Abstract
Occlusal splints are a common and effective therapy for temporomandibular joint disorder. Latest hypotheses on the impact of occlusal splints suggest an altered cerebral control on the occlusion movements after using a splint. However, the impact of using a splint during chewing on its cerebral representation is quite unknown. We used functional magnetic resonance imaging (fMRI) to investigate brain activities during occlusal function in centric occlusion on natural teeth or on occlusal splints in fifteen healthy subjects. Comparisons between conditions revealed an increased activation for the bilateral occlusion without a splint in bilateral primary and secondary sensorimotor areas, the putamen, inferior parietal and prefrontal cortex (left dorsal and bilateral orbital) and anterior insular. In contrast, using a splint increased activation in the bilateral prefrontal lobe (bilateral BA 10), bilateral temporo‐parietal (BA 39), occipital and cerebellar hemispheres. An additionally applied individually based evaluation of representation sites in regions of interest demonstrated that the somatotopic representation for both conditions in the pre‐ and postcentral gyri did not significantly differ. Furthermore, this analysis confirmed the decreasing effect of the splint on bilateral primary and secondary motor and somatosensory cortical activation. In contrast to the decreasing effect on sensorimotor areas, an increased level of activity in the fronto‐parieto‐occipital and cerebellar network might be associated with the therapeutic effect of occlusal splints. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: oclusal splint, motor economization, somatosensory integration
INTRODUCTION
The use of occlusal splints is a common method to treat temporomandibular joint (TMJ) disorders and associated pain symptoms [Carlsson, 2009; Ommerborn et al., 2010]. Management of myofascial face pain with an occlusal splint worn at night is likely to lead to an improvement when compared with no treatment [Türp et al., 2004], but the proposed mechanisms explaining the treatment effects range from occlusal disengagement, neurophysiologic effects on the masticatory system, change of vertical dimension or caput‐fossa relation, cognitive awareness of harmful behavior, up to stress reduced load on masticatory components or placebo effects [Carlsson, 2009]. The effect of occlusal splints on the cerebral representation of chewing is coming more and more into focus [Otsuka et al., 2009]. However, no study on altered cerebral representation of chewing with occlusal splints is available.
With functional magnetic resonance imaging (fMRI) a noninvasive mapping of different occlusal movements is achievable and some recent functional neuroimaging studies have raised important data on this topic. Mastication activates several cerebral regions, with the greatest activation in the bilateral primary motor cortex (M1) and somatosensory cortex (S1), secondary motor areas [the supplementary motor area (SMA) and the premotor area (PMC)] and secondary somatosensory cortex (S2), insula, thalamus, basal ganglia, and anterior cerebellar hemispheres [Onozuka et al., 2002; Shinagawa et al., 2004; Takada and Miyamoto, 2004; Tamura et al., 2003]. In addition, it has been shown that chewing representation in M1 and S1 of healthy subjects is lateralized to the dominant hemisphere [Foki et al., 2007]. Recently, Otsuka et al. [2009] investigated occlusal movements with a malfunctioning splint and found additional activation in the anterior cingulate cortex (ACC) and the amygdala. Activation magnitude in these areas was positively associated with scores of discomfort.
We sought to identify cerebral regions associated with a possible beneficial effect of occlusal splints by using an individually adapted splint (Michigan technique) in healthy volunteers. We expected that the cerebral changes in fMRI representation associated with the splint might be comparable to the beneficial effect of splints in patients with craniomandibular dysfunction (CMD). In these patients, beside a decrease in pain, predominantly three physiologic changes have been reported: first, a decrease in electromyographic activity of masticatory muscles has been described after short term [Ferrario, 2002] and long term use of an occlusal splint [Tecco et al., 2008]. Second, a more symmetric masticatory activation after usage of the splint has been shown [Botelho et al., 2010; Ferrario et al., 2002]. Third, an altered temporomandibular joint position [Ekberg et al., 1998; Ettlin et al., 2008] has been noted. The first might be explained by a reduction of activation in primary sensorimotor areas, the second might be associated with a more symmetric activation pattern and the findings in the third might be associated with increased attentional control of movement performance and possibly with a more prefrontal involvement.
To compare changes in cerebral activation we used fMRI during centric occlusion by natural teeth (“bilateral”) and by using occlusal splints (“splint”). To detect possible representational differences associated solely with a clinically used splint, but not with abnormal occlusion, we investigated healthy subjects with individually comfortably fitted occlusal splints. To avoid possible representational differences between subjects due to normalization processes, we additionally performed a between‐condition comparison on the individual activation maxima for M1, S1, S2, and anterior cerebellar hemisphere.
METHODS
Subjects
Fifteen healthy subjects (mean age: 25.3 years; six female) were investigated. Informed consent was obtained from these subjects and approval was given by the ethical committee of the medical faculty of the University of Greifswald. All of the subjects were screened by dentists to rule out dysfunction symptoms such as cranio‐mandibular dysfunction (CMD) or any medical, psychiatric, or neurological disorders. For each subject a maxillary occlusal splint was made with the Michigan technique using an individual articulator after mounting of the upper jaw cast with a face bow, and the lower jaw cast with a centric relation record. The occlusal splint opened the bite 2–3 mm (see Fig. 1B).
Figure 1.
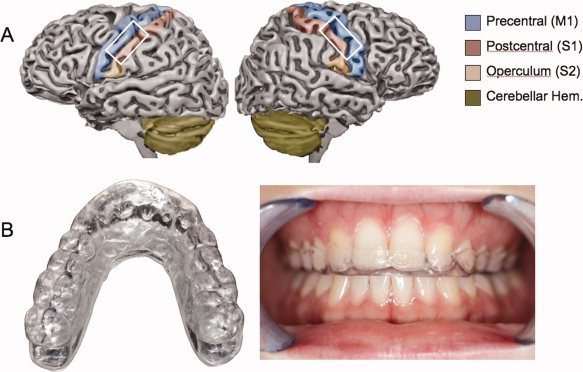
Illustration on an individually segmented brain of the anatomical ROIs selected. Blue: precentral gyrus, Red: postcentral gyrus, orange: secondary somatosensory cortex (S2); green: area of the superior cerebellum selected. Picture of the Michigan splint: left in an overview, right: implemented for the upper alignment. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Task
Subjects performed repetitive (1) opening from centric occlusion and closing into centric dental occlusion of natural teeth (“bilateral” tapping: range capacity was ∼5–7 mm with a frequency of 1–1.5 Hz) and (2) tapping movements of the mandible as in (1), but with incorporated occlusal “splint.” Before scanning we trained the subjects with a visual signal to perform a precise frequency of the tapping movements. Some subjects found 1.5 Hz more comfortable, some 1 Hz. To ensure that exactly the same frequency between conditions was performed, we allowed a slight interindividual variation between the tapping frequencies. Subjects were instructed to follow the onset and end of repetitive movements by a visual signal presented by a multimedia projector and observed via a mirror affixed on the head coil. All tasks began with a 30‐s rest followed by nine alternating 30‐s epochs of “do” (green color) and “rest” (red color). Task presentation was randomized between the different conditions to correct for habituation effects in the scanner. After each task subjects received new appliances due to the next task without changing head position. This change had a duration of 2–4 min. The position of the head was fixed using special pillars to fill out spatial distances between head and coil.
fMRI‐Scanning
All examinations were performed on a 1.5 Tesla MRI‐System (Magnetom Symphony, Quantum gradients, SIEMENS Medical Systems, Erlangen, Germany) using an eight‐channel head coil. Before functional data were acquired, anatomical images were obtained using a flash‐3D‐sequence (TR = 368 ms, TE = 4.88 ms, flip angle 40°, FoV 192 mm, matrix = 256 × 256, voxel size = 1 × 1 × 1 mm3). We performed BOLD‐imaging using an EPI T2* weighted imaging technique covering the whole brain (33 slices, slice thickness 3 mm, 1 mm gap, TE 50 ms TR 3,000 ms, flip angle 90°, Matrix 64 × 64, FOV 192 mm, voxel size = 3 × 3 × 3 mm3, 8 initial dummy volumes). Per task 100 volumes were measured including five rest and five activation periods with ten volumes respectively. Overall 216 whole head EPI‐volumes were recorded and 200 were used for statistical analysis.
fMRI‐Data Processing
We used Brainvoyager QX 2.0 (Comp. Brain Innovations B.V., Netherlands) for preprocessing of fMRI‐data including motion correction with sinc‐interpolator and linear trend removal. The brain volumes were calculated and transformed into the standardized Talairach space [Talairach and Tournoux, 1988] and an individual segmentation of the gray matter was performed.
Statistical analysis was performed using the general linear model as implemented in Brain Voyager QX. For each subject a design matrix was created using a canonical hemodynamic response function for modelling the response to each of the conditions “bilateral” and “splint” and the interaction “bilateral > splint” and “splint > bilateral.” Contrast images of each subject (first level) were subsequently used for group statistics. A statistical group‐map was calculated using a random effects analysis (second level), which takes variance between subjects into account. The statistical threshold for comparisons of the main effect of each condition was set at P < 0.01 (t > 3.02), using the false‐discovery‐rate‐method for correction of the multiple comparison problem [FDR; Genovese et al., 2002]. Additionally, we set the cluster level to 10. Between group comparisons were performed with P < 0.05; FDR‐correction which resulted in the same t‐threshold for between group comparisons (t > 3.27). Group activation maps were projected on the segmented Talairach normalized high‐resolution brain included as a template in Brain Voyager (CG‐brain) for visualizing the representation maps of the cortical surface; for presenting subcortical activations on slices we used the normalized T1‐image averaged for all subjects (Figs. 2 and 3).
Figure 2.
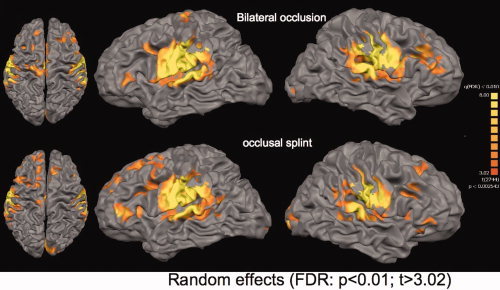
Main effect of both conditions; projection of group maps on a high‐resolution segmented brain (CG‐brain; P < 0.01; FDR corrected). Top row: Bilateral occlusal movements without a splint; Bottom row: Group representation for occlusion with the usage of an occlusal splint. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
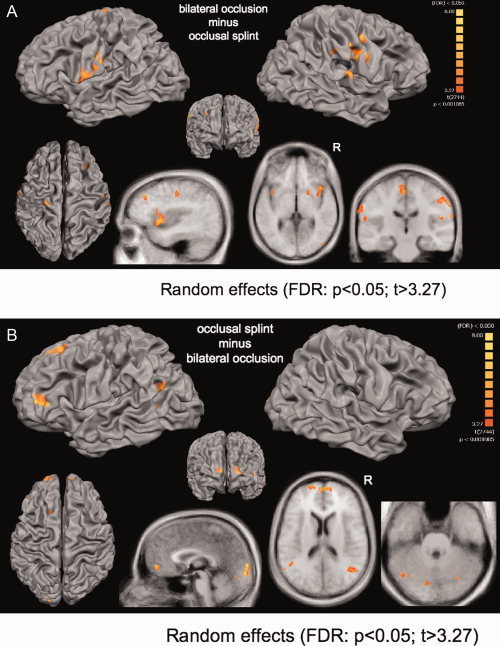
Comparison between conditions; Top row: Cortical differences projected on a segmented high‐resolution brain (CG‐brain; P < 0.05; FDR corrected). Bottom row: slices projected on the averaged T1‐weighted structural datasets of all 15 subjects. Occlusion (“bilateral”) minus occlusional splint (“splint”): The bilateral occlusion without a splint showed increased activation in bilateral M1, S1, and S2, anterior insula, inferior parietal lobe, putamen and medial cingulate cortex. Occlusional splint (“splint”) minus occclusion (“bilateral”): The usage of a occlusal splint involved increasing activation in bilateral prefrontal lobe (BA 46, left BA 10, right BA 45), bilateral temporo‐parietal junction, bilateral occipital lobe and bilateral cerebellar hemispheres.
Furthermore, we performed an additional approach for the comparison between conditions, which was based on the selection of individual activation maxima within anatomically selected regions of interest (ROIs). In this second approach we selected activation maxima based on the individual anatomy of each subject. One aim of this analysis was to separate activation sites within the pre‐ and postcentral gyri which might be no more distinguishable after the linear Talairach normalization approach [Talairach and Tournoux, 1988]. Another aim was to detect possible differences in the somatotopic site of representation maxima between subjects. Furthermore, only the individual analysis allowed statistical test lateralization for differences in somatotopic representation between conditions in the pre‐ and postcentral gyri.
We selected anatomical regions of interest (pre‐ and postcentral gyri, supramarginal gyrus, anterior cerebellar hemispheres) in the nonnormalized datasets and additionally identified the proper location of the activation cluster within ROI in the denormalized segmented data of every subject. For the pre‐ and postcentral gyri we only used the somatotopic representation below the hand knob and above the area assumed to be S2. S2 activation was assigned to the region of the inferior postcentral gyrus [see Ruben et al., 2001]. Because this differentiation was performed in non‐normalized datasets an orientation in Talairach coordinates and confidence intervals of representation sites, or an overlay on standardized cytoarchitectural masks was not possible. For the anterior cerebellar hemisphere we used previous findings to orientate the somatotopic representation [Grodd et al., 2001]. This analysis was performed by a neuroscientist (ML) who has been experienced in the detection of regions of interest for several years. A schematic illustration for the ROIs selected can be seen in Figure 1A.
We described t values and coordinates for activation maxima in these anatomically defined regions and compared these data in a second level analysis using SPSS 16.0. Activation intensity was compared in a three factorial repeated measurements ANOVA with the factors “ROI” (M1, S1, S2, and cerebellum), “Hemisphere” (left and right) and “Condition” (bilateral occlusion and occlusal splint). Significant results in the ANOVA were followed by multivariate tests, corrected for multiple comparisons (Bonferroni).
We then selected the appropriate Talairach coordinates for the normalized dataset to compare the precise location in the pre‐ and postcentral gyri of individual normalization with those of the voxel based group statistics. Euclidian distances between activation maxima, calculated by the two evaluation methods (“random effects group analysis” versus “individually based ROI‐analysis”) and the two conditions (“bilateral” versus “splint”) were calculated.
Significant differences between representation maxima could only be calculated for the ROI‐analysis using t tests corrected for eight comparisons. Comparisons between methods were only descriptive.
RESULTS
The two conditions did not differ with respect to the head movement during measurement as detected with the realignment procedure (directions: x, y, z; and spatial translations: pitch, yaw, and roll; paired t tests between the occlusion and the splint condition: t(14) < 1.40; n.s.).
fMRI‐Data: Main Effect
Both occlusal movements without and with the splint were associated with activations in bilateral primary and secondary somatosensory and motor areas, bilateral anterior cerebellar hemispheres, thalamus, anterior and posterior insula, primary visual cortex and prefrontal cortex (see Fig. 2 and Supporting Information Tables I and II). The Euclidian distances between representation maxima obtained with the two evaluation methods (“random effects group analysis” and “individually based ROI analysis”) during the condition “bilateral” revealed an average spatial difference of 6.94 mm (M1 ri: 2.23 mm; M1le: 13.38 mm; S1 ri: 3.00 mm; S1 le: 9.43 mm; S2 ri: 6.00 mm; S2 le: 10.49 mm; cerebellar ri: 5.09 mm; cerebellar le: 5.92 mm; Supporting Information Tables I and III). Additional activation sites for the “bilateral” condition were observed in the left insula, bilateral putamen and the anterior (ACC) and medial (MCC) cingulate cortex. In contrast, the “splint” condition showed additional activation in the bilateral superior temporal gyrus, the pallidum, the orbitofrontal lobe and the inferior parietal cortex.
fMRI‐Data: Comparison Between Conditions
Random effects group analysis
Bilateral occlusion without a splint (“bilateral”) revealed increased activation in the bilateral M1 and S1, left S2, bilateral anterior insula, SMA, and MCC (Table I; Fig. 3A). Additionally, the bilateral supramarginal gyrus (BA 40), bilateral inferior (BA 46) and bilateral medial frontal gyri (BA 9), bilateral putamen, and the right posterior cerebellar hemisphere were increasingly involved in “bilateral” occlusal movements without the splint. The “splint” condition increased activation in the bilateral inferior prefrontal lobe (bilateral BA 10), bilateral occipital (BA 18) and temporoparietal (BA 39) lobe, and bilateral anterior and posterior cerebellar hemispheres (Table II, Fig. 3B).
Table I.
Occlusion (“bilateral”) minus occlusional splint (“splint”)
| Anatomical landmark | Brodman's/ Larsell's area | t value | x | y | z |
|---|---|---|---|---|---|
| Precentral right (M1) | BA 4 | 7.84 | 51 | −9 | 46 |
| Postcentral right (S1) | BA 3 | 7.43 | 55 | −19 | 39 |
| Postcentral right (S1) | BA 3 | 6.68 | 56 | −20 | 33 |
| Postcentral left (S1) | BA 3 | 6.26 | −58 | −8 | 30 |
| Postcentral gyrus left (S2) | OP4 | 5.85 | −59 | −7 | 20 |
| Anterior insula right | BA 13 | 6.85 | 38 | 7 | 0 |
| Anterior insula left | BA 13 | 5.03 | −39 | 11 | 7 |
| Supramarginal gyrus right | BA 40 | 6.26 | 47 | −35 | 44 |
| Supramarginal gyrus left | BA 40 | 5.84 | −48 | −31 | 37 |
| Lateral prefrontal cortex right | BA 46 | 5.66 | 32 | 35 | 31 |
| Lateral prefrontal cortex left | BA 46 | 4.89 | −33 | 46 | 31 |
| Medial frontal gyrus right (supplementary motor area, SMA) | BA 6 | 5.60 | 2 | 0 | 51 |
| Precental gyrus left | BA 6 | 5.47 | −18 | −19 | 64 |
| Inferior frontal gyrus right | BA 44 | 5.50 | 50 | −1 | 20 |
| Medial cingulate cortex (MCC) right | BA 32 | 5.28 | 6 | 11 | 34 |
| Medial frontal gyrus right | BA 9 | 5.61 | 39 | 32 | 37 |
| Medial frontal gyrus left | BA 9 | 4.81 | −35 | 45 | 31 |
| Putamen right | 4.61 | 21 | 6 | 9 | |
| Putamen left | 4.18 | −18 | 9 | 5 | |
| Posterior cerebellar hemisphere right | Crus 2 | 6.60 | 27 | −78 | −36 |
Table II.
Occlusional splint (“splint”) minus occlusion (“bilateral”)
| Anatomical landmark | Brodman's/ Larsell's area | t value | x | y | z |
|---|---|---|---|---|---|
| Frontal medial gyrus right | BA 9 | 6.82 | 27 | 53 | 31 |
| Frontomedial lobe | BA 10 | 6.36 | 18 | 48 | −2 |
| Frontal inferior gyrus (triangular) right | BA 45 | 6.09 | 48 | 44 | −2 |
| Frontal inferior gyrus (triangular) left | BA 46 | 5.52 | −36 | 36 | 11 |
| Occipital lobe right | BA 18 | 6.59 | 25 | −82 | 1 |
| Occipital lobe left | BA 18 | 7.03 | −45 | −76 | −9 |
| Temporoparietal right | BA 39 | 5.90 | 40 | −60 | 18 |
| Temporoparietal left | BA 39 | 5.50 | −49 | −54 | 20 |
| Precuneus right | BA 19 | 5.97 | 5 | −49 | 9 |
| Anterior cerebellar hemisphere right | Larsell H6 | 7.61 | 34 | −60 | −23 |
| Anterior cerebellar hemisphere left | Crus 1 | 5.71 | −31 | −66 | −25 |
| Posterior cerebellar hemisphere right | Crus 2 | 6.58 | −21 | −81 | −29 |
fMRI‐Data: Individually Based ROI‐Evaluation
The analysis based on the individual anatomic localization of representation sites in ROIs confirmed the decreasing effect of the splint on bilateral primary and secondary motor and somatosensory cortical activation, but no effect for cerebellar representation sites (Fig. 4, Supporting Information Table III). In detail, the ANOVA showed a main effect for “condition” F(1,14) = 5.34; 0.037 and an effect for the interaction between “region” (4 ROIs) דcondition” (occlusion with versus occlusion without splint): F(3,42) = 5.05; P < 0.005. There was a significant main effect for “side” (F(1,14) = 4.80; 0.046; a later t test on the direction of the effect revealed a lateralization to the left), which was not influenced by any other factors (no significant interaction). Post‐hoc multivariate tests for the significant region × condition interaction showed a splint usage associated decrease in activation of M1 (F(14) = 11.80; P < 0.005), S1 (F(14) = 4.91; P < 0.05), and S2 (F(14) = 5.66; P < 0.05) but not for the cerebellar hemispheres (F(14) = 0.19; n.s.; see Fig. 4).
Figure 4.
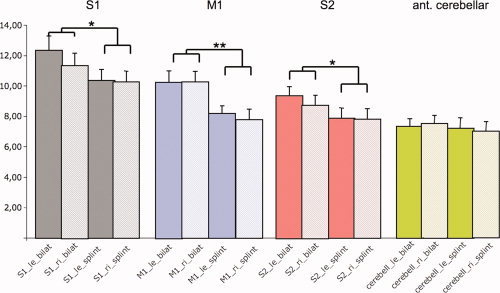
Group analysis based on individual activation sites and magnitude revealed differential activation magnitude in bilateral M1 (P < 0.05), S1 (P < 0.005), and S2 (P < 0.05) but not in the anterior cerebellar hemispheres (n.s.). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Euclidian differences of representation maxima in the pre‐ and postcentral gyri between the two conditions (“bilateral,” “splint”) were varying in a very small range of 2–5 mm (3.16 mm in M1 ri; 1.41 mm in M1 le; 4.69 mm in S1 right; 2.00 mm in S1 le; average: 2.82 mm). They therefore showed no significant differences between conditions in the statistical comparison.
DISCUSSION
Short Repetition of Main Results
Our study demonstrated that an individually fitted splint economizes representational load in the primary sensorimotor and secondary somatosensory cortex during occlusion. Additionally, the random‐effects analysis revealed further decreases of activation magnitude in the bilateral anterior insula (BA 13), bilateral putamen, bilateral prefrontal and supramarginal gyrus (BA 40), the medial cingulate gyrus (MCC) and the SMA. Using the splint involved additional resources in the bilateral prefrontal cortex (predominantly in BA 10), the temporoparietal (BA 39) and occipital lobe (BA 18). Interestingly, the anterior and posterior cerebellar hemispheres were more strongly involved for the “splint” condition.
Overall our investigation on bilateral occlusal movements support the results of others describing a representation map of primary and secondary motor areas [Onozuka et al., 2002; Shinagawa et al., 2004; Tamura et al., 2003], thalamus and cerebellar hemispheres [Onozuka et al., 2002], a frontoparietal network [Takada and Miyamato, 2004], bilateral insula [Onozuka et al., 2002], and cingulate cortex [Otsuka et al., 2009]. Additionally, a network consisting of prefrontal (BA 9, BA 46), inferior parietal (BA 40) and posterior cerebellar areas have been associated with attentional control during voluntarily performed motor tasks [Geier et al., 2007]. An overall increase in dorsolateral prefrontal activation during chewing has been associated with working memory processing [Hirano et al., 2008].
Lateralization and Somatotopy
Our “individually based ROI‐analysis” confirmed previous data of a left hemispheric lateralization of bilateral occlusion in M1 and S1 of the dominant left hemisphere in healthy participants [Foki et al., 2007]. This important result cannot be verified by a conventional voxel‐based group analysis. However, both evaluation methods yielded almost comparable results with respect to the localization of the activation maxima. Additionally, the representation maxima between conditions (“splint” versus “bilateral”)—as tested with the individually based ROI‐analysis—were not different, suggesting a similar representation pool for both conditions in the pre‐ and postcentral gyri. Overall, the representation maxima of bilateral occlusion in the bilateral precentral gyrus were located in the somatotopic height between lip and tongue representation on average about 10‐mm Euclidian distance from the representation maxima of pursing the lip and 16 mm from moving the tip of the tongue [compared with Lotze et al., 2000].
Changes of Sensorimotor Activity by Using the Splint
Motor activation and sensorimotor feedback decrease when using an individually fitted splint. This finding was quite robust in both evaluation methods. The economization of sensorimotor activation by the usage of the splint might help to decrease mastication associated motor activity. It has been demonstrated that increasing muscular force increases the activation magnitude in M1, S1, PMC, SMA, the MCC and the anterior cerebellar hemisphere in a linear mode [e.g., Cramer et al., 2002; Dettmers et al., 1995; van Duinen et al., 2008]. Comparable tight associations have recently been described for the direct evaluation of the muscular output (electromyography) and fMRI activation [Sehm et al., 2010]. Additional decrease of activation during the usage of an occlusal splint in the medial cingulate cortex and the SMA, underline that the effort of occlusion with a splint might be decreased in relation to movements without the splint. It has been demonstrated that increased mental effort without an actual impact on motor performance is highly associated for instance in older subjects with activation in the SMA but also with increases in bilateral sensorimotor activity [Mattay et al., 2002]. Because the temporal characteristics between conditions were kept constant, we would rather suggest that BA 6 activation decrease is associated with an unspecific effort effect but not with special functional contribution of the SMA for instance in temporal sequencing of the task [Lang et al., 1991]. Furthermore, the supramarginal gyrus showed decreased activation when using the splint, which might be associated with the decreased somatosensory response by less occlusal contact, but also by a decrease of temporomandibular movement amplitude. The inferior parietal cortex is associated with movements in relation to one's own body [Halsband et al., 2001] and seems to be more critically involved when bilateral occlusion is not restricted by a splint. More generally, the inferior parietal cortex has been associated with the integration of sensory and motor signals for sensory guidance of movements [Fogassi and Luppino, 2005] also utilizing somatosensory information [Jancke et al., 2001].
However, in the present study a decrease of sensorimotor activation seems to be limited on the cortex and was not observed in the cerebellar anterior hemisphere. Particularly the spinocere‐bellum is concerned with sensorimotor tasks [Gao et al., 1997]. This area showed increased activation during occlusal movements with the splint. Our finding points to an increased sensorimotor integration effort by using the splint without longer training. This increased sensorimotor integration might also be associated with increased temporo‐parieto‐occipital activation observed during usage of the splint. When interacting with a strange object in one's mouth haptic exploration might interfere with visual associations. This has been described for the right occipital lobe already by other investigators during vibrotactile stimulation of the teeth [Ettlin et al., 2004]. They discussed that this activation might be associated with visual coassociations during unusual somatosensory feedback. It fits quite well in this concept, that tactile exploration of objects has been also reported to involve visual representation in BA 18 [Deibert et al., 1999]. However, a satisfactory functional interpretation of this representation site usually associated with visual feedback is not possible in this state of research.
Interestingly, the usage of a splint decreased the activation in the anterior insula cortex, an area which is highly interconnected to limbic and prefrontal regions. The anterior insula is involved in autonomic reactions, affective‐motivational functions, and the association of emotions with former painful experiences [Dubé et al., 2009]. The subjects investigated here did not show pain syndromes during occlusion. Therefore, a more detailed differentiation of anterior insular activation in patients with occlusal dysfunction might be highly interesting.
An increased representation using the individually fitted occlusal splint generally might be associated with the nontrained and unusual condition of moving with the splint. This question can only definitely be solved when subjects before and after training with an occlusal splint are investigated. However, areas increasingly involved with the splint are not those areas which are associated with increased effort during task performance. In contrast, these areas are predominantly associated with complex retrieval of haptic information [BA 10 and temporoparietal areas; Stock et al., 2009] which might be associated with the splint but also with sensorimotor interaction [cerebellum; Gao et al., 1997].
Limitations
With respect to the decrease of BOLD‐signal magnitude with splint use, it has to be considered that the splint is reducing the amplitude of the movement which might have additionally influenced BOLD‐magnitude in M1. In addition, a global activation decrease might be less for somatosensory areas than motor areas since there is additional somatosensory stimulation with the splint. However, there seems to be an additional effect beyond that since the cerebellar representation magnitude for the hemispheric ROI was overall not altered whereas activation in the spinocerebellum was increased.
Additionally, a change of movement pattern might also induce differential forces in the temporomandibular joint. This might be actually one effect induced by behavioral feedback therapy, which has been demonstrated to show equivalent therapeutic results to the usage of occlusal splints [Luther et al., 2010]. However, there is probably a modulation of the splint for movements near the occlusal contact plane since this end of the movement range is highly dependent on the surface of the teeth. Furthermore, the splint has an impact on the movement pattern and the EMG‐amplitude and therefore even a control of both parameters during scanning might not ensure a comparable movement performance with and without a splint.
At the least, different patterns of the jaw movement might induce different head movements which might also have an impact on the statistical results of the fMRI‐analysis. However, after testing possible differences of head movements between both conditions we saw no significant effect.
CONCLUSIONS
The use of an occlusional maxillar splint resulted in a significant reduction in the cerebral representation of the temporomandibular tapping movements. For the ROI‐analysis this reduction was significant for the bilateral primary and secondary somatosensory cortices and the primary motor cortex but not for the cerebellar ROI. However, somatotopic representation of mastication in M1 and S1 was not altered by splint use. The splint increased activation in the prefrontal lobe and in areas associated with somatosensory integration and precise differentiation of the unusual object in the participant's mouth. This somatosensory training and the motor economization might be associated with the therapeutic effect of occlusal splits for temporomandibular disorder. Further investigations on these patients and longer training periods with occlusal splints are in progress.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Tables.
Acknowledgements
The authors thank Flavia Di Pietro for carefully checking the manuscript for the English language.
REFERENCES
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H ( 1999): A fronto‐parietal circuit for object manipulation in man: Evidence from an fMRI‐study. Eur J Neurosci 11: 3276–3286. [DOI] [PubMed] [Google Scholar]
- Botelho AL, Silva BC, Gentil FH, Sforza C, da Silva MA ( 2010): Immediate effect of the resilient splint evaluated using surface electromyography in patients with TMD. Cranio 28: 266–273. [DOI] [PubMed] [Google Scholar]
- Carlsson GE ( 2009): Critical review of some dogmas in prosthodontics. J Prosthodont Res 53: 3–10. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Weisskoff RM, Schaechter JD, Nelles G, Foley M, Finklestein SP, Rosen BR ( 2002): Motor cortex activation is related to force squeezing. Hum Brain Mapp 16: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibert E, Kraut M, Kremen S, Hart J ( 1999): Neural pathways in tactile object recognition. Neurology 52: 1413–1417. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS ( 1995): Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol 74: 802–815. [DOI] [PubMed] [Google Scholar]
- Doyon J, Owen AM, Petrides M, Sziklas V, Evans AC ( 1996): Functional anatomy of visuomotor skill learning in human subjects examined with positron emission tomography. Eur J Neurosci 8: 637–648. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG ( 2002): Experience‐dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA 99: 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé AA, Duquette M, Roy M, Lepore F, Duncan G, Rainville P ( 2009): Brain activity associated with the electrodermal reactivity to acute heat pain. Neuroimage 45: 169–180. [DOI] [PubMed] [Google Scholar]
- Ekberg E, Sabet ME, Petersson A, Nilner M ( 1998): Occlusal appliance therapy in a short‐term perspective in patients with temporomandibular disorders correlated to condyle position. Int J Prosthodont 11: 263–268. [PubMed] [Google Scholar]
- Ettlin DA, Zhang H, Lutz K, Järmann T, Meier D, Gallo LM, Jäncke L, Palla S ( 2004): Cortical activation resulting from painless vibrotactile dental stimulation measured by functional magnetic resonance imaging (FMRI). J Dent Res 83: 757–761. [DOI] [PubMed] [Google Scholar]
- Ettlin DA, Mang H, Colombo V, Palla S, Gallo LM ( 2008): Stereometric assessment of TMJ space variation by occlusal splints. J Dent Res 87: 877–881. [DOI] [PubMed] [Google Scholar]
- Ferrario VF, Sforza C, Tartaglia GM, Dellavia C ( 2002): Immediate effect of a stabilization splint on masticatory muscle activity in temporomandibular disorder patients. J Oral Rehabil 29: 810–815. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Luppino G ( 2005): Motor functions of the parietal lobe. Curr Opin Neurobiol 15: 626–631. [DOI] [PubMed] [Google Scholar]
- Foki T, Geissler A, Gartus A, Pahs G, Deecke L, Beisteiner R ( 2007): Cortical lateralization of bilateral symmetric chin movements and clinical relevance in tumor patients—A high field BOLD‐FMRI study. Neuroimage 37: 26–39. [DOI] [PubMed] [Google Scholar]
- Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT ( 1996): Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science 272: 545–547. [DOI] [PubMed] [Google Scholar]
- Geier CF, Garver KE, Luna B ( 2007): Circuitry underlying temporally extended spatial working memory. Neuroimage 35: 904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese R, Lazar NA, Nichols Th ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discorvery rate. NeuroImage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Grodd W, Hülsmann E, Lotze M, Wildgruber D, Erb M ( 2001): Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp 13: 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsband U, Schmitt J, Weyers M, Binkofski F, Grützner G, Freund HJ ( 2001): Recognition and imitation of pantomimed motor acts after unilateral parietal and premotor lesions: A perspective on apraxia. Neuropsychologia 39: 200–216. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Obata T, Kashikura K, Nonaka H, Tachibana A, Ikehira H, Onozuka M ( 2008): Effects of chewing in working memory processing. Neurosci Lett 436: 189–192. [DOI] [PubMed] [Google Scholar]
- Jancke L, Kleinschmidt A, Mirzazade S, Shah NJ, Freund HJ ( 2001): The role of the inferior parietal cortex in linking the tactile perception and manual construction of object shapes. Cereb Cortex 11: 114–121. [DOI] [PubMed] [Google Scholar]
- Jellema T, Baker CI, Wicker B, Perrett DI ( 2000): Neural representation for the perception of the intentionality of actions. Brain Cogn 44: 280–302. [DOI] [PubMed] [Google Scholar]
- Lang W, Cheyne D, Kristeva R, Beisteiner R, Lindinger G, Deeke L ( 1991): Three‐dimensional localization of SMA activity preceding voluntary movement: A study of electric and magnetic fields in a patient with infarction of the right SMA. Exp Brain Res 87: 688–695. [DOI] [PubMed] [Google Scholar]
- Lotze M, Seggewies G, Erb M, Grodd W, Birbaumer N ( 2000): The representation of articulation in the primary sensorimotor cortex. NeuroReport 11: 2985–2989. [DOI] [PubMed] [Google Scholar]
- Luther F, Layton S, McDonald F ( 2010): Orthodontics for treating temporomandibular joint (TMJ) disorders. Cochrane Database Syst Rev 7: CD006541. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR ( 2002): Neurophysiological correlates of age‐related changes in human motor function. Neurology 58: 630–635. [DOI] [PubMed] [Google Scholar]
- Ommerborn MA, Kollmann C, Handschel J, Depprich RA, Lang H, Raab WH ( 2010): A survey on German dentists regarding the management of craniomandibular disorders. Clin Oral Invest 14: 137–144. [DOI] [PubMed] [Google Scholar]
- Onozuka M, Fujita M, Watanabe K, Hirano Y, Niwa M, Nishiymana K, Saito S ( 2002): Mapping brain region activity during chewing: A functional magnetic resonance imaging study. J Dent Res 81: 743–746. [DOI] [PubMed] [Google Scholar]
- Otsuka T, Watanabe K, Hirano Y, Kubo K, Miyake S, Sato S, Sasaguri K ( 2009): Effects of mandibular deviation on brain activation during clenching: An fMRT preliminary study. J Craniomand Practice 27: 88–93. [DOI] [PubMed] [Google Scholar]
- Ruben J, Schwiemann J, Deuchert M, Meyer R, Krause T, Curio G, Villringer K, Kurth R, Villringer A ( 2001): Somatotopic organization of human secondary somatosensory cortex. Cereb Cortex 11: 463–473. [DOI] [PubMed] [Google Scholar]
- Sehm B, Perez MA, Xu B, Hidler J, Cohen LG ( 2010): Functional neuroanatomy of mirroring during a unimanual force generation task. Cereb Cortex 20: 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa H, Ono T, Honda E, Sasaki T, Taira M, Iriki A, Kuroda T, Ohyama K ( 2004): Chewing‐side preference is involved in differential cortical activation patterns during tongue movements after bilateral gum‐chewing: A functional magnetic resonance imaging study. J Dent Res 83: 762–766. [DOI] [PubMed] [Google Scholar]
- Stock O, Röder B, Burke M, Bien S, Rösler F ( 2009): Cortical activation patterns during long‐term memory retrieval of visually or haptically encoded objects and locations. J Cogn Neurosci 21: 58–82. [DOI] [PubMed] [Google Scholar]
- Takada T, Miyamoto T ( 2004): A fronto‐parietal network for chewing of gum: A study on human subjects with functional magnetic resonance imaging. Neurosci Lett 360: 137–140. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain. New York: Thieme. [Google Scholar]
- Tamura T, Kanayama T, Yoshida S, Kawasaki T ( 2003): Functional magnetic resonance imaging of human jaw movements. J Oral Rehabil 30: 614–622. [DOI] [PubMed] [Google Scholar]
- Tecco S, Tetè S, D'Attilio M, Perillo L, Festa F ( 2008): Surface electromyographic patterns of masticatory, neck, and trunk muscles in temporomandibular joint dysfunction patients undergoing anterior repositioning splint therapy. Eur J Orthod 30: 592–597. [DOI] [PubMed] [Google Scholar]
- Türp JC, Komine F, Hugger A ( 2004): Efficacy of stabilization splints for the management of patients with masticatory muscle pain: A qualitative systematic review. Cin Oral Invest 8: 179–195. [DOI] [PubMed] [Google Scholar]
- van Duinen H, Renken R, Maurits NM, Zijdewind I ( 2008): Relation between muscle and brain activity during isometric contractions of the first dorsal interosseus muscle. Hum Brain Mapp 29: 281–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Tables.


