Abstract
Areas of expertise that cultivate specific sensory domains reveal the brain's ability to adapt to environmental change. Perfumers are a small population who claim to have a unique ability to generate olfactory mental images. To evaluate the impact of this expertise on the brain regions involved in odor processing, we measured brain activity in novice and experienced (student and professional) perfumers while they smelled or imagined odors. We demonstrate that olfactory imagery activates the primary olfactory (piriform) cortex (PC) in all perfumers, demonstrating that similar neural substrates were activated in odor perception and imagination. In professional perfumers, extensive olfactory practice influences the posterior PC, the orbitofrontal cortex, and the hippocampus; during the creation of mental images of odors, the activity in these areas was negatively correlated with experience. Thus, the perfumers' expertise is associated with a functional reorganization of key olfactory and memory brain regions, explaining their extraordinary ability to imagine odors and create fragrances. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: expertise, functional plasticity, olfaction, fMRI, perfumers
INTRODUCTION
The brain adapts to new abilities obtained through extensive practice. In experts with enhanced visual, auditory or motor skills, such as musicians and athletes, superior performance is associated with functional brain changes in modality‐specific brain regions [Cross et al.,2006; Kleber et al.,2009; Lotze and Halsband,2006; Lotze et al.,2003; Margulis et al.,2009; Milton et al.,2007; Ohnishi et al.,2001; Ross et al.,2003]. We thus questioned whether the effects observed in other modalities could be generalized to olfaction. This topic has not been previously addressed, probably because olfactory abilities are less developed in humans than other animals. Accordingly, olfactory experts are rare; in fact, there are only about 500 perfumers (known informally as “noses”) worldwide. Nevertheless, a recent study investigated the effect of sommelier expertise on brain activity related to wine tasting and showed that brain activation in olfactory/gustatory areas was substantially different in experts relative to naïve drinkers [Castriota‐Scanderbeg et al.,2005].
Several behavioral and functional studies have suggested that we can “imagine” smells by reactivating percept‐like memory representations [Bensafi et al.,2007; Carrasco and Ridout,1993; Djordjevic et al.,2005; Lyman and McDaniel,1990]. For instance, the detection of perithreshold odors was improved after imagining a matched rather than a mismatched odor [Djordjevic et al.,2005]. However, for the average person it is much easier to imagine visual images than olfactory ones. In contrast, perfumers have learned to form olfactory sensory representations through daily practice and extensive training. Furthermore, they claim to have the ability to produce perceptual images of smells in the total absence of odorants. Forming mental images of odors is thus a crucial component of the perfumer's expertise.
In this study, we use functional magnetic resonance imaging (fMRI) to determine the impact of expertise on the neural substrates of odor‐relevant mental imagery in olfaction experts. We scan both perfumers renowned for creating perfumes and students from an international school of perfumery while they either perceive odors or imagine them from their chemical labels. At the perfumery school, the student perfumers learn to recognize the smells of about 300 chemical molecules over the course of 2 years. The professional perfumers typically improve their knowledge of these molecules during subsequent vocational courses or business experience. We took advantage of this variability in expertise among professional perfumers to identify the functional brain areas associated with their experience. Indeed, recent findings have suggested that the extent of functional reorganization is proportional to the length of training—even when this training lasts for a short period of time [Ilg et al.,2008]. Because naïve (untrained) subjects are largely unfamiliar with the chemical labels of odorants used in the current study (e.g., dihydromyrcenol, triplal, benzyl acetate, and alpha‐damascone), we did not include naïve subjects in our study. We hypothesize that, during the imagination of odors, the perfumer's expertise would be associated with a modification of the activation patterns in olfactory and memory‐processing regions such as the piriform cortex (PC) and the hippocampus. Such a modification of activation in experts would be the result of a cortical reorganization due to experience, which is the sign of a functional plasticity [Jancke,2009] in the olfactory brain.
MATERIALS AND METHODS
Subjects
Twenty‐eight healthy, right‐handed subjects participated in our study, including 14 student perfumers (11 female; mean age: 23 years; range: 21−26 years) from an international school of perfumery (Institut Supérieur International de la Parfumerie, de la Cosmétique et de l'Aromatique, Versailles, France) and 14 professional perfumers famous for creating perfumes (four females; mean age: 42 years; range: 29−59 years). Although the student experts had, at most, been trained for 2 years as part of their education, the professional experts all had 5–35 years of career‐relevant business experience. The functional data from three students were discarded because of technical problems with the MRI scanner. The exclusion criteria were rhinal disorders (colds, active allergies, a history of nasal‐sinus surgery, or asthma), pregnancy, ferrous implants (e.g., pacemakers, cochlear implants), claustrophobia, or any neurological disease. Handedness was assessed by the Edinburgh Handedness Inventory [Oldfield,1971]. This study was conducted according to French regulations on biomedical experiments using healthy volunteers and according to the principles outlined in the Declaration of Helsinki. All subjects gave written informed consent as required by the local Institutional Review Board.
Experimental Design
The subjects were scanned during four functional runs. For two runs, the subjects were asked to passively smell odors (“Perception”); for the other two, the subjects had to actively imagine odors based on the visual presentation of the odor's chemical name (“Imagery”). The order of task presentation was counterbalanced among subjects.
The perception runs followed a mixed‐block/event‐related design. Each run consisted of 20 12‐s blocks of odor perception alternated with 12‐s blocks without stimuli (Fig. 1). During those runs, 20 odors were presented, one every sniff. Because the mean duration of a breathing cycle ranged from 3 to 5 s depending on the subject, and because we waited for the beginning of the inspiration before administering odors [see Vigouroux et al.,2005 for details], the number of odor stimulations varied between 2 and 4 during each odor perception block. The odors were presented in a loop following a pre‐determined order. The imagery runs consisted of 40 15‐s blocks that included 12 s of mental imagery (a 4‐s presentation of the odor name followed by an 8‐s presentation of black fixation cross‐hairs), 2 s of response during which the subjects used a key pressed to indicate whether they could imagine the odor (the word “response” was presented on the screen), and a 1 s delay before the next block (Fig. 1). During each imagery run, each of the 20 odor names were presented twice. The same 40 names were presented in a different order during the second imagery run. During both the perception and imagery runs, the subjects were instructed to breathe naturally and regularly without sniffing or holding their breath.
Figure 1.
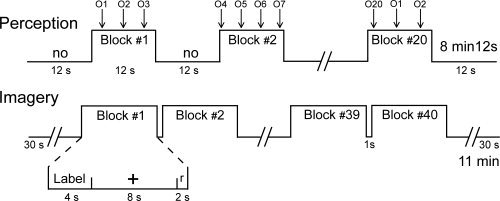
Experimental design. During the perception condition, 20 odors (from O1 to O20) were delivered in synchrony with breathing during 20 12‐s blocks (from Block #1 to Block #20) interleaved with 12‐s no‐odor (no) blocks. During the imagery condition, 20 odor labels were presented twice within 40 15‐s blocks (from Block #1 to Block #40). Each block consisted of the presentation of the odor label, then a cross‐hair followed by the presentation of the word “response” (r) and a brief rest.
Before scanning, the subjects participated in a training session. To familiarize the subjects with the experimental conditions, they were trained to create olfactory images while lying on the fMRI bed. During this session, the 20 odor names used in the imagery runs were presented visually, and the subjects pressed a button when they began perceiving the odor image. This session allowed us to measure the latency of image creation.
Stimuli and Experimental Setup
Twenty odor stimuli provided by Créations Aromatiques (Haarman & Reimer, Grasse, France) were employed during scanning. These odorants were aldehyde C‐11, alpha‐damascone, benzyl acetate, beta‐ionone, citronellol, dihydromyrcenol, eucalyptol, eugenol, lavandin (essential oil: EO), lemon (EO), linalyl acetate, linanol, methyl anthranylate, orange (EO), phenylethyl alcohol, sandalwood (EO), spearmint, tangerine (EO), triplal, and vanillin. These are the 20 most common odors among the 300 learned by student perfumers. The odorants were diluted in mineral oil (Sigma Aldrich, Saint‐Quentin‐Fallavier, France) to a concentration of 10%. For stimuli presentation, 5 ml of this solution was absorbed into compressed polypropylene filaments that were inside a 100 ml white polyethylene squeeze‐bottle equipped with a dropper (Fisher Scientific, Illkirch, France).
During perception, the odorants were presented using an airflow olfactometer, which allows the stimulation to be synchronized with breathing [Vigouroux et al.,2005]. The stimulation equipment consisted of two modules: the non‐ferrous (Duralumin®) air‐dilution injection head (placed in the magnet room) and the electronic component of the olfactometer (positioned outside the magnet room). Compressed air (10 l/min) was pumped into the olfactometer and delivered continuously through a standard oxygen mask (positioned on the subject's face). At the beginning of an inspiration phase, an odorant was injected into the olfactometer by rapidly squeezing the odor bottle into the injection head, thereby transmitting the odorant to the mask. Information regarding the onset of stimulation was transmitted by optical fibers to analog‐to‐digital converters located outside the magnetically shielded room and powered by nickel‐cadmium batteries. During imaging, the chemical names of these odors were displayed on a screen that could be viewed in a mirror secured directly in the subject's line of sight. The presentation timing was monitored using commercially available Psyscope® software on a Mac OS computer and was synchronized with the scanner.
Breathing was recorded using polyvinyl‐chloride foot bellows (Herga Electric Limited, Suffolk, UK) that were secured to the subject's abdomen with a cotton belt. Breathing data, stimulation onset, and trigger signals from the MRI scanner were recorded online (100 Hz sampling rate) on a personal computer equipped with a DAQCard‐500 digital acquisition board (National Instruments®, Austin, TX) using the LabVIEW software package (National Instruments®). Data were further analyzed using WinDaq Waveform Browser 2.36 software (DataQ Instruments®, Akron, OH) and custom‐developed routines that were created using Matlab (The Mathworks, Natick, MA).
The amplitudes of the inspiratory and expiratory waveforms were estimated by integrating the curves from one side of baseline to the other. The mean inspiratory volumes were computed for each 12‐s odor stimulation block and mental‐imagery block, and these data were entered into a three‐way (Group × Task × Stimuli) analysis of variance (ANOVA) with repeated measurements on the last two factors using Statistica 6.0 (StatSoft®, Tulsa, OK).
Functional and Structural Data Acquisition
We acquired T2*‐weighted functional images sensitive to blood oxygen level‐dependent contrast using a Philips NT 1.5 Tesla MRI scanner (Philips Medical Systems, Best, Netherlands) with a birdcage head coil. The imaging volume was composed of 25 adjacent, 5‐mm‐thick axial slices based on scout images acquired in the sagittal plane. This imaging volume covered the entire brain and was in an axial orientation parallel to the bicommissural plane. To acquire functional images, we used a three‐dimensional, four‐shot PRESTO MRI sequence [Liu et al.,1993] with the following parameters: repetition time (TR) = 26 ms, echo time (TE) = 12 ms, flip angle = 14°, field‐of‐view = 256 × 205 mm2, and imaging matrix = 64 × 51 (voxel size: 4 × 4 × 5 mm3). This sequence is less prone to magnetic susceptibility artifacts than the usual single‐shot, gradient‐echo planar imaging sequence [van Gelderen et al.,1995] and thus prevents most of the signal loss—especially in the orbitofrontal cortex and the mesial temporal region. The acquisition time per volume was 2.5 s. We collected a total of 384 volumes for the perception condition and 528 volumes for the imagery condition over the 4 runs. Finally, a high‐resolution (1 mm3) structural image was acquired using a three‐dimensional, T1‐weighted gradient echo sequence (repetition time = 23.7 ms, echo time = 6.9 ms, flip angle = 28°, number of accumulations [NA] = 2).
Functional Data Analysis
We processed all functional images using Statistical Parametric Mapping software [Friston et al.,1995] (SPM2, Wellcome Department of Cognitive Neurology, London, UK). For each subject, all functional volumes were realigned to the median volume, coregistered to the anatomical image, spatially normalized to the Montreal Neurological Institute (MNI) standard brain, and smoothed with an 8 × 8 × 10 mm full‐width, half‐maximum Gaussian kernel. Preprocessed data were statistically analyzed on a subject‐by‐subject basis using the General Linear Model, which convolves a neural model derived from the stimuli onsets with a hemodynamic response function (hrf). A high‐pass filter (with a cut‐off frequency of 1/128 Hz) was used to eliminate instrumental and physiological signal fluctuations at very low frequencies.
Because the hrf varies depending on the subject and the area of interest [Handwerker et al.,2004], we attempted to better estimate this function by using both the canonical hrf and its time derivative [Hopfinger et al.,2000]. We used the amplitude of the hrf in the group random‐effect analysis, which removes potential bias in the results caused by latency [Calhoun et al.,2004]. For the statistical analysis of images acquired during the perception condition, stimulus‐onset asynchronies were determined relative to the maximum amplitude of the inspiratory phases that followed odor stimulation. For the statistical analysis of images acquired during the imagery condition, stimulus onset asynchronies were fixed 2 s after the beginning of name presentation. We retained only those odor names that resulted in mental imagery. The homogeneity of the individual data inferred from both canonical and time‐derivative models were confirmed with the Cook test using the ‘DISTANCE’ software package (http://www.madic.org).
Random‐effect analyses were performed to identify patterns of brain activation at the population level. We first led one‐way ANOVAs to explore the activation pattern associated with the tasks or groups and, second, performed regression analyses to identify the brain region in which the level of activation varied with the level of expertise. Both analyses were performed first on the whole brain and then on specific regions of interest.
First of all, activations common to both the imagery and perception condition and found in both professional and student perfumers were determined in a conjunction of the [Imagery] and [Perception] simple contrasts for each group. A second analysis was performed to separately investigate areas that were differentially activated under the imagery and perception conditions in the [Imagery minus Perception] contrast in student and professional perfumers. A third analysis was then performed in the [(Imagery Student minus Perception Student) minus (Imagery Professional minus Perception Professional)] double contrast to display differences between the two groups. For those analyses, the level of significance was set at P < 0.001, uncorrected at the cluster level for multiple comparisons across the much larger volume of the whole brain (i.e., whole‐brain correction: WBC). The activation cluster size (k) is also reported. The anatomic atlases created by Mai et al. and Duvernoy [Duvernoy,1999; Mai et al.,2008] were used to localize and describe activated regions. Voxels were reported in terms of the MNI coordinate space. Following neurological convention, the right side of an image corresponds to the right side of the brain.
Specific analyses were performed on brain regions known to play a role in olfactory and memory processing. Anatomical Volumes‐Of‐Interest (VOIs) in the anterior PC (aPC), posterior PC (pPC), the amygdala, the orbitofrontal cortex, and the hippocampus were defined in both hemispheres using MRIcro (http://www.mricro.com) and human brain atlases [Duvernoy,1999; Mai et al.,2008]. These regions were drawn for each subject in both hemispheres from coronal slices for the PC (from 12.5 mm anterior to −2.7 mm posterior to the anterior commissure), the amygdala (from 0 mm anterior to −13.3 mm posterior to the anterior commissure), and from sagittal slices for the hippocampus (from −24.5 mm to −38 mm on the left hemisphere and from 24 mm to 38 mm on the right hemisphere). For the PC, the beginning of the amygdala was used as anatomical landmark to determine the utmost caudal part of the pPC located 4 mm before. For the amygdala and the hippocampus, delineations were based on the visual differentiation of structures (T1 images) and on the detailed diagrams and pictures from the Mai et al. and Duvernoy's atlases. Orbitofrontal VOIs were spheres of 10‐mm radius located at coordinates previously identified by Gottfried and Zald [2005] in the left and right hemispheres (−22, 30, −17, and 24, 33, −12). The activation levels were extracted in the VOIs using the MarsBar toolbox (http://marsbar.sourceforge.net). For activation arising from these VOIs, we tested differences in activation levels as a function of Task (imagery vs. perception), Group (student vs. professional), and Hemisphere (right vs. left) using a within‐subject, repeated measures ANOVA (Statistica 6.0, StatSoft®, Tulsa, OK).
To investigate whether the level of expertise affected the level of activation in neural networks involved in olfactory and memory processing, two multiple‐regression analyses (Imagery and Perception) with constant term were performed on professional perfumers. The subject's age served as a covariate reflecting expertise (from 5 to 35 years of career‐relevant business experience), while the gender effect was covaried out (entered as variable of no‐interest). These regression analyses were only performed on professional perfumers because the range of expertise in students was too small to be considered a covariate. The level of significance was set at P < 0.001, uncorrected at the cluster level for multiple comparisons. In regions with strong, a priori significance, the level of significance was set at P < 0.05, corrected at the voxel level for multiple comparisons. Spheres of 10‐mm diameter were then used to define small volumes of interest (small‐volume correction: SVC) [Worsley et al.,1996] based on coordinates from previous human‐imaging studies on olfactory and memory processing [Gottfried et al.,2004; Gottfried and Zald,2005; Plailly et al.,2005; Royet et al.,2003; Zald and Pardo,1997].
Regression analyses were also performed to detect relationships between the level of experience and the level of activation in the VOIs. For each VOI and each subject, mean activation from all voxels was extracted using Marsbar. Within both groups of subjects, the gender effect was suppressed by normalizing data (X = X 0 − X where X was the mean value as a function of gender). Linear regression analyses were then performed using Statistica 6.0 (StatSoft®, Tulsa, OK).
Statistical Analysis
Results are expressed as Mean ± SEM for subjects or conditions. Where not otherwise indicated in the text, statistical significance was determined using a two‐tailed Student's t test (when comparing two conditions) or ANOVA with repeated measurements when comparing more than two conditions. The normality of the samples and the homogeneity of their variance were controlled using the Lilliefors and Hartley tests. In all statistical tests, a value of P < 0.05 (corrected for multiple comparisons) was considered significant.
RESULTS
Ability of Perfumers to Imagine Odors
On the basis of these subjects' self‐reported ability to imagine odors throughout the experiment, the professional perfumers scored an average of 92.9% (range: 80−100) and student perfumers scored an average of 92.0% (range: 70−100). We did not take psychophysical measurements (such as image richness or vividness, or latency to image creation or its duration) during the imagery period, which would have allowed investigation of the difference in quality of the images between groups, because the subjects claimed that providing responses disturbed their mental imagery. During the training period, however, the subjects were asked to imagine each of the 20 odors sequentially and press a button when they began to “smell” each odor mentally. The professionals created odor images more successfully than the students [0.99 ± 0.01 and 0.89 ± 0.02, respectively; t (38) = 4.09, P < 0.001] and faster than the students [4.32 s ± 0.10 and 6.15 s ± 0.20, respectively; t (38) = 8.26, P < 0.001]. These results confirm that the professionals had a superior ability to generate odor‐relevant mental imagery when compared to student perfumers.
Common Neural Network for Imagery and Perception of Odors
The conjunction analysis revealed a common pattern of activation between both student and professional perfumers and between both imagery and perception conditions. This pattern included a bilateral activation of the PC that reached the anterior part of the amygdala (WBC; Fig. 2a; Table I), a finding in accordance with previous imaging studies [Gottfried et al.,2002; Royet and Plailly,2004; Savic et al.,2000; Sobel et al.,2000; Zatorre et al.,1992]. This result showed that mental imagery can induce the activation of the primary olfactory brain region. Clusters of activated voxels were also observed in the superior frontopolar gyrus, the precuneus, the cerebellum, and the inferior portion of the middle occipital gyrus (WBC; Table I).
Figure 2.
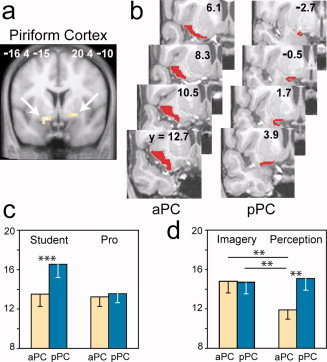
Neural responses in the piriform cortex. (a) Piriform cortex activation common to both the imagery and perception conditions and detected in both student and professional (Pro) perfumers. The activations are superimposed on a coronal section of the normalized, T1‐weighted structural scan averaged among the entire pool of subjects (MNI coordinate system). (b) The aPC and pPC VOI mappings of a single subject depicted in eight serial coronal sections. (c and d) Plots of the signal change in the PC VOIs as a function of (c) groups and areas or (d) tasks and areas. Error bars represent SEM. aPC, anterior piriform cortex; pPC, posterior piriform cortex; **P < 0.01, ***P < 0.001.
Table I.
Neural activations common to both imagery and perception conditions in both student and professional perfumers
| Anatomical regions | R/L | k | T | x | y | z |
|---|---|---|---|---|---|---|
| Middle occipital gyrus, inferior part | L | 6 | 7.49 | −48 | −72 | −15 |
| Middle occipital gyrus, inferior part | R | 16 | 7.24 | 44 | −76 | −10 |
| Posterior Piriform cortex | L | 13 | 7.12 | −16 | 4 | −15 |
| Anterior Piriform cortex | L | 7.01 | −20 | 8 | −20 | |
| Amygdala | L | 6.25 | −12 | −4 | −20 | |
| Cerebellum | R | 11 | 7.11 | 8 | −40 | −10 |
| Superior frontopolar gyrus | R | 17 | 7.10 | 8 | 64 | 25 |
| Cerebellum | L | 29 | 7.02 | −16 | −52 | −30 |
| Amygdala | R | 9 | 6.79 | 12 | −4 | −15 |
| Posterior Piriform cortex | R | 6.74 | 20 | 4 | −10 | |
| Anterior Piriform cortex | R | 6.52 | 24 | 8 | −15 | |
| Precuneus | L | 9 | 6.78 | −4 | −64 | 60 |
L, left; R, right; k, size of the cluster in number of connected voxels; T, Student's t value; x, y, z, MNI coordinates (in mm) of the maximum peak.
Imagery‐Specific Neural Network
To determine which brain regions were involved in odor imagery in each group, we subtracted the images acquired during the perception condition from those acquired during the imagery condition. The results showed that the left parahippocampal gyrus (WBC; k = 6, T = 4.31) was significantly more activated during the imagery condition in the professionals (Fig. 3a), whereas the right anterior insula (WBC; k = 14, T = 4.11) was more strongly activated during the imagery condition in the students (Fig. 3b). To test whether the level of expertise affected the pattern of activation generated by creating odor images, we compared imagery‐specific brain activation between professional and student perfumers. The right anterior insula was the only imagery‐specific region to survive the statistical test and was more activated in students than in perfumers (WBC; k = 14, T = 4.40).
Figure 3.
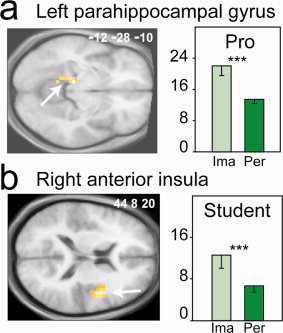
Imagery‐specific brain regions differently activated between groups. Significantly higher activation was detected for the imagery condition in (a) the parahippocampal gyrus in professionals (Pro) and (b) the anterior insula in students. The activations are depicted on axial sections of the normalized mean brain image. The bar graphs indicate the levels of activation in odor imagery (Ima) and perception (Per) in students and professionals. Error bars reflect SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Differential Involvement of Subregions in the Piriform Cortex
Following the regional analysis of mental imagery, we used VOI analyses to focus on the role of the PC. The PC VOI was separated into anterior (aPC) and posterior (pPC) parts (Fig. 2b) based on the anatomical and functional heterogeneity of this structure, which has been established in both animals and humans [Gottfried et al.,2002,2006; Litaudon et al.,1997; Mouly and Di Scala,2006]. We measured the activation levels in these anatomical VOIs under each condition and tested for the effects of task, group, area and hemisphere. The results showed that, overall, the pPC was more activated than the aPC [F (1, 23) = 9.80, P = 0.005] and the left PC was more activated than the right PC [F (1, 23) = 6.74, P = 0.016]. The significant group × area interaction [F (1, 23) = 5.62, P = 0.027] was caused by the higher activation of the pPC in students (P = 0.001) relative to professional perfumers, regardless of the task and the hemisphere (Fig. 2c). These results suggest that the two groups used different strategies to process odors. The significant task × area interaction [Fig. 2d; F (1, 23) = 5.54, P = 0.028] revealed that the aPC plays a crucial role in the generation of odor mental images; regardless of the perfumer's level of expertise or the brain hemisphere, the aPC was more activated when odors were imagined than when the same odors were actually perceived (P = 0.002; the activation recorded during perception was used as a reference). The importance of the PC in processing mental images of odors was reinforced by the absence of any effect of Task, Group, and Hemisphere on the activation levels in the amygdala, orbitofrontal cortex, or hippocampus (VOIs).
Effect of Expertise on Activation Pattern
Because brain activity in musicians or athletes is related to prolonged, intensive practice [Cross et al.,2006; Lotze and Halsband,2006; Lotze et al.,2003; Milton et al.,2007], we subsequently examined whether the functional activity in perfumers during perception and imagery depends on the duration of practice (and therefore the level of expertise). Interestingly, the multiple regression analysis only produced significant results for Imagery. No significant result was observed for the passive odor perception condition. In the imagery condition, an increased level of expertise was associated with the decreased activation of regions typically associated with olfactory and memory functions: the right pPC, the left orbitofrontal cortex and the left hippocampus (SVC; Table II; Fig. 4). In other words, the greater the level of expertise, the less these three key brain regions were activated. In addition, this analysis showed activity in a small group of regions that included the bilateral cerebellum, the left inferior temporal gyrus, the left postcentral gyrus and the right middle frontal gyrus (WBC; Table II). No regions that are involved in odor imagery showed a significant activation increase with increasing expertise.
Table II.
Neural activations decreased with expertise during the imagery condition
| Anatomical regions | R/L | k | T | x | y | z |
|---|---|---|---|---|---|---|
| Cerebellum | L | 21 | 8.50 | −24 | −76 | −25 |
| Postcentral gyrus | L | 8 | 7.74 | −36 | −24 | 65 |
| Cerebellum/Inferior temporal gyrus | R | 12 | 5.83 | 40 | −36 | −25 |
| Middle frontal gyrus | R | 8 | 5.62 | 44 | 56 | 5 |
| Inferior temporal gyrus | L | 12 | 5.41 | −52 | −68 | −5 |
| Hippocampus (subiculum)* | L | 2 | 4.73 | −24 | −20 | −15 |
| Olfactory orbitofrontal cortex* | L | 1 | 4.31 | −20 | 40 | −10 |
| Posterior frontal piriform cortex* | R | 1 | 4.27 | 16 | 0 | −10 |
L, left; R, right; k, size of the cluster in number of connected voxels; T, Student's t value; x, y, z, MNI coordinates (in mm) of the maximum peak; *, small‐volume correction.
Figure 4.
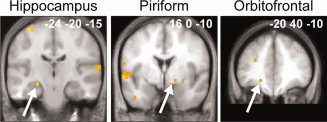
Whole‐brain multiple regression analysis. Significant negative correlations between the activation levels and the length of expertise were detected in the left hippocampus, the right PC, and the left orbitofrontal cortex in professionals during the imagery condition. The activations are depicted on coronal sections of the normalized, mean T1‐weighted brain image.
The analysis of pre‐defined VOIs (aPC, pPC, amygdala, orbitofrontal cortex, and hippocampus) confirmed the results of the random‐effect analysis. Significant negative correlations were observed between the level of expertise of the professional perfumers and the level of activation during odor imagery in the right pPC (r = −0.717, F = 12.698, P = 0.004). Strong, but not significant, negative correlations were found in the left (r = −0.532, F = 4.730, P = 0.050) and right (r = −0.521, F = 4.471, P = 0.056) aPC (Fig. 5a). During imagery, significant negative correlations were also found in the right (r = −0.540, F = 4.930, P = 0.046) and left (r = −0.582, F = 6.142, P = 0.029) hippocampus (Fig. 5b). No significant correlations were observed for perception.
Figure 5.
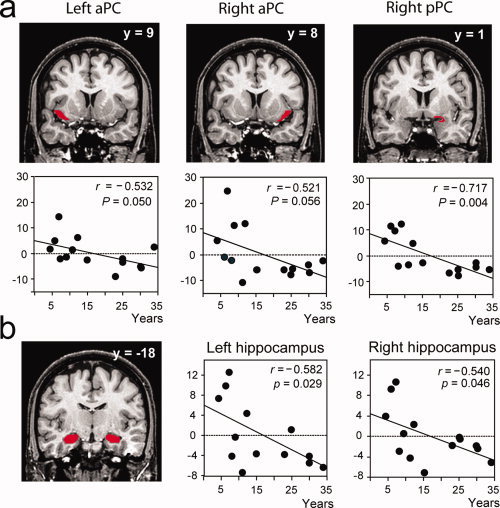
VOI regression analyses. Significant negative correlations between the activation levels and the length of expertise were detected in (a) the left and right aPC and right pPC and (b) the left and right hippocampus. VOIs mappings are represented in red on coronal sections of a normalized, T1‐weighted, unsmoothed, structural scan of a professional perfumer. Scatter diagrams represent variability in the activation levels of VOIs during the imagery condition as a function of length of experience. Data reported in scatter diagrams were normalized as a function of gender to coincide with those of the multiple regression analysis (Fig. 4). aPC, anterior piriform cortex; pPC, posterior piriform cortex; r, correlation coefficient; P, probability value.
Control of Breathing Activity
Throughout this experiment, the subjects were asked to breathe in a natural way during all tasks to minimize bias caused by sniffing‐related brain activation. However, because sniffing fluctuations can result in a confounding activation of the PC [Sobel et al.,1998], nasal airflows were systematically controlled throughout the experiment. No differences in breathing activity were observed between the groups [F (1,23) = 0.051, P = 0.824] or between the tasks [F (1,23) = 0.415, P = 0.526], and there was no interaction between these two factors [F (1,23) = 1.439, P = 0.243]. Thus, the activation evoked during odor imagery cannot be explained by variations in nasal airflow.
DISCUSSION
To our knowledge, our study is the first to investigate the neural basis of olfactory perception and mental imagery in perfumers. The ability to create olfactory mental images is rare, and studying olfactory experts is the best way to accurately identify the mental processes underlying the creation of these images. By analyzing functional data in relation to the subjects' expertise, we were able to investigate functional reorganization resulting from prolonged, intensive practice.
Our results demonstrate the robust activation of a large neural network, including the olfactory primary cortex, when perfumers mentally imagine odors in the total absence of odorants. The PC is believed to be unimodal, meaning that it does not receive direct verbal, visual or semantic inputs. Its activation in the present study unambiguously proves that olfactory mental imagery can reactivate memory traces within the PC via a top‐down process. This finding is consistent with the general view that similar neural networks are activated during both mental imagery and the actual perception of sensory stimuli, a phenomenon that is well‐documented in vision, audition and motor processes [Kosslyn et al.,2001]. Our study demonstrates that these neurological similarities between perception and imagery also apply to olfaction. The use of perfumers, who are renowned for their expertise in olfaction and their ability to mentally imagine odors, was fundamental in supporting this theory.
The anterior and posterior areas of the PC were differentially involved in olfactory mental imagery and odor perception, further indicating the heterogeneous nature of the PC [Gottfried et al.,2002,2006; Litaudon et al.,1997; Mouly and Di Scala,2006]. Whereas the pPC was activated during both tasks identically, the aPC was activated more strongly during imagery than during perception. The aPC is reportedly involved in the synthetic encoding of odorant identity in animals [Kadohisa and Wilson,2006] and in processing structure‐based information in humans [Gottfried et al.,2006]. In contrast, the pPC is believed to process information regarding odor quality [Gottfried et al.,2006; Kadohisa and Wilson,2006]. These data suggest that, rather than the quality of the odorant, experts use highly specific information (such as the chemical identity) in order to accurately reactivate odor‐related memory traces in response to the chemical label of the odorant.
Although both students and professionals were able to imagine odors, our data show marked differences between these groups. The professionals succeeded more frequently and responded more rapidly than students in mentally imagining odors, and these behavioral differences were associated with functional differences. The pPC was more strongly activated than the aPC in students, but not in professionals, suggesting that the students focused more on odor quality [Gottfried et al.,2006; Howard et al.,2009; Kadohisa and Wilson,2006] than the professionals when perceiving or imagining an odor. It has been previously demonstrated that the parahippocampal gyrus is involved in memory recall and mental imagery [Kreiman et al.,2000], and this region was significantly more activated in professionals than in students. In contrast, the right anterior insula, a region involved in multisensory integration [Dade et al.,2001; Slotnick and Schacter,2004], was significantly more activated in students than in professionals. Our findings thus demonstrate that training can alter the neural processes that are activated when imagining an odor. Whereas the creation of an odor mental image was a more automatic mnestic process in professional perfumers, this process needed to be supported by previously encoded associations in student perfumers.
More strikingly, by comparing the functional data obtained during odor imagery with the level of experience in professional perfumers, we revealed the functional reorganization of several brain regions. When imagining an odor, the experienced perfumers exhibited lower levels of activity in key regions involved in olfactory and memory processing: the primary olfactory cortex (bilateral aPC and right pPC), the left and right hippocampus and the left olfactory orbitofrontal cortex. For the pPC and the hippocampus, these effects were observed using both whole‐brain and VOI analyses. A decrease in the activation of these structures cannot be attributed to age because these variations in activity were not observed during odor perception or in other brain regions. Because these functional differences were observed in subjects who differed in age and experience by several decades, they further support the view that the mental imaging of odors develops from daily practice and is not an innate skill.
How brain activity relates to practice and learning is complex and multifactorial. A few studies have indicated that experience increases activity in specific brain regions [Cross et al.,2006; Kleber et al.,2009; Lotze et al.,2003; Ohnishi et al.,2001]; however, decreases in activity have also been reported in the primary and secondary motor areas of professional pianists and golfers [Haslinger et al.,2004; Jancke et al.,2000; Koeneke et al.,2004; Krings et al.,2000; Lotze et al.,2003; Ross et al.,2003]. These decreases in activity have been associated with performance gains. It has been claimed that professional piano players control their movements with more automation, better manual dexterity, and less motor cortical activation. Researchers have suggested that this mechanism may result in enhanced efficiency, reduced effort, increased spontaneity, and/or the liberation of additional resources for other aspects of artistic and motor performance [Jancke et al.,2001; Krings et al.,2000; Lotze et al.,2003]. Here, professional perfumers were able to quickly—and even instantaneously—imagine most odors, whereas students had difficulty with this task and could only imagine odors by deliberately focusing their attention. By easily reactivating memory representations of pure or complex odors, professional perfumers can compare and mix different scents to create new fragrances.
To our knowledge, functional plasticity within the PC resulting from long‐term training has not been observed in humans. However, the rapid reorganization of human PC activation patterns has been observed following enhanced discrimination and aversive conditioning [Li et al.,2008]. In rats, olfactory learning is accompanied by an increase in dendritic spine density in the primary olfactory cortex, suggesting an increase in the number of excitatory synapses [Knafo et al.,2001]. Our data thus confirm the involvement of the PC in memory processes. In professional subjects, we also observed a negative correlation between functional activity in the hippocampus and the level of expertise. This is consistent with the theory that repeated information‐recall reduces the involvement of the hippocampus. A recent study has suggested that remembering previously heard data can activate the hippocampus and that this activation decreases as the stimulus is repeated [Svoboda and Levine,2009].
CONCLUSION
In summary, we observed that the perfumers' level of expertise influences functional brain plasticity in regions involved in odor‐relevant mental imagery. We showed that the PC is activated in response to olfactory imagery in student and professional perfumers, but that this activity is proportionally reduced as the duration of experience increases in professional perfumers. Our results suggest that experts progressively develop more efficient strategies in their field of expertise, allowing them to liberate additional resources for other aspects of artistic performance such as the creation of new fragrances. Taken together, these findings demonstrate the extraordinary ability of the brain to adapt to environmental demands and reorganize with experience.
Acknowledgements
The authors thank perfumers J.C. Ellena and D. André for their helpful advice regarding experimental design, M. Hugentobler for assistance with student recruitment, and S. Garcia for his assistance with breathing data analysis. They are greatly indebted to students from the Institut Supérieur International de la Parfumerie, de la Cosmétique et de l'Aromatique, and to professionals from France and Switzerland (Azur Fragrances, Créations Aromatiques, Givaudan, Haarman & Reimer, IFF, Luzi SA, Parfum Concept, Quest, Takasago).
REFERENCES
- Bensafi M, Sobel N, Khan RM ( 2007): Hedonic‐specific activity in piriform cortex during odor imagery mimics that during odor perception. J Neurophysiol 98: 3254–3262. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Stevens MC, Pearlson GD, Kiehl KA ( 2004): fMRI analysis with the general linear model: Removal of latency‐induced amplitude bias by incorporation of hemodynamic derivative terms. Neuroimage 22: 252–257. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Ridout JB ( 1993): Olfactory perception and olfactory imagery: A multidimensional analysis. J Exp Psychol Hum Percept Perform 19: 287–301. [DOI] [PubMed] [Google Scholar]
- Castriota‐Scanderbeg A, Hagberg GE, Cerasa A, Committeri G, Galati G, Patria F, Pitzalis S, Caltagirone C, Frackowiak R ( 2005): The appreciation of wine by sommeliers: A functional magnetic resonance study of sensory integration. Neuroimage 25: 570–578. [DOI] [PubMed] [Google Scholar]
- Cross ES, Hamilton AF, Grafton ST ( 2006): Building a motor simulation de novo: Observation of dance by dancers. Neuroimage 31: 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dade LA, Zatorre RJ, Evans AC, Jones‐Gotman M ( 2001): Working memory in another dimension: Functional imaging of human olfactory working memory. Neuroimage 14: 650–660. [DOI] [PubMed] [Google Scholar]
- Djordjevic J, Zatorre RJ, Petrides M, Boyle JA, Jones‐Gotman M ( 2005): Functional neuroimaging of odor imagery. Neuroimage 24: 791–801. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM ( 1999): The Human Brain‐Surface, Three Dimensional Sectional Anatomy and MRI. Wien (Austria): Springer. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith C, Frackowiak RS ( 1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Gottfried JA, Deichmann R, Winston JS, Dolan RJ ( 2002): Functional heterogeneity in human olfactory cortex: An event‐related functional magnetic resonance imaging study. J Neurosci 22: 10819–10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Smith AP, Rugg MD, Dolan RJ ( 2004): Remembrance of odors past: Human olfactory cortex in cross‐modal recognition memory. Neuron 42: 687–695. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Winston JS, Dolan RJ ( 2006): Dissociable codes of odor quality and odorant structure in human piriform cortex. Neuron 49: 467–479. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Zald DH ( 2005): On the scent of human olfactory orbitofrontal cortex: Meta‐analysis and comparison to non‐human primates. Brain Res Brain Res Rev 50: 287–304. [DOI] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, D'Esposito M ( 2004): Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage 21: 1639–1651. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Altenmuller E, Hennenlotter A, Schwaiger M, Grafin von Einsiedel H, Rummeny E, Conrad B, Ceballos‐Baumann AO ( 2004): Reduced recruitment of motor association areas during bimanual coordination in concert pianists. Hum Brain Mapp 22: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, Buchel C, Holmes AP, Friston KJ ( 2000): A study of analysis parameters that influence the sensitivity of event‐related fMRI analyses. Neuroimage 11: 326–333. [DOI] [PubMed] [Google Scholar]
- Howard JD, Plailly J, Grueschow M, Haynes JD, Gottfried JA ( 2009): Odor quality coding and categorization in human posterior piriform cortex. Nat Neurosci 12: 932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg R, Wohlschlager AM, Gaser C, Liebau Y, Dauner R, Woller A, Zimmer C, Zihl J, Muhlau M ( 2008): Gray matter increase induced by practice correlates with task‐specific activation: A combined functional and morphometric magnetic resonance imaging study. J Neurosci 28: 4210–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancke L ( 2009): The plastic human brain. Restor Neurol Neurosci 27: 521–538. [DOI] [PubMed] [Google Scholar]
- Jancke L, Shah NJ, Peters M ( 2000): Cortical activations in primary and secondary motor areas for complex bimanual movements in professional pianists. Brain Res Cogn Brain Res 10: 177–183. [DOI] [PubMed] [Google Scholar]
- Jancke L, Gaab N, Wustenberg T, Scheich H, Heinze HJ ( 2001): Short‐term functional plasticity in the human auditory cortex: An fMRI study. Brain Res Cogn Brain Res 12: 479–485. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Wilson DA ( 2006): Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc Natl Acad Sci USA 103: 15206–15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber B, Veit R, Birbaumer N, Gruzelier J, Lotze M ( 2010): The brain of opera singers: Experience‐dependent changes in functional activation. Cereb Cortex 20:1144–1152. [DOI] [PubMed] [Google Scholar]
- Knafo S, Grossman Y, Barkai E, Benshalom G ( 2001): Olfactory learning is associated with increased spine density along apical dendrites of pyramidal neurons in the rat piriform cortex. Eur J Neurosci 13: 633–638. [DOI] [PubMed] [Google Scholar]
- Koeneke S, Lutz K, Wustenberg T, Jancke L ( 2004): Long‐term training affects cerebellar processing in skilled keyboard players. Neuroreport 15: 1279–1282. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, Thompson WL ( 2001): Neural foundations of imagery. Nat Rev Neurosci 2: 635–642. [DOI] [PubMed] [Google Scholar]
- Kreiman G, Koch C, Fried I ( 2000): Imagery neurons in the human brain. Nature 408: 357–361. [DOI] [PubMed] [Google Scholar]
- Krings T, Topper R, Foltys H, Erberich S, Sparing R, Willmes K, Thron A ( 2000): Cortical activation patterns during complex motor tasks in piano players and control subjects. A functional magnetic resonance imaging study. Neurosci Lett 278: 189–193. [DOI] [PubMed] [Google Scholar]
- Li W, Howard JD, Parrish TB, Gottfried JA ( 2008): Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science 319: 1842–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litaudon P, Datiche F, Cattarelli M ( 1997): Optical recording of the rat piriform cortex activity. Prog Neurobiol 52: 485–510. [DOI] [PubMed] [Google Scholar]
- Liu G, Sobering G, Duyn J, Moonen CT ( 1993): A functional MRI technique combining principles of echo‐shifting with a train of observations (PRESTO). Magn Reson Med 30: 764–768. [DOI] [PubMed] [Google Scholar]
- Lotze M, Halsband U ( 2006): Motor imagery. J Physiol Paris 99: 386–395. [DOI] [PubMed] [Google Scholar]
- Lotze M, Scheler G, Tan HR, Braun C, Birbaumer N ( 2003): The musician's brain: Functional imaging of amateurs and professionals during performance and imagery. Neuroimage 20: 1817–1829. [DOI] [PubMed] [Google Scholar]
- Lyman BJ, McDaniel MA ( 1990): Memory for odors and odor names: Modalities of elaborating and imagery. J Exp Psychol: Learn Mem Cogn 16: 656–664. [Google Scholar]
- Mai JK, Paxinos G, Voss T ( 2008): Atlas of the Human Brain. San Diego, CA: Academic Press. [Google Scholar]
- Margulis EH, Mlsna LM, Uppunda AK, Parrish TB, Wong PC ( 2009): Selective neurophysiologic responses to music in instrumentalists with different listening biographies. Hum Brain Mapp 30: 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton J, Solodkin A, Hlustik P, Small SL ( 2007): The mind of expert motor performance is cool and focused. Neuroimage 35: 804–813. [DOI] [PubMed] [Google Scholar]
- Mouly AM, Di Scala G ( 2006): Entorhinal cortex stimulation modulates amygdala and piriform cortex responses to olfactory bulb inputs in the rat. Neuroscience 137: 1131–1141. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Asada T, Aruga M, Hirakata M, Nishikawa M, Katoh A, Imabayashi E ( 2001): Functional anatomy of musical perception in musicians. Cereb Cortex 11: 754–760. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Plailly J, Bensafi M, Pachot‐Clouard M, Delon‐Martin C, Kareken DA, Rouby C, Segebarth C, Royet JP ( 2005): Involvement of right piriform cortex in olfactory familiarity judgments. Neuroimage 24: 1032–1041. [DOI] [PubMed] [Google Scholar]
- Ross JS, Tkach J, Ruggieri PM, Lieber M, Lapresto E ( 2003): The mind's eye: Functional MR imaging evaluation of golf motor imagery. AJNR Am J Neuroradiol 24: 1036–1044. [PMC free article] [PubMed] [Google Scholar]
- Royet JP, Plailly J ( 2004): Lateralization of olfactory processes. Chem Senses 29: 731–745. [DOI] [PubMed] [Google Scholar]
- Royet JP, Plailly J, Delon‐Martin C, Kareken DA, Segebarth C ( 2003): fMRI of emotional responses to odors: Influence of hedonic valence and judgment, handedness, and gender. Neuroimage 20: 713–728. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyas B, Larsson M, Roland P ( 2000): Olfactory functions are mediated by parallel and hierarchical processing. Neuron 26: 735–745. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Schacter DL ( 2004): A sensory signature that distinguishes true from false memories. Nat Neurosci 7: 664–672. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JD ( 1998): Sniffing and smelling: Separate subsystems in the human olfactory cortex. Nature 392: 282–286. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Zhao Z, Desmond JE, Glover GH, Sullivan EV, Gabrieli JD ( 2000): Time course of odorant‐induced activation in the human primary olfactory cortex. J Neurophysiol 83: 537–551. [DOI] [PubMed] [Google Scholar]
- Svoboda E, Levine B ( 2009): The effects of rehearsal on the functional neuroanatomy of episodic autobiographical and semantic remembering: A functional magnetic resonance imaging study. J Neurosci 29: 3073–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelderen P, Ramsey NF, Liu G, Duyn JH, Frank JA, Weinberger DR, Moonen CT ( 1995): Three‐dimensional functional magnetic resonance imaging of human brain on a clinical 1.5‐T scanner. Proc Natl Acad Sci USA 92: 6906–6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigouroux M, Bertrand B, Farget V, Plailly J, Royet JP ( 2005): A stimulation method using odors suitable for PET and fMRI studies with recording of physiological and behavioral signals. J Neurosci Methods 142: 35–44. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC ( 1996): A unified statistical approach for determining signals of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV ( 1997): Emotion, olfaction, and the human amygdala: Amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA 94: 4119–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Jones‐Gotman M, Evans AC, Meyer E ( 1992): Functional localization and lateralization of human olfactory cortex. Nature 360: 339–340. [DOI] [PubMed] [Google Scholar]


