Abstract
Previous studies suggest the importance of medial temporal lobe, areas of parietal cortex, and retrosplenial cortex in human spatial navigation, though the exact role of these structures in representing the relations of elements within a spatial layout (“allocentric” representation) remains unresolved. Hippocampal involvement, in particular, during memory processing is affected by whether a previously formed representation is employed in a novel fashion (“flexible” usage) or in a manner comparable with how it was encoded originally (“rigid” usage). To address whether brain systems are differentially involved during flexible vs. rigid utilization of a pre‐existing allocentric representation, subjects encoded the position of six different target buildings relative to a centrally located landmark building in a virtual city seen from an aerial view. They then actively searched for the locations of these target buildings using the landmark (rigid retrieval) or using a previously shown target building in a novel fashion (flexible retrieval) while undergoing fMRI. Activations in posterior superior parietal cortex and precuneus were greater during more rigid than flexible forms of allocentric retrieval while activation in the hippocampus decreased linearly over blocks during flexible allocentric retrieval. A functional connectivity analysis further revealed significant interactions between hippocampus and these parietal areas during flexible compared with rigid allocentric retrieval. These results extend previous models of the neural basis of spatial navigation by suggesting that while the posterior superior parietal cortex/precuneus play an important role in allocentric representation, the hippocampus, and interactions between hippocampus and these parietal areas, are important for flexible utilization of these representations. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: hippocampus, parahippocampus, retrosplenial, posterior superior parietal cortex, spatial navigation, spatial memory, partial learning paradigm
INTRODUCTION
Navigation is a central component of our daily lives and the ability to locate ourselves in space is important to our survival. Navigation, particularly successful way‐finding, has been hypothesized to rely in part on “cognitive maps,” which refer to spatial representations referenced to external coordinate systems. Repeated trajectories through an environment allow us to build up a representation independent of our heading that becomes referenced to external landmarks (“allocentric”) rather than our body position (“egocentric”), thus providing one means of forming a cognitive map [Klatzky, 1998; O'Keefe and Nadel, 1978; Sholl, 1987; Tolman, 1948]. An example of this would be remembering the location of a restaurant you enjoy based on the fact that it is located 1/3 of the way between a street corner and a supermarket you frequent. Another means of extracting the relations between landmarks in a spatial layout is by viewing or learning their interrelations from an aerial perspective or cartographic map [Siegel and White, 1975; Thorndyke and Hayes‐Roth, 1982; Moeser, 1988; Taylor and Tversky, 1992]. The processes of building allocentric representations has been suggested to depend on brain regions such as the hippocampus [Brown et al., 2010; Goodrich‐Hunsaker and Hopkins, 2010; Goodrich‐Hunsaker et al., 2010; Hartley et al., 2003; Igloi et al., 2010; Maguire et al., 1998b; O'Keefe and Nadel, 1978; Parslow et al., 2004, 2005; Suthana et al., 2009], retrosplenial/superior posterior parietal cortex [Burgess et al., 2002; Epstein, 2008; Rosenbaum et al., 2004; Shelton and Gabrieli, 2002; Shelton and Pippitt, 2007; Wolbers and Buchel, 2005], parahippocampal cortex [Committeri et al., 2004; Galati et al., 2010; Jordan et al., 2004; Maguire et al., 1998a; Shipman and Astur, 2008], and precuneus [Committeri et al., 2004; Galati et al., 2010]. These previous studies have employed environments with which subjects have had varying degrees of familiarity (e.g., a well‐learned environment vs. a novel environment) and have utilized different degrees of first‐person vs. map‐based learning (e.g., subjects directly navigating or subjects viewing layouts from an aerial perspective and/or attempting to draw maps), all of which could potentially influence the relevant brain regions involved reported. Thus, the extent to which first‐person vs. aerial perspectives and familiar vs. novel spatial environments influence regional brain involvement in allocentric representation remains an open question.
Previous fMRI studies demonstrating the involvement of retrosplenial and superior posterior parietal cortex in allocentric coding often, explicitly or implicitly, involved some degree of reliance on cartographic maps. Wolbers and Buchel [2005] had subjects view videos of navigation through a virtual environment and then draw aerial maps of the environment following their fMRI session, providing a putative measure of the geometric relation of landmarks to each other (“survey knowledge”). Following each encoding session, subjects also indicated the relative location of a building that appeared next to a landmark (right, left, or behind). Retrosplenial activation correlated with improvements in performance across sessions, leading the authors to infer a greater involvement of retrosplenial cortex in map representation compared with hippocampus. In a similar vein, Rosenbaum et al [2004] looked at relative and absolute distance judgments (as well as other measures) of experienced Toronto navigators. Participants, who had extensive experience navigating Toronto (and likely viewed maps at some points as part of learning the city) showed significant retrosplenial and parahippocampal activation, but no hippocampal activation, during all three types of judgment tasks relative to a perceptual baseline task [see also Epstein and Higgins, 2007; Epstein et al., 2007; Shelton and Pippitt, 2007]. These findings argue for the importance of cortical rather than direct hippocampal involvement in allocentric representation.
In contrast, several fMRI studies involving free navigation of a spatial environment provided evidence for hippocampal and parahippocampal involvement in forming “on‐the‐fly” spatial representations of the environment. In a study by Hartley et al. [2003], subjects freely navigated in a virtual environment and then explored the environment again while undergoing fMRI. When subjects employed novel paths to get around obstacles, path accuracy within and between subjects was associated with greater hippocampal activation. Similarly, when subjects used a spatial strategy (remembering the locations of landmarks in a virtual maze), Iaria et al [2003] found greater hippocampal activation compared with randomly searching for objects placed around the maze [see also: Aguirre et al., 1996; Parslow et al., 2004; Shipman and Astur, 2008; Suthana et al., 2009; Brown et al., 2010]. Similar results have also been obtained for “free” mental navigation of recently learned spatial environments, although the inferences of these studies are necessarily limited by the fact that it is impossible to precisely monitor subjects' actual movements during imagination [Ghaem et al., 1997; Mellet et al., 2000]. As a whole, the inference from these studies is that subjects with better topographical representations formed “on‐the‐fly” during free navigation utilized their hippocampus to a greater degree [see also Voss et al., 2010].
Consistent with the idea that map‐learning and free navigation possibly induce different types of representations, previous cognitive work suggests differences between information learned from route‐based navigation vs. learning and retrieving information from maps. Evans and Pezdek [1980], in a finding subsequently replicated in several studies, found that recalling the positions of elements within both familiar maps (United states) and maps of recently learned layouts (an unfamiliar campus) showed a strong degree of dependence on the original learned perspective, such as north vs. south orientation [Evans and Pezdek, 1980; Levine et al., 1984; Presson et al., 1989; Richardson et al., 1999; Shelton and McNamara, 2004]. In contrast, recalling information following free navigation showed less perspective dependence [McNamara et al., 2003; Sholl, 1987]. These findings argue for the idea that map‐learning may result in view‐dependent representations that are comparatively inflexible to manipulation in contrast to route learning. Together, these studies suggests that map‐learning and route learning could have differential effects on how we structure our spatial representations [Thorndyke and Hayes‐Roth, 1982].
Because the degree of map‐learning and route learning may differentially affect spatial representations, this could, in turn, possibly affect neural representations of spatial layouts [Shelton and Gabrieli, 2002; Shelton and Pippitt, 2007]. In attempt to better understand how map‐learning vs. free (first‐person) navigation affect neural representations, we wished to study the converse situation of what has often been studied previously [Rosenbaum et al., 2004; Wolbers and Buchel, 2005]: how people navigate a spatial environment following learning a spatial layout using aerial‐view snap‐shots. We reasoned that this manipulation could potentially provide insight into how subjects utilize a novel representation formed during simulated map‐learning (learning from an aerial perspective) when they subsequently utilize these representations “on‐the‐fly” during free navigation. We did this by having subjects first study aerial views of the spatial relations between buildings (“stores”) arranged around a centrally placed landmark building (“the landmark”). We then employed fMRI to study neural activity while they freely searched for these stores during two critical searching conditions. A rigid retrieval condition involved finding the hidden target store by referencing to its position relative to the landmark learned previously from the aerial viewpoint. Thus, other than having to transfer knowledge from the aerial to first‐person viewpoint, this condition closely matched how they had learned it from the previous aerial view. A flexible retrieval condition also involved finding the hidden target store but referenced to the location of a previously learned store rather than the landmark. In this condition, subjects utilized their pre‐existing spatial representation in a novel fashion, because, in addition to transferring their knowledge from the aerial to first‐person viewpoint, they had to extrapolate between different encoding trials to derive the location of one store relative to the other. We additionally conducted two control conditions and a baseline task, which we describe in more detail in the methods. We analyzed concurrently acquired fMRI data to determine brain regions active during flexible vs. rigid allocentric retrieval and additionally employed a functional connectivity to assess interactions between relevant brain regions.
MATERIALS AND METHODS
Participants
Seventeen subjects (seven male, ten female) were recruited from the general population in Davis, CA area. Subjects were free of significant neurological deficits and had no history of psychiatric disorders. All were right‐handed and had normal or corrected‐to‐normal vision and gave written informed consent to participate in the study, which was approved by the institutional review board at the University of California, Davis. One of the subjects was excluded in the further data analysis because of excess head movement; another subject was excluded due to technical issues with the scanner. The final data set was comprised of fifteen subjects.
Experimental Stimuli
We used Panda3D software (Entertainment Technology Center, Carnegie Mellon University) to present a virtual environment consisting of a set of rectangular stores (∼3.8 × 2.6 × 2.3 virtual units, length × wide × height), a circular wall (40 virtual units in radius and 5 virtual units in height), sky, and clouds. A landmark store was located at the center of the circle; the four sides of the landmark were different colors (black, blue, pink, and green) and could easily be distinguished from both the aerial view and first‐person navigation (see Fig. 1A–C). Surrounding the landmark were six stores, which were arranged irregularly on an invisible concentric circle (30 virtual units in radius). The entire layout was contained within the circular wall (see Fig. 1C).
Figure 1.
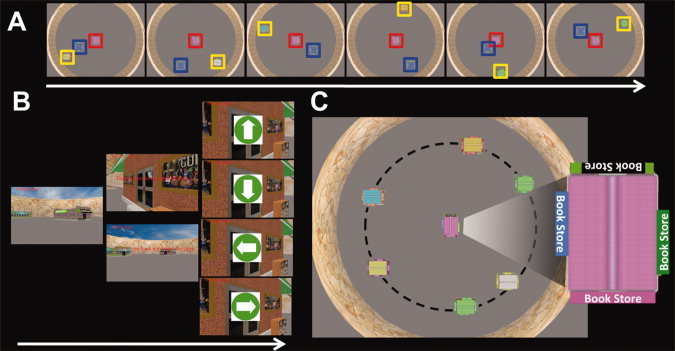
Experimental design. A: Example layouts subjects experienced during encoding. The red‐box indicates the landmark, the blue box indicates the randomly located store used as part of one of the control conditions, and the yellow box indicates the store subjects were encoding on that trial. B: An example of a retrieval trial in the landmark condition. Subjects navigated to the hidden target stores using the landmark; navigation occurred from a first‐person perspective. Subjects then performed the baseline task, which involved following arrows with the joystick. C: Schematic of the layout of the city from an aerial view. The store at the center of the circle was the landmark. The locations of stores on the dashed line were fixed relative to the landmark. Zoomed inset shows actual view of the landmark from an aerial view experienced by subjects. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The locations of the stores on the invisible circle relative to the landmark store were the same every trial; however, a seventh store, used as part of our control condition, appeared in a random location every encoding trial and therefore was not possible to represent relative to the landmark. The rules regarding the position of the randomly located store were that (a) its location was randomized each trial that it appeared in the scene during both the encoding and retrieval session (b) its position never overlapped with the position of other stores. Subjects learned the positions of stores relative to the central landmark from an aerial view 78 virtual units above the center of the virtual city (Fig. 1A). We employed a partial learning paradigm in which subjects viewed a set of configurations one by one, comprised of a store seen in conjunction with the centrally located landmark and a randomly located store (see Fig. 1A). There were six configurations in each encoding block with one configuration for each store. There were six encoding blocks in total; the order of configurations within each encoding block was randomized and each configuration appeared for 6 s.
Retrieval During Active Navigation
After encoding, we tested knowledge of the spatial layout of the virtual city by having subjects drive to the locations of the stores they had just encoded while they underwent functional imaging. For each trial, the target store was rendered invisible such that they had to reference to external landmarks to find its location. Subjects drove to the target store in four randomly interspersed conditions.
The two primary conditions of interest were the landmark condition and store‐reference conditions (Fig. 2B). The landmark condition was designed to replicate, as closely as possible, the conditions under which subjects had encoded this information from the aerial perspective. At the beginning of each trial, subjects were positioned to face the landmark and then navigated to a hidden target store using the landmark. The target store was hidden until subjects were 14 virtual units from it; when they were within this distance from the target store, it appeared on the screen and subjects drove directly to it. In the store‐reference condition, at the beginning of each trial, subjects were positioned to face one of the six stores (see Figs. 1C and 2B). Subjects then navigated to the hidden target store using the other store as a reference (the target store was again invisible until subjects were within 14 virtual units of its location). Subjects had not previously experienced these two stores in the same encoding configuration and had to extract the location of the hidden target store from knowledge of the different encoding configurations (see Fig. 2A,B for a schematic comparison of the two conditions).
Figure 2.
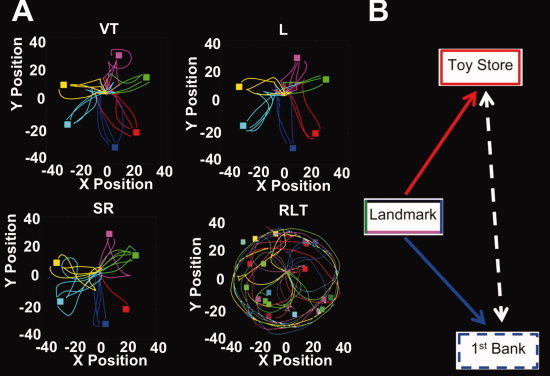
Example subject paths and schematic of store‐reference and landmark conditions. A: Examples of paths taken in each condition of one representative subject. The colored squares were the positions of each store on the invisible circle (dashed line in Fig. 1c.) while the colored lines were routes the subject employed to find the target store. VT, the visible target condition (control condition); L, the landmark condition; SR, the store‐reference condition; RLT, the randomly located target store condition (control condition). B: Schematic illustrating the landmark vs. store‐reference conditions. As an example, in the landmark condition, subjects navigated to 1st Bank using the landmark. In contrast, in the store‐reference condition, subjects needed to use Toy Store as a reference to find the 1st Bank. Thus, in the store reference conditions, subjects inferred the position of Toy Store based on their knowledge of its location relative to 1st Bank on the circle (dashed white line). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In the randomly located store control condition, which involved finding the seventh randomly placed store they had seen during encoding trials, subjects searched as best as they could for the randomly located hidden target. This condition was designed to control for the use of an allocentric representation because in this condition, subjects navigated randomly and could not reference to other stores to find the target store. This condition, however, still required continuous updating of one's bearing to avoid searching in the same locations they had previously visited.
In a second control condition, the visible target condition, subjects navigated directly to the target store while it was continuously visible to subject; this condition was designed to control for the presence of visually salient objects in our task.
Finally, in a baseline task, subjects moved the joystick following the direction of an arrow shown on the screen (Fig. 1B) for 10 s while movement remained stopped; the arrow pointed randomly either left, right, up, and down [Ekstrom et al., 2009; Stark and Squire, 2001]. The baseline task was designed to control for movement unrelated to active navigation.
General Procedure
We first placed subjects in a “mock” scanner to accustom them to virtual navigation in the MRI environment. They performed 20 min of practice driving in an unrelated virtual environment. Subjects then viewed three‐dimensional images of all stores they would subsequently view on a gray background to familiarize them with store names and images. Each store appeared for eight seconds and subjects read the name of the store outloud to make sure they could distinguish between the different stores. Subjects then began the encoding session. After encoding, subjects were placed in the scanner for the retrieval task.
Subjects started each trial in a random position in the virtual city. This was done to avoid alignment in facing direction between learning and test phases, which might inadvertently serve as an implicit egocentric retrieval cue [McNamara et al., 2003]. We determined the subjects starting position on each trial according to the following criteria: (1) the location was randomly generated for each trial, (2) the initial position was at least six virtual units from all the stores in the scene, (3) the initial position was a minimum of 15 virtual units from the target store, (4) subjects always faced the landmark at the beginning of the trial in the landmark and two control conditions and faced the reference‐store in the store‐reference condition. While searching for the target store, subjects were encouraged to take a path as direct as possible and to find a target store as quickly as possible. Each trial during the retrieval phase lasted 40 seconds in total. Subjects searched for the target store for 30 s and then performed the baseline task for 10 s. In cases in which subjects found the target store before expiration of the allotted time, they performed the baseline task for the remaining period. There were three trials for each condition in a block resulting in 12 trials in each test block with six test blocks in total. The order of trials in each test block was randomized.
Behavioral Data Acquisition and Analysis
During active navigation (the retrieval session in which we scanned brain activity), we recorded the position and facing direction of subjects every 20 ms into a log file. We analyzed the response accuracy, latency and excess path length for each of the four conditions. The response accuracy was the number of trials that subjects successfully found the target store within the allotted time (30 s) for each condition. Latency was the time interval between the onset of the trial and the onset of the baseline task following that trial (for correct trials only). The excess path length was computed by taking the deviation of the subject's actual path from the straightest path to the target, based on the idea that a better representation of the position of the target would lead to lower excess path length [Newman et al., 2007].
MRI Acquisition
Scanning was performed at the Imaging Research Center at the University of California, Davis on a 3T Siemens (Erlangen, Germany) Trio equipped with a thirty‐two‐channel head coil. Forty‐three contiguous axial slices were acquired using a gradient‐echo echo‐planar T2*‐sensitive sequence [repetition time (TR), 3000 ms; echo time (TE), 29 ms; voxel size, 2.5 × 2.5 × 2.5 mm3; matrix size, 88 × 88 × 43]. Structural T1‐weighted images for anatomical localization were acquired using a three‐dimensional magnetization‐prepared rapid‐acquisition gradient echo pulse sequence [TR, 1,900 ms; TE, 2.88 ms; inversion time (TI), 1,100 ms; voxel size, 1 × 1 × 1 mm3; matrix size, 256 × 256 × 208]. Visual stimuli were projected onto a screen at the end of the scanner with an Digital Projection Mercury 5000HD projector equipped with a three‐chip DLP technology (Edmund Optics, Barrington, NJ 08007‐1380) and viewed through a custom in‐bore screen (Mag Design and Engineering, Inc. Sunnyvale, CA 94086) mounted to the head coil. Responses were recorded using a MR‐compatible joystick (Current Design, Philadelphia, PA). The linear and turning acceleration of the joystick were 28 virtual units/s2 and 22 deg/s2, respectively; the full linear and turning speed were 28 virtual units/s and 100 deg/s, respectively.
fMRI Data Analysis
Image processing and statistical analysis were performed using Statistical Parametric Mapping (SPM8, The Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK). Functional images were corrected for differences in slice timing by resampling slices in time to match the first slice of each volume, realigned with respect to the first image of the scan, and high‐pass filtered to remove baseline drifts. After resampling the functional data at 1 x 1 x 1 mm3 and normalizing to the MNI template, the functional data were resliced to their original 2.5 × 2.5 × 2.5 mm3 resolution and smoothed with a 6 mm full‐width at half‐maximum (FWHM) isotropic Gaussian kernel. Data were first modeled for each subject individually using a general linear model (GLM) [Friston et al., 1995]; only correct trials were modeled. This first‐level analysis provided parameter estimates for each condition and each subject. These parameter estimates were then entered into a repeated‐measures, whole brain, random‐effects 1 × 4 condition (landmark, store reference, randomly located, and visually guided conditions) ANOVA to determine activation patterns across the group. We extracted the mean signal based on the intersection of clusters revealed by the whole brain ANOVA and specific brain areas of interest. We then conducted post hoc t‐tests comparing the percent signal change within these large clusters between individual conditions. We also conducted an additional ANOVA on these signals to see how relative activations between regions might change due to our task. Because statistical tests on signal change extracted from a large cluster might fail to reveal smaller clusters of significant activity within that larger cluster, we additionally conducted voxelwise post hoc t‐tests within clusters of activation revealed by the ANOVA.
fMRI Linear BOLD Signal Analysis
We searched for linear decreases in BOLD across each of the four conditions based on previous work suggesting decreases in hippocampal activation over learning blocks in particular during navigation [Wolbers and Buchel, 2005]. We did this by convolving the HRF with a decreasing linear function over trials.
fMRI Functional Connectivity Analysis
We used the beta‐series correlation method [Rissman et al., 2004] to analyze whole brain functional connectivity relative to seed ROIs. For this analysis, a new GLM matrix was constructed to model each trial individually for each subject. Whole brain maps of functional connectivity were generated for each subject by extracting beta values for each trial from each seed ROI and correlating the mean beta values across trials with every other voxel in the brain [Rissman et al., 2004]. Four seed ROIs were chosen: parahippocampus, precuneus, retrosplenial, and superior parietal lobe. To avoid the possibility of selection bias (“double dipping”) [Kriegeskorte et al., 2009], we selected clusters of activation with our ROIs based on three previous studies of navigation using fMRI. No single previous study reported clusters of activation in all four ROIs so we selected clusters from most relevant papers that contained activations in these areas, all of which we have mentioned previously in the Introduction. A study by Wolbers and Buchel [2005] found activation in retrosplenial cortex [cluster centered at (18 ‐68 60)] and superior parietal cortex (cluster centered at [‐14 ‐54 58)], a study by Hartley et al. [2003] found activation in precuneus [cluster centered at (6 ‐66 33)], and a study by Epstein and Higgins [2007] reported activation in parahippocampal cortex [cluster with maximum activation centered in parahippocampal cortex at (20 ‐36 ‐6)]. We then created seeds of activation by averaging mean signal change based on a sphere of radius 20 mm centered on these coordinates and intersected with our anatomical regions of interest.
Correction For Multiple Comparisons
For all whole brain analyses (ANOVA and post hoc voxel‐wise t‐tests), we used a threshold of P FWE < 0.05, i.e., corrected for family‐wise error (FWE). We did this by calculating the cluster size needed for a threshold of P FWE < 0.05 using Monte Carlo simulations [Forman et al., 1995] with alphaSim software based on an uncorrected, voxelwise P < 0.005. These simulations showed that our threshold of P FWE < 0.05 corresponded to P < 0.005 with a voxel extent (k) of 38. For behavioral analyses and post hoc comparisons on percent signal change extracted from ROIs, we employed an uncorrected P‐value of 0.05.
RESULTS
Behavioral Results
For all the behavioral indices, there were no differences between the landmark and store‐reference conditions (response latency: [t(14) = 0.74, P = 0.47]; accuracy: [t(14) = 1.20, P = 0.25]; excess path length: [t(14) = 1.08, P = 0.30]; see Table I; sample paths shown in Fig. 2A,B). Speed and accuracy were highly negatively correlated across subjects (r = −0.99, df = 14), indicating that the higher the accuracy in finding a hidden target store, the faster the subject found the target. This indicates there was no speed‐accuracy trade‐off in our data [Fitts, 1954], justifying analysis of our reaction time data. As might be expected for the two control conditions, we found differences in performance in the randomly located and visually guided condition. Performance was significantly different in both conditions compared with the experimental conditions, which was likely due to the fact that both experimental conditions required retrieving and manipulating a learned representation while the control conditions did not necessitate these. We discuss differences in performance, excess path, and latency in the control conditions in detail in the Supporting Information. We accounted for the differences in performance in terms of functional activation patterns by including only correct trials in each condition; including subject performance as covariates separately for each condition led to identical results. Thus, we include only analyses using correct trials for further consideration.
Table I.
The mean (standard deviation) response latency, accuracy, and excess path length of the behavioral data during test phase
| Visible target condition | Landmark condition | Store reference condition | Randomly located Target Store Condition | |
|---|---|---|---|---|
| Response Latency (s) | 14.22 (1.96) | 18.49 (1.75) | 18.89 (2.47) | 16.01 (1.43) |
| Accuracy | 0.91 (0.12) | 0.68 (0.18) | 0.63 (0.18) | 0.78 (0.16) |
| Excess path length | 6.78 (3.87) | 8.10 (3.91) | 10.07 (4.46) | 15.67 (7.76) |
To ensure that subjects transferred knowledge from the aerial to the first‐person perspective, as hypothesized, we analyzed their performance on the first trial in which they navigated to each target store. We divided their trajectories for each trial into four different quadrants (the target quadrant, two adjacent quadrants, and the opposite quadrant) to assess whether they searched the target quadrant at a level that would be expected to exceed a chance level of searching [Morris et al., 1982]. We averaged the time spent and distance traveled in each quadrant over all trials separately for each subject. We found that in the landmark condition, subjects spent 39% of their total searching time and traveled 42% of their total path in the same quadrant as the target, which was significantly higher than a chance level of 25% (time: t(14)=4.42, P < 0.001; path length: t(14)=5.43, P < 0.001). Similarly, in the store‐reference condition, subjects spent 33% of their total searching time and traveled 33% of their total path in the same quadrant as the target, which was significantly greater than chance (time: t(14)=2.98, P < 0.01; path length: t(14) = 2.97, P < 0.05). Because one possibility is that subjects simply avoided the most distant quadrant rather than searching in the target quadrant, we directly compared searching time and total distance traveled in the target quadrant compared with the two adjacent quadrants. We found that subjects searched in the target quadrant at significantly higher levels than the adjacent quadrants (landmark condition: time: t(14) = 3.53, P < 0.005, path length: t(14) = 4.27, P < 0.001; store‐reference condition: time: t(14) = 2.19, P < 0.05, path length: t(14)=2.30, P < 0.05). These findings support the idea that in both the landmark and store‐reference condition, subjects transferred knowledge from the aerial view to the first‐person perspective to successfully locate target stores. We discuss possible explanations for their relatively low accuracy levels on the first blocks of finding the target in the discussion.
We then looked at whether performance improved over trials during navigation and whether this changed differentially between the landmark and store‐reference condition as a function of retrieval trial. Both conditions showed a significant linear increase in accuracy over blocks [see Supporting Information Figure 1S, F(1,14) = 14.49, P < 0.005 in the store‐reference condition; F(1,14) = 4.48, P < 0.05 in the landmark condition], consistent with the idea that the retrieval conditions involved some degree of new learning as subjects repeatedly navigated to target stores over blocks. Degrees of learning over blocks did not differ between the two conditions [no interaction effect between conditions and blocks, F(5,70) = 0.903, P = 0.484], suggesting equivalent degrees of new learning in both conditions.
fMRI Analysis: Involvement of Precuneus and Superior Posterior Parietal Cortex in Rigid Allocentric Representation
Our whole brain ANOVA analysis revealed a large cluster of activation centered in the precuneus and medial temporal gyrus (Fig. 3E and Table 1S). We did not find significant activation in the hippocampus with our ANOVA comparison. Using these clusters, we then conducted post hoc tests on clusters identified with our ANOVA analysis in parahippocampus, retrosplenial, precuneus, and superior posterior parietal lobe.
Figure 3.
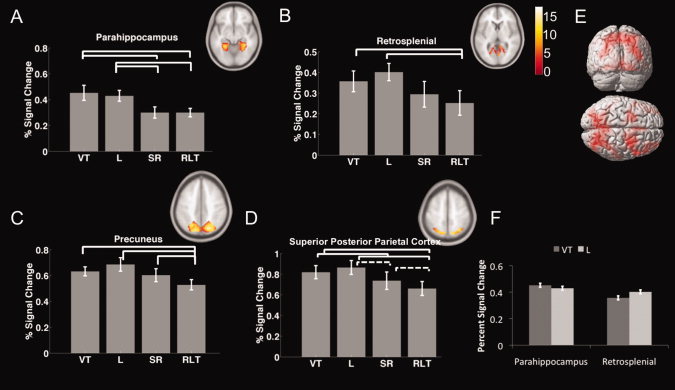
ROI analyses illustrating brain regions involved in experimental manipulations. VT, the visible target condition; L, the landmark condition; SR, the store‐reference condition; RLT, the randomly located target store condition. A–D: Region of interest analysis for (A) parahippocampal cortex, (B) retrosplenial cortex, (C) precuneus, and (D) superior posterior parietal lobe. E: Clusters of activation identified by ANOVA (Table 1S) (P FWE < 0.05). F: Interaction effect between parahippocampus and retrosplenial cortex in the visible target and landmark condition. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Mean percent signal change within all clusters within regions of interest (Fig. 3) showed greater activity in the landmark condition to be higher than the randomly located store control condition [t(14) = 2.86, 2.29, 2.77, and 2.85, respectively; P < 0.05]. Activation during the store‐reference compared with the randomly located store control was significantly higher [t(14) = 2.96, P < 0.05] in precuneus and marginally significantly higher [t(14) = 2.05, P = 0.059] in superior posterior parietal lobe. Voxelwise post hoc t‐tests (conducted at P FWE < 0.05; see Methods) confirmed the significance of the effect in superior posterior parietal lobe (‐24, ‐70, 42) and additionally revealed smaller clusters of activation in retrosplenial cortex (‐11, ‐65, 9) during the store‐reference vs. randomly located store control condition (Fig. 4A and Table II). Activation during the landmark compared with the store‐reference condition was significantly higher [t(14) = 2.63, P < 0.05] in the parahippocampal cortex and marginally significantly higher [t(14) = 2.01, P = 0.06] in superior posterior parietal cortex. Voxelwise post hoc t‐tests again confirmed the significance of activation in superior posterior parietal cortex (30, ‐60, 45) and additionally revealed smaller clusters of activation in precuneus (‐23, ‐62, 38) during the landmark vs. store‐reference contrast (see Fig. 4B and Table II). Activation for the visible target control condition was higher than the randomly located store control condition in parahippocampus, precuneus, retrosplenial, and superior parietal lobe [t(14) =3.42, 2.66, 3.26, and 3.41, P < 0.05 for all the comparisons]. In the parahippocampal cortex activation in the visible target control condition was also higher than the store‐reference condition [t(14) = 2.92, P < 0.05]; this was not the case in any other brain regions.
Figure 4.
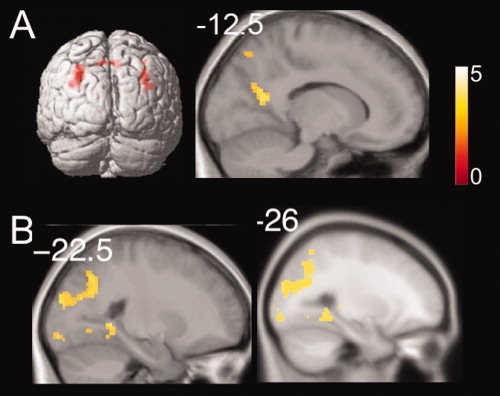
Post hoc voxel‐wise t‐tests between conditions of interest. A: Store‐reference vs. control contrast showing significant activation in retrosplenial cortex (P FWE < 0.05). B: Landmark vs. store‐reference contrast showing precuneus and superior posterior parietal activation (P FWE < 0.05). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table II.
Spatial coordinates of brain regions of post hoc t‐test between conditions of interested (P FWE < 0.05)
| Region | Coordinate (x, y, z; in mm) | Voxel level (z‐score) | |
|---|---|---|---|
| LH | RH | ||
| Paired t‐test between the landmark and the store‐reference condition | |||
| Inferior Frontal Gyrus | −38, 3, 30 | 3.66 | |
| Superior Parietal Lobule | 30, −60, 48 | 2.92 | |
| Inferior Parietal Lobule | −33, −54, 45 | 3.99 | |
| Middle Occipital Gyrus | 37, −77, 2 | 4.21 | |
| −30, −80, 20 | 3.71 | ||
| −36, −87, 5 | 3.66 | ||
| Inferior Occipital Gyrus | −33, −77, −8 | 3.67 | |
| Parahippocampal Gyrus | −28, −44, −8 | 4.15 | |
| Fusiform Gyrus | −38, −70, −15 | 4.49 | |
| 37, −44, −18 | 3.84 | ||
| 42, −64, −12 | 3.78 | ||
| Angular Gyrus | 30, −64, 32 | 3.17 | |
| Paired t‐test between the landmark and the randomly located target condition | |||
| Inferior Frontal Gyrus | −40, 3, 30 | 5.23 | |
| −38 10 28 | 4.76 | ||
| 40, 8, 30 | 3.56 | ||
| 54, 36, 15 | 3.00 | ||
| Middle Frontal Gyrus | 42, 28, 18 | 3.56 | |
| Medial Frontal Gyrus | −8, 16, 52 | 3.81 | |
| Superior Parietal Lobule | −28, −64, 40 | 5.76 | |
| 32, −60, 48 | 4.72 | ||
| Precentral Gyrus | −40, −10, 42 | 3.57 | |
| Precuneus | −33, −54, 45 | 5.65 | |
| Posterior Cingulate | 14, −57, 22 | 4.44 | |
| Angular Gyrus | 32, −64, 32 | 5.00 | |
| Paired t‐test between the store‐reference and the randomly located target condition | |||
| Precentral Gyrus | −43, 3, 35 | 3.28 | |
| Precuneus | −6, −74, 50 | 4.38 | |
| 7, −62, 45 | 3.97 | ||
| 40, −70, 35 | 3.33 | ||
| 22, −57, 22 | 3.48 | ||
| Superior parietal Lobe | 37, −57, 52 | 3.67 | |
| Middle Temporal Gyrus | 40, −70, 25 | 3.38 | |
| Angular Gyrus | −36, −77, 32 | 4.40 | |
RH, right hemisphere; LH, left hemisphere.
Although the absolute magnitude of activations may be problematic to compare between different brain regions because areas may differ in terms of raw signal strength (e.g., a main effect of ROI), the presence of an interaction effect indicates a relative difference in terms of activations produced by different conditions [Ekstrom and Bookheimer, 2007; Epstein and Higgins, 2007; Epstein et al., 2007]. We found a significant interaction effect in the visible target control and landmark condition between parahippocampal and retrosplenial cortex (Fig. 3F, F(1,14) = 5.177, MSE = 0.004, P < 0.05]); this interaction effect was also present between parahippocampal cortex and precuneus (F(1,14)=18.8, P < 0.001) and parahippocampal cortex and superior posterior parietal cortex (F(1,14)=42.8, P < 0.001). This indicated that retrosplenial, precuneus, and posterior parietal cortex activated to a higher degree in the landmark condition than the visible target control condition, while the parahippocampal cortex activated to a higher degree in the visible target control condition than the landmark condition. We conclude from this that the two conditions resulted in a different pattern of relative activation in the parahippocampal cortex compared with the other three regions, with parahippocampal cortex showing a greater relative involvement in the visible target control condition compared with the landmark condition.
To summarize the principle results from these analyses, we found (1) greater activation in retrosplenial cortex, posterior superior parietal cortex, and precuneus in the two allocentric conditions compared with the randomly located control condition, implicating these regions specifically in allocentric retrieval (2) greater activation in superior posterior parietal cortex and precuneus for the rigid form of allocentric retrieval compared with the flexible form, implicating these regions specifically in rigid forms of allocentric retrieval (3) greater activation in parahippocampal cortex for the rigid form of retrieval compared with the randomly located store control condition and the flexible condition, coupled with greater relative activation in parahippocampal cortex during the visible target control condition compared with the landmark condition, implicating parahippocampal cortex more specifically in rigid scene processing compared with the other three areas we investigated.
Linear‐Effects Analysis Showing Involvement of the Hippocampus in Flexible‐Allocentric Retrieval
As described in the methods, we modeled a linear change in activation over blocks to assess whether there was a systematic decrease in brain activation that paralleled retrieval in the different conditions [Wolbers and Buchel, 2005]. Activations are described in Table 2S. As shown in Figure 5A,B, we found a negative linear correlation with learning blocks in the hippocampus and parahippocampal cortex across trials in the store‐reference condition but not in the other three conditions. To explore the extent to which these changes over blocks correlated with behavioral performance, we extracted the percent signal change in these two clusters and calculated the correlation with response accuracy in each corresponding block in the store‐reference condition. As shown in the Figure 5C, in the store‐reference condition, hippocampal activation and accuracy were significantly correlated (r = −0.937, P < 0.01), an effect we also observed in the parahippocampus (r = −0.875, P < 0.05). To ensure that this effect did not generalize to the landmark condition, we applied the same cluster mask to the pattern of activations in the landmark condition; we did not find a significant correlation with accuracy in either the hippocampus (r = −0.299, P = 0.565) or parahippocampal cortex (r = 0.562, P = 0.246). These data thus support the idea that the hippocampus and parahippocampal cortex are involved early in learning in the store‐reference condition. We note that because we did not find differences in learning rate between the landmark and store reference conditions, this argues that the linear decrease we observed in the store reference but not landmark condition was not due to differences in performance but likely related to differential involvement of these two brain regions over trials.
Figure 5.
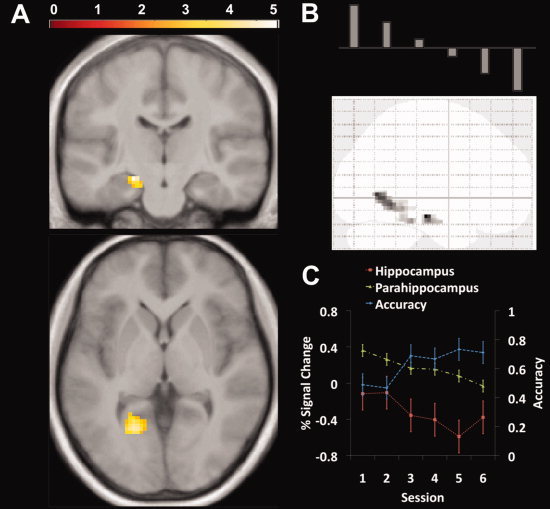
Decreasing linear activation in hippocampus during store‐reference condition. A: Brain areas that had a negative linear correlation with trials in the store‐reference condition. The hippocampus (−17.7 −15,9 −18) and parahippocampus (−10.1 −46.8 −6.66) showed significant clusters of activation in the whole brain analysis (P FWE < 0.05). B: The model used to perform the negative linear correlation analysis. C: Correlation between accuracy and activation in the hippocampus and parahippocampal cortex over testing blocks. We observed a significant negative correlation between accuracy and activation in both hippocampus and parahippocampal cortex. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Functional Connectivity Analyses Showing Significant Connectivity Between Hippocampus and Extrahippocampal Cortical Areas During the Store‐Reference Condition
We additionally performed a beta‐series correlation analysis to assess functional connectivity between regions in the landmark and store‐reference conditions [Rissman et al., 2004]. Because hippocampal activation was present only transiently over blocks, we used the extrahippocampal regions (parahippocampus, retrosplenial, precuneus, and superior posterior parietal cortical clusters; see Methods for details) as seeds in our connectivity analysis. The retrosplenial cortex had significantly higher connectivity with the inferior parietal lobe and the superior temporal gyrus in the landmark compared with the store‐reference condition (Table III). We also found significant connectivity between superior posterior parietal cortex and medial frontal gyrus and the precentral gyrus during the landmark > store‐reference contrast. See Table III for a summary of functional connectivity during the landmark > store‐reference contrast. No other seed regions showed significant functional interactions in the landmark > store‐reference contrast. These findings argue for the involvement of functional interactions between the retrosplenial cortex and superior posterior parietal cortex with other cortical areas in rigid‐forms of spatial representation.
Table III.
Spatial coordinates of brain regions showing higher functional connectivity for landmark vs. store‐reference condition with seed region indicated (P FWE < 0.05)
| Region | Coordinate (x, y, z; in mm) | Voxel level (z‐score) | |
|---|---|---|---|
| LH | RH | ||
| Seed = retrosplenial cortex | |||
| Superior temporal gyrus | 37, −40, 5 | 4.07 | |
| Inferior parietal lobule | 14, 8, 22 | 3.40 | |
| Sublobar | 44, −37, 28 | 3.44 | |
| Seed = superior posterior parietal cortex | |||
| Medial Frontal Gyrus | 20, −14, 52 | 3.70 | |
| Paracentral Lobule | 14, −27, 52 | 3.42 | |
| Precentral Gyrus | 27, −20, 45 | 3.13 | |
| 37, −20, 58 | 3.24 | ||
| 32, −32, 52 | 3.13 | ||
RH, right hemisphere; LH, left hemisphere
We then looked for brain areas that showed higher functional connectivity during the store‐reference vs. landmark condition. Both precuneus and superior posterior parietal cortex had significantly higher connectivity with the posterior hippocampus in this contrast (Fig. 6A,B). We did not find any significant connectivity between parahippocampal cortex and hippocampus nor retrosplenial cortex and hippocampus (Table IV). Retrosplenial cortex, however, showed significant functional connectivity with prefrontal cortex during the store‐reference > landmark contrast. We summarize areas showing significantly greater connectivity in the store‐reference vs. landmark contrast in Table IV. Our findings indicate that precuneus and superior posterior parietal cortex interacted functionally with the posterior hippocampus during retrieval in the store‐reference condition. Our functional connectivity results thus support the involvement of the hippocampus in the flexible store‐reference condition via interactions with cortical areas involved in more rigid forms of scene representation.
Figure 6.
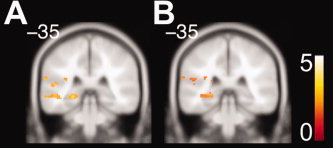
Functional connectivity analyses. Connectivity between hippocampus and extrahippocampal brain areas during the store‐reference condition (Tables III and IV). A: Significantly greater connectivity in hippocampus with precuneus for store‐reference > landmark (P FWE < 0.05). B: Significantly greater connectivity in hippocampus with superior posterior parietal cortex for store‐reference > landmark (P FWE < 0.05). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table IV.
Spatial coordinates of brain regions showing higher functional connectivity for store‐reference vs. landmark condition with seed region indicated (P FWE < 0.05)
| Region | Coordinate (x, y, z; in mm) | Voxel level (z‐score) | |
|---|---|---|---|
| LH | RH | ||
| Seed = parahippocampal cortex | |||
| Medial frontal gyrus | 14, 33, 48 | 3.74 | |
| −6, 26, 42 | 3.24 | ||
| 2, 48, 42 | 3.72 | ||
| Middle frontal gyrus | 22, 30, 45 | 3.28 | |
| Superior frontal gyrus | 14, 50, 40 | 2.95 | |
| Seed = precuneus | |||
| Insula | −36, −40, 20 | 4.14 | |
| Superior temporal gyrus | −43, −24, 2 | 3.73 | |
| Middle temporal gyrus | −40, −54, 8 | 3.18 | |
| −58, −57, 10 | 3.18 | ||
| −50, −27, −10 | 3.46 | ||
| Superior frontal gyrus | 22, 26, 52 | 3.94 | |
| 2, 50, 35 | 3.49 | ||
| Middle frontal gyrus | 40, 20, 50 | 3.82 | |
| 37, 28, 48 | 3.37 | ||
| −28, 20, 38 | 3.54 | ||
| Medial frontal gyrus | 10, 40, 42 | 3.50 | |
| Parahippocampal gyrus | −23, −37, −5 | 3.21 | |
| −23, −42, 5 | 2.75 | ||
| Hippocampus | −23, −34, −5 | 3.14 | |
| Seed = retrosplenial cortex | |||
| Superior frontal gyrus | 12, 48, 38 | 3.78 | |
| Middle frontal gyrus | −20, 33, 40 | 3.98 | |
| −30, 20, 40 | 3.44 | ||
| −36, 28, 40 | 3.32 | ||
| Medial frontal gyrus | −6, 26, 42 | 4.85 | |
| Cingulate gyrus | −20, 10, 38 | 3.42 | |
| Precentral gyrus | −40, 13, 40 | 3.33 | |
| Seed= superior posterior parietal cortex | |||
| Insula | −36, −44, 20 | 3.73 | |
| −38, −24, 28 | 3.71 | ||
| Hippocampus | −23, −42, −2 | 3.04 | |
RH, right hemisphere; LH, left hemisphere.
DISCUSSION
The goals of this paper were to (1) to investigate the effects of prior learning from an aerial perspective on the neural systems underlying active navigation; (2) to compare flexible and rigid implementations of allocentric representations acquired from an aerial view during first‐person navigation. Our two primary contrasts of interest involved subjects finding a target store in which they utilized a landmark to navigate to a hidden store (the “rigid” landmark condition) or utilized a store in a flexible fashion to find the hidden target (the “flexible” store‐reference condition). Both conditions necessitated computing the position of the hidden target based on its relative direction and position to the landmark or another store, therefore involving an allocentric computation [Klatzky, 1998; O'Keefe and Nadel, 1978; Tolman, 1948]. Furthermore, because subjects had to transfer knowledge from the aerial to first‐person perspective, they could not rely on previously learned viewpoint (egocentric) knowledge and instead required an allocentric strategy to find target stores. Thus, what is particularly novel in our approach is that because subjects learned representations prior to navigation, our contrasts allowed us to directly compare utilization of a spatial representation allocentrically in a more rigid compared with more flexible fashion. Our behavioral results indicated that subjects performed above chance on the first trial of retrieval for finding a specific store in both the landmark and store‐reference conditions, both when we compared the time spent in a quadrant and the distance traveled within a quadrant. We note, though, that the time spent and distance traveled within the target quadrant on the first trial of searching for the target store, particularly in the store‐reference condition, was numerically low (Landmark: 39%; Store‐reference: 33%). Transferring knowledge from the aerial perspective to a first‐person view‐point involved inferring information from one perspective to a different one and could be expected to be challenging [Hartley et al., 2007] and previous studies have noted that allocentric representations may be less precise and less readily employed than egocentric representations [Wang and Spelke, 2000; Waller and Hodgson, 2006]. Thus, because our paradigm involved subjects inferring information from one perspective to another while simultaneously employing an allocentric representation, subject performance, at least initially, could be expected to be numerically low.
In addition to transferring from one perspective to another, the store‐reference condition involved an additional inference in that subjects had to extrapolate the location of one store relative to another by inferring this information across different encoding trials. Despite this important and critical difference between these conditions, the store‐reference and landmark conditions did not differ on any of our behavioral measures (accuracy, latency, and excess path). Furthermore, improvements in performance over trials during first‐person navigation did not differ between the two conditions. Thus, although a challenging task, our data indicate that subjects did indeed utilize information from the aerial perspective to find their way during active navigation, and, despite the differences in transferring knowledge during the rigid and flexible conditions, these two conditions did not differ overall in performance. Our approach and behavioral findings thus positioned us to explore the neural correlates of rigid vs. flexible allocentric retrieval.
Roles of Superior Posterior Parietal, Retrosplenial Cortex, and Precuneus During Allocentric Representation
We first identified broad areas of activation related to our task using a whole brain ANOVA by comparing activation patterns across our four conditions of interest: the rigid and flexible allocentric retrieval conditions, navigating to a randomly located target store (randomly located store control condition), and finding a the target store when it appeared at the beginning of the trial (visible target control condition). We then performed post hoc tests to identify specific differences within conditions based on these activation clusters. Retrosplenial cortex, superior parietal lobe, and precuneus activated in both the landmark vs. control and store‐reference vs. control contrasts, indicating their general importance to allocentric retrieval. These findings are broadly consistent with previous studies demonstrating the involvement of retrosplenial cortex, precuneus, and superior posterior parietal cortex in allocentric representation [Committeri et al., 2004; Galati et al., 2010; Jordan et al., 2004; Rosenbaum et al., 2004; Shelton and Gabrieli, 2002; Shelton and Pippitt, 2007; Wolbers and Buchel, 2005). These findings are further consistent with the proposal of Galati et al. [2010] that the precuneus and retrosplenial cortex form a network of brain regions subserving retrieval of object locations within the context of a stable reference frame. Previous studies though have also implicated parts of posterior parietal cortex in egocentric representation [Galati et al., 2000, 2010; Neggers et al., 2006]. In our study, we looked specifically at an area of posterior parietal cortex, superior posterior parietal cortex, implicated in previous studies in contrasts of aerial view vs. first‐person encoding of spatial layouts and hypothesized to play a role in translation from multiple view points into a holistic object representation supporting an allocentric representation [Shelton and Gabrieli, 2002; Shelton and Pippitt, 2007]. Thus, it is possible that the activations we reported in superior posterior parietal cortex could potentially differ from those classically associated with egocentric representation in posterior parietal cortex.
Roles of Precuneus, Superior Parietal, and Retrosplenial Cortex in Rigid Forms of Spatial Representation
Because the landmark condition involved utilizing a spatial representation based on similar conditions to which it had been encoded while the store‐reference condition additionally involved reference to another store by inferring across encoding trials, the two conditions allowed us to directly contrast a more rigid form of spatial representation with a more flexible version. We observed that activation was higher in the rigid landmark condition compared with the flexible store‐reference condition in two key regions: precuneus and superior posterior parietal cortex. We also observed significantly higher connectivity between superior posterior parietal cortex/retrosplenial cortex with parts of temporal and parietal cortex during rigid compared with flexible allocentric retrieval (Table III). These data thus extend the model of Galati et al. [2010] to suggest the specific involvement of precuneus, superior posterior parietal cortex, and retrosplenial cortex in more rigid forms compared with more flexible forms of allocentric spatial representation.
Parahippocampal Cortex in Rigid Scene Representation
We also observed a significant interaction between the landmark and visible target conditions and their respective activation levels in parahippocampal cortex compared with the other three regions we investigated, with parahippocampal cortex showing relatively more activation in the visible‐target control compared with the landmark condition. The visible‐target condition differed from the other control condition in that it involved navigating directly to a visible store. A subject could potentially use the visible‐target condition to update the existing representation of the target locations and thus we cannot rule out that this condition involved some retrieval of a previously learned representation. Broadly speaking, though, the visible‐target condition provided an index into the neural systems involved in visually guided navigation without the need for direct use of an internal representation [Hartley et al., 2003; Iaria et al., 2003]. Thus, the presence of greater relative activation in the parahippocampal cortex in the visibly guided condition, compared with the three other regions, indicates greater involvement for parahippocampal cortex in visually guided scene‐based navigation. The parahippocampal cortex also showed greater activation in the landmark vs. control contrast but not the store‐reference vs. control contrast, suggesting its specificity to more rigid forms of scene representation. These findings are broadly consistent with data showing greater parahippocampal activation during viewing of visual scenes from specific view‐points [Epstein et al., 2003] and together suggest the possibility of less direct parahippocampal involvement during retrieval of recently learned spatial representations and a more direct involvement in active scene processing [Aguirre et al., 1996; Epstein and Kanwisher, 1998; Epstein et al., 2007; Janzen and van Turennout, 2004].
Role of Hippocampus, and Interactions Between Hippocampus and Cortical Areas, in Flexible Allocentric Retrieval of Recently Learned Spatial Layouts
We found greater functional connectivity between precuneus and posterior hippocampus and between superior posterior parietal cortex and posterior hippocampus during the store‐reference compared with the landmark condition. These findings indicate the potential importance of the hippocampus in the store‐reference condition and thus a role in extracting new spatial relations based on previously learned metric information residing in extrahippocampal regions. We also found hippocampal activation in the store‐reference condition but not landmark condition, which decreased linearly over blocks. This demonstrates that the hippocampus was engaged early in trials during the store‐reference condition but that it was less involved in later learning blocks, consistent with previous findings on both navigation [Wolbers and Buchel, 2005] and paired‐associate learning [Poldrack et al., 2001; Zeineh et al., 2003]. One possible explanation for the presence of significant functional connectivity in the store‐reference vs. landmark condition over blocks in the absence of continuing activation during later blocks could be due to the presence of coupled rather than significantly increased activity later in learning [Rissman et al., 2004]. Finally, we noted that the degree of hippocampal activation early in learning trials correlated positively with overall performance on the store‐reference task. These findings all suggest the importance of hippocampal involvement in the store‐reference condition.
In both the landmark and the store‐reference condition, subjects needed to retrieve the encoded knowledge of the position of each store relative to the landmark. However, compared with the landmark condition, the store‐reference condition involved inferring the spatial relationship of the target and based on the reference store despite the fact that they had never seen these two stores on the same trial. From this perspective, both the rigid and flexible conditions involved similar processes supporting the retrieval of relationships between stores and the static landmark except that the flexible condition involved an additional relational inference task across encoding trials [Konkel and Cohen, 2009]. Our findings showed brain areas involved in rigid representation retrieval (precuneus and superior parietal lobe) were highly connected to hippocampus in the flexible retrieval condition. Our findings regarding hippocampal involvement in the flexible but not rigid retrieval condition are thus consistent with rodent and human studies showing a critical role for the hippocampus in flexible and relational coding of information [Eichenbaum et al., 1992; Eichenbaum and Cohen, 2001; Konkel et al., 2008; Konkel and Cohen, 2009]. Our findings, however, potentially extend the results from these studies by emphasizing the importance of functional interactions between hippocampus and extrahippocampal regions during flexible retrieval of spatial information, suggesting not just the importance of the hippocampus to this process but rather its active interactions with extrahippocampal regions involved in representing spatial layouts.
Comparable with our results, Hartley et al. and Iaria et al. also found greater hippocampal activation when subjects used existing allocentric representations in a novel fashion, although these studies did not systematically compare rigid and flexible forms of representations as we did in our study, instead relying on inferences based on navigation strategies post hoc. One possible implication of our results, in light of those by Hartley et al and Iaria et al, is that hippocampal involvement is not specific to allocentric retrieval but rather specific to utilizing that representation in a flexible manner [Cohen and Eichenbaum, 1991; Konkel and Cohen, 2009]. This interpretation provides possible additional insight into why hippocampal lesions often affect some forms of allocentric memory [Goodrich‐Hunsaker et al., 2010; Goodrich‐Hunsaker and Hopkins, 2010; Hartley et al., 2007; Parslow et al., 2005] but not others [Bohbot and Corkin, 2007; Bohbot et al., 1998; Ploner et al., 1999, 2000]. Namely, conditions requiring utilization of previously learned spatial information flexibly invoke the hippocampus [Eichenbaum et al., 1992] while utilizing an allocentric representation on its own, particularly when consistent with how it was originally encoded, may not involve or require the hippocampus directly.
Wolbers and Buchel [2005] also showed hippocampal involvement as subjects learned the distance between landmarks in a virtual environment. Hippocampal activation in their study correlated most strongly with periods of high‐learning, which were most pronounced during early testing blocks; retrosplenial activation, in contrast, correlated with performance on the landmark‐distance task over all learning trials. Our findings are consistent with the idea that hippocampal activation is strongest when a spatial representation might be expected to be employed most flexibly, and similar to Wolbers and Buchel, the first block of retrieval was also the period when learning was the most pronounced in our task (see Supporting Information Figure 1S). Our results, though, extend the findings of Wolbers and Buchel to include (1) utilization of previously learned representation during active navigation (as opposed to the Wolbers and Buchel study, where the test phase involved a paired‐associate comparison of landmarks) (2) utilization of a representation specific to the flexible‐store condition rather than generally to learning the distance of landmarks in a layout (3) continued interaction of hippocampus and extrahippocampal areas during retrieval requiring a flexible representation, as indicated by our functional connectivity analysis (which Wolbers and Buchel did not perform).
In contrast to our results and those of Wolbers and Buchel [2005], Rosenbaum et al. found little hippocampal and primarily retrosplenial and parahippocampal activation in navigators highly familiar with a spatial environment they had navigated for several years. Because this study looked at a highly familiar environment, which likely would benefit little from relearning and on‐line manipulation, it is possible they did not see hippocampal activation because little flexible usage of the spatial representation was required. Another possibility is that spatial layout representations were consolidated to cortical areas in the flexible condition, thus obviating the need for hippocampal involvement [McClelland et al., 1995; Rosenbaum et al., 2004; Tse et al., 2007]. This would also explain why, in our study, the hippocampus was not involved in the landmark condition, as perhaps, by the time of retrieval, it was sufficiently consolidated to no longer require hippocampal involvement. Unfortunately, we can only speculate on issues related to consolidation as our paradigm was not set‐up to address this idea directly. Future studies will need to address the importance of consolidation in the context of human allocentric representation.
Encoding vs. Retrieval During Navigation
In the current study, subjects were shown the spatial relation of each store relative to the landmark during encoding and had no direct view of the relationship between any two stores on the invisible circle (Fig. 1c). After the encoding session, subjects were immediately put into the scanner for the retrieval task. Thus, they had no chance to learn the relationship of any two stores on the invisible circle before the task. During the navigation task in the store‐reference condition, subjects thus had to calculate the spatial relations of stores on the invisible circle. During this retrieval procedure, subjects possibly encoded new information of the relation between stores on the invisible circle, especially during earlier trials in the store‐reference condition (see also the discussion about the role of hippocampus above). Early reports suggested that there might be differences in hippocampal activation during encoding and retrieval (Lepage et al., 1998); however, more recent reports demonstrate hippocampal activation during both encoding (Cansino et al., 2002; Davachi et al., 2003; Uncapher et al., 2006; Diana et al., 2009; Staresina and Davachi, 2009) and retrieval (Hayes et al., 2004; Daselaar et al., 2006; Ekstrom and Bookheimer, 2007; Mugikura et al., 2010) of item and general contextual information. These data, in addition to others, suggest that encoding and retrieval likely tap into largely intersecting brain regions, including areas such as the hippocampus (see: Rugg et al., 2008 for a review). Baumann et al. 2010 also found hippocampal activation in a navigation task involving encoding of landmark locations and subsequently finding hidden versions of these landmarks during retrieval [Baumann et al., 2010].
It should be noted though that during active navigation, the line between encoding and retrieval is necessarily less defined than verbal memory paradigms because active navigation involves a continual updating of representations based on available sensory input and the current navigational goal. While some paradigms explicitly attempted to separate encoding and retrieval during navigation [Baumann et al., 2010; Shelton and Pippitt, 2007], landmarks may serve as a potential cues to recollect and update spatial representations [Sturz et al., 2009], even during retrieval. Thus, while it may be possible to separate encoding and retrieval components for navigation, as has been done in previous verbal memory paradigms, it is not clear that this distinction holds as strongly during navigation as it might for verbal memory paradigms. Furthermore, previous findings from verbal memory paradigms and navigation studies suggest the involvement of several key brain regions, such as the hippocampus, during both encoding and retrieval, as part of forming and utilizing a spatial representation. Importantly, because our behavioral results indicated that subjects transferred knowledge from the aerial to active navigation condition, while learning occurred during both encoding and retrieval in our paradigm, there can be little doubt that subjects did in fact utilize information during retrieval they had acquired during encoding, albeit with some modification. These findings thus argue for the validity of our brain activation patterns regarding rigid vs. flexible forms of spatial representation.
CONCLUSION
Our results provide an important extension to previous work on human allocentric representation. Our findings demonstrate increased activation during allocentric retrieval in retrosplenial, superior posterior parietal cortex, and precuneus, particularly during retrieval that relies primarily on utilizing an existing representation with little modifications. We also found greater activation in precuneus and superior posterior parietal cortex, and greater functional connectivity between superior posterior parietal/retrosplenial cortex and other cortical areas (e.g., superior temporal and medial frontal gyrus) during rigid compared with flexible allocentric representation. Our results also confirmed the importance of parahippocampal cortex to more rigid forms of scene representation. In contrast, we found greater connectivity between precuneus/superior posterior parietal and the hippocampus during flexible utilization of an existing representation, suggesting the hippocampus as a possible mediator of accessing previously stored rigid scene information to permit its flexible engagement. We also found a linear decrease in hippocampal involvement in the flexible allocentric condition over trials, suggesting its initial more direct involvement in this process early in trials. Finally, the degree of hippocampal activation on early trials correlated significantly with performance during flexible retrieval. Our results are consistent with previous models of human spatial navigation suggesting the importance of superior posterior parietal cortex and precuneus in allocentric spatial representation and extend these findings to suggest their specific involvement in more rigid forms of representations. Our results also highlight the role of the hippocampus in flexible forms of retrieval, extending these findings to human spatial navigation following learning from an aerial perspective.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
The authors wish to thank Weimin Mou, Dan Ragland, Andrew Watrous, and the UC‐Davis Memory Group for helpful comments on the manuscript.
REFERENCES
- Aguirre GK, Detre JA, Alsop DC, D'Esposito M ( 1996): The parahippocampus subserves topographical learning in man. Cereb Cortex 6: 823–829. [DOI] [PubMed] [Google Scholar]
- Baumann O, Chan E, Mattingley JB ( 2010): Dissociable neural circuits for encoding and retrieval of object locations during active navigation in humans. Neuroimage 49: 2816–2825. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Corkin S ( 2007): Posterior parahippocampal place learning in H.M. Hippocampus 17: 863–872. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L ( 1998): Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia 36: 1217–1238. [DOI] [PubMed] [Google Scholar]
- Brown TI, Ross RS, Keller JB, Hasselmo ME, Stern CE ( 2010): Which way was I going? Contextual retrieval supports the disambiguation of well learned overlapping navigational routes. J Neurosci 30: 7414–7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J ( 2002): The human hippocampus and spatial and episodic memory. Neuron 35: 625–641. [DOI] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD ( 2002): Brain activity underlying encoding and retrieval of source memory. Cereb Cortex 12: 1048–1056. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H ( 1991): The theory that wouldn't die: A critical look at the spatial mapping theory of hippocampal function. Hippocampus 1: 265–268. [DOI] [PubMed] [Google Scholar]
- Committeri G, Galati G, Paradis AL, Pizzamiglio L, Berthoz A, LeBihan D ( 2004): Reference frames for spatial cognition: different brain areas are involved in viewer‐, object‐, and landmark‐centered judgments about object location. J Cogn Neurosci 16: 1517–1535. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R ( 2006): Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. J Neurophysiol 96: 1902–1911. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD ( 2003): Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA 100: 2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C ( 2009): Medial temporal lobe activity during source retrieval reflects information type, not memory strength. J Cogn Neurosci 22: 1808–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ ( 2001): From Conditioning to Conscious Recollection: Memory Systems of the Brain. New York: Oxford University Press. [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ ( 1992): The hippocampus—What does it do? Behav Neural Biol 57: 2–36. [DOI] [PubMed] [Google Scholar]
- Ekstrom A, Suthana N, Millett D, Fried I, Bookheimer S ( 2009): Correlation Between BOLD fMRI and theta‐band local field potentials in the human hippocampal area. J Neurophysiol 101: 2668–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Bookheimer SY ( 2007): Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learn Mem 14: 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N ( 1998): A cortical representation of the local visual environment. Nature 392: 598–601. [DOI] [PubMed] [Google Scholar]
- Epstein R, Graham KS, Downing PE ( 2003): Viewpoint‐specific scene representations in human parahippocampal cortex. Neuron 37: 865–876. [DOI] [PubMed] [Google Scholar]
- Epstein RA ( 2008): Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci 12: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Higgins JS ( 2007): Differential parahippocampal and retrosplenial involvement in three types of visual scene recognition. Cereb Cortex 17: 1680–1693. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Parker WE, Feiler AM ( 2007): Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. J Neurosci 27: 6141–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Pezdek K ( 1980): Cognitive mapping: knowledge of real‐world distance and location information. J Exp Psychol Hum Learn 6: 13–24. [PubMed] [Google Scholar]
- Fitts PM ( 1954): The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47: 381–391. [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC ( 1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R ( 1995): Characterizing dynamic brain responses with fMRI: A multivariate approach. Neuroimage 2: 166–172. [DOI] [PubMed] [Google Scholar]
- Galati G, Pelle G, Berthoz A, Committeri G ( 2010): Multiple reference frames used by the human brain for spatial perception and memory. Exp Brain Res 206: 109–120. [DOI] [PubMed] [Google Scholar]
- Galati G, Lobel E, Vallar G, Berthoz A, Pizzamiglio L, Le Bihan D ( 2000): The neural basis of egocentric and allocentric coding of space in humans: A functional magnetic resonance study. Exp Brain Res 133: 156–164. [DOI] [PubMed] [Google Scholar]
- Ghaem O, Mellet E, Crivello F, Tzourio N, Mazoyer B, Berthoz A, Denis M ( 1997): Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport 8: 739–744. [DOI] [PubMed] [Google Scholar]
- Goodrich‐Hunsaker NJ, Hopkins RO ( 2010): Spatial memory deficits in a virtual radial arm maze in amnesic participants with hippocampal damage. Behav Neurosci 124: 405–413. [DOI] [PubMed] [Google Scholar]
- Goodrich‐Hunsaker NJ, Livingstone SA, Skelton RW, Hopkins RO ( 2010): Spatial deficits in a virtual water maze in amnesic participants with hippocampal damage. Hippocampus 20: 481–491. [DOI] [PubMed] [Google Scholar]
- Hartley T, Maguire EA, Spiers HJ, Burgess N ( 2003): The well‐worn route and the path less traveled: Distinct neural bases of route following and wayfinding in humans. Neuron 37: 877–888. [DOI] [PubMed] [Google Scholar]
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha‐Khadem F, Burgess N ( 2007): The hippocampus is required for short‐term topographical memory in humans. Hippocampus 17: 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Ryan L, Schnyer DM, Nadel L ( 2004): An fMRI study of episodic memory: Retrieval of object, spatial, and temporal information. Behav Neurosci 118: 885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD ( 2003): Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: Variability and change with practice. J Neurosci 23: 5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igloi K, Doeller CF, Berthoz A, Rondi‐Reig L, Burgess N ( 2010): Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proc Natl Acad Sci USA 107: 14466–14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen G, van Turennout M ( 2004): Selective neural representation of objects relevant for navigation. Nat Neurosci 7: 673–677. [DOI] [PubMed] [Google Scholar]
- Jordan K, Schadow J, Wuestenberg T, Heinze HJ, Jancke L ( 2004): Different cortical activations for subjects using allocentric or egocentric strategies in a virtual navigation task. Neuroreport 15: 135–140. [DOI] [PubMed] [Google Scholar]
- Klatzky R ( 1998) Allocentric and egocentric spatial representations: Definitions, distinctions, and interconnections In: Freksa C, Habel C, Wender CF, editors. Spatial cognition: An Interdisciplinary Approach to Representation and Processing of Spatial Knowledge. Berlin: Springer‐Verlag; pp 1–17. [Google Scholar]
- Konkel A, Cohen NJ ( 2009): Relational memory and the hippocampus: Representations and methods. Front Neurosci 3: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ ( 2008): Hippocampal amnesia impairs all manner of relational memory. Front Hum Neurosci 2: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI ( 2009): Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci 12: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E ( 1998): Hippocampal PET activations of memory encoding and retrieval: The HIPER model. Hippocampus 8: 313–322. [DOI] [PubMed] [Google Scholar]
- Levine M, Marchon I, Hanley G ( 1984): The placement and misplacement of you‐are‐here maps. Environ Behav 16: 139. [Google Scholar]
- Maguire EA, Frith CD, Burgess N, Donnett JG, O'Keefe J ( 1998a) Knowing where things are parahippocampal involvement in encoding object locations in virtual large‐scale space. J Cogn Neurosci 10: 61–76. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J ( 1998b) Knowing where and getting there: a human navigation network. Science 280: 921–924. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC ( 1995): Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102: 419–457. [DOI] [PubMed] [Google Scholar]
- McNamara TP, Rump B, Werner S ( 2003): Egocentric and geocentric frames of reference in memory of large‐scale space. Psychon Bull Rev 10: 589–595. [DOI] [PubMed] [Google Scholar]
- Mellet E, Briscogne S, Tzourio‐Mazoyer N, Ghaem O, Petit L, Zago L, Etard O, Berthoz A, Mazoyer B, Denis M ( 2000): Neural correlates of topographic mental exploration: The impact of route versus survey perspective learning. Neuroimage 12: 588–600. [DOI] [PubMed] [Google Scholar]
- Moeser S ( 1988) Cognitive mapping in a complex building. Environ Behav 20: 21–48. [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O'Keefe J ( 1982): Place navigation impaired in rats with hippocampal lesions. Nature 297: 681–683. [DOI] [PubMed] [Google Scholar]
- Mugikura S, Abe N, Suzuki M, Ueno A, Higano S, Takahashi S, Fujii T ( 2010): Hippocampal activation associated with successful external source monitoring. Neuropsychologia 48: 1543–1550. [DOI] [PubMed] [Google Scholar]
- Neggers SF, Van der Lubbe RH, Ramsey NF, Postma A ( 2006): Interactions between ego‐ and allocentric neuronal representations of space. Neuroimage 31: 320–331. [DOI] [PubMed] [Google Scholar]
- Newman EL, Caplan JB, Kirschen MP, Korolev IO, Sekuler R, Kahana MJ ( 2007): Learning your way around town: How virtual taxicab drivers learn to use both layout and landmark information. Cognition 104: 231–253. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L ( 1978): The Hippocampus as a Cognitive Map. Oxford: Clarendon Press. [Google Scholar]
- Parslow DM, Morris RG, Fleminger S, Rahman Q, Abrahams S, Recce M ( 2005): Allocentric spatial memory in humans with hippocampal lesions. Acta Psychol (Amst) 118: 123–147. [DOI] [PubMed] [Google Scholar]
- Parslow DM, Rose D, Brooks B, Fleminger S, Gray JA, Giampietro V, Brammer MJ, Williams S, Gasston D, Andrew C, Vythelingum GN, Loannou G, Simmons A, Morris RG ( 2004): Allocentric spatial memory activation of the hippocampal formation measured with fMRI. Neuropsychology 18: 450–461. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Gaymard BM, Rivaud‐Pechoux S, Baulac M, Clemenceau S, Samson S, Pierrot‐Deseilligny C ( 2000): Lesions affecting the parahippocampal cortex yield spatial memory deficits in humans. Cereb Cortex 10: 1211–1216. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Gaymard BM, Ehrle N, Rivaud‐Pechoux S, Baulac M, Brandt SA, Clemenceau S, Samson S, Pierrot‐Deseilligny C ( 1999): Spatial memory deficits in patients with lesions affecting the medial temporal neocortex. Ann Neurol 45: 312–319. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare‐Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck MA ( 2001): Interactive memory systems in the human brain. Nature 414: 546–550. [DOI] [PubMed] [Google Scholar]
- Presson CC, DeLange N, Hazelrigg MD ( 1989): Orientation specificity in spatial memory: What makes a path different from a map of the path? J Exp Psychol Learn Mem Cogn 15: 887–897. [DOI] [PubMed] [Google Scholar]
- Richardson AE, Montello DR, Hegarty M ( 1999): Spatial knowledge acquisition from maps and from navigation in real and virtual environments. Mem Cognit 27: 741–750. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M ( 2004): Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage 23: 752–763. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Ziegler M, Winocur G, Grady CL, Moscovitch M ( 2004): “I have often walked down this street before”: fMRI studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus 14: 826–835. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Johnson JD, Park H, Uncapher MR ( 2008): Encoding‐retrieval overlap in human episodic memory: A functional neuroimaging perspective. Prog Brain Res 169: 339–352. [DOI] [PubMed] [Google Scholar]
- Shelton AL, Gabrieli JD ( 2002): Neural correlates of encoding space from route and survey perspectives. J Neurosci 22: 2711–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton AL, McNamara TP ( 2004): Orientation and perspective dependence in route and survey learning. J Exp Psychol Learn Mem Cogn 30: 158–170. [DOI] [PubMed] [Google Scholar]
- Shelton AL, Pippitt HA ( 2007): Fixed versus dynamic orientations in environmental learning from ground‐level and aerial perspectives. Psychol Res 71: 333–346. [DOI] [PubMed] [Google Scholar]
- Shipman SL, Astur RS ( 2008): Factors affecting the hippocampal BOLD response during spatial memory. Behav Brain Res 187: 433–441. [DOI] [PubMed] [Google Scholar]
- Sholl MJ ( 1987): Cognitive maps as orienting schemata. J Exp Psychol Learn Mem Cogn 13: 615–628. [DOI] [PubMed] [Google Scholar]
- Siegel AW, White SH ( 1975): The development of spatial representations of large‐scale environments In Reese HW, editor. Advances in Child Development and Behavior. New York: Academic. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L ( 2009): Mind the gap: Binding experiences across space and time in the human hippocampus. Neuron 63: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR ( 2001) When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci USA 98: 12760–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturz BR, Brown MF, Kelly DM ( 2009): Facilitation of learning spatial relations among locations by visual cues: Implications for theoretical accounts of spatial learning. Psychon Bull Rev 16: 306–312. [DOI] [PubMed] [Google Scholar]
- Suthana NA, Ekstrom AD, Moshirvaziri S, Knowlton B, Bookheimer SY ( 2009): Human hippocampal CA1 involvement during allocentric encoding of spatial information. J Neurosci 29: 10512–10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HA, Tversky B ( 1992): Spatial mental models derived from survey and route descriptions. J Mem Lang 31: 261–292. [Google Scholar]
- Thorndyke PW, Hayes‐Roth B ( 1982): Differences in spatial knowledge acquired from maps and navigation. Cogn Psychol 14: 560–589. [DOI] [PubMed] [Google Scholar]
- Tolman EC ( 1948): Cognitive maps in rats and men. Psychol Rev 55: 189–208. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RG ( 2007): Schemas and memory consolidation. Science 316: 76–82. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD ( 2006): Episodic encoding is more than the sum of its parts: An fMRI investigation of multifeatural contextual encoding. Neuron 52: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ ( 2010): Hippocampal brain‐network coordination during volitional exploratory behavior enhances learning. Nat Neurosci 14: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller D, Hodgson E ( 2006): Transient and enduring spatial representations under disorientation and self‐rotation. J Exp Psychol Learn Mem Cogn 32: 867–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RF, Spelke ES ( 2000): Updating egocentric representations in human navigation. Cognition 77: 215–250. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Buchel C ( 2005): Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. J Neurosci 25: 3333–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY ( 2003): Dynamics of the hippocampus during encoding and retrieval of face‐name pairs. Science 299: 577–580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


