Abstract
Objective: Enhanced memory for emotionally charged events helps us to remember potentially vital information. There are large interindividual differences in emotional‐memory enhancement, but little is known about their neurobiological basis. Recently, a functional deletion variant of the gene that codes for the α2b‐adrenoceptor (ADRA2B) has been shown to affect memory for emotional experiences. Initial neuroimaging evidence linked this behavioral effect to increased amygdala activity, but its influence on successful memory processing remains unknown. Therefore, the aim of this study was to investigate the effect of the common deletion in the ADRA2B gene on neural activity related to specific mnemonic processing, successful memory formation, and retrieval. Methods: Twenty‐three noncarriers (10 males) and 28 deletion carriers (13 males) with a mean age of 24 years were investigated while performing an emotional‐learning task with sad and happy scenes. Functional magnetic resonance imaging was acquired both during memory formation and retrieval. Results: Although there were no differences in memory performance between groups, the common deletion variant of ADRA2B was related to enhanced activity in the amygdala and inferior frontal gyrus during successful emotional memory formation, but not retrieval. Deletion carriers showed a larger differential response in these brain regions between later‐remembered and later‐forgotten stimuli than nondeletion carriers did. Conclusion: Our results demonstrate that the ADRA2B polymorphism influences emotional memory formation but not memory retrieval in the amygdala and left inferior frontal gyrus. Hum Brain Mapp, 2011. © 2011 Wiley Periodicals, Inc.
Keywords: amygdala, emotional memory, functional MRI, mood disorders, noradrenaline
INTRODUCTION
Enhanced memory for emotional events is a well‐recognized phenomenon that has an obvious adaptive value in evolutionary terms, because it is vital to remember both dangerous and favorable situations [McGaugh,2004]. Across individuals, differences in the strength of emotional memories are associated with the vulnerability to develop mood disorders and to maintain them [Haas and Canli,2008]. However, little is known about the neurobiological mechanisms underlying these individual differences. Recently, a functional deletion variant of three glutamic acids (residues 301–303) in the third intracellular loop of the α2b‐adrenoceptor (ADRA2B) gene that codes for the ADRA2B [Small et al.,2001] has been shown to affect memory for emotional experiences [de Quervain et al.,2007]. Carriers of the deletion variant showed enhanced memory for emotional pictures compared to nondeletion carriers, and civil war survivors with the deletion variant have higher trauma re‐experiencing symptoms than noncarriers. Taken together, these findings suggest that the deletion variant of the ADRA2B gene may affect emotional memory processing, whereby one allele leads to enhanced memory for emotional events.
Recently, Rasch and colleagues [Rasch et al.,2009] showed that carriers of the deletion variant of the ADRA2B gene have increased amygdala responses compared to nondeletion carriers when processing emotionally negative photographs. However, Rasch and colleagues did not observe any interaction between this genetic effect on amygdala activity and successful memory formation and did not investigate ADRA2B effects on memory retrieval. Additionally, Cousijn and colleagues [2010] used acute psychological stress, which increases noradrenergic activity, and probed its effect on tonic activity and phasic responses in the amygdala. The authors found that only deletion carriers displayed increased phasic amygdala responses under stress while amygdala perfusion, reflecting tonic activity, increased independently of genotype after stress induction [Cousijn et al.,2010]. Hence, there is initial neural evidence that the deletion variant in the ADRA2B gene may influence emotional memory via altered amygdala functioning.
The amygdala, in particular, its basolateral complex, is associated with the enhancement of memory for emotional events [McGaugh,2004]. It stimulates memory processes in other brain regions such as the hippocampus, inferior temporal cortex, and prefrontal cortex [Cahill et al.,1995; Kilpatrick and Cahill,2003; Morris et al.,1998; Richardson et al.,2004]. Besides its central role for memory formation and consolidation, it is also involved in memory retrieval [Dolcos et al.,2005]. The amygdala contributes via noradrenergic activation to emotional memory formation, but less to retrieval [Cahill et al.,1994; McGaugh and Roozendaal,2002; Strange and Dolan,2004]. Yet, it remains unknown whether the genetic variation influences memory formation only, or whether memory retrieval is affected as well. Therefore, we set out to investigate the influence of the variation in the ADRA2B gene on the activity in brain structures associated with both emotional memory formation and retrieval by means of functional magnetic resonance imaging (fMRI); we focused on brain activity associated with processes underlying successful memory formation and retrieval.
The behavioral data of de Quervain and colleagues suggest that the behavioral effect of the deletion is independent of emotional valence of the stimuli [de Quervain et al.,2007]. However, the fMRI study of Rasch and colleagues [Rasch et al.,2009] suggests that the genotype may bias neural processes more for negative than positive stimuli, as the genotype effect was not significant for positive pictures. Valence‐specific effects may be larger when mood at the time of learning or retrieval matches the valence of the emotional stimulus [Bower et al.,1981]. For that reason, we used a negative mood induction procedure in combination with emotional negative and positive stimuli for all subjects to explore further valence‐specific memory effects. During scanning, negative mood was induced by showing negative movie clips, which was interleaved with a memory task that included pictures with either positive or negative emotional valence. The participants initially memorized those pictures while making an emotional valence decision and subsequently performed a recognition memory test. On the basis of the aforementioned background, we hypothesized that deletion carriers would show higher activity in the amygdala and possibly also in connected hippocampal, inferior temporal, and prefrontal regions, compared to nondeletion carriers during successful memory formation, but not retrieval, of emotional stimuli.
MATERIALS AND METHODS
Participants
Fifty‐eight right‐handed healthy volunteers (31 males) with a mean age of 24 years (range, 18–43) participated in the study. This sample size is similar to that of Rasch and colleagues [Rasch et al.,2009] and in line with sample size recommendations based on power analyses [Mier et al., in press; Munafo et al.,2008]. All participants were physically and mentally healthy as determined by a self‐report questionnaire and reported no history of psychiatric or somatic diseases potentially affecting the brain. To avoid any confound due to threshold depressive symptoms, subjects were screened using the Dutch version of the standard Beck Depression Inventory [Beck et al.,1996], and all had a score below 10. Data of seven participants were excluded because their memory performance was at ceiling, resulting in too few miss trails for the fMRI analysis (<10). Therefore, the reported results are based on the data of 51 participants, including 23 noncarriers (10 males) and 28 deletion carriers (13 males). In line with the previous studies, heterozygous and homozygous (three males and three females) deletion carriers were treated as one group [de Quervain et al.,2007]. The groups did not reveal significant differences with respect to age {t(49) = −1.2, P > 0.3), gender [X 2(1) = 0.0, P > 0.8]}, and level of education [X 2(3) = 2.3, P > 0.5]. Possible changes in mood in response to the experiment were examined by the Dutch shortened five scale version of the Profile and Mood States Questionnaire (POMS; [McNair et al.,1971]). The study was approved by the local ethics committee (CMO region Arnhem‐Nijmegen, The Netherlands), and all volunteers gave written informed consent before participating in the study.
Negative Mood Induction Procedure
Based on a previous study [Kernis et al.,1997], negative mood induction consisted of participants watching and listening to six movie clips interspersed throughout the scanning session. Clips were taken from the movie Sophie's Choice (1982) about the holocaust and lasted between 3 and 7:30 min. Before and after each clip, participants rated their current mood on a visual analogous scale (ranging from −10 to 10).
Memory Paradigm
During scanning, participants completed a memory task using positive and negative pictures. During the study phase, participants were asked to memorize 240 pictures in total while making a valence decision for each picture. Following a video of about four and a half minutes, subjects were required to recognize the old and reject the same amount of randomly intermixed new pictures during the recognition phase (480 pictures in total). Participants were encouraged to make a decision between old and new, but also had the option to make an unsure decision. The pictures showed emotional scenes displaying one or more humans. The 480 pictures were taken from a pool of positive and negative pictures, which had been rated during a behavioral pilot study (five‐point scale ranging from “emotionally positive” to “emotionally negative”; mean valence rating of negative pictures was ≤2 and mean valence rating of positive pictures was ≥4).
Throughout the memory paradigm under negative mood induction, half of the 480 pictures (including 120 positive and 120 negative pictures) were taken as items to be learned during the study phase of the experiment. The other half of the 480 pictures (including 120 positive and 120 negative pictures) was taken as lures for the recognition memory test phase of the experiment. The assignment of which pictures were used as study items and which as lures for memory testing was counterbalanced across participants and their gender. The content of positive and negative pictures [i.e., individual or group, child or adult, and male or female person(s)] was equally distributed across stimulus sets. To maximize the possibility to observe valence‐specific effects, positive and negative pictures were chosen. Neutral pictures were not used, because they represent mood‐indifferent stimuli. The order of picture presentation was randomized. Pictures were presented for 0.5 s with a jittered interstimulus interval of 3.7–4.7 s. The fixation period between pictures was used as baseline condition during statistical analysis.
Image Acquisition
Data were acquired with a 1.5‐T Siemens Sonata MR scanner (Siemens, Erlangen, Germany), equipped with a CP head array coil. T2*‐weighted blood oxygenation level‐dependent images were acquired using echo‐planar imaging (EPI), with each volume consisting of 32 slices (voxel size, 3.3 × 3.3 × 3.5 mm3; TR = 2,340 ms, TE = 35 ms, 64 × 64 matrix, FOV = 212 mm, and FA = 90°). High‐resolution T1‐weighted structural MR images were acquired for spatial normalization procedures (MPRAGE, TR = 2,250 ms, TE = 2.95 ms, TI = 850 ms, 176 one‐mm slices, 256 × 256 matrix, and FOV = 256 mm).
Image Analysis
Image analysis was performed with SPM5 (Wellcome Department of Imaging Neuroscience, London, UK). The first five EPI volumes were discarded to allow for T1 equilibration, and the remaining images were realigned to the first volume. Images were then corrected for slice acquisition time, coregistered to the anatomical scan, spatially normalized to the Montreal Neurological Institute (MNI) T1 template, resampled into 2 × 2 × 2‐mm3 voxels, and spatially smoothed with a Gaussian kernel of 8‐mm FWHM.
Statistical analysis was performed within the framework of the general linear model. Later‐remembered and later‐forgotten stimuli were separately modeled for the study phase, while hits, misses, correct rejections, and false alarms were separately modeled for the recognition phase. Furthermore, all stimuli were differentially modeled according to their valence. Stimuli with an incorrect valence decision or omission during study or with an unsure response or omission during the recognition test were included in a condition of no interest. The explanatory variables (0.5 s) were temporally convolved with the hemodynamic response function of SPM5. In addition, the realignment parameters were included to model potential movement artifacts, as was a high‐pass filter (cut‐off at 1 of 128 Hz). The data was proportionally scaled to account for various global effects, and temporal autocorrelation was modeled with an AR(1) process. The relevant parameter images contrasting each condition to the interstimulus baseline were entered in a random‐effect mixed‐model ANOVA with a nonsphericity correction. Statistical tests were family‐wise error rate corrected for multiple comparisons across the entire brain (P < 0.05) or the search volume for regions of interests using a small volume correction [Worsley et al.,1996]. The amygdala (basolateral group) and hippocampus were anatomically defined, based on probabilistic cytoarchitectonic maps implemented in the SPM Anatomy toolbox [Amunts et al.,2005; Eickhoff et al.,2005]. In addition, we also report activations of clusters exceeding 50 voxels at P < 0.001 uncorrected. Peak voxels of activated clusters are reported in MNI coordinates.
Behavioral Performance Analysis
The discrimination index d′ was used as a measure of recognition accuracy [Snodgrass and Corwin,1988]. These measures are based on the hit and false alarm rates, which were defined as the number of hits or false alarms divided by the number of old or new trials for which an old or new decision was made. Behavioral data were analyzed with SPSS 16 (SPSS, Chicago IL), and Greenhouse–Geisser corrections were used when appropriate.
Genotyping
For the analysis of the deletion polymorphism in ADRA2B, we used fragment length analysis on a genetic analyzer. In short, amplification of a fragment containing the variant was performed in a total volume of 10 μl containing 50 ng of DNA and 1 × PCR buffer II (Applied Biosystems, Nieuwerkerk aan de IJssel, The Netherlands), 2.5 mM MgCl2 (Applied Biosystems), 0.25 mM dNTPs (GE Healthcare, Zeist, The Netherlands), 0.5 μl (10 pmol/μl) of each primer (Applied Biosystems), that is, a NED‐labeled forward primer (NED‐AGA AGG AGG GTG TTT GTG GGG) and a reverse primer carrying a “PIG tail” (ACC TAT AGC ACC CAC GCC CCT‐GTTTCTT), 0.5 M betaine (Sigma, Zwijndrecht, The Netherlands), and 0.04 U AmpliTaq Gold (Applied Biosystems). The amplification protocol consisted of an initial 12 min at 95°C, followed by 32 cycles of 1 min at 94°C, 1 min at 66°C, and 1 min at 72°C, and finishing with a step of 7 min at 72°C. For the fragment length analysis on an ABI Prism 3730 Genetic Analyser, 1 μl of diluted PCR product was added to 8.7‐μl formamide (Applied Biosystems) and 0.3‐μl Liz600 standard (Applied Biosystems). The results were analyzed using GeneMapper® Software, version 4.0 (Applied Biosystems). Testing for Hardy–Weinberg equilibrium did not show deviations from the expected distribution [X 2(1) = 0.04, P = 0.83].
RESULTS
Questionnaires and Behavioral Performance
The entire procedure including the negative mood induction decreased vigor [F(1,48) = 132.9, P < 0.001] and tension [F(1,48) = 12.1, P = 0.001] and increased anger [F(1,48) = 6.7, P = 0.01], fatigue [F(1,48) = 53.5, P < 0.001], and depression [F(1,48)) = 8.0, P = 0.007], as measured by the POMS. No significant genotype effects were observed [F(1,48) < 2.8, P > 0.1]. The mood ratings during the experiment show that the negative mood induction was successful in both groups, leading to significantly lower mood ratings throughout the experiment than before the mood‐induction procedure [F(12,37) = 21.5, P < 0.001]. Again, no significant effect of genotype was observed (F < 1).
As expected on the basis of the current sample size and the effect size observed in a previous study [de Quervain et al.,2007], no significant difference in recognition memory accuracy between deletion carriers and nondeletion carriers was observed [d′ (means ± SEM); deletion carriers: 1.70 ± 0.49; nondeletion carriers: 1.83 ± 0.49; t(45) = 0.9, P = 0.37]. Reaction times during the recognition memory test were significantly shorter for hits than correct rejections than false alarms and misses [F(3,45) = 30.9, P < 0.001; paired t‐tests, P < 0.001], but did not significantly differ between genotypes (F < 1). Also, valence judgments during the study phase did not differ significantly between genotypes [i.e., decision accuracy and reaction times (mean ± SEM); deletion carriers: 0.94% ± 0.04%, 1,098 ± 290 ms; nondeletion carriers: 0.96% ± 0.02%, 1,144 ± 272 ms, both P > 0.05].
Functional Imaging Data
Memory formation
Brain regions involved in the successful formation of emotional memories were identified by comparing responses to subsequently remembered and subsequently forgotten items across genotype groups. This analysis revealed significant bilateral activations in the amygdala, hippocampus, fusiform gyrus, and inferior frontal gyrus as well as left middle temporal gyrus, left inferior temporal gyrus, and right parahippocampal gyrus (see Table I). The results showed no significant main effect of genotype, suggesting that ADRA2B variation did not influence neural responses irrespective of memory (P > 0.9, corrected).
Table I.
Brain regions showing a main effect of successful memory formation (i.e., subsequent hits vs. subsequent misses) and successful recognition (i.e., hits vs. misses)
| Region | MNI coordinates | Z‐value | P‐value (FWE) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Memory formation | |||||
| L/R orbitofrontal cortex | 2 | 48 | −16 | >8 | <0.001 |
| R fusiform gyrus | 42 | −50 | −18 | >8 | <0.001 |
| L fusiform gyrus | −36 | −48 | −18 | >8 | <0.001 |
| R hippocampus | 20 | −10 | −18 | 7.4 | <0.001 |
| L hippocampus | −20 | −10 | −16 | 6.7 | <0.001 |
| R amygdalaa | 22 | −8 | −20 | 6.6 | <0.001 |
| L amygdalaa | −20 | −8 | −18 | 6.4 | <0.001 |
| L inferior frontal gyrus | −38 | 32 | −14 | 6.7 | <0.001 |
| R fusiform gyrus | 42 | −14 | −26 | 5.8 | <0.001 |
| L middle temporal gyrus | −46 | −66 | 20 | 5.7 | 0.001 |
| L inferior frontal gyrus | −50 | 32 | 14 | 5.5 | 0.001 |
| R frontal inferior gyrus | 48 | 34 | 12 | 5.4 | 0.002 |
| R frontal inferior gyrus | 38 | 10 | 28 | 5.4 | 0.003 |
| R frontal inferior gyrus | 46 | 18 | 22 | 5.2 | 0.008 |
| Memory retrieval | |||||
| L amygdalaa | −22 | −4 | −14 | 3.8 | 0.013 |
| R amygdalaa | 24 | −4 | −16 | 3.3 | 0.063 |
| R angular gyrus | 38 | −72 | 40 | 4.9 | 0.024 |
Statistics for the amygdala region of interest (SVC).
R, right; L, left.
Data are reported for peak voxels of activated clusters (P < 0.05, FWE corrected) and a cluster size >10 voxels.
The effect of the ADRA2B deletion polymorphism on emotional memory formation was investigated using a genotype × memory interaction. This analysis showed a significant interaction in the left amygdala [(x = −36, y = −8, z = −22), Z = 3.5, P = 0.036, and SVC] and the left inferior frontal gyrus [(x = −40, y = 40, z = −2), Z = 4.2, uncorrected at P < 0.001, and cluster size = 70 voxels]. As is evident from Figure 1, this interaction arose, because the difference between later remembered and later forgotten pictures was larger in deletion than nondeletion carriers. No significant interaction with emotional valence was observed in the aforementioned regions (P > 0.3, corrected).
Figure 1.
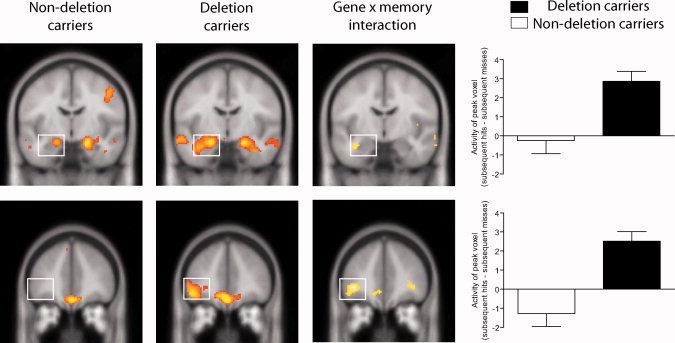
The ADRA2B genotype affects memory formation in the amygdala and inferior frontal gyrus. Coronal slices showing amygdala (upper row) and inferior frontal gyrus (lower row) effects (i.e., subsequent hits—subsequent misses) at the peak of the interaction. Columns show activations for nondeletion carriers, deletion carriers, the interaction between genotype, and memory, as well as the parameter estimates for the peak voxel of the activated cluster for illustration of the interaction (subsequent hits—subsequent misses; mean ± SEM). In all images, the left side corresponds to the left hemisphere, and the figures are displayed at P < 0.005, uncorrected for illustration purposes. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
An exploratory whole‐brain analysis showed no additional genotype × memory interactions in other brain regions. The genotype × memory × valence interaction showed a cluster in the anterior cingulate cortex (P < 0.001, cluster size >50 voxels; see Supporting Information table).
Memory retrieval
The brain regions involved in the successful recognition of emotional pictures were identified by comparing responses for hits and misses. In line with earlier studies investigating emotional memory retrieval (e.g., [Dolcos et al.,2005], this analysis revealed bilateral activation of the amygdala as well as activation of the right angular gyrus (see Table I). The results showed no significant main effect of genotype, suggesting that ADRA2B variation did not influence neural responses irrespective of memory (P > 0.5, corrected).
The effect of the ADRA2B deletion polymorphism on memory retrieval was investigated using a genotype × memory interaction, but no significant effects were found (P > 0.9, corrected). Exploratory whole‐brain analyses showed a genotype × memory × valence interaction in the caudate nucleus (P < 0.001, cluster size >50 voxels; see Supporting Information table).
DISCUSSION
Our results demonstrate that the common ADRA2B deletion affects the neural correlates underlying memory formation of emotional stimuli: deletion carriers showed increased neural activity related to successful memory formation compared to nondeletion carriers in the amygdala and the inferior frontal gyrus. In contrast, we did not find an effect of genotype during successful memory retrieval. However, when comparing memory formation and retrieval, no significant genotype × memory phase × memory success interaction was found. Therefore, our results suggest that the variation in human ADRA2B gene modulates emotional memory formation, presumably via increased noradrenergic availability [Small et al.,2001]. However, a possible role of the genotype for emotional memory retrieval cannot be excluded and needs further investigation.
Our findings are in line with earlier studies suggesting that noradrenergic neurotransmission plays an important role during memory formation. Blockade of noradrenergic signaling during memory formation reduces the memory enhancement for emotional events [Cahill et al.,1994] and is mediated by the reduction of amygdala activity [Strange and Dolan,2004]. Our results support these findings and, additionally, show that genetic variation in noradrenaline signaling clearly affects memory formation, whereas we were unable to detect an influence on memory retrieval.
Besides the increased amygdala activation during successful memory formation of emotional stimuli, deletion carriers also showed a larger contribution of the inferior frontal gyrus. This brain region is involved in emotional memory formation [Erk et al.,2003; Smith et al.,2004], and its function is thought to be related to semantic elaboration [Demb et al.,1995; Kapur et al.,1994]. Hence, its heightened activity might reflect increased semantic elaboration of emotional content eventually leading to more elaborate memory formation. This hypothesis is supported by a recent study showing that prefrontal regions contribute to deep‐encoding processes during emotional memory formation [Ritchey et al., in press]. Given that the amygdala modulates emotional memory processing in other brain regions [Kilpatrick and Cahill,2003], the prefrontal effect could also be a consequence of increased amygdala activity. However, the ADRA2B is also expressed in the frontal cortex [Wang et al.,2002], suggesting that the larger contribution of the inferior frontal gyrus is potentially mediated by locally increased noradrenergic signaling as well.
Our patterns of results confirm the previous findings [Rasch et al.,2009] by showing that deletion carriers have stronger amygdala responses than nondeletion carriers. We were able to add cognitive specificity to this effect by showing an interaction with subsequent memory and extending the identification of brain regions involved in gene × memory interactions to the prefrontal cortex. Most likely, Rasch and colleagues failed to find a gene × memory interaction due to the small number of trials that was used. Their experiment consisted of 24 pictures per valence category, whereas we used 120 pictures per valence category. Furthermore, other differences in the study designs might have contributed to the variability of results as well. On the general level, our procedure including the specificity of the induced mood state and the intentional learning instruction combined with a subsequent memory recognition test was quite different from the procedure used by Rasch and colleagues. Furthermore, we did not use neutral pictures as baseline condition, but the interstimulus fixation period. This latter difference relates to the different focus of the studies: Rasch and colleagues focused on effects of general emotional enhancement, whereas we examined possible mood‐congruent effects. Importantly, neutral pictures represent mood‐indifferent pictures that would have reduced the impact of mood‐congruency. Presumably, all these differences have contributed to differential mnemonic processes.
We used a negative mood‐induction procedure, because valence‐specific memory appears strongest for mood‐congruent information [Bower et al.,1981]. However, in line with the behavioral results from the initial study by de Quervain and colleagues [2007], we did not find any evidence for valence‐specific effects of the deletion. Thus, the changed noradrenergic signaling due to the deletion seems to result in a general increase of emotional memory processing without any valence‐specificity. This pattern of results is in line with the modulation hypothesis, stating that, in particular, the basolateral part of the amygdala modulates the formation and consolidation of memories of emotionally arousing experiences independently of the valence [Erk et al.,2003; Kensinger and Schacter,2006; McGaugh,2004; Sergerie et al.,2008]. This is in line with the finding of increased neural activity during the processing of emotionally negative compared to neutral stimuli in deletion compared to nondeletion carriers, as observed by Rasch and colleagues [2009], as the negative stimuli were more arousing. This claim is supported by the findings of Cousijn and colleagues [2010] who showed that increased arousal as a result of stress induction resulted in increased amygdala responses only in deletion carriers.
Previous in vitro studies have reported both agonistic and antagonistic effects of the deletion variant [Small et al.,2001], making it difficult to discern whether the deletion leads to increased or decreased noradrenergic signaling. Given that the propranolol reaches the brain and blocks the subsequent memory effect for emotional stimuli in the amygdala, presumably via a reduction of noradrenergic signaling [Liang et al.,1986], our results are in line with the idea that deletion carriers have higher noradrenaline availability compared to nondeletion carriers [Cousijn et al.,2010; de Quervain et al.,2007]. In this study, we observed a larger contribution of the amygdala to successful memory formation in deletion carriers. Because this appears to be dependent on noradrenergic signaling [Strange and Dolan,2004], our results provide additional evidence for the idea that the ADRA2B deletion variant potentiates noradrenergic activity.
In summary, our results show that a common deletion variant in the ADRA2B gene coding for a noradrenergic receptor leads to larger contributions of the amygdala and inferior frontal gyrus to successful formation of emotional memories. Interestingly, the amygdala and the prefrontal cortex are key brain structures implicated in mood disorders [Drevets,2003; Philipps et al.,2003]. For example, depressed patients show increased amygdala activity during successful memory formation for faces and negative pictures [Hamilton and Gotlib,2008; Roberson‐Nay et al.,2006]. Furthermore, increased prefrontal activity during verbal emotional memory formation has been observed in individuals at risk for mood disorders, such as anxiety and depression [Wolfensberger et al.,2008]. This suggests that the larger contribution of these brain regions during emotional memory formation in deletion carriers might indicate a marker of vulnerability in the development of mood disorders.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Table ADDRA2B genotype effects on successful memory formation (i.e. subsequent hits vs. subsequent misses) and successful recognition (i.e. hits vs. misses).
Acknowledgements
We thank Sabine Kooijman for general assistance, Marlies Naber and Jesse Eernstman for genotyping, Paul Gaalman for technical support, and Tari Awipi for comments on the manuscript. In particular, we also thank Dominique de Quervain for constructive suggestions concerning the project and interpretation of the results. The authors have no potential conflict of interest.
REFERENCES
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K ( 2005): Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl) 210: 343–352. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK ( 1996): In: Antonio S, editor. Manual for the Beck Depression Inventory‐II. Texas: Psychological Corporation. [Google Scholar]
- Bower GH, Gilligan SG, Monteiro KP ( 1981): Selectivity of learning caused by affective states. J Exp Psychol Gen 110: 451–473. [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL ( 1994): β‐Adrenergic activation and memory for emotional events. Nature 371: 702–704. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL ( 1995): The amygdala and emotional memory. Nature 377: 295–296. [DOI] [PubMed] [Google Scholar]
- Cousijn H, Rijpkema M, Qin S, van Marle HJ, Franke B, Hermans EJ, van Wingen G, Fernandez G ( 2010): Acute stress modulates genotype effects on amygdala processing in humans. Proc Natl Acad Sci USA 107: 9867–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ ( 2007): Preventive effect of β‐adrenoceptor blockade on glucocorticoid induced memory retrieval deficits. Am J Psychiatry 164: 967–969. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Kolassa IT, Ertl V, Onyut PL, Neuner F, Elbert T, Papassotiropoulos A ( 2007): A deletion variant of the α2b‐adrenoceptor is related to emotional memory in Europeans and Africans. Nat Neurosci 10: 1137–1139. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE ( 1995): Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task‐difficulty and process specificity. J Neurosci 15: 5870–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R ( 2005): Remembering one year later: Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci USA 102: 2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC ( 2003): Neuroimaging abnormalities in the amygdala in mood disorders. Ann NY Acad Sci 985: 420–444. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Erk S, Kiefer M, Grothe J, Wunderlich AP, Spitzer M, Walter H ( 2003): Emotional context modulates subsequent memory effect. Neuroimage 18: 439–447. [DOI] [PubMed] [Google Scholar]
- Haas BW, Canli T ( 2008): Emotional memory function, personality structure and psychopathology: A neural system approach to the identification of vulnerability markers. Brain Res Rev 58: 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Gotlib IH ( 2008): Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry 63: 1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Tulving E, Wilson AA, Houle S, Brown GM ( 1994): Neuroanatomical correlates of encoding in episodic memory: Levels of processing effect. Proc Natl Acad Sci USA 91: 2008–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL ( 2006): Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. J Neurosci 26: 2564–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernis MH, Greenier KD, Herlocker CE, Whisenhunt CR, Abend TR ( 1997): Self‐perceptions of reactions to doing well or poorly: The roles of stability and level of self‐esteem. Person Indiv Diff 22: 845–854. [Google Scholar]
- Kilpatrick L, Cahill L ( 2003): Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage 20: 2091–2099. [DOI] [PubMed] [Google Scholar]
- Liang KC, Juler RG, McGaugh JL ( 1986): Modulating effects of post‐training epinephrine on memory involvement of the amygdala noradrenergic system. Brain Res 368: 125–133. [DOI] [PubMed] [Google Scholar]
- McGaugh JL ( 2004): The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27: 1–28. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B ( 2002): Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol 12: 205–210. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF ( 1971): Manual: Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service. [Google Scholar]
- Mier D, Kirsch P, Meyer‐Lindenberg A: Neural substrates of pleiotropic action of genetic variation in COMT: A meta‐analysis. Mol Psychiatry 15: 918–927. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, C. B.,Frith CD, Young AW, Calder AJ, Dolan RJ ( 1998): A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 121: 47–57. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR ( 2008): Serotonin transporter (5‐HTTLPR) genotype and amygdala activation: A meta‐analysis. Biol Psychiatry 63: 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipps ML, Drevets WC, Rauch SL, Lane R ( 2003): Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry 54: 515–528. [DOI] [PubMed] [Google Scholar]
- Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Papassotiropoulos A, de Quervain DJ ( 2009): A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proc Natl Acad Sci USA 106: 19191–19196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ ( 2004): Encoding of emotional memories depends on amygdala and hippocampus and their interaction. Nat Neurosci 7: 278–285. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Labar KS, Cabeza R: Level of processing modulates the neural correlates of emotional memory formation. J Cogn Neurosci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson‐Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, Charney DS, Lebenluft E, Blair J, Ernst M, et al. ( 2006): Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An fMRI study. Biol Psychiatry 60: 966–973. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Lepage M, Armony JL ( 2008): A face to remember: Emotional expression modulates prefrontal activity during memory formation. Neuroimage 24: 580–585. [DOI] [PubMed] [Google Scholar]
- Small KM, Brown KM, Forbes SL, Liggett SB ( 2001): Polymorphic deletion of three intracellular acidic residues of the α 2B‐adrenergic receptor decreases G protein‐coupled receptor kinase‐mediated phosphorylation and desensitization. J Biol Chem 276: 4917–4922. [DOI] [PubMed] [Google Scholar]
- Smith APR, Henson RNA, Dolan RJ, Rugg MD ( 2004): fMRI correlates of the episodic retrieval of emotional contexts. NeuroImage 22: 868–878. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J ( 1988): Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J Exp Psychol Gen 117: 34–50. [DOI] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ ( 2004): β‐Adrenergic modulation of emotional memory‐evoked human amygdala and hippocampal responses. Proc Natl Acad Sci USA 101: 11454–11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GS, Chang N‐C, Wu S‐C, Chang AC ( 2002): Regulated expression of α2B adrenoceptor during development. Dev Dynam 225: 142–152. [DOI] [PubMed] [Google Scholar]
- Wolfensberger SP, Veltman DJ, Hoogendijk WJ, De Ruiter MB, Boomsma DI, de Geus EJ ( 2008): The neural correlates of verbal encoding and retrieval in monozygotic twins at low or high risk for depression and anxiety. Biol Psychol 79: 80–90. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC ( 1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Table ADDRA2B genotype effects on successful memory formation (i.e. subsequent hits vs. subsequent misses) and successful recognition (i.e. hits vs. misses).


