Abstract
The self has been the topic of philosophical inquiry for centuries. Neuropsychological data suggest that the declarative self can be fractionated into three functionally independent systems processing personal information at several levels of abstraction, including episodic memories of one's own life (episodic autobiographical memory, EAM), semantic knowledge of facts about one's own life (semantic autobiographical memory, SAM), and semantic summary representations of one's personal identity (conceptual self, CS). Our proposal here was to present a comprehensive description of the neural networks underpinning self‐representations. To this aim, we performed three meta‐analyses, one each for EAM, SAM, and CS, using the activation likelihood estimation (ALE) method. We expected a shift from posterior to anterior structures associated with the incrementally increasing level of abstraction of self‐representations. The key finding was that EAM predominantly activates posterior and limbic regions including hippocampus. SAM is associated with anterior activations and also posterior and limbic activations in a lesser degree than EAM. CS mainly recruits medial prefrontal structures. Interestingly, medial prefrontal cortex is activated irrespective of the level of abstraction, but a more caudal part is recruited during CS, while SAM and EAM activate more rostral portions. To conclude, in line with the previous proposals, our results corroborate the idea that the declarative self is not monolithic but a multidimensional construct comprising distinct representations at different levels of abstraction. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: self, autobiographical memory, episodic memory, semantic memory, neuroimaging, meta‐analysis, MPFC
INTRODUCTION
The study of the neural bases of the self has attracted increasing attention in the last decades. One of the challenges for the scientific study of this topic is to give a coherent and operational definition of the self. The concept of self is indeed very complex and has been the topic of philosophy for centuries. Its representation encompasses different aspects ranging from low‐level bodily perception to highly cognitive processes and social values such as the body schema, body ownership, agency, self‐traits, expectancies and values [Klein,2010]. These processes of self‐representation can be practically divided into implicit and declarative aspects of the self. Although implicit processes such as body ownership and agency are linked to proprioception and action planning and remain outside of awareness most of the time, other aspects of the self are tightly connected with explicit memory processes and require high‐level cognitive and metacognitive functions [Klein,2010].
This “declarative self” can be further analyzed as a set of three functionally independent but highly connected systems: episodic memories of one's own life, semantic knowledge of facts about one's own life, and semantic summary representations of one's personal identity. This proposal has been confirmed and developed by several authors based on experimental and neuropsychological studies [Conway,2005; Haslam et al.,2010; Klein,2010], although their terminology differs slightly (e.g., episodic memories in Conway's model versus episodic self‐knowledge in Klein and Haslam's models). All these authors consider that one's sense of self fundamentally depends on memories of one's past experiences. Klein [2010] for example argued that episodic memories and semantic self‐knowledge have an essential role in accounting for a person's knowledge that he or she possesses some traits but not others. For clarity, we will refer to this distinction using the following terminology: episodic autobiographical memory (EAM), semantic autobiographical memory (SAM) [Addis et al.,2004; Levine et al.,2004; Murphy et al.,2008], and the conceptual self (CS) [Conway,2005; Duval et al.,2007; Fitts and Warren,1996]. EAM consists of concrete and specific items of personal information that are closely related to unique autobiographical events situated in a specific time and place [Piolino et al.,2009; Tulving,2002], which refer to the individual in relation to a specific episodic context (e.g., “The first time I kissed my beloved in a wonderful small village in Italy, it was a warm evening in August…”). SAM contains semantic personal information, comprising general knowledge of personal facts (e.g. “My name is X,” information about friends and common locations), but also general events encompassing both repeated and extended events, (e.g., “first job”, “weekends at the country house,” and “that holiday in Italy”). SAM is associated with noetic consciousness involving the awareness of general facts about personal events accompanied by a sense of simply “knowing” without contextual details, while EAM is associated with autonoetic consciousness that gives rise to the sense of phenomenal recollection in the mental re‐enactment of previous personal events [Tulving et al.,1988,2002]. CS is stored in semantic memory in the form of summaries of personal beliefs, values, and attitudes [Conway,2005], self‐knowledge of personality traits [Klein,2010], and judgments on a number of categories of self‐identity [Haslam et al.,2010] that represent our personal identity. We will focus our investigation on the semantic summaries of self‐knowledge of personality traits. The reason for this choice is explained in more detail in the following. For a schematic illustration of the three levels of self‐representation, see Figure 1.
Figure 1.
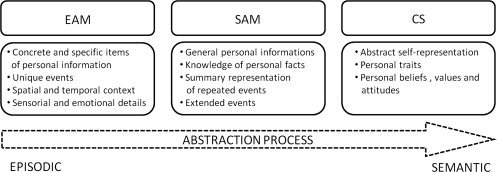
Schematic representation of the three levels of abstraction of the self with the corresponding cognitive processes.
These multiple systems and their functional independence have been reported in numerous neuropsychological studies of patients with memory disorders [Klein and Gangi,2010]. In most of these neuropsychological cases, EAM is deficient while SAM and trait‐knowledge are preserved. Tulving showed that K.C., an amnesic patient, possessed accurate and detailed knowledge about his postaccident facts and personality traits despite having no conscious access to any episodic memories from which he could infer that knowledge [Tulving et al.,1988,1993]. Studies on semantic dementia, a pathological state characterized by a gradual breakdown in general semantic knowledge in which patients gradually lose their knowledge of objects, concepts, famous people, and public events [Hodges and Patterson,2007], showed the reverse pattern (i.e., deficits of SAM and spared EAM [Piolino et al.,2003]). In the same vein, CS seems to be partially independent from SAM and EAM. In a recent case study, Klein and Lax [2010] presented results supporting the idea that personality trait‐knowledge is a specific type of semantic knowledge that can be preserved even when EAM and SAM are altered (see also Duval et al. [revision]). Additionally, studies on Alzheimer's disease have shown that the progressive loss of SAM in addition to EAM deficits leads to an inability to upgrade one's trait self‐concept and impacts the integrity of identity [Addis and Tippet,2004; Klein et al.,2003]. Klein and Lax [2010] suggested that EAM and SAM may constitute a potential source for CS but that judgments about one's own personality may be immediately available and precomputed summaries of the dispositions that one has manifested in various behavioral episodes. All these neuropsychological cases favor the view that the self is composed of multiple systems—i.e., EAM, SAM, and CS—which, while functionally isolable in neuropsychological patients, normally operate in interconnection.
According to Conway's model [2005,2009], these systems are organized hierarchically from highly abstract self‐concepts such as personal beliefs, attitudes, and self‐images (CS) through semantic self‐knowledge (SAM) to specific and experience‐near knowledge on unique events (EAM). During AM retrieval, most EAM are indirectly accessed via a chain of activation from CS and SAM. Moreover, most semantic self‐representations (SAM and CS) emerge from the summary of episodes that yield abstracted scripts and concepts (see also Klein and Gangi [2010] and Haslam et al. [2010]). For example, general events knowledge is supposed to be generated by the repetition of similar events producing a shift from knowledge about specific to general events, that is, from episodic memory to semantic knowledge [Cermak,1982; Conway et al.,1997; Piolino et al.,2006]. Also, according to this model, information about one's own personality traits is abstracted from episodes and behaviors [Klein and Lax,2010]. Therefore, AM plays a fundamental role in the formation of self‐identity and the experience of personhood, as AM retrieval can sustain or change aspects of the self [Conway and Pleydell‐Pearce,2000; Klein and Lax,2010].
Although the interconnection of the self and memory systems is behaviorally well established [Rogers et al.,1977; Symons and Johnson,1997] and theoretically described [Conway,2005; Haslam et al.,2010; Klein,2010], at present, little is known about their interconnections at the neural level. Work on the neural correlates of the self has generally studied mainly self‐referential processing, involving the more abstract level of self‐representation, while the more concrete levels of the self have been quite exclusively considered within the scope of AM research itself. Neural correlates of the different levels of self‐representations, from episodic memories to the CS, are briefly summarized in the following.
The conceptual proximity of AM and self‐referential processing is often evoked but rarely examined, especially at the neural level. Svoboda et al. [2006] proposed in their meta‐analysis that AM is supported by a predominantly left‐lateralized network comprising the medial and ventrolateral prefrontal cortices, medial and lateral temporal cortex, posterior cingulate cortex (PCC), temporo‐parietal junction, and the cerebellum. Beyond this core network, activations have also been reported, albeit less frequently, in dorsolateral prefrontal cortex (dLPFC), superior medial and lateral frontal cortex (Ba 6), anterior cingulate cortex (ACC), medial orbitofrontal cortex, polar temporal cortex, the occipital cortices, the thalamus, and the amygdala [Daselaar et al.,2008; Maguire,2001; Maguire and Frith,2003b; Svoboda et al.,2006]. Differences in activations between studies may be principally explained by the type of control task and the nature of autobiographical representations retrieved (i.e., semantic or episodic).
Based on a neurophysiological study, Conway et al. [2003] suggested that when searching for EAM, left fronto‐temporal activations reflect the initial strategic and generative process via SAM and CS while reliving specific EAM preferentially engages the right hemisphere, especially the posterior cortical regions (see also Cabeza and St Jacques [2007]). More recently, Conway [2009] suggested that fronto‐temporal and temporo‐occipital regions are responsible for personal knowledge and episodic details, respectively. Indeed, left prefrontal and middle temporal activations seem to be associated with the initial search for semantic information at the beginning of the AM retrieval process [Svoboda et al.,2006], while reliving episodic experience seems to be directly linked to posterior structures and hippocampal formations, apparently regardless of the age of memories [Nadel and Moscovitch,1997; Moscovitch et al.,2005; Piolino et al.,2009; Viard et al.,2007,2010]. The network underpinning SAM encompasses the right inferior temporal gyrus, medial frontal cortex, and left thalamus [Addis et al.,2004]. Several experiments have reported that the neural network that sustains the processing of the familiarity of general personal information is linked to ACC, PCC, and retrosplenial cortex [Donix et al.,2010a; Gobbini et al.,2004]. In a study distinguishing between EAM and SAM activations, Levine et al. [2004] found that both EAM and SAM engage left anteromedial prefrontal cortex associated with self‐reference processes, but EAM retrieval did so to a greater extent and specifically engaged medial temporal, posterior cingulate, and diencephalic regions. Moreover, Addis et al. [2004] showed that EAM was more strongly associated with the activation of regions involved in imagery, including the left precuneus, left superior parietal lobule, and right cuneus, while SAM was linked to the activation of the right inferior temporal gyrus, right medial frontal cortex, and left thalamus. In a recent study, Holland et al. [2011] showed that prefrontal cortex and the lateral temporal lobe were mainly engaged by EAM during the initial search process and by SAM during the elaboration process.
The neural correlates of CS are frequently investigated by asking participants to judge whether a trait adjective describes their personality: the operations required are referred to as self‐referential processing. Self‐referential processing involves a complex set of cognitive functions involved in the processing of stimuli that are experienced as strongly related to one's own person [Northoff et al.,2006]. However, given its broad definition, CS could also be recruited in virtually any aspect of real life such as decision making, feeling, or attribution of social emotions as guilt, shame or pride, and mental state attribution as belief. Each of these aspects constitutes a well‐defined and independent research topic in neuroimaging literature. Here, it is important to distinguish between the content and the process of self‐reference. The former refers to representations concerning the self stored in memory, and the latter involves the very process of experiencing and interacting with the world in first person and is intrinsic to all the processes listed earlier. For example, basic emotions, but also social emotions [Shin et al.,2000; Takahashi et al.,2008], are studied by inducing different moods by means of pictures, faces, videos, or scripts presentation depicting different emotions (for a recent review, see [Vytal and Hamann,2010]). Usually, the crucial difference between conditions in these studies is the emotionality (e.g., positive vs. neutral situation) and not the self‐referential aspect (e.g., me vs. others). This is mainly due to the process of interest, emotion, but it is also the consequence of the very nature of this process, which is intrinsically self‐referential and for which it is quite difficult to disentangle a self from a non‐self emotional processing. This is in line with the findings on the neural correlates of empathy showing a common network for personal feeling and seeing emotions in others (for a recent meta‐analysis, see Fan et al. [2011]). For these reasons, in this work, we focused on self‐referential processes based on self‐trait judgment as a paradigm to study the abstracted representational content of one's personal identity (CS), because this paradigm allows, compared to non‐self judgment using similar material, to isolate the self‐dimension per se. Converging evidence suggests an essential role for the medial prefrontal cortex (MPFC) [D'Argembeau et al.,2007; Kelley et al.,2002] and other medial posterior regions (PCC and the precuneus) [Fossati et al.,2003; Johnson et al.,2002; Kircher et al.,2002; Schmitz et al.,2004]. However, only ventromedial prefrontal cortex (vMPFC) seems to be specifically involved in self‐referential processing (for a recent meta‐analysis, see van der Meer et al. [2010]), while other midline cortical regions seem to be involved in reflective processes more generally [Legrand and Ruby,2009]. Van der Meer et al. [2010] argued that the vMPFC may be specifically linked to the affective processing of self‐relevant information, the dorsal MPFC (dMPFC) involved in evaluation, decision‐making related to determining whether a certain stimulus is applicable to the self or to another person, and the posterior cingulate involved in the access to AM. To summarize, on this view, MPFC, and particularly its ventral component, plays a pivotal role in processing information related to the CS. Moreover, several studies have especially pointed to the role of left‐hemispheric superior and inferior prefrontal cortex and lateral temporal cortex in semantic memory when people are required to judge trait adjectives for self‐descriptiveness [Craik et al.,1999; Kelley et al.,2002; D'Argembeau et al.,2007].
Here, we wanted to give a unitary account of the neural bases of these three different levels of self‐representation using a meta‐analytic procedure. To this end, we searched for neuroimaging studies investigating the functions of interest—EAM, SAM, and CS—and included only studies that compared these functions with high‐level processes that differ in terms of self‐relevance. Previous meta‐analyses have been conducted separately on CS and AM material.
Previous quantitative meta‐analyses concerning EAM have focused on the neural substrates of episodic memories formed in the laboratory context [Spaniol et al.,2009] or on the differences between EAM and laboratory episodic memory [McDermott et al.,2009]. Although the latter study included experiments using several types of control condition including either low‐ or high‐level cognitive tasks, we selected only studies that compared EAM with a control condition that required memory processes. Although various meta‐analyses explore the neural substrate of several aspects of AM processes, no study to date has fully addressed the brain correlates of SAM [Svoboda et al.,2006]. Here, we pooled together studies requiring subjects to access memories for general personal information (e.g., familiar people, places, names, and faces) and generalized events (e.g., information about extended and repeated events).
Legrand and Ruby [2009] offered a qualitative synthesis of major results on self‐representation, other‐representation, and recall. Northoff et al. [2006] compared neural correlates during processing of stimuli related to the self with those of non‐self‐referential stimuli with a special focus on cortical midline structures. In another recent study, van der Meer et al. [2010] used a quantitative method to shed light on self‐reflective processes, focusing uniquely on self‐referential processing. In our meta‐analysis of the CS condition, because we were interested in structures engaged in the treatment of strictly self‐related material, we reproduced the part of this meta‐analysis comparing self versus other trait judgments, excluding studies that did not fit the purpose and inclusion criteria of this work as listed earlier (e.g., we excluded studies requiring episodic retrieval of information learned in the laboratory).
To examine the functional independence of the three levels of representation [Conway,2009; Haslam et al.,2010; Klein and Gangi,2010], we performed three separate meta‐analyses using the activation likelihood estimation (ALE) method [Laird et al.,2005; Turkeltaub et al.,2002] for EAM, SAM, and CS (see Fig. 1). We also tested statistical differences in brain activations between each condition, using meta‐analytic subtraction to explore the distinct structures involved in each of these processes. Our main hypothesis was a distinct pattern of activation for each type of self‐content, with a shift from posterior to anterior structures associated to the gradually increasing abstraction of the relevant level of representation (from EAM to CS). In sum, the main goal of this work was to study common and distinct substrates of self‐representations from experience‐grounded events (EAM) to semantic self information (SAM) to completely abstract representation (CS), filling a gap in the literature between self‐referential processing and AM.
MATERIALS AND METHODS
Studies Selection
Articles included in the present meta‐analysis were identified by a literature search using specific terms, depending on the condition, in the PubMed database and recent meta‐analyses published up to March 2011. For the EAM condition, the query terms used were “(autobiographical memory) AND Episodic AND (fMRI OR PET).” Articles for the SAM were identified using a PubMed search with “(autobiographical memory) AND (Semantic OR personal information) AND (fMRI OR PET)” as keywords. For the CS condition, articles were identified through two recent meta‐analyses [Northoff et al.,2006; van der Meer et al.,2010] and a PubMed search “(Personal Traits) AND (fMRI OR PET).” For all three categories, we identified additional studies by searching through the reference lists of studies obtained through the initial search.
General Inclusion Criteria
-
1
Studies measuring regional cerebral blood flow (PET), glucose metabolism (PET), or blood oxygenation (fMRI). Studies including whole brain statistics were included, while studies reporting only region‐of‐interest analyses were excluded.
-
2
Articles reporting results as coordinates in a standard reference frame (Talairach and Tournoux or MNI).
-
3
Studies including healthy subjects with no neurological, medical, or psychiatric disorders or substance abuse. Articles including patients were also selected if they reported results for the control group separately.
-
4
Studies including young adults (mean range, 18–59 years) were included to avoid effects due to aging in self‐memory processes [Gutchess et al.,2007; Levine et al.,2002; Piolino et al.,2002].
-
5
Experiments using both auditory and visual cues for memory retrieval (e.g., words, sentences, pictures, photographs, or faces) were included.
-
6
Studies were included independently of the emotional valence of the memory retrieved or of the cue (positive, negative, or neutral).
-
7
If several instances of the same dataset were encountered, only one was used in the meta‐analysis.
Specific Inclusion Criteria
EAM criteria
-
1
Studies that measured brain activity during the retrieval of a personal past event recollected in the context of a particular time and place and with some reference to oneself as a participant in the episode.
-
2
Studies assessing brain correlates of the retrieval of both remote and recent memories were included (from childhood to recent past events).
SAM criteria
-
1
Studies assessing brain correlates of the retrieval of general personal events (both extended in time and repeated) or personal information (including familiar people, objects, places, names, voices, and autobiographical facts).
CS criteria
-
1
Studies measuring brain activity during the judgment of the self‐ and other‐applicability of personal trait descriptions (word or sentence).
Contrast Selection
Thirty‐eight studies (EAM = 13, SAM = 13, and CS = 12) met our criteria, comprising a total of 575 subjects (EAM = 171, SAM = 186, and CS = 210) and reporting 444 foci of activation (EAM = 190, SAM = 184, and CS = 83). All foci were accepted when reported as significant according to the criteria designated in each individual study. Coordinates originally published in MNI space were converted to Talairach space using the Lancaster transformation [Lancaster et al.,2007]. Only activation data were included, while deactivations were not considered. Separate ALE meta‐analyses were conducted to investigate the brain activations related to each condition. An overview of studies and contrasts included is provided in Table I.
Table I.
Overview of studies included in the three meta‐analyses
| Articles | Category | Method | Subjects | Mean age | Exp Cond, Cont | Contrast |
|---|---|---|---|---|---|---|
| Addis et al.,2004 | EAM | fMRI | 14 | 28 | EAM, SAM, Cont, GS | EAM > SAM |
| Cabeza et al.,2004 | EAM | fMRI | 13 | 20.8 | EAM,LEM | EAM > LEM |
| Conway et al.,1999 | EAM | PET | 6 | 34.3 | EAM, LEM, Cont | EAM > LEM |
| Denkova et al.,2006 | EAM | fMRI | 10 | 40.6 | EAM, Cont | EAM > FF |
| Donix et al.,2010b | EAM | fMRI | 15 | 28 | EAM, SAM | EAM > SAM |
| Gilboa et al.,2004 | EAM | fMRI | 9 | 50.7 | EAM, Cont | vivid > nonvivid |
| Greenberg et al.,2005 | EAM | fMRI | 11 | 18–25 | EAM, Cont | EAM > GS |
| Levine et al.,2004 | EAM | fMRI | 5 | 26–37 | EAM, SAM, GS, other events | EAM > SAM + GS + other events |
| Mayes et al.,2004 | EAM | fMRI | 9 | 22 | EAM, GS | EAM > GS |
| Oddo et al.,2010 | EAM | fMRI | 15 | 20.8 | EAM, PE | EAM > PE |
| Okuda et al.,2003 | EAM | fMRI | 12 | 20.7 | EAM, Future Events, GS | EAM > Cont |
| Summerfield et al., 2008 | EAM | fMRI | 18 | 25.1 | EAM, LEM, IE, Cont | EAM‐Cont > IE‐Cont |
| Vandekerckhove et al.,2005 | EAM | fMRI | 16 | 21–32 | EAM, Cont | EAM > Cont |
| Addis et al.,2004 | SAM | fMRI | 14 | 28 | EAM, SAM, Cont, GS | SAM > EAM |
| Donix et al.,2010a | SAM | fMRI | 12 | 30.4 | FF, FP, UF, UP | FF + FP > UF + UP |
| Gobbini et al.,2004 | SAM | fMRI | 10 | 26.8 | FF, UF, FamF | FF > UF |
| Leibenluft et al.,2004 | SAM | fMRI | 7 | 20–40 | FF, UF | FF > UF |
| Levine et al.,2004 | SAM | fMRI | 5 | 26–37 | EAM, SAM, GS, other events | SAM > EAM |
| Maddock et al.,2001 | SAM | fMRI | 8 | 22–45 | FN, UN | FN > UN |
| Maguire et al.,2003a | SAM | fMRI | 12 | 32.4 | EAM, SAM, PE, GS, Cont | SAM > Cont |
| Nakamura et al.,2000 | SAM | PET | 7 | 23–29 | FF, FP, Cont | (FF‐Cont) + (FP‐Cont) |
| Shah et al.,2001 | SAM | fMRI | 10 | 28.5 | FV, FF, UV, UF | FF + FV > UF + UV |
| Sugiura et al.,2005 | SAM | fMRI | 25 | 18–31 | FP, FO, UP, UO | FO + FP > UF + UO |
| Sugiura et al.,2006 | SAM | fMRI | 24 | 18–25 | FN, UN, FamN | FN > UN |
| Sugiura et al.,2009 | SAM | fMRI | 28 | 18–32 | FN, UN, FamN | FN > UN |
| Sugiura et al.,2011 | SAM | fMRI | 24 | 19–31 | FF, UF, FamF | FF > UF |
| D'Argembeau et al.,2008 | CS | fMRI | 16 | 21 | STJ, OTJ | STJ > OTJ |
| Gutchess et al.,2007 | CS | fMRI | 19 | 23.11 | STJ, OTJ, PT | STJ > OTJ |
| Heatherton et al.,2006 | CS | fMRI | 30 | 24 | STJ, OTJ, PT | STJ > OTJ |
| Jenkins et al.,2008 | CS | fMRI | 13 | 20.7 | Sop, Oop | Sop > Oop |
| Kelley et al.,2002 | CS | fMRI | 24 | 20 | STJ, OTJ, PT | STJ > OTJ |
| Kjaer et al.,2002 | CS | PET | 7 | 22–27 | STJ,OTJ | STJ > OTJ |
| Modinos et al.,2009 | CS | fMRI | 16 | 20.8 | STJ,OTJ | STJ > OTJ |
| Ochsner et al.,2005 | CS | fMRI | 16 | 29.95 | STJ, OTJ, PT | STJ > OTJ |
| Pfeifer et al.,2007 | CS | fMRI | 12 | 26.1 | STJ, OTJ | STJ > OTJ |
| Schmitz et al.,2004 | CS | fMRI | 19 | 24 | STJ, OTJ, VJ | STJ > OTJ |
| Seger et al.,2004 | CS | fMRI | 12 | 20–32 | STJ, OTJ | STJ > OTJ |
| Zhu et al.,2007 | CS | fMRI | 26 | 22.3 | STJ, OTJ | STJ > OTJ |
EAM, episodic autobiographical memory; SAM, semantic autobiographical memory; CS, conceptual self; Cont, control; GS, general semantic; LEM, laboratory episodic memory; PE, public events; IE, imagined events; FF, famous faces; FP, familiar places; UP, unfamiliar places; FO, familiar objects; UO, unfamiliar objects; FN, familiar names; UF, unfamiliar names; FF, familiar faces; UF, unfamiliar faces; FamF, famous faces; FV, familiar voices; UV, unfamiliar voices; STJ, self traits judgment; OTJ, other traits judgment; PT, perception task; VJ, valence judgment; Sop, self‐opinion; Oop, other opinion.
In the EAM condition, autobiographical retrieval had to be compared to a control task that was a memory task (e.g., semantic knowledge, laboratory episodic memory, famous face recognition, retrieval of public events, and general semantic memory including category generation and sentence completion). Contrasts between EAM and a low‐level task were excluded (e.g., rest, perception task, and mental reading). In the SAM condition, contrasts identified had to compare personal general events or personal information with nonpersonal general information (e.g., laboratory episodic memory, general semantic knowledge, unfamiliar faces, names, voices, places, and objects). For the CS meta‐analysis, we selected only contrasts directly comparing self versus other judgment for an adjectival trait.
Statistical Analysis
The technique of ALE meta‐analysis has been recently described [Laird et al.,2005; Turkeltaub et al.,2002]. The original ALE algorithm was modified for the current version (GingerALE 2.0, http://brainmap.org) [Eickhoff et al.,2009]. In short, all reported foci (coordinates of maximum activation) for a given study are modeled as the peaks of a 3D Gaussian probability distribution. A “modeled activation” (MA) map is computed, representing a summary of the results of that specific study. ALE scores are then calculated on a voxel‐by‐voxel basis by taking the union of these individual MA maps. This revised analysis tests for convergence between studies (random effects) rather than foci (fixed effects). Statistical significance was assessed using the analytic solution implemented in the new version of GingerALE [Eickhoff et al.,2009]. To assess statistical significance, a P threshold corrected for multiple comparisons using the false discovery rate was fixed at 0.05 [Genovese et al.,2002; Laird et al.,2005], and a minimum cluster size of 200 mm3 was used. To assess brain correlates for EAM, SAM, and CS, we ran three separate meta‐analyses and overlaid the corresponding ALE map onto an anatomical template generated by spatially normalizing the International Consortium for Brain Mapping template to the Talairach space [Kochunov et al.,2002]. To estimate statistical differences in brain activations between conditions, we used the meta‐analytic subtraction procedure implemented in a previous version of GingerALE (GingerALE 1.2) that uses the permutation technique described in Laird et al. [2005]. The number of spatial permutations was set to 5,000, as generally reported in previous studies (e.g. [Fusar‐Poli et al.,2009; Vytal and Hamann,2010]). Statistical threshold and cluster size were the same used in the three separate meta‐analyses for each condition.
RESULTS
In the EAM meta‐analysis, we found evidence for activations encompassing anterior and posterior cortical regions and subcortical structures. More precisely, we found activation in limbic structures (left hippocampus and bilateral parahippocampal formation), midline cortical structures (MPFC, precuneus, and PCC), and left middle temporal gyrus. The activations revealed by the ALE for SAM tasks fall within ACC and PCC, MPFC, left middle and inferior frontal gyrus, left superior and middle temporal gyrus, left thalamus, left fusiform gyrus, and parahippocampus. The meta‐analysis for CS revealed activations in vMPFC and dMPFC, lateral frontal cortex in both hemispheres, and ACC. The results of the three meta‐analyses are summarized in Figures 2 and 3 and Table II.
Figure 2.
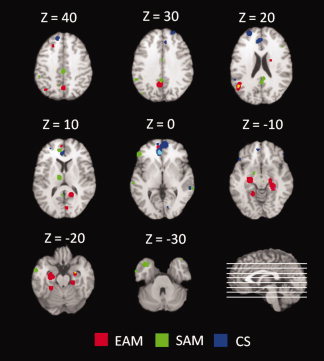
Results of the separate meta‐analyses for EAM, SAM and CS superimposed to axial slices. All activations are significant at P < 0.05 corrected for multiple comparisons using the false discovery rate (FDR). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
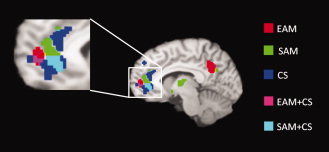
Sagittal view of differential and common MPFC activations for the three levels of self‐representation (EAM, SAM, and CS). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table II.
Peaks of activation for EAM, SAM, and CS
| Region | Brodmann area | Volume (mm3) | Talairach coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| EAM | |||||
| R parahippocampal gyrus | BA 36 | 2776 | 26 | −36 | −10 |
| R parahippocampal gyrus | BA 35 | 20 | −22 | −14 | |
| R culmen | 22 | −30 | −18 | ||
| L hippocampus | 2512 | −26 | −20 | −16 | |
| L precuneus | BA 31 | 2080 | −6 | −58 | 26 |
| R precuneus | BA 7 | 2 | −64 | 40 | |
| L middle temporal gyrus | BA 39 | 1352 | −48 | −64 | 22 |
| L middle temporal gyrus | BA 19 | −48 | −62 | 18 | |
| L parahippocampal gyrus | BA 19 | 760 | −16 | −42 | −4 |
| L culmen | −16 | −50 | −10 | ||
| R posterior cingulate | BA 30 | 672 | 16 | −52 | 8 |
| L medial frontal gyrus | BA 10 | 432 | −6 | 50 | 6 |
| L cerebellum | 328 | −24 | −42 | −20 | |
| SAM | |||||
| R posterior cingulate | BA 23 | 1520 | 6 | −50 | 26 |
| R posterior cingulate | BA 23 | 2 | −50 | 24 | |
| R posterior cingulate | BA 29 | 6 | −42 | 20 | |
| L anterior cingulate | BA 32 | 1096 | −6 | 40 | 4 |
| L medial frontal gyrus | BA 10 | −10 | 44 | 14 | |
| Thalamus | 856 | −6 | −10 | 4 | |
| L superior temporal gyrus | BA 38 | 784 | −32 | 10 | −28 |
| L middle temporal gyrus | BA 21 | −44 | 6 | −26 | |
| L superior temporal gyrus | BA 38 | −38 | 18 | −26 | |
| L middle frontal gyrus | BA 47 | 776 | −48 | 36 | −2 |
| L inferior frontal gyrus | BA 47 | −54 | 26 | −6 | |
| L fusiform gyrus | BA 20 | 712 | −56 | −4 | −24 |
| L middle temporal gyrus | BA 21 | 520 | −60 | −26 | −4 |
| R cingulate gyrus | BA 31 | 400 | 2 | −28 | 38 |
| R parahippocampal gyrus | 392 | 26 | −10 | −16 | |
| R middle frontal gyrus | BA 46 | 368 | 46 | 24 | 24 |
| L parahippocampal gyrus | 208 | −22 | −8 | −16 | |
| CS | |||||
| R medial frontal gyrus | BA 10 | 3288 | 6 | 54 | 2 |
| L anterior cingulate | BA 32 | −6 | 40 | −2 | |
| L medial frontal gyrus | BA 10 | −10 | 56 | 4 | |
| L middle frontal gyrus | BA 10 | 1280 | −24 | 52 | 20 |
| L anterior cingulate | BA 32 | 1128 | −8 | 28 | 4 |
| L anterior cingulate | BA 32 | −2 | 38 | 16 | |
| L medial frontal gyrus | BA 8 | 832 | −12 | 40 | 36 |
| R superior frontal gyrus | BA 9 | 432 | 22 | 52 | 30 |
| R superior frontal gyrus | BA 9 | 28 | 52 | 36 | |
| R anterior cingulate | BA 32 | 368 | 6 | 30 | 26 |
L = Left, R = Right, BA = Brodmann Area
MPFC activations were shared by all conditions, but in slightly different locations along a rostrocaudal gradient from EAM to SAM to CS (Fig. 3 ).
With regard to activations specific to each condition, our meta‐analytic subtraction revealed greater activations in left temporo‐parietal junction, bilateral parahippocampus, precuneus, and PCC in EAM than in the other two conditions (SAM and CS). SAM showed greater activation in MPFC, bilateral middle frontal gyrus, left middle and superior temporal gyrus, left inferior parietal lobe, PCC, and left thalamus compared to CS and EAM. Moreover, in SAM, left parahippocampus was more activated in comparison with CS only. Finally, CS compared to the other two conditions recruited vMPFC and dMPFC, ACC, and left lateral prefrontal cortex to a larger extent than the other two conditions. Activations for each comparison are reported in Figure 4, and the local maxima of activation clusters are detailed in Table III.
Figure 4.
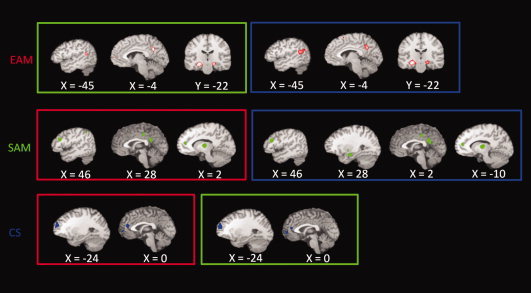
Results of meta‐analytic subtractions between each pair of conditions. First row EAM versus SAM (green) and CS (blue). Second row SAM versus EAM (red) and CS (blue). Third row CS versus EAM (red) and SAM (green). All activations are significant at P < 0.05 corrected for multiple comparisons using the false discovery rate (FDR). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table III.
Peaks of activation of meta‐analytic subtractions between EAM, SAM, and CS
| Region | Brodmann area | Volume (mm3) | Talairach coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| EAM‐SAM | |||||
| R parahippocampal gyrus | BA 36 | 3064 | 26 | −36 | −10 |
| R culmen | 22 | −30 | −18 | ||
| R parahippocampal gyrus | BA 35 | 20 | −22 | −14 | |
| L hippocampus | 1840 | −26 | −20 | −16 | |
| L precuneus | BA 31 | 1344 | −6 | −58 | 28 |
| R precuneus | BA 7 | 2 | −64 | 40 | |
| L culmen | 872 | −16 | −50 | −10 | |
| L parahippocampal gyrus | BA 19 | −16 | −42 | −4 | |
| R posterior cingulate | BA 30 | 808 | 16 | −52 | 8 |
| L superior temporal gyrus | BA 22 | 584 | −44 | −54 | 16 |
| L middle temporal gyrus | BA 19 | −48 | −62 | 14 | |
| L fusiform gyrus | BA 20 | 488 | −30 | −38 | −18 |
| EAM‐CS | |||||
| R parahippocampal gyrus | BA 36 | 3392 | 24 | −34 | −12 |
| Culmen | 20 | −30 | −18 | ||
| R parahippocampal gyrus | BA 35 | 20 | −22 | −14 | |
| R hippocampus | 26 | −12 | −20 | ||
| L hippocampus | 3160 | −26 | −20 | −16 | |
| L middle temporal gyrus | BA 19 | 2080 | −48 | −62 | 18 |
| L superior temporal gyrus | BA 22 | −46 | −56 18 | 18 | |
| L precuneus | BA 31 | 2056 | −6 | −58 | 28 |
| R precuneus | BA 7 | 2 | −64 | −40 | |
| L culmen | 912 | −26 | −42 | −20 | |
| L culmen | 888 | −16 | −50 | −10 | |
| L parahippocampal gyrus | BA 19 | −16 | −42 | −4 | |
| R posterior cingulate | BA 30 | 760 | 16 | −52 | 8 |
| R precuneus | BA 31 | 320 | 12 | −60 | 24 |
| L precuneus | BA 19 | 224 | −38 | −68 | 38 |
| L superior frontal gyrus | BA 6 | −6 | 12 | 60 | |
| R superior frontal gyrus | BA 6 | 2 | 6 | −56 | |
| R caudate | 208 | 20 | −4 | 20 | |
| SAM‐EAM | |||||
| Thalamus | 1176 | −10 | −14 | 6 | |
| R middle frontal gyrus | BA 46 | 608 | 46 | 24 | 24 |
| L middle frontal gyrus | BA 47 | 560 | −48 | 36 | −2 |
| L superior temporal gyrus | BA 38 | 552 | −32 | 8 | −28 |
| L anterior cingulate | BA 24 | 504 | −6 | 38 | 2 |
| L medial frontal gyrus | BA 9 | −10 | 42 | 14 | |
| L posterior cingulate | BA 23 | 496 | 0 | −50 | 24 |
| L fusiform gyrus | BA 20 | 384 | −58 | −4 | −24 |
| L middle temporal gyrus | BA 21 | −60 | −26 | −4 | |
| L superior frontal gyrus | BA 8 | 368 | −26 | 26 | 54 |
| L inferior parietal lobule | BA 40 | 312 | −50 | −40 | 42 |
| L supramarginal gyrus | BA 40 | −52 | −46 | 32 | |
| R cingulate gyrus | BA 31 | 304 | 2 | −28 | 38 |
| L medial frontal gyrus | BA 11 | 280 | −2 | 56 | −14 |
| L lentiform nucleus | 248 | −14 | −4 | −8 | |
| R superior frontal gyrus | BA 6 | 224 | 12 | 12 | 62 |
| SAM‐CS | |||||
| R posterior cingulate | BA 23 | 1776 | 2 | −50 | 24 |
| L posterior cingulate | BA 30 | −4 | −56 | 6 | |
| Thalamus | 1312 | −10 | −14 | 6 | |
| L middle frontal gyrus | BA 47 | 888 | −48 | 34 | −2 |
| L fusiform gyrus | BA 20 | 784 | −56 | −4 | −24 |
| R middle frontal gyrus | BA 46 | 712 | 46 | 24 | 24 |
| L middle temporal gyrus | BA 21 | 560 | −60 | −26 | −4 |
| L middle temporal gyrus | BA 39 | 552 | −52 | −68 | 26 |
| L middle temporal gyrus | BA 39 | −46 | −28 | ||
| L lentiform nucleus | 536 | −14 | −4 | −8 | |
| L parahippocampal gyrus | −22 | −6 | −16 | ||
| R hippocampus | 528 | 26 | −10 | −16 | |
| L superior temporal gyrus | BA 38 | 416 | −36 | 10 | −30 |
| L inferior parietal lobule | BA 40 | 376 | −50 | −40 | 42 |
| L supramarginal gyrus | BA 40 | −50 | −48 | 34 | |
| R cingulate gyrus | BA 31 | 336 | 2 | −28 | 38 |
| L medial frontal gyrus | BA 10 | 248 | −10 | 44 | 14 |
| R middle temporal gyrus | BA 21 | 240 | 54 | 4 | −26 |
| L superior parietal lobule | BA 7 | −40 | −60 | 52 | |
| R lentiform nucleus | 232 | 24 | −6 | −2 | |
| L medial frontal gyrus | BA 11 | 200 | −2 | 58 | −14 |
| CS‐EAM | |||||
| R medial frontal gyrus | BA 10 | 880 | 6 | 56 | 4 |
| L anterior cingulate | BA 32 | 672 | −2 | 38 | 16 |
| L medial frontal gyrus | BA 8 | 592 | −12 | 40 | 36 |
| L middle frontal gyrus | BA 10 | 568 | −24 | 52 | 20 |
| L anterior cingulate | BA 24 | 280 | −6 | 38 | 0 |
| CS‐SAM | |||||
| R medial frontal gyrus | BA 10 | 1344 | 6 | 54 | 2 |
| L middle frontal gyrus | BA 10 | 920 | −24 | 52 | 20 |
| L medial frontal gyrus | BA 38 | 456 | −10 | 42 | 36 |
| R superior frontal gyrus | BA 39 | 256 | 22 | 52 | 30 |
| L anterior cingulate | BA 32 | 216 | 0 | 38 | 16 |
L, left; R, right; BA, Brodmann area.
DISCUSSION
In this study, we conducted a quantitative meta‐analysis of 38 studies for a total of 575 subjects to investigate the neural correlates of different levels of declarative self‐representation. In particular, we carried out three separate analyses to study differences and commonalities in brain activity between EAM, SAM, and CS. The existence of these three interdependent systems that support self‐representation was proposed by Conway [2005] and recently integrated into Klein [2010]'s and Haslam et al. [2010]'s theoretical models of the self as a multiplicity of related contents comprising both personal identity and AM. Our main hypotheses, based on neuropsychological and neuroimaging studies were (1) dissociable activations for the three categories, suggesting some functional independence of the three systems and (2) a posterior‐to‐anterior gradient of activations with the movement from experience‐near to abstract personal information.
The main findings confirmed our hypotheses, showing that each category uniquely activated specific cortical regions as evidenced by meta‐analytic subtractions, with a shift from posterior to anterior structures associated with gradually increasing abstraction of representation from EAM to CS. Indeed, EAM predominantly activated posterior and limbic structures, including hippocampus, whereas CS recruited medial prefrontal structures. SAM was associated with anterior, posterior, and limbic activations, although in a lesser degree than EAM. Moreover, we found differential recruitment of medial prefrontal areas. MPFC was indeed activated irrespective of the abstraction of the self‐representation, but in slightly different locations.
In what follows, we will first discuss the functional role of the specific structures activated in each condition as evidenced by meta‐analytic subtractions. Thereafter, we will focus on MPFC as a core system for self‐representation, as indicated by its engagement in all three conditions, and we will propose a tentative interpretation of the different MPFC activations associated with each condition. We will then describe the postero‐anterior shift in cortical activation associated with the abstraction level of representation. Finally, we will briefly describe the contribution of our results to the understanding of neuropsychological diseases in which different aspects of personal information are dysfunctional.
Functional Independence and Commonality Within the Self‐Memory System
Neural correlates of EAM
In our EAM meta‐analysis, we reported activations encompassing anterior and posterior cortical regions and subcortical structures. More precisely, we found activation in the limbic system (hippocampus and bilateral parahippocampal formation), midline cortical structures (MPFC, precuneus, and PCC), and left middle temporal gyrus. Our results are congruent with the proposal in Maguire [2001]'s meta‐analysis that left hippocampus is more highly activated during EAM retrieval than in SAM, retrieval of public events, or general semantic knowledge [Maguire,2001; Maguire and Mummery,1999; Maguire et al.,2000]. It is important to note that hippocampus was specifically activated in EAM compared to the other two conditions. This is coherent with its role as a pointer to sensory and perceptual details as well as temporal context [Holland et al.,2011].
Voluntary retrieval of EAM, like SAM, depends on self‐referential processing involving MPFC for the construction of personal memory as well as control processes [Cabeza and St Jacques,2007]. During EAM retrieval, monitoring processes are commonly associated with vMPFC [Moscovitch et al.,2005]. The activations of vMPFC during EAM that our results suggest are consistent with Graham et al. [2003] who found vMPFC activations in EAM retrieval compared to general semantic memory. Gilboa et al. [2004] confirmed these results, showing that BA 10 activations were greater with retrieval of episodic autobiographical memories than episodic memories related to laboratory material. Moscovitch and Winocur [2002] suggested the interpretation of BA 10 activations during EAM as the sign of a typical intuitive and preconscious form of monitoring called “feeling of rightness,” very different from the monitoring associated with episodic retrieval of laboratory material (see also Cabeza and St Jacques [2007]). EAM remembering also includes specific vividness and visuospatial processes linked to the uniqueness of past event evocation. These processes are usually associated to the activation of more posterior regions such as precuneus, PCC, and hippocampal formations [Addis et al.,2004; Gardini et al.,2006; Gilboa et al.,2004], as reported in this study. On the other hand, we did not find activations in dLPFC, which is often reported for memory search and controlled retrieval [Cabeza and St Jacques,2007]. This could be due to our choice of contrasts of interest, whose aim was to isolate the “selfness” of the episodic memory processing and that may thus have excluded other processes commonly engaged in episodic memory retrieval more generally.
Neural correlates of SAM
Our meta‐analysis revealed an association between SAM and activations in ACC and PCC, MPFC, bilateral ventrolateral prefrontal cortex (vLPFC), left superior and middle temporal gyrus, left thalamus, left fusiform gyrus, and parahippocampus. SAM engaged left anteromedial prefrontal cortex associated with self‐reference [Levine et al.,2004] and more posterior regions such as temporo‐parietal and parieto‐frontal systems that are known to be involved in spatial egocentric processing and top‐down attentional control, respectively [Levine et al.,2004] and activations in the PCC related to the processing of personal familiarity [Donix et al.,2010a; Epstein et al.,2007; Shah et al.,2001; Sugiura et al.,2005]. Interestingly, our results specifically linked SAM to thalamic activation, compared to EAM and CS. The involvement of the thalamus in SAM, although frequently reported, has seldom been debated. The thalamus is frequently reported to be activated in studies that test general semantic or linguistic processes (i.e., verbal fluency [Senhorini et al.,2011]) and frequency effect [Vannest et al.,2011]); thalamic activation was also reported in a recent study on chronesthesia [Nyberg et al.,2010]. Nyberg et al. [2010] asked trained participants to imagine themselves in a familiar place, varying the moment of imagined time (past, present, and future). Their results demonstrated the implication of bilateral thalamus in the past and future conditions of familiar scene imagination. Indeed, these results may suggest that the thalamus is involved in the evocation of general events. This tempting interpretation is consistent with our results, but it must be taken with caution, and further research is needed before drawing a firm conclusion.
Neural correlates of CS
In neuroimaging studies of CS based on self‐referential processes, activations are frequently reported in medial cortical structures, lateral prefrontal cortex, lateral parietal cortex, bilateral temporal poles, insula, and subcortical regions [D'Argembeau et al.,2007; Northoff et al.,2006; van der Meer et al.,2010]. In our meta‐analysis, we found activations only in MPFC, including both ventral and dorsal prefrontal regions, and in ACC. This was probably due to our choice of contrasts of interest, because we were specifically interested in self‐referential processes and not in reflection processes more generally, we included only conditions contrasting self‐ versus other‐referential processing. The role of the ventral and dorsal parts of the MPFC during self‐referential processing is still under debate. On the one hand, vMPFC has been implicated in emotional processes such as the specific affective processing of self‐referential stimuli [van der Meer et al.,2010], the assessment of the salience of a stimuli [Gusnard et al.,2001], the coupling of emotional and cognitive processes in decision‐making [Bechara et al.,1997], the detection of the self‐relevance of a perceived stimulus [Schmitz and Johnson,2006], and the processing of the emotional component inherent to self‐processing [Northoff and Bermpohl,2004]. On the other hand, dMPFC has been implicated in multiple cognitive processes, including the evaluation of self‐referential stimuli [Northoff and Bermpohl,2004], introspection processes [Schmitz and Johnson,2007], as well as reflection processes per se, such as in evaluation and decision‐making on whether a stimulus is applicable to the self or to another person [van der Meer et al.,2010]. Schmitz and Johnson [2007] showed that vMPFC is consistently activated only during the presentation of stimuli requiring appraisal (e.g., affective or arousing) of informational features that convey implications for one's own survival, well‐being, and potential goals. In line with these results, Schmitz and Johnson [2007] proposed to distinguish between two top‐down systems involved in the processing of self‐relevant stimuli: the vMPFC‐vACC system, responsible for an automatic preattentive biasing for salient or explicitly self‐relevant information, and the dMPFC, engaged in introspective processes (e.g., self‐reflection, evaluation, and recollection). The presence of both vMPFC and dMPFC activation in our meta‐analysis fits with this proposal, taking vMPFC and dMPFC, respectively, as the emotional and cognitive counterparts of self‐referential processes.
A Core System: MPFC Activation
MPFC was the only region activated in all conditions. This result confirms the pivotal role of MPFC in self‐representation as discussed in the previous sections. Nevertheless, differential activations were revealed in MPFC, corresponding to the level of abstraction of the material involved. The results suggested that EAM only activates a rostral region of MPFC, SAM a more caudal part, and CS both rostral and caudal MPFC. These results were confirmed by subtractions between conditions. MPFC activations entirely disappeared when CS or SAM was subtracted from EAM. SAM activated a rostral subregion of MPFC (BA 10) when compared with EAM and CS. Finally, CS, compared to EAM and SAM, led to greater activation in a more caudal part of MPFC (BA 10) at the border with ACC. MPFC is one of the least understood regions of the human brain [Christoff et al.,2003; Gilbert et al.,2006; Ramnani and Owen,2004]. Ramnani and Owen [2004] argued that rostral MPFC is a functionally homogeneous region involved in the “processing of internal states,” “memory retrieval models,” “prospective memory,” “branching and reallocation of attention,” and “relational integration.” On the other hand, there is evidence for a functional differentiation within MPFC [Bush et al.,2000; Steele and Lawrie,2004] with emotional and cognitive tasks represented in more rostral and more caudal parts, respectively. Moreover, Gilbert et al. [2006] reported a functional distinction within medial rostral MPFC (BA10), with the more caudal portion, at the border with the paracingulate cortex, being activated for mentalizing emotional tasks, while a more rostral part was more highly activated when coordination between different tasks was required. Our results are in line with these findings and corroborate the hypothesis of a functional differentiation within MPFC. In particular, a more caudal part seems to be recruited during CS, while SAM and EAM seem to activate progressively more rostral portions. We think that the investigation of functional differentiation within MPFC is of great interest and hope that our findings will stimulate experimental research on this topic in relation to self‐representation.
To summarize, our results show a core region for self‐representations in MPFC, as evidenced by the activation of this region in all conditions, and they are also in line with a functional differentiation between the three levels of representation, as evidenced by the specific activation for each condition reported in our meta‐analytic subtractions. Specifically, EAM seems to activate regions linked to memory retrieval, scene construction, and reviviscence, probably supporting continuity in subjective time. SAM seems to recruit basically the same structures (to a lesser extent), excluding the hippocampus and precuneus, which are specifically linked to the re‐experiencing of a particular moment. This data shows that SAM is supported by memory processes that do not attain the specificity of EAM. Finally, CS only recruits MPFC, suggesting that at this level of abstraction, a memory query is not necessary and that this region may subserve crystallized and ready‐to‐use self‐representations.
The Postero‐Anterior Distinction
We hold that the shift from posterior to anterior structures associated with gradually increasing abstraction of representation is due to the involvement of posterior regions in reviviscence processes and access to autobiographical information, whereas prefrontal cortical regions are involved in self‐referential assessment. Indeed, our results showed a clear shift from posterior parieto‐temporal regions for EAM to anterior MPFC structures for CS. However, we did not find that CS specifically engages regions underpinning storage or access to semantic knowledge, as might have been expected [Craik et al.,1999; Kelley et al.,2002; D'Argembeau et al.,2007]. This point could be explained by the lack in this meta‐analysis of neuroimaging experiments concerning the retrieval of highly abstract conceptual knowledge, more abstract than the knowledge involved in self‐referential processing (see below).
This result is also in line with previous work on the precise timing of activations during AM retrieval. Conway et al. [2003] demonstrated a pattern of activation in PFC during the first seconds of retrieval followed later by additional temporo‐occipital activation once a memory was formed. This was interpreted as resulting from the activation of PFC during initial access to CS and SAM in the construction of AM, and, once a memory was formed, the activation of posterior regions was related to the sensory and perceptual features of EAM. On this view, PFC activity is characteristic of the generative phase of retrieval, during which CS and SAM are accessed, reflecting the operation of control processes related to the elaboration of cues, the probing of the personal knowledge base, and the evaluation of the relevance of accessed knowledge [Conway et al.,2003]. In Conway's model, the most abstract and conceptual knowledge (goals, attitudes, desires, and life summary or life‐story) is represented in frontal and anterior temporal regions involved in semantic processes, whereas sensory perceptual details of specific events are associated with more posterior structures. In this meta‐analysis, we confirm and extend Conway et al. [2003]'s results showing a gradual shift of the pattern of activation from frontal to posterior and limbic structures depending on the level of specificity of self‐related memory retrieval.
Our findings may offer further insights about neuropsychological reports (for review, see Conway and Fthenaki [2003], Klein and Gangi [2010], and Kopelman and Kapur [2001]). Klein and Lax [2010] proposed the existence of a subsystem within semantic memory that they called “trait self‐knowledge” (which corresponds to CS) that is functionally specialized. On one hand, they suggest that this CS system is stored independently of episodic memory [Klein and Lax,2010], and this could explain why CS information is rapidly accessible with no systematic consultation of each pertinent EAM to determine personality traits. Tulving [1993] showed that the amnesic patient K.C. presented accurate and detailed knowledge about his postaccident personality despite the fact that he had no conscious access to any EAM from which he could infer that knowledge. Our results are in line with these neuropsychological data, because we found CS activations to be relatively independent of EAM activations. On the other hand, CS as personality trait‐knowledge may be a specific type of semantic knowledge that is partly independent of other forms of semantic memory [Klein and Lax,2010]. Klein and Lax [2010] tested amnesic patients and suggest that within semantic memory, SAM can be impacted by cognitive or neural damage in patients with a preserved CS. These authors presented the case of patient D.B., who had preserved CS but whose SAM appeared to be only partially intact (42 vs. 92% of correct responses in control subjects on the modified Autobiographical Memory Interview [Klein and Lax,2010]). Our results are in line with these neuropsychological findings, which go beyond the traditional episodic/semantic distinction to suggest that there are content‐specific dissociations within semantic self‐knowledge [Klein and Lax,2010]. These results demonstrate the existence of a subsystem within semantic memory that processes specifically self‐related information based on trait‐knowledge and seems to be associated with specific regions of MPFC. Further studies should extend these findings to other kinds of information within the CS and explore the distinction from general semantic memory.
LIMITATIONS AND CONCLUSION
As pointed out in the “Introduction” section, research included in the CS category of our study did not cover the broader theoretical definition of CS, because we were only able to include experiments investigating self‐referential processes by means of personality traits, opinion, or tastes. This is due to the lack of published neuroimaging experiments suiting our inclusion criteria on CS‐knowledge, such as beliefs, goals, and desires. In the same vein, due to the restricted number of studies, we included experiments using different materials (visual, acoustic, verbal, picture, etc.). Thus, we cannot rule out that different kinds of stimuli differently affect brain activity. We tentatively performed three new separate meta‐analyses for each category comprising only verbal stimuli, as this condition was the most represented across the three categories. Results for CS and EAM were close to those presented earlier, while results for SAM slightly differed (MPFC, left thalamic, and right parahippocampal activations were no longer found). These differences could be explained, however, by low‐statistical power for SAM, as only 7 of the 13 originally included studies on SAM were included in this supplementary analysis. A summary of these results is reported in Supporting Information Table I.
To conclude, in line with the previous reports, our results corroborate that the “self” is not monolithic, but rather a multidimensional construct comprising representations at different levels of abstraction that are supported by different neural correlates. MPFC seems to play a crucial role in self‐representation independently of the level of abstraction, with different areas within this region differentially activated at different levels, while other structures such as the limbic system and posterior medial regions appear to be gradually recruited when specific information has to be retrieved in order to build a more experience‐near representation.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Table 1. Peaks of activation for SAM and EAM
REFERENCES
- Addis DR, Tippett LJ ( 2004): Memory of my Self: autobiographical memory and identity in Alzheimer's disease. Memory 12: 56–74. [DOI] [PubMed] [Google Scholar]
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP ( 2004): Characterizing spatial and temporal features of autobiographical memory retrieval networks: A partial least squares approach. Neuroimage 23: 1460–1471. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR ( 1997): Deciding advantageously before knowing the advantageous strategy. Science 275: 1293–1295. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P ( 2007): Functional neuroimaging of autobiographical memory. Trends Cogn Sci 11: 219–227. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, Rubin DC ( 2004): Brain activity during episodic retrieval of autobiographical and laboratory events: An fMRI study using a novel photo paradigm. J Cogn Neurosci 16: 1583–1594. [DOI] [PubMed] [Google Scholar]
- Cermak L ( 1982): The Episodic Semantic Distinction in Amnesia In: Squire LR, Butters LN, editors. The Neuropsychology of Memory. New York: Guilford; pp 52–62. [Google Scholar]
- Christoff K, Ream JM, Geddes LP, Gabrieli JD ( 2003): Evaluating self‐generated information: Anterior prefrontal contributions to human cognition. Behav Neurosci 117: 1161–1168. [DOI] [PubMed] [Google Scholar]
- Conway MA ( 2005): Memory and the self. J Mem Lang 53: 594–628. [Google Scholar]
- Conway MA ( 2009): Episodic memories. Neuropsychologia 47: 2305–2313. [DOI] [PubMed] [Google Scholar]
- Conway MA, Fthenaki A ( 2003): Disruption of inhibitory control of memory following lesions to the frontal and temporal lobes. Cortex 39: 667–686. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell‐Pearce CW ( 2000): The construction of autobiographical memories in the self‐memory system. Psychol Rev 107: 261–288. [DOI] [PubMed] [Google Scholar]
- Conway MA, Gardiner JM, Perfect TJ, Conway MA, Anderson SJ, Cohen GM ( 1997): Changes in memory awareness during learning: The acquisition of knowledge by psychology undergraduates. J Exp Psychol: Gen 126: 393–413. [DOI] [PubMed] [Google Scholar]
- Conway MA, Turk DJ, Miller SL, Logan J, Nebes RD, Meltzer CC, Becker JT ( 1999): A positron emission tomography (PET) study of autobiographical memory retrieval. Memory 7: 679–702. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell‐Pearce CW, Whitecross SE, Sharpe H ( 2003): Neurophysiological correlates of memory for experienced and imagined events. Neuropsychologia 41: 334–340. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, Kapur S ( 1999). In search of the self: A positron emission tomography study. Psychol Sci 10: 26–34. [Google Scholar]
- D'Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E ( 2007): Distinct regions of the medial prefrontal cortex are associated with self‐referential processing and perspective taking. J Cogn Neurosci 19: 935–944. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Feyers D, Majerus S, Collette F, Van der Linden M, Maquet P, Salmon E ( 2008): Self‐reflection across time: Cortical midline structures differentiate between present and past selves. Social Cogn Affect Neurosci 3: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC ( 2008): The spatiotemporal dynamics of autobiographical memory: Neural correlates of recall, emotional intensity, and reliving. Cereb Cortex 18: 217–229. [DOI] [PubMed] [Google Scholar]
- Denkova E, Botzung A, Scheiber C, Manning L ( 2006): Implicit emotion during recollection of past events: A nonverbal fMRI study. Brain Res 1078: 143–150. [DOI] [PubMed] [Google Scholar]
- Donix M, Petrowski K, Jurjanz L, Huebner T, Herold U, Baeumler D, Amanatidis EC, Poettrich K, Smolka MN, Holthoff VA ( 2010a): Age and the neural network of personal familiarity. PLoS One 5: e15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donix M, Poettrich K, Weiss PH, Werner A, von Kummer R, Fink GR, Holthoff VA ( 2010b): Age‐dependent differences in the neural mechanisms supporting long‐term declarative memories. Arch Clin Neuropsychol 25: 383–395. [DOI] [PubMed] [Google Scholar]
- Duval C, Eustache F, Piolino P ( 2007): Multidimensional Self, autobiographical memory and aging. Psychol NeuroPsychiatr Vieil 5: 179–192. [PubMed] [Google Scholar]
- Duval C, Desgranges B, de la Sayette V, Belliard S, Eustache F, Piolino P. What happens to personal identity when semantic knowledge degrades: Insights from semantic dementia. Neuropsychologia (revision). [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT ( 2009): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30: 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Higgins JS, Jablonski K, Feiler AM ( 2007): Visual scene processing in familiar and unfamiliar environments. J Neurophysiol 97: 3670–3683. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G ( 2011): Is there a core neural network in empathy? An fMRI based quantitative meta‐analysis. Neurosci Biobehav Rev 35: 903–911. [DOI] [PubMed] [Google Scholar]
- Fitts WH, Warren WL ( 1996): Tennessee Self‐Concept Scale: 2 Manual, 2nd ed Los Angeles: Western Psychological Services. [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H ( 2003): In search of the emotional self: An fMRI study using positive and negative emotional words. Am J Psychiatry 160: 1938–1945. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli P, Placentino A, Carletti F, Allen P, Landi P, Abbamonte M, Barale F, Perez J, McGuire P, Politi PL ( 2009): Laterality effect on emotional faces processing: ALE meta‐analysis of evidence. Neurosci Lett 452: 262–267. [DOI] [PubMed] [Google Scholar]
- Gardini S, Cornoldi C, De Beni R, Venneri A ( 2006): Left mediotemporal structures mediate the retrieval of episodic autobiographical mental images. Neuroimage 30: 645–655. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW ( 2006): Functional specialization within rostral prefrontal cortex (area 10): A meta‐analysis. J Cogn Neurosci 18: 932–948. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M ( 2004): Remembering our past: Functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex 14: 1214–1225. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Leibenluft E, Santiago N, Haxbya JV ( 2004): Social and emotional attachment in the neural representation of faces. Neuroimage 22: 1628–1635. [DOI] [PubMed] [Google Scholar]
- Graham KS, Lee AC, Brett M, Patterson K ( 2003): The neural basis of autobiographical and semantic memory: New evidence from three PET studies. Cogn Affect Behav Neurosci 3: 234–254. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, Labar KS ( 2005): Co‐activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia 43: 659–674. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME ( 2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL ( 2007): Aging, self‐referencing, and medial prefrontal cortex. Soc Neurosci 2: 117–133. [DOI] [PubMed] [Google Scholar]
- Haslam C, Jetten J, Haslam A, Pugliese C, Tonks J ( 2010): I remember therefore I am, and I am therefore I remember': Exploring the contributions of episodic and semantic self‐knowledge to strength of identity. Br J Psychol 102: 184–203. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM ( 2006): Medial prefrontal activity differentiates self from close others. Social Cogn Affect Neurosci 1: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Patterson K ( 2007): Semantic dementia: A unique clinicopathological syndrome. Lancet Neurology 6: 1004–1014. [DOI] [PubMed] [Google Scholar]
- Holland C, Addis DR, Kensinger EA ( 2011): The neural correlates of specific versus general autobiographical memory construction and elaboration. Neuropsychologia 49: 3164–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins AC, Macrae CN, Mitchell JP ( 2008): Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proc Natl Acad Sci 105: 4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP ( 2002): Neural correlates of self‐reflection. Brain 125( Pt 8): 1808–1814. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF ( 2002): Finding the self? An event‐related fMRI study. J Cogn Neurosci 14: 785–794. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Brammer M, Bullmore E, Simmons A, Bartels M, David AS ( 2002): The neural correlates of intentional and incidental self processing. Neuropsychologia 40: 683–692. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC ( 2002): Reflective self‐awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage 17: 1080–1086. [PubMed] [Google Scholar]
- Klein SB ( 2010): The self: As a construct in psychology and neuropsychological evidence for its multiplicity. WIREs Cogn Sci 1: 172–183. [DOI] [PubMed] [Google Scholar]
- Klein SB, Gangi CE ( 2010): The multiplicity of self: Neuropsychological evidence and its implications for the self as a construct in psychological research. Ann NY Acad Sci 1191: 1–15. [DOI] [PubMed] [Google Scholar]
- Klein SB, Lax ML ( 2010): The unanticipated resilience of trait self‐knowledge in the face of neural damage. Memory 18: 918–948. [DOI] [PubMed] [Google Scholar]
- Klein SB, Cosmides L, Costabile K ( 2003): Preserved knowledge of self in a case of Alzheimer's dementia. Social Cogn 21: 157–165. [Google Scholar]
- Kochunov P, Lancaster JL, Thompson P, Toga AW, Brewer P, Hardies J ( 2002): An optimized individual target brain in the Talairach coordinate system. Neuroimage 17: 922–927. [PubMed] [Google Scholar]
- Kopelman MD, Kapur N ( 2001): The loss of episodic memories in retrograde amnesia: Single‐case and group studies. Philos Trans R Soc Lond B Biol Sci 356: 1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox M, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT ( 2005): ALE meta‐analysis: Controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT ( 2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28: 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand D, Ruby P ( 2009): What is self‐specific? Theoretical investigation and critical review of neuroimaging results. Psychol Rev 116: 252–282. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV ( 2004): Mothers' neural activation in response to pictures of their children and other children. Biol Psychiatr 56: 225–232. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M ( 2002): Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychol Aging 17: 677–689. [PubMed] [Google Scholar]
- Levine B, Turner GR, Tisserand D, Hevenor SJ, Graham SJ, McIntosh AR ( 2004): The functional neuroanatomy of episodic and semantic autobiographical remembering: A prospective functional MRI study. J Cogn Neurosci 16: 1633–1646. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH ( 2001): Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104: 667–676. [DOI] [PubMed] [Google Scholar]
- Maguire EA ( 2001): Neuroimaging, memory and the human hippocampus. Rev Neurol (Paris) 157( 8–9, Pt 1): 791–794. [PubMed] [Google Scholar]
- Maguire EA, Frith CD ( 2003a): Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain 126( Pt 7): 1511–1523. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD ( 2003b): Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J Neurosci 23: 5302–5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ ( 1999): Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus 9: 54–61. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ, Buchel C ( 2000): Patterns of hippocampal‐cortical interaction dissociate temporal lobe memory subsystems. Hippocampus 10: 475–482. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Montaldi D, Spencer TJ, Roberts N ( 2004): Recalling spatial information as a component of recently and remotely acquired episodic or semantic memories: An fMRI study. Neuropsychology 18: 426. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Szpunar KK, Christ SE ( 2009): Laboratory‐based and autobiographical retrieval tasks differ substantially in their neural substrates. Neuropsychologia 47: 2290–2298. [DOI] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A ( 2009): Activation of anterior insula during self‐reflection. PLoS One 4: e4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G ( 2002): The Frontal Cortex and Working with Memory In: Stuss DTE, Knight RTE, editors. New York, NY: Oxford University Press; 616 p. [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L. ( 2005): Functional neuroanatomy of remote episodic, semantic and spatial memory: A unified account based on multiple trace theory. J Anat 207: 35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KJ, Troyer AK, Levine B, Moscovitch M ( 2008): Episodic, but not semantic, autobiographical memory is reduced in amnestic mild cognitive impairment. Neuropsychologia 46: 3116–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M ( 1997): Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol 7: 217–227. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Sato N, Nakamura A, Sugiura M, Kato T, Hatano K, Ito K, Fukuda H, Schormann T ( 2000): Functional delineation of the human occipito‐temporal areas related to face and scene processing. Brain 123: 1903. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F ( 2004): Cortical midline structures and the self. Trends Cogn Sci 8: 102–107. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J ( 2006): Self‐referential processing in our brain—A meta‐analysis of imaging studies on the self. Neuroimage 31: 440–457. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Kim AS, Habib R, Levine B, Tulving E ( 2010): Consciousness of subjective time in the brain. Proc Natl Acad Sci USA 107: 22356–22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oschner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JDE, Kihsltrom JF, D'Esposito M ( 2005): The neural correlates of direct and reflected self‐knowledge. Neuroimage 28: 797–814. [DOI] [PubMed] [Google Scholar]
- Oddo S, Lux S, Weiss PH, Schwab A, Welzer H, Markowitsch HJ, Fink GR ( 2010): Specific role of medial prefrontal cortex in retrieving recent autobiographical memories: An fMRI study of young female subjects. Cortex 46: 29–39. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, Kawashima R, Fukuda H, Itoh M, Yamadori A ( 2003): Thinking of the future and past: The roles of the frontal pole and the medial temporal lobes. Neuroimage 19: 1369–1380. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M ( 2007): “I Know You Are But What Am I?”: Neural Bases of Self‐and Social Knowledge Retrieval in Children and Adults. J Cogn Neurosci 19: 1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Benali K, Eustache F ( 2002): Episodic and semantic remote autobiographical memory in ageing. Memory 10: 239–257. [DOI] [PubMed] [Google Scholar]
- Piolino P, Belliard S, Desgranges B, Perron M, Eustache F ( 2003): Autobiographical memory and autoneotic consciousness in a case of semantic dementia. Cogn Neuropsychol 20: 619–639. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Clarys D ( 2006): Autobiographical memory, autonoetic consciousness, and self‐perspective in aging. Psychol Aging 21: 510–525. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Eustache F ( 2009): Episodic autobiographical memories over the course of time: Cognitive, neuropsychological and neuroimaging findings. Neuropsychologia 47: 2314–2329. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM ( 2004): Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nat Rev Neurosci 5: 184–194. [DOI] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS ( 1977): Self‐reference and the encoding of personal information. J Pers Soc Psychol 35: 677–688. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC ( 2006): Self‐appraisal decisions evoke dissociated dorsal‐ventral aMPFC networks. Neuroimage 30: 1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC ( 2007): Relevance to self: A brief review and framework of neural systems underlying appraisal. Neurosci Biobehav Rev 31: 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara‐Baccus TN, Johnson SC ( 2004): Metacognitive evaluation, self‐relevance, and the right prefrontal cortex. Neuroimage 22: 941–947. [DOI] [PubMed] [Google Scholar]
- Seger CA, Stone M, Keenan JP ( 2004): Cortical activations during judgments about the self and an other person. Neuropsychologia 42: 1168–1177. [DOI] [PubMed] [Google Scholar]
- Senhorini MC, Cerqueira CT, Schaufelberger MS, Almeida JC, Amaro E, Sato JR, Barreiros MA, Ayres AM, Castro CC, Scazufca M, Menezes PR, Busatto GF ( 2011): Brain activity patterns during phonological verbal fluency performance with varying levels of difficulty: A functional magnetic resonance imaging study in Portuguese‐speaking healthy individuals. J Clin Exp Neuropsychol 33: 864–873. [DOI] [PubMed] [Google Scholar]
- Shah NJ, Marshall JC, Zafiris O, Schwab A, Zilles K, Markowitsch HJ, Fink GR ( 2001): The neural correlates of person familiarity. A functional magnetic resonance imaging study with clinical implications. Brain 124: 804–815. [DOI] [PubMed] [Google Scholar]
- Shin LM, Dougherty DD, Orr SP, Pitman RK, Lasko M, Macklin ML, Alpert NM, Fischman AJ, Rauch SL ( 2000): Activation of anterior paralimbic structures during guilt‐related script‐driven imagery. Biol Psychiatry 48: 43–50. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL ( 2009): Event‐related fMRI studies of episodic encoding and retrieval: Meta‐analyses using activation likelihood estimation. Neuropsychologia 47: 1765–1779. [DOI] [PubMed] [Google Scholar]
- Symons CS, Johnson BT ( 1997): The self‐reference effect in memory: A meta‐analysis. Psychol Bull 121: 371–394. [DOI] [PubMed] [Google Scholar]
- Steele JD, Lawrie SM ( 2004): Segregation of cognitive and emotional function in the prefrontal cortex: A stereotactic meta‐analysis. Neuroimage 21: 868–875. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Shah NJ, Zilles K, Fink GR ( 2005): Cortical representations of personally familiar objects and places: Functional organization of the human posterior cingulate cortex. J Cogn Neurosci 17: 183–198. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Sassa Y, Watanabe J, Akitsuki Y, Maeda Y, Matsue Y, Fukuda H, Kawashima R ( 2006): Cortical mechanisms of person representation: Recognition of famous and personally familiar names. Neuroimage 31: 853–860. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Sassa Y, Watanabe J, Akitsuki Y, Maeda Y, Matsue Y, Kawashima R ( 2009): Anatomical segregation of representations of personally familiar and famous people in the temporal and parietal cortices. J Cogn Neurosci 21: 1855–1868. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Mano Y, Sasaki A, Sadato N ( 2011): Beyond the memory mechanism: Person‐selective and nonselective processes in recognition of personally familiar faces. J Cogn Neurosci 23: 699–715. [DOI] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA ( 2009): Cortical midline involvement in autobiographical memory. Neuroimage 44: 1188–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B ( 2006): The functional neuroanatomy of autobiographical memory: A meta‐analysis. Neuropsychologia 44: 2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons CS, Johnson BT ( 1997): The self‐reference effect in memory: A meta‐analysis. Psychol Bull 121: 371–394. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Matsuura M, Koeda M, Yahata N, Suhara T, Kato M, Okubo Y ( 2008): Brain activations during judgments of positive self‐conscious emotion and positive basic emotion: Pride and joy. Cereb Cortex 18: 898–903. [DOI] [PubMed] [Google Scholar]
- Tulving E ( 1993): Self‐Knowledge of an Amnesic Individual Is Represented Abstractly In: Srull TK, Wyer RS, editors. Hillsdale, NJ: Erlbaum; 147–156 p. [Google Scholar]
- Tulving E ( 2002): Episodic memory: From mind to brain. Annu Rev Psychol 53: 1–25. [DOI] [PubMed] [Google Scholar]
- Tulving E, Schacter DL, McLachlan DR, Moscovitch M ( 1988): Priming of semantic autobiographical knowledge: A case study of retrograde amnesia. Brain Cogn 8: 2–20. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA ( 2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. Neuroimage 16( 3, Pt 1): 765–780. [DOI] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS ( 2010): Self‐reflection and the brain: A theoretical review and meta‐analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev 34: 935–946. [DOI] [PubMed] [Google Scholar]
- Vandekerckove MM, Markowitsch HJ, Mertens M, Woermann FG ( 2005): Bi‐hemispheric engagement in the retrieval of autobiographical episodes. Behav Neurol 16: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannest J, Newport EL, Newman AJ, Bavelier D ( 2011): Interplay between morphology and frequency in lexical access: The case of the base frequency effect. Brain Res 1373: 144–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard A, Piolino P, Desgranges B, Chetelat G, Lebreton K, Landeau B, Young A, De La Sayette V, Eustache F ( 2007): Hippocampal activation for autobiographical memories over the entire lifetime in healthy aged subjects: An fMRI study. Cereb Cortex 17: 2453–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard A, Lebreton K, Desgranges B, Chételat G, Landeau B, Young A, De La Sayette V, Eustache F, Piolino P ( 2010): Patterns of hippocampal‐neocortical connectivity in the retrieval of autobiographical memories across the entire life‐span of older adults. Hippocampus 20: 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal K, Hamann S ( 2010): Neuroimaging support for discrete neural correlates of basic emotions: A voxel‐based meta‐analysis. J Cogn Neurosci 22: 2864–2885. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Fan J, Han S ( 2007): Neural basis of cultural influence on self‐representation. Neuroimage 34: 1310–1316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Table 1. Peaks of activation for SAM and EAM


