Abstract
Background: Cerebral small vessel disease (SVD) and hippocampal atrophy are related to verbal memory failures and may ultimately result in Alzheimer's disease. However, verbal memory failures are often present before structural changes on conventional MRI appear. Changes in microstructural integrity of the hippocampus, which cannot be detected with conventional MRI, may be the underlying pathological substrate. With diffusion tensor imaging (DTI), we investigated the relation between the microstructural integrity of the hippocampus and verbal memory performance in 503 nondemented elderly with SVD. Methods: The Radboud University Nijmegen Diffusion tensor and Magnetic resonance imaging Cohort study is a prospective cohort study among 503 nondemented elderly with cerebral SVD aged between 50 and 85 years. All participants underwent T1 MPRAGE, fluid‐attenuated inversion recovery, DTI scanning and the Rey Auditory Verbal Learning Test. After manual segmentation of the hippocampi, we calculated the mean diffusivity (MD) and fractional anisotropy in both hippocampi. The relation between memory performance and hippocampal DTI parameters was adjusted for age, sex, education, depressive symptoms, hippocampal, and white‐matter lesions volume and lacunar infarcts. Results: We found inverse relations between hippocampal MD and verbal memory performance (β = −0.22; P < 0.001), immediate recall (β = −0.22; P < 0.001), delayed recall (β = −0.20; P < 0.001), and forgetting rate (β = −0.13; P = 0.025), most pronounced in participants with a normal hippocampal volume. Conclusion: Microstructural integrity of the hippocampus assessed by DTI is related to verbal memory performance in elderly with SVD, also in participants with an intact appearing hippocampus. Changes in hippocampal microstructure may be an early marker of underlying neurodegenerative disease, before macrostructural (i.e., volumetric) changes occur. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: cognition, memory, dementia, elderly, cerebral small vessel disease, hippocampus, imaging, magnetic resonance imaging, diffusion tensor imaging
INTRODUCTION
Cerebral small vessel disease (SVD) includes white‐matter lesions (WML) and lacunar infarcts. The prevalence of SVD in the general population over 60 years is high [de Leeuw et al.,2001; Vernooij et al.,2007]. Both patient and population‐based studies have shown that cerebral SVD is an important cause of verbal memory failure and may ultimately result in cognitive decline associated with Alzheimer's disease (AD) in some [de Groot et al.,2000b,2002; Vermeer et al.,2003].
In the brains of patients with AD, atrophy of the hippocampus is one of the first observed changes [Braak and Braak,1991]. Interestingly, the progression of hippocampal atrophy is related to the presence and progression of cerebral SVD [de Leeuw et al.,2004,2006]. Generally, its presence is supportive for the diagnosis of AD and indicative for the future development of AD in patients with mild cognitive impairment (MCI) [Dubois et al.,2007; Korf et al.,2004]. Hippocampal atrophy underlies the profound deficit in the consolidation of memory in AD [Squire et al.,2004], that is, long‐term encoding and storage of relevant new information.
However, these macroscopic structural hippocampal changes on conventional MRI occur at a relatively late stage and are usually preceded by clinical symptoms including subjective cognitive failures, verbal memory decline, and problems in other cognitive domains [van Norden et al.,2008]. Conceptually, there must be changes in the microstructural integrity of the hippocampus before macroscopic loss of volume occurs. Diffusion tensor imaging (DTI) can provide more detailed information on the microstructural integrity of the hippocampus. DTI provides amongst other possibilities two scalar parameters: mean diffusivity (MD), a measure of the magnitude of diffusion of water averaged in all spatial directions, and fractional anisotropy (FA), which provides information about the directionality of water diffusion [Basser et al.,1994; Pierpaoli et al.,1996]. DTI is usually used to assess microstructural integrity of the white matter in which damage to the white matter has found to be accompanied by an increase in MD and a decrease in FA [Charlton et al.,2006; Kraus et al., 2007], but there are a number of reports showing its usefulness in the assessment of (predominantly) gray‐matter structures [Fellgiebel et al.,2004; Kantarci et al.,2002,2005; Muller et al.,2007]. There is some evidence of a higher diffusion in the hippocampus in MCI and AD patients that are independent of hippocampal atrophy and WML [Fellgiebel et al.,2004; Kantarci et al.,2002].
More importantly, two studies have shown that hippocampal MD measures were superior to volume measures in predicting clinical progression to dementia in MCI patients [Kantarci et al.,2005; Muller et al.,2007]. However, to date, the role of hippocampal DTI measurements has never been investigated in the light of specific processes of verbal memory function in nondemented elderly, focusing on encoding, storage, and retrieval and to detect early changes in hippocampal microstructure even before macrostructural changes occur and the relation with early impairment of verbal memory.
In the present study, the relation between hippocampal DTI measures and verbal memory processes is examined, independent of hippocampal volume (HV) and SVD, in 503 nondemented, independently living elderly with cerebral SVD, aged between 50 and 85 years.
METHODS
Study Population
The Radboud University Nijmegen Diffusion tensor and Magnetic resonance imaging Cohort (RUN DMC) study is a prospective cohort study that was designed to investigate risk factors and cognitive, motor, and mood consequences of functional and structural brain changes as assessed by structural MRI, DTI, and resting state fMRI among independently living nondemented elderly with cerebral SVD.
In people with cerebral SVD symptoms are due to either complete (lacunar syndromes) or incomplete infarction (WML) of subcortical structures that might lead to acute symptoms as transient ischaemic attacks (TIAs) or lacunar syndromes, or subacute manifestations as cognitive, motor (gait), and/or mood disturbances [Roman et al.,2002]. As the onset of cerebral SVD is often insidious, clinically heterogeneous, and typically with mild symptoms, it has been suggested that the selection of people with cerebral SVD in clinical studies should be based on the more consistent brain‐imaging features [Erkinjuntti,2002].
Accordingly, in 2006, consecutive individuals who visited the department of neurology between October 2002 and November 2006 were selected for possible participation. Inclusion criteria were (a) age between 50 and 85 years; (b) cerebral SVD on neuroimaging; and (c) acute (n = 219) or subacute (n = 284) clinical symptoms of SVD as assessed by standardized structured assessments. Patients who were eligible because of a lacunar syndrome were included only >6 months after the event to avoid acute effects on the outcomes.
Exclusion criteria were (a) dementia; (b) parkinson(‐ism) according to the international diagnostic criteria [Gelb et al.,1999; McKhann et al.,1984; Roman et al.,1993]; (c) life expectancy of less than 6 months; (d) intracranial space occupying lesion; (e) (psychiatric) disease interfering with cognitive testing or follow‐up; (f) recent or current use of acetylcholine‐esterase inhibitors, neuroleptic agents, L‐dopa, or dopa‐a(nta)gonists; (g) WML mimics (e.g., multiple sclerosis and irradiation induced gliosis); (h) prominent visual or hearing impairment; (i) language barrier; and (j) MRI contraindications or known claustrophobia.
From 1,004 invited individuals by letter, 727 were eligible after contact by phone of whom 525 agreed to participate. In 22 individuals, exclusion criteria were found during their visit to our research center (14 with unexpected claustrophobia, one died before MRI scanning, one was diagnosed with multiple sclerosis, in one there was a language barrier, one participant fulfilled the criteria for Parkinson's disease, and four met the dementia criteria) [Gelb et al.,1999; McKhann et al.,1984; Roman et al.,1993], yielding a response of 71.3% (503/705). These 503 individuals had symptoms of TIA or lacunar syndrome (n = 219), cognitive disturbances (n = 245), motor disturbances (n = 97), depressive symptoms (n = 100), or a combination thereof. All participants signed an informed consent form. The Medical Review Ethics Committee region Arnhem‐Nijmegen approved the study.
Conventional MRI Scanning Protocol
All participants underwent a 1.5‐T MRI scanning on the same Magnetom scanner (Siemens, Erlangen, Germany). The protocol included the following whole brain scans: T1 3D MPRAGE imaging (TR/TE/TI 2,250/3.68/850 ms; flip angle 15°; voxel size 1.0 × 1.0 × 1.0 mm); fluid‐attenuated inversion recovery (FLAIR) pulse sequences (TR/TE/TI 9,000/84/2,200 ms; voxel size 1.0 × 1.2 × 5.0 mm, with an interslice gap of 1 mm); DTI (TR/TE 10,100/93 ms; voxel size 2.5 × 2.5 × 2.5 mm; four unweighted scans, 30 diffusion‐weighted scans with b‐value 900 s/mm2). The complete protocol took 31 min.
Conventional MRI Analysis
Hippocampus and intracranial volumetry
One experienced investigator, blinded to clinical data (IvU), manually segmented the left and right hippocampus on the MPRAGE image using the interactive software program “ITK‐SNAP” [Yushkevich et al.,2006]. Anatomical boundaries were determined in coronal sections with the aid of neuroanatomical atlases [Duvernoy,1997; Mai et al.,2007], and actual segmentation was performed using a previously published protocol [Geuze et al.,2005] in which segmentation was performed from posterior to anterior. The posterior border of the hippocampus was identified in the slice before the level in which the crurae fornices appeared in full view. The anterior border of the hippocampus was defined as the slice in which the hippocampus was no longer present, and the amygdala fully covered the hippocampus [General Brain Segmentation‐Method and Utilization, Version 3. Center for Morphometric Analysis 2004; Geuze et al.,2005; van de Pol et al.,2006]. The superior border was the inferior horn of the lateral ventricle, and the inferior border was determined by the white‐matter boundary. The lateral border was defined by the temporal horn of the lateral ventricle and the white‐matter adjacent to the hippocampus.
Volumes were calculated for the left and right hippocampus separately by summing all voxel volumes of the segmented areas. Interrater studies on a random sample of 10% showed an intraclass correlation coefficient for the left hippocampus of 0.73 and for the right hippocampus of 0.79. Intrarater studies on this sample showed an intraclass correlation coefficient for the left and right hippocampus of 0.97 and 0.96, respectively.
For the same image, gray (GM) and white‐matter (WM) tissue and cerebrospinal fluid (CSF) probability maps were computed using SPM5 routines (Wellcome Department of Cognitive Neurology, University College London, UK). Total GM, WM, and CSF volumes were calculated by summing all voxel volumes that had a P > 0.5 for belonging to the tissue class. Intracranial volume (ICV) was taken as the sum of total GM, WM, and CSF volume.
HV measurements were normalized to the total ICV. The normalized hippocampal volume (NHV) is defined as NHV = ICVm × HVp/ICVp, where ICVm is the average total ICV of all participants, ICVp is the ICV of the participant, and the HVp is the HV of the participant [Colliot et al.,2008].
WML volumetry
WML was manually segmented on transversal FLAIR images. WML was defined as hyperintense lesions on FLAIR and not CSF like hypointense lesions on T1‐weighted image [Herve et al.,2005; Wahlund et al.,2001]. Two trained raters, blinded for all clinical, cognitive, and DTI information, segmented all scans. WML volume was calculated as lesion surface multiplied by slice thickness. Interrater variability was determined in a random sample of ten percent and yielded an intraclass correlation coefficient of 0.99 for total WML volume. The FLAIR image was used to compute the co‐registration parameters to the anatomical T1 image, which was then applied to the segmentation results. All images were visually checked for co‐registration errors.
Lacunar infarcts
Lacunar infarcts were defined as hypointense areas >2 mm and ≤15 mm on FLAIR and T1, ruling out enlarged perivascular spaces (≤2 mm, except around the anterior commissure, where perivascular spaces can be large) and infraputaminal pseudolacunes [Herve et al.,2005; Wahlund et al.,2001]. Evaluation of infarcts was performed by a resident in neurology blinded to all clinical data with good intrarater variability with a weighted κ of 0.80. In 10% of the scans, interrater variability was calculated with a weighted κ of 0.88.
DTI Analysis
The diffusion‐weighted images of each patient were realigned on the unweighted image using mutual information based co‐registration routines from SPM5. Then, the diffusion tensor [Basser et al.,1994] and its eigenvalues were estimated using linear regression (spurious negative values were set to zero), after which the tensor derivates MD and FA were calculated [Basser and Jones,2002]. The mean unweighted image was used to compute the co‐registration parameters to the anatomical T1 image (SPM5 mutual information co‐registration), which was then applied to all diffusion‐weighted images and derivates. The mean MD and FA were then calculated in both hippocampi. All images were visually checked for motion artifacts and co‐registration errors, especially for not including perihippocampal CSF.
Measurement of cognitive function
Cognitive function was assessed by two trained investigators (AvN and KdL). For this substudy, the Mini‐Mental State Examination (MMSE) (range, 0–30) [Folstein et al.,1975] was used as an index of overall cognitive performance. The three‐trial version of the Rey Auditory Verbal Learning Test (RAVLT) [Van der Elst et al.,2005] was administered to examine episodic memory formation.
We defined five memory indices based on RAVLT performance (e.g., immediate recall, learning rate, forgetting rate, delayed recall, and delayed recognition), as described previously [Vakil and Blachstein,1993]. Immediate recall was calculated by the mean of the total number of words remembered in the three learning trials of the RAVLT. Learning rate was also determined by the three learning trials of the RAVLT, in which a learning curve was estimated with the following formula: [(trial 2 − trial 1/trial 1) plus (trial 3 − trial 2/trial 2)]/2. Delayed recall was the number of words recalled 30 min after the learning trials. Forgetting rate, as a measure of decay over time, was obtained by the scores in the delayed recall trial corrected for the score obtained in the third learning trial (delayed recall – trial 3/trial 3). The delayed recognition score was calculated by computing the total of each correctly recognized word (the 15 target words among 15 new distracter items) 30 min after the learning trials.
Performance across tests was made comparable by transforming the raw test scores into Z‐scores as described elsewhere [de Groot et al.,2000b], for which the assumption of normality of the distribution was examined. For data reduction purposes, a compound score for global verbal memory function was calculated, as described previously [de Groot et al.,2000b] by taking the mean of two Z‐scores from the RAVLT; one for the added scores on three learning trials of this test and one for the delayed recall of this test. If the test assistant encountered problems, a code was given for test status, and the result was not used in the calculation of the Z‐scores. Separate codes were given for lack of motivation (0.8%) or not following the instructions (0.9%). For 98% of all participants, reliable compound scores for global verbal memory performance could be calculated without any recording of test problems.
Other measurements
The following characteristics were considered possible confounders: age, sex, educational level (classified using seven categories, one being less than primary school and seven reflecting an academic degree) [Hochstenbach et al.,1998] and depressive symptoms. Depressive symptoms were defined as a score ≥ 16 on the Center of Epidemiologic Studies on Depression Scale [de Groot et al.,2000a; Radloff,1977] and/or current use of antidepressive medication [de Groot et al.,2000a].
Statistical analysis
Statistical analyses were performed with SPSS 16.0 for Windows (SPSS, Chicago, IL). Baseline characteristics were summarized as means (standard deviations; SD) or proportions for skewed variability parameters the median, and the interquartile range was calculated.
We used multiple linear regression analyses to investigate the relation between hippocampal DTI (FA/MD) parameters (left and right separately) and global verbal memory performance as well as with subprocesses of verbal memory performance. Adjustments were made for potential confounders including age, sex, educational level, depressive symptoms, hippocampal, and WML volume and the presence of lacunar infarcts. Next, we calculated estimated mean scores of global verbal memory performance per tertile of hippocampal MD by the analysis of covariance (ANCOVA), adjusted for the same potential confounders. To identify whether these microstructural changes precede macroscopic HV loss, we investigated the relation (linear regression analysis) between hippocampal DTI measures and global verbal memory performance stratified on low (lowest tertile of the distribution) versus normal HV (upper two tertiles) adjusting for the same confounders as the previous analysis. If so, we would expect a significant relation between hippocampal DTI measures and global verbal memory performance, particularly in the group with still intact (normal) HVs. Regression coefficients are presented as standardized β‐values.
All assumptions for ANCOVA analysis were tested and verified for all measures. For the trend analysis of the ANCOVA results, tertiles of hippocampal MD were considered as a continuous variable in a multiple linear regression model.
RESULTS
Of the 503 participants, three were excluded because of DTI motion artefacts and one because of an automatic segmentation problem that could not be solved manually. Demographic and neuroimaging characteristics of 499 participants are shown in Table I. Mean age of the population was 65.6 years (SD 8.8), 56.5% were males, and mean MMSE was 28.1 (SD 1.6).
Table I.
Demographic characteristics of the 499 participants
| Baseline characteristic | |
| Age (years) | 65.6 (8.8) |
| Sex (male, n (%)) | 282 (56.5%) |
| Participants with only primary education | 49 (9.8%) |
| CES‐D | 11.0 (9.4) |
| Participants with depressive symptomsa | 166 (33%) |
| Cognitive function | |
| MMSE | 28.1 (1.6) |
| Rey auditory verbal learning test: number of words recalled | |
| Immediate recall | |
| Trial 1 | 5.1 (1.8) |
| Trial 2 | 7.2 (2.3) |
| Trial 3 | 8.6 (2.6) |
| Total trial 1–3 | 20.9 (6.0) |
| Learning rate | 0.36 (0.24) |
| Delayed recall | 5.8 (3.1) |
| Forgetting rate | −0.34 (0.25) |
| Delayed recognition | 26.7 (3.5) |
| Neuroimaging characteristics | |
| WML volume | 7.1 (3.4;18.1) |
| Normalized hippocampal volume | 6.8 (0.9) |
| Left normalized hippocampal volume | 3.5 (0.5) |
| Right normalized hippocampal volume | 3.3 (0.5) |
| Presence of lacunar infarcts (n, %) | 171 (34) |
MMSE, Mini‐Mental State Examination; CES‐D, Centre of Epidemiological Studies on Depression Scale; WML, White matter lesions.
Values are means (SD), n (%), medians (interquartile range) or ml for the neuroimaging characteristics.
Defined as CES‐D scores ≥16 and/or the current use of antidepressive medication.
The relation between left and right hippocampal DTI parameters and the performance on global verbal memory as well as on the subprocesses of memory is shown in Table II. The model that included all confounders explained about 22.3% of the variance of the global memory performance, adding hippocampal MD increased the R square to about 0.25, a relative increase of about 12%. MD in the left and right hippocampus showed a significant relation with the compound score of the global verbal memory performance (β = −0.18, P = 0.001 and β = −0.21, P < 0.001). Analyses of the specific components of the RAVLT demonstrated significant relations with immediate recall (β = −0.19, P = 0.001 and β = −0.19, P < 0.001), delayed recall (β = −0.16, P = 0.005 and β = −0.20, P < 0.001), and delayed recognition (β = −0.17, P = 0.003 and β = −0.14, P = 0.013) and the MD of the left and right hippocampus, independent of the confounders. The MD in the right hippocampus and total hippocampus showed a relation with forgetting rate (β = −0.15, P = 0.013 and β = −0.14, P = 0.027). The relation between hippocampal FA values and memory performance was less evident, but were most pronounced for the left hippocampus (Table II). We did not find essential differences in standardized betas and P‐values between men and women separately performing the same analyses. We could not demonstrate a sex difference in lateralization of verbal memory performance.
Table II.
The relation between hippocampal DTI parameters and the sub‐processes of verbal memory function as measured with the Rey Auditory Verbal Learning Test (RAVLT)
| Hippocampus | ||||||
|---|---|---|---|---|---|---|
| Left | Right | Total | ||||
| MD | FA | MD | FA | MD | FA | |
| Global verbal memory performance | −0.18** | 0.10* | −0.21** | 0.04 | −0.22** | 0.07 |
| Immediate memory | ||||||
| Trial 1 | −0.16** | 0.03 | −0.12* | 0.01 | −0.16** | 0.03 |
| Trial 2 | −0.16** | 0.07 | −0.18** | 0.03 | −0.19** | 0.06 |
| Trial 3 | −0.18** | 0.12* | −0.20** | 0.09* | −0.21** | 0.12* |
| Total trials 1–3 | −0.19** | 0.09* | −0.19** | 0.06 | −0.21** | 0.08 |
| Learning rate | −0.08 | 0.02 | −0.01 | 0.05 | 0.04 | 0.04 |
| Delayed recall | −0.16** | 0.09* | −0.20** | 0.03 | −0.20** | 0.06 |
| Forgetting ratea | −0.10 | 0.04 | −0.15* | 0.03 | −0.14* | 0.01 |
| Delayed recognition | −0.17** | 0.03 | −0.14* | 0.01 | −0.17** | 0.02 |
Numbers represent regression coefficients (standardized beta's), adjusted for age, sex, educational level, depressive symptoms, hippocampal, and WML volume and the presence of lacunar infarcts.
P < 0.05
**P < 0.01
Higher test scores reflect a less negative forgetting rate, that is, a better performance.
There was no relation between hippocampal DTI parameters and learning rate.
When stratified on tertiles of hippocampal MD, higher MD values, represented by a higher MD tertile, for both left and right hippocampus were related to worse performances on immediate recall (P trend = 0.001 and P trend < 0.001), delayed recall (P trend = 0.006 and P trend = 0.001), and forgetting rate (P trend = 0.057 and P trend = 0.035) after adjusting for possible confounders (see Fig. 1). Again, no significant relation was found for the learning rate.
Figure 1.
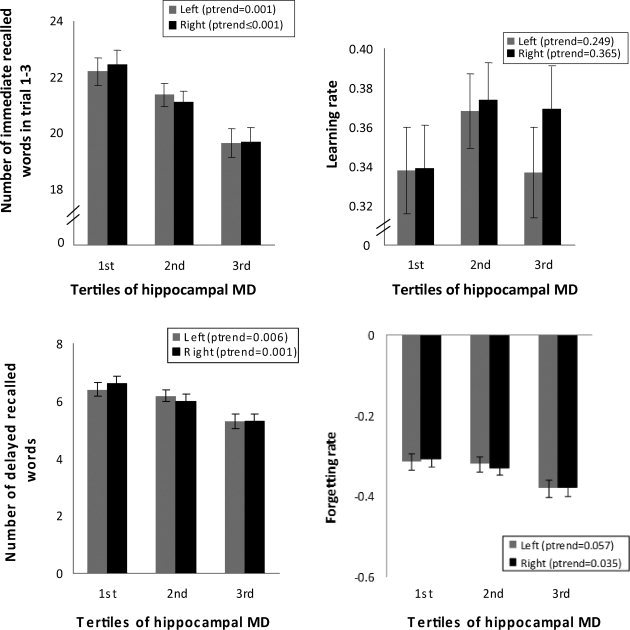
Mean scores on subprocesses of memory performance per tertile of hippocampal MD adjusted for age, sex, educational level, depressive symptoms, hippocampal, and WML volume and the presence of lacunar infarcts.
Table III shows the relation between hippocampal DTI parameters and global verbal memory performance in participants with a low or normal HV. The relation was most striking for MD values in participants with normal HVs for left (β = −0.15, P = 0.02), right (β = −0.24, P < 0.001), and total (β = −0.21, P = 0.002) hippocampus, independent of earlier mentioned confounders. This relation was also found for MD in the left hippocampus in participants with a low HV. The FA of the hippocampus in participants with low or normal HV did not correlate with global memory function.
Table III.
The relation between hippocampal DTI parameters and global verbal memory performance in participants with low and normal hippocampal volume
| Hippocampus | ||||||
|---|---|---|---|---|---|---|
| Left | Right | Total | ||||
| MD | FA | MD | FA | MD | FA | |
| Global verbal memory performance | ||||||
| Low‐hippocampal volume (n = 166) | −0.20 (0.044) | 0.11 (0.169) | −0.13 (0.162) | 0.03 (0.706) | −0.19 (0.073) | 0.07 (0.368) |
| Normal hippocampal volume (n = 333) | −0.15 (0.020) | 0.08 (0.108) | −0.24 (<0.001) | 0.05 (0.390) | −0.21 (0.002) | 0.07 (0.194) |
Numbers represent regression coefficients standardized beta's (P values), adjusted for age, sex, educational level, depressive symptoms, hippocampal, and WML volume and the presence of lacunar infarcts.
Low‐hippocampal volume is defined as the lowest tertile of the hippocampal volume distribution; normal hippocampal volume is defined as the upper two tertiles of the hippocampal volume distribution.
DISCUSSION
Our findings demonstrate that early changes in hippocampal integrity are related to reduced global verbal memory function, specifically immediate and delayed recall, delayed recognition and forgetting rate, independent of depressive symptoms, hippocampal, and WML volume and lacunar infarcts in 499 participants with cerebral SVD. These findings are in line with the notion that the hippocampus is crucial for the acquisition of new knowledge that has to be recalled (i.e., episodic memory formation). Our data demonstrate that microstructural integrity of the hippocampus beyond the detection limit of conventional MRI is related with episodic memory formation. The findings clearly show that episodic memory formations—specifically, the encoding and storage of new information—are related to hippocampal integrity. Although it is difficult to isolate encoding as a process using a standard word‐list paradigm, the encoding stage is most prominently reflected in the immediate recall index of the RAVLT [Babiloni et al.,2009; Vakil and Blachstein,1993]. We demonstrate that hippocampal integrity is related to immediate recall, which is in line with neuroimaging studies showing that the hippocampus is implicated in verbal encoding, predicting later successful recall [Strange et al.,2002]. Storage of information is reflected by various indices on the RAVLT [Vakil and Blachstein,1993]. In general, successful recall after learning depends on adequate storage of information. However, the forgetting rate (delayed free recall performance compared to the amount of previously learned information) may also be confounded by a retrieval deficit, that is, the inability to access previously stored information. Cued‐recall paradigms have been developed to facilitate access to previously acquitted information (e.g., the recognition trial on the RAVLT). Our findings show a relation between hippocampal integrity and free delayed recall, forgetting rate, and delayed recognition, clearly showing a crucial role for the hippocampus in the storage of information that is independent from a retrieval deficit. These findings support recent neuroimaging evidence indicating that the hippocampus is crucial for the successful performance on recognition memory paradigms [Heun et al.,2006; Wais et al.,2006]. With respect to lateralization effects, our results suggest a mild lateralization for hippocampal DTI measures and verbal memory performance. This is in line with previous findings from functional neuroimaging studies and lesion studies [Strange et al.,2002]. Additional analyses on possible sex differences in lateralization of verbal memory performance showed no significant findings related to sex. Research investigating the effects of sex on lateralization of verbal (memory) function shows some mixed results, with some studies demonstrating sex differences in which men have a left predominance and women a more bilateral representation [Shaywitz et al.,1995]. However, these findings have not been replicated in an extensive meta‐analysis demonstrating no consistent evidence for more bilateral representation of verbal function in women than men [Sommer et al.,2004] as well as in a functional MR imaging study [Haut and Barch,2006].
Some methodological issues need to be considered. Although our data are derived from the largest DTI study thus far, they are cross‐sectional in nature preventing us from drawing conclusions with respect to causality. Follow‐up examination of this cohort is already planned to investigate whether early microstructural changes in the hippocampus correlate with the development of hippocampal atrophy, MCI, and eventually AD.
We found that the relation between FA and memory performance is less strong than for MD. This may be due to the fact that FA is a measure reflecting the dominant directionality of diffusion of water. Because of the size of our region of interest (the hippocampus), multiple fibers are present that may all have different directions, which influence the FA. Because of that intrahippocampal fiber incoherence, low FA may not necessarily reflect underlying lower structural integrity [Pierpaoli et al.,1996]. In contrast, MD is affected by fiber crossing to a lesser extent, because it reflects magnitude of water diffusion, which is not influenced by direction. Subsequently, MD remains relatively constant [Pierpaoli et al.,1996]. This might be the explanation for the lack of finding with FA but not with MD. These complications in the analyses of FA in the hippocampus were also reported by others looking at MCI and AD patients [Fellgiebel et al.,2004]. In that study, they tried to take this into account by considering smaller regions of interest in the hippocampus, but still found a low sensitivity for FA values in contrast to MD [Fellgiebel et al.,2006].
The strengths of our study include its large size with a high‐response rate of over 70% to participate of all those were eligible. In addition, it is a single‐center study in which MRI data were identically acquired on a single scanner, and the hippocampus and WML were assessed volumetrically in a reliable, sensitive, and objective way by two trained experts, who were blinded to all clinical data. Furthermore, we used extensive adjustment for possible confounders such as educational level, depressive symptoms, a well‐known confounder in cognitive performance in elderly with SVD [de Groot et al.,2000a], hippocampal, and WML volume and lacunar infarcts.
To the best of our knowledge, there are no other studies investigating the relation between hippocampal DTI parameters and verbal memory function in nondemented elderly. A few studies have assessed DTI measurements in the hippocampus of MCI and AD patients [Fellgiebel et al.,2006; Muller et al.,2007]. In line with these studies, we found that a measurable change in structural integrity is observed together with a decline in verbal memory performance before changes in HV are observed. DTI parameters in the hippocampus were found to discriminate between early phases of cognitive decline (MCI) and normal aging. Patients with more advanced cognitive decline (i.e., AD) could only be discriminated based on their lower HV compared to controls and not by differences in diffusivity [Kantarci et al.,2002]. The same investigators also found that high diffusivity in the hippocampus and low HV was related to an increased risk for conversion of MCI in AD, with diffusivity being a stronger predictor than volume [Kantarci et al.,2005]. In these studies, additional adjustments were made only for age, sex, and education. Specific processes of episodic memory performance as in our study, which represent a more sensitive measure for cognitive decline than a clinical diagnosis of MCI or AD based on clinical diagnostic criteria [McKhann et al.,1984; Morris et al.,2001], were not taken into account. A further difference with our study was the mean age of their study group that was around 80 years [Kantarci et al.,2005]. Furthermore, they demonstrated that baseline hippocampal MD is associated with conversion to AD rather than HV [Fellgiebel et al.,2006]. Others demonstrated a higher sensitivity of hippocampal DTI parameters than HV measures in the diagnosis of MCI [Muller et al.,2007]. However, participants with relevant cerebrovascular disease (e.g., cortical infarcts, multiple lacunar infracts, and leukoaraiosis) were excluded in these studies in contrast to our study. Consequently, these may have a limited external validity as over 50% of AD patients have WML to some extent [Launer et al.,2008; Neuropathology group, MRC CFAS,2001]. In our study population, SVD was present in some degree in all participants, making our findings more representative.
Interestingly, the relation between hippocampal integrity and verbal memory function was also found in participants without loss of HV as assessed by structural MRI. Although there is abundant evidence that episodic memory formation is impaired by patients with hippocampal lesions or atrophy, such as early AD, and functional neuroimaging studies also indicate a crucial role for the medial temporal lobe including the hippocampus [Squire et al.,2004], this is the first study to demonstrate, using DTI, such a relationship in older people without apparent hippocampal pathology using DTI. This may indicate that microstructural changes assessed by DTI in the hippocampus indeed precede structural changes as assessed by conventional MRI, this must be unraveled by the follow‐up examination.
The relation between DTI parameters and verbal memory function was less striking in participants with low HVs. This could be because at the time that atrophy occurs, the structural changes (atrophy) are more prominently correlated with verbal memory performance than the microstructural changes. This line of reasoning is in agreement with the results of others demonstrating that diffusion parameters of the hippocampus can differentiate between MCI and controls, but could not classify AD [Kantarci et al.,2002], as well as studies showing that hippocampal DTI parameters in patients with MCI correlated with disease progression to AD [Fellgiebel et al.,2006; Kantarci et al.,2005].
Future studies should prospectively investigate the predictive value of DTI parameters of the hippocampus before the development of hippocampal atrophy and incident cognitive decline and dementia. To extrapolate our results, future studies should include participants from the general population. When proven, DTI of the hippocampus could possibly play a role as a surrogate marker for disease progression and could as such be used in therapeutic trials.
REFERENCES
- Babiloni C, Vecchio F, Mirabella G, Buttiglione M, Sebastiano F, Picardi A, Di Gennaro G, Quarato PP, Grammaldo LG, Buffo P, Esposito V, Manfredi M, Cantore G, Eusebi F ( 2009): Hippocampal, amygdala, and neocortical synchronization of theta rhythms is related to an immediate recall during Rey auditory verbal learning test. Hum Brain Mapp 30: 2077–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Jones DK ( 2002): Diffusion‐tensor MRI: Theory, experimental design and data analysis—A technical review. NMR Biomed 15: 456–467. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D ( 1994): Estimation of the effective self‐diffusion tensor from the NMR spin echo. J Magn Reson B 103: 247–254. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E ( 1991): Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol 82: 239–259. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O'Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS ( 2006): White matter damage on diffusion tensor imaging correlates with age‐related cognitive decline. Neurology 66: 217–222. [DOI] [PubMed] [Google Scholar]
- Colliot O, Chetelat G, Chupin M, Desgranges B, Magnin B, Benali H, Dubois B, Garnero L, Eustache F, Lehericy S ( 2008): Discrimination between Alzheimer disease, mild cognitive impairment, and normal aging by using automated segmentation of the hippocampus. Radiology 248: 194–201. [DOI] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM ( 2000a): Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry 57: 1071–1076. [DOI] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM ( 2000b): Cerebral white matter lesions and cognitive function: The Rotterdam Scan Study. Ann Neurol 47: 145–151. [DOI] [PubMed] [Google Scholar]
- De Groot JC, De Leeuw FE, Oudkerk M, Van Gijn J, Hofman A, Jolles J, Breteler MM ( 2002): Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 52: 335–341. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, Hofman A, Jolles J, van Gijn J, Breteler MM ( 2001): Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 70: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw FE, Barkhof F, Scheltens P ( 2004): White matter lesions and hippocampal atrophy in Alzheimer's disease. Neurology 62: 310–312. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, Korf E, Barkhof F, Scheltens P ( 2006): White matter lesions are associated with progression of medial temporal lobe atrophy in Alzheimer disease. Stroke 37: 2248–2252. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger‐Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, et al. ( 2007): Research criteria for the diagnosis of Alzheimer's disease: Revising the NINCDS‐ADRDA criteria. Lancet Neurol 6: 734–746. [DOI] [PubMed] [Google Scholar]
- Duvernoy H ( 1997): The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. New York: Springer‐Verlag. [Google Scholar]
- Erkinjuntti T ( 2002): Subcortical vascular dementia. Cerebrovasc Dis 13( Suppl 2): 58–60. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Wille P, Muller MJ, Winterer G, Scheurich A, Vucurevic G, Schmidt LG, Stoeter P ( 2004): Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: A diffusion tensor imaging study. Dement Geriatr Cogn Disord 18: 101–108. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Dellani PR, Greverus D, Scheurich A, Stoeter P, Muller MJ ( 2006): Predicting conversion to dementia in mild cognitive impairment by volumetric and diffusivity measurements of the hippocampus. Psychiatry Res 146: 283–287. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR ( 1975): “Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S ( 1999): Diagnostic criteria for Parkinson disease. Arch Neurol 56: 33–39. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Bremner JD ( 2005): MR‐based in vivo hippocampal volumetrics. II. Findings in neuropsychiatric disorders. Mol Psychiatry 10: 160–184. [DOI] [PubMed] [Google Scholar]
- Haut KM, Barch DM ( 2006): Sex influences on material‐sensitive functional lateralization in working and episodic memory: Men and women are not all that different. Neuroimage 32: 411–422. [DOI] [PubMed] [Google Scholar]
- Herve D, Mangin JF, Molko N, Bousser MG, Chabriat H ( 2005): Shape and volume of lacunar infarcts: A 3D MRI study in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 36: 2384–2388. [DOI] [PubMed] [Google Scholar]
- Heun R, Freymann K, Erb M, Leube DT, Jessen F, Kircher TT, Grodd W ( 2006): Successful verbal retrieval in elderly subjects is related to concurrent hippocampal and posterior cingulate activation. Dement Geriatr Cogn Disord 22: 165–172. [DOI] [PubMed] [Google Scholar]
- Hochstenbach J, Mulder T, van Limbeek J, Donders R, Schoonderwaldt H ( 1998): Cognitive decline following stroke: A comprehensive study of cognitive decline following stroke. J Clin Exp Neuropsychol 20: 503–517. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Xu Y, Shiung MM, O'Brien PC, Cha RH, Smith GE, Ivnik RJ, Boeve BF, Edland SD, Kokmen E, Tangalos EG, Petersen RC, Jack CR Jr, Kokmen E ( 2002): Comparative diagnostic utility of different MR modalities in mild cognitive impairment and Alzheimer's disease. Dement Geriatr Cogn Disord 14: 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Petersen RC, Boeve BF, Knopman DS, Weigand SD, O'Brien PC, Shiung MM, Smith GE, Ivnik RJ, Tangalos EG, Jack CR Jr ( 2005): DWI predicts future progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology 64: 902–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf ES, Wahlund LO, Visser PJ, Scheltens P ( 2004): Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology 63: 94–100. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Petrovitch H, Ross GW, Markesbery W, White LR ( 2008): AD brain pathology: Vascular origins? Results from the HAAS autopsy study. Neurobiol Aging 29: 1587–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J, Paxinos G, Voss T. 2007. Atlas of the Human Brain, 3rd ed. Elsevier, San Diego, CA. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM ( 1984): Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34: 939–944. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L ( 2001): Mild cognitive impairment represents early‐stage Alzheimer disease. Arch Neurol 58: 397–405. [DOI] [PubMed] [Google Scholar]
- Muller MJ, Greverus D, Weibrich C, Dellani PR, Scheurich A, Stoeter P, Fellgiebel A ( 2007): Diagnostic utility of hippocampal size and mean diffusivity in amnestic MCI. Neurobiol Aging 28: 398–403. [DOI] [PubMed] [Google Scholar]
- Neuropathology Group of the Medical Research Counsil Cognitive Function and Ageing Study (MRC CFAS) ( 2001): Pathological correlates of late‐onset dementia in a multicentre, community‐based population in England and Wales. Lancet 357: 169–175. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G ( 1996): Diffusion tensor MR imaging of the human brain. Radiology 201: 637–648. [DOI] [PubMed] [Google Scholar]
- Radloff LS ( 1977): The CES‐D ScaleL: A self‐report depression scale for research in the general population. Appl Psychol Measurem 1: 385–401. [Google Scholar]
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, Moody DM, O'Brien MD, Yamaguchi T, Grafman J, Drayer BP, Bennett DA, Fisher M, Ogata J, Kokmen E, Bermejo F, Wolf PA, Gorelick PB, Bick KL, Pajeau AK, Bell MA, DeCarli C, Culebras A, Korczyn AD, Bogousslavsky J, Hartmann A, Scheinberg P ( 1993): Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology 43: 250–260. [DOI] [PubMed] [Google Scholar]
- Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC ( 2002): Subcortical ischaemic vascular dementia. Lancet Neurol 1: 426–436. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L, Gore JC ( 1995): Sex differences in the functional organization of the brain for language. Nature 373: 607–609. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Aleman A, Bouma A, Kahn RS ( 2004): Do women really have more bilateral language representation than men? A meta‐analysis of functional imaging studies. Brain 127: 1845–1852. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE ( 2004): The medial temporal lobe. Annu Rev Neurosci 27: 279–306. [DOI] [PubMed] [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ ( 2002): Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. J Neurosci 22: 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakil E, Blachstein H ( 1993): Rey Auditory‐Verbal Learning Test: Structure analysis. J Clin Psychol 49: 883–890. [DOI] [PubMed] [Google Scholar]
- van de Pol LA, Hensel A, van der Flier WM, Visser PJ, Pijnenburg YA, Barkhof F, Gertz HJ, Scheltens P ( 2006): Hippocampal atrophy on MRI in frontotemporal lobar degeneration and Alzheimer's disease. J Neurol Neurosurg Psychiatry 77: 439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J ( 2005): Rey's verbal learning test: Normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc 11: 290–302. [DOI] [PubMed] [Google Scholar]
- van Norden AG, Fick WF, de Laat KF, van Uden IW, van Oudheusden LJ, Tendolkar I, Zwiers MP, de Leeuw FE ( 2008): Subjective cognitive failures and hippocampal volume in elderly with white matter lesions. Neurology 71: 1152–1159. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM ( 2003): Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 348: 1215–1222. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, Niessen WJ, Breteler MM, van der Lugt A ( 2007): Incidental findings on brain MRI in the general population. N Engl J Med 357: 1821–1828. [DOI] [PubMed] [Google Scholar]
- Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, Wallin A, Ader H, Leys D, Pantoni L, Pasquier F, Erkinjuntti T Scheltens P, European Task Force on Age Related White Matter Changes ( 2001): A new rating scale for age‐related white matter changes applicable to MRI and CT. Stroke 32: 1318–1322. [DOI] [PubMed] [Google Scholar]
- Wais PE, Wixted JT, Hopkins RO, Squire LR ( 2006): The hippocampus supports both the recollection and the familiarity components of recognition memory. Neuron 49: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G ( 2006): User‐guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 31: 1116–1128. [DOI] [PubMed] [Google Scholar]


