Abstract
It is widely accepted that abnormalities in the frontal area of the brain underpin the pathophysiology of obsessive‐compulsive disorder (OCD). Fundamental to this investigation is the delineation of frontal white matter tracts including dorsal and ventral frontal projections of interhemispheric connections. While previous investigations of OCD have examined the dorsal and ventral frontal regions, the corresponding callosal connections have not been investigated, despite their importance. We recruited twenty patients with OCD (15 drug‐naïve and 5 currently unmedicated) and demographically similar healthy controls, and conducted fiber tractography and post hoc quantitative analysis using diffusion tensor imaging. We extracted fractional anisotropy (FA) of the fronto‐callosal fibers along the entire length of the tract. Function‐specific [by the Brodmann area region‐of‐interest (ROI) approach] and region‐specific (by the length‐parameterization approach) tracts were defined. In addition, we devised a new index of dorsal‐ventral imbalance (DVII) of fiber integrity. Significant FA decreases were observed in orbitofrontal and dorsolateral prefrontal projections of the corpus callosum (P < 0.05, false discovery rate‐corrected) with higher function/region sensitivity than voxel‐based or ROI‐based approaches. Importantly, OCD patients also exhibited significantly higher ventral‐greater‐than‐dorsal asymmetry of FA values than normal controls (P < 0.05, FDR‐corrected). This study is the first to investigate fiber integrity in the dorsal/ventral frontal parts of the callosal tractography in unmedicated OCD patients. Using a more quantitative method in terms of functional and regional specificity than previous studies, we report abnormalities in interhemispheric connectivity of both dorsal and ventral networks in the pathophysiology of OCD. Hum Brain Mapp 33:2441–2452, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: obsessive‐compulsive disorder, corpus callosum, diffusion tractography, orbitofrontal, dorsolateral prefrontal, fiber integrity
INTRODUCTION
Obsessive‐compulsive disorder (OCD) is a chronic anxiety disorder marked by intense, recurrent, and intrusive thoughts (obsession) in conjunction with ritualistic behaviors (compulsion) [American Psychiatric Association, 1994]. From a neuroanatomical perspective, much evidence supports the hypothesis that abnormalities in frontostriatal regions, including dysfunctional prefrontal cortex‐basal ganglia circuits, mediate the etiology of OCD [Rosenberg et al., 1997a; Saxena and Rauch, 2000]. In recent studies, dorsal and ventral networks of frontostriatal cognitive/affective networks have been of particular interest in OCD [Gu et al., 2008; Han et al., to appear; Harrison et al., 2009; Kwon et al., 2009; Saxena and Rauch, 2000]. Interestingly, both functional MRI studies [Fitzgerald et al., 2010; Gu et al., 2008; Han et al., 2011; Harrison et al., 2009] and anatomical connectivity studies [Cannistraro et al., 2007; Ha et al., 2009; Nakamae et al., 2008; Saito et al., 2008; Yoo et al., 2007] of OCD have suggested that a functional distinction of the dorsal and ventral frontal areas is important for differentiating OCD symptoms from normal controls. Interestingly, recent studies have pointed to a dorsal/ventral frontal network imbalance (i.e., hyperactivation of ventral frontostriatal system and reduced inhibitory activation of dorsal frontostriatal system) in the etiology of OCD [Mataix‐Cols and van den Heuvel, 2006; Saxena and Rauch, 2000]. More specifically, Harrison et al. [ 2009] revealed that the patients with OCD exhibit increased functional connectivity of the ventral corticostriatal axis as well as reduced functional connectivity of dorsal areas of the striatum and the frontal area.
The ventral frontostriatal pathway is known as the direct pathway, as it is directly connected to basal ganglia [Stein, 2002]. The ventral frontostriatal region consists of the orbitofrontal cortex (OFC), rostral anterior cingulate cortex, ventral striatum, and limbic structures. These regions have figured as one of the major neural circuits involved in OCD pathophysiology [Cheyette and Cummings, 1995]. More recently, some investigators have focused on the dorsal frontostriatal regions in their OCD research. The dorsal frontostriatal regions primarily consist of the dorsolateral prefrontal cortex (DLPFC) and dorsal striatum [Mataix‐Cols and van den Heuvel, 2006]. Patients with OCD are generally not able to disengage from previous stimuli, which in turn interfere with the processing of new stimuli (cognitive inflexibility) [Stein, 2002]. The dorsal frontal regions are thought to be hypoactive to cognitive loading during executive functional process in OCD patients compared with normal controls [Kwon et al., 2009; Shin et al., 2006]. Many researchers have interpreted this as meaning that the pathological hyperactivity of the OFC results from reduced inhibitory activation of the DLPFC known as disinhibition [Baxter et al., 1996; Gu et al., 2008; Han et al., 2011; Kwon et al., 2009].
Abbreviations
- BA
Brodmann area
- CC
corpus callosum
- DLPFC
dorsolateral prefrontal cortex
- DTI
diffusion tensor imaging
- DVII
dorsal‐ventral imbalance index
- EEG
electroencephalogram
- FA
fractional anisotropy
- FDR
false discovery rate
- LI
lateralization index
- OCD
obsessive‐compulsive disorder
- OFC
orbitofrontal cortex
- ROI
region‐of‐interest
- TBSS
tract‐based spatial statistics
Whereas research to date has focused on abnormalities in dorsal/ventral frontostriatal regions in patients with OCD [Baxter et al., 1987; Cheyette and Cummings, 1995; Gu et al., 2008; Han et al., to appear; Shin et al., 2006], the interhemispheric integration of the dorsal and ventral frontal regions has not been addressed sufficiently. The corpus callosum (CC) is the largest white matter tract in the human brain, and is the predominant axonal fiber structure (more than 200 million fibers), interconnecting the cerebral hemispheres [Aboitiz et al., 1992]. More importantly, the CC plays a primary role in the interhemispheric integration of many brain functions, from basic sensorimotor functions to high‐level cognitive integration [Clarke and Zaidel, 1994; de Lacoste et al., 1985; Witelson, 1989]. Among the substructures of the CC, the rostrum and genu are important cross‐sectional elements that enable prefrontal interhemispheric integration by interconnecting the bilateral prefrontal cortices that are associated with affective and cognitive brain functions [Denenberg et al., 1991; Witelson, 1989]. Indeed, many studies of other psychiatric diseases have focused on the CC. As affective and cognitive impairments are known to be associated also with OCD symptoms, abnormalities in frontal interhemispheric integration may play a pivotal role in OCD pathophysiology. Despite its importance, little research has been carried out to investigate white matter abnormalities [e.g., fractional anisotropy (FA) decreases] of the frontal CC, which could lead to functional abnormalities of the frontal networks in patients with OCD. A study of pediatric patients with OCD reported larger‐than‐normal volumes of genu, body, and splenium in those with OCD [Rosenberg et al., 1997b]. Another pediatric OCD study reported genu CC signal intensity decreases in treatment‐naïve pediatric OCD, which might indirectly indicate increased genu myelination in pediatric OCD patients [MacMaster et al., 1999]. Saito et al. [ 2008] reported diminished callosal fiber integrity in patients with OCD compared with normal control (NC) subjects, in the rostrum of the CC. These rostral callosal fibers mostly travel through the OFC and in turn permit interhemispheric integration of the ventral frontal regions. However, these previous studies were limited to the investigation of structural abnormalities in the midsagittal CC cross‐section, thus not fully addressing the investigation of callosal fibers projecting into dorsal and ventral frontal areas.
Diffusion tensor imaging (DTI) and fiber tractography have been widely used to investigate white matter structures in the brain in vivo. Although the tractographic visualization technique has developed to a good degree of sophistication, the quantification of tractographic output (such as extraction of FA values on the fiber tractography) has not been fully explored. However, recent approaches to quantitative DTI methods, such as tract parameterization [Gong et al., 2005; Lin et al., 2006; Oh et al., 2007, 2009] and tract‐based spatial statistics (TBSS) [Smith et al., 2006] are representative classes of these quantitative methods. Using these methods, quantitative diffusion measures, including FA of white matter tracts, can be traced and used for differentiating psychiatric patients from NCs. Recently, we developed a function‐ and location (region)‐specific analysis framework using a Brodmann area (BA) atlas‐based tractography and tract parameterization [Oh et al., 2009]. In this study, we applied this quantitative technique to investigate frontal interhemispheric fibers of OCD patients that are specific for dorsal and ventral frontostriatal networks.
In this study, we investigated the integrity of the dorsal and ventral white matter projections from the CC that connect the DLPFC and OFC, respectively. Based on previous studies [Gu et al., 2008; Han et al., to appear; Kwon et al., 2009; Mataix‐Cols and van den Heuvel, 2006; Rosenberg et al., 1997a; Saxena and Rauch, 2000], we hypothesized that (1) the dorsal and ventral projections emanating from the CC will exhibit decreased fiber integrity in OCD patients compared with NCs, and more importantly, that (2) an imbalance of these two networks, i.e., ventral‐greater‐than‐dorsal connectivity might exist [Mataix‐Cols and van den Heuvel, 2006; Saxena and Rauch, 2000].
METHODS
Participants
The study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital, and all participants provided written informed consent. Because research has demonstrated marked gender‐associated differences in CC shape [DeLacoste‐Utamsing and Holloway, 1982] and CC fiber integrity [Oh et al., 2007; Shin et al., 2005], we decided to recruit NCs who were sex‐matched with the OCD patients. Twenty right‐handed OCD patients (13 men, 7 women; mean age 25.95 ± 6.79 years) and 19 age, sex, handedness, education, and IQ‐matched healthy NCs (13 men, 6 women; mean age 24.68 ± 3.77 years) were examined. We recruited the OCD patients from the OCD clinic at Seoul National University Hospital (South Korea), and the NCs via an internet advertisement. All patients met the criteria of OCD based on the Structured Clinical Interview for DSM‐IV [First et al., 1996]. The Structured Clinical Interview for DSM‐IV Non‐patient Version was used to assess Axis I psychiatric disorders for NCs. Exclusion criteria included a lifetime history of psychosis, bipolar disorder, major depressive disorder, substance dependence, Tourette's disorder, attention‐deficit hyperactivity disorder (ADHD), learning disabilities, eating disorder, significant head injury, seizure disorder, or mental retardation. Comorbidity was found for two subjects with OC personality disorder, and one with schizotypal personality disorder. To estimate intelligence quotient (IQ), the Korean version of the Wechsler Adult Intelligence Scale (K‐WAIS) [Yum et al., 1992] was administered to all subjects, and it revealed no significant difference between groups. Fifteen of the 20 patients were drug‐naïve (DNO), and the remaining five had been unmedicated for at least 4 weeks (unmedicated OCD patients: UMO). In most cases, the periods of past medication of UMO patients were very short, being less than or equal to 2 months, but one subject had received past medication for 8 months.
Image Acquisition
Single‐shot diffusion‐weighted Echo Planar Imaging (EPI) was used to acquire DTI volumes on a 1.5T magnet whole‐body imaging system (Siemens Avanto, Germany). Diffusion‐weighted images were acquired with diffusion gradients (b‐factor 1000 s/mm2) along twelve non‐collinear directions. Ten images were acquired with no diffusion gradient (B0 images) to increase the signal‐to‐noise ratio (SNR). Two‐millimeter axial slices were acquired with no gap, aligned to the AC‐PC line and interhemispheric fissure. Other parameters were as follows: TR/TE 9200/83 ms, field of view (FOV) 224 × 256 mm2, acquisition matrix 112 × 128, and axial slice thickness 2 mm (2 × 2 × 2 mm3 voxel dimensions). To compensate for a relatively low spatial resolution, cubic spline interpolation was conducted, producing a 1 mm3 voxel dimension.
To define anatomically precise Brodmann region‐of‐interests (ROIs), 176‐208 contiguous (variable per individual brain sizes) axial images were acquired using a 3D Magnetization‐Prepared Rapid Gradient‐Echo (MP‐RAGE) MR sequence with the following parameters: TR/TE 1160/4.76 ms, flip angle 15°, FOV 230 × 230 mm2, 3 NEX, matrix 256 × 256, and axial slice thickness 0.9 mm (0.45 × 0.45 × 0.9 mm3 voxel dimensions). A board‐certified radiologist (C.H.C.) reviewed all scans and found no gross abnormalities in any subjects.
ROI Definition and Preprocessing
To extract dorsal/ventral projections of the frontal CC, we adopted a whole‐brain seeding tractography technique and a multiple‐ROI approach [Conturo et al., 1999; Mori et al., 1999; Oh et al., 2009]. To extract the midsagittal CC automatically, we used a color‐coded DTI map‐based method similar to that of Kubicki et al. [ 2002, 2003]. In fact, the fibers in the rostrum, genu, and rostral body of the CC do not exclusively connect bilaterally in either OFCs or DLPFCs. To better extract the CC projections related to these networks, we adopted the BA atlas. From the resulting bulky callosal fibers of whole‐brain tractography, fibers interconnecting bilateral DLPFCs or bilateral OFCs were extracted separately. To this end, the second set of ROIs within the frontal lobe was selected using a BA atlas available in MRIcro 1.4 (http://www.sph.sc.edu/comd/rorden/mricro.html), BA 11(12)/47 for the OFC and BA 9/46 for the DLPFC.
For the convenience of interpretation of functionally similar BAs, and taking into account the marked intersubject anatomical variability [Amunts et al., 2007; Rajkowska and Goldman‐Rakic, 1995], we merged areas 9 and 46 into one ROI. Similarly, we merged areas 11 and 47, which correspond to the OFC, and more lateral parts (i.e., the ventrolateral prefrontal cortex), respectively. As the atlas is defined in common template space, it needs to be registered to each individual subject's space. As in our previous study [Oh et al., 2009], we first performed a nonlinear registration of the BA template to the individual anatomical T1 MP‐RAGE image, and then performed an affine registration to DTI. This process was done using parts of Statistical Parametric Mapping 2 (Welcome Department of Imaging Neuroscience, London, UK). To compensate for mild EPI‐induced distortions in DTI, we used in‐house software based on cortical surface‐based nonlinear registration of the affine‐registered T1 image to the DTI using a technique similar to that of ANIMAL software [Collins and Evans, 1997], which matches a source volume to a target volume by estimating local deformation fields defined on a set of equally spaced nodes. To extract cortical surfaces of T1 and B0 images, we used an automated skull stripping process called Brain Extraction Tool in MRIcro 1.4 software. Using deformation fields between two images, a final BA label map could be obtained in each individual DTI space. To keep the ROIs intact during coregistration, nearest‐neighbor interpolation was employed in all spatial transformation steps [Thottakara et al., 2006]. All together, we generated CC ROIs and cortical Brodmann ROIs, which were used for the fiber tract extraction (see Fig. 1A, B).
Figure 1.
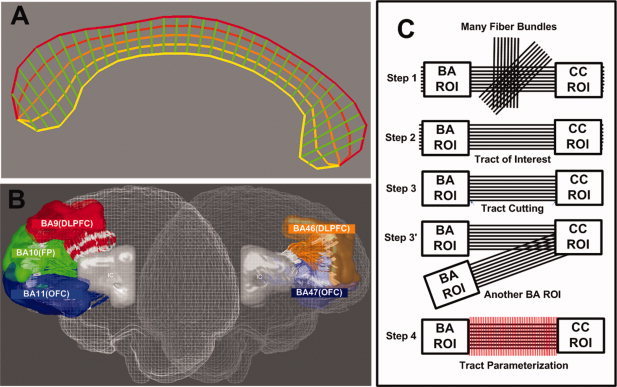
Callosal fiber extraction, parcellation, and parameterization. The parameterized mCC template, i.e., the mCC‐specific coordinate system constructed (A); Frontal Brodmann ROIs (B); Processing pipeline of the tract parcellation and parameterization (see Oh et al. [ 2009] for more details) (C); B and C have been adapted from Oh et al. [ 2009]. Abbreviations: mCC, midsagittal corpus callosum; BA, Brodmann area; ROI, region of interest); DLPFC, dorsolateral prefrontal cortex; OFC, orbitofrontal cortex.
Fiber Tractography
First, raw diffusion‐weighted images were corrected for motion and eddy current‐induced distortions using affine registration to the mean individual B0 images. Diffusion tensor estimation, fiber tractography, and post processing were conducted using in‐house fiber‐tracking software [Oh et al., 2007, 2009] based on Interactive Data Language (IDL‐ITT Visual Information Solutions, Boulder, CO) version 8. For tensor estimation, we used a general log‐ratio least square algorithm. Subsequently, we performed matrix diagonalization, resulting in three eigenvalues and corresponding eigenvectors. As mentioned above, we used a whole‐brain seeding strategy. Our experience [Oh et al., 2007, 2009] and that of others suggest that this is a robust way of tracing fiber paths in DTI in the presence of noise and fiber crossings [Conturo et al., 1999; Mori et al., 1999; Oh et al., 2009]. We used an FA threshold of 0.15 for both the seeding and stopping criterion for the fiber tracing, and also stopped when curvature exceeded 50 degrees per millimeter. After all the brain white matter tracts were constructed, we applied a multiple‐ROI selection method to segment parts of the CC that project to each individual BA.
Postprocessing
To minimize parameterization errors due to variable lengths and shapes of white matter tracts adjacent to the gray matter, all surviving fiber tracts were cut using the boundaries of the two anatomical landmarks (i.e., Brodmann ROIs in both hemispheres). Then, a small percentage of unwanted tracts produced by DTI noises, were regularized using the sensitivity tool developed by Estepar et al. [ 2006] (part of Slicer package—http://www.slicer.org) and applied to a schizophrenia study [Fitzsimmons et al., 2009]. Resulting tracts were subjected to tract parameterization along the direction of principal diffusion (tract length‐parameterization—see Fig. 1C) [Lin et al., 2006; Oh et al., 2009]. Specifically, each tract in each subject was divided into percentiles (i.e., 50 segments of equal length in each hemisphere, originating from the midsagittal CC), thus providing further correspondence between specific locations along the tracts and subjects.
In addition, a probabilistic CC connection map [Park et al., 2008] was created for each subject and used later for comparing probabilistic connection patterns of OCD patients with those of NCs (See Supporting Information for details).
Statistical Analysis
Between‐group comparisons of continuous demographic variables (age, IQ, and education) were performed using independent t‐tests, and χ2 tests were used to assess differences in joint classifications of discrete demographic variables (i.e., sex and handedness). In addition, we tested all patients with OCD using the Yale‐Brown Obsessive‐Compulsive Scale (Y‐BOCS) [Goodman et al., 1989a, b] and Beck Depression/Anxiety Inventory (BDI/BAI) [Beck et al., 1961, 1988] to assess OCD symptom severity and anxiety/depression levels, which were subjected to correlation analysis with FA values.
Between‐group comparison of FA values was straightforward, as we already had well‐established correspondences between specific locations along the tracts and subjects by tract parameterization. We conducted a stepwise statistical analysis. First, we performed independent t‐tests to assess group effects for FA values for each (dorsal/ventral) projection. Then, we conducted post hoc independent t‐tests along the parameterized tracts for each subject to determine region‐specific group differences. For the multiple‐comparison correction purpose, we controlled the false discovery rate (FDR) to be less than 5%. All statistical analyses were conducted using statistical procedures in IDL.
To better detect any aforementioned imbalance between dorsal and ventral networks, we performed a post hoc analysis (after all FA comparisons) of dorsal‐ventral imbalances between both DLPFC and OFC projections from the CC. In many previous studies, the lateralization index (LI) has been defined for assessing hemispheric asymmetry of electroencephalogram (EEG) [de Toffol and Autret, 1991] or functional MRI [Golby et al., 2002] activations, as well as FA values [Parker et al., 2005]. Similarly, we devised a new index of dorsal‐ventral imbalance (DVII) in Eq. (1) below:
| (1) |
where FAL and FAR stand for FA values in homologous regions in left and right hemispheres, and FAD and FAV stand for FA values in DLPFC and OFC projections from the CC, respectively. The DVII values were also observed along entire tracts as well as regionally (at each location of the parameterized tract).
We considered the possibility that past medication treatment might already have affected the patients' biology as well as comorbidity. To investigate this issue, we conducted an additional analysis excluding one UMO patient with a relatively long (i.e., 8 months) period of past medication. In addition, the OCD patients had a fair amount of depression, which may influence the neurobiology of OCD [Saxena et al., 2001]. To remove the potential influence of depression level on the statistical results, we regressed out the depression level of all patients from FA values in the further statistical analysis.
RESULTS
Demographic and Clinical Data
Although significant group differences were found in BDI and BAI scores, no significant group difference in age, sex, handedness, IQ, or education were observed. (Table I)
Table I.
Demographic characteristics of the patients with obsessive‐compulsive disorder and the normal controls
| Controls | OCD | Analysis | ||
|---|---|---|---|---|
| (n = 19) | (n = 20) | χ2, T, F | P | |
| Sex (male/female) | 13/6 | 13/7 | 1.43 | 0.49 |
| Handedness (right/left) | 19/0 | 18/2 | 4.62 | 0.10 |
| Age (years) | 24.68 ± 3.77 | 25.95 ± 6.79 | −0.72 | 0.48 |
| IQa | 111.95 ± 10.91 | 108.65 ± 12.93 | 0.86 | 0.40 |
| Illness duration (years) | 7.79 ± 5.17 | |||
| Age of onset | 18.60 ± 7.23 | |||
| BDI | 3.58 ± 5.87 | 16.55 ± 11.37 | 8.22 | 0.01b |
| BAI | 4.53 ± 4.74 | 17.15 ± 15.45 | 4.69 | 0.01b |
| Y‐BOCS | ||||
| Obsession | 11.20 ± 4.66 | 2.26 | 0.03 | |
| Compulsion | 9.30 ± 5.53 | 0.96 | 0.35 | |
| Total score | 20.50 ± 6.66 | 2.25 | 0.03 | |
Estimated by Korean‐Wechsler Adult Intelligence Scale‐Revised (K‐WAIS‐R).
Values are presented as mean ± SD.
Analysis of variance (ANOVA).
IQ, Intelligence Quotient; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; Y‐BOCS, Yale‐Brown Obsessive Compulsive Scale.
Statistical Analysis
The first‐level t‐tests revealed significant FA decreases of both DLPFC projection (t = 3.84, P < 0.001, mean ± SD FA: 0.37 ± 0.03 for NC; 0.34 ± 0.03 for OCD) and OFC projection (t = 2.7, P = 0.01, mean ± SD FA: 0.40 ± 0.02 for NC; 0.38 ± 0.03 for OCD) from the CC of the OCD patients as compared with NCs (see Fig. 2A for more details). In addition, correlation analysis of FA values with aforementioned clinical scores (Y‐BOCS, BAI, and BDI) revealed that (1) the fiber integrity of the CC projection to DLPFC exhibited a significant negative correlation with the Y‐BOCS compulsion subtotal score (r = −0.63, P = 0.003), and (2) the fiber integrity of the CC projection to OFC exhibited a significant positive correlation with BAI (r = 0.48, P = 0.03); other scales did not exhibit any significant correlation with the fiber integrity of either CC projection (see Fig. 2B,C).
Figure 2.
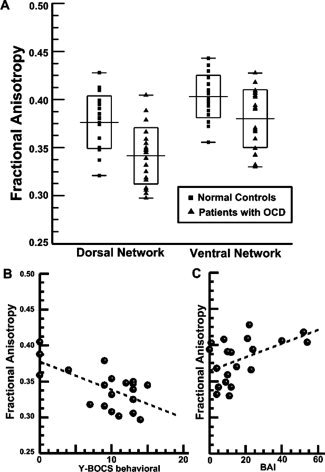
Scatter plot of FA values used in the first level independent t‐tests and dot plots of FA values versus symptom scales used in the correlation analysis for OCD patients. Significant group differences in fiber integrity (i.e., FA values) are shown in CC projections for both DLPFC and OFC (A). The boxes present the range, mean, and SD of FA values of each ROI in each group. We also visualized a significant negative correlation of FA values of the DLPFC projection of the CC with Y‐BOCS compulsive subtotal scores (r = −0.63, P = 0.003) and a significant positive correlation of FA values of the OFC projection of the CC with BAI scores (r = 0.48; P = 0.03) in B and C, respectively. Vertical axes (FA values) have the same range across B and C. Abbreviations: OCD, obsessive‐compulsive disorder; CC, corpus callosum; FA, fractional anisotropy; SD, standard deviation; Y‐BOCS, Yale‐Brown Obsessive‐Compulsive Scale; BAI, Beck Anxiety Inventory; DLPFC, dorsolateral prefrontal cortex; OFC, orbitofrontal cortex.
The results became clearer after removing the aforementioned confounds of comorbidity and past medication, and depression level. We found a significant negative correlation of FA values of DLPFC projection of the CC with Y‐BOCS compulsive subtotal scores (r = −0.67, P = 0.004) as well as trend level and significant positive correlations of FA values of OFC projections of the CC with BAI scores (r = 0.48, P = 0.058) and with Y‐BOCS obsessive subtotal scores (r = 0.54, P = 0.03), respectively.
Regarding cortical mapping of DLPFC and OFC projections from the CC, the results exhibited no significant differences on the connection probability map; thus we presented normal control data only (see Supporting Information).
More importantly, post hoc independent t‐tests revealed regionally specific group differences for FA values, where most FA decreases were observed near midsagittal CC, in both projections (See Fig. 3A,B). In addition, compared with NCs, we found significantly higher DVII in OCD patients in the midsagittal CC and lower DVII in OCD patients in small portions of parasagittal CC. In other words, the relative integrity of DLPFC projection (vs. OFC projection) from the CC was, for the most part, significantly lower in OCD patients than in NCs in the midsagittal portion of fronto‐callosal fibers [See Eq. (1) and Fig. 3].
Figure 3.
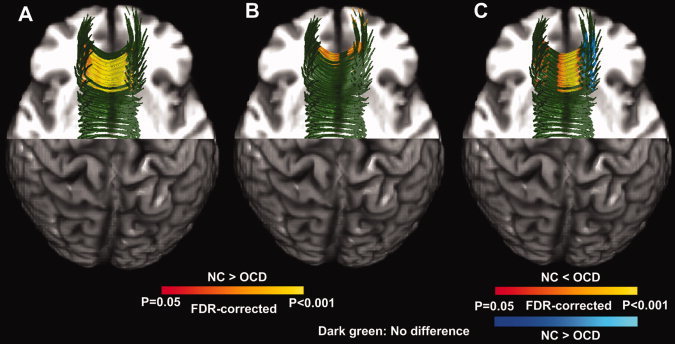
Statistical results of post hoc tract parameterization‐based FA and DVII analyses. We represent the significant FA decreases (P < 0.05, FDR‐corrected) of the OCD group (vs. NC group) in red to yellow for the callosal projections to the OFC (A) and DLPFC (B). In addition, we depict the significant DVII group differences in red to yellow (NC < OCD) and blue to light blue (NC > OCD). The structural background image is the same as for Figure 3. As this figure is not sufficient for understanding the location of dorsal and ventral CC tracts, readers may wish to refer to Supporting Information Figure S1 for more details. Abbreviations: FA, fractional anisotropy; DVII, dorsal‐ventral imbalance index; OCD, obsessive compulsive disorder; NC, normal control; FDR, false discovery rate; DLPFC, dorsolateral prefrontal cortex; OFC, orbitofrontal cortex.
DISCUSSION
A Quantitative Tractography Analysis in Drug‐Naïve or Unmedicated OCD
Recently, DTI has been introduced as a quantitative tool for investigating white matter tracts in OCD. For example, recent findings have revealed FA decreases in the anterior cingulum [Garibotto et al., 2010; Szeszko et al., 2005], rostrum/splenium of the CC [Garibotto et al., 2010; Saito et al., 2008], superior longitudinal fasciculus, and inferior fronto‐occipital fasciculus [Garibotto et al., 2010]. On the other hand, increased FA in the internal capsule has also been reported [Yoo et al., 2007].
To the best of our knowledge, this study is the first to investigate unmedicated (mostly drug‐naïve) patients with OCD using a tract‐oriented approach, and in particular, applying Brodmann ROI and tract parameterization approaches. On the basis of increased sensitivity and specificity provided by the tractographic analysis approach [Kubicki et al., 2008; Oh et al., 2009], particularly the tract parameterization approach [Oh et al., 2009], our results demonstrate global and local FA decreases in OCD versus NCs within callosal fibers traveling through either DLPFC or OFC. The disrupted fiber integrity (i.e., FA decreases) in the OFC projections concur with results of a previous DTI finding [Gong et al., 2005] as well as findings from functional imaging studies that reported abnormalities of activation in the OFC in a resting state [Shin et al., 2006; Swedo et al., 1989]. In addition, our results may implicate some involvement of inhibited DLPFC function in OCD patients [Shin et al., 2006].
We enhanced the regional sensitivity to FA decreases in the DLPFC circuitry with the assistance of BA‐specific ROI selection, as compared with a previous study that conducted an FA analysis of the midsagittal CC using Witelson's method [Saito et al., 2008]. Using a Brodmann ROI approach, we were able to extract fiber tracts that carry information to‐and‐from functionally homogenous regions of gray matter (i.e., BA) such that the associated fiber integrity inevitably correlates with particular brain functions of the specific BA [Oh et al., 2009]. By this framework, we were able to establish the relationship between anatomy and function and the associated abnormalities in OCD. In addition, we incorporated tract parameterization in our analysis, which allowed us to perform group comparisons at any given position along the extracted fiber bundles, thus further increasing spatial specificity.
Clinical Implications
The DLPFC (BA 9/46) projection of callosal fibers exhibited FA decreases. This bundle plays important roles in working memory and executive function, reported to be abnormal in OCD [Gu et al., 2008; Shin et al., 2006]. Furthermore, the OFC (BA 11/47) projection of callosal fibers also exhibited decreased FA. Reduced integrity in this fiber bundle is important, as it underlies executive functions such as task switching [Gu et al., 2008], which are reported to be abnormal in OCD.
The interhemispheric fibers of the CC develop in a rostral‐caudal pattern until early adulthood with myelination of white matter fibers [Thompson et al., 2000]. A recent family‐based genetic study reported an association between OCD and an oligodendrocyte lineage transcription factor 2 (OLIG2) gene, which is an essential regulator in the development of cells producing myelin [Stewart et al., 2007]. Abnormal processes in these developments might affect white matter integrity of the CC in patients with OCD.
In addition, results of the DVII analysis provide us with evidence of an abnormal imbalance between dorsal and ventral networks in OCD, which supports the hypothesis of dorsal‐ventral imbalance in the etiology of OCD [Harrison et al., 2009; Mataix‐Cols and van den Heuvel, 2006; Saxena and Rauch, 2000]. In addition, our analysis of the correlation of DVII with symptom scales revealed opposite‐signed results between two networks, i.e., a negative correlation between FA in the DLPFC projections from the CC and Y‐BOCS, and positive correlation of between FA in the OFC projection and BAI.
We can interpret the FA analysis results in two parts. First, frontal callosal fiber integrity in general is lower in the OCD patients than the normal controls. In other words, both dorsal and ventral frontal callosal fiber integrity are decreased for OCD patients. Second, OCD patients exhibited the less disintegrated fibers connecting ventral frontal areas and the more interrupted fiber integrity of dorsal frontal areas that are associated with increased anxiety in OCD patients. Regarding negative correlation of dorsal FA and YBOCS compulsive subtotal score, we can interpret that the higher the dorsal FA values (i.e., more inhibition of OCD symptom), the lower the compulsion symptom level. However, the positive correlation between ventral FA and BAI as well as reduced ventral frontal FA indicate the anxiety symptom seems to be associated with both the dorsal and ventral FA at the same time, not the ventral FA in itself. In accordance with our hypothesis, when the ventral frontal CC fibers are more intact than the dorsal frontal CC fibers, the “disinhibition” induced by the imbalanced dorsal/ventral network can originate the anxiety symptom of patients.
Comparison with Previous Studies
Several reports have been made regarding disrupted fiber integrity in OCD using more generic approaches [Cannistraro et al., 2007; Garibotto et al., 2010; Nakamae et al., 2008; Saito et al., 2008; Szeszko et al., 2005]. Saito et al. [ 2008], for example, investigated callosal fiber integrity using the Witelson's geometric division approach and reported FA decreases within the rostrum. Another class of studies used voxel‐based analysis to investigate the integrity of the cingulate cortex [Szeszko et al., 2005] and whole brain [Yoo et al., 2007] using voxel‐based morphometry (VBM) approaches. These various findings of FA decreases and increases in several white matter tracts might be due to developmental differences across studies and populations as well as methodological differences in the diffusion property measurements. Moreover, all the previous studies using voxel‐based or ROI‐based approaches observed diffusion properties in indirectly defined white matter tracts.
In contrast, we present here a more tract‐oriented investigation, i.e., fiber tractography in conjunction with post hoc tract parameterization [Gong et al., 2005; Lin et al., 2006; Oh et al., 2007, 2009]. Although fiber tractography has been utilized in many psychiatric neuroimaging studies, to the best of our knowledge, only a few previous studies have illuminated OCD by means of fiber tractography. As mentioned above, the tractographic approach has great advantages in terms of tract specificity versus conventional VBM or ROI approaches [Kubicki et al., 2008]. Regarding functional specificity, our investigation sought to separate dorsal and ventral projections of callosal fibers based on approximate functional segmentation (i.e., Brodmann ROIs), [Oh et al., 2009; Thottakara et al., 2006] rather than anatomical cortical segmentation [Park et al., 2008].
Recently, diffusion properties (i.e., FA and water diffusivity) of the CC were investigated using the classical Witelson's method [Witelson et al., 1989], revealing significant FA decreases in the rostrum, which mostly connects bilateral OFCs, in OCD patients [Saito et al., 2008]. Whereas Witelson's method separates the CC into seven divisions using geometric vertical lines with respect to the anterior‐most and posterior‐most points, novel approaches have introduced finer subdivisions [Jancke et al., 1997; Oh et al., 2007]. According to reports by several researchers [Abe et al., 2004; Jancke et al., 1997; Park et al., 2008; Saito et al., 2008], the callosal topography has complicated patterns with high intersubject variability [Park et al., 2008] and considerable overlap [Park et al., 2008; Saito et al., 2008]. Since our method provides a more CC shape‐specific division with finer scale [Oh et al., 2007] than conventional methods, we believe our approach is more useful for taking advantage of diffusion tractography‐based DTI analysis.
Furthermore, previous studies have not utilized DTI's capacity for reconstructing and parcellating white matter tracts, particularly callosal tractography and its parcellation [Huang et al., 2005; Park et al., 2008], nor have they investigated subsequent useful information (e.g., hemispheric asymmetry). With the novel DTI technique, use of fiber tractography is an advantageous approach. This approach has been shown to have higher sensitivity and specificity [Kubicki et al., 2008] compared with conventional VBM or ROI approaches. In particular, fiber tractography has demonstrated more promising results when combined with gray matter parcellation methods, e.g., lobar parcellation [Behrens et al., 2003; Kubicki et al., 2008], the BA ROI approach [Oh et al., 2009; Thottakara et al., 2006], and sulcal‐gyral parcellation of individual brains [Park et al., 2008].
Interestingly, we were able to separate the DLPFC‐specific portion of the genu and rostral body (and, partially, the rostrum) using fiber tractography, revealing significant FA decreases in this callosal projection, which had not been found using previous methods [Saito et al., 2008].
A common approach is to average the FA values for the entire fiber bundle. However, this method is not sensitive to local subtle changes in fiber integrity along the tract [Oh et al., 2009]. Recently, tract parameterization methods have been introduced to measure diffusion properties and to compare those properties between populations. Most of these studies have focused on length‐based division (i.e., parameterization) of fiber tracts [Lin et al., 2006; Oh et al., 2007, 2009], which are based on a transformation that converts rectangular Cartesian coordinate systems to a normalized tract‐oriented coordinate system. These methods are commonly tract‐dedicated, dividing fibers into several segments and thereby providing better regional specificity than is achieved by the conventional and cumbersome ROI approach. In particular, our previous studies demonstrated the usefulness of tract parameterization in conjunction with Brodmann ROI parcellation in terms of greater regional specificity [Oh et al., 2007, 2009] by tract parameterization and greater functional specificity [Oh et al., 2009; Thottakara et al., 2006] by the Brodmann ROI approach.
Limitations
Our study has some limitations. As noted in our previous study [Oh et al., 2009], we should also consider individual variability of BAs [Amunts et al., 2007; Rajkowska and Goldman‐Rakic, 1995], which cannot be captured by an atlas‐based approach. In this context, one study has conducted sulcal/gyral borderline‐based parcellation of callosal fibers [Park et al., 2008]. As each approach has its own advantages and drawbacks (more functional specificity for Brodmann ROIs vs. better anatomical precision for gyral ROIs), we are biased toward neither of them. Nonetheless, Brodmann ROIs are easy to define by inverse normalization, i.e., simple spatial transformation of templates to individual brains, very similar to VBM analysis, a more popular neuroimaging method. Another limitation of this study is that the most of our OCD patients had late age of onset. Although there have been reports regarding clinical and neurobiological differences between early vs. late onset OCD patient groups [Lomax et al., 2009; Pauls et al., 1995], our study could not measure this early vs. late onset difference. Interestingly, a recent PET study reported more pronounced dysfunctions of the serotonin system in the ventral network, such as limbic, paralimbic, nuclesus accumbens, and striatal regions, in late onset OCD compared with early onset OCD and normal controls (Hesse et al., to appear). However, no significant association between clinical variables and serotonin system dysfunction was found in that study. Further research is needed to clarify the relationship between serotonin system dysfunction and the integrity of ventral frontal fibers.
CONCLUSION
In summary, we conducted (1) dorsal/ventral frontal region‐specific investigation of frontal callosal fiber integrity using a precise definition of white matter tracts on the basis of the Brodmann ROI approach, and (2) region (location)‐specific investigation of frontal callosal fiber integrity was performed using a tract parameterization approach. On the basis of our findings, we suggest that our quantitative DTI analysis methods, with the assistance of improved functional specificity (by Brodmann ROI approach) and regional specificity (by tract parameterization approach), have successfully validated a widely held view that abnormalities in the dorsal and ventral frontal regions are involved in OCD pathophysiology. In addition, we found imbalanced fiber integrity between the callosal fibers within the two (i.e., dorsal and ventral) regions, supporting a recent hypothesis on the pathophysiology of OCD in terms of a “dorsal‐ventral network imbalance” [Mataix‐Cols and van den Heuvel, 2006; Saxena et al., 2000], namely, hyperactivation of the ventral frontostriatal system and inhibition of the dorsal frontostriatal system, from the white matter development perspective.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Supporting Information
REFERENCES
- Abe O, Masutani Y, Aoki S, Yamasue H, Yamada H, Kasai K, Mori H, Hayashi N, Masumoto T, Ohtomo K ( 2004): Topography of the human corpus callosum using diffusion tensor tractography. J Comput Assist Tomogr 28: 533–539. [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E ( 1992): Fiber composition of the human corpus callosum. Brain Res 598: 143–153. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association ( 1994): Diagnostic and statistical manual of mental disorders 4th ed (DSM‐IV) Washington, DC: American Psychiatric Association. [Google Scholar]
- Amunts K, Schleicher A, Zilles K ( 2007): Cytoarchitecture of the cerebral cortex‐more than localization. Neuroimage 37: 1061–1065. [DOI] [PubMed] [Google Scholar]
- Baxter LR Jr, Phelps ME, Mazziotta JC, Guze BH, Schwartz JM, Selin CE ( 1987): Local cerebral glucose metabolic rates in obsessive‐compulsive disorder: A comparison with rates in unipolar depression and in normal controls. Arch Gen Psychiatry 44: 211–218. [DOI] [PubMed] [Google Scholar]
- Baxter LR Jr, Saxena S, Brody AL, Ackermann RF, Colgan M, Schwartz JM, Allen‐Martinez Z, Fuster JM, Phelps ME ( 1996): Brain mediation of obsessive‐compulsive disorder symptoms: Evidence from functional brain imaging studies in the human and nonhuman primate. Semin Clin Neuropsychiatry 1: 32–47. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward C, Mendelson M, Mock J, Erbaugh J ( 1961): An inventory for measuring depression. Arch Gen Psychiatry 4: 561–571. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA ( 1988): An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol 56: 893–897. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen‐Berg H, Woolrich MW, Smith SM, Wheeler‐Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM ( 2003): Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6: 750–757. [DOI] [PubMed] [Google Scholar]
- Cannistraro PA, Makris N, Howard JD, Wedig MM, Hodge SM, Wilhelm S, Kennedy DN, Rauch SL ( 2007): A diffusion tensor imaging study of white matter in obsessive‐compulsive disorder. Depress Anxiety 24: 440–446. [DOI] [PubMed] [Google Scholar]
- Cheyette SR, Cummings JL ( 1995): Encephalitis lethargica: Lessons for contemporary neuropsychiatry. J Neuropsychol Clin Neurosci 7: 125–134. [DOI] [PubMed] [Google Scholar]
- Clarke JM, Zaidel E ( 1994): Anatomical‐behavioral relationships: Corpus callosum morphometry and hemispheric specialization. Behav Brain Res 64: 185–202. [DOI] [PubMed] [Google Scholar]
- Collins DL, Evans AC ( 1997): ANIMAL: Validation and application of nonlinear registration‐based segmentation. Int J Patt Rec Art Int 8: 1271–1294. [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME ( 1999): Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci 96: 10422–10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lacoste MC, Kirkpatrick JB, Ross ED ( 1985): Topography of the human corpus callosum. J Neuropathol Exp Neurol 44: 578–591. [DOI] [PubMed] [Google Scholar]
- de Toffol B, Autret A ( 1991): Influence of lateralized neuropsychological activities with and without sensorimotor components on EEG spectral power. Int J Psychophysiol 11: 109–114. [DOI] [PubMed] [Google Scholar]
- DeLacoste‐Utamsing C, Holloway RL ( 1982): Sexual dimorphism in the human corpus callosum. Science 216: 1431–1432. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Kertesz A, Cowell PE ( 1991): A factor analysis of the human's corpus callosum. Brain Res 548: 126–132. [DOI] [PubMed] [Google Scholar]
- Estepar RS, Kubicki M, Shenton ME, Westin CF ( 2006): A kernel‐based approach for user‐guided fiber bundling using diffusion tensor data. Paper presented at: IEEE Engineering in Medicine and Biology Society. [DOI] [PMC free article] [PubMed]
- First MB, Spitzer RL, Gibbon M ( 1996): Structured Clinical Interview for DSM‐IV Axis I Disorders. New York: New York State Psychiatric Institute. [Google Scholar]
- Fitzgerald KD, Stern ER, Angstadt M, Nicholson‐Muth KC, Maynor MR, Welsh RC, Hanna GL, Taylor SF ( 2010): Altered function and connectivity of the medial frontal cortex in pediatric obsessive compulsive disorder. Biol Psychiatry 68: 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons J, Kubicki M, Smith K, Bushell G, Estepar RS, Westin CF, Nestor PG, Niznikiewicz MA, Kikinis R, McCarley RW, Shenton ME ( 2009): Diffusion tractography of the fornix in schizophrenia. Schizophr Res 107: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibotto V, Scifo P, Gorini A, Alonso CR, Brambati S, Bellodi L, Perani D ( 2010): Disorganization of anatomical connectivity in obsessive compulsive disorder: A multi‐parameter diffusion tensor imaging study in a subpopulation of patients. Neurobiol Dis 37: 468–476. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Illes J, Chen D, Desmond JE, Gabrieli JD ( 2002): Memory lateralization in medial temporal lobe epilepsy assessed by functional MRI. Epilepsia 43: 855–863. [DOI] [PubMed] [Google Scholar]
- Gong G, Jiang T, Zhu C, Zang Y, Wang F, Xie S, Xiao J, Guo X ( 2005): Asymmetry analysis of cingulum based on scale‐invariant parameterization by diffusion tensor imaging. Hum Brain Mapp 24: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS ( 1989a) The Yale‐Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 46: 1006–1011. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS ( 1989b) The Yale‐Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry 46: 1012–1016. [DOI] [PubMed] [Google Scholar]
- Gu BM, Park JY, Kang DH, Lee SJ, Yoo SY, Jo HJ, Choi CH, Lee JM, Kwon JS ( 2008): Neural correlates of cognitive inflexibility during task‐switching in obsessive‐compulsive disorder. Brain 131: 155–164. [DOI] [PubMed] [Google Scholar]
- Ha TH, Kang DH, Park JS, Jang JH, Jung WH, Choi JS, Jung MH, Choi CH, Lee JM, Ha K, Kwon JS ( 2009): White matter alterations in male patients with obsessive‐compulsive disorder. Neuroreport 20: 735–739. [DOI] [PubMed] [Google Scholar]
- Han JY, Kang DH, Gu BM, Jung WH, Choi JS, Jung MH, Choi CH, Jang JH, Kwon JS ( 2011): Altered brain activation in ventral frontal‐striatal regions following a 16‐week pharmacotherapy in unmedicated obsessive‐compulsive disorder. J Korean Med Sci 26: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Soriano‐Mas C, Pujol J, Ortiz H, Lopez‐Sola M, Hernandez‐Ribas R, Deus J, Alonso P, Yucel M, Pantelis C, Menchon JM, Cardoner N ( 2009): Altered corticostriatal functional connectivity in obsessive‐compulsive disorder. Arch Gen Psychiatry 66: 1189–1200. [DOI] [PubMed] [Google Scholar]
- Hesse S, Stengler K, Regenthal R, Patt M, Becker GA, Franke A, Knupfer H, Meyer PM, Luthardt J, Jahn I, Lobsien D, Heinke W, Brust P, Hegerl U, Sabri O ( 2011): The serotonin transporter availability in untreated early‐onset and late‐onset patients with obsessive‐compulsive disorder. Int J Neuropsychopharmcol 14: 606–617. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, van Zijl PC, Hillis AE, Wytik AE, Mori S ( 2005): DTI tractography based parcellation of white matter: Application to the mid‐sagittal morphology of corpus callosum. Neuroimage 26: 195–205. [DOI] [PubMed] [Google Scholar]
- Jancke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H ( 1997): The relationship between corpus callosum size and forebrain volume. Cereb Cortex 7: 48–56. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME ( 2002): Uncinate fasciculus findings in schizophrenia: A magnetic resonance diffusion tensor imaging study. Am J Psychiatry 159: 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, Kikinis R, Jolesz FA, McCarley RW, Shenton ME ( 2003): Cingulate fasciculus integrity disruption in schizophrenia: A magnetic resonance diffusion tensor imaging study. Biol Psychiatry 54: 1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Styner M, Bouix S, Gerig G, Markant D, Smith K, Kikinis R, McCarley RW, Shenton ME ( 2008): Reduced interhemispheric connectivity in schizophrenia‐tractography based segmentation of the corpus callosum. Schizophr Res 106: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, Jang JH, Choi JS, Kang DH ( 2009): Neuroimaging in obsessive‐compulsive disorder. Expert Rev Neurother 9: 255–269. [DOI] [PubMed] [Google Scholar]
- Lin F, Yu C, Jiang T, Li K, Li X, Qin W, Sun H, Chan P ( 2006): Quantitative analysis along the pyramidal tract by length‐normalized parameterization based on diffusion tensor tractography: Application to patients with relapsing neuromyelitis optica. Neuroimage 33: 154–160. [DOI] [PubMed] [Google Scholar]
- Lomax CL, Oldfield VB, Salkovskis PM ( 2009): Clinical and treatment comparisons between adults with early‐ and late‐onset obsessive‐compulsive disorder. Behav Res Ther 47: 99–104. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Keshavan MS, Dick EL, Rosenberg DR ( 1999): Corpus callosum signal intensity in treatment‐naïve pediatric obsessive compulsive disorders. Prog Neuropsychopharmacol Biol Psychiatry 23: 601–612. [DOI] [PubMed] [Google Scholar]
- Mataix‐Cols D, van den Heuvel OA ( 2006): Common and distinct neural correlates of obsessive‐compulsive and related disorders. Psychiatr Clin North Am 29: 391–410. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC ( 1999): Three‐dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45: 265–269. [DOI] [PubMed] [Google Scholar]
- Nakamae T, Narumoto J, Shibata K, Matsumoto R, Kitabayashi Y, Yoshida T, Yamada K, Nishimura T, Fukui K ( 2008): Alteration of fractional anisotropy and apparent diffusion coefficient in obsessive‐compulsive disorder: A diffusion tensor imaging study. Prog Neuropsychopharmacol Biol Psychiatry 32: 1221–1226. [DOI] [PubMed] [Google Scholar]
- Oh JS, Song IC, Lee JS, Kang H, Park KS, Kang E, Lee DS ( 2007): Tractography‐guided statistics (TGIS) in diffusion tensor imaging for the detection of gender difference of fiber integrity in the midsagittal and parasagittal corpora callosa. Neuroimage 36: 606–616. [DOI] [PubMed] [Google Scholar]
- Oh JS, Kubicki M, Rosenberger G, Bouix S, Levitt JJ, McCarley RW, Westin CF, Shenton ME ( 2009): Thalamo‐frontal white matter alterations in chronic schizophrenia: A quantitative diffusion tractography study. Hum Brain Mapp 30: 3812–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Kim JJ, Lee SK, Seok JH, Chun J, Kim DI, Lee JD ( 2008): Corpus callosal connection mapping using cortical gray matter parcellation and DT‐MRI. Hum Brain Mapp 29: 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GJ, Luzzi S, Alexander DC, Wheeler‐Kingshott CA, Ciccarelli O, Lambon Ralph MA ( 2005): Lateralization of ventral and dorsal auditory‐language pathways in the human brain. Neuroimage 24: 656–666. [DOI] [PubMed] [Google Scholar]
- Pauls DL, Alsobrook JP, Goodman W, Rasmussen S, Leckman JF ( 1995): A family study of obsessive‐compulsive disorder. Am J Psychiatry 152: 76–84. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman‐Rakic PS ( 1995): Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of Area 9 and 46 and relationship to the Talairach coordinate system. Cereb Cortex 5: 323–337. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS, O'Hearn KM, Dick EL, Bagwell WW, Seymour AB, Montrose DM, Pierri JN, Birmaher B ( 1997a) Frontostriatal measurement in treatment‐naive children with obsessive‐compulsive disorder. Arch Gen Psychiatry 54: 824–830. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS, Dick EL, Bagwell WW, MacMaster FP, Birmaher B ( 1997b) Corpus callosal morphology in treatment‐naive pediatric obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 21: 1269–1283. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nobuhara K, Okugawa G, Takase K, Sugimoto T, Horiuchi M, Ueno C, Maehara M, Omura N, Kurokawa H, Ikeda K, Tanigawa N, Sawada S, Kinoshita T ( 2008): Corpus callosum in patients with obsessive‐compulsive disorder: Diffusion‐tensor imaging study. Radiology 246: 536–542. [DOI] [PubMed] [Google Scholar]
- Saxena S, Rauch SL ( 2000): Functional neuroimaging and the neuroanatomy of obsessive‐compulsive disorder. Psychiatr Clin North Am 23: 563–586. [DOI] [PubMed] [Google Scholar]
- Shin YW, Kim DJ, Ha TH, Park HJ, Moon WJ, Chung EC, Lee JM, Kim IY, Kim SI, Kwon JS ( 2005): Sex differences in the corpus callosum: Diffusion tensor imaging study. Neuroreport 16: 795–798. [DOI] [PubMed] [Google Scholar]
- Shin YW, Kwon JS, Kim JJ, Kang DH, Youn T, Kang KW, Kang E, Lee DS, Lee MC ( 2006): Altered neural circuit for working memory before and after symptom provocation in patients with obsessive‐compulsive disorder. Acta Psychiatr Scand 113: 420–429. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE ( 2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- Stein DJ ( 2002): Obsessive‐compulsive disorder. Lancet 360: 397–405. [DOI] [PubMed] [Google Scholar]
- Stewart SE, Platko J, Fagerness J, Birns J, Jenike E, Smoller JW, Perlis R, Leboyer M, Delorme R, Chabane N, Rauch SL, Jenike MA, Pauls DL ( 2007): A genetic family‐based association study of OLIG2 in obsessive‐compulsive disorder. Arch Gen Psychiatry 64: 209–214. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Schapiro MB, Grady CL, Cheslow DL, Leonard HL, Kumar A, Friedland R, Rapoport SI, Rapoport JL ( 1989): Cerebral glucose metabolism in childhood‐onset obsessive‐compulsive disorder. Arch Gen Psychiatry 46: 518–523. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Malhotra AK, Robinson DG, Bilder RM, Lim KO ( 2005): White matter abnormalities in obsessive‐compulsive disorder: A diffusion tensor imaging study. Arch Gen Psychiatry 62: 782–790. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW ( 2000): Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature 404: 190–193. [DOI] [PubMed] [Google Scholar]
- Thottakara P, Lazar M, Johnson SC, Alexander AL ( 2006): Application of Brodmann's area templates for ROI selection in white matter tractography studies. Neuroimage 29: 868–878. [DOI] [PubMed] [Google Scholar]
- Witelson SF ( 1989): Hand and sex differences in the isthmus and genu of the human corpus callosum: A postmortem morphological study. Brain 112: 799–835. [DOI] [PubMed] [Google Scholar]
- Yoo SY, Jang JH, Shin YW, Kim DJ, Park HJ, Moon WJ, Chung EC, Lee JM, Kim IY, Kim SI, Kwon JS ( 2007): White matter abnormalities in drug‐naïve patients with obsessive‐compulsive disorder: A diffusion tensor study before and after citalopram treatment. Acta Psychiatr Scand 116: 211–219. [DOI] [PubMed] [Google Scholar]
- Yum TH, Park YS, Oh KJ ( 1992): The manual of Korean‐Wechsler adult intelligence scale. Seoul: Korean Guidance Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Supporting Information


