Abstract
Impaired understanding of others' sensations and emotions as well as abnormal experience of their own emotions and sensations is frequently reported in individuals with Autism Spectrum Disorder (ASD). It is hypothesized that these abnormalities are based on altered connectivity within “shared” neural networks involved in emotional awareness of self and others. The insula is considered a central brain region in a network underlying these functions, being located at the transition of information about bodily arousal and the physiological state of the body to subjective feelings. The present study investigated the intrinsic functional connectivity properties of the insula in 14 high‐functioning participants with ASD (HF‐ASD) and 15 typically developing (TD) participants in the age range between 12 and 20 years by means of “resting state” or “nontask” functional magnetic resonance imaging. Essentially, a distinction was made between anterior and posterior regions of the insular cortex. The results show a reduced functional connectivity in the HF‐ASD group, compared with the TD group, between anterior as well as posterior insula and specific brain regions involved in emotional and sensory processing. It is suggested that functional abnormalities in a network involved in emotional and interoceptive awareness might be at the basis of altered emotional experiences and impaired social abilities in ASD, and that these abnormalities are partly based on the intrinsic functional connectivity properties of such a network. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: fMRI, emotion, sensory, awareness, feelings, homeostasis, simulation, social cognition, resting state, autism
INTRODUCTION
Autism Spectrum Disorders (ASD) are chronic developmental neurobiological disorders, whose onset normally occurs in the first years of life and are characterized by deficits in social interaction, in verbal and nonverbal communication, and by restricted and stereotyped patterns of behavior, interests, and activities [e.g. Minshew and Williams,2007]. With variable degrees of severity, people with ASD are impaired in social cognition, like establishing meaningful social communications and bonds, and to establish visual contact with the world of others, to share attention with others, and show reduced capabilities of imitating others' behavior or understanding others' intentions, emotions, and sensations. As put forward by Gallese [2006], the early manifestations of ASD share a common root: “the cognitive skills required to establish meaningful bonds with others are missing or seriously impaired.” However, although it is a well‐accepted idea that impaired social cognition is a defining feature of ASD, the dysfunctional neural and cognitive mechanisms underlying these social deficits are poorly understood.
The capacity to recognize mental states underlying others' behavior, is considered one of the most important abilities for intersubjectivity and, hence, social survival. In particular, emotional awareness of self and others has an essential role in social understanding, providing basic information to explain intentional behavior in terms of mental states. Emotional awareness allows the individual to give a meaning to others' intentional behavior and, therefore, to appropriately adapt one's own behavior.
A putative mechanism underlying intersubjectivity and social understanding is embodied simulation. Current neuroscientific models of embodied simulation propose that the same neural structures involved in our own body‐related experiences also contribute to an implicit, automatic, bottom‐up understanding of what we observe in the world around us, like, for instance, others' emotions, sensations, and intentions [Gallese,2005, 2006]. This model of social understanding is supported by extensive empirical evidence for a shared neural circuitry in the sensory‐motor system of humans and primates for first and third person experience in the domains of actions, emotions, pain, and tactile sensations [e.g. Adolphs et al.,1994; Avenanti et al.,2005; Carr et al.,2003; Ebisch et al.,2008; Fogassi et al.,2005; Gallese et al.,1996; Jackson et al.,2006; Keysers et al.,2004; Rizzolatti et al.,1996; Saarela et al.,2007; Singer et al.,2004; Sprengelmeyer et al.,1999; Wicker et al.,2003]. Interestingly, the brain regions that have been implicated most often in ASD [cerebellum, amygdala, insula, medial prefrontal cortex, premotor cortex/ inferior frontal gyrus (IFG)] have mirror/simulation capacities involved in social cognition, not coincidentally impaired in ASD [Dapretto et al.,2006; Fecteau et al.,2006; Hadjikhani et al.,2006, 2007; Pfeifer et al.,2008; Théoret et al.,2005]. Hence, it has been postulated that impaired simulation processes in shared neural circuits are at the root of social deficits in ASD, such as impaired affective and motor empathy [Gallese,2003, 2006; Minio‐Paluello et al.,2009; Oberman and Ramachandran,2007].
In addition to impaired understanding of others' experiences, abnormal experience of their own emotions and sensations is frequently reported in individuals with ASD, too [Ben Shalom et al.,2006; Blakemore et al.,2006; Hill et al.,2004; Hubert et al.,2009; Rieffe et al.,2007]. For example, autistic children become aroused when exposed to trivial objects and events, while they often ignored stimuli that normally trigger connected emotional responses in typically developing children [Hirstein et al.,2001]. Evidence from neuroimaging studies suggests that a reduced emotional awareness in ASD patients is associated with a dysfunctional limbic system, in particular anterior insula and amygdala [Silani et al.,2008]. Though this issue has received much less attention in neuroscientific research, it may be little surprising in the light of models of embodied simulation sustaining that emotional awareness of both self and others share largely similar neural mechanisms. Based on empirical evidence, some authors have proposed that connections within neural circuits involved in emotional processing, like connections between sensory regions and amygdala, may be altered in ASD, eventually leading to distorted emotional experiences as well as impaired abilities to recognize others' emotions correctly [Ramachandran and Oberman,2006]. Connectivity or temporal binding deficits have been proposed underlying reduced integrative information processing in general between brain regions in ASD [e.g. Brock et al.,2002; Just et al.,2004; Minshew and Williams,2007]. However, although previous studies in ASD showed functional deficits in brain areas within the limbic network and associated behavioral measures, the functional connectivity between regions in the limbic network has received little attention. For instance, it is unknown whether alterations are present in the intrinsic organization of such a network, perhaps underlying functional deficits in emotional awareness of self and others.
The insula is considered a central brain region in sensorimotor, visceral, interoceptive processing, homeostatic/allostatic functions, and emotional awareness of self and others, interacting with limbic, somatosensory, and motor regions [Craig,2002; Critchley,2005; Seminowicz and Davis,2007]. Recently, a “nontask” or “resting state” functional magnetic resonance imaging (fMRI) study with healthy adult participants investigated functional connectivity of the insular cortices and identified distinct functional networks for the insula, in accordance with anatomical and functional evidence [Taylor et al.,2008]. The authors suggested that a first network comprising the anterior insula and anterior‐mid cingulate cortex (pACC/aMCC) could be involved in emotional salience monitoring, whereas a second network linking the midposterior insula with MCC could underlie general environmental monitoring, response selection, and skeletomotor body orientation. A related distinction between anterior and posterior insula is also described by Craig [2002] proposing that bilateral midposterior insular cortices receive interoceptive information about the physiological state of the body. This information is then projected to the (right) anterior insula involved in subjective evaluations of internal conditions, in interaction with other structures underlying subjective feelings and emotional awareness, like, for example, ACC. Within a framework provided by peripheral theories of emotions [Lange,1885; James,1894] and the somatic‐marker hypothesis [Damasio,1994], the insular cortex, located at the transition of afferent homeostatic information to subjective feelings, could be a central node in a neural circuit underlying emotional awareness.
For this reason, the present study aims at investigating the intrinsic functional connectivity properties of the insula in high‐functioning participants with ASD (HF‐ASD) by means of “resting state” fMRI, essentially distinguishing between anterior and posterior regions of the insular cortex. Functional connectivity is operationally defined as the statistical dependence between blood‐oxygen‐level‐dependent (BOLD) signals in distant brain regions, and is considered to represent an index of brain function [Biswal et al.,1995; Fox and Raichle,2007; Horwitz,2003]. Analysis of functional connectivity during a “nontask” or “resting” state regards spontaneous low‐frequency BOLD fluctuations in brain voxels, and identifies strong temporally correlated patterns of neural activity across brain regions that subserve similar or related functions [Birn,2007].
It can be hypothesized that, compared with typically developing (TD) individuals, individuals with ASD show altered functional connectivity patterns of the insular cortices being at the transition/gateway of sensory‐visceromotor‐interoceptive and emotional‐limbic processing. Alternatively, activation levels, but not intrinsic connectivity may be altered in ASD individuals.
The present results verify the presence of altered functional connectivity of insular cortices with amygdala and somatosensory regions in HF‐ASD and suggest that abnormalities are present in the intrinsic functional connectivity properties of a network involved in emotional awareness.
METHODS
Participants
Fourteen HF‐ASD (four females) and 15 healthy TD participants (two females; age 12–20 years) matched for intelligence quotient (IQ), age, gender, handedness participated in the present study. All participants and their parents gave written informed consent to the procedures approved by the local ethics committee and conducted in accordance with the 1964 Declaration of Helsinki. Demographic and diagnostic information of the participants is presented in Table I. No significant differences were present between the TD and HF‐ASD group with respect to these demographic variables. Handedness was determined by means of the Edinburgh Handedness Inventory [Oldfield,1971]. Depending on the age of the participant, IQ was measured using the Wechsler Adult Intelligence Scale or Wechsler Intelligence Scale for Children (WAIS‐R/WISC‐III) [Wechsler,1981, 1991]. To control for differences in head movement measures between the TD and HF‐ASD groups, participants with excessive movement during fMRI scanning were excluded from data analysis and the groups were compared with respect to motion parameters (root mean squared variance, rmsvariance) [Church et al.,2009]. Average subject rmsvariance of motion was 0.259 mm (SD = 0.125) in the TD group and 0.214 mm (SD = 0.163) in the HF‐ASD group, and not significantly different between the groups (P = 0.41). Clinical diagnosis of ASD was performed by an experienced child psychiatrist according to standard criteria of autistic disorder (N = 10) or Asperger syndrome (N = 4) (DSM‐IV) [American Psychiatric Association, 1994]. In line with current practice, clinical diagnosis of the participants in the HF‐ASD group was confirmed by means of a structured diagnostic interview with the caretakers, the Autism Diagnostic Interview—Revised (ADI‐R) [Lord et al.,1994]. With respect to comorbidity and medication in the HF‐ASD group, one patient was also diagnosed with Gilles de la Tourette syndrome, two with Attention Deficit Hyperactivity Disorder, and one with dysthemia, two patients were on Fluoxetine, one on Risperidone, and one on Concerta. To verify the absence of psychiatric symptoms related to ASD in TD participants the Social Communication Questionnaire (SCQ) [Berument et al.,1999] was administered to their parents, using a cutoff score of 9 as exclusion criterion for participating in the present study.
Table I.
Demographic information about the typically developing (TD) and high‐functioning ASD (HF‐ASD) group
| TD group (N = 15) | HF‐ASD group (N = 14) | |
|---|---|---|
| Age | 15.95 (±1.65) | 15.79 (±1.93) |
| IQ | 104.9 (±8.45) | 106.5 (±20.01) |
| Handedness score | 78.7 (±47.5) | 66.8 (±58.1) |
| Male/female | 13/2 | 10/4 |
| Diagnosis | NA | Autistic disorder (N = 10), Asperger syndrome (N = 4) |
| Social interaction | ||
| ADI‐R scale A (subj. 1–14) | NA | 10‐12‐22‐14‐21‐19‐24‐19‐10‐13‐19‐13‐9‐28 |
| Comunication and language | ||
| ADI‐R scale B (subj. 1–14) | NA | 12‐7‐14‐4‐15‐16‐20‐14‐9‐10‐14‐6‐9‐19 |
| Nonverbal | ||
| ADI‐R scale B N.V. (subj. 1–14) | NA | 10‐6‐9‐1‐10‐7‐14‐10‐6‐6‐8‐4‐6‐16 |
| Repetitive, restricted and stereotyped interests and behavior | ||
| ADI‐R scale C (subj. 1–14) | NA | 0‐1‐5‐5‐5‐4‐3‐3‐0‐1‐6‐4‐9‐8 |
| Other | ||
| ADI‐R scale D (subj. 1–14) | NA | 1‐1‐1‐3‐2‐2‐2‐2‐1‐1‐3‐3‐2‐5 |
| Co‐morbitity | NA | ADHD (N = 2), Gilles de la Tourette (N = 1), dysthemia (N = 1) |
| Medication | NA | Fluoxetine (N = 2), Risperidone (N = 1), Concerta (N = 1) |
Data Acquisition
For each subject, BOLD contrast functional imaging was performed with a Siemens Magnetom Avanto syngo MR B15 scanner at the Donders Institute for Brain, Cognition and Behavior with 1.5T field strength by T2*‐weighted echo‐planar imaging (EPI) free induction decay (FID) sequences with the following parameters: TR = 1,990 ms, TE = 45 ms, matrix size 64 × 64, Field of View (FoV) = 224 mm, 23 transaxial slices with interleaved acquisition order, in‐plane voxel size = 3.5 × 3.5 mm, flip angle = 83 degrees, slice thickness = 5.0 mm, and no gap. A standard “birdcage” head coil was used and the subject's head was fixed with foam pads to reduce involuntary movement. Two hundred sixty functional volumes were acquired for a duration of 8.6 minutes. All participants were instructed to relax (but not sleep), keep their eyes closed, and think of nothing in particular during fMRI scanning.
A high‐resolution structural volume was acquired at the end of the session via an ascending single shot sequence with the following features: TR = 2,250 ms, TE = 2.95 ms, 176 sagittal slices, FoV 256 mm, slice thickness 1.0 mm, in‐plane voxel size 1.0 × 1.0 mm, flip angle 15 degrees.
Data Analysis
Raw data were analyzed with the Brain Voyager QX 1.9 software (Brain Innovation, Maastricht, The Netherlands) at the ITAB. Because of T1 saturation effects, the first five scans of each run were discarded from the analysis. Preprocessing of functional data included slice scan time correction, three‐dimensional motion correction, and removal of linear trends from voxel time series. Preprocessed functional volumes of a participant were coregistered with the corresponding structural dataset. As the four‐dimensional functional and three‐dimensional structural measurements were acquired in the same session, the coregistration transformation was determined using the Siemens slice position parameters of the functional images and those of the structural volume. Both structural and functional volumes were then transformed into the Talairach space [Talairach and Tournoux,1988] using a piecewise affine and continuous transformation. Functional volumes were resampled at a voxel size of 3 × 3 × 3 mm3. A modest spatial smoothing with a Gaussian kernel of 6.0 mm full‐width half‐maximum was applied to functional images corresponding to two voxels in the resampled data to account for intersubject variability, though maintaining a relatively high spatial resolution.
Before functional connectivity analysis, the BOLD time series were preprocessed by means of MATLAB software (The Mathworks Inc., Natick, MA) according to the procedure described in Fox et al. [2005, see also Fox and Raichle,2007]. The preprocessing steps included (1) band‐pass filtering between 0.009 and 0.08 Hz; (2) regression of global, white matter, and ventricle signals, and their first derivatives; and (3) regression of three‐dimensional motion parameters, and their first derivatives.
Functional connectivity analysis in the present study was restricted to the frequency band between 0.009 and 0.08 Hz in accordance with a large body of literature reporting that long‐range communication processes in resting state networks as detected from fMRI signals oscillate in this ultra‐low frequency range [e.g. Cordes et al.,2001; Fox and Raichle,2007; Fox et al.,2005, 2006a, b; Vincent et al.,2006, 2008]. Band‐pass filtering is applied to attenuate the contribution of nonphysiological noise. For example, frequencies below 0.01 Hz may be partly related to very slow drifts in hardware, and are also excluded in classical fMRI analysis for task‐related experiments. On the other hand, higher frequencies (e.g. >0.1 Hz) are likely to receive the contribution of cardiac and respiratory activity [e.g. Auer,2008], and should be possibly attenuated [Fox and Raichle,2007].
After this preprocessing, a selection of regions of interest (ROIs) was performed; among them, right hemisphere anterior insula (RH aIC), left hemisphere anterior insula (LH aIC), RH posterior insula (RH pIC), and LH posterior insula (LH pIC) were determined using the Talairach coordinates reported by Taylor et al. [2008] (Table II). As control measures, connectivity maps were calculated for seed ROIs that did not result in different connectivity maps between HF‐ASD and TD participants in previous studies [Kennedy and Courchesne,2008]: left intraparietal sulcus (−25,−57,46), right superior precentral sulcus (25,−13,50), and left middle temporal region (−45,−69,−2). For each ROI, a representative BOLD time‐course was obtained by averaging the signals of the voxels within a sphere of 6 mm radius corresponding to two voxels in the resampled data. This size has been shown to be a reliable and efficient size for seed ROIs [e.g. Fox et al.,2005], whereas a larger radius also would increase the risk to include other adjacent brain regions, like those close to the insular cortices.
Table II.
Brain regions showing seed‐based functional connectivity at a significance level of P < 0.05 (FDR corrected) with seed regions of interest (ROIs)
| Seed ROI | Brain region functionally connected with seed ROI | Talairach coordinates peak value (x, y, z) | t‐Score TD group | t‐Score HF‐ASD group | t‐Score TD vs. HF‐ASD | P value TD vs. HF‐ASD |
|---|---|---|---|---|---|---|
| aIC RH (Talairach coordinates: 36, 16, 2) | ||||||
| aIC LH | −40, 12, 5 | 18.43 | 11.68 | |||
| dACC RH | 5, 8, 44 | 13.96 | 6.36 | |||
| pMSFG RH | 6, 9, 52 | 12.74 | 6.80 | |||
| pIC RH | 36, −8, −4 | 9.76 | 5.26 | |||
| ACC RH | 4, 33, 26 | 10.66 | 6.03 | |||
| SFG RH | 34, 44, 28 | 14.06 | 7.85 | |||
| PreCG RH | 39, −5, 48 | 8.74 | 5.57 | |||
| IFG RH | 38, 2, 30 | 9.41 | NS | |||
| Amygd RH | 29, −6, −22 | 3.43 | NS | 4.35 | 0.0009 | |
| aMCC LH | −13, 2, 40 | 10.82 | 3.30 | |||
| pMCC RH | 4, −24, 38 | 8.25 | NS | |||
| SFG LH | −32, 49, 29 | 7.23 | 5.48 | |||
| OFC RH | 23, 49, 3 | 6.87 | 3.69 | |||
| IPS RH | 41, −52, 47 | 4.78 | NS | |||
| Thal RH | 7, −16, 9 | 7.32 | NS | |||
| aIC LH (Talairach coordinates: −34, 14, 2) | ||||||
| aIC RH | 36, 24, 5 | 15.24 | 10.06 | |||
| dACC RH | 7, 25, 34 | 11.75 | 9.95 | |||
| dMSFG RH | 4, 18, 50 | 12.63 | 5.59 | |||
| aMCC RH | 2, 7, 44 | 11.40 | 6.44 | |||
| aMCC LH | −3, 13, 41 | 11.30 | 6.06 | |||
| SFG RH | 32, 45, 25 | 10.62 | 4.66 | |||
| SFG LH | −33, 51, 25 | 9.36 | 9.38 | |||
| vPreCG LH | −55, 0, 20 | 7.35 | 3.54 | |||
| mIC LH | −42, −12,11 | 6.64 | 4.68 | |||
| OFC LH | −30, 48, −1 | 9.25 | NS | |||
| Thal LH | −9, −12, 2 | 7.15 | 3.57 | |||
| pMCC RH | 9, −25, 38 | 6.13 | 6.26 | |||
| vPreCG RH | 37, 2, 29 | 5.89 | 8.30 | |||
| IFG RH | 35, 11, 28 | 5.81 | 5.64 | |||
| DLPFC LH | −43, 11, 33 | 6.22 | NS | |||
| pIC LH | −38, −26, 14 | 4.92 | NS | |||
| pIC LH (Talairach coordinates: −38, −12, 7) | ||||||
| mIC LH | 43, −2, 12 | 14.22 | 8.70 | |||
| SII LH | −46, −27, 25 | 16.18 | 4.33 | |||
| MCC LH | −10, −6, 42 | 13.00 | 5.20 | |||
| MCC RH | 6, −7, 49 | 14.29 | 5.63 | |||
| dPostCG LH | −46, −28, 45 | 6.23 | NS | 3.62 | 0.002 | |
| vPostCG LH | −46, −26, 24 | 15.55 | 4.33 | 3.44 | 0.002 | |
| vPostCG RH | 63, −14, 29 | 11.11 | 3.47 | |||
| dPostCG LH | −38, −21, 51 | 9.71 | NS | 3.38 | 0.003 | |
| dPreCG RH | 51, −15, 51 | 11.53 | 4.62 | |||
| SII RH | 53, −21, 19 | 11.98 | 5.80 | 3.48 | 0.002 | |
| Thal LH | −10, −20, 1 | 6.59 | NS | |||
| Thal RH | 11, −18, 1 | 7.34 | 3.99 | |||
| SPC LH | −38, −43, 61 | 6.90 | NS | |||
| pIC RH (Talairach coordinates: 38, −10, 7) | ||||||
| pIC LH | −45, −23, 16 | 13.88 | 6.61 | |||
| pIC RH | 40, −18, 14 | 14.74 | 10.44 | |||
| mIC LH | −46, −9, 6 | 14.09 | 12.34 | |||
| vPostCG RH | 54, −14, 37 | 9.79 | 3.72 | |||
| vPostCG RH | 50, −19, 25 | 9.68 | 3.61 | |||
| vPostCG RH | 63, −6, 24 | 9.63 | 5.27 | |||
| vPostCG LH | −54, 0, 28 | 8.90 | 3.69 | 3.30 | 0.01 | |
| MCC RH | 3, −8, 39 | 7.54 | 5.13 | |||
| MCC RH | 6, 4, 39 | 5.71 | 3.66 | |||
| Thal RH | 17, −26, 4 | 6.31 | 3.22 | |||
| MCC LH | −8, −4, 42 | 5.15 | 5.13 | |||
| dPostCG RH | 41, −20, 57 | 4.48 | NS | |||
| mIC LH | −38, −1, 1 | 11.03 | 6.88 | |||
LH, left hemisphere; RH, right hemisphere; aIC, anterior insular cortex; mIC, midinsular cortex; pIC, posterior insular cortex; (a/p)MCC, (anterior/posterior) midcingulate cortex; (d)ACC, (dorsal) anterior cingulate gyrus; (v/d)PreCG, (ventral/dorsal) precentral gyrus; Amygd, amygdala; Thal, thalamus; SFG, superior frontal gyrus; IPS, intraparietal sulcus; MSFG, medial superior frontal gyrus; IFG, inferior frontal gyrus; OFC, orbitofrontal cortex; DLPFC, dorsolateral prefrontal cortex; (v/d)PostCG, (ventral/dorsal) postcentral gyrus; SII, secondary somatosensory cortex.
Specific whole‐brain seed‐based connectivity maps of the aforementioned ROIs were created for all individual participants, by calculating correlations between the ROI time‐course (i.e. time‐course in each of the insula ROIs) and all the time‐courses of the brain voxels [Fox et al.,2005]. After applying Fisher's r‐to‐z transformation [Zar,1996] to each correlation map, random‐effect analysis was performed independently for each of the two groups of participants in order to reveal consistent functional connectivity patterns that were consistent for both the groups of participants for each of the insula ROIs [Fox et al.,2006a]. A similar independent‐samples t‐test, according to a random‐effect analysis, between the whole‐brain connectivity maps for the different insula seed ROIs of the healthy TD control and HF‐ASD groups was used to calculate direct contrasts between HF‐ASD patients and the TD control group.
Statistical significance was assessed using a statistical threshold corrected by the False Discovery Rate (FDR) [q < 0.05, approximately corresponding to t > 3.203 and P < 0.003 at the voxel level; Genovese et al.,2002] and a minimal cluster volume threshold of 100 mm3.
ROI‐based functional connectivity analyses were applied to verify the results of the seed‐based functional connectivity analysis. In this case, a cross‐correlation matrix was calculated for each subject and each insula network using the ROI time‐courses of all clusters functionally connected with the insula seed ROI. These clusters were defined as spheres with a 6‐mm radius and functionally based on the peak coordinates of the previously described seed‐based group analysis. Subsequently, the correlations were converted to z‐scores by means of the Fisher's r‐to‐z transformation [Zar,1996]. Group‐level analyses for HF‐ASD patients and TD controls were performed by means of independent‐samples t‐tests between the cross‐correlation matrices for the different insula seed ROIs of the healthy TD control and HF‐ASD groups.
RESULTS
Functional connectivity analyses based on the seed ROIs resulted in significantly different connectivity maps for distinct insula regions in TD participants (FDR corrected). LH aIC and RH aIC showed significant functional connectivity with anterior midcingulate cortex (aMCC) extending to the border with supplementary motor area (SMA) and anterior cingulate cortex (ACC), posterior midcingulate cortex (pMCC), superior frontal gyrus (SFG), dorsolateral prefrontal cortex (DLPFC), precentral gyrus (PreCG), thalamus, posterior insula, orbitofrontal cortex (OFC), and contralateral aIC. Specifically for RH aIC, significant connectivity was also found with midposterior insula, RH intraparietal sulcus (IPS), and RH amygdala. LH pIC and RH pIC showed significant functional connectivity with midcingulate cortex (MCC) extending to SMA, primary motor cortex (dPreCG), ventral and dorsal postcentral gyrus (v/dPostCG, SI), parietal operculum (SII), IFG, thalamus, and contralateral midposterior insula.
The functional connectivity maps in the HF‐ASD group were largely similar to those of the TD group, although some differences could be observed qualitatively in the group statistical maps (i.e. by separate inspection of the statistical maps of the TD and HF‐ASD group): using the same statistical threshold as for the TD group statistical maps, no significant connectivity was observed in the HF‐ASD group connectivity maps between RH aIC, and amygdala and thalamus; between LH pIC, and dPostCG and SPC; between RH pIC and dPostCG; between LH aIC, and OFC and pIC. Table II shows the brain regions significantly connected with the seed ROI for the TD as well as for the HF‐ASD group, and statistical values of the peak coordinates and between‐group differences.
Statistical comparisons between the whole‐brain connectivity group maps of RH aIC, RH pIC, and LH pIC showed significant connectivity differences between the TD and HF‐ASD (Fig. 1). No significant between‐group differences are found for functional connectivity of LH aIC and the control seed ROIs (left intraparietal sulcus, right superior precentral sulcus; left middle temporal region). Multivariate tests regarding the cross‐correlation matrices confirmed significant differences in connectivity of the RH aIC, RH pIC, and LH pIC with other network nodes between the TD and HF‐ASD group (P < 0.05).
Figure 1.
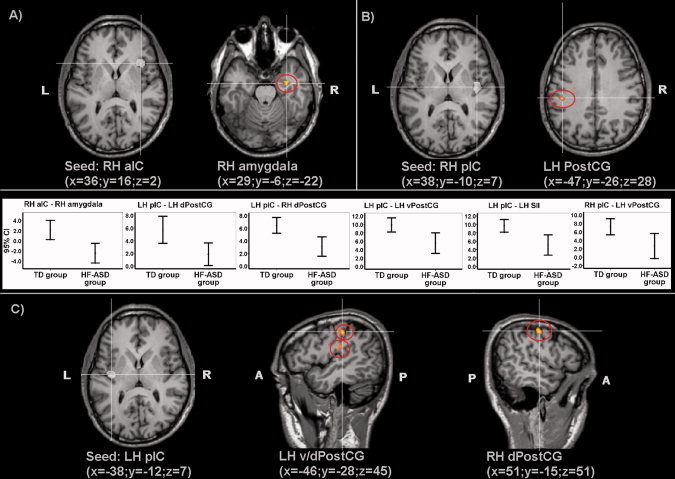
Group statistical maps showing brain regions with significant differences (random effect analysis, P < 0.05 FDR corrected) between connectivity maps of the TD and HF‐ASD group based on: (A) seed ROI “right anterior insular cortex” (RH aIC) (x = 36, y = 16, z = 2); (B) seed ROI “right posterior insular cortex” (RH pIC) (x = 38, y = −10, z = 7); (C) seed ROI “left posterior insular cortex” (LH pIC) (x = −38, y = −12, z = 7); corresponding graphics showing 95% confidence intervals (CI) of functional connectivity in the TD and HF‐ASD group.
Contrasts between the RH aIC connectivity maps of the TD group and the HF‐ASD group showed decreased functional connectivity for the HF‐ASD group between RH aIC and RH amygdala (Fig. 1a). Statistical contrasts between the RH pIC connectivity maps of the TD group and HF‐ASD group showed reduced functional connectivity for the HF‐ASD group between RH pIC and LH ventral PostCG/somatosensory cortex (Fig. 1b). Statistical comparisons between the LH pIC connectivity maps of the TD group and HF‐ASD group showed reduced functional connectivity for the HF‐ASD group between LH pIC, and LH ventral and dorsal PostCG/somatosensory cortex and RH dorsal PostCG (Fig. 1c). Graphics representing 95% confidence intervals (CI) for connectivity measures in both groups are shown in Figure 2.
Figure 2.
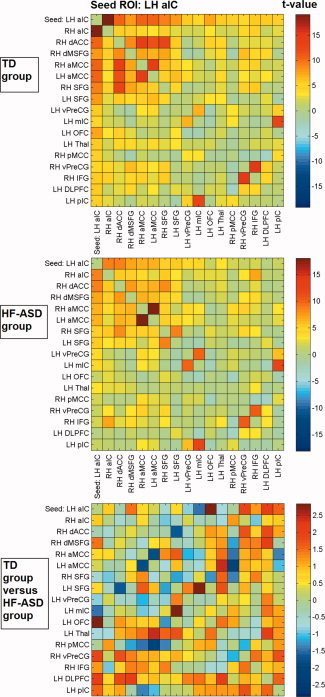
Cross‐correlation matrices showing color‐coded t‐values of connectivity between brain regions within the LH aIC network for the typically developing (TD) and high‐functioning autism spectrum disorder (HF‐ASD) group, based on the peak coordinates and 6 mm radius of the main network nodes.
With respect to the control ROI‐based functional connectivity analysis, Figures 2 to 5 show t‐values of cross‐correlation connectivity matrices in the TD and HF‐ASD group, functionally based on the peak coordinates and 6 mm radius of the main network nodes within the RaIC, LaIC, RpIC, and LpIC networks, respectively (the cross‐correlation values between the same ROIs on the x‐ and y‐axis are set to zero). Contrasts between the cross‐correlation connectivity matrices of the TD and HF‐ASD group of the main network nodes within the RH aIC, RH pIC, and LH pIC networks confirmed the between‐group differences described above for the seed‐based analysis (Figs. 2, 3, 4, 5). To control for the possibility that the results could be specific for the choice of ROIs with a particular size, the analyses also were repeated with various radii in the range of 4 to 8 mm. However, these provided similar results.
Figure 5.
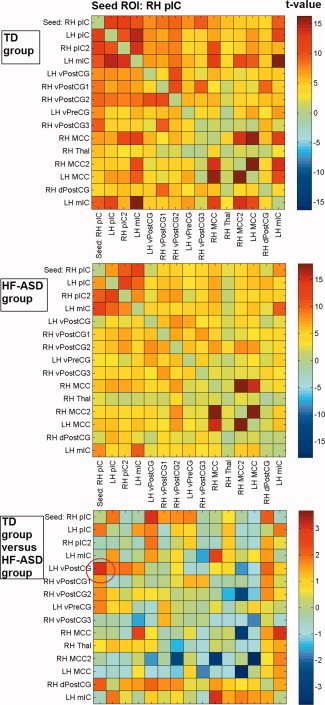
Cross‐correlation matrices showing color‐coded t‐values of connectivity between brain regions within the RH pIC network for the typically developing (TD) and high‐functioning autism spectrum disorder (HF‐ASD) group, based on the peak coordinates and 6 mm radius of the main network nodes.
Figure 3.
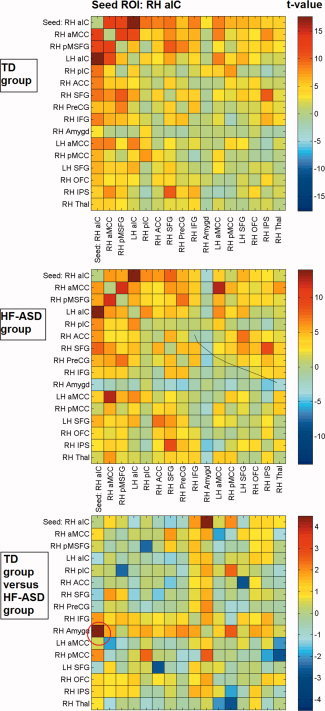
Cross‐correlation matrices showing color‐coded t‐values of connectivity between brain regions within the RH aIC network for the typically developing (TD) and high‐functioning autism spectrum disorder (HF‐ASD) group, based on the peak coordinates and 6 mm radius of the main network nodes.
Figure 4.
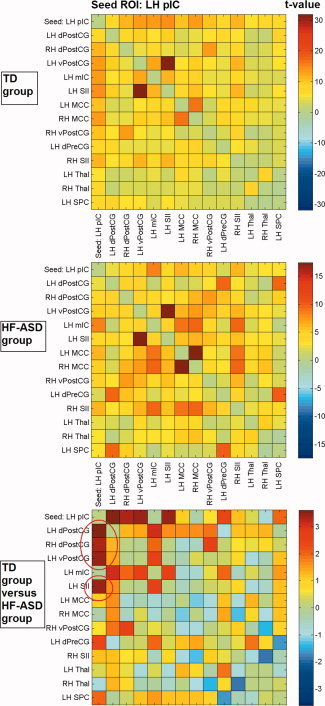
Cross‐correlation matrices showing color‐coded t‐values of connectivity between brain regions within the LH pIC network for the typically developing (TD) and high‐functioning autism spectrum disorder (HF‐ASD) group, based on the peak coordinates and 6 mm radius of the main network nodes.
Importantly, significant positive functional connectivity was found with the insula seed ROIs in the TD group, and most single TD participants, for all the brain regions showing between‐group differences, whereas connectivity was consistently decreased or even absent in the HF‐ASD group (see also Table II and CI graphics in Fig. 1). Covariance analysis in both the TD and HF‐ASD group detected no correlations between age and connectivity in the brain regions that were altered in the HF‐ASD group.
The HF‐ASD group in the present study included autistic disorder as well as Asperger syndrome. To take into account possible differences in brain function between these two groups, direct ROI‐based comparisons were performed between the cross‐correlation matrices for the different insula seed‐ROIs of these groups. Cross‐correlation matrices were functionally based on the peak coordinates and 6 mm radius of the main network nodes within the RaIC, LaIC, RpIC, and LpIC networks. A significant difference could be detected in the RH aIC network. Participants with autistic disorder showed significantly decreased functional connectivity between RH aIC, and RH dACC (t = 4.16, 0.004; Talairach coordinates: 5, 8, 44), compared with participants with Asperger syndrome as well as compared with the TD group, whereas functional connectivity between RH aIC and amygdala was significantly reduced in both HF‐ASD subgroups. Functional connectivity between RH aIC and dACC in all four patients with Asperger syndrome was in the same range as the TD group.
DISCUSSION
The present study investigated the intrinsic functional connectivity properties of the insula in HF‐ASD by means of “resting state” or “nontask” fMRI taking into account an anterior‐posterior distinction of the insular cortex. The results show different functional connectivity networks for anterior and posterior insular cortices in the TD group. The networks obtained for the different insula regions are largely consistent with those reported by Taylor et al. [2008] in healthy adults, principally showing a similar anterior‐posterior distinction of the cingulate cortices and connectivity between posterior insula and somatosensory cortices; in accordance with other studies, additional functional connectivity in the present study was found between anterior insula, and thalamus and amygdala [Robinson et al.,2010; Seeley et al.,2007]. The functional connectivity patterns reported here also correspond to the anatomical connections of the insular cortices [Augustine,1996; Craig,2002, 2009; Critchley,2005]. Extending previous studies, this study reveals altered intrinsic functional connectivity patterns for different insula regions in HF‐ASD, compared with TD participants. HF‐ASD participants showed reduced intrinsic functional connectivity of bilateral posterior insular cortices with ventral and dorsal somatosensory cortices, and between anterior insular cortices (RH, but not LH) and amygdala. These brain regions showing differential connectivity patterns between TD and HF‐ASD participants in this study all have clearly described afferent (e.g. dPostCG) or reciprocal connections (e.g. amygdala, vPostCG, SII) with the insular cortices and, therefore, plausibly reflect anatomical connectivity [Augustine,1996; Craig,2009; Heimer and Van Hoesen,2006; Höistad and Barbas,2008]. Moreover, in accordance with previous studies [Kennedy and Courchesne,2008] differences between the TD and HF‐ASD group were specific for the insular seed regions and were not detected for the control seed ROIs.
The insula is considered a multimodal region involved in sensorimotor, allostatic/homeostatic, emotional, and cognitive functions. According to Craig [2002], thalamocortical pathways provide a direct representation of homeostatic afferent information to the posterior insular cortex that engenders distinct bodily or interoceptive feelings by projections onto the right anterior insula for an emotional evaluation [see also Cechetto and Saper,1987; Crichley,2005; Saper,2002]. In convergence with the somatic‐marker hypothesis of consciousness [Damasio,1994], these feelings could represent the “material me” in the sense of a mental interoceptive representation of the sentient self [Craig,2002]. This idea can be traced back to 19th century peripheral theories of emotion, postulating that afferent bodily arousal information is intrinsically related to emotional experiences. For example, the James‐Lange theory argues that changes in afferent feedback about bodily arousal are necessary for emotional awareness and determining for the intensity of our emotional experiences [Lange,1885; James,1894]. Although emotional experiences certainly involve a more extended network that comprises also OFC, ACC, and amygdala, all anatomically connected with the anterior insula [Augustine,1996; Heimer and Van Hoesen,2006; Höistad and Barbas,2008], the right anterior insula could be at the basis of emotional awareness originating from interoceptive feelings processed in posterior insula [Craig,2009; Critchley et al.,2004]. Correspondingly, recent anatomical and functional neuroimaging evidence suggests that anterior insula is responsible for the degree to which healthy individuals and patients with ASD are more or less aware of, or susceptible to, their emotions [Borsci et al.,2009; Iaria et al.,2008; Silani et al., 2008]. Quite relevantly, in the light of models of embodied simulation and impaired empathic abilities in ASD, the anterior insula and related emotional network, including amygdala, OFC, and ACC, are also involved in the understanding of others' emotional experiences, like pain, disgust, or fear [Carr et al.,2003; Jackson et al., 2006; Saarela et al.,2007; Singer et al.,2004; Sprengelmeyer et al.,1999; Wicker et al., 2003].
Only recently intrinsic functional connectivity of the insular cortex has been investigated by some research groups. Slightly different functional explanations have been offered for the role of the insula in neural networks. For example, Seeley et al. [2007] proposed that the insula is part of a “salience network” comprising also ACC, OFC, and limbic structures responding to the amount of personal salience. Also Dosenbach et al. [2007] distinguished a similar network comprising insula and ACC calling it “core network.” An alternative possibility is offered by Taylor et al. [2008] investigating the intrinsic functional connectivity of the insular cortex based on an anatomical anterior‐posterior distinction. They identified an anterior insula network involved in emotional salience detection, and a midposterior insula network believed to be involved in more general detection of salient perceptual stimuli, skeletomotor orientation, and response selection.
In this study, a reduced functional connectivity was found for the HF‐ASD group between posterior insular cortices and somatosensory cortices. This result was largely consistent for LH pIC and RH pIC. In line with previous evidence for functional interactions described above and reciprocal anatomical connections [Augustine,1996] between posterior insula MCC, thalamus, somatosensory (SI and SII), and motor cortices, the TD group showed strong functional connectivity among these regions. Within the framework provided by the somatic marker hypothesis and the James‐Lange theory for emotional awareness, this could reflect a network involved in homeostasis and interoceptive awareness at the basis of a subsequent emotional evaluation in right anterior insula [Craig,2002; Damasio,1994; James,1894; Khalsa et al., 2009; Lange,1885]. In support of this close relationship between emotions and changes in bodily arousal, functional neuroimaging and neurophysiological studies show engagement of somatosensory, insula, and brainstem regions during self‐generated as well as stimulus‐induced emotions, and a correlation between interoceptive awareness and intensity of induced feelings [Damasio et al.,2000; Pollatos et al.,2007]. In accordance with the model of embodied simulation, somatosensory cortices play a role in the visual recognition of others' emotions and feelings, too [Adolphs et al.,2000; Avenanti et al.,2005; Ebisch et al.,2008; Gallese,2003, 2006]. Altered intrinsic functional connectivity between posterior insula and somatosensory cortices in the HF‐ASD group as found in this study could therefore be interpreted as evidence for a dysfunctional network underlying interoceptive awareness in ASD, likely to ground altered subjective feelings.
Regarding the anterior insula, the current results show a reduced functional connectivity between right anterior insula and amygdala in the HF‐ASD group. Functional connectivity between anterior insular cortex and amygdala is likely to reflect anatomical connectivity [Augustine,1996; see also Höistad and Barbas,2008, for a more extensive discussion of information transfer between insula and amygdala]. Given the putative role of the anterior insula underlying subjective feelings and emotional salience detection, it could be argued that this may lead to alterations in attributing emotional valence to external events. This valence could be grounded in past experiences, given the strong relationship between amygdala and memory of emotional experiences [e.g. Buchanan et al.,2006]. A more abstract valence function is also proposed for the amygdala in the detection of particularly salient, like emotionally relevant, stimuli [Adolphs,2008; see also Anderson and Phelps,2001]. For example, a relationship has been demonstrated between both amygdala lesions and volumetric measures, and the inability to fixate the eye region of others' faces spontaneously, and fear recognition from facial expressions [Adolphs et al., 1994; Spezio et al.,2007a]. Interestingly, both the ability to make spontaneously eye contact with other individuals and the understanding of others' emotional expressions are impaired in ASD [Dalton et al.,2005; Spezio et al.,2007b]. Therefore, differently from the posterior insula, it could be argued that the anterior insula network may rather function as part of a valence network, coding in particular emotional saliency in the context of personal interests, needs, and desires [see also Seeley et al.,2007]. Such functions could be reasonably reconciled with evidence for a role of the right anterior insula in switching between a resting state Default Mode Network (DMN) and central executive networks (for instance, reflecting a switch from a resting state to an active state for interaction with the environment) [Sridharan et al.,2008]. In the context of the vital representation of the physiological state of the body in the insular cortex and its interaction with “limbic motor cortex”/ACC [Craig,2009], this could have an obvious evolutionary value for survival.
Hence, the present results confirm the hypothesis of altered connectivity between insular cortex, and amygdala and sensory regions in ASD. As proposed by Ramachandran and Oberman [2006], these altered connections, with an eventual mediating role of the insula between sensory and limbic processing [Craig,2009], could cause a distorted “salience landscape” in ASD. The insular cortex is considered a principal node in a neural mechanism integrating bodily arousal, autonomic, and valence information from sensory, limbic, memory, and motor regions [e.g. Craig,2002]. Disruptions of connections of the insular cortex with somatosensory and limbic brain regions could plausibly underlie altered awareness of emotions and feelings of self frequently observed in ASD. A similar, “shared,” mechanism for emotional awareness might explain an impaired capacity to resonate and understand these mental states in other individuals as well. This is a principal characteristic of ASD related to dysfunctional simulation of others' emotional experiences [Gallese,2003, 2006]. The specificity of the current findings for right anterior insula (but not left), in contrast to bilateral posterior insula, is also consistent with the neuroanatomical pathways in interoception and afferent visceral control described by Craig [2009].
Previous fMRI studies in ASD investigated intrinsic functional connectivity within the DMN comprising brain regions reportedly involved in “mentalizing” or Theory of Mind (ToM) tasks and within so‐called task positive networks (TPN), like those involved in sustained attention and goal‐directed cognition. They reported specific alterations within the DMN, but not in the TPN, although not directly linked it with relevant functions [e.g. Kennedy and Courchesne,2008]. The results from this study cannot be directly related to these observations. However, we do not think that they are conflicting, but may be related to different aspects of ASD pathology. Moreover, it is not intended that the present results are the underlying cause of general ASD pathology. ASD, most likely, is due to concurrent and multiple physiopathological mechanisms, not confined to ToM or “mentalizing” regions, like medial prefrontal cortex [Frith,2001]. Indeed, underconnectivity [Just et al.,2004; Minshew and Williams,2007] or reduced temporal binding [Brock et al.,2002] between brain regions has been proposed as a more general integrative processing deficit in ASD that may not be confined to specific functions.
Different from the DMN, the networks investigated in this study likely contribute to integrative processing underlying emotional awareness and valence monitoring, and automatic bottom‐up aspects of affective empathy, putatively grounded in “resonance” or simulation mechanisms. Accordingly, neuroimaging studies suggest that anterior insula and amygdala might be involved in emotional awareness of self and others, and its disruption in ASD [Pfeifer et al.,2008; Silani et al.,2008], but not DMN/ToM regions. The latter could be involved in more inferential and cognitive aspects of empathy, such as “mentalizing” [Baron‐Cohen,2009; Frith,2001]. However, these affective/simulation and cognitive/inferential networks possibly subserve complementary functions underlying full‐blown social cognition abilities, that are clearly distinct, but both affected in ASD [e.g. Baron‐Cohen2009; de Lange et al.,2008; Minio‐Paluello et al.,2009]. In this sense, this study complements previous functional connectivity studies that specifically investigated the functional connectivity properties of ToM/DMN regions in ASD. Moreover, neuroimaging evidence suggested a close, eventually antagonistic, interaction between the insula network (in particular, right anterior insula) and the DMN [Sridharan et al.,2008; Uddin et al.,2008]. The functionality of this interaction deserves further investigation in this context.
Some other issues should be mentioned. First, the participants in this study were in the age range between 12 and 20 years. Although this age range includes different developmental stages, it is not plausible that the detected differences between the TD and HF‐ASD group can be explained by this relatively wide range because the groups were well matched and the results consistent across the participants in the different groups. Furthermore, although developmental changes in functional connectivity have been reported between childhood and adulthood [Fair et al.,2007], the absence of a relationship between age and altered functional connectivity in this study suggests that the between‐group differences are not due to developmental changes in the TD‐group or a deviant development in the HF‐ASD group, at least during adolescence. The results rather suggest that these alterations could already be present before the onset of adolescence and emerge during preceding developmental stages. The interaction between developmental changes and alterations of functional connectivity in developmental disorders is a crucial topic for further investigation and it is suggested that also earlier developmental stages should be included.
Second, one could argue that HF‐ASD is characterized by a heterogeneous manifestation plausibly reflecting differences in brain function. Symptoms and their severity may vary between patients, and the HF‐ASD group in this study included autistic disorder as well as Asperger syndrome. Importantly, social deficits are defining features for both diagnoses, although they differ on other domains. Indeed, significant social dysfunction was reported for all participants in the HF‐ASD group and altered functional connectivity between the insular cortices, somatosensory cortices, and amygdala appeared consistent across patients (e.g. Fig. 1). Nevertheless, direct comparisons between participants with autistic disorder (N = 10) and Asperger syndrome (N = 4) suggested additional alterations of functional connectivity in cases of autistic disorder, in particular between RH anterior insula and dACC, whereas functional connectivity between these regions seemed unaffected in all cases of Asperger syndrome. However, given the small sample size, these differences remain preliminary.
Third, it should be noted that the results of this study regard spontaneous BOLD fluctuations, analyzed in terms of intrinsic functional connectivity within specific networks. Some caution is required in generalizing them to specific tasks and functions involving these networks; BOLD response in the implicated regions was neither explicitly manipulated by specific tasks nor investigated in relationship with measurements of emotional or social personality characteristics in this study. However, the direct relationship between the reported alterations in spontaneous functional connectivity and their functional relevance for human behavior needs to be clarified and would be an important topic for further studies [see, for instance, Fox et al.,2006b]. However, this approach led to successful identification of functional connectivity within several meaningful networks during resting state strongly overlapping with networks commonly modulated during the performance of cognitive tasks [e.g. Dosenbach et al.,2007; Fox et al.,2005, 2006a; Fransson et al.,2005; Mantini et al., 2007; Seeley et al.,2007]. Moreover, a series of studies established that the identification of these functional networks during a resting state are consistent across individual subjects, repeated measurements [e.g. Damoiseaux et al.,2006] and analysis methods [Mantini et al.,2008] are consistent with anatomical connectivity patterns [Buckner et al.,2008; Greicius et al.,2009; Honey et al.,2009; van den Heuvel et al.,2009], and are likely related to ongoing neural activity (e.g. De Luca et al.,2006; Mantini et al.,2008]. It also has been proven useful in the identification of deviant brain functioning in various neurological and psychiatric disorders, like ASD, schizophrenia, Tourette syndrome, and Alzheimer disease [e.g. Buckner et al.,2008; Church et al.,2009; Kennedy and Courchesne,2008].
Finally, although reduced functional connectivity in ASD was found between insula and other brain regions involved in emotional and sensory processing, the low temporal resolution of the BOLD signal as measured by fMRI is a limiting factor for distinguishing between alternative accounts for these connectivity differences as well as for the determination of the specific frequency bands of neuronal oscillations that drive the observed correlations of the BOLD signals in different brain regions. For example, reduced functional connectivity may result from time lags in neural activation or a lack of communication between brain regions, and specific ultra‐low frequency neural oscillations might be related to long‐range communication processes within the brain [e.g. Linkenkaer‐Hansen et al.,2004; Poil et al.,2008]. Because fMRI signals are typically sampled between 1 and 3 seconds, frequency‐domain analyses of resting state fMRI signals are limited to a spectral range including only very slow fluctuations, while attenuating nonphysiological noise likely to contribute to lower and higher frequencies [e.g. Fox and Raichle,2007]. We argue that magnetoencephalography/electroencephalography studies may potentially reveal the alteration of intrinsic functional connectivity in terms of frequencies and time‐delays in ASD (e.g. de Pasquale et al., in press).
In conclusion, the alterations in intrinsic functional connectivity reported in the HF‐ASD group, compared with TD participants, are consistent with empirical neuroscientific evidence as well as with current hypotheses regarding emotional awareness of self and others. Principally, HF‐ASD participants showed disrupted intrinsic functional connectivity between insular cortex and amygdala as well as somatosensory regions. The insula is located at the transition of bodily arousal information to subjective feelings. In the light of peripheral theories of emotion and the somatic marker hypothesis, it is suggested that these alterations could be at the basis of some characteristic aspects of ASD symptomatology, like altered emotional experiences and impaired social abilities. Furthermore, the present results suggest that functional abnormalities in a network involved in emotional and interoceptive awareness in ASD are partly based on the intrinsic functional connectivity properties of such a network.
Acknowledgements
The authors thank Maurizio Corbetta for his valuable advise regarding the data analyses and Florian Krause for his assistance with scanning.
REFERENCES
- Adolphs R, Tranel D, Damasio H, Damasio A ( 1994): Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372: 669–672. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR ( 2000): A role for somatosensory cortices in the visual recognition of emotion as revealed by three‐dimensional lesion mapping. J Neurosci 20: 2683–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R ( 2008): Fear, faces, and the human amygdala. Curr Opin Neurobiol 18: 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA ( 2001): Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature 411: 305–309. [DOI] [PubMed] [Google Scholar]
- Auer DP ( 2008): Spontaneous low‐frequency blood oxygenation level‐dependent fluctuations and functional connectivity analysis of the ‘resting’ brain. Magn Reson Imaging 26: 1055–1064. [DOI] [PubMed] [Google Scholar]
- Augustine JR ( 1996): Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev 22: 229–244. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM ( 2005): Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci 8: 955–960. [DOI] [PubMed] [Google Scholar]
- Baron‐Cohen S ( 2009): Autism: The empathizing‐systemizing (E‐S) theory. Ann NY Acad Sci 1156: 68–80. [DOI] [PubMed] [Google Scholar]
- Ben Shalom D, Mostofsky SH, Hazlett RL, Goldberg MC, Landa RJ, Faran Y, McLeod DR, Hoehn‐Saric R ( 2006): Normal physiological emotions but differences in expression of conscious feelings in children with high‐functioning autism. J Autism Dev Disord 36: 395–400. [DOI] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A ( 1999): Autism screening questionnaire: Diagnostic validity. Br J Psychiatry 175: 444–451. [DOI] [PubMed] [Google Scholar]
- Birn RM ( 2007): The behavioral significance of spontaneous fluctuations in brain activity. Neuron 56: 8–9. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS ( 1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34: 537–541. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Tavassoli T, Calò S, Thomas RM, Catmur C, Frith U, Haggard P ( 2006): Tactile sensitivity in Asperger syndrome. Brain Cogn 61: 5–13. [DOI] [PubMed] [Google Scholar]
- Borsci G, Boccardi M, Rossi R, Rossi G, Perez J, Bonetti M, Frisoni GB ( 2009): Alexithymia in healthy women: A brain morphology study. J Affect Disord 114: 208–215. [DOI] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G ( 2002): The temporal binding deficit hypothesis of autism. Dev Psychopathol 14: 209–224. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R ( 2006): Memories for emotional autobiographical events following unilateral damage to medial temporal lobe. Brain 129: 115–127. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL ( 2008): The brain's default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL ( 2003): Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA 100: 5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB ( 1987): Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol 262: 27–45. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME ( 2001): Frequencies contributing to functional connectivity in the cerebral cortex in “resting‐state” data. AJNR Am J Neuroradiol 22: 1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Craig AD ( 2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2009): How do you feel—Now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ ( 2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7: 189–195. [DOI] [PubMed] [Google Scholar]
- Critchley HD ( 2005): Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 493: 154–166. [DOI] [PubMed] [Google Scholar]
- Church JA, Fair DA, Dosenbach NU, Cohen AL, Miezin FM, Petersen SE, Schlaggar BL ( 2009): Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain 132: 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ ( 2005): Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci 8: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR ( 1994): Descartes' Error: Emotion, Reason and the Human Brain. New York: Grosset Putnam. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD ( 2000): Subcortical and cortical brain activity during the feeling of self‐generated emotions. Nat Neurosci 3: 1049–1056. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M ( 2006): Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci 9: 28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF ( 2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103: 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H ( 2008): Complementary systems for understanding action intentions. Curr Biol 18: 454–457. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM ( 2006): fMRI resting state networks define distinct modes of long‐distance interactions in the human brain. Neuroimage 29: 1359–1367. [DOI] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Lewis C, Mantini D, Marzetti L, Belardinelli P, Ciancetta L, Pizzella V, Romani GL, Corbetta M ( 2010): Temporal dynamics of spontaneous MEG activity in brain networks. Proc Natl Acad Sci USA 107: 6040–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE ( 2007): Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104: 11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJ, Perrucci MG, Ferretti A, Del Gratta C, Romani GL, Gallese V ( 2008): The sense of touch: Embodied simulation in a visuotactile mirroring mechanism for observed animate or inanimate touch. J Cogn Neurosci 20: 1611–1623. [DOI] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL ( 2007): Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA 104: 13507–13512.17679691 [Google Scholar]
- Fecteau S, Lepage JF, Théoret H ( 2006): Autism spectrum disorder: Seeing is not understanding. Curr Biol 16: R131–R133. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME ( 2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME ( 2006a): Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103: 10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME ( 2006b): Coherent spontaneous activity accounts for trial‐to‐trial variability in human evoked brain responses. Nat Neurosci 9: 23–25. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME ( 2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G ( 2005): Parietal lobe: From action organization to intention understanding. Science 308: 662–667. [DOI] [PubMed] [Google Scholar]
- Fransson P ( 2005): Spontaneous low‐frequency BOLD signal fluctuations: An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U ( 2001): Mind blindness and the brain in autism. Neuron 32: 969–979. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G ( 1996): Action recognition in the premotor cortex. Brain 119: 593–609. [DOI] [PubMed] [Google Scholar]
- Gallese V ( 2003): The roots of empathy: The shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology 36: 171–180. [DOI] [PubMed] [Google Scholar]
- Gallese V ( 2005): Embodied simulation: From neurons to phenomenal experience. Phenomenol Cogn Sci 4: 23–48. [Google Scholar]
- Gallese V ( 2006): Intentional attunement: A neurophysiological perspective on social cognition and its disruption in autism. Brain Res 1079: 15–24. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF ( 2009): Resting‐state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19: 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager‐Flusberg H ( 2006): Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex 16: 1276–1282. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager‐Flusberg H ( 2007): Abnormal activation of the social brain during face perception in autism. Human Brain Mapp 28: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW ( 2006): The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev 30: 126–147. [DOI] [PubMed] [Google Scholar]
- Hill E, Berthoz S, Frith U ( 2004): Brief report: Cognitive processing of own emotions in individuals with autistic spectrum disorder and in their relatives. J Autism Dev Disord 34: 229–235. [DOI] [PubMed] [Google Scholar]
- Hirstein W, Iversen P, Ramachandran VS ( 2001): Autonomic responses of autistic children to people and objects. Proc Biol Sci 268: 1883–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höistad M, Barbas H ( 2008): Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. Neuroimage 40: 1016–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P ( 2009): Predicting human resting‐state functional connectivity from structural connectivity. Proc Natl Acad Sci USA 106: 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B ( 2003): The elusive concept of brain connectivity. Neuroimage 19: 466–470. [DOI] [PubMed] [Google Scholar]
- Hubert BE, Wicker B, Monfardini E, Deruelle C ( 2009): Electrodermal reactivity to emotion processing in adults with autistic spectrum disorders. Autism 13: 9–19. [DOI] [PubMed] [Google Scholar]
- Iaria G, Committeri G, Pastorelli C, Pizzamiglio L, Watkins KE, Carota A ( 2008): Neural activity of the anterior insula in emotional processing depends on the individuals' emotional susceptibility. Hum Brain Mapp 29: 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J ( 2006): Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia 44: 752–761. [DOI] [PubMed] [Google Scholar]
- James W ( 1894): Physical basis of emotion. Psychol Rev 1: 516–529. Reprinted (1994) Psychol Rev 101:205–210. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ ( 2004): Cortical activation and synchronization during sentence comprehension in high‐functioning autism: Evidence of underconnectivity. Brain 127: 1811–1821. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E ( 2008): The intrinsic functional organization of the brain is altered in autism. Neuroimage 39: 1877–1885. [DOI] [PubMed] [Google Scholar]
- Keysers C, Wicker B, Gazzola V, Anton JL, Fogassi L, Gallese V ( 2004): A touching sight: SII/PV activation during the observation and experience of touch. Neuron 42: 335–346. [DOI] [PubMed] [Google Scholar]
- Lange CG ( 1885): The mechanism of the emotions In: Rand B, editor. The Classical Psychologist. Boston: Houghton Mifflin; pp 672–685. [Google Scholar]
- Linkenkaer‐Hansen K, Nikulin VV, Palva JM, Kaila K, Ilmoniemi RJ ( 2004): Stimulus‐induced change in long‐range temporal correlations and scaling behaviour of sensorimotor oscillations. Eur J Neurosci 19: 203–211. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A ( 1994): Autism Diagnostic Interview‐Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24: 659–685. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M ( 2008): Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA 104: 13170–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minio‐Paluello I, Baron‐Cohen S, Avenanti A, Walsh V, Aglioti SM ( 2009): Absence of embodied empathy during pain observation in Asperger syndrome. Biol Psychiatry 65: 55–62. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL ( 2007): The new neurobiology of autism: Cortex, connectivity, and neuronal organization. Arch Neurol 64: 945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Ramachandran VS ( 2007): The simulating social mind: The role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychol Bull 133: 310–327. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Iacoboni M, Mazziotti JC, Dapretto M ( 2008): Mirroring others' emotions relates to empathy and interpersonal competence in children. Neuroimage 39: 2076–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poil SS, van Ooyen A, Linkenkaer‐Hansen K ( 2008): Avalanche dynamics of human brain oscillations: Relation to critical branching processes and temporal correlations. Hum Brain Mapp 29: 770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Gramann K, Schandry R ( 2007): Neural systems connecting interoceptive awareness and feelings. Hum Brain Mapp 28: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS, Oberman LM ( 2006): Broken mirrors: A theory of autism. Sci Am 295: 62–69. [DOI] [PubMed] [Google Scholar]
- Rieffe C, Meerum Terwogt M, Kotronopoulou K ( 2007): Awareness of single and multiple emotions in high‐functioning children with autism. J Autism Dev Disord 37: 455–465. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L ( 1996): Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res 3: 131–141. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT ( 2010): Metaanalytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Hum Brain Mapp 31: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarela MV, Hlushchuk Y, Williams AC, Schürmann M, Kalso E, Hari R ( 2007): The compassionate brain: Humans detect intensity of pain from another's face. Cereb Cortex 17: 230–237. [DOI] [PubMed] [Google Scholar]
- Saper CB ( 2002): The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 25: 433–469. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD ( 2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD ( 2007): Interactions of pain intensity and cognitive load: the brain stays on task. Cereb Cortex 17: 1412–1422. [DOI] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U ( 2008): Levels of emotional awareness and autism: An fMRI study. Soc Neurosci 3: 97–112. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD ( 2004): Empathy for pain involves the affective but not sensory components of pain. Science 303: 1157–1162. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Huang PY, Castelli F, Adolphs R ( 2007a): Amygdala damage impairs eye contact during conversations with real people. J Neurosci 27: 3994–3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RS, Piven J ( 2007b): Abnormal use of facial information in high‐functioning autism. J Autism Dev Disord 37: 929–939. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Young AW, Schroeder U, Grossenbacher PG, Federlein J, Büttner T, Przuntek H ( 1999): Knowing no fear. Proc Biol Sci 266: 2451–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V ( 2008): A critical role for the right fronto‐insular cortex in switching between central‐executive and default‐mode networks. Proc Natl Acad Sci USA 105: 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐planar Stereotaxic Atlas of the Human Brain: 3‐Dimensional Proportional System—An Approach to Cerebral Imaging. New York, NY: Thieme Medical Publishers. [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD ( 2008): Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 30: 2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théoret H, Halligan E, Kobayashi M, Fregni F, Tager‐Flusberg H, Pascual‐Leone A ( 2005): Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Curr Biol 15: R84–R85. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Clare Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP ( 2008): Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp 30: 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE ( 2009): Functionally linked resting‐state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp 30: 3127–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL ( 2006): Coherent spontaneous activity identifies a hippocampal‐parietal memory network. J Neurophysiol 96: 3517–3531. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL ( 2008): Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100: 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D ( 1981): Manual for the Wechsler Adult Intelligence Scale‐Revised. New York: Psychological Corporation. [Google Scholar]
- Wechsler D ( 1991): Wechsler Intelligence Scale for Children, 3rd ed. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G ( 2003): Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron 40: 655–664. [DOI] [PubMed] [Google Scholar]
- Zar JH ( 1996): Biostatistical Analysis. Upper Saddle River, NJ: Prentice‐Hall. [Google Scholar]


