosci 40: 271–280. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. ( 1959): On methods in the analysis of profile data. Psychometrika 24: 95–111. [Google Scholar]
- Hirata M, Goto T, Barnes G, Umekawa Y, Yanagisawa T, Kato A, Oshino S, Kishima H, Hashimoto N, Saitoh Y, Tani N, Yorifuji S, Yoshimine T. ( 2010): Language dominance and mapping based on neuromagnetic oscillatory changes: Comparison with invasive procedures. J Neurosurg 112: 528–538. [DOI] [PubMed] [Google Scholar]
- Jones SE, Mahmoud SY, Phillips MD. ( 2011): A practical clinical method to quantify language lateralization in fMRI using whole‐brain analysis. NeuroImage 54: 2937–2949. [DOI] [PubMed] [Google Scholar]
- Kamada K, Sawamura Y, Takeuchi F, Kuriki S, Kawai K, Morita A, Todo T. ( 2007): Expressive and receptive language areas determined by a non‐invasive reliable method using functional magnetic resonance imaging and magnetoencephalography. Neurosurgery 60: 296–306. [DOI] [PubMed] [Google Scholar]
- Kim J, Chung C. ( 2008): Language lateralization using MEG beta frequency desynchronization during auditory oddball stimulation with one‐syllable words. NeuroImage 42: 1499–1507. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein EB, Henningsen H. ( 2000): Handedness and hemispheric language dominance in healthy humans. Brain 123: 2512–2518. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kuriki S. ( 1999): Principal component elimination method for the improvement of in evoked neuromagnetic field measurements. IEEE Trans Biomed Eng 46: 951–958. [DOI] [PubMed] [Google Scholar]
- Kujala A, Alho K, Service E, Ilmoniemi RJ, Connolly JF. ( 2004): Activation in the anterior left auditory cortex associated with phonological analysis of speech input: Localization of the phonological mismatch negativity response with MEG. Brain Res Cogn Brain Res 21: 106–13. [DOI] [PubMed] [Google Scholar]
- Lagerlund TD, Sharbrough FW, Busacker NE. ( 1997): Spatial filtering of multichannel electroencephalographic recordings through principal component analysis by singular value decomposition. J Clin Neurophysiol 14: 73–82. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Thesen T, Hagler DJ Jr, Carlson C, Devinksy O, Kuzniecky R, Barr W, Gharapetian L, Trongnetrpunya A, Dale AM, Halgren E. ( 2009): Distributed source modeling of language with magnetoencephalography: Application to patients with intractable epilepsy. Epilepsia 50: 2256–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses SN, Ryan JD, Bardouille T, Kovacevic N, Hanlon FM, McIntosh AR. ( 2009): Semantic information alters neural activation during transverse patterning performance. NeuroImage 46: 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AJ, Pancheva R, Ozawa K, Neville HJ, Ullman MT. ( 2001): An event‐related fMRI study of syntactic and semantic violations. J Psycholinguist Res 30: 339–364. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Sarkari S, Pataraia E, Billingsley RL, Buchanan S, Wheless J, Maggio V, Maggio WW. ( 2004): Magnetocephalography: A noninvasive alternative to the Wada procedure. J Neurosurg 100: 867–876. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. ( 2004): Revisiting Snodgrass and Vanderwart's object pictorial set: The role of surface detail in basic‐level object recognition. Perception 33: 217–236. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Swanson SJ, Hammeke TA, Spanaki MV, Possing ET, Morris GL III, Mueller WM, Binder JR. ( 2003): Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology 60: 1788–1792. [DOI] [PubMed] [Google Scholar]
- Sharan A, Ooi YC, Langfitt J, Sperling MR. ( 2011): Intracarotid amobarbital procedure for epilepsy surgery. Epilepsy Behav 20: 209–213. [DOI] [PubMed] [Google Scholar]
- Stufflebeam SM, Tanaka N, Ahlfors SP. ( 2009): Clinical applications of magnetoencephalography. Hum Brain Mapp 30: 1813–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Kajola M, Simola J. ( 2004): Suppression of interference and artifacts by the signal space separation method. Brain Topogr 16: 269–275. [DOI] [PubMed] [Google Scholar]
- Van der Haegen L, Cai Q, Seurinck R, Brysbaert M. ( 2011): Further fMRI validation of the visual half field technique as an indicator of language laterality: A large‐group analysis. Neuropsychologia 49: 2879–2888. [DOI] [PubMed] [Google Scholar]
- Wada J. ( 1949): A new method for the determination of the side of cerebral speech dominance. A preliminary report of the intra‐carotid injection of sodium amytal in man. Igaku to Seibutsugaki 14: 221–222. [Google Scholar]
- Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, Okujava M, Wolf P, Tuxhorn I, Ebner A. ( 2003): Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology 61: 699–701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1
Supporting Information Figure 2


