Abstract
Fine surface texture is best discriminated by touch, in contrast to macro geometric features like shape. We used functional magnetic resonance imaging and a delayed match‐to‐sample task to investigate the neural substrate for working memory of tactile surface texture. Blindfolded right‐handed males encoded the texture or location of up to four sandpaper stimuli using the dominant or non‐dominant hand. They maintained the information for 10–12 s and then answered whether a probe stimulus matched the memory array. Analyses of variance with the factors Hand, Task, and Load were performed on the estimated percent signal change for the encoding and delay phase. During encoding, contralateral effects of Hand were found in sensorimotor regions, whereas Load effects were observed in bilateral postcentral sulcus (BA2), secondary somatosensory cortex (S2), pre‐SMA, dorsolateral prefrontal cortex (dlPFC), and superior parietal lobule (SPL). During encoding and delay, Task effects (texture > location) were found in central sulcus, S2, pre‐SMA, dlPFC, and SPL. The Task and Load effects found in hand‐ and modality‐specific regions BA2 and S2 indicate involvement of these regions in the tactile encoding and maintenance of fine surface textures. Similar effects in hand‐ and modality‐unspecific areas dlPFC, pre‐SMA and SPL suggest that these regions contribute to the cognitive monitoring required to encode and maintain multiple items. Our findings stress both the particular importance of S2 for the encoding and maintenance of tactile surface texture, as well as the supramodal nature of parieto‐frontal networks involved in cognitive control. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: human, fMRI, cognition, touch perception, short‐term memory
INTRODUCTION
Human grasping and skillful object manipulation are dependent on accurate memory encoding and maintenance of tactile object properties, like shape, weight, and surface texture [Jenmalm et al., 2006; Johansson and Westling, 1984, 1987]. The tactile system processes information about material properties (e.g., roughness) more efficiently than information about macro geometrical properties [e.g., orientation; Klatzky et al., 1987]. When participants were judging object properties which could be seen and touched, they used vision alone for macro geometric properties and coarse material judgments. However, they used touch to perceive subtle differences in material properties [Klatzky et al., 1993]. Furthermore, tactile judgments of fine surface texture have been shown to be superior to visual judgments [Heller, 1989; Jones and O'Neil, 1985]. Incongruous tactile stimulation modulated visual assessment of roughness, whereas tactile roughness judgments were insensitive to visual interference even when the visual distractors were more discriminable than the tactile targets [Guest and Spence, 2003]. Therefore, stimuli that differ in material properties are especially suited to reveal the neural bases of memory processes specific to the somatosensory modality and crucial to object manipulation skills.
Previous studies have investigated tactile memory for temporal (vibrotactile) information in human [Numminen et al., 2004] and non‐human primates [Harris et al., 2001, 2002; Koch and Fuster, 1989; Romo et al., 1999]. Human fMRI studies have explored working memory for macro geometrical information [shape, orientation; Kaas et al., 2007; Stoeckel et al., 2003, 2004]. However, except for a study in non‐human primates [Zhou and Fuster, 1996], the neural substrates of working memory for tactile material properties, which are of importance to, for example, grip force planning, have rarely been studied.
There is substantial evidence that the processing of material properties such as roughness is supported by neural networks that differ from those involved in processing of macro geometrical aspects, such as shape, orientation, or spatial layout [Bodegard et al., 2001; Bohlhalter et al., 2002; Klatzky et al., 1987; Merabet et al., 2004; Randolph and Semmes, 1974; Roland et al., 1998; Stoesz et al., 2003; Zhang et al., 2005]. Right hand discrimination of macro geometrical object features recruited the left anterior intraparietal sulcus [Bodegard et al., 2001; Roland et al., 1998; Zhang et al., 2005] and left anterior supramarginal gyrus [Bodegard et al., 2001]. In contrast, right hand discrimination of roughness specifically activated left secondary somatosensory [S2; Roland et al., 1998] and right angular gyrus [Sathian et al., 1997; Zhang et al., 2005]. A double dissociation was found in the cortical areas involved in judgments of roughness or spacing of raised dot patterns [Merabet et al., 2004]. Whereas low frequency repetitive transcranial magnetic stimulation (rTMS) over the occipital cortex impaired tactile judgments of dot spacing but not roughness, rTMS over primary somatosensory regions only impaired judgments of roughness but not dot spacing. These findings suggest that working memory for tactile surface texture might involve different cortical regions than those revealed by previous studies using tactile shape and orientation stimuli.
In the current study, blindfolded right‐handed participants were presented with sandpaper stimuli of different roughness (Fig. 1). Tactile discrimination of subtle differences in this material property most likely involves mainly somatosensory processing, rather than visual imagery or verbal strategies. Functional magnetic resonance images were collected while participants performed a texture match‐to‐sample task with a 10–12 s delay and a memory load varying to up to four stimuli. To our knowledge, this is the first study revealing the cortical areas involved in tactile working memory for fine surface texture. We assumed that areas supporting tactile texture working memory would show increased activity when encoding and maintaining the fine surface texture of the stimuli compared to encoding and maintaining the location of the stimuli. Furthermore, we expected that areas supporting cognitive monitoring would respond to the parametrical variation of stimulus load. On the basis of previous reports on the involvement of primary somatosensory cortex [Bodegard et al., 2001; Merabet et al., 2004] and secondary somatosensory cortex [Roland et al., 1998], and the angular gyrus of the posterior parietal cortex [Sathian et al., 1997; Zhang et al., 2005] in tactile discrimination of material properties, as well as activations in prefrontal cortex in tactile working memory [Klingberg et al., 1996; Numminen et al., 2004] and cognitive monitoring [Smith and Jonides, 1999], we hypothesized that the encoding and maintenance of tactile surface texture would involve the above mentioned areas.
Figure 1.
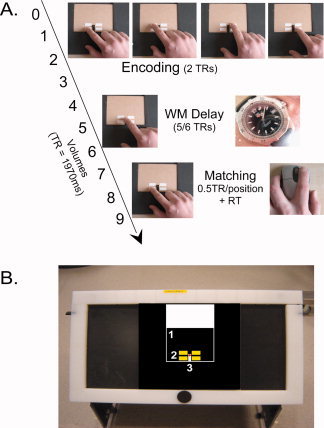
Experimental design and set‐up. A: The temporal succession of the different trial phases for the texture task. While only the first probe position was explored in the matching phase of the texture task, all four positions had to be explored in the location matching task, taking 2 TRs (3940 ms) in total. B: The table used in the scanner, with the stimulus board, and stimulus card (1) on top. For clarity, the four stimulus positions are marked by yellow squares (2) in the picture. Likewise, a red dot marks the wooden pin which served as the resting position for the finger during delay and inter trial interval. In these intervals, the stimulus cards were exchanged by carefully pulling the old card away and sliding the new card around the wooden pin, which fitted exactly in the card's recess (3). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
MATERIALS AND METHODS
Participants
Twelve healthy male participants were paid to participate in the present fMRI experiment. Data from one participant were discarded from further analyses, due to excessive head movement. The average age of the remaining eleven participants was 24 (SD 2 years). Only male participants were recruited for the fMRI experiment, to eliminate potential gender effects that have been reported in studies of tactile performance and lateralization [e.g., Hiscock et al., 1999; Sadato et al., 2000; Toga and Thompson, 2003]. All participants were right‐handed as assessed by a Dutch translation of the Edinburgh Handedness Inventory [Oldfield, 1971]. They were unfamiliar with the purpose of the study and never saw the set‐up and the stimuli. There was a behavioral training session prior to scanning, including one or more runs to practice the task and become proficient at correctly pacing the movements, as well as a behavioral experiment with the same tasks that were to be performed in the scanner. Before the fMRI experiment was conducted, a separate group of 15 healthy right‐handed participants (seven female, eight male) took part in a behavioral experiment to test the discriminability of the texture stimuli to be used in the fMRI experiment. Informed consent was obtained from all participants prior to the experiments. The study was approved by the local ethics committee and was performed in accordance with the declaration of Helsinki.
Tactile Stimuli and Task
The study involved a texture and a location delayed match‐to‐sample task. The latter served as a control condition. In the texture task, participants judged whether the texture of a sandpaper probe (presented after a delay) matched with one of the textures in the encoded sample array. In the location task participants judged whether the location of a sandpaper probe matched with a location that was filled in the previously encoded sample array. Memory load was varied by increasing the number of texture stimuli in the sample array from zero to four. Load zero was included as a motor control condition. Identical sample arrays were used for the texture and location (control) task. To investigate a potential hemispheric asymmetry for areas involved in working memory encoding and maintenance, stimulus encoding and matching was performed with the dominant and non‐dominant hand during separate sessions, with the hand used varied across sessions.
Sample arrays were created by gluing four 2 × 1 cm2 pieces of sandpaper and/or smooth paper in a square arrangement to small wooden cards (12 × 10 cm2). Each wooden card contained a rectangular 1 × 2 cm2 recess in the middle at the bottom of the card. This allowed the card to be slid around a wooden pin which marked the default position for the participant's index finger. To ensure that participants had no trouble finding the stimulus strips, they were fixed at four standard positions with respect to the recess and by consequence also with respect to the wooden pin (Fig. 1).
Before fMRI experiment, a selection of six sandpaper types had been tested for reliable discrimination in a separate group of 15 participants (Supporting Information). Each combination of two strips of different sandpaper types was presented four times. Participants explored each strip (2 × 1 cm2) by making two lateral sweeps with the right index finger for approximately half a TR (985 ms), subsequently responding whether the textures were the same or different. Results for each combination of sandpapers were analyzed across participants. Five different types of sandpaper (grit designation P60, P80, P120, P180, and P280, corresponding to average particle diameters of 269, 201, 125, 82, and 52 μm) showing mutual above chance discrimination were selected to create the stimulus arrays for the current fMRI experiment. The stimulus load was varied by increasing the amount of sandpaper strips in the sample array from zero to four. Load zero arrays were included to control for exploration movements. Positions that were not occupied by a sandpaper strip were filled with strips of smooth plastic foil in order to provide participants with feedback that they were performing the exploration of the stimulus positions correctly. Although exploration of the plastic foil might involve slightly different tactile processing, this is not expected to affect the load manipulation during the working memory maintenance. Any differential low‐level stimulus effect during the encoding phase would be expected to cancel out when contrasting performance with left and right hand and the texture and location control task as these conditions used identical sample arrays. The specific stimuli used in the matching phase were not expected to influence the activation observed in the encoding and delay phases, which were the focus of our study. Eight different sample arrays were created for each load, four for the behavioral pretest and four for the fMRI session. Each sample array was presented twice, once with a matching probe and once with a non‐matching probe. To make sure performance would not be affected by erosion of the stimulus textures, several cards were created for each sample array, and exchanged between participants.
The sample arrays for the texture and the location control task were identical, but the probes were different; this in order to prevent participants from matching sample and probe based on a conjunction of texture and location. In the location task, the probe stimulus could be in any of the four stimulus positions and always had the same texture (grit designation P220, average particle diameter of 68 μm). In the texture task, the probe stimulus always appeared in the same location (the first, bottom left stimulus position), and was drawn from the set of five textures used to create the different sample arrays.
Experimental Set‐Up and Procedure
Participants were blindfolded and lay supine in the scanner. Their head and arms were stabilized using foam padding. A small table was placed over the scanner bed below the waistline. The position of the table and the angle of the tabletop were adjusted to a comfortable position for each participant. The wooden pin on the table top marked the center of the stimulus array, and served as the default position for the index finger of the hand used for stimulus exploration (Fig. 1). A button box was attached to the other hand.
Each trial consisted of an encoding phase, a delay phase and a matching phase. The start of the encoding phase was indicated by an auditory warning signal (2000 Hz). Participants were instructed to move the index finger of the exploration hand, keeping pace with four auditory stimuli (1000 Hz, 1/2 TR = 985 ms duration), and making two lateral left‐to‐right sweeps along each of the four potential stimulus positions. During the delay phase, participants kept the index finger at the default position, on the wooden pin in the middle of the stimulus board (Fig. 1). The start of the matching phase was indicated by a second warning signal, alerting participants that they would have to feel the probe with two lateral left‐to‐right sweeps of the index finger, again paced by an auditory stimulus. In the location control condition, participants explored all four locations of the probe stimulus array, paced by auditory stimuli. In the texture condition, subjects only explored a single probe stimulus in the lower left position of the array. This was done to limit the length of the study, and to prevent that subjects would mistakenly perform the location task in the texture condition and vice versa.
After feeling the probe, participants responded whether it was a match (left button) or a non‐match (right button) by a button press with the index or middle finger of the other hand. In the load zero trials, participants responded by randomly pressing the left or right button. For the load one to four trials, the ratio of matching and non‐matching probes was set to 50% in each run. The delay interval between sample and probe was 5 or 6 TRs (TR = 1970 ms; so either 9850 or 11,820 ms respectively) to reduce expectancy effects. The SOA between the probe and the next sample was a multiple of the TR, between 5 and 8 TRs (9850–15,760 ms). Presentation of the auditory warning signal at the beginning of each trial was synchronized with the fMRI sequence (Presentation version 9.2, Neurobehavioral Systems Inc., Albany CA; http://nbs.neuro-bs.com).
All participants took part in two scanning sessions, one performing the task with the right hand and one performing with the left hand. Five participants used their right hand in the first session; six participants started with their left hand. Because we were interested in areas that would be involved in working memory independently of the hand used for stimulus exploration, we studied task performance with the non‐dominant left as well as the dominant right hand. By entering the factor hand explicitly in our analysis, regions showing just an effect of task or load or an interaction of these two could be studied. We expected that regions showing differential activation for the hands would show a main effect for hand. Regions showing a differentiation between the hands related to a difference between tasks would show up in the task by hand interaction. Regions showing a difference between the hands in the effect of load would show up in the load by hand interaction.
The behavioral pretest and each MRI session contained four runs, during which the experimenter presented the samples and the probes manually. The texture task and the location control task were performed using identical stimulus samples. The tasks were presented in two separate blocks of 10 trials (two repetitions for each load) within one run. Two random sequences of ten sample stimuli were created using the random number generator in Microsoft Office Excel (part of Microsoft Office professional Edition, 2003). Each ten‐stimulus sequence was then concatenated with its mirrored version, resulting in two sequences of 20 stimuli. Each of these 20‐stimulus sequences was subsequently used in two runs, once starting with the texture task, and once starting with the location task. An auditory cue in the middle of the run indicated that participants had to switch to the other task.
Behavioral Data Analysis
For nine participants, behavioral data were available during the scanning session for the right hand, and for six participants behavioral data were also available for the left hand session. For the other subjects, behavioral data were lacking due to technical problems with the response button box. The average percentage correct was calculated per task and load and t‐tests were performed to determine whether the overall average percentage correct was significantly different from chance. The effect of task and load on percentage correct was analyzed in a 2 (task) by 4 (load) within‐subjects ANOVA. In addition, the effect of hand on texture task performance was investigated in a 2 (hand) by 4 (load) within‐subjects ANOVA on the fMRI behavioral data from six participants for whom left hand data were available.
Image Acquisition
Participants took part in two scanning sessions in a 3 Tesla MR scanner (Siemens Allegra, Erlangen, Germany) at the Maastricht Brain Imaging Center (Maastricht, The Netherlands). To limit the time in the scanner to a reasonable amount, data for each hand were acquired in separate scanning sessions, balancing the order across participants. Each session contained one anatomical run and four functional runs of 301 volumes. The high‐resolution anatomical image was recorded using a T1‐weighted MDEFT sequence (matrix: 256 × 256 × 176, voxel size: 1 mm3). Functional MRI data were acquired using a T2*‐weighted echo‐planar sequence (matrix: 256 × 256 × 27, voxel size: 3 mm3, gap: 1 mm, TE/TR = 30/1970 ms, FA = 90°) covering the whole brain with the exception of the lower cerebellum. Each functional run included one block of the texture task and one block of the location task, composed of a pseudorandom series of 10 trials.
fMRI Data Analysis
The first two volumes of each run were discarded to remove T1 saturation effects. Standard preprocessing was performed, including slice scan time correction, motion correction, temporal smoothing (high pass filtering at 0.01 Hz) and linear trend removal, as implemented in the BrainVoyager QX software package version 1.6 [Goebel et al., 2006]; (Brain Innovation B.V. Maastricht, the Netherlands; http://www.brainvoyager.com). Further analyses were performed in BrainVoyager QX version 2.08. Functional images from different runs were coregistered with the anatomical images and transformed into Talairach coordinate space [Talairach and Tournoux, 1988], interpolating the functional images to obtain a volume time course with a resolution of 3 × 3 × 3 mm3. Motion correction parameters exceeded 2 mm for all runs for one of the 12 participants, leading to his exclusion from further analyses. For the same reason, one left hand run had to be removed in two other participants, and one left and one right hand run were discarded in a third participant.
Separate boxcar predictors were defined for the three trial phases (encoding/delay/matching) for each hand, task, and stimulus load (0–4), resulting in a total of 60 predictors. All trials of the included runs were entered in the analysis. During encoding, texture stimuli were equally likely to occur in each of the four positions. Hence, after averaging trials for each load, encoding processes would cover the full 2 TRs of the encoding phase, although the strength of these processes is expected to be lower for the lower loads. The activation difference for the encoding of different stimulus loads is expected to be reflected in larger beta values for larger loads. Each predictor's box car function was convolved with a gamma distribution, accounting for the shape and delay of the hemodynamic response [Boynton et al., 1996]. A functional mask was created based on the positive F‐map [thresholded and corrected for multiple comparisons at q(FDR) = 0.05]; [Genovese et al., 2002] obtained from a random effects (RFX) general linear model (GLM) analysis using one run from each participant and each hand (22 runs in total). The mask was used to reduce the multiple comparison problem by limiting the analysis to voxels containing brain tissue showing modulation by the model predictors. Within this mask, a RFX GLM was computed on the remaining 62 runs. Two separate analyses of variance (ANOVA) with within‐subject factors hand (2: left vs. right), task (2: texture vs. location) and stimulus load (5: 0–4) were performed on the beta values extracted for the 20 encoding and delay predictors. This way, our design was optimized to investigate the neural substrates of tactile working memory maintenance involved in the encoding and delay phase. The matching phase was not further analyzed due to the fact that this phase was not the same for the texture and location (control) condition. For the latter, it involved exploration of all four stimulus positions whereas it involved only exploration of the lower left position for the texture condition. This was done to prevent that subjects would mistakenly perform the location task in the texture condition and vice versa and to limit the length of the experiment.
The resulting whole brain F‐maps [thresholded and corrected for multiple comparisons at q(FDR) = 0.01] for each of the main and interaction effects were projected on an anatomical image. Average beta values were extracted from each cluster of significant voxels and visualized in bar charts to show the direction of the effects.
RESULTS
Behavioral Data
The average percentage correct differed significantly from chance for all combinations of load and task (P < 0.05). A 2 (task) by 4 (load) within‐subject ANOVA revealed a significant main effect for task [F (1, 8) = 9.95; P = 0.014] with better performance in the location control task, and an interaction between task and load [F (3, 24) = 3.01; P = 0.05]. As can be seen in Figure 2, the task effect was strongest in load 1 and 2. The interaction was caused by a differential response for the texture and location task for load 2 and 3 [within‐subjects contrast: F (1, 8) = 14.18; P = 0.006]. For a subgroup of six participants data were also available for the left hand. The 4 (load) by 2 (hand) within‐subjects ANOVA on the texture task data from this subgroup revealed a significant load effect [F (3, 15) = 4.05; P < 0.05] related to the decrease in performance from load 1 to load 2, but no significant effect of hand, nor a load by hand interaction.
Figure 2.
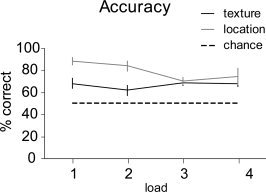
Behavioral results. Average percentage correct and (between‐subjects) standard error bars for each task and load, as obtained from nine participants in the MR scanner.
Imaging Data
Encoding
The repeated measures ANOVA on the betas extracted for the encoding predictors in the RFX GLM revealed an effect of Hand [q(FDR) = 0.01]. This was related to greater activation for the right hand in the left pericentral cortex, corresponding to the primary somatosensory and motor cortex (S1 and M1), and greater activation for the left hand in the right thalamus, right pericentral cortex (S1 and M1) and the right medial frontal gyrus (supplementary motor area; SMA) (Table I and Fig. 3).
Table I.
ANOVA tactile encoding and delay
| Brain region | BA (COG) | Task phase | Effect | Sign level q(FDR) | Talairach coordinates (COG) | Number of voxels | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left hemisphere | Right hemisphere | ||||||||||
| x | y | z | x | y | z | ||||||
| Pericentral cortex (M1/S1) | 3/4 | Encode | Task | 0.01 | 35 | −23 | 52 | 4453 | |||
| 3/4 | Encode | Hand | 0.01 | 34 | −25 | 55 | 2393 | ||||
| 3/4 | Encode | Hand | 0.01 | −38 | −23 | 54 | 1615 | ||||
| 3/4 | Delay | Task | 0.01 | −40 | −23 | 52 | 11523 | ||||
| 3/4 | Delay | Task | 0.01 | 33 | −25 | 54 | 5293 | ||||
| Precentral gyrus (M1/PMA) | 6 | Encode | Task | 0.01 | −29 | −14 | 61 | 1250 | |||
| 4 | Encode | Load | 0.05 | 40 | −13 | 57 | 126 | ||||
| Postcentral sulcus (S1) | 2/40 | Encode | Task | 0.01 | −48 | −27 | 44 | 2263 | |||
| 2/40 | Encode | Load | 0.05 | −45 | −33 | 47 | 441 | ||||
| 2/40 | Encode | Load | 0.05 | 47 | −30 | 48 | 187 | ||||
| Parietal operculum (S2) | 43 | Encode | Task | 0.01 | 54 | −18 | 16 | 1975 | |||
| Encode | Task | 0.01 | −50 | −17 | 12 | 1955 | |||||
| 3 | Encode | Load | 0.05 | −58 | −17 | 27 | 220 | ||||
| 43 | Delay | Task | 0.01 | 52 | −19 | 19 | 2992 | ||||
| Delay | Task | 0.01 | −50 | −15 | 12 | 3671 | |||||
| Parietal operculum (S2)/Insula | Encode | Task | 0.01 | 39 | −3 | 5 | 454 | ||||
| Encode | Task | 0.01 | −39 | −4 | 9 | 251 | |||||
| Encode | Task | 0.01 | 43 | −18 | 16 | 119 | |||||
| Encode | Load | 0.05 | −39 | −6 | 14 | 365 | |||||
| Superior parietal lobule | 7 | Encode | Task | 0.01 | 32 | −42 | 54 | 224 | |||
| 7 | Encode | Load | 0.05 | −32 | −54 | 53 | 179 | ||||
| 7 | Delay | Task | 0.01 | −25 | −55 | 56 | 364 | ||||
| 7 | Delay | Task | 0.01 | 27 | −66 | 37 | 147 | ||||
| Medial frontal cortex (SMA) | 6 | Encode | Hand | 0.01 | 6 | −12 | 48 | 374 | |||
| Medial frontal cortex (pre‐SMA) | 6 | Encode | Task | 0.01 | 1 | −3 | 54 | 2194 | |||
| 6 | Encode | Load | 0.05 | −3 | 11 | 45 | 1416 | ||||
| 6 | Delay | Task | 0.01 | 1 | −1 | 50 | 9019 | ||||
| Inferior frontal gyrus (dlPFC) | 9 | Encode | Task | 0.01 | 54 | 5 | 23 | 287 | |||
| 9 | Encode | Load | 0.05 | −48 | 5 | 30 | 1159 | ||||
| 9 | Delay | Task | 0.01 | −57 | 3 | 27 | 329 | ||||
| 9 | Delay | Task | 0.01 | 54 | 4 | 22 | 154 | ||||
| Inferior frontal gyrus/Anterior Insula | Delay | Task | 0.01 | 38 | 19 | 7 | 826 | ||||
| Insula/putamen | Delay | Task | 0.01 | −28 | 9 | 8 | 2624 | ||||
| Putamen | Delay | Task | 0.01 | 32 | −4 | 3 | 236 | ||||
| Delay | Task | 0.01 | 22 | −1 | 14 | 199 | |||||
| Thalamus | Encode | Hand | 0.01 | 13 | −19 | 9 | 452 | ||||
| Encode | Task | 0.01 | −12 | −18 | 13 | 272 | |||||
| Encode | Task | 0.01 | 11 | −14 | 12 | 173 | |||||
| Delay | Task | 0.01 | −12 | −16 | 8 | 1578 | |||||
| Delay | Task | 0.01 | 10 | −15 | 9 | 936 | |||||
BA, Brodmann area; q(FDR), false discovery rate; COG, center of gravity; Sign., significance; M1, primary motor area; S1, primary somatosensory area; PMA, pre‐motor area; S2, secondary somatosensory area; SMA, supplementary motor area; dlPFC, dorsolateral prefrontal cortex.
Figure 3.
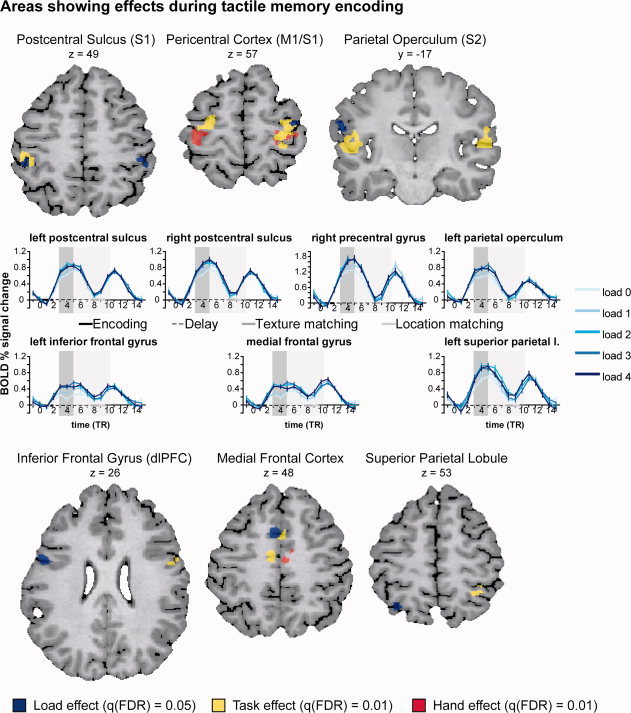
Areas showing effects during tactile memory encoding. Colors indicate the different effects: red = Hand effect; yellow = Task effect; blue = Load effect. All hand effects were related to greater activation for the contralateral than the ipsilateral hand. All Task effects were related to greater activation in the texture task than in the control task. The Load effects are illustrated by the event related average plots in the middle rows. The percent signal change was computed with respect to the average signal level at time point zero. The different load levels are shown in different shades of blue (darker blue for higher loads). The actual timing of the different trial events is indicated on the x‐axis (encoding: black solid line; delay: black striped line; texture matching: dark gray solid line; location matching: light gray solid line). The indicated delay length corresponds to the average delay length (5.5 TRs or 10,835 ms). The shaded areas show the encoding (dark gray) and delay intervals (light gray) with a 3 TR (5910 ms) hemodynamic shift. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The Task effects [q(FDR) = 0.01] were due to higher activation during the texture task. Bilateral effects were localized in the parietal operculum (secondary somatosensory cortex; S2), precentral gyrus, thalamus and medial frontal gyrus (pre‐SMA). Lateralized effects were found in the right superior parietal lobule, right inferior frontal gyrus (dorsolateral prefrontal cortex) and left postcentral sulcus (BA2; Fig. 3).
No significant main effect of load or interaction effects were found at q(FDR) = 0.01. Lowering the threshold to q(FDR) = 0.05 revealed areas responsive to the load variation (Fig. 3). The effects consisted of increasing activation levels up to load 2. The activation increase levelled off for stimulus load 3 and 4. Bilateral load effects occurred in the postcentral sulcus. Lateralized effects were found in the left inferior frontal gyrus (dorsolateral prefrontal cortex), the medial frontal gyrus (pre‐SMA), the left superior parietal lobule, the left parietal operculum (S2) and the right precentral gyrus. Interaction effects did not reach statistical significance.
Delay
For the delay interval, the repeated measures ANOVA only revealed a main effect for Task at q(FDR) = 0.01 (Table I and Fig. 4). This was due to greater activation during the texture task. The effects were localized in bilateral pericentral cortex, parietal operculum (S2), superior parietal cortex, thalamus, medial frontal gyrus (pre‐SMA), basal ganglia (putamen) and right inferior frontal gyrus (dorsolateral prefrontal cortex). Lowering the threshold to q(FDR) = 0.05 did not reveal additional main or interaction effects.
Figure 4.
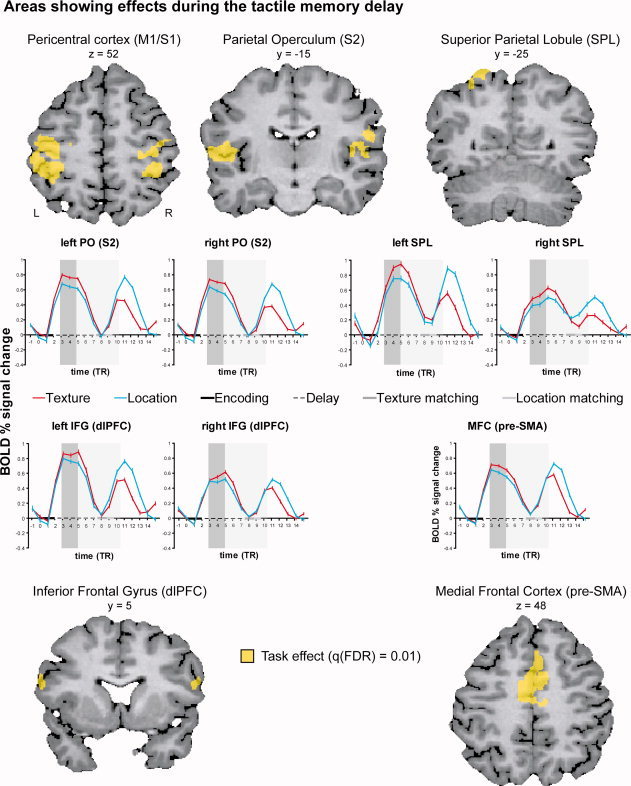
Areas showing effects during the tactile memory delay. Yellow indicates the effect of Task. All Task effects were related to greater activation in the texture task than in the control task. The event‐related time courses for each task (red = texture; turquoise = location) are shown on the bottom rows. The percent signal change was computed with respect to the average signal level at time point zero. The actual timing of the different trial events is indicated on the x‐axis (encoding: black solid line; delay: black striped line; texture matching: dark gray solid line; location matching: light gray solid line). The indicated delay length corresponds to the average delay length (5.5 TRs or 10,835 ms). The shaded areas show the encoding (dark gray) and delay intervals (light gray) with a 3 TR (5910 ms) hemodynamic shift. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The event related average plots of the cortical regions with load effects during encoding (Fig. 3) and task effects during the delay (Fig. 4 and Supporting Information Fig. 4) show that, in all regions, the response during the delay is smaller than the response during the encoding and matching phases which involve sensory stimulation and motor activity. The response in the matching phase is larger for the location task with extended probe exploration. As can be seen from the graphs, the cortical areas around the central sulcus (S1/M1), the parietal operculum (S2) and the right IFG (dlPFC) show a prolonged response. The left inferior frontal gyrus (dlPFC) and the medial frontal gyrus (pre‐SMA/SMA) give a mixed picture: a more sustained response is observed when taking into account the event related average in the region with significant load effects during encoding; a prolonged response is observed in the smaller sub region showing task effects during the delay. The SPL shows a sustained response throughout the delay.
DISCUSSION
The current study investigated the neural substrate for tactile working memory of fine surface texture, using a delayed match‐to‐sample task, in which the texture or location (control condition) of sandpaper stimuli had to be maintained in working memory during a 10–12 s delay. Participants explored sandpaper strips with their left or right hand. Memory load was varied by changing the number of texture stimuli from zero (control) to four. Two separate analyses of variance with the factors Hand (2), Task (2), and Load (5) were performed on the estimated percent signal change in the encoding and delay phases. The analysis of the encoding phase revealed effects for Hand in the pericentral cortex, thalamus and supplementary motor area (SMA). For Load, we found predominantly left‐hemispheric cortical activation in the dorsolateral prefrontal cortex, pre‐SMA, the parietal operculum (secondary somatosensory cortex; S2) and the superior parietal lobule; right hemispheric cortical activation was found in precentral gyrus and bilateral activation in postcentral sulcus. The analyses of the encoding and delay phases both demonstrated Task effects due to stronger activation for the texture task. These effects were found in cortical regions around the central sulcus, the parietal operculum (S2), the inferior frontal gyrus (dorsolateral prefrontal cortex), the medial frontal gyrus (pre‐SMA) and the superior parietal lobule (Table I).
We assumed that areas supporting tactile texture working memory would show increased activation in the texture task compared to the location control task. Indeed, we only found Task effects due to higher activation for the texture task. This might be attributable to the greater difficulty of the latter task, as indicated by the lower performance levels. To remember the subtle differences between pieces of sandpaper participants could not easily resort to a verbal or visual recoding strategy, while this could be easily done for the location (control) task. Maintenance of the tactile memory trace probably requires additional attentional resources as compared to maintaining the information on filled or unfilled locations in the control task. Higher activation in the texture task could therefore be interpreted as reflecting the specific tactile nature of the memory trace as well as the increased attentional demands. We do not expect that the stimuli used for the probe affected the encoding‐ or delay‐related activations (which were the focus of our study), although they might have affected decisional processing in the matching phase due to increased difficulty of the stimuli
To disentangle the regions involved in general attentional processes from those related to tactile memory processing, a load manipulation was added, to which areas supporting cognitive monitoring were expected to respond. The relatively stable behavioral performance for texture matching across different loads suggests that participants recruited additional resources to compensate for the increased memory load. However, the stable behavioural performance for texture load 3 and 4 could also have arisen in the matching phase, and reflect that it is somewhat easier to decide whether the probe was a match or a non‐match with a larger range of textures in the remembered sample, especially given that the number of textures in the set was limited (the overall percentage of matches was set to 50% for each sample). The lack of a load effect for the higher stimulus loads could be due to the difficulty of the tactile texture task, for which load level 1 already proved to be quite hard. As a result, there was not much room left for a decrease in performance for higher loads. The tactile sandpaper stimuli were not easy to categorize, visualize or verbalize, especially for sighted people not used to making very fine tactile discriminations in daily life. Therefore, higher‐order working memory maintenance strategies might have been unavailable, and subjects would have had to rely on attention‐based processing with limited capacity. This interpretation is supported by the fact that at the neurophysiological level, the clearest sustained delay activation was observed in the SPL, which has been linked to the capacity limit of visual WM maintenance [Fusser et al., 2011; Linden et al., 2003; Mayer et al., 2007; Todd and Marois, 2004] as well as the capacity limit of visual attention [Fusser et al., 2011; Mayer et al., 2007] and not in the prefrontal cortex (dlPFC) typically associated with strategic processing in working memory maintenance [Fusser et al., 2011; Linden et al., 2003; Mayer et al., 2007]. In the fMRI data, main effects of Load were only found during the encoding phase and not during the delay. The matching phase was not part of our analysis because it was not identical for the texture and location condition. The lack of a Load main effect during the delay in the fMRI data can not be explained by a differential effect of the Load increase in the texture and control tasks, because no interaction of Task and Load was found.
Regions Involved in Tactile Discrimination of Material Properties
The primary and secondary somatosensory cortex, the angular gyrus and dorsolateral prefrontal cortex were hypothesized to be involved in the encoding and maintenance of tactile surface texture because of their role in tactile discrimination of material properties.
Primary and secondary somatosensory cortex
These areas showed effects during both encoding and maintenance. More specifically, we found that the primary somatosensory cortex (S1) showed contralateral effects of Hand, bilateral Task effects (texture > control) and bilateral effects of Load during tactile encoding. The contralateral Hand effects in S1 during encoding are in accordance with the known contralateral dominance in somatosensory and motor processing found in previous imaging studies of tactile discrimination [Roland et al., 1998; Stoeckel et al., 2004; Zhang et al., 2005]. The greater S1 activation for the texture task during encoding could indicate deeper processing of the tactile features of the texture stimuli. The effects of load during encoding were observed in a separate region in the postcentral sulcus. 96% of the voxels of the left cluster and 98% of the right cluster (Supporting Information Table 1 and Fig. 1) overlapped with a probability map for Brodmann's area 2 (BA2) based on microstructurally defined population maps [Grefkes et al., 2001]. BA2 neurons are responsive to direction and orientation of cutaneous stimuli and to active tactile discrimination [Costanzo and Gardner, 1980; Gardner and Costanzo, 1980; Hyvarinen and Poranen, 1978; Ruiz et al., 1995]. Whereas both left and right postcentral sulcus showed effects of Load during encoding, only the region in the left postcentral sulcus showed a stronger response for the texture task, indicating that this region might be of specific importance for higher‐level processing of tactile features during encoding in the texture task.
Secondary somatosensory cortex (S2) showed left sided Load effects during encoding, characterized by increasing activation levels up to stimulus load two [at a lower threshold q(FDR) = 0.05], and bilateral effects of Task during both encoding and delay. Four out of five left hemispheric clusters showed greatest overlap (56–100%; Supporting Information Table 1 and Supporting Information Figs. 2 and 3) with the cytoarchitectonic probability map of a subdivision of S2 which was coined OP4 (Eickhoff et al., 2006b). This is thought to correspond to a subdivision of S2 called area PV (Parietal Ventral) in the non‐human primate (Eickhoff et al., 2006a). Area OP 4 was found to be more closely integrated with areas responsible for basic sensorimotor processing and action control [Eickhoff et al., 2010]. Three out of four right hemispheric clusters showed greatest correspondence (89–96%; Supporting Information Table 1 and Supporting Information Figs. 2 and 3) with region OP1, which is believed to be the human homologue of the non‐human primate SII (Eickhoff et al., 2006b) and was found to be more closely connected to the parietal networks for higher order somatosensory processing [Eickhoff et al., 2010]. The parametric effect of Load and the increased activation in S2 during texture encoding correspond to the known involvement of this region in the sensory processing of tactile roughness [Kitada et al., 2005; Roland et al., 1998; Sathian et al., 2011], and effects of attentional modulation which were found to be stronger in S2 [Johansson and Westling, 1987]. The levelling off of the Load effects in the fMRI data corresponds to the drop in performance after load two in the behavioural data.
During the delay, the regions around the central sulcus (S1) as well as S2 showed a greater activation for the texture task bilaterally. Greater activation in the delay could signal that these regions are involved in the early stages of tactile memory [Burton and Sinclair, 2000; Harris et al., 2002; Zhou and Fuster, 1996], possibly involving tactile imagery [Yoo et al., 2003]. However, previous studies showed that S1 neurons in non‐human primates only reliably represented stimulus properties during encoding and not during the delay of sequential vibrotactile discrimination tasks [Salinas et al., 2000]. S2 neurons, on the other hand, were found to encode the stimulus frequency for a few ms into the delay between the first and second stimulus [Salinas et al., 2000].
Angular gyrus of the posterior parietal cortex
Although previous studies of microspatial discrimination have reported effects in the angular gyrus [Sathian et al., 1997; Zhang et al., 2005], we did not find this region to be active during encoding and working memory of fine surface texture. This might be due to stimulus‐related differences: the sandpaper stimuli in the current study had a microspatial structure at a sub millimeter scale, whereas the spacing of the gratings used in previous studies varied between one and three mm. In the studies by Sathian et al. and Zhang et al. subjects might have performed a judgment of the distance between line elements, involving computations similar to those involved in judging macro geometric object characteristics. In fact, activation in the angular gyrus has previously been reported in a study where subjects performed a delayed discrimination task of object oblongness [Stoeckel, et al., 2003].
Dorsolateral prefrontal cortex (dlPFC)
This area showed an increasing response with Load during encoding, and increased activation in the texture task during encoding and delay. The texture task effect was right lateralized during encoding whereas the effect of Load was left lateralized. Kitada et al. [2005] found that a (more anterior) region in right lateral prefrontal cortex showed a graded response with stimulus roughness in a roughness estimation task. Right hemispheric dlPFC has been suggested to be of special importance in cognitive tasks challenging the limits of working memory capacity [Hillary et al., 2006]. The current tactile texture matching task fits this description, in using relatively uncommon non‐object and non‐verbal tactile stimuli.
In the delay phase, Task effects were bilateral. DlPFC has been argued to contribute to executive control processes in working memory, for example, updating, monitoring and manipulating new information, instead of storing memory representations per se [Blumenfeld and Ranganath, 2006; Hillary et al., 2006; Petrides, 2000; Postle et al., 2003; Smith and Jonides, 1999]. Activity in dlPFC has been reported in studies of tactile short term memory in humans [Klingberg et al., 1996; Numminen et al., 2004]. In non‐human primates, the firing rates of neurons in ventrolateral PFC scaled with the flutter frequency of the first stimulus in the delay of a sequential vibrotactile discrimination tasks [Romo et al., 1999]. In Romo's study, dorsolateral PFC did not show task‐related activity (but neurons from this region were only recorded in a pilot with a single monkey). DlPFC was also proposed to be involved in skilled force production for precision grips, in which isometric grip force has to be adjusted to the object's weight and surface characteristic [Ehrsson et al., 2000].
Effects Found in Other Cortical Areas
Apart from the expected and observed activations in the somatosensory cortex and dorsolateral prefrontal cortex, we found additional effects in the primary motor cortex, medial frontal cortex and superior parietal lobule.
Primary motor cortex
Although it is not unexpected to find motor‐related areas during active stimulus exploration, we had not expected to find these areas to be involved differentially in the texture and location task, because participants were instructed to make the same explorative movements for both tasks. The bilateral Task effects (texture > control) during the encoding and delay period extended into primary motor cortex. There is some evidence for tactile receptive fields in Brodmann areas 4 and 6 in monkeys [Gentilucci et al., 1988; Rizzolatti et al., 1981; Strick and Preston, 1982] and humans [Moore et al., 2000], which have been hypothesized to support the execution of movements that require tactile feedback. Our task involved active stimulus exploration instead of more commonly used passive stimulation. A memory representation of actively explored tactile textures might involve a representation linking the specific movements during exploration to the tactile inputs obtained by those movements.
Medial frontal cortex
We found effects of Hand and Load during encoding and Task effects during encoding and delay. The focus of the activation related to the higher activation for the non‐dominant hand during encoding was posterior to the vertical plane passing through the anterior commissure (AC), in a region that most likely corresponds to the supplementary motor area (SMA). The SMA is a motor area directly connected to M1 and to the spinal cord, areas concerned with concrete aspects of movement [Picard and Strick, 2001]. Increased SMA activation during non‐dominant compared to dominant hand performance during non‐visually guided movements has also been observed by Van Mier et al. [1998]. Activation foci for the Load and Task effects were found more anteriorly, extending into the pre‐SMA, rostral to the vertical AC plane. The pre‐SMA has been classified as a prefrontal area based on its connectivity and physiology [Luppino and Rizzolatti, 2000] as well as function, supporting modality‐ and effector‐independent sensory‐motor associations [Picard and Strick, 2001] and the selection of action sets [Rushworth et al., 2004]. In visual working memory studies, pre‐SMA showed sustained activation in the delay of both spatial and non‐spatial tasks [Petit et al., 1998]. It was suggested to reflect “a state of preparedness for selection of a motor response based on the information held online”, dissociated from motor aspects of the task [Picard and Strick, 2001]. The pre‐SMA has also been proposed to facilitate and inhibit the selection of wanted and unwanted actions, respectively, when the environment provides conflicting information [Rushworth et al., 2004]. In our study, the pre‐SMA involvement might be related to cognitive monitoring processes during encoding and delay.
Superior parietal lobule
This parietal area was activated during encoding and delay. While the left hemispheric foci related to the Load effect during encoding and the Task effect (texture > control) during the delay overlapped, the foci in the right hemisphere were more distributed. The focus of the Task effect was located more anterior during encoding and more posterior in the delay phase.
At first sight, the texture task might be classified as a perceptual “what” task and as such it might be surprising that it induced stronger activation in the superior parietal cortex than the location task that could be characterized as a spatial “where” task. However, in the texture task specific details of the interaction between hand (movements) and object surface as well as the precise surface characteristics have to be remembered. Conversely, the location task does not entail complex hand‐object interaction and might therefore involve a perceptual rather than an action‐related spatial representation. Moreover, gross motor planning does not differ compared to the texture task.
The superior parietal lobule has been related to tactile object‐ and action‐related representations [Dijkerman and de Haan, 2007] and to tactile (and visual) spatial attention specifically when attention was focused close to the hands, in peripersonal space [Macaluso et al., 2000; Macaluso et al., 2002]. The greater activation found in the right aSPL in our study during the active exploration of textures might correspond to the right aSPL involvement previously observed during the haptic discrimination of object shape (oblongness) by Stoeckel et al. [2004], who interpreted this as being related to kinesthetic attention. During the delay, we observed more posterior foci in the bilateral SPL with increased activation for the maintenance of texture compared to the location control task. While previous studies have often implicated the superior parietal cortex in action‐related representations [van Mier, 2000; van Mier et al., 1998], the current study shows that it is also involved in working memory representations of actively explored tactile textures. This is in agreement with findings for kinesthetic working memory [Fiehler et al., 2008] and retrieval of haptically encoded information from long term memory [Stock et al., 2009]. Ricciardi et al. [2006] and Stoeckel et al. [2003, 2004] also reported superior parietal activations in both discrimination and maintenance of tactile stimuli that were actively explored. Hence, the SPL might be of particular importance for the storage of kinesthetic sensations evoked by the exploration of tactile stimuli. On the surface, the involvement of SPL in memory‐related processes appears to contradict with results from clinical studies demonstrating that patients with parietal lesions improve on grasping and pointing tasks when a delay is imposed [Milner et al., 1999; Revol et al., 2003; Rossetti et al., 2005]. This is explained by assuming that they can rely on grasping‐related memories supported by the ventral stream. However, a crucial difference is that such delay effects have been mainly observed for visually guided grasping, whereas the current study investigated purely haptic processing, for which the action and recognition related processing streams—given that such streams exist—might have a different anatomical basis. It has been proposed that somatosensory processing related to recognition engages the posterior parietal cortex, the posterior insula and the secondary somatosensory cortex [Dijkerman and de Haan, 2007].
Summary and Comparison to Previous Studies of Tactile Working Memory
Based on our findings for fine surface texture, we propose that tactile working memory encoding and maintenance of material properties involve the primary sensorimotor cortex (S1/M1), the secondary somatosensory cortex (S2) in coordination with the parietal (SPL) and pre‐frontal cortex (PFC). The event related averages of the sensory regions showed more transient responses whereas the parietal and frontal regions showed a more sustained response, corresponding to previous findings from visual [Courtney et al., 1997; Linden et al., 2003] and active tactile [Kaas et al., 2007] working memory studies. In all regions, the response during the delay was smaller than the response during the encoding and matching phases which involve sensory stimulation and motor activity, as reported in previous studies [Fiehler et al., 2008; Kaas et al., 2007; Schluppeck et al., 2006]. Possible explanations for the lower delay activation observed in fMRI studies compared to single cell recordings might be the different neurophysiological bases of the signals, the longer delay lengths employed in fMRI, and the selective measuring of neurons preselected for showing delay‐related activation in single‐unit studies [Schluppeck et al., 2006].
In contrast to results form previous studies of active tactile working memory [Kaas et al., 2007; Ricciardi et al., 2006; Stoeckel et al., 2003] the tactile texture working memory delay in the current study did not recruit occipital or occipitotemporal regions, for example, lateral occipital cortex or fusiform gyrus, traditionally associated with visual object perception and imagery. In both monkeys and humans, parieto‐occipital regions and caudal IPS have been shown to be specifically involved in the coding of 3D macro geometric object features. Haptic location and texture discrimination were found to activate segregated pathways in a human fMRI study [Sathian et al., 2011]. Whether the neural substrate for working memory of tactile texture and other material properties is indeed distinct from the neural correlates maintaining 3D object information or other tactile macro geometric properties as suggested by our data remains to be tested in future experiments, directly comparing the maintenance of these properties.
CONCLUSION
The current study revealed activity in a parieto‐frontal network including the dorsolateral prefrontal cortex, the superior parietal lobule, the pre‐SMA, S1 and S2 related to the maintenance of tactile texture information in a delayed match‐to‐sample task.
After extraction of tactile texture information by the primary and secondary somatosensory cortices, the tactile sensory trace might be converted to a higher‐order somatosensory representation, maintained in the secondary somatosensory cortex. We suggest that SPL might be involved in maintenance of kinesthetic memory representations obtained from active stimulus exploration. Additional regions in the pre‐SMA and dlPFC might subserve abstract cognitive monitoring. Our results provide supporting evidence for the existence of specialized areas in the secondary somatosensory cortex for processing of tactile surface texture, and extend previous findings by showing that these regions are also involved in short term memory.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Figure 3.
Supporting Information Figure 4.
Supporting Information Table 1.
Acknowledgements
The authors gratefully acknowledge the support of the BrainGain Smart Mix Programme of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science. They thank Nadia Mueller, Anneke Hinzen, Martin Frost, and all participants for their help collecting the data. In addition, they thank J. Reithler, J. Peters, A. Sack, and the Brain Innovation team for useful comments and help in preparing the manuscript.
REFERENCES
- Blumenfeld RS, Ranganath C ( 2006): Dorsolateral prefrontal cortex promotes long‐term memory formation through its role in working memory organization. J Neurosci 26: 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodegard A, Geyer S, Grefkes C, Zilles K, Roland PE ( 2001): Hierarchical processing of tactile shape in the human brain. Neuron 31: 317–328. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Fretz C, Weder B ( 2002): Hierarchical versus parallel processing in tactile object recognition: A behavioural‐neuroanatomical study of aperceptive tactile agnosia. Brain 125 ( Part 11): 2537–2548. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ ( 1996): Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci 16: 4207–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ ( 2000): Attending to and remembering tactile stimuli: A review of brain imaging data and single‐neuron responses. J Clin Neurophysiol 17: 575–591. [DOI] [PubMed] [Google Scholar]
- Costanzo RM, Gardner EP ( 1980): A quantitative analysis of responses of direction‐sensitive neurons in somatosensory cortex of awake monkeys. J Neurophysiol 43: 1319–1341. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV ( 1997): Transient and sustained activity in a distributed neural system for human working memory. Nature 386: 608–611. [DOI] [PubMed] [Google Scholar]
- Dijkerman HC, de Haan EH ( 2007): Somatosensory processes subserving perception and action. Behav Brain Sci 30: 189–201; discussion 201–239. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H ( 2000): Cortical activity in precision‐ versus power‐grip tasks: An fMRI study. J Neurophysiol 83: 528–536. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K ( 2006a): The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex 16: 268–279. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TE ( 2010): Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci 30: 6409–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K ( 2006b): The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex 16: 254–267. [DOI] [PubMed] [Google Scholar]
- Fiehler K, Burke M, Engel A, Bien S, Rosler F ( 2008): Kinesthetic working memory and action control within the dorsal stream. Cereb Cortex 18: 243–253. [DOI] [PubMed] [Google Scholar]
- Fusser F, Linden DE, Rahm B, Hampel H, Haenschel C, Mayer JS ( 2011): Common capacity‐limited neural mechanisms of selective attention and spatial working memory encoding. Eur J Neurosci 34: 827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EP, Costanzo RM ( 1980): Neuronal mechanisms underlying direction sensitivity of somatosensory cortical neurons in awake monkeys. J Neurophysiol 43: 1342–1354. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G ( 1988): Functional organization of inferior area 6 in the macaque monkey. I. Somatotopy and the control of proximal movements. Exp Brain Res 71: 475–490. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E ( 2006): Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single‐subject to cortically aligned group general linear model analysis and self‐organizing group independent component analysis. Hum Brain Mapp 27: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K ( 2001): Human somatosensory area 2: Observer‐independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage 14: 617–631. [DOI] [PubMed] [Google Scholar]
- Guest S, Spence C ( 2003): Tactile dominance in speeded discrimination of textures. Exp Brain Res 150: 201–207. [DOI] [PubMed] [Google Scholar]
- Harris JA, Harris IM, Diamond ME ( 2001): The topography of tactile working memory. J Neurosci 21: 8262–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Miniussi C, Harris IM, Diamond ME ( 2002): Transient storage of a tactile memory trace in primary somatosensory cortex. J Neurosci 22: 8720–8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller MA ( 1989): Texture perception in sighted and blind observers. Percept Psychophys 45: 49–54. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Genova HM, Chiaravalloti ND, Rypma B, DeLuca J ( 2006): Prefrontal modulation of working memory performance in brain injury and disease. Hum Brain Mapp 27: 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock M, Inch R, Hawryluk J, Lyon PJ, Perachio N ( 1999): Is there a sex difference in human laterality? III. An exhaustive survey of tactile laterality studies from six neuropsychology journals. J Clin Exp Neuropsychol 21: 17–28. [DOI] [PubMed] [Google Scholar]
- Hyvarinen J, Poranen A ( 1978): Movement‐sensitive and direction and orientation‐selective cutaneous receptive fields in the hand area of the post‐central gyrus in monkeys. J Physiol 283: 523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenmalm P, Schmitz C, Forssberg H, Ehrsson HH ( 2006): Lighter or heavier than predicted: Neural correlates of corrective mechanisms during erroneously programmed lifts. J Neurosci 26: 9015–9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Westling G ( 1984): Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res 56: 550–564. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G ( 1987): Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip. Exp Brain Res 66: 141–154. [DOI] [PubMed] [Google Scholar]
- Jones B, O'Neil S ( 1985): Combining vision and touch in texture perception. Percept Psychophys 37: 66–72. [DOI] [PubMed] [Google Scholar]
- Kaas AL, van Mier H, Goebel R ( 2007): The neural correlates of human working memory for haptically explored object orientations. Cereb Cortex 17: 1637–1649. [DOI] [PubMed] [Google Scholar]
- Kitada R, Hashimoto T, Kochiyama T, Kito T, Okada T, Matsumura M, Lederman SJ, Sadato N ( 2005): Tactile estimation of the roughness of gratings yields a graded response in the human brain: An fMRI study. Neuroimage 25: 90–100. [DOI] [PubMed] [Google Scholar]
- Klatzky RL, Lederman SJ, Matula DE ( 1993): Haptic exploration in the presence of vision. J Exp Psychol Hum Percept Perform 19: 726–743. [DOI] [PubMed] [Google Scholar]
- Klatzky RL, McCloskey B, Doherty S, Pellegrino J, Smith T ( 1987): Knowledge about hand shaping and knowledge about objects. J Mot Behav 19: 187–213. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Kawashima R, Roland PE ( 1996): Activation of multi‐modal cortical areas underlies short‐term memory. Eur J Neurosci 8: 1965–1971. [DOI] [PubMed] [Google Scholar]
- Koch KW, Fuster JM ( 1989): Unit activity in monkey parietal cortex related to haptic perception and temporary memory. Exp Brain Res 76: 292–306. [DOI] [PubMed] [Google Scholar]
- Kurth F, Eickhoff SB, Schleicher A, Hoemke L, Zilles K, Amunts K ( 2010): Cytoarchitecture and probabilistic maps of the human posterior insular cortex. Cereb Cortex 20: 1448–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DE, Bittner RA, Muckli L, Waltz JA, Kriegeskorte N, Goebel R, Singer W, Munk MH ( 2003): Cortical capacity constraints for visual working memory: Dissociation of fMRI load effects in a fronto‐parietal network. Neuroimage 20: 1518–1530. [DOI] [PubMed] [Google Scholar]
- Luppino G, Rizzolatti G ( 2000): The Organization of the Frontal Motor Cortex. News Physiol Sci 15: 219–224. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith C, Driver J ( 2000): Selective spatial attention in vision and touch: Unimodal and multimodal mechanisms revealed by PET. J Neurophysiol 83: 3062–3075. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith CD, Driver J ( 2002): Directing attention to locations and to sensory modalities: Multiple levels of selective processing revealed with PET. Cereb Cortex 12: 357–368. [DOI] [PubMed] [Google Scholar]
- Mayer JS, Bittner RA, Nikolic D, Bledowski C, Goebel R, Linden DE ( 2007): Common neural substrates for visual working memory and attention. Neuroimage 36: 441–453. [DOI] [PubMed] [Google Scholar]
- Merabet L, Thut G, Murray B, Andrews J, Hsiao S, Pascual‐Leone A ( 2004): Feeling by sight or seeing by touch? Neuron 42: 173–179. [DOI] [PubMed] [Google Scholar]
- Milner AD, Paulignan Y, Dijkerman HC, Michel F, Jeannerod M ( 1999): A paradoxical improvement of misreaching in optic ataxia: New evidence for two separate neural systems for visual localization. Proc Biol Sci 266: 2225–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CI, Stern CE, Corkin S, Fischl B, Gray AC, Rosen BR, Dale AM ( 2000): Segregation of somatosensory activation in the human rolandic cortex using fMRI. J Neurophysiol 84: 558–569. [DOI] [PubMed] [Google Scholar]
- Numminen J, Schurmann M, Hiltunen J, Joensuu R, Jousmaki V, Koskinen SK, Salmelin R, Hari R ( 2004): Cortical activation during a spatiotemporal tactile comparison task. Neuroimage 22: 815–821. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Petit L, Courtney SM, Ungerleider LG, Haxby JV ( 1998): Sustained activity in the medial wall during working memory delays. J Neurosci 18: 9429–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M ( 2000): The role of the mid‐dorsolateral prefrontal cortex in working memory. Exp Brain Res 133: 44–54. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL ( 2001): Imaging the premotor areas. Curr Opin Neurobiol 11: 663–672. [DOI] [PubMed] [Google Scholar]
- Postle BR, Druzgal TJ, D'Esposito M ( 2003): Seeking the neural substrates of visual working memory storage. Cortex 39: 927–946. [DOI] [PubMed] [Google Scholar]
- Randolph M, Semmes J ( 1974): Behavioral consequences of selective subtotal ablations in the postcentral gyrus of Macaca mulatta. Brain Res 70: 55–70. [DOI] [PubMed] [Google Scholar]
- Revol P, Rossetti Y, Vighetto A, Rode G, Boisson D, Pisella L ( 2003): Pointing errors in immediate and delayed conditions in unilateral optic ataxia. Spat Vis 16: 347–364. [DOI] [PubMed] [Google Scholar]
- Ricciardi E, Bonino D, Gentili C, Sani L, Pietrini P, Vecchi T ( 2006): Neural correlates of spatial working memory in humans: A functional magnetic resonance imaging study comparing visual and tactile processes. Neuroscience 139: 339–349. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Scandolara C, Matelli M, Gentilucci M ( 1981): Afferent properties of periarcuate neurons in macaque monkeys. I. Somatosensory responses. Behav Brain Res 2: 125–146. [DOI] [PubMed] [Google Scholar]
- Roland PE, O'Sullivan B, Kawashima R ( 1998): Shape and roughness activate different somatosensory areas in the human brain. Proc Natl Acad Sci U S A 95: 3295–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Brody CD, Hernandez A, Lemus L ( 1999): Neuronal correlates of parametric working memory in the prefrontal cortex. Nature 399: 470–473. [DOI] [PubMed] [Google Scholar]
- Rossetti Y, Revol P, McIntosh R, Pisella L, Rode G, Danckert J, Tilikete C, Dijkerman HC, Boisson D, Vighetto A and others ( 2005): Visually guided reaching: Bilateral posterior parietal lesions cause a switch from fast visuomotor to slow cognitive control. Neuropsychologia 43: 162–177. [DOI] [PubMed] [Google Scholar]
- Ruiz S, Crespo P, Romo R ( 1995): Representation of moving tactile stimuli in the somatic sensory cortex of awake monkeys. J Neurophysiol 73: 525–537. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM ( 2004): Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 8: 410–417. [DOI] [PubMed] [Google Scholar]
- Sadato N, Ibanez V, Deiber MP, Hallett M ( 2000): Gender difference in premotor activity during active tactile discrimination. Neuroimage 11 ( 5 Part 1): 532–540. [DOI] [PubMed] [Google Scholar]
- Salinas E, Hernandez A, Zainos A, Romo R ( 2000): Periodicity and firing rate as candidate neural codes for the frequency of vibrotactile stimuli. J Neurosci 20: 5503–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathian K, Lacey S, Stilla R, Gibson GO, Deshpande G, Hu X, Laconte S, Glielmi C ( 2011): Dual pathways for haptic and visual perception of spatial and texture information. Neuroimage 57: 462–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A, Hoffman JM, Grafton ST ( 1997): Feeling with the mind's eye. Neuroreport 8: 3877–3881. [DOI] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ ( 2006): Sustained activity in topographic areas of human posterior parietal cortex during memory‐guided saccades. J Neurosci 26: 5098–5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J ( 1999): Storage and executive processes in the frontal lobes. Science 283: 1657–1661. [DOI] [PubMed] [Google Scholar]
- Stock O, Roder B, Burke M, Bien S, Rosler F ( 2009): Cortical activation patterns during long‐term memory retrieval of visually or haptically encoded objects and locations. J Cogn Neurosci 21: 58–82. [DOI] [PubMed] [Google Scholar]
- Stoeckel MC, Weder B, Binkofski F, Buccino G, Shah NJ, Seitz RJ ( 2003): A fronto‐parietal circuit for tactile object discrimination: An event‐related fMRI study. Neuroimage 19: 1103–1114. [DOI] [PubMed] [Google Scholar]
- Stoeckel MC, Weder B, Binkofski F, Choi HJ, Amunts K, Pieperhoff P, Shah NJ, Seitz RJ ( 2004): Left and right superior parietal lobule in tactile object discrimination. Eur J Neurosci 19: 1067–1072. [DOI] [PubMed] [Google Scholar]
- Stoesz MR, Zhang M, Weisser VD, Prather SC, Mao H, Sathian K ( 2003): Neural networks active during tactile form perception: Common and differential activity during macrospatial and microspatial tasks. Int J Psychophysiol 50: 41–49. [DOI] [PubMed] [Google Scholar]
- Strick PL, Preston JB ( 1982): Two representations of the hand in area 4 of a primate. II. Somatosensory input organization. J Neurophysiol 48: 150–159. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R ( 2004): Capacity limit of visual short‐term memory in human posterior parietal cortex. Nature 428: 751–754. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM ( 2003): Mapping brain asymmetry. Nat Rev Neurosci 4: 37–48. [DOI] [PubMed] [Google Scholar]
- van Mier H ( 2000): Human learning In: Toga AW, Mazziotta JC, editors. Brain Mapping: The Systems. San Diego: Academic Press; pp 605–621. [Google Scholar]
- van Mier H, Tempel LW, Perlmutter JS, Raichle ME, Petersen SE ( 1998): Changes in brain activity during motor learning measured with PET: Effects of hand of performance and practice. J Neurophysiol 80: 2177–2199. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Freeman DK, McCarthy JJ, 3rd , Jolesz FA ( 2003): Neural substrates of tactile imagery: A functional MRI study. Neuroreport 14: 581–585. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mariola E, Stilla R, Stoesz M, Mao H, Hu X, Sathian K ( 2005): Tactile discrimination of grating orientation: fMRI activation patterns. Hum Brain Mapp 25: 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YD, Fuster JM ( 1996): Mnemonic neuronal activity in somatosensory cortex. Proc Natl Acad Sci USA 93: 10533–10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Figure 3.
Supporting Information Figure 4.
Supporting Information Table 1.


