Abstract
Electroencephalography (EEG) can directly monitor the temporal progression of cortical changes induced by repetitive Transcranial Magnetic Stimulation (rTMS) and facilitate the understanding of cortical and subcortical influences in the genesis of oscillations. In this combined rTMS/EEG study, we aimed to investigate changes in oscillatory activity after high‐frequency (∼11 Hz) rTMS relative to the number of applied pulses. Twenty intermittent trains of 20 or 60 rTMS pulses were delivered over the human primary motor cortex at rest and tuned to individual mu frequency. The regional and interregional oscillatory neural activity after stimulation were evaluated using event‐related power (ERPow) and event‐related coherence (ERCoh) transformations. The most prominent changes for ERPow were observed in the theta band (4–7 Hz), as an increase in ERPow up to 20 s following 60 rTMS pulses, whereas ERPow increases were smaller in mu (10–12 Hz) and beta (13–30 Hz). ERCoh revealed that rTMS 60 modulated the connectivity in the theta band for up to 20 s. The topography of mu and theta changes were not identical; mu was more focal and theta was more global. Our data suggested the presence of independent cortical theta and mu generators with different reactivity to rTMS but could not rule out possible thalamocortical contributions in generating theta and mu over the motor network. Hum Brain Mapp 33:2224–2237, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: EEG‐TMS combination, event‐related power, event‐related coherence, motor cortex, oscillations, synaptic plasticity
INTRODUCTION
Recent findings indicate that network oscillations through various brain rhythms temporally link neurons into assemblies and facilitate synaptic plasticity, but the exact mechanisms remain uncertain [Buzsáki and Draguhn, 2004; Hutcheon and Yarom, 2000]. Much of what we know about network oscillations and brain rhythms comes from animal studies [Buzsáki, 2004; Steriade, 2006]. Understanding the physiology of neuronal oscillations in humans will provide insight into the role of brain rhythms and may assist in the diagnosis and treatment of brain disorders [Miniussi and Thut, 2010; Thut and Pascual‐Leone, 2010].
A noninvasive method that can help uncover the mechanisms of human brain oscillations in specific frequency bands is the combination of repetitive Transcranial Magnetic Stimulation (rTMS) and Electroencephalography (EEG). rTMS allows transient modulation of cortical excitability with effects lasting beyond its application, emulating the patterns of synaptic plasticity in the hippocampus [Gilio et al., 2009; Hallett, 2007]. Unlike other neuroimaging tools such as functional magnetic resonance imaging (fMRI) or positron emission tomography (PET) that rely on hemodynamic responses, EEG records neural activity directly with excellent temporal resolution in the range of milliseconds [Sauseng and Klimesch, 2008]. Therefore, rTMS‐EEG integration has the potential to provide real‐time information on the dynamics of human brain oscillations and the role of brain rhythms. It also has the potential to help us understand the neurophysiological basis for the effects of rTMS which still baffles neuroscientists [Komssi and Kahkonen, 2006; Thut and Miniussi, 2009].
There are limited human electrophysiological studies highlighting the relationship between the number of rTMS pulses applied and the EEG after‐effect‐size although combination of TMS‐EEG can provide more robust and sensitive cortical read‐out than behavioural measurements [Miniussi and Thut, 2010; Thut and Pascual‐Leone, 2010]. Recently, Thut and Pascual‐Leone [ 2010] performed a meta‐analysis of many previous TMS‐EEG studies. Their results suggested linear correlations between EEG after‐effect‐size and total number of TMS pulses applied in high‐frequency protocols (5–20 Hz rTMS). However, there is no high‐frequency rTMS‐EEG study so far that examines EEG oscillatory phenomena in the healthy human brain by manipulating the number of applied magnetic pulses within a train of stimulation while holding all other TMS parameters constant. To date, Brignani et al. [ 2008] has investigated the cumulative effects of the duration of magnetic stimulation on human EEG oscillatory activity by using a low frequency TMS protocol (1 Hz). Their results showed that modulation of cortical oscillations increased linearly with the duration of stimulation, with higher synchronization seen in alpha (8–12 Hz) more than beta (12–30 Hz) brain rhythms [Brignani et al., 2008]. Moreover, studies involving delivery of high‐frequency stimulation manipulating the number of applied magnetic pulses have tended to focus on indirect measurement of cortical read‐out such as MEP with contradictory results [Fitzgerald et al., 2006]. Several studies have shown an increase in MEP size after high‐frequency magnetic stimulation dependent on the number of pulses [Maeda et al., 2000; Modugno et al., 2001; Peinemann et al., 2004], while other studies showed no effects on MEP size regardless of the total number of pulses [Daskalakis et al., 2006; Romeo et al., 2000; Quartarone et al., 2005; Wassermann et al., 1996].
In this article, we first aimed to investigate the EEG oscillatory activity in healthy human brains induced by rTMS trains of different numbers of pulses while holding all other TMS parameters constant. Thus, we delivered rTMS at individual μ‐frequency (∼11 Hz) at 100% resting motor threshold over left human primary motor cortex (M1) and manipulated rTMS along one dimension: the number of applied pulses for each train of magnetic stimulation. We used twenty intermittent trains of 20 rTMS pulses (rTMS 20), and twenty intermittent trains of 60 rTMS pulses (rTMS 60). Our second aim was to look at the presence of cumulative effects defined as the state at which later portions of an experimental session may differ from early portions as a function of the number of applied TMS pulses [Hamidi et al., 2011] across different rTMS trains. To address this question, we compared the first ten intermittent trains of stimulation with the subsequent 10 intermittent trains of stimulation, for both rTMS 20 and rTMS 60 protocols, respectively. EEG was analyzed in terms of immediate responses up to 60 s after each magnetic train. EEG responses were evaluated using spectral analysis of event‐related power (ERPow) and event‐related coherence (ERCoh) transformations, which reflect the regional neural activity and the interregional functional coupling between cortical areas, respectively.
We hypothesized that trains with a larger number of applied pulses (rTMS 60) would produce a larger EEG power modulation compared with trains of fewer pulses (rTMS 20). In an elegant experiment combining TMS‐EEG, Rosanova et al. [ 2009] demonstrated that each cortical area tended to preserve its own natural frequency; TMS consistently evoked dominant alpha oscillations in the occipital cortex, beta oscillations in the parietal cortex and fast beta/gamma oscillations in the frontal cortex. Therefore, we hypothesized that the dominant frequency in our study would be mu (10–12 Hz) since the stimulation was given over the M1 at rest and tuned to each participant's Rolandic mu rhythm, i.e., the natural frequency of the resting motor cortex. Mu is a distinct alpha frequency rhythm that is present over central cortical areas during quiet wakefulness. It is generally accepted as the idling rhythm derived from the synchronized neurons involved in the thalamo‐cortical loop and shown to be blocked by motor movements or somatosensory stimuli [Pfurtscheller et al., 2006; Pfurtscheller and Lopes da Silva, 1999]. Taken as a whole, during high‐frequency magnetic stimulation, we expected that trains with a greater number of pulses would be more effective than trains with fewer pulses in producing pronounced cumulative effects.
METHODS
Subjects and Experimental Design
Twelve healthy volunteers (6 men, 6 women; mean age 22.18 ± 1.07 years) with no history of neurological disorder or head injury participated in the study. All subjects were right‐handed as assessed by the Edinburgh handedness inventory [Oldfield, 1971]. Written informed consent was given by all subjects in accordance with the declaration of Helsinki. The study was approved by the Local Ethics Committee.
Subjects were seated in a comfortable armchair with elbows flexed at 90°, hands pronated in a relaxed position, eyes opened, watching a computer screen. At the beginning of the experimental session, 3 min of EEG recordings were taken at rest. Fast‐Fourier Transform (FFT) was performed looking at the discrete frequency with the maximum power within the range of mu rhythm (8–13 Hz) at C3 electrode, which is covering the activity of the pericentral (Rolandic) oscillatory phenomena. High‐frequency rTMS was then delivered at the frequency of individual's mu rhythm (mean 11.05 Hz ± 0.56) over the left M1 at 100% resting motor threshold (RMT) simultaneously with multichannel EEG recording. The spectral distribution of the mu rhythm usually has an average peak of 10–11 Hz in healthy adults which appear maximally over the central Rolandic or sensorimotor area during a relaxed state, but the frequency varies among individuals [Pfurtscheller and Lopes da Silva, 1999]. Therefore, in this study, we decided to deliver the trains of rTMS at the frequency of individual mu rhythm to make sure that this parameter of rTMS was constant across all subjects. This takes into account the individual differences in the natural frequency of the resting motor cortex so that the interpretation of the findings is related only to the manipulation of the number of applied rTMS pulses. Each subject underwent three experimental conditions: 20 intermittent trains of 20 pulses (400 stimuli, rTMS 20), 20 intermittent trains of 60 pulses (1200 stimuli, rTMS 60) and sham rTMS with 20 trains of 20 pulses (400 stimuli). Because event‐related changes in ongoing EEG need time to develop and to recover, the inter‐train‐interval (ITI) was 68 s for all experimental conditions. The order of presentation of the three experimental conditions was counterbalanced across participants to avoid order effects. Each experimental condition was followed by a 20‐min rest before the next administration to avoid carry‐over effects from one experimental condition to the next. Fitzgerald et al. [ 2007] demonstrated that multiple short‐trains of high‐frequency rTMS did not modify the cortical excitability 15 min poststimulation, supporting the view of an absence of a post‐train effect. However, Thut and Pascual‐Leone [ 2010] in a review of combined TMS‐EEG studies, showed a lasting effect of a variety of rTMS protocols on EEG measurement. Therefore, to rule out possible carry‐over effects between the experimental conditions, we compared the different baseline periods in‐between protocols. Baseline MEPs were also recorded before and after each experimental condition to assess conditioning effects of rTMS. Figure 1 shows the experimental paradigm.
Figure 1.
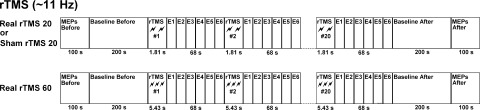
Experimental design: the study design consisted of three experimental conditions of twenty intermittent trains of 20 or 60 high frequency (∼11 Hz) rTMS pulses delivered over the human primary motor cortex at rest (rTMS 20, rTMS 60, and sham rTMS 20). It comprised epochs of EEG recorded continuously before, during, and after the trains of stimulation (Baseline Before, E1, E2, E3, E4, E5, E6, and Baseline After, respectively). MEPs were also recorded at the beginning and at the end of each experimental condition.
In this study, by applying trains of rTMS at 11 Hz for 5.36 s with 60 pulses, we slightly exceeded the parameters of stimulation suggested by the safety and ethical guidelines for the use of TMS in clinical practice and research [Rossi et al., 2009; Wassermann, 1998]. In the guidelines, the maximum duration of a train of rTMS at 10 Hz and 100% MT is fixed at 5 s with 50 pulses. Our choice was made to triple the length of each train of stimulation (60 vs 20 pulses) and the total number of applied active pulses (1200 vs. 400 pulses) between the two experimental conditions of rTMS 60 and rTMS 20. The most serious side effect of using high‐frequency rTMS is the potential induction of a seizure, especially in the case of neurological disorders. All the participants of our study were healthy with no history of neurological disorders or head injury. In addition, to be sure that there was no sign of impending seizure, we were constantly monitoring the participant's EEG during the whole experimental session.
TMS Procedure and MEP Data Acquisition
TMS was performed using a high‐power Magstim‐Rapid stimulator (Magstim, Whitland, UK). The magnetic stimulus had a biphasic waveform with a pulse width of 300 μs. TMS was delivered through a figure‐of‐eight coil (70 mm standard coil; Magstim), oriented so that the induced electric current flowed in a posterior–anterior direction over the underlying motor cortex. The coil was placed tangentially to the scalp with the handle pointing backward and laterally at a 45° angle away from the midline perpendicular to the line of the central sulcus to achieve the lowest motor threshold.
MEPs were recorded from the right thenar eminence (TE) muscle using Ag/AgCl surface electrodes in a belly‐tendon montage. The amplified and bandpass‐filtered (50 Hz–5 kHz) EMG signal was fed into a Basis Esaote Machine (Esaote Company, Florence, Italy) at a sampling rate of 5000 Hz. The optimal position for right TE activation was determined by moving the coil in 0.5‐cm steps around the motor hand area of the left motor cortex. The optimal position was defined as the site where stimuli of slightly suprathreshold intensity consistently produced the largest MEPs with the steepest negative slope in the target muscle (referred to as “motor hot spot”). The individual RMT was determined by reducing the stimulus intensity in 1% steps. RMT was defined as the lowest stimulus intensity to produce minimum five MEPs of at least 50 μV in ten successive stimuli [Rossini et al., 1994]. The MEPs recorded from the right TE were computed as the amplitude between the two largest peaks of opposite polarity after 20 ms from the TMS pulse. To assess conditioning effects of rTMS, in this study, we delivered 10 single‐pulse TMS within 100 s at the beginning and at the end of each of the three experimental conditions. The intensity of single‐pulse TMS was set to 120% of individual RMT.
The participants were naive to the differences between sham and active rTMS before the study. The sham condition was performed with an intensity of 100% RMT with the coil tilted at 90° to the skull in order to avoid real stimulation to the motor cortex. Finding a valid sham condition in rTMS research is vital to produce valid outcomes [Arana et al., 2008; Herwig et al., 2010]. Better devices that can provide sensory artefacts by electrical stimulation and can mimic the effects of magnetic stimulation are not yet available [Rossi et al., 2007]. A recent study by Herwig et al. [ 2010] highlighted that using a “real” coil with a modified stimulation condition such as angling and dislocating the coil and reducing the stimulation intensity can be used for a reliable sham condition in randomized rTMS trials.
EEG Data Acquisition and Analysis
The EEG data were acquired using a MR compatible EEG amplifier (SD MRI 32, Micromed, Treviso, Italy) and a cap providing 30 Ag/AgCl electrodes positioned according to a 10/20 system with impedance kept below 10 kΩ. The activities in the right (TE) muscle and in the right eye vertical electroculogram (vEOG) were bipolarly registered from two surface electrodes in two EMG channels. The reference electrode was placed anterior to Fz, and the ground electrode posterior to Fz as in previous studies using the same system [Formaggio et al., 2008; Fuggetta et al., 2008]. To ensure subjects' safety, the wires were carefully arranged to avoid loops and physical contact with the subject. To avoid electrical saturation of EEG channels induced by TMS, the EEG amplifier had a resolution of 22 bits with a range of ±25.6 mV and an antialiasing hardware band‐pass filter was applied with a bandwidth between 0.15 and 269.5 Hz. EEG data were sampled at a frequency of 1024 Hz using the software package SystemPlus (Micromed, Treviso, Italy).
EEG data were analyzed with commercial software (Vision Analyzer, BrainVision) to characterize rTMS‐induced effects. The EEG analyses started at 1 s after magnetic stimulation to avoid large TMS artefacts contaminating the EEG signal. Data were segmented into temporal windows of equal length (1000 ms containing 1024 data points) for eight time intervals. The intervals were as follows: Reference or baseline (200 s) before trains of stimulation, first epoch (1–5 s), second epoch (6–10 s), third epoch (11–15 s), fourth epoch (16–20 s), fifth epoch (36–40 s), sixth epoch (56–60 s), baseline (200 s) after trains of stimulation. For all experimental conditions (rTMS 20, rTMS 60, and sham rTMS 20), each epoch consisted of 80 trials. EEG signals were filtered (1–40 Hz, slope 24 dB/octave) and a notch filter (50 Hz) applied to all channels. A semi‐automatic epoch inspection‐rejection procedure was applied to remove TMS artefacts, muscle or EOG activity. Segments with values outside the range of ±70 μV were rejected for semiautomatic epoch rejection criterion. A mean of 51.0 ± 17.8 of clean data were extracted from each experimental condition.
Power spectra analyses were performed using FFT for all frequency bins between 1 and 40 Hz (1 Hz of maximum bin width). Recordings were Hamming‐windowed to control spectral leakage. Broad‐band power changes were obtained by averaging the power values for theta (4–7 Hz), mu (10–12 Hz) and beta (13–30 Hz) frequency ranges chosen for analysis. The output data were imported into Microsoft Excel, to calculate: (i) event‐related power (ERPow) and (ii) event‐related coherence (ERCoh). EEG power changes were quantified using event‐related desynchronization and event‐related synchronization (ERD/ERS) using a standard formula: [(band power after rTMS) − (band power baseline before)/(band power baseline before) × 100]. ERCoh was obtained by subtracting ERCoh baseline before from ERCoh after rTMS [Pfurtscheller and Andrew, 1999; Fuggetta et al., 2008]. The baseline band power before stimulation was used as a reference.
To provide valuable information on effect duration, and whether there was a carry‐over effect in the experimental conditions, we compared the different baseline values in‐between protocols. ERPow values were submitted to repeated measures analysis of variance (ANOVAs) for theta (4–7 Hz), mu (10–12 Hz) and beta (13–30 Hz) frequencies, respectively. Two factors were tested within subjects: ‘time’ (baseline before the first rTMS condition, baseline before the second rTMS condition, and baseline before the third rTMS condition) and “electrode” (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4).
Both event‐related transformations were submitted to repeated measures ANOVAs for the three frequency bands of theta (4–7 Hz), mu (10–12 Hz) and beta (13–30 Hz) to test the relationship between EEG after‐effect‐size and the number of rTMS applied pulses. Four factors were tested within subjects. These factors were: “condition” (rTMS 20 pulses, rTMS 60 pulses, sham rTMS 20 pulses); “epoch” (epoch one to six); “part” (part A, during the first 10 trains of stimulation; part B, during the subsequent 10 trains of stimulation) and “electrode” (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4). Cumulative effects induced by rTMS were examined by looking at the difference in EEG oscillations between part A and part B for the rTMS protocols (rTMS 20 part A, 200 stimuli; rTMS 20 part B, 400 stimuli; rTMS 60 part A, 600 stimuli; rTMS 60 part B, 1200 stimuli). For each ANOVA, the sphericity assumption was assessed with Mauchly's test. Greenhouse‐Geisser epsilon adjustments for nonsphericity were applied where appropriate. Posthoc paired t‐tests (adjusted for multiple comparisons using the Bonferroni method) were used for significant main effects and interactions of ANOVAs. P < 0.05 was considered significant for all statistical tests.
MEPs Analysis
The average of the 10 MEP pulses before and after each of the three experimental conditions was used for statistical analysis of MEPs to assess conditioning effects of rTMS. Mean MEP peak‐to‐peak amplitudes (mV) and latencies (ms) were normalized with the baseline before and submitted to repeated measures ANOVAs with the factor of “condition” (rTMS 20 pulses, rTMS 60 pulses, sham rTMS 20 pulses).
RESULTS
The initial sample consisted of twelve healthy volunteers of whom eleven subjects were considered suitable for reliable EEG analysis. We removed one subject's data due to an excessive number of blinks and muscle activities that drastically decreased the amount of data needed to obtain a reliable spectral estimate. None of the participants had any adverse side effects during the experiment. RMT for subjects ranged from 65 to 89% of maximum stimulator output with mean of 77.3% ± 8.5. The statistical analyses performed to rule out carry‐over effects in‐between the three rTMS protocols, did not show any significant main effect of “time” or two‐way interaction “time × electrode” for the three frequency bands of theta, mu and beta, respectively. Theta [“time” (F 2,20 = 1.23; P = 0.32, η p 2 = 0.1) and “time × electrode” (F 16,160 = 0.18; P = 1.0, η p 2 = 0.02)]; mu [“time” (F 1.16,11.6 = 1.74; P = 0.22, η p 2 = 0.15) and “time × electrode” (F 16,160 = 1.67; P = 0.06, η p 2 = 0.1)]; beta [“time” (F 2,20 = 0.36; P = 0.703, η p 2 = 0.04) and “time × electrode” (F 16,160 = 1.09; P = 0.37, η p 2 = 0.1)].
Event Related Power in the Theta Band
Figure 2 shows the grand average of ERPow for theta band (4‐7 Hz). The ANOVA showed the following statistically significant interactions: “condition × epoch” (F 2,24 = 10.4; P < 0.001, η p 2 = 0.5) and “condition × epoch × electrode” (F 80,800 = 3.16; P < 0.001, η p 2 = 0.2). Post‐hoc comparisons for the significant two‐way interaction of “condition × epoch” revealed that rTMS 60 had a significant increase in power compared to sham until epoch four, up to 20 s after each train of stimulation (Fig. 2A). Epoch five and six did not revealed significant results. The highest synchronization was during epoch one up to 5 s after the train of stimulation for rTMS 60 versus rTMS 20 and sham (120.0 vs. 76.87, 17.8%). Post‐hoc comparisons for “condition × epoch × electrode” exhibited a greater synchronization for rTMS 60 compared to rTMS 20 and sham across all electrodes until 20 s post stimulation (Fig. 2B1–B4). C3 was the most sensitive electrode in epoch one with higher cortical oscillations for rTMS 60 versus sham (287.88 vs. 74.64%) and rTMS 20 versus sham (223.19 vs. 74.64%) (Fig. 2B1).
Figure 2.
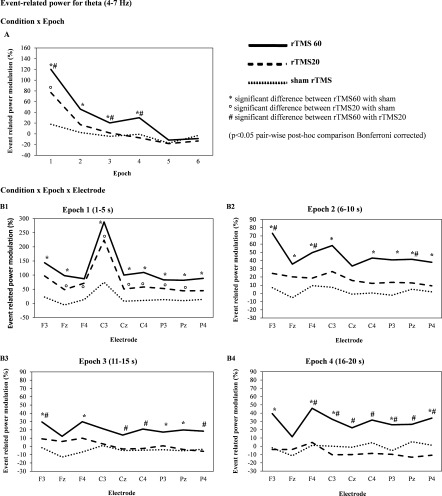
Grand average of event‐related power (ERPow) transformation (n = 11) for theta (4–7 Hz) analyzed as a function of the experimental conditions (rTMS 20, rTMS 60, and sham rTMS 20), electrodes (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4) and epoch of time [first epoch (1–5 s), second epoch (6–10 s), third epoch (11–15 s), fourth epoch (16–20 s), fifth epoch (36–40 s), sixth epoch (56–60 s)] after each train of magnetic stimulation. ‘Condition x Epoch’ showed higher power modulation for rTMS 60 compared to rTMS 20 and sham. ‘Condition x Epoch x Electrode’ demonstrated that rTMS 60 pulses induced higher cortical oscillations of theta (4–7 Hz) for 20 s across all electrodes after high frequency stimulation (∼11 Hz) at rest.
Event Related Power in the mu Band
Figure 3 shows the grand average of ERPow for mu band (10–12 Hz). The ANOVA showed the following statistically significant interactions: “condition × epoch” (F 10,100 = 7.06; P < 0.001, η p 2 = 0.4), “condition × epoch × electrode” (F 80,800 = 1.77; P < 0.001, η p 2 = 0.2), and “condition × epoch × part” (F 16,160 = 3.16; P < 0.001, η p 2 = 0.2). Post‐hoc comparisons for “condition × epoch” showed that rTMS 20 induced a higher EEG oscillation compared to rTMS 60 at epoch one (Fig. 3A). Post‐hoc comparisons for “condition × epoch × electrode” demonstrated a higher synchronization in electrode C3 at epoch one for rTMS 20 versus sham (80.15 vs. 21.5%) and rTMS 60 versus sham (64.0 vs. 21.5%) (Fig. 3B1). At epoch two, rTMS 60 had a significant higher EEG oscillation versus sham rTMS for F3, Fz and P3 (Fig. 3B2). Post‐hoc comparisons for “condition × epoch × part” indicated that for rTMS 20, there was a significant increase in synchronization from part A to B at epoch one (25.28 vs. 40.23%) and two (3.63 vs. 9.64%)(Fig. 3C1). rTMS 60 had a significant increase in synchronization from part A to B at epoch two (4.67 vs. 16.53%) (Fig. 3C2).
Figure 3.
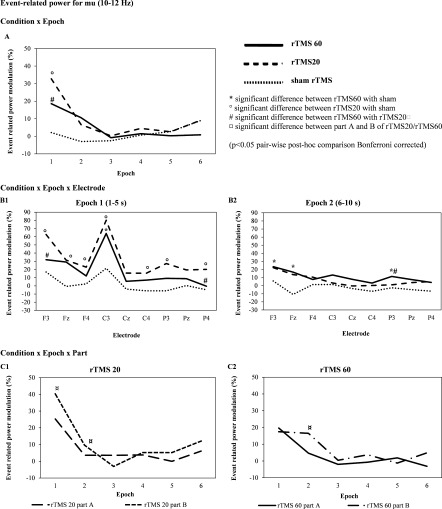
Grand average of event‐related power (ERPow) transformation (n = 11) for mu (10–12 Hz) analyzed as a function of the experimental conditions (rTMS 20, rTMS 60, and sham rTMS 20), electrodes (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4) and epoch of time [first epoch (1–5 s), second epoch (6–10 s), third epoch (11–15 s), fourth epoch (16–20 s), fifth epoch (36–40 s), sixth epoch (56–60 s)] after each train of magnetic stimulation. rTMS 20 pulses induced higher cortical oscillations of mu (10–12 Hz) for 5 s with focal distribution of electrodes after high frequency stimulation (∼11 Hz) at rest. A cumulative effect was seen in mu (10–12 Hz) band where rTMS 20 part B (400 stimuli) induced a higher EEG oscillation compared with rTMS 20 part A (200 stimuli) mainly in epoch one. Shorter trains of rTMS 20 part B (400 pulses) were more efficient at inducing a higher synchronization compared to longer trains of rTMS 60 part B (1200 pulses).
Event Related Power in the Beta Band
Figure 4 shows the grand average of ERPow for mu band (10–12 Hz). The ANOVA showed the following statistically significant interactions: “condition × epoch” (F 10,100 = 4.23; P < 0.001, η p 2 = 0.3) and “condition × epoch × electrode” (F 80,800 = 2.14; P < 0.001, η p 2 = 0.2). Post‐hoc comparisons of “condition × epoch” revealed higher cortical oscillations for rTMS 20 compared to rTMS 60 and sham at epoch one (15.14 vs. 3.65, 2.31%) (Fig. 4A). Post‐hoc analyses of “condition × epoch × electrode” revealed a higher oscillation mainly in epoch one for rTMS 20 compared to sham for C3, Cz, P3 and Pz and rTMS 20 versus rTMS 60 for F4, Cz, C4, Pz, P4 (Fig. 4B1). C3 was the most sensitive electrode at epoch one with higher oscillations for rTMS 20 compared to sham (40.27 vs. 10.0%). Event‐related power modulation of beta was seen up to 20 s post magnetic stimulation but this was less than event‐related power modulation of theta and mu (Fig. 4B1–B4).
Figure 4.
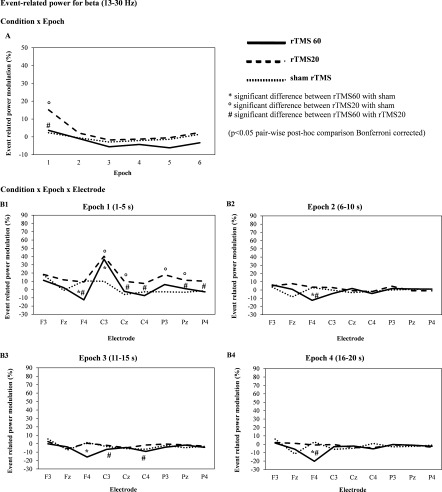
Grand average of event‐related power (ERPow) transformation (n = 11) for beta (13–30 Hz) analyzed as a function of the experimental conditions (rTMS 20, rTMS 60, and sham rTMS 20), electrodes (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4) and epoch of time [first epoch (1–5 s), second epoch (6–10 s), third epoch (11–15 s), fourth epoch (16–20 s), fifth epoch (36–40 s), sixth epoch (56–60 s)] after each train of magnetic stimulation. rTMS 20 pulses induced higher cortical oscillations of beta (13–30 Hz) than sham rTMS for 5 s with widespread distribution across electrodes after high frequency stimulation (∼11 Hz) at rest.
Event Related Coherence in the Theta Band
Figure 5 shows the grand average of ERCoh for theta band (4–7 Hz). The ANOVA showed the following statistically significant interactions: “condition × epoch × electrode” (F 80,800 = 1.55; P < 0.01, η p 2 = 0.1). Post‐hoc comparisons of “condition × epoch × electrode” initially showed a decrease in functional coupling for rTMS 60 compared with rTMS 20 and sham during epoch one [F3], but subsequently a higher coherence for rTMS 60 versus rTMS 20 during epoch two [Fz], epoch three [Fz, F4] and epoch four [Cz, C4] (Fig. 5A1‐4).
Figure 5.
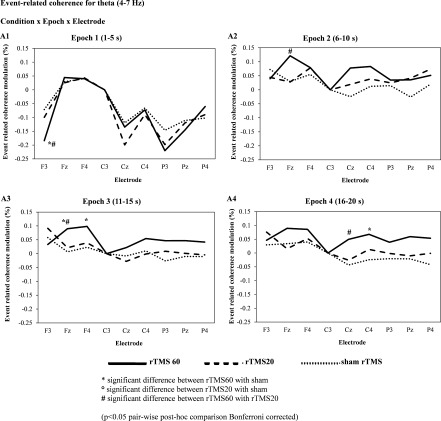
Grand average of event‐related coherence (ERCoh) transformation (n = 11) for theta (4–7 Hz) frequency band of nine electrodes analyzed (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4) referenced to C3 electrode, as a function of the experimental conditions (rTMS 20, rTMS 60, and sham rTMS 20) and epoch of time [first epoch (1–5 s), second epoch (6–10 s), third epoch (11–15 s), fourth epoch (16–20 s), fifth epoch (36–40 s), sixth epoch (56–60 s)] after each train of magnetic stimulation. A decrease of coherence in rTMS 60 for theta band was seen mainly in the frontal region ipsilateral to the stimulation site (coupling between C3 and F3) during the first epoch, 5 s post magnetic stimulation. However, this decrease in functional coupling was followed subsequently by an increase in coherence modulation from epoch two to four, up to 20 s post stimulation in frontal (Fz, F4) and central electrodes (Cz, C4). These results suggest a rebound phenomenon for rTMS 60 during high‐frequency magnetic stimulation.
Event Related Coherence in the mu and Beta Bands
The ANOVA for mu (10–12 Hz) and beta (13–30 Hz) did not show significant results.
Motor Evoked Potentials (MEPs)
The ANOVA for MEPs with normalized amplitude showed no significant main effect of “condition” (F 2,30 = 2.9; P = 0.07, η p 2 = 0.16). The MEPs with normalized latency also did not show significant difference for the main effect of “condition” (F 2,30 = 0.03; P = 0.97, η p 2 = 0.002).
DISCUSSION
The main finding of this study was the higher EEG power modulation of theta (4‐7 Hz) compared to mu (10–12 Hz) and beta (13–30 Hz) during trains of rTMS 60 pulses for 20 s after high frequency stimulation (∼11 Hz) at human primary motor cortex which to our knowledge has not been reported previously. We also found that the topography of mu and theta changes was not identical (mu became more focal and theta became more global) with focal mu enhancement dominating early after the train of stimulation (5 s) versus global theta enhancement which lasted longer (20 s). The negative result was the lack of cumulative lasting after‐effects (more than one minute) on the modulation of cortical oscillations after intermittent trains of high frequency rTMS.
We found that trains of rTMS with 60 pulses produced greater ERPow modulation than shorter trains of 20 pulses, supporting our main hypothesis. However, the dominant frequency was theta (4–7 Hz) instead of mu (10–12 Hz). Previous studies of combined TMS and EEG mostly showed higher EEG after‐effect sizes on the alpha band [Brignani et al., 2008; Jing and Takigawa, 2000; Oliviero et al., 2003; Romei et al., 2008; Sauseng et al., 2009; Zarkowski et al., 2006], or triggered transient cortical modulations in the alpha and/or beta bands [Chen et al., 2003; Fuggetta et al., 2005, 2008; Paus et al., 2001a; Van Der Werf et al., 2006; Van Der Werf and Paus, 2006]. The frequency bands of alpha and beta oscillations were affected more often after sensory or motor cortex stimulation than after dorsolateral prefrontal cortex (DLPFC) stimulation. After‐effects in other frequency bands (theta, delta) were associated more often with DLPFC than sensory or motor cortex stimulation [Thut and Miniussi, 2009]. Schutter et al. [ 2001] performed a study combining rTMS and EEG and found an increase in EEG theta activity. The authors performed low frequency rTMS (1 Hz) at suprathreshold intensities to the right DLPFC for 20 min to look at the subsequent effects on mood. EEG spectral analysis revealed left hemisphere increase in EEG theta activity at 25–35 and 55–65 min after stimulation with a significant reduction in anxiety. They concluded that an increase in contralateral theta activity was associated with reduced anxiety but they did not explore the neurophysiologic basis of rTMS‐induced oscillations.
We propose that the increase in theta power modulation seen in our study might be due to the presence of independent theta generators near the brain surface. Animal studies have demonstrated that theta oscillations in the rat were not confined to the hippocampus but could also be recorded in many sensory regions of the rat cortex [Leung and Borst, 1987; Silva et al., 1991]. External or internal stimuli are able to produce theta oscillations in various part of the brain due to the selectively distributed parallel processing theta system throughout the entire brain [Başar et al., 2001]. Multielectrode intracranial EEG (iEEG) recordings have provided evidence that theta oscillations also occur in the human cortex [Caplan et al., 2001; Kahana et al., 2001; Raghavachari et al., 2006; Rizzuto et al., 2006]. A study by Cantero et al. [ 2003] using depth recordings from epileptic patients showed an absence of coupling between hippocampus and any neocortical region during the emergence of theta oscillations in quiet wakefulness. This observation strongly supports the hypotheses that the synchronous neuronal firing associated with neocortical theta waves in humans is cortically specific and independent from theta waves generated within the hippocampus. It also suggests that human theta does not appear to be restricted to hippocampal sites but appears over widespread regions of neocortex and reflects generators near the surface of the brain [Kahana et al., 2001; Mitchell et al., 2008; Raghavachari et al., 2006]. These cortical theta oscillations, when synchronized over large regions, may account for the changes in oscillatory power that can be observed using noninvasive scalp EEG techniques.
Besides the influence of independent cortical theta generators, we could not rule out by scalp EEG that the increase in theta oscillations seen in our study might also involve the thalamus through large recursive loops of excitation and inhibition between the cortex and the thalamus [Hughes et al., 2004]. The precise cellular thalamic mechanisms of theta and alpha rhythm in animal studies were investigated through rhythmic‐burst firing termed high‐threshold (HT) bursting in thalamocortical (TC) neurons; cellular thalamic mechanisms for alpha synchronization that occurs in the range of 2 to 13 Hz. In a study by Hughes et al. [ 2004], it was discovered that both alpha and theta waves are underpinned by the same intrinsic neuronal behaviour at the thalamic level as observed in an in vitro slice preparation of the cat lateral geniculate nucleus (LGN). It has been found that strong activation of metabotropic glutamate receptor (mGluR), mGluR1a, that is located postsynaptically to corticothalamic fibres, leads to alpha frequency rhythm through increase TC depolarization. A reduction in activation intensity is found when the TC neurons are less depolarized bringing about theta waves [Hughes and Crunelli, 2005; Hughes et al., 2004].
Patterns of power modulation for mu (10–12 Hz) revealed larger amplitude over the stimulated hemisphere compared to the beta (13–30 Hz) frequency band. This result was in line with an rTMS/EEG study by Fuggetta et al. [ 2008] who applied 20 intermittent rTMS trains of 20 pulses at 5 Hz over the primary motor cortex. They observed a higher synchronization in alpha (10–12 Hz) compared to beta (18–22 Hz). This TMS‐related α‐synchronization seen at rest is thought to be due to the resetting of the stimulated neurons to oscillate at the frequencies of the motor cortex [Fuggetta et al. 2005; Paus et al., 2001a]. A key hypothesis supporting these phenomena is that TMS might induce a synchronous activation of neurons via the modulation of the cortex‐thalamus‐cortex pathways, through which cortical oscillations are generated [Fuggetta et al., 2008; Llinas and Steriade, 2006; Steriade and Timofeev, 2003]. Although TMS activates superficial regions, such as the cerebral cortex, cerebellum, and spinal cord directly, the effects may indirectly reach deeper structures [Allen et al., 2007; Bestmann et al., 2008]. Thalamic involvement in generation of alpha rhythms are suggested by several human studies using PET and fMRI which show a correlation between EEG alpha band power and thalamic metabolic activity [Goldman et al., 2002; Danos et al., 2001]. In a study by Van Der Werf et al. [ 2006] on patients with Parkinson's disease who underwent unilateral ventrolateral nucleus thalamotomy, they found that beta band (15–30Hz) oscillations after M1 TMS are of higher amplitude in the unoperated hemisphere. This illustrates the importance of thalamus in facilitating cortically generated oscillations.
Our study also demonstrated a different topography of mu and theta rhythms. Mu was focally distributed and dominated early (5 s) after the magnetic stimulation, whereas high‐amplitude theta oscillations showed widespread distribution across EEG electrodes, which lasted longer (20 s). These different dynamics of mu and theta enhancement of oscillations indeed suggest the presence of independent mu and theta generators with different reactivity to rTMS. Because theta and alpha rhythms have fundamentally different functional roles [Başar et al., 2001; Sauseng and Klimesch, 2008], the different topography and dynamics of mu and theta oscillations seen in our study, suggest that their generations might result from different origins. A study by Sarnthein and Jeanmonod, [ 2007] of thalamic local field potentials and scalp EEG in patients with Parkinson's disease showed thalamocortical theta coherence concentrated mainly in the frontal electrodes. Studies using human intracranial EEG recordings have found theta scattered across multiple locations in the brain of the same subject with the absence of coupling between hippocampus and neocortical theta [Caplan et al., 2001; Cantero et al., 2003; Kahana et al., 2001]. Therefore, based on the different topography of theta and mu seen in our investigation, we propose that the cortical theta generators may account for the global changes in high‐amplitude theta oscillatory power observed using noninvasive scalp EEG [Kahana et al., 2001], whereas the focal mu enhancement seen could be generated by thalamocortical networks [Nunez et al., 2001]. Nevertheless, EEG can only allow inferences about the synchronicity of a large population of neurons in the cortex near the electrodes and using this technique, it is not possible to investigate network properties on a microlevel. Therefore, we are unable to know for certain whether the interactions of rTMS with cortical theta generators are in parallel with thalamocortical loops generating theta and mu over M1.
To look at the cumulative effect between the 20 intermittent trains of rTMS 20 and rTMS 60 pulses, we analyzed the difference in EEG modulation between the first 10 trains (part A) and the subsequent 10 trains of stimulations (part B). Initially, we hypothesized that a stronger cumulative effect would be observed during the “longer” train of rTMS 60. Instead, we saw a higher cumulative effect in the relatively “shorter” train of rTMS 20 in mu rhythm mainly around 5 s post magnetic stimulation. Our results extend the finding of Aydin‐Abidin et al. [ 2006] on visual cortex excitability in anaesthetized and paralysed cats who demonstrated that short high‐frequency rTMS trains (10 Hz, 600 pulses) were more effective than longer rTMS trains (10 Hz, 1200 pulses). Our results suggest that synchronization was reached faster with relatively fewer numbers of pulses during high‐frequency rTMS. A TMS/PET study by Paus et al. [ 2001b] looking at corticocortical connectivity of the middorsolateral prefrontal cortex (MDL‐FC) after brief periods of rTMS, showed that a mere 30 trains (300 pulses) of 10 Hz rTMS can induce changes in cortical excitability and connectivity of the stimulated region. The relatively higher number of pulses in rTMS 60 did not result in more pronounced cumulative effects compared to fewer stimuli in rTMS 20. This result could be due to the brain's compensatory regulatory mechanisms. rTMS like any other artificial stimulation will be subjected to homeostatic mechanisms invoked in underlying regions to maintain normal brain functions [Thickbroom, 2007; Turrigiano and Nelson, 2004; Turrigiano, 2007]. Besides homeostatic mechanisms, the local inhibitory interneuronal network involving the complex interplay of ionotropic GABAA receptors and the metabotropic GABAB receptors might also play a role to limit the after‐effects of high‐frequency magnetic stimulation [McDonnell et al., 2007; Chen, 2004].
The physiological changes of the neuronal networks and interhemispheric connections caused by rTMS can be determined through EEG coherence analysis that measure the information transmitted between two sites in the cortex [Leocani, 1997]. It allows the detection of small oscillatory effects in shared variance of signals in the frequency domains and reveals the spatio‐temporal correlation between a pair of signals [Jing and Takigawa, 2000]. In this study, we observed a decrease of coherence in rTMS 60 for theta band mainly in the frontal region ipsilateral to the stimulation site (coupling between C3 and F3) during the first epoch, 5 s post magnetic stimulation. However, this decrease in functional coupling was followed subsequently by an increase in coherence modulation from epoch two to four, up to 20 s post stimulation in frontal and central electrodes. These results suggest a rebound phenomenon for rTMS 60 during high‐frequency magnetic stimulation. Previous studies mainly found coherence modulation in the alpha or beta frequency bands [Chen et al., 2003; Fuggetta et al., 2008; Jing and Takigawa, 2000; Oliviero et al., 2003; Strens et al., 2002]. The differences in results suggested that different frequency bands could have different sources and functions in response to rTMS and different sensitivities of the frequency ranges analyzed.
We demonstrated from our combined rTMS/EEG study that both short and long trains of high frequency rTMS stimulations modulated the regional activity and interregional functional connectivity of oscillatory neural phenomenon despite absence of MEP changes. Maeda et al. [ 2000] reported that 10 Hz rTMS had no lasting effect on MEP size after administration of 240 pulses but found an increase in MEP amplitude with 1600 pulses. Several studies of MEPs have not found any changes in MEP amplitude despite high frequency magnetic stimulations [Daskalakis et al., 2006; Romeo et al., 2000; Wassermann et al., 1996]. This illustrates that high density EEG is a more sensitive and robust method of measuring cortical activity than MEP measurement [Thut and Pascual‐Leone, 2010]. A recent combined TMS/EEG study by Mäki and Ilmoniemi, [ 2010] has found that MEP and EEG oscillation amplitudes were overall not strongly correlated. The authors argued that the cortical excitability component of MEP amplitude fluctuations was specific to the neurons controlling the target muscle, whereas the EEG signal reflects the sum of activity from a large neuronal population of cortical areas including those that control different muscles. Furthermore, MEP which is commonly used in TMS experiments as an indicator of cortical excitability—is a polysynaptic measurement, separated by at least three synapses from the TMS source (synapses onto corticospinal neurons; synapses onto motor neurons of the spinal cord; and the neuromuscular synapses) [Huerta and Volpe, 2009; Siebner and Rothwell, 2003]. On the other hand, the EEG signal is driven by the brain's own electrical activity through the synchronous excitatory and inhibitory input of pyramidal dendrites, making it a more powerful tool than MEPs in providing a more accurate interpretation of cortical output [Huerta and Volpe, 2009; Sauseng and Klimesch, 2008; Thut and Miniussi, 2009; Taylor et al., 2008].
Overall, our study has provided a new insight into the presence of independent human cortical theta generators during high‐frequency rTMS via noninvasive electrophysiological measurement. The study provides an important bridge between animal theta studies and direct evidence for cortical oscillatory generators using invasive human intracranial recordings. More research using combined rTMS/EEG methods is needed to investigate the role of rTMS‐induced human cortical theta and other brain rhythms in reflecting the basic modes of dynamic brain organization. It will prove useful not only for the understanding of the neurophysiology underlying various brain rhythms but also for therapeutic manipulations of brain plasticity.
Acknowledgements
The authors would like to thank two anonymous reviewers for providing comments, Dr Paolo Manganotti for the possibility he gave to collect the data in his laboratory, Dr Luisa Sartori for her assistance in collecting the data from participants of this “Ryanair” study, and Dr Phil Duke for revisions of the language of manuscript. Nor Azila Noh is a PhD student sponsored by Ministry of Higher Education, Malaysia and Universiti Sains Islam Malaysia.
REFERENCES
- Allen EA, Pasley BN, Duong T, Freeman RD ( 2007): Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science 317: 1918–1921. [DOI] [PubMed] [Google Scholar]
- Arana AB, Borckardt JJ, Ricci R, Anderson B, Li X, Linder KJ, Long J, Sackeim HA, George MS ( 2008): Focal Electrical Stimulation as a Sham Control for rTMS: Does it truly mimic the cutaneous sensation and pain of active prefrontal rTMS? Brain Stimulat 1: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin‐Abidin S, Moliadze V, Eysel UT, Funke K ( 2006): Effects of repetitive TMS on visually evoked potentials and EEG in the anaesthetized cat: Dependence on stimulus frequency and train duration. J Physiol 574: 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başar E, Schurmann M, Sakowitz O ( 2001): The selectively distributed theta system: Functions. Int J Psychophisiol 39: 197–212. [DOI] [PubMed] [Google Scholar]
- Bestmann S ( 2008): The physiological basis of transcranial magnetic stimulation. Trends Cogn Sci 12: 81–83. [DOI] [PubMed] [Google Scholar]
- Brignani D, Manganotti P, Rossini PM, Miniussi C ( 2008): Modulation of cortical oscillatory activity during transcranial magnetic stimulation. Hum Brain Mapp 29: 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G ( 2004): Large‐scale recording of neuronal ensembles. Nat Neurosci 7: 446–451. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A ( 2004): Neuronal oscillations in cortical networks. Science 304: 1926–1929. [DOI] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Stickgold R, Kahana MJ, Madsen JR, Kocsis B ( 2003): Sleep‐dependent theta oscillations in the human hippocampus and neocortex. J Neurosci 23: 10897–10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Raghavachari S, Kahana MJ ( 2001): Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. J Neurophysiol 86: 368–380. [DOI] [PubMed] [Google Scholar]
- Chen R ( 2004): Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res 154: 1–10. [DOI] [PubMed] [Google Scholar]
- Chen WH, Mima T, Siebner HR, Oga T, Hara H, Satow T, Begum T, Nagamine T, Shibasaki H ( 2003): Low‐frequency rTMS over lateral premotor cortex induces lasting changes in regional activation and functional coupling of cortical motor areas. Clin Neurophysiol 114: 1628–1637. [DOI] [PubMed] [Google Scholar]
- Danos P, Guich S, Abel L, Buchsbaum MS ( 2001): EEG alpha rhythm and glucose metabolic rate in the thalamus in schizophrenia. Neuropsychobiology 43: 265–272. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Moller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R ( 2006): The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res 174: 403–412. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ ( 2006): A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 117: 2584–2596. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Hoy K, Maller J, Enticott P, Laycock R ( 2007): A comparative study of the effects of repetitive paired transcranial magnetic stimulation on motor cortical excitability. J Neurosci Meth 165: 265–269. [DOI] [PubMed] [Google Scholar]
- Formaggio E, Storti SF, Avesani M, Cerini R, Milanese F, Gasparini A, Acler M, Pozzi Mucelli R, Fiaschi A, Manganotti P ( 2008): EEG and fMRI coregistration to investigate the cortical oscillatory activities during finger movement. Brain Topogr 21: 100–111. [DOI] [PubMed] [Google Scholar]
- Fuggetta G, Fiaschi A, Manganotti P ( 2005): Modulation of cortical oscillatory activities induced by varying single‐pulse transcranial magnetic stimulation intensity over the left primary motor area: A combined EEG and TMS study. Neuroimage 27: 896–908. [DOI] [PubMed] [Google Scholar]
- Fuggetta G, Pavone EF, Fiaschi A, Manganotti P ( 2008): Acute modulation of cortical oscillatory activities during short trains of high‐frequency repetitive transcranial magnetic stimulation of the human motor cortex: A combined EEG and TMS study. Hum Brain Mapp 29: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilio F, Iacovelli E, Frasca V, Gabriele M, Giacomelli E, De Lena C, Cipriani AM, Inghilleri M ( 2009): Electrical and magnetic repetitive transcranial stimulation of the primary motor cortex in healthy subjects. Neurosci Lett 455: 1–3. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J Jr., Cohen MS ( 2002): Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport 13, 2487–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M ( 2007): Transcranial magnetic stimulation: A primer. Neuron 55: 187–199. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Johson JS, Feredoes E, Postle BR ( 2011): Does high‐frequency repetitive transcranial magnetic stimulation produce residual and/or cumulative effects within an experimental session? Brain Topogr 23: 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U, Cardenas‐Morales L, Connemann BJ, Kammer T, Schonfeldt‐Lecuona C ( 2010): Sham or real–post hoc estimation of stimulation condition in a randomized transcranial magnetic stimulation trial. Neurosci Lett 471: 30–33. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Volpe BT ( 2009): Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J Neuroeng Rehabil 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Crunelli V ( 2005): Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist 11: 357–372. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Lorincz M, Cope DW, Blethyn KL, Kekesi KA, Parri HR, Juhasz G, Crunelli V ( 2004): Synchronized oscillations at alpha and theta frequencies in the lateral geniculate nucleus. Neuron 42: 253–268. [DOI] [PubMed] [Google Scholar]
- Hutcheon B, Yarom Y ( 2000): Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci 23: 216–222. [DOI] [PubMed] [Google Scholar]
- Jing H, Takigawa M ( 2000): Observation of EEG coherence after repetitive transcranial magnetic stimulation. Clin Neurophysiol 111: 1620–1631. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR ( 2001): Theta returns. Curr Opin Neurobiol 11: 739–744. [DOI] [PubMed] [Google Scholar]
- Komssi S, Kahkonen S ( 2006): The novelty value of the combined use of electroencephalography and transcranial magnetic stimulation for neuroscience research. Brain Res Rev 52: 183–192. [DOI] [PubMed] [Google Scholar]
- Leocani L, Toro C, Manganotti P, Zhuang P, Hallett M ( 1997): Event‐related coherence and event‐related desynchronization/synchronization in the 10 Hz and 20 Hz EEG during self‐paced movements. Electroencephalogr Clin Neurophysiol 104: 199–206. [DOI] [PubMed] [Google Scholar]
- Leung LW, Borst JG ( 1987): Electrical activity of the cingulate cortex. I. Generating mechanisms and relations to behavior. Brain Res 407: 68–80. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Steriade M ( 2006): Bursting of thalamic neurons and states of vigilance. J Neurophysiol 95: 3297–3308. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual‐Leone A ( 2000): Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol 111: 800–805. [DOI] [PubMed] [Google Scholar]
- Mäki H, Ilmoniemi RJ ( 2010): EEG oscillations and magnetically evoked motor potentials reflect motor system excitability in overlapping neuronal populations. Clin Neurophysiol 121: 492–501. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U ( 2007): Suppression of LTP‐like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp Brain Res 180: 181–186. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Thut G ( 2010): Combining TMS and EEG offers new prospects in cognitive neuroscience. Brain Topogr 22: 249–256. [DOI] [PubMed] [Google Scholar]
- Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ ( 2008): Frontal‐midline theta from the perspective of hippocampal “theta”. Prog Neurobiol 86: 156–185. [DOI] [PubMed] [Google Scholar]
- Modugno N, Nakamura Y, MacKinnon CD, Filipovic SR, Bestmann S, Berardelli A, Rothwell JC ( 2001): Motor cortex excitability following short trains of repetitive magnetic stimuli. Exp Brain Res 140: 453–459. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Wingeier BM, Silberstein RB ( 2001): Spatial‐temporal structures of human alpha rhythms: Theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp 13: 125–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Strens LH, Di Lazzaro V, Tonali PA, Brown P ( 2003): Persistent effects of high frequency repetitive TMS on the coupling between motor areas in the human. Exp Brain Res 149: 107–113. [DOI] [PubMed] [Google Scholar]
- Paus T, Sipila PK, Strafella AP ( 2001a): Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: An EEG study. J Neurophysiol 86: 1983–1990. [DOI] [PubMed] [Google Scholar]
- Paus T, Castro‐Alamancos MA, Petrides M ( 2001b): Cortico‐cortical connectivity of the human mid‐dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci 14: 1405–1411. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Reimer B, Loer C, Quartarone A, Munchau A, Conrad B, Siebner HR ( 2004): Long‐lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol 115: 1519–1526. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Brunner C, Schlogl A, Lopes da Silva FH ( 2006): Mu rhythm (de)synchronization and EEG single‐trial classification of different motor imagery tasks. Neuroimage 31: 153–159. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Andrew C ( 1999): Event‐related changes of band power and coherence: Methodology and interpretation. Clin Neurophysiol 16: 512–526. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH ( 1999): Event‐related EEG/MEG synchronization and desynchronization: Basic principles. Clin Neurophysiol 110: 1842–1857. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Morgante F, Sant'angelo A, Battaglia F, Messina C, Siebner HR, Girlanda P ( 2005): Distinct changes in cortical and spinal excitability following high‐frequency repetitive TMS to the human motor cortex. Exp Brain Res 161: 114–124. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ ( 2006): Theta oscillations in human cortex during a working‐memory task: evidence for local generators. J Neurophysiol 95: 1630–1638. [DOI] [PubMed] [Google Scholar]
- Rizzuto DS, Madsen JR, Bromfield EB, Schulze‐Bonhage A, Kahana MJ ( 2006): Human neocortical oscillations exhibit theta phase differences between encoding and retrieval. Neuroimage 31: 1352–1358. [DOI] [PubMed] [Google Scholar]
- Romei V, Rihs T, Brodbeck V, Thut G ( 2008): Resting electroencephalogram alpha‐power over posterior sites indexes baseline visual cortex excitability. Neuroreport 19: 203–208. [DOI] [PubMed] [Google Scholar]
- Romeo S, Gilio F, Pedace F, Ozkaynak S, Inghilleri M, Manfredi M, Berardelli A ( 2000): Changes in the cortical silent period after repetitive magnetic stimulation of cortical motor areas. Exp Brain Res 135: 504–510. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M ( 2009): Natural frequencies of human corticothalamic circuits. J Neurosci 29: 7679–7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Ferro M, Cincotta M, Ulivelli M, Bartalini S, Miniussi C, Giovannelli F, Passero S ( 2007): A real electro‐magnetic placebo (REMP) device for sham transcranial magnetic stimulation (TMS). Clin Neurophysiol 118: 709–716. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual‐Leone A, Safety of TMSCG ( 2009): Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120: 2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Martino G, Narici L, Pasquarelli A, Peresson M, Pizzella V, Tecchio F, Torrioli G, Romani GL ( 1994): Short‐term brain ‘plasticity’ in humans: Transient finger representation changes in sensory cortex somatotopy following ischemic anesthesia. Brain Res 642: 169–77. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Jeanmonod D ( 2007): High thalamocortical theta coherence in patients with Parkinson's disease. J Neurosc 27: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gerloff C, Hummel FC ( 2009): Spontaneous locally restricted EEG alpha activity determines cortical excitability in the motor cortex. Neuropsychologia 47: 284–288. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W ( 2008): What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci Biobehav Rev 32: 1001–1013. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J, d'Alfonso AA, Postma A, de Haan EH ( 2001): Effects of slow rTMS at the right dorsolateral prefrontal cortex on EEG asymmetry and mood. Neuroreport 12: 445–447. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J ( 2003): Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res 148: 1–16. [DOI] [PubMed] [Google Scholar]
- Silva LR, Amitai Y, Connors BW ( 1991): Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science 251: 432–435. [DOI] [PubMed] [Google Scholar]
- Steriade M ( 2006): Grouping of brain rhythms in corticothalamic systems. Neuroscience 137: 1087–1106. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I ( 2003): Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron 37: 563–576. [DOI] [PubMed] [Google Scholar]
- Strens LH, Oliviero A, Bloem BR, Gerschlager W, Rothwell JC, Brown P ( 2002): The effects of subthreshold 1 Hz repetitive TMS on cortico‐cortical and interhemispheric coherence. Clin Neurophysiol 113: 1279–1285. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Walsh V, Eimer M ( 2008): Combining TMS and EEG to study cognitive function and cortico‐cortico interactions. Behav Brain Res 191: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickbroom GW ( 2007): Transcranial magnetic stimulation and synaptic plasticity: Experimental framework and human models. Exp Brain Res 180: 583–593. [DOI] [PubMed] [Google Scholar]
- Thut G, Miniussi C ( 2009): New insights into rhythmic brain activity from TMS‐EEG studies. Trends Cogn Sci 13: 182–189. [DOI] [PubMed] [Google Scholar]
- Thut G, Pascual‐Leone A ( 2010): Integrating TMS with EEG: How and what for? Brain Topogr 22: 215–218. [DOI] [PubMed] [Google Scholar]
- Turrigiano G ( 2007): Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol 17: 318–324. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB ( 2004): Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5: 97–107. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Paus T ( 2006): The neural response to transcranial magnetic stimulation of the human motor cortex. I. Intracortical and cortico‐cortical contributions. Exp Brain Res 175: 231–245. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Sadikot AF, Strafella AP, Paus T ( 2006): The neural response to transcranial magnetic stimulation of the human motor cortex. II. Thalamocortical contributions. Exp Brain Res 175: 246–255. [DOI] [PubMed] [Google Scholar]
- Wassermann EM ( 1998): Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol 108: 1–16. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M ( 1996): Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res 109: 158–163. [DOI] [PubMed] [Google Scholar]
- Zarkowski P, Shin CJ, Dang T, Russo J, Avery D ( 2006): EEG and the variance of motor evoked potential amplitude. Clin EEG Neurosci 37: 247–251. [DOI] [PubMed] [Google Scholar]


