Abstract
The nucleus accumbens and medial frontal cortex (MFC) are part of a loop involved in modulating behavior according to anticipated rewards. However, the precise temporal landscape of their electrophysiological interactions in humans remains unknown because it is not possible to record neural activity from the nucleus accumbens using noninvasive techniques. We recorded electrophysiological activity simultaneously from the nucleus accumbens and cortex (via surface EEG) in humans who had electrodes implanted as part of deep‐brain‐stimulation treatment for obsessive–compulsive disorder. Patients performed a simple reward motivation task previously shown to activate the ventral striatum. Spectral Granger causality analyses were applied to dissociate “top–down” (cortex → nucleus accumbens)‐ from “bottom–up” (nucleus accumbens → cortex)‐directed synchronization (functional connectivity). “Top–down”‐directed synchrony from cortex to nucleus accumbens was maximal over medial frontal sites and was significantly stronger when rewards were anticipated. These findings provide direct electrophysiological evidence for a role of the MFC in modulating nucleus accumbens reward‐related processing and may be relevant to understanding the mechanisms of deep‐brain stimulation and its beneficial effects on psychiatric conditions. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: nucleus accumbens, medial frontal cortex, EEG, reward, motivation, obsessive compulsive disorder
INTRODUCTION
The nucleus accumbens is widely implicated in motivation, learning, and adaptive behavior [Haber and Knutson, 2010]. Activity within the human nucleus accumbens increases during reward anticipation [Knutson et al., 2001] and feedback that signals the need to adjust behavior [Cohen et al., 2009b; O'Doherty et al., 2004]. Dysfunctional nucleus accumbens functioning has been implicated in a range of disorders, including obsessive–compulsive disorder (OCD), depression, schizophrenia, drug abuse, and ADHD [Juckel et al., 2006; Kienast and Heinz, 2006; Wise, 1996]. Clearly, this relatively small brain area plays a large role in cognitive and emotional processing.
Invasive tract tracing results in primates demonstrate a unidirectional monosynaptic connection from areas of the medial frontal cortex (MFC) to the nucleus accumbens [Haber et al., 1995], suggesting that the MFC provides top–down signals that regulate nucleus accumbens processing. Here, we examined the dynamics of interactions between the nucleus accumbens and the cortex by recording local field potentials directly from the nucleus accumbens, simultaneously with surface EEG electrodes that measure cortical activity, of patients undergoing deep brain stimulation (DBS) surgery for the treatment of OCD. DBS is a technique in which stimulating electrodes are placed into the brain to alleviate symptoms of various diseases, including movement disorders and acute psychiatric conditions. DBS to the nucleus accumbens is a promising treatment option for major depression [Schlaepfer et al., 2008) and OCD (Denys and Mantione, 2009]. Relevant to scientific investigation, there is a brief period of time in which electrophysiological signals can be recorded directly in the nucleus accumbens from implanted but distally externalized DBS electrodes, while patients are awake and able to participate in cognitive tasks. Though issues of disease influence on the activity arise because there is no healthy control group, these recordings provide a unique and unparalleled opportunity to examine the fine temporal structure of electrical activity of the nucleus accumbens and its interactions with electrical activity in the cortex in a way not otherwise possible in humans.
We simultaneously recorded nucleus accumbens electrical activity and surface EEG activity in six patients while they performed a simplified version of the monetary‐incentive‐delay task [Knutson et al., 2001], which has been used to elicit ventral striatal activity in healthy individuals and a range of patient groups. These findings provide unique evidence for the rapid electrophysiological interactions between the cortex and ventral striatum during reward processing, with a temporal resolution not normally possible in humans.
MATERIALS AND METHODS
Patients
Six patients (aged 34–56, mean = 41.5) undergoing DBS surgery for treatment of OCD participated in the task. The implantation of the electrodes was performed according to standard stereotactic procedures using frame‐based MRI for target determination, as described elsewhere [Denys et al., in press]. The location of electrode placement was made entirely on clinical grounds. The ethics committee at the University of Amsterdam Academic Medical Center approved this experiment.
Task
Patients performed a simplified version of the monetary‐incentive‐delay task [Knutson et al., 2001]. In the task (Fig. 1A), patients saw one of two visual cues indicating that a reward either was or was not possible on that trial, then, after a delay of 2 s, pressed a response button as quickly as possible to a target stimulus. The experiment took place in a quiet testing room after implantation of the DBS electrodes, and patients were without anesthesia or sedation at the time. The task was run on a desktop computer, which was equipped with a cable that sent TTL pulse triggers to the EEG acquisition with millisecond‐precision information about when events in the experiment occurred. Because of technical issues, response button triggers were not sent to the EEG.
Figure 1.
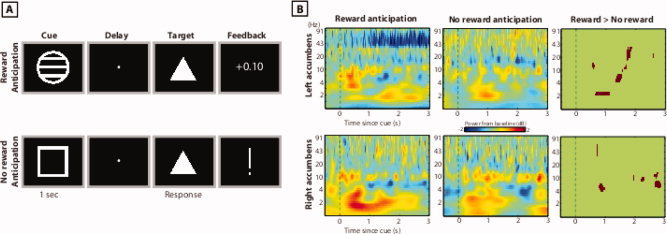
Overview of task (A) and nucleus accumbens oscillation power results (B). The right‐most plots show pixels in which oscillation power was significantly greater during reward anticipation compared to no‐reward anticipation (P < 0.05 with cluster thresholding). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
EEG Recording
EEG data were collected in a quiet testing room after implantation of the DBS electrodes. The DBS electrodes are Medtronic model 3389. Each DBS probe (one per hemisphere) contains four electrode contacts (1.27 mm diameter, 1.5 mm length, and 0.5 mm spacing between contacts). Continuous EEG from these electrodes was sampled at 1,000 Hz. Simultaneous recordings were taken from 12 surface EEG electrodes (F3, F4, Fz, C3, C4, Cz, FCz, P3, P4, Pz, FC3, and FC4). Because precise source localization is difficult with such few electrodes, we use the term “MFC” as a general anatomical descriptor without implying precise anatomical origin of the topographic activity.
Offline, surface EEG data were rereferenced to linked mastoids, and nucleus accumbens activity in each hemisphere was rereferenced to the average activity of all four contacts in that hemisphere. This subtracts potentially volume‐conducted activity from distant sources. Data from the ventral‐most contact, which anatomically is located in the nucleus accumbens shell, were used in the analyses presented in the main text. Results from other contacts were similar. Trials were manually inspected and rejected if they contained artifacts. Surface EEG data were subjected to independent components analysis using EEGLAB [Delorme and Makeig, 2004], and components containing blinks/ocular artifacts were removed from the data.
Oscillation Power and Phase Synchrony Analyses
Time–frequency decomposition was conducted via wavelet analysis, in which the complex power spectrum of the single‐trial EEG time series (obtained from FFT) was multiplied by the complex power spectrum of a family of complex Morlet wavelets, and then the inverse Fourier transform was taken. This is equivalent to, but faster than, time‐domain convolution. The wavelets were defined as Gaussian‐windowed complex sine waves,
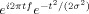 , in which t is time and f is frequency, which increased from 1 to 100 Hz in 50 logarithmically spaced steps. σ defines the width of each frequency band and is set according to 4/(2πf), which provides an adequate trade‐off between time and frequency resolution. After convolution of the wavelet with the EEG, power is defined as the modulus of the resulting complex signal Z[t] (power time series: p(t) = real[z(t)]2 + imag[z(t)]2). The baseline was defined as average frequency power from −300 to −100 ms prior to the onset of the cue. Finally, stimulus‐induced power time courses were normalized by converting the baseline‐corrected signal to a decibel (dB) scale (10 log 10[power/baseline]); this allows for direct comparison of effects across frequency bands and patients.
, in which t is time and f is frequency, which increased from 1 to 100 Hz in 50 logarithmically spaced steps. σ defines the width of each frequency band and is set according to 4/(2πf), which provides an adequate trade‐off between time and frequency resolution. After convolution of the wavelet with the EEG, power is defined as the modulus of the resulting complex signal Z[t] (power time series: p(t) = real[z(t)]2 + imag[z(t)]2). The baseline was defined as average frequency power from −300 to −100 ms prior to the onset of the cue. Finally, stimulus‐induced power time courses were normalized by converting the baseline‐corrected signal to a decibel (dB) scale (10 log 10[power/baseline]); this allows for direct comparison of effects across frequency bands and patients.
Spectral Granger causality estimates the influence of activity in one region over activity in another region. It is defined as the ratio of error variances resulting from a univariate autoregression, in which nucleus accumbens activity is predicted from previous nucleus accumbens activity, to the those resulting from a bivariate autoregression, in which nucleus accumbens activity is predicted from previous activity in both the nucleus accumbens and surface EEG electrodes. This ratio (termed “Granger gain” in the main text) provides an estimate of the directed synchrony from the cortex to the nucleus accumbens (“top–down”). Estimating directed synchrony from the accumbens to the cortex (“bottom–up”) is done using the same procedure but swapping the order of the data in the analyses. This analysis is computed repeatedly over sliding windows, thus estimating the time course of directed synchrony. We used an autoregression order of 5 and a sliding window of 200 ms. Preliminary analyses using a range of parameters suggest that those parameter settings were optimal; nonetheless, because the same parameters are applied to all conditions, timepoints, and patients, parameter selection could not have influenced the overall patterns of results. Note that the spectral Granger causality analyses were not optimized for frequency resolution, but rather for sensitivity to differences in directionality and across conditions. Thus, in these analyses, the differences in the direction of the effects and their modulation by reward anticipation are more important than the precise frequency ranges in which the effects occur.
Statistics
Because of the small number of patients, we opted for nonparametric permutation testing for statistical significance. This allowed us to rely on observed characteristics of the dataset rather than to rest the analyses on assumptions regarding the distribution of the data and the characteristics of the error covariance matrices. In the permutation tests, the labeling of condition and patient was randomly shuffled, and the difference between randomized conditions was computed. Values were not shuffled over time or frequency, thus conserving inherent time–frequency smoothing and scaling. One thousand permuted difference maps were computed, creating a distribution at each time–frequency point of the differences expected under the null hypothesis. Then, at each pixel, a Z value was computed as the difference between the true observed difference and the average permuted difference, scaled by the standard deviation of the permuted differences. This time–frequency map of Z values thus reflects the distance of the observed data from a distribution of data expected under the null hypothesis of no differences between conditions. These maps were then thresholded such that pixels with a Z value corresponding to a P value of 0.05 two‐tailed were set to zero (green color). Finally, a cluster threshold was applied such that clusters comprising fewer than 100 contiguous pixels were also eliminated. Based on permutation testing, this corresponds roughly to a cluster‐level threshold of P < 0.05.
The correlation with hand electromyographic (EMG) activity was done by computing Granger causality during the anticipation period of each trial and correlating this activity (across trial within each patient) with the average EMG activation from a 150‐ms window surrounding the postresponse peak in the rectified data.
RESULTS
We first examined local oscillatory activity within the left and right nucleus accumbens using a complex wavelet convolution analysis in combination with group‐level nonparametric permutation testing for statistical thresholding (see Materials and Methods). We found that nucleus accumbens oscillation power in the theta frequency band increased significantly compared to baseline during the anticipation period and that there was a significant increase in oscillation power during anticipation of rewards compared to anticipation of no rewards (Fig. 1B). These findings suggest that hemodynamic activity observed during reward anticipation [Knutson et al., 2001] may be driven by enhanced theta frequency oscillations.
We next applied spectral Granger causality to estimate the influence of cortical activity on subsequent nucleus accumbens activity (“top–down”‐directed synchrony) and the influence of nucleus accumbens activity on subsequent cortical activity (“bottom–up”‐directed synchrony). Top–down‐directed synchrony was strongest over medial frontal sites (see Fig. 2) in low frequencies, whereas bottom‐up‐directed synchrony from the nucleus accumbens to the cortex was weaker and with a less well‐defined topography. These findings suggest that the MFC has a strong influence over accumbens activity, whereas accumbens activity has little direct influence over cortical activity.
Figure 2.
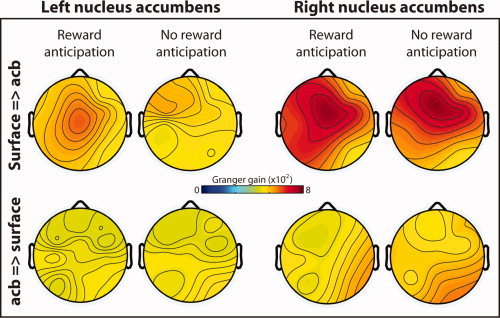
Topographical distribution of directed synchrony during the anticipation delay, estimated through Granger causality. Hotter colors indicate increased directed synchrony from the surface EEG electrodes to the left and right nucleus accumbens (top row) and weaker directed synchrony from the nuclei accumbens to the surface EEG electrodes (bottom row). Data are averaged from 600 to 1,800 ms, 1–4 Hz. This time window was selected to exclude transient stimulus‐evoked dynamics. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
On the basis of these topographical distributions, we selected electrode Fz (located over MFC) for further statistical analyses. Top–down‐directed synchrony was stronger during reward anticipation compared to no reward anticipation (Fig. 3A,B). This was statistically significant in the left accumbens (Fig. 3C) and was numerically in the same direction in the right accumbens (Fig. 3E–G). Statistical analyses also confirmed that top–down‐directed synchrony was stronger than bottom–up‐directed synchrony (Fig. 3D,H).
Figure 3.
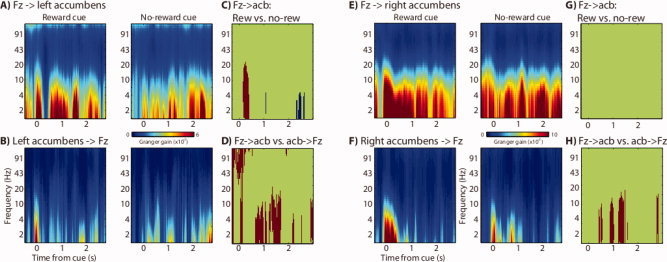
Spectral Granger time–frequency plots show a sustained increase in directional synchrony from electrode Fz (located over MFC) to the nucleus accumbens, which was stronger during anticipation of rewards compared to anticipation of no rewards. (A) Time–frequency plots of “top–down” (Fz → left accumbens) for no reward (right) and reward (left) conditions. (B) Similar plots of “bottom–up” (left accumbens → Fz). (C) Time–frequency points in which top–down synchrony was significantly stronger for anticipation of reward compared to anticipation of no reward (red colors). (D) Time–frequency points in which top–down synchrony was significantly stronger than bottom–up during the reward anticipation condition. (E–H) Same as A–D but for the right nucleus accumbens. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In a final analysis, we tested whether this Fz → accumbens synchrony was related to preparation of the upcoming motor response. Specifically, we correlated the strength of “top–down”‐directed synchrony during the anticipation delay with the rectified and filtered EMG recording from the hand used to indicate the response. Correlation coefficients were not consistent or significant across patients (Spearman's rank correlation, all rs <0.1).
DISCUSSION
The nucleus accumbens is a major convergence zone for disparate inputs from MFC and orbitofrontal cortex, hippocampus, amygdala, the thalamus, and midbrain dopamine regions [Groenewegen et al., 1996] and may integrate across these inputs to compute reward prediction signals [e.g., Cromwell et al., 2005; Knutson et al., 2001; Small et al., 2001]. By recording simultaneously from the cortex (via surface EEG) and directly from the nucleus accumbens with high temporal precision, we could examine the rapid flow of information between them. We found that top–down‐directed synchrony from the cortex to the nucleus accumbens was maximal over medial frontal scalp sites and increased during reward anticipation compared to no reward anticipation. Bottom–up‐directed synchrony, in contrast, was relatively weak and not modulated by reward condition. Indeed, this pattern of MFC → accumbens flow of information is consistent with known anatomical connectivity: Regions of the MFC including anterior cingulate directly project to, and can modulate activity and dopamine levels in, the ventral striatum [Brady and O'Donnell, 2004; Jackson et al., 2001]. In contrast, the nucleus accumbens can send information back to cortex only indirectly, via the thalamus or ventral tegmental area [Carr and Sesack, 2000; Haber and Calzavara, 2009; Haber et al., 2000]. Nonetheless, our findings do not necessarily imply a monosynaptic connection: In theory, it is possible that a third region (e.g., mediodorsal nucleus of the thalamus, hippocampus, and amygdala) acts as a mediator between the cortex and nucleus accumbens. It is also possible that a third region projects to both the MFC and the nucleus accumbens but to the MFC faster. In relation, Granger causality may not be ideally suited to detect possible indirect modulations of the nucleus accumbens on MFC activity via its influence over the midbrain dopamine system, which in turn projects diffusely to the prefrontal cortex.
Because of spatial limitations of low‐density surface EEG, it is not possible to determine the precise cortical region within the MFC that generates the observed top–down signal. Based on anatomical studies in nonhuman primates, ventromedial prefrontal cortex and dorsal anterior cingulate provide prominent input into the nucleus accumbens [Haber et al., 2006]. Studies of human hemodynamic imaging also suggest functional connectivity between the ventral striatum and the anterior cingulate [Cohen et al., 2005; Harrison et al., 2009; Ma et al., 2010]. The present findings are less likely to be driven by ventromedial prefrontal regions due to its depth and distance from the electrodes. Future recordings with a denser surface EEG array (e.g., 64 or more electrodes) or MEG recordings may improve cortical localization. Nonetheless, despite the limitation on precise source localization, these findings demonstrate a strong top–down modulatory role of the prefrontal cortex over the ventral striatum.
These and other findings [Cohen et al., 2009a, b; Munte et al., 2007] suggest that electrophysiological oscillatory synchrony may be the mechanism that allows the MFC and other limbic regions to bias processing in the ventral striatum according to goals, context, and reinforcements. We have previously suggested that when signaling the need to adjust behavior, the functional organization of a limbic network in which the nucleus accumbens plays a key role shifts from a local to a more global configuration [Cohen et al., 2009b]. The present findings buttress this idea and suggest that the MFC provides a top–down biasing signal that may modulate reward signaling in the nucleus accumbens. Further, by recording simultaneously from the finger muscles, we could rule out a simple alternative hypothesis that the top–down signal reflected a direct motor process.
These findings are also interesting from a clinical perspective because DBS to the MFC (white matter of the subgenual cingulate area 25) has proven effective at alleviating psychiatric conditions, specifically major depression [Mayberg et al., 2005]. It is possible that part of this effect is related to DBS‐induced top–down modulation of the ventral striatum. Even though these two DBS targets may have unique patterns of connectivity on their own [Gutman et al., 2009], because DBS can stimulate distant regions due to antidromic activation and modulatory effects [McCracken and Grace, 2009], DBS to either nucleus accumbens or cingulate area 25 may have overlapping functional consequences.
Generalizability of these findings to healthy individuals is important to consider. We have previously discussed limitations of invasive nucleus accumbens DBS recordings in more depth [Cohen et al., 2009a, b]. As in previous studies, stimulation had not begun before the recordings, and so long‐lasting effects of DBS could not have influenced our results. The principal issue with regard to generalization to healthy brains is that there is no adequate control group because DBS surgery will always target circuits thought to be dysfunctional. Nonetheless, findings supporting a directional flow of information from the MFC to the nucleus accumbens are consistent with the known direction of anatomical fiber pathways. Thus, we expect that the magnitude of these effects—and not their qualitative patterns—are stronger in healthy controls compared to patients with OCD. For example, such a quantitative but not qualitative difference in cortical–striatal functional connectivity between OCD patients and controls has been observed with functional MRI [Remijnse et al., 2006].
REFERENCES
- Brady AM, O'Donnell P ( 2004): Dopaminergic modulation of prefrontal cortical input to nucleus accumbens neurons in vivo. J Neurosci 24: 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR ( 2000): Projections from the rat prefrontal cortex to the ventral tegmental area: Target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci 20: 3864–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Heller AS, Ranganath C ( 2005): Functional connectivity with anterior cingulate and orbitofrontal cortices during decision‐making. Brain Res Cogn Brain Res 23: 61–70. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE ( 2009a): Good vibrations: Cross‐frequency coupling in the human nucleus accumbens during reward processing. J Cogn Neurosci 21: 875–889. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE ( 2009b): Nuclei accumbens phase synchrony predicts decision‐making reversals following negative feedback. J Neurosci 29: 7591–7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Hassani OK, Schultz W ( 2005): Relative reward processing in primate striatum. Exp Brain Res 162: 520–525. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S ( 2004): EEGLAB: An open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21. [DOI] [PubMed] [Google Scholar]
- Denys D, Mantione M ( 2009): Deep brain stimulation in obsessive–compulsive disorder. Prog Brain Res 175: 419–427. [DOI] [PubMed] [Google Scholar]
- Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, Bosch A, Schuurman R: Deep brain stimulation of the nucleus accumbens for therapy‐refractory obsessive–compulsive disorder (in press). [DOI] [PubMed]
- Groenewegen HJ, Wright CI, Beijer AV ( 1996): The nucleus accumbens: Gateway for limbic structures to reach the motor system? Prog Brain Res 107: 485–511. [DOI] [PubMed] [Google Scholar]
- Gutman DA, Holtzheimer PE, Behrens TE, Johansen‐Berg H, Mayberg HS ( 2009): A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry 65: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Calzavara R ( 2009): The cortico‐basal ganglia integrative network: The role of the thalamus. Brain Res Bull 78( 2/3): 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B ( 2010): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd‐Balta E ( 1995): The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci 15( 7 Pt 1): 4851–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR ( 2000): Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 20: 2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R ( 2006): Reward‐related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive‐based learning. J Neurosci 26: 8368–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD ( 2009): Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 66: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Frost AS, Moghaddam B ( 2001): Stimulation of prefrontal cortex at physiologically relevant frequencies inhibits dopamine release in the nucleus accumbens. J Neurochem 78: 920–923. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Wrase J, Heinz A ( 2006): Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage 29: 409–416. [DOI] [PubMed] [Google Scholar]
- Kienast T, Heinz A ( 2006): Dopamine and the diseased brain. CNS Neurol Disord Drug Targets 5: 109–131. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D ( 2001): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR ( 2010): Addiction related alteration in resting‐state brain connectivity. Neuroimage 49: 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH ( 2005): Deep brain stimulation for treatment‐resistant depression. Neuron 45: 651–660. [DOI] [PubMed] [Google Scholar]
- McCracken CB, Grace AA ( 2009): Nucleus accumbens deep brain stimulation produces region‐specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci 29: 5354–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munte TF, Heldmann M, Hinrichs H, Marco‐Pallares J, Kramer UM, Sturm V, Heinze HJ ( 2007): Nucleus accumbens is involved in human action monitoring: Evidence from invasive electrophysiological recordings. Front Hum Neurosci 1: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ ( 2004): Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304: 452–454. [DOI] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, Veltman DJ ( 2006): Reduced orbitofrontal–striatal activity on a reversal learning task in obsessive–compulsive disorder. Arch Gen Psychiatry 63: 1225–1236. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V ( 2008): Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33: 368–377. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones‐Gotman M ( 2001): Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain 124( Pt 9): 1720–1733. [DOI] [PubMed] [Google Scholar]
- Wise RA ( 1996): Addictive drugs and brain stimulation reward. Annu Rev Neurosci 19: 319–340. [DOI] [PubMed] [Google Scholar]


