Abstract
The hallucinogenic brew Ayahuasca, a rich source of serotonergic agonists and reuptake inhibitors, has been used for ages by Amazonian populations during religious ceremonies. Among all perceptual changes induced by Ayahuasca, the most remarkable are vivid “seeings.” During such seeings, users report potent imagery. Using functional magnetic resonance imaging during a closed‐eyes imagery task, we found that Ayahuasca produces a robust increase in the activation of several occipital, temporal, and frontal areas. In the primary visual area, the effect was comparable in magnitude to the activation levels of natural image with the eyes open. Importantly, this effect was specifically correlated with the occurrence of individual perceptual changes measured by psychiatric scales. The activity of cortical areas BA30 and BA37, known to be involved with episodic memory and the processing of contextual associations, was also potentiated by Ayahuasca intake during imagery. Finally, we detected a positive modulation by Ayahuasca of BA 10, a frontal area involved with intentional prospective imagination, working memory and the processing of information from internal sources. Therefore, our results indicate that Ayahuasca seeings stem from the activation of an extensive network generally involved with vision, memory, and intention. By boosting the intensity of recalled images to the same level of natural image, Ayahuasca lends a status of reality to inner experiences. It is therefore understandable why Ayahuasca was culturally selected over many centuries by rain forest shamans to facilitate mystical revelations of visual nature. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: Ayahuasca; mental imagery; functional magnetic resonance imaging; psychedelic substance; N,N‐dimethyltryptamine; monoamine oxidase inhibitors
INTRODUCTION
Ayahuasca, a central pillar of Amerindian traditional medicine, has been traditionally used by rain forest populations during religious ceremonies. Since the 1980s, Ayahuasca‐based religions have been spreading to urban centers in South as well as in North America [Santos et al., 2007]. In 1992, the Brazilian government approved a resolution legalizing the use of Ayahuasca within a religious context, and similar action was taken by the United States Supreme Court in 2006.
Ayahuasca is served as a tea prepared as a decoction of a bush (Psychotria viridis) and a liana (Banisteriopsis caapi). Psychotria viridis is a rich source of the psychedelic substance N,N‐dimethyltryptamine (DMT), whereas Banisteriopsis caapi contains β‐carbolines such as harmine, harmaline, and tetrahydroharmine, which are potent monoamine oxidase inhibitors (MAOi). The synergistic interaction of these alkaloids determines the psychotropic action of Ayahuasca [Buckholtz and Boggan 1977]. DMT is a serotonergic agonist that acts mainly on 5‐HT2A and 5‐HT2C receptors [Smith et al., 1998], but in itself it is not orally active, since it is inactivated by MAO. However, the inhibition of MAO by β‐carbolines allows DMT to be psychoactive when ingested. MAOi also contribute directly to the neuropharmacological effects of Ayahuasca by increasing extracellular levels of 5‐HT.
The effects of Ayahuasca begin ∼ 30–40 min after oral intake, and last up to 4 hours. Autonomic responses include increases in cardiac and respiratory rates, blood pressure, temperature, and pupil diameter [Riba et al., 2003]. Ayahuasca users report many psychological effects, which include changes in self‐perception, spatio‐temporal scaling [Shanon 2003], and sensory hallucination. Among all perceptual changes induced by Ayahuasca, the most remarkable one are vivid visual hallucinations called mirações (seeings) [Riba et al., 2001; Shanon 2002]. Individuals report a variety of scenarios, as exuberant as a colored dream. Such seeings appear in different forms and context, ranging from simple to complex situations, from the vision of an animal to a long conversation with somebody unknown. However, despite their visual nature, Ayahuasca seeings are all internally generated, without the need of external stimuli [Shanon 2002]. Although enhanced imagery occupies center stage in Ayahuasca‐based rituals, very little is known about the neural mechanisms underlying such seeings.
The ability to generate visual mental imagery has long been a subject of research [Denis 1991; Richardson 1969]. Apart from the debate that this theme has provoked among psychologists and philosophers [Kosslyn et al., 2001; Pylyshyn 1981], we will herein consider mental imagery as the action of mentally summoning a visual representation of the world. Previous studies using functional Magnetic Resonance Imaging (fMRI) have investigated the neural basis of imagery (reviewed in Kosslyn et al., 2001; Mellet et al., 1998] and demonstrated a substantial overlap among brain regions involved with imagery and perception [Ganis et al., 2004]. The involvement of associative visual areas during imagery has been consistently reported in the literature (DEsposito et al., 1997], but the participation of the primary visual area (V1; Brodmann area 17, BA17) remains controversial [Ishai and Sagi 1995; Ishai et al., 2000; Kosslyn and Thompson 2003].
To investigate the neural basis of imagery induced by Ayahuasca, we used BOLD (Blood Oxygenation Level Dependent) fMRI during three sequential conditions: natural image of pictures, imagery of the same pictures, and natural image of scrambled versions of the same pictures. The same paradigm was applied before and after the oral intake of Ayahuasca.
MATERIALS AND METHODS
Subjects
Ten frequent Ayahuasca users participated in the study (mean age: 29 years, from 24 to 48 years, 5 female), after informed consent was obtained from all subjects in accordance with the guidelines approved (No 14672/2006) by the Human Research Committee and the Ethics Committee of the University of Sao Paulo. One subject was excluded from analyses due to uncorrectable amounts of head movement, leaving 9 participants (5 women) in the final dataset.
Ayahuasca Dosage
Each subject drank 120–200 mL of Ayahuasca (2.2 mL/kg of body weight). The Ayahuasca batch used in the experiment contained 0.8 mg/mL of DMT and 0.21 mg/mL of harmine. No harmaline was present in the batch, at the chromatography detection threshold of 0.02 mg/mL. To quantify these amounts, a 1 mL sample of the Ayahuasca batch taken by the subjects was homogenized with sodium acetate buffer solution pH = 9, extracted with 5 mL of diethyl ether in a shaker (20 min), and centrifuged at 3,000 rpm for 15 min. The organic phase was collected and evaporated under a nitrogen stream. The residue was dissolved in 1 mL of methanol and 1 μL was analyzed by gas chromatography/mass spectrometry (GC/MS). GC/MS analyses were performed using a Varian CP3800 gas chromatograph coupled to a Varian Saturn 2000 ion trap mass spectrometer (Varian Inc.). A capillary column (DB‐5MS 30 m × 0.25 mm i.d. × 0.25 μm film thickness (Agilent) was used. The chromatographic conditions were as follows: injector temperature was 250°C in the splitless mode, oven temperature program was 80°C for 1 min ramped at 5°C/min to 220°C and held for 10 min, and then to 300°C for 5 min. Helium at a flow rate of 0.8 mL/min was used as carrier gas.
MR Image Acquisition
All images were acquired in a 1.5T scanner (Siemens, Magneton Vision). The functional dataset was acquired using EPI‐BOLD like sequences, and comprises 147 brain volumes with the following parameters: TR = 3,000 ms; TE = 60 ms; flip angle = 90°, FOV = 220 mm; matrix = 128 × 128, slice thickness = 5 mm, and number of slices = 16. High spatial resolution images were obtained from typical Gradient Recalled Echo sequences, constituted of 156 sagital slices, covering both hemispheres, with a 1 mm3 of voxel resolution.
Experimental Design
Each subject performed two fMRI sessions. In the first session, subjects were scanned before Ayahuasca intake. Immediately after the first scan, subjects drank the tea. Psychiatric scales were applied at intervals of 0, 40, 80, and 200 min after Ayahuasca intake. The second fMRI session began 40 min after intake, when subjects were engaged in the same set of tasks of the first session. Figure 1 shows a diagram of the experimental design (one session). In each fMRI session, subjects were submitted to three conditions in a block design (21 sec per condition; 3 conditions per block, 7 blocks per session). In the first condition (natural image), subjects passively viewed images of people, animals or trees (one different image per block, no repetition). The second condition (imagery) consisted of an imagery task, during which subjects were asked to close their eyes and mentally generate the same image they had just seen. During the last condition (scrambled image) subjects passively viewed a scrambled version of the image previously presented in the natural image condition. The scrambled image served as baseline for the analysis. Each scanning session lasted 441 sec (7 blocks × 3 conditions × 21 sec). The stimulation protocol was designed in Presentation® software (Version 0.60, Neurobehavioral Systems). Images were projected onto a translucent screen and reached the eyes of the subjects by reflection on a mirror system adapted to the head coil.
Figure 1.
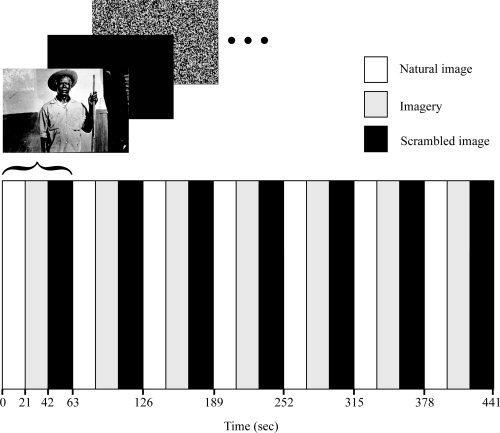
Experimental design of one fMRI scanning session. Subjects were submitted to three conditions (natural image, imagery with closed eyes, and scrambled image) in a block design (21 sec per condition; 3 conditions per block, 7 blocks per session). Each subject performed two fMRI sessions. In the first session, subjects were scanned before Ayahuasca intake. Immediately after the first scan, subjects drank Ayahuasca. The second fMRI session began 40 min after intake.
Retinotopic Mapping
We used a flickering white and black checkerboard formed into a ray‐shaped configuration subtending 22.5° (1/8) of polar angle. The disk segment started at the right horizontal meridian and slowly rotated anticlockwise for a full cycle of 360°. Each mapping session consisted of thirteen repetitions of a full rotation, which lasts 67 sec. Retinotopy of polar angle was revealed with cross‐correlation analysis. The BOLD signal was correlated with an ideal response function that assumes the first 1/8 of a stimulation cycle as being the reference section (corrected for a hemodynamic delay). Areas activated at particular polar angles were revealed through selecting the lag value that resulted in the highest cross‐correlation value for a particular voxel[Linden et al., 1999]. Lag values were color‐coded and used to compute the cross‐correlation maps, to find the retinotopic boundaries of early visual areas V1, V2, and V3.
Image Processing
Images were processed in Brain Voyager QX 1.9 (Brain Innovation, Maastricht, The Netherlands). Data analysis consisted of preprocessing steps, which included, 3D head motion correction (sinc interpolation), slice scan time correction (sinc interpolation), spatial smoothing (4.0 mm FWHM, 3D Gaussian filter), a high pass filtered at 0.01 Hz, and linear trend removal. The contrasts, in each condition, were evaluated using a general linear model (GLM) taking into account the hemodynamic response function (modeled by a two‐gamma function) and baseline state. Group differences were analyzed using a fixed effect GLM with separate subject predictors, and statistical threshold was set taking into account a correction for multiple comparison based on the false discovery rate (FDR). Within and between multi‐subjects corrections were made by setting q(FDR) <0.05. After transformation into Talairach space, the group analysis model included three orthogonal contrasts: mental imagery before intake versus baseline, mental imagery before intake versus mental imagery after intake and real imagery before intake versus real imagery after intake. Clusters of activation were then segregated into regions of at least 50 mm3.
Image Analyses
The anatomical MRI of all subjects were investigated by an experienced neuroradiologist, and all the reports were classified as normal. The analysis of fMRI data was based on the GLM. The hemodynamic response function was modeled based on the conventional difference between two gamma functions. Unless otherwise specified, the GLM analysis was based on a fixed effect (FFX) assumption. Statistical maps were corrected for multiple comparison based on the FDR, and a threshold of q(FDR <0.05) was set for all group‐level contrasts. Contrast maps were overlaid onto a coregistered anatomical image. The individual beta values used in the co‐variance analyses were extracted from all statistically significant voxels in the ROI. Moreover, BOLD signal averages were computed for imagery as well as natural image conditions, both before and after potion intake. The strategy used is similar to computing EEG evoked potential averages along a series of trials. To generate BOLD signal time course averages Brain Voyager QX 1.9 (Brain Innovation, Maastricht, The Netherlands) was used. In brief, the averaged signal was computed for the selected ROI based on the average of all trials of particular conditions of interest (imagery before and after, natural image before and after), encompassing a baseline period (scrambles image). These time courses were then averaged across subjects.
Functional Connectivity Analysis
This analysis was used to capture temporal correlations between areas, as a simplified snapshot of the dynamical state of the system. The correlations are represented by a graph, with nodes corresponding to areas, and links to functional connections. The time traces fed into the algorithm were, for each area, the fold activation over noise. Area‐to‐area correlations were computed as
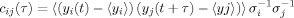 , where
, where
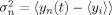 . The maximum correlation, and the delayed at which it was met, were computed as
. The maximum correlation, and the delayed at which it was met, were computed as
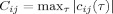 and
and
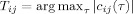 . A link was considered to be present whenever
. A link was considered to be present whenever
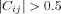 . A link was considered to be present if the P‐value of the correlation survived FDR correction at 0.05; with only one exception, all the links were found to be significant. If the delay at which this threshold was met exceeded 5 TR's (i.e., 15 sec), then the link was considered to be directed, with the direction pointing from the “leader” to the “follower”; otherwise, the link was considered as undirected, that is, no temporal precedence relationship could be determined. The choice of 5 TR's was based on the auto‐correlation of the activations, which on average drop significantly for this time delay (see Supporting Information for details on this analysis).
. A link was considered to be present if the P‐value of the correlation survived FDR correction at 0.05; with only one exception, all the links were found to be significant. If the delay at which this threshold was met exceeded 5 TR's (i.e., 15 sec), then the link was considered to be directed, with the direction pointing from the “leader” to the “follower”; otherwise, the link was considered as undirected, that is, no temporal precedence relationship could be determined. The choice of 5 TR's was based on the auto‐correlation of the activations, which on average drop significantly for this time delay (see Supporting Information for details on this analysis).
RESULTS
Psychological Effects
To estimate the time course of the psychological changes associated with Ayahuasca intake, we applied two psychiatric scales at intervals of 0, 40, 80, and 200 min after Ayahuasca intake: the brief psychiatric ratings scale (BPRS) to detect psychotic symptoms [Crippa et al., 2001], and the Young Mania Rating Scale (YMRS), to measure mania symptoms [Vilela et al., 2005]. Figure 2 shows that all subjects experienced increases in the two psychiatric scales following Ayahuasca ingestion. The effects were significant at 40 and 80 min post‐intake (P = 0.036 for BPRS and P = 0.036 YMRS), Wilcoxon corrected for multiple comparisons of 4 time points), with respect to baseline (T = 0 min), in agreement with the time course of psychological [Riba et al., 2003], electroencephalographic changes induced by Ayahuasca [Riba et al., 2002] and single photon emission computed tomography [Riba et al., 2006]. Importantly, all subjects reported a marked increase in the ability to perform the imagery task after tea intake, corroborating that imagined scenes become much more vivid and detailed after Ayahuasca [Shanon 2002].
Figure 2.
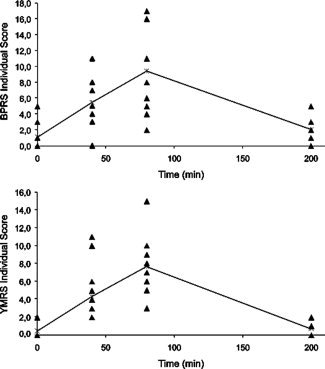
Individual scores reached on the psychiatric scales BPRS and YMRS at 0, 40, 80, and 200 min for all subjects. Ayahuasca intake took place at T = 0 min. The psychological effects with respect to baseline (T = 0 min) reached significance at 40 min (P = 0.05 for BPRS, P < 0.036 for YMRS, Wilcoxon corrected for multiple comparisons), immediately before scanning, and peaked at 80 min (P = 0.036 for BPRS and P = 0.036 for YMRS, Wilcoxon corrected for multiple comparisons). Line connects mean values at different time points. Note that the variability is very low at 0 and 200 min, when most subjects presented identical scores.
BOLD Signal Modulation by Ayahuasca
To test for the modulation of brain activity during imagery by Ayahuasca, fMRI data from individual subjects were analyzed using the following additional contrast: imagery after intake (IA) > imagery before intake (IB). Statistically significant areas of increased BOLD signal (q(FDR) < 0.05) comprised bilateral precuneus (BA7, 18, 19, 31), cuneus (BA 17, 18, 19, 30), lingual gyrus (BA 17, 18, 19, 30), fusiform gyrus (BA 19 and 37), middle occipital gyrus (BA 18, 19, 37), parahippocampal gyrus (BA 30), posterior cingulate gyrus (BA 23, 29, 30, 31), superior temporal gyrus (BA22 and 42), superior and middle frontal gyrus (BA 8, 9, 10, 47), and inferior frontal gyrus (BA 47).
To identify which areas were specifically modulated by Ayahuasca during the imagery task, and not by a generalized, nonspecific effect of Ayahuasca across all conditions, a further contrast was built in which the main effect of the imagery task (IA > IB) was subtracted from the main effect of natural image task (natural image after intake (NIA) > natural image before intake (NIB)). To determine the extent and borders of the early visual areas (V1, V2, and V3), a phase‐encoded retinotopic mapping was performed in an additional fMRI session [Linden et al., 1999]. As shown in Figure 3, BOLD signal increase was most prominent in the occipital cortex, extending into inferior and mesial temporal lobe, and to portions of the frontal lobe. Statistically significant voxels (q(FDR) < 0.05) were found bilaterally in the occipital cortex (BA 17, 18, 19), comprising the three early visual areas with their respective retinotopic representations. Statistically significant modulation was also detected bilaterally in the parahippocampal gyrus (BA 30), middle temporal cortex (BA 37) and frontal cortex (BA 10; Fig. 3; Table I).
Figure 3.
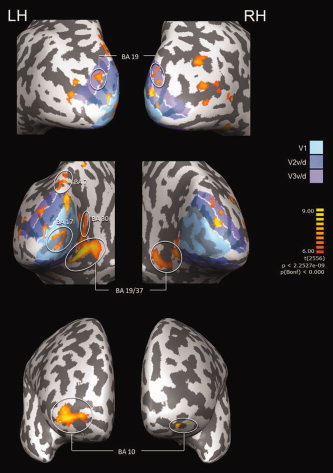
Main effect of the imagery task (q(FDR) < 0.05). The contrast corresponds to (imagery after > imagery before)‐(natural image after > natural image before). Retinotopic mapping appears in light blue, blue and purple corresponding to areas V1, V2, and V3, respectively.
Table I.
Areas modulated by Ayahuasca during the imagery task
| Region | Hem | BA | % Change | Cluster Vol. | x | y | z | σx | σy | σz |
|---|---|---|---|---|---|---|---|---|---|---|
| Middle Occipital Gyrus | Left | 18, 19 | 1.18% | 138 mm3 | −28 | −88 | 4 | 1 | 1 | 1 |
| Right | 19, 37 | 1.02% | 30 mm3 | 43 | −66 | 5 | 1 | 2 | 2 | |
| Fusiform Gyrus | Left | 19 | 1.52% | 30 mm3 | −22 | −54 | −8 | 1 | 2 | 1 |
| Right | 37 | 0.51% | 264 mm3 | 43 | −56 | −16 | 1 | 2 | 1 | |
| Middle Frontal Gyrus | Right | 10 | −0.47% | 519 mm3 | 33 | 51 | 1 | 2 | 1 | 1 |
| Superior Frontal Gyrus | Right | 10 | −0.47% | 216 mm3 | 27 | 50 | 1 | 3 | 2 | 1 |
| Lingual Gyrus | Left | 17, 18, 19 | 1.52% | 597 mm3 | −16 | −57 | 1 | 5 | 15 | 2 |
| Right | 17, 18, 19 | 1.35% | 417 mm3 | 12 | −54 | 1 | 1 | 8 | 1 | |
| Parahippocampal Gyrus | Left | 30 | 1.37% | 837 mm3 | −14 | −46 | 1 | 3 | 8 | 3 |
| Right | 30 | 1.19% | 237 mm3 | 13 | −41 | −2 | 2 | 5 | 3 | |
| Cuneus | Left | 17, 18 | 1.53% | 1578 mm3 | −6 | −90 | 5 | 3 | 6 | 6 |
| Right | 17, 18 | 1.21% | 294 mm3 | 13 | −94 | 4 | 2 | 2 | 1 | |
| Precuneus | Left | 7 | 1.29% | 174 mm3 | −3 | −67 | 39 | 1 | 2 | 1 |
| Middle Temporal Gyrus | Right | 37 | 0.51% | 108 mm3 | 45 | −63 | 4 | 1 | 1 | 1 |
Hem = hemisphere; Cluster volume = volume of the cluster in mm3; % Change = ROI maximum percent BOLD signal change related to the same contrast used to generate the current table; x, y, and z = coordinates of the center of the cluster; (σx,σy,σz) = respective standard deviation.
The contrast subtracts the main effect of the imagery task (IA > IB) from the main effect of natural image (NIA > NIB). The Talairach coordinates correspond to the center of the cluster of activity with its respective standard deviation.
BOLD Signals Averages Before and After Ayahuasca Intake
To further inspect the modulation elicited by Ayahuasca, we computed the averaged percent BOLD signal changes for the same ROI that were specifically modulated by Ayahuasca during the imagery task (BA 10, 17, 18, 19, 30, and 37). Figure 4 shows the extracted mean values and standard deviations were based on all trials. These included a baseline interval (5 sec–scrambled image, presented in Fig. 4 as a shaded gray area) and the whole periods for the following conditions: imagery before (Fig. 4, blue line), imagery after (Fig. 4, red line), natural image before (Fig. 4, light gray line), natural image after (Fig. 4, green line). As can be observed, the averaged BOLD responses for scrambled image (Fig. 4, shaded gray area) were similar to the ones from the natural image conditions (before intake–Fig. 4, light gray line), in all ROI from the visual cortex (BA 17, 18, and 19). This was expected since the BOLD signal in visual cortex should not be different in the scrambled condition when compared to the natural image condition. The same pattern was preserved when observing the averaged signals after Ayahuasca intake. However, the average BOLD response during the imagery condition shows a remarkable increase after Ayahuasca intake. This can be observed by comparing the traces of the imagery before (blue lines) with imagery after intake (red lines). It is important to note that the averaged BOLD signal amplitude on visual areas during the imagery task before Ayahuasca intake (blue line) is expected to be smaller than during the scrambled period, when the visual system is actually being stimulated. After Ayahuasca intake, however, the averaged signal reaches amplitudes compatible to the ones obtained during the scrambled conditions. The same pattern of positive modulation was observed in all ROI, in the occipital (BA17, BA19, and BA7), temporal (BA30 and BA37) and frontal areas (BA10). The effect of Ayahuasca in occipital areas was particularly noteworthy, because the signal amplitude after intake increased markedly during imagery, but not during natural image (see Fig. 4). Supported by retinotopic mapping (see Fig. 3), the specific BA17 location modulated by Ayahuasca corresponds to the cuneus and lingual gyrus, which are related to the peripheral visual field [Yoshor et al., 2007]. Also worth mentioning is the modulation by Ayahuasca of the parahipocampal cortex and the retrosplenial cortex (BA30 and BA37) during the imagery task. These structures are important for the retrieval of episodic memories [de Araujo et al., 2002], and have recently been implicated in the processing and representation of contextual associations [Bar 2004]. As shown in Figure 4, in addition to occipital and temporal areas, Ayahuasca also potentiated parts of the frontopolar cortex (BA10) known to be involved in imagery [Kosslyn et al., 1999]. Among all regions modulated by Ayahuasca, BA10 was the only one that showed a positive BOLD signal during the imagery task even before Ayahuasca intake (see Fig. 4), and it was potentiated after tea ingestion during the imagery task (see Fig. 4).
Figure 4.
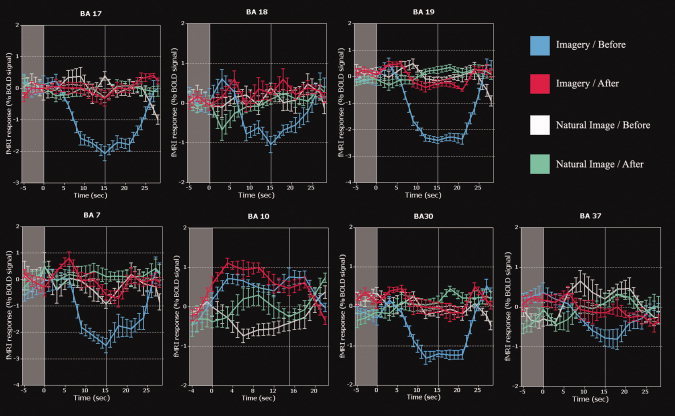
Average time courses of BOLD responses before and after Ayahuasca intake (blue for imagery before intake, red for imagery after intake, white for natural image before intake, and green for natural image after intake). Each time point corresponds to an fMRI TR = 3 sec. Note the marked increase in BOLD signal during imagery following Ayahuasca intake. Shaded gray area represents baseline periods (scrambled image condition). Blue line–imagery before intake; red line–imagery after intake; light gray line–natural image before intake; green line–natural image after intake.
Correlation Between BOLD Amplitude and Psychiatric Scales
To investigate the relationship between neural and psychological changes, we calculated correlations between BOLD signal amplitude in different brain areas during post‐intake sessions, and the values reached on the two psychiatric scales 40 min after intake, that is, immediately before subjects entered the scanner (see Fig. 5). A significant correlation was observed exclusively between BA17 activation and BPRS data (Spearmann's Rho = 0.78, P = 0.037, two‐tailed, corrected for multiple comparisons of 7 brain areas; BA7, BA10, BA17, BA18, BA19, BA30, and BA37). No significant correlations were observed between BPRS individual scores and activation of any other brain region, nor between YMRS scores and BOLD signal modulation in any brain region. This indicates that BA17 activation levels during post‐Ayahuasca imagery are specifically correlated with the occurrence of increased manifestation of perceptual changes, which included visual and auditory, as measured by the psychiatric scales.
Figure 5.
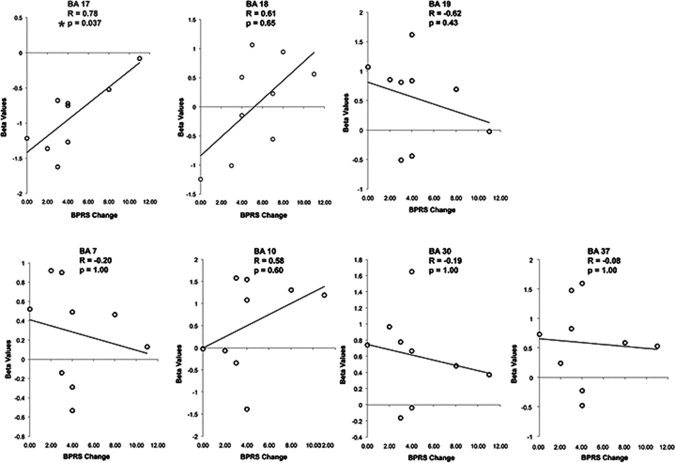
Scatter plot and trend lines of the beta values against individual BPRS scores at 40 min, with respect to baseline (T = 0 min). Statistically significance was found only for BA17 (P = 0.037, corrected for multiple comparisons).
Functional Connectivity
Is the Ayahuasca potentiation of intentional imagery accompanied by changes in the coordination of frontal, temporal, and occipital cortical areas? To address this issue, we implemented a functional connectivity analysis based on delayed correlations [Cecchi et al., 2007]. The results are presented in Figure 6. The top row shows the full connectivity in the four conditions: imagery and natural image, pre/post intake. To understand the changes in connectivity, we sorted apart the links for areas BA17, BA10, and BA19, and showed them individually in separate rows. An interesting change observed for imagery is that BA17 becomes a leader of BA7 and BA37 after intake (Fig. 6, second row), while maintaining its leadership with respect to BA19 and BA30. Overall, the connectivity pattern centered on BA17 for post‐intake imagery seems to be a superposition of connectivity features observed during pre‐intake natural image and imagery. Other effect associated with imagery after Ayahuasca intake is the change from “leader” to “follower” for BA10 with respect to BA7 and BA30, by their turn “followers” of BA17. Furthermore, both for natural image and for imagery, BA19 becomes a “follower” area, lagging behind BA10 and BA17 (fourth row from the top).
Figure 6.
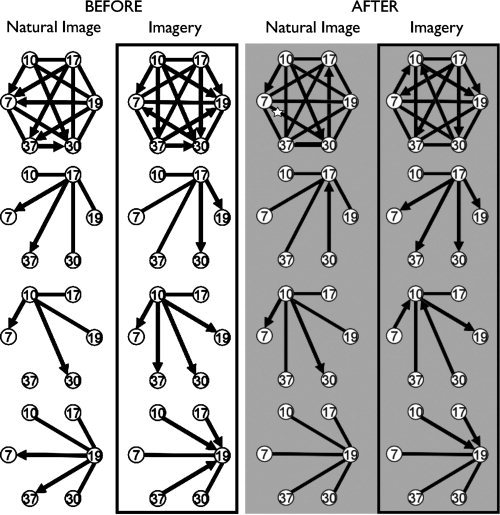
BOLD signal correlations between brain areas before and after Ayahuasca intake. The labels correspond to the same Brodmann areas defined in Figure 3. Arrow direction indicates that the source precedes the target by at least 5 TR's (15 sec); undirected links indicate that there is no such temporal delay between the areas. The top row shows the full connectivity for the four conditions. The second, third, and fourth rows from the top correspond to links involving only BA17, BA10, and BA19, respectively.
DISCUSSION
The effects of Ayahuasca reported here are putatively mediated by the activation of serotonergic receptors widely distributed in the brain, including the several cortical areas modulated during post‐Ayahuasca imagery [Petit‐Taboue et al., 1999]. Interestingly, Ayahuasca did not enhance occipital BOLD signal during the natural image condition in comparison with the scrambled image condition. Perhaps activity in BA17 and other visual areas reaches a ceiling when subjects see with open eyes, irrespective of Ayahuasca ingestion. In contrast, the activation levels of BA17 during imagery were very different depending on whether Ayahuasca had been previously administered or not. While BA17 activation was very low during pre‐Ayahuasca imagery, post‐intake imagery was concomitant with very high BA17 activity, comparable to BOLD signal amplitude during natural image condition (see Fig. 4). Our study did not control for potential effects related to the order of the sessions with and without Ayahuasca ingestion. Although this question was not addressed in our study, it seems very not parsimonious to attribute the remarkable BOLD signal modulations described here to an order effect. Importantly, the effects in BA17 were positively and quite selectively correlated with the individual scores reached on the BPRS psychiatric scale (Spearman's Rho = 0.78, P = 0.037, two‐tailed, corrected for multiple comparisons of 7 brain areas), pointing to a tight relationship between neural changes in BA17 and psychotic effects caused by Ayahuasca intake (see Fig. 5).
In addition to BA17, Ayahuasca had a strong effect in other brain areas related to vision. The nonprimary visual areas strongly modulated by Ayahuasca (BA7, BA18, and BA19) are known to be activated during psychopathological hallucinations [Allen et al., 2008] as well as during normal dreaming, within rapid‐eye‐movement (REM) sleep [Braun et al., 1998; Wehrle et al., 2007]. Although these areas showed BOLD signal time courses similar to that of BA17 (see Fig. 4), no statistically significant correlation was found between their signal modulation during imagery and the psychiatric scales used here (see Fig. 5).
The activity of cortical areas BA30 and BA37, known to be involved with episodic memory retrieval and the processing of contextual associations, was also potentiated by Ayahuasca intake during imagery. Within this framework, Ayahuasca engages cortical regions necessary for the integration of separate visual elements into a whole scene [Chadwick et al., 2010]. This suggests that the seeings induced by the tea are associated with an endogenous engagement of mnemonic circuits, possibly feeding visual areas with the content of the Ayahuasca seeings.
Activation of BA 10 during mental imagery has already been reported [Kosslyn 1999], but its exact role in mental imagery is yet to be characterized. Previous studies have shown that BA10 activity correlates with the amount of intentional effort involved in self‐awareness and the imagination of future events [Goldberg et al., 2006]. Furthermore, neuroimaging and lesion studies reported that BA10 plays an important role in prospective memory [Burgess et al., 2001]. It has been recently proposed that this region is involved with the temporal direction of an imaginary event [Addis and Schacter 2008], and there is evidence of its engagement when internally generated information needs to be evaluated [Christoff and Gabrieli 2000; Turner et al., 2008]. At present, it is believed that working memory depends on the interaction of BA10 and the dorsolateral prefrontal cortex (DLPFC): While the former processes information from internal sources, the latter is concerned with information generated externally [Vinogradov et al., 2006].
It is noteworthy to mention that although all subjects studied were experienced Ayahuasca users, their hemodynamic responses have a canonical shape. Such observation comes from another experiment conducted on the same subjects when performing a classical verbal fluency task, before and after Ayahuasca ingestion [Prado et al., 2009]. The results show a consistent and expected response in expressive language centers, such as Broca's area (BA 44), with a classical hemodynamic response function.
In this study, subjects were asked to intentionally imagine visual scenes. The broad range of neuroanatomical sites significantly affected by Ayahuasca during intentional imagery underlies the remarkable psychological changes produced by the tea. The findings from the connectivity analysis indicate that Ayahuasca intake strongly alters fronto‐occipital relationships, producing marked changes in the temporal ordering of events across several brain regions. In particular, Ayahuasca intake is accompanied by an increased capacity of BA17 to lead other cortical areas during imagery. The functional prevalence and temporal precedence of BA17 during post‐Ayahuasca imagery suggest that the seeings caused by Ayahuasca ingestion, robust even with the eyes shut, may in fact be initiated in the primary visual cortex.
Ayahuasca‐induced seeings have been traditionally used within religious contexts to give access to a deeply meaningful internal world. Altogether, our results indicate that these seeings stem from the activation, during voluntary imagery, of an extensive network of occipital, temporal, and frontal cortical areas respectively involved with vision, memory, and intention. By boosting the intensity of recalled images to the same level of natural image, Ayahuasca lends a status of reality to inner experiences. It is therefore understandable why Ayahuasca was culturally selected over many centuries by rain forest shamans to facilitate mystical revelations of visual nature.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
The authors would like to express our gratitude to the Santo Daime Church, especially to Mestre Irineu, Pelicano, Jacy, and Fernandes, and to all the subjects who participated in the study. They thank Prof. Ronald T. Wakai for important discussions.
REFERENCES
- Addis DR, Schacter DL ( 2008): Constructive episodic simulation: Temporal distance and detail of past and future events modulate hippocampal engagement. Hippocampus 18: 227–237. [DOI] [PubMed] [Google Scholar]
- Allen P, Laroi F, McGuire PK, Aleman A ( 2008): The hallucinating brain: A review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev 32: 175–191. [DOI] [PubMed] [Google Scholar]
- Bar M ( 2004): Visual objects in context. Nat Rev Neurosci 5: 617–629. [DOI] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesensten NJ, Gwadry F, Carson RE, Varga M, Baldwin P, Belenky G, Herscovitch P ( 1998): Dissociated pattern of activity in visual cortices and their projections during human rapid eye movement sleep. Science 279: 91–95. [DOI] [PubMed] [Google Scholar]
- Buckholtz NS, Boggan WO ( 1977): Monoamine‐Oxidase Inhibition in Brain and Liver Produced by Beta‐Carbolines‐Structure‐Activity‐Relationships and Substrate‐Specificity. Biochem Pharmacol 26: 1991–1996. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD ( 2001): Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia 39: 545–555. [DOI] [PubMed] [Google Scholar]
- Cecchi GA, Rao AR, Centeno MV, Baliki M, Apkarian AV, Chialvo DR. 2007. Identifying directed links in large scale functional networks: application to brain fMRI. BMC Cell Biol 8: S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick MJ, Hassabis D, Weiskopf N, Maguire EA ( 2010): Decoding individual episodic memory traces in the human hippocampus. Curr Biol 20: 544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JDE ( 2000): The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology 28: 168–186. [Google Scholar]
- Crippa JAS, Sanches RF, Hallak JEC, Loureiro SR, Zuardi AW ( 2001): A structured interview guide increases Brief Psychiatric Rating Scale reliability in raters with low clinical experience. Acta Psychiatr Scand 103: 465–470. [DOI] [PubMed] [Google Scholar]
- de Araujo DB, Baffa O, Wakai RT ( 2002): Theta oscillations and human navigation: A magnetoencephalography study. J Cogn Neurosci 14: 70–78. [DOI] [PubMed] [Google Scholar]
- Denis M. 1991. Image and Cognition. Greenbaum C, translator. New York: Prentice Hall/Harvester Wheatsheaf. [Google Scholar]
- DEsposito M, Detre JA, Aguirre GK, Stallcup M, Alsop DC, Tippet LJ, Farah MJ ( 1997): A functional MRI study of mental image generation. Neuropsychologia 35: 725–730. [DOI] [PubMed] [Google Scholar]
- Ganis G, Thompson WL, Kosslyn SM ( 2004): Brain areas underlying visual mental imagery and visual perception: an fMRI study. Brain Res Cogn Brain Res 20: 226–241. [DOI] [PubMed] [Google Scholar]
- Goldberg II, Harel M, Malach R ( 2006): When the brain loses its self: Prefrontal inactivation during sensorimotor processing. Neuron 50: 329–339. [DOI] [PubMed] [Google Scholar]
- Ishai A, Sagi D ( 1995): Common mechanisms of visual‐imagery and perception. Science 268: 1772–1774. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Haxby JV ( 2000): Distributed neural systems for the generation of visual images. Neuron 28: 979–990. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM ( 1999): The role of Area 17 in visual imagery: Convergent evidence from PET and rTMS. Science 284: 917–917. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL ( 2003): When is early visual cortex activated during visual mental imagery? Psychol Bull 129: 723–746. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Pascual‐Leone A, Felician O, Camposano S, Keenan JP, Thompson WL, Ganis G, Sukel KE, Alpert NM ( 1999): The role of Area 17 in visual imagery: Convergent evidence from PET and rTMS. Science 284: 167–170. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, Thompson WL ( 2001): Neural foundations of imagery. Nat Rev Neurosci 2: 635–642. [DOI] [PubMed] [Google Scholar]
- Linden DEJ, Kallenbach U, Heinecke A, Singer W, Goebel R ( 1999): The myth of upright vision. A psychophysical and functional imaging study of adaptation to inverting spectacles. Perception 28: 469–481. [DOI] [PubMed] [Google Scholar]
- Mellet E, Petit L, Mazoyer B, Denis M, Tzourio N ( 1998): Reopening the mental imagery debate: Lessons from functional anatomy. Neuroimage 8: 129–139. [DOI] [PubMed] [Google Scholar]
- Petit‐Taboue MC, Landeau B, Barre L, Onfroy MC, Noel MH, Baron JC ( 1999): Parametric PET imaging of 5HT(2A) receptor distribution with F‐18‐setoperone in the normal human neocortex. J Nucl Med 40: 25–32. [PubMed] [Google Scholar]
- Prado DA, Pinto J, Crippa J, Santos A, Ribeiro S, Araujo D, Zuardi A, Chaves C, Hallak J ( 2009): Effects of the Amazonian psychoactive plant beverage ayahuasca on prefrontal and limbic regions during a language task: A fMRI study. Eur Neuropsychopharmacol 19: S314–S315. [Google Scholar]
- Pylyshyn ZW ( 1981): Psychological explanations and knowledge‐dependent processes. Cognition 10: 267–274. [DOI] [PubMed] [Google Scholar]
- Riba J, Rodriguez‐Fornells A, Urbano G, Morte A, Antonijoan R, Montero M, Callaway JC, Barbanoj MJ ( 2001): Subjective effects and tolerability of the South American psychoactive beverage Ayahuasca in healthy volunteers. Psychopharmacology 154: 85–95. [DOI] [PubMed] [Google Scholar]
- Riba J, Anderer P, Morte A, Urbano G, Jane F, Saletu B, Barbanoj MJ ( 2002): Topographic pharmaco‐EEG mapping of the effects of the South American psychoactive beverage ayahuasca in healthy volunteers. Br J Clin Pharmacol 53: 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ ( 2003): Human pharmacology of ayahuasca: Subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J Pharmacol Exp Ther 306: 73–83. [DOI] [PubMed] [Google Scholar]
- Riba J, Romero S, Grasa E, Mena E, Carrio I, Barbanoj MJ ( 2006): Increased frontal and paralimbic activation following ayahuasca, the pan‐amazonian inebriant. Psychopharmacology 186: 93–98. [DOI] [PubMed] [Google Scholar]
- Richardson A. 1969. Mental Imagery. London: Routledge & Kegan Paul PLC; p 192. [Google Scholar]
- Santos RG, Landeira‐Fernandez J, Strassman RJ, Motta V, Cruz APM ( 2007): Effects of ayahuasca on psychometric measures of anxiety, panic‐like and hopelessness in Santo Daime members. J Ethnopharmacol 112: 507–513. [DOI] [PubMed] [Google Scholar]
- Shanon B ( 2002): Ayahuasca visualizations‐A structural typology. J Conscious Stud 9: 3–30. [Google Scholar]
- Shanon B ( 2003): Altered states and the study of consciousness‐The case of ayahuasca. J Mind Behav 24: 125–153. [Google Scholar]
- Smith RL, Canton H, Barrett RJ, Sanders‐Bush E ( 1998): Agonist properties of N,N‐Dimethyltryptamine at serotonin 5‐HT2A and 5‐HT2C receptors. Pharmacol Biochem Behav 61: 323–330. [DOI] [PubMed] [Google Scholar]
- Turner MS, Simons JS, Gilbert SJ, Frith CD, Burgess PW ( 2008): Distinct roles for lateral and medial rostral prefrontal cortex in source monitoring of perceived and imagined events. Neuropsychologia 46: 1442–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilela JAA, Crippa JAS, Del‐Ben CM, Loureiro SR ( 2005): Reliability and validity of a Portuguese version of the Young Mania Rating Scale. Braz J Med Biol Res 38: 1429–1439. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Luks TL, Simpson GV, Schulman BJ, Glenn S, Wong AE ( 2006): Brain activation patterns during memory of cognitive agency. Neuroimage 31: 896–905. [DOI] [PubMed] [Google Scholar]
- Wehrle R, Kaufmann C, Wetter TC, Holsboer F, Auer DP, Pollmacher T, Czisch M ( 2007): Functional microstates within human REM sleep: First evidence from fMRI of a thalamocortical network specific for phasic REM periods. Eur J Neurosci 25: 863–871. [DOI] [PubMed] [Google Scholar]
- Yoshor D, Bosking WH, Ghose GM, Maunsell JHR ( 2007): Receptive fields in human visual cortex mapped with surface electrodes. Cereb Cortex 17: 2293–2302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


