Abstract
Excessive intake of dietary salt (sodium chloride) may increase the risk of chronic diseases. Accordingly, various strategies to reduce salt intake have been conducted. This study aimed to investigate whether a salty‐congruent odor can enhance saltiness on the basis of psychophysical (Experiment 1) and neuroanatomical levels (Experiment 2). In Experiment 1, after receiving one of six stimulus conditions: three odor conditions (odorless air, congruent, or incongruent odor) by two concentrations (low or high) of either salty or sweet taste solution, participants were asked to rate taste intensity and pleasantness. In Experiment 2, participants received the same stimuli during the functional magnetic resonance imaging scan. In Experiment 1, compared with an incongruent odor and/or odorless air, a congruent odor enhanced not only taste intensity but also either pleasantness of sweetness or unpleasantness of saltiness. In Experiment 2, a salty‐congruent combination of odor and taste produced significantly higher neuronal activations in brain regions associated with odor–taste integration (e.g., insula, frontal operculum, anterior cingulate cortex, and orbitofrontal cortex) than an incongruent combination and/or odorless air with taste solution. In addition, the congruent odor‐induced saltiness enhancement was more pronounced in the low‐concentrated tastant than in the high‐concentrated one. In conclusion, this study demonstrates the congruent odor‐induced saltiness enhancement on the basis of psychophysical and neuroanatomical results. These findings support an alternative strategy to reduce excessive salt intake by adding salty‐congruent aroma to sodium reduced food. However, there are open questions regarding the salty‐congruent odor‐induced taste unpleasantness. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: congruency, functional magnetic resonance imaging, odor–taste integration, salt intake reduction
INTRODUCTION
Excessive intake of dietary salt (mainly as sodium chloride, NaCl) may increase the risk of chronic diseases including stroke, cardiovascular disease, hypertension, renal diseases, or gastric cancer [Cutler and Follmann, 1997; Doyle and Glass, 2010; He and MacGregor, 2009; Strazzullo et al., 2009]. Specifically, using a meta‐analysis, Cutler and Follmann [ 1997] found that sodium intake reduction decreases blood pressure in not only hypertensive but also normotensive participants. Another meta‐analysis by Strazzullo et al. [ 2009] reported that participants with high intake of salt (i.e., >5 g/day) showed a higher prevalence of stroke (23%) or cardiovascular disease (17%).
Many campaigns aiming to reduce salt intake have been initiated: for example, Consensus Action on Salt and Health (CASH) in the UK [MacGregor and Sever, 1996] and World Action on Salt and Health (WASH) of global organization [He et al., 2010]. Additionally, some countries have set their own national recommendation on salt intake, and effects from these actions are spreading across countries [He and MacGregor, 2009; He et al., 2010; Mohan et al., 2009]. For example, in the UK the Committee on Medical Aspects of Food and Nutrition Policy (COMA) recommended to reduce salt intake to less than 6 g/day in adults [He and MacGregor, 2009; He et al., 2010]. Furthermore, the World Health Organization (WHO) has set a maximum daily intake of salt for adults to 5 g. Nevertheless, the average amount of salt intake indeed exceeds the recommended daily intakes in children as well as in adults of worldwide population [Brown et al., 2009]. In particular, the daily salt consumption in many European and Asian countries seems to be higher than 12 g [Strazzullo et al., 2009].
A large amount (estimated 75%) of sodium intake comes from processed or restaurant‐prepared foods in European and Northern American countries [He et al., 2010; Mattes and Donnelly, 1991]. Accordingly, food industry has been challenged to reduce the amount of salt added to food products around the world [Desmond, 2006; Doyle and Glass, 2010; He and MacGregor, 2009; He et al., 2010; Mohan et al., 2009]. However, the low‐sodium products seem to decrease acceptance of consumers for the products in many cases [Breslin and Beauchamp, 1997]. Consequently, several strategies have emerged to decrease the sodium content of processed foods without consumer rejection [Desmond, 2006; Doyle and Glass, 2010].
First, many studies have reported that processed foods using alternative salts, such as potassium chloride (KCl), could partially reduce sodium contents with keeping pleasantness of consumers [Katsiari et al., 1998]. However, KCl is not applicable to certain population whose potassium intake is strictly controlled [FSAI Scientific Committee, 2005]. Rather, due to its negative characteristics (e.g., strong bitterness, weak saltiness, and metallic attribute), KCl is not preferred to be used in foods [Desmond, 2006]. Second, umami tasting substances may play a role in reducing salt intake based on the findings that umami taste may magnify saltiness [Mojet et al., 2004]. Indeed, many studies have demonstrated that umami tasting substances such as monosodium glutamate (MSG) and soy sauce could decrease sodium contents in various foods [Kremer et al., 2009; Yamaguchi and Takahashi, 1984]. However, the enhancing effects of MSG and soy sauce on saltiness and palatability seem to be dependent on food matrices [Barylko‐Pikielna and Kostyra, 2007; Kremer et al., 2009]. Rather, in that MSG itself contains sodium, the overall concentration of sodium in the food matrices added MSG should be considered.
Another possible approach to reduce sodium contents is to use a salty‐congruent odor to food products. Indeed, a series of studies has demonstrated that sweet‐congruent odors (e.g., strawberry or vanilla) magnify sweetness [Bingham et al., 1990; Burseg et al., 2010; Clark and Lawless, 1994; de Araujo et al., 2003; Djordjevic et al., 2004; Frank and Byram, 1988; Frank et al., 1989; Frank et al., 1993; Sakai et al., 2001; Schifferstein and Verlegh, 1996; Small et al., 2004]. Specifically, Frank and Byram [ 1988] showed that strawberry flavor (without evoking gustatory sensation), but not peanut butter flavor, increased sweetness in whipped‐cream.
Only a few studies have addressed the idea that salty‐congruent odors (e.g., soy sauce or bacon odor) increase perceived saltiness [Busch et al., 2009; Djordjevic et al., 2004; Lawrence et al., 2009; Lawrence et al., 2011]. For example, Djordjevic et al. [ 2004] found that orthonasally presented soy sauce odor but not strawberry odor could increase the saltiness of a NaCl solution. Interestingly, this saltiness enhancement was also to some extent obtained only by imagining the soy sauce odor by participants. Recently, Lawrence et al. [ 2009] demonstrated that participants were able to estimate saltiness of foods on the basis of their written names. Rather, their estimated saltiness was significantly correlated with the reported sodium content of these foods. In addition, the authors showed that specific salt‐associated odors (e.g., bacon or anchovy odor) administrated by retronasal route (i.e., flavor addition) could amplify saltiness in a low‐concentrated NaCl solution. Lawrence et al. [ 2011] extended the odor‐induced saltiness enhancement to a solid‐food model system (e.g., lipoproteic matrix; similar to mozzarella cheese) containing a low amount of salt. That is, salt‐associated odors such as comté cheese and sardine increased saltiness in the solid‐food system, whereas non‐salt‐associated odor (e.g., carrot) produced no enhanced saltiness.
To build on these findings, this study aimed to investigate whether a salty‐congruent odor can enhance saltiness and/or taste preference on psychophysical (Experiment 1) and neuroanatomical levels (Experiment 2). Indeed, in most studies reporting olfactory and gustatory integration, olfactory stimuli were delivered via the retronasal route; flavors were added into aqueous solution or semi‐solid/solid samples. Little is known about an influence of orthonasal odor on taste intensity and/or preference. Considering the process of eating, the association of orthonasal odor and taste is important. That is, humans look at the food and often try smelling that via an orthonasal route before they experience retronasal odor and taste during the process of mastication and swallowing. Therefore, in Experiment 1, we examined the odor–taste integration with a focus on the initial step of eating. That is, we presented an orthonasal odor before and during gustatory stimulation.
In Experiment 2, we attempted to assess the congruent odor‐induced saltiness enhancement in the neuroanatomical level using functional magnetic resonance imaging (fMRI). Previous brain imaging studies have revealed that odor–taste integration takes place in multiple brain regions including anterior insular, frontal operculum (FO), anterior cingulate cortex (ACC), and orbitofrontal cortex (OFC) [de Araujo et al., 2003; Small et al., 1997; Small et al., 2004; for a review see Small and Prescott, 2005], although the exact areas activated were not completely identical (e.g., insular and OFC) across the studies. Only a few brain imaging studies have addressed an odor–taste integration using salty taste. Even though Small et al. [ 1997] presented either matched or mismatched odors with four aqueous tastes including NaCl solution during their positron emission tomography (PET) scanning, they did not report results specifically associated with the interaction between salty taste and odor.
In addition, we wanted to examine whether neural activations in the brain regions associated with the congruent odor‐induced saltiness are different in the low‐ and high‐concentrated taste solutions. This appeared to be necessary because Djordjevic et al. [ 2004] showed that a congruent odor‐enhanced saltiness existed in the presence of low‐concentrated NaCl solution but not when salt was presented at a high concentration.
The protocol of this study (EK285112008) was approved by the Ethics Committee of the University of Dresden Medical School and was conducted in accordance with the Declaration of Helsinki.
EXPERIMENT 1
Materials and Methods
Participants
A total of 25 right‐handed volunteers (19 females) with an age ranging from 19 to 39 years (mean age ± standard deviation [SD] = 25 ± 4 years) participated in Experiment 1. Handedness was determined using a translated version of the Edinburgh inventory [Oldfield, 1971]. Participants were recruited via leaflet. All participants confirmed that they had no clinical history of major diseases and that they had normal senses of smell and taste. To screen participants for impairments in olfactory, gustatory, or cognitive function, the following tests were used: the ‘Sniffin’ Sticks” screening test [Burghart Instruments, Wedel, Germany; for details see Hummel et al., 2001], the “Taste Strips” test [Burghart Instruments, Wedel, Germany; for details see Landis et al., 2009], and the “Mini‐Mental‐State Examination” [Folstein et al., 1975], respectively. The experiment was explained to all participants in great detail and informed written consent was obtained.
Olfactory and gustatory stimuli
As olfactory stimuli, we used two odors: 1% dilution of bacon odor (#202970, Symrise AG, Holzminden, Germany) and 10% dilution of strawberry odor (#221047, Symrise AG, Germany) in 1,2‐propanediol (Sigma‐Aldrich Chemie GmbH, Munich, Germany). Additionally, odorless air was used as control condition. All olfactory stimuli were delivered using a computer‐controlled air‐dilution olfactometer (OM6b, Burghart, Wedel, Germany). To minimize mechanical stimulation, the olfactory stimuli (10%, v/v) diluted by humidified air were embedded in a constantly flowing air stream (7.0 L/min) with controlled temperature (36°C) and humidity (80% relative humidity [RH]). The intensity of two odors was matched. The odors were provided for 3 s via a tube placed in the right nostril of the participants. This method was based on previous studies determining that olfactory performance is better (e.g., improved odor sensitivity and discrimination) when the odors are presented to the right than to the left nostril [Kobal et al., 2000; Zatorre and Jones‐Gotman, 1990].
As gustatory stimuli, we used two aqueous taste solutions: NaCl solution and sucrose solution. Both taste solutions consisted of low (0.16 M) and high (0.64 M) concentration, respectively and these concentrations were established based on earlier studies [Cerf‐Ducastel and Murphy, 2004; Singh et al., 2011; Spetter et al., 2010]. Gustatory stimuli were delivered using a computer‐controlled gustometer (GU001, Burghart, Wedel, Germany). They were embedded in repetitively pulsed water stream (11.8 mL/min) with controlled temperature (36°C). The gustatory stimuli were presented via a tube placed 3 cm in front of the participant's tongue.
Procedure
To examine an influence of congruent odor on saltiness and sweetness, respectively, this study consisted of two sessions for either saltiness or sweetness. Sessions were conducted on different days, with a maximum of 7 days in between. The order of two sessions was randomized across participants.
During each session six combinations between olfactory and gustatory stimuli were tried; that is, three odor conditions (congruent or incongruent odor and odorless air) by two concentrations (low or high) of tastant. Each combination of stimuli was repeated 12 times during the session.
Participants were seated on a chair 1 m from the computer monitor, with their heads and necks supported by a headrest. The delivery tube of the olfactometer was inserted into the right nostril of participants. Next, participants were asked to stick out their tongue and then the delivery tube of gustometer was established 3 cm in front of tongue. During the experiment, the gustatory stimuli and rinsing water were presented on the anterior part of tongue with the mouth opened. Because participants could not swallow the presented stimuli under this experimental condition, the presented stimuli on the tongue were subsequently dropped down to the funnel placed below the chin of participants.
Participants received one of three olfactory stimuli for 3 s. Subsequently, 2.75 s after the onset of olfactory presentation, one of two tastant concentrations (i.e., low or high) was provided for 0.25 s. Following stimulus presentation, participants were asked to immediately rate taste intensity on a visual analogue scale (VAS) ranging from 0 (extremely weak) to 10 (extremely strong). They were also asked to rate taste pleasantness on a VAS ranging from (5 (extremely unpleasant) to +5 (extremely pleasant). Instructions and scales were presented on the monitor. To minimize the olfactory and gustatory desensitization, 24–28 s were allowed before the next stimulus. Meanwhile, an odorless humidified air stream (7.0 L/min, 36(C, 80% RH) and continuously pulsed water stream (11.8 mL/min, 36°C) were presented to minimize residual effects of previous olfactory and gustatory stimuli. In addition, white noise (∼60 dB) was presented via headphones to dampen environmental sounds (e.g., the stimulus‐related valve‐switching sound of the olfactometer and gustometer).
Data Analysis
Statistical software, SPSS 16.0 (SPSS Inc., USA) for Windows, was used to analyze the data. Descriptive analyses were used wherever appropriate. To determine whether odor congruency and/or tastant concentration can influence ratings of taste intensity or pleasantness, data were analyzed by using two‐way repeated measures analyses of variance (RM‐ANOVAs). If the sphericity assumption was violated via the Mauchly's sphericity test, degrees of freedom were adjusted using the “Huynh–Feldt” correction. If a significant difference of means was indicted by RM‐ANOVAs, post hoc comparisons between independent variables were conducted using Bonferroni t‐tests. The alpha level was 0.05.
Results
Effects of congruent odor on intensity ratings of taste solution
Two‐way (“tastant concentration” and “odor congruency”) RM‐ANOVAs revealed that the concentration of taste solution significantly influenced intensity ratings of either saltiness [F(1,24) = 89.42, P < 0.001] or sweetness [F(1,24) = 46.76, P < 0.001]. As expected, participants rated the high‐concentrated tastant as more intense than low‐concentrated one, indicating that participants apparently discriminated the taste intensity in both salty and sweet taste solutions.
The odor congruency significantly modulated intensity ratings of either saltiness [F(1.60,38.46) = 16.98, P < 0.001; with Huynh‐Feldt correction] or sweetness [F(2,48) = 6.87, P < 0.01]. Specifically, post hoc Bonferroni t‐tests showed that participants rated the NaCl solution as significantly more salty in the presence of a congruent odor (i.e., bacon odor; mean (SD = 6.7 ± 1.5) than in the presence of an incongruent odor (i.e., strawberry odor; 6.2 ± 1.5), P < 0.05, or odorless air (5.9 ± 1.6), P < 0.001. In addition, participants judged the sucrose solution as significantly sweeter in the presence of a congruent odor (6.3 ± 1.5) than in the presence of odorless air (5.7 ± 1.7), P < 0.001, but not than in the presence of an incongruent odor (6.0 ± 1.6), P = 0.37.
There was a significant interaction between tastant concentration and odor congruency on the intensity ratings of either saltiness [F(2,48) = 5.51, P < 0.01] or sweetness [F(2,48) = 5.08, P = 0.01]. As shown in Figure 1, a congruent odor‐induced saltiness enhancement appeared to be more pronounced in the low‐concentrated taste solution than in the high‐concentrated solution. Specifically, in the low‐concentrated NaCl solution, a salty‐congruent odor enhanced saltiness significantly more than either a salty‐incongruent odor [P < 0.05] or odorless air [P < 0.001]. However, in the high‐concentrated solution, the congruent odor increased saltiness significantly more than an odorless air [P < 0.01] but not than an incongruent odor [P = 0.07]. Rather, the pronounced effect of congruent odor on taste intensity was also observed for the sucrose solution. That is, while a congruent odor magnified sweetness of low‐concentrated sucrose solution than odorless air [P < 0.001], there was no significant influence of odor congruency on intensity ratings in the high‐concentrated sucrose solution [P = 0.10], as seen in Figure 1b.
Figure 1.
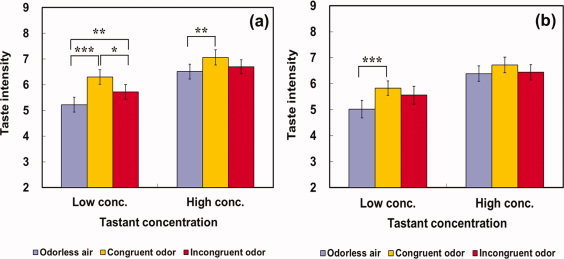
Modulatory effect of odor stimuli on taste intensity in relation to tastant concentration (low and high). After receiving one of three odor stimuli together with one of two concentrations in the NaCl (a) or sucrose (b) solution, participants rated taste intensity. *, **, and *** indicate a significance at P < 0.05, P < 0.01, and P < 0.001, respectively. Error bars indicate standard error of the mean. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Effects of congruent odor on pleasantness ratings of taste solution
Two‐way (“tastant concentration” and “odor congruency”) RM‐ANOVAs revealed that the concentration of taste solution significantly influenced pleasantness ratings of either saltiness [F(1,24) = 14.89, P = 0.001] or sweetness [F(1,24) = 4.53, P < 0.05]. Specifically, while participants preferred low‐concentrated NaCl solution to high‐concentrated one, they liked high‐concentrated sucrose solution significantly more than low‐concentrated one.
Odor congruency significantly influenced pleasantness ratings for either saltiness [F(2,48) = 43.50, P < 0.001] or sweetness [F(2,48) = 55.51, P < 0.001] of taste solutions. Specifically, post hoc Bonferroni t‐tests found that participants rated the NaCl solution as significantly more unpleasant when they were presented with a congruent odor (mean (SD = 2.6 ± 1.3) than when presented with either an incongruent odor (4.1 ± 1.7), P < 0.05, or odorless air (3.8 ± 1.5), P < 0.001. In contrast, participants judged the sucrose solution as more pleasant in the presence of a congruent odor (6.1 ± 1.5) than in the presence of either an incongruent odor (4.0 ± 1.8), P < 0.001, or odorless air (5.7 ± 1.5), P = 0.01.
There was a significant interaction between tastant concentration and odor congruency on the pleasantness ratings of either saltiness [F(2,48) = 5.86, P < 0.01] or sweetness [F(2,48) = 8.11, P = 0.001]. As shown in Figure 2a, a congruent odor increased taste unpleasantness significantly more than either an incongruent odor [P < 0.001] or odorless air [P < 0.001] in both low‐ and high‐concentrated NaCl solutions. Furthermore, while an incongruent odor (i.e., strawberry odor) produced significantly higher pleasantness than odorless air in the presence of high‐concentrated NaCl solution, no significant difference between them was observed in the presence of low‐concentrated NaCl solution. In addition, in the high‐concentrated sucrose solution a congruent odor amplified taste pleasantness than either an incongruent odor [P < 0.001] or odorless air [P = 0.01]. However, in the low‐concentrated sucrose solution, while a congruent odor increased taste pleasantness significantly more than an incongruent odor [P < 0.001] but not than odorless air [P = 0.07].
Figure 2.
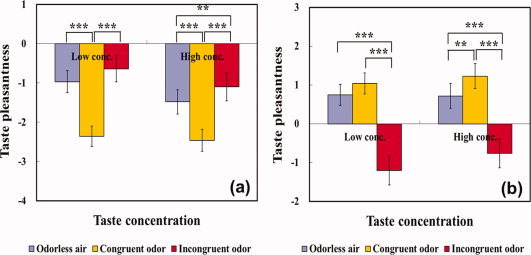
Modulatory effect of odor stimuli on taste pleasantness in relation to tastant concentration (low and high). After receiving one of three odor stimuli together with one of two concentrations in the NaCl (a) or sucrose (b) solution, participants rated taste pleasantness. ** and *** indicate a significance at P < 0.01 and P < 0.001, respectively. Error bars indicate standard error of the mean. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Taken together, Experiment 1 demonstrated that a congruent odor significantly increases intensity ratings of either saltiness or sweetness than an incongruent odor and/or odorless air. In addition, a congruent odor significantly enhanced either unpleasantness of saltiness or pleasantness of sweetness. Furthermore, the modulatory effects of congruent odors on taste intensity and pleasantness are dependent on tastant concentration presented.
EXPERIMENT 2
In Experiment 2, we attempted to investigate the congruent odor‐induced saltiness enhancement at a neuroanatomical level using fMRI. Based on earlier findings that odor–taste integration takes place in the brain areas of anterior insular, FO, ACC, and OFC [de Araujo et al., 2003; Small et al., 1997; Small et al., 2004], we hypothesized that the salty‐congruent odor yielded significantly higher brain activations in these brain areas than an incongruent odor and/or odorless air.
Materials and Methods
Participants
Another 25 right‐handed volunteers (16 females, mean age ± SD = 23 ± 2 years) participated in Experiment 2. Handedness was determined using a translated version of the Edinburgh inventory [Oldfield, 1971]. All participants confirmed that they had no clinical history of major diseases and that they had normal senses of smell and taste. Participants underwent screening tests for olfactory, gustatory, and cognitive function similar to those conducted in Experiment 1. In addition, participants who had difficulty in swallowing aqueous solutions when laid on the bed (i.e., similar to fMRI condition) were excluded. The experiment was explained to all participants in great detail and informed written consent was obtained.
Olfactory and gustatory stimuli
There were three olfactory stimuli: odorless air, salty‐congruent odor (i.e., bacon odor), and salty‐incongruent odor (i.e., strawberry odor). All olfactory stimuli were delivered using a computer‐controlled air dilution olfactometer to right nostril of participant according to the identical procedure as in Experiment 1.
In addition, salty taste (NaCl) solutions with two different concentrations: low (0.16 M; STlow) and high (0.64 M; SThigh) were used as gustatory stimuli. The gustatory stimuli were presented to participants' mouth via dedicated Teflon tubing fed through a small outlet of the wall in the scanner room according to the previous study [Hummel et al., 2007]. Three separate tubes delivered low‐ and high‐concentrated NaCl solutions and rinsing water. The outer and inner diameters of tubes were 3 and 2 mm, respectively.
Experimental Design
The fMRI paradigm was built in a six‐session block design. In each session six stimuli conditions (three odor conditions by two tastant concentrations) were randomized. Accordingly, each stimulus condition was repeated six times through six sessions. Each session consists of six ON‐blocks (8 scans for 20 s) and OFF‐blocks (8 scans for 20 s). During every ON‐block, participants received one of three olfactory stimuli (stimulus duration: 1 s; interval between stimuli: 3 s) five times together with one of two NaCl solutions (0.1 mL). During every OFF‐block, participants received odorless humidified air (7.0 L/min, 36(C, 80% RH) with tasteless rinsing water (2 mL) to minimize a residual effect of previous stimuli. In addition, they received no additional information during the OFF‐blocks.
After each session, participants randomly received one of six stimuli combination. Subsequently, they were asked to rate saltiness intensity (0 = extremely weak; 10 = extremely strong) and taste pleasantness (−5 = extremely unpleasant; +5 = extremely pleasant).
fMRI Data Acquisition and Analysis
For fMRI data (both functional and anatomical imaging) acquisition, a 1.5‐T MR‐scanner (Sonata; Siemens, Erlangen, Germany) was used. The functional images (96 volumes per session) were acquired by means of a 26 axial‐slice matrix 2D Spin Echo (SE)/Echo Planar (EP) sequence with echo time = 40 ms, repetition time = 2500 ms, flip angle = 90°, matrix = 64 × 64, and voxel size = 3 × 3 × 3.75 mm3. After the experimental session high‐resolution (1 × 1 × 1 mm3) T 1‐weighted anatomical images were obtained using a “magnet prepared rapid gradient echo” sequence.
The fMRI data analyses were done using Statistical Parametric Mapping 8 (SPM 8, http://www.fil.ion.ucl.ac.uk/spm/) implemented in MATLAB R2007b (The Mathworks Inc., USA) and the WFU Pickatlas tool version 2.4 [Maldjian et al., 2003]. For functional images, a series of spatial preprocessing including registration, realignment, coregistration between functional and anatomical images, normalization, and smoothing (8 × 8 × 8 mm3 FWHM Gaussian kernel) was performed. Coordinates of the activation are presented according to the Montreal Neurological Institute (MNI) coordinates [Evans et al., 1993]. The brain responses to rinsing and swallowing (i.e., OFF‐blocks) were not included in data analyses. To compare brain activations between stimulus conditions, group analyses using the t‐test were performed based on cluster size of three voxels and P < 0.005 (uncorrected).
In addition, behavioral data (i.e., ratings of saltiness intensity and pleasantness) were analyzed by using RM‐ANOVAs. Because of technical and behavioral problems, 15 participants' behavioral data were analyzed. If a significant difference of mean ratings was indicated by RM‐ANOVAs, post hoc comparisons between independent variables were conducted using Bonferroni t‐tests. The alpha level was 0.05. Statistical analyses of the behavioral data were done using SPSS 16.0 for Windows.
Results
Behavioral data
Two‐way (“odor congruency” and “tastant concentration”) RM‐ANOVAs revealed a significant interaction between odor congruency and tastant concentration in terms of saltiness intensity [F(2,28) = 3.62, P = 0.04] but not pleasantness [P = 0.78]. Specifically, the significant effect of odor condition on taste intensity was obtained in the presence of low‐concentrated NaCl solution [F(2,28) = 4.81, P = 0.02] but not in that of high‐concentrated one [F(2,28) = 1.82, P = 0.18]. That is, post hoc Bonferroni t‐tests showed that participants rated the low‐concentrated NaCl solution as less salty when they were presented with incongruent odor (mean ±SD = 2.87 ± 2.92) than when presented with odorless air (5.37 ± 2.72) [P = 0.02]. As seen in the Supporting Information Figure 1a, the intensity rating of low‐concentrated NaCl solution was not different between in the congruent (4.33 ± 3.06) and incongruent odor (2.87 ± 2.92) conditions [P > 0.05]. There was no significant main effect of odor congruency and tastant concentration on the ratings of either saltiness intensity or pleasantness [P > 0.05] (Supporting Information Fig. 1).
Neuroimaging data
Congruent odor‐induced brain activation in comparison with odorless air
Compared with a combination of odorless air and salty taste (ST), a salty‐congruent combination of odor and taste significantly increased neural activations in multiple brain regions [(congruent odor + STlow + high) − (odorless air + STlow + high)]. As shown in Table I, the salty‐congruent odor induced significantly greater activations in right inferior FO, right inferior frontal gyri, right middle frontal gyrus, and right ACC than odorless air.
Table I.
Brain regions where salty‐congruent combination of odor and taste induced significantly higher neural activations than odorless air combination
| Brain region | Voxel size | x | y | z | T value | Z score | P value |
|---|---|---|---|---|---|---|---|
| (Congruent odor + STlow+high) − (odorless air + STlow+high) | |||||||
| Inferior FO | 7 | 48 | 14 | 4 | 3.09 | 3.04 | 0.001 |
| Anterior cingulate cortex | 8 | 6 | 41 | 4 | 2.91 | 2.87 | 0.002 |
| Middle frontal gyrus | 9 | 27 | 53 | 13 | 2.92 | 2.88 | 0.002 |
| Inferior frontal gyrus | 28 | 36 | 35 | 16 | 3.30 | 3.23 | 0.001 |
| 48 | 35 | 16 | 2.89 | 2.85 | 0.002 | ||
| Extranuclear | 13 | −24 | −25 | −2 | 3.27 | 3.20 | 0.001 |
| (Congruent odor + STlow) − (odorless air + STlow) | |||||||
| Rolandic operculum | 18 | 60 | −4 | 13 | 3.29 | 3.23 | 0.001 |
| Anterior cingulate cortex | 3 | 6 | 44 | 4 | 2.76 | 2.72 | 0.003 |
| Insula | 12 | 27 | 14 | −20 | 3.16 | 3.11 | 0.001 |
| Superior frontal gyrus | 6 | 24 | 50 | 13 | 2.92 | 2.87 | 0.002 |
| Middle frontal gyrus | 4 | 27 | 47 | −5 | 2.89 | 2.85 | 0.002 |
| Superior temporal gyrus | 3 | 45 | −13 | −8 | 2.79 | 2.75 | 0.003 |
| Inferior occipital gyrus | 5 | 30 | −88 | −14 | 3.01 | 2.96 | 0.002 |
| Angular gyrus | 8 | 57 | −55 | 25 | 3.07 | 3.02 | 0.001 |
| (Congruent odor + SThigh) − (odorless air + SThigh) | |||||||
| Inferior frontal gyrus | 26 | 36 | 35 | 16 | 3.55 | 3.47 | 0.000 |
| 48 | 32 | 16 | 2.85 | 2.81 | 0.002 | ||
| Medial frontal gyrus | 7 | 9 | 59 | −5 | 2.88 | 2.83 | 0.002 |
| Medial frontal gyrus | 5 | 9 | 59 | 13 | 2.73 | 2.69 | 0.004 |
| Extranuclear | 8 | −24 | −28 | −5 | 3.23 | 3.16 | 0.001 |
STlow and SThigh: low and high concentrations of salty taste solution, respectively. x, y, z indicates MNI coordinates. All reported activations were significant at P uncorrected < 0.005 (≥3 voxels).
The brain regions activated by congruent combinations of odor and taste was different depending on tastant concentration. As seen in Table I, when presented with low‐concentrated NaCl solution, a salty‐congruent odor produced significantly higher activation in multiple brain regions associated with olfactory and/or gustatory processing (e.g., right anterior ventral insula, right rolandic operculum, right ACC, and right parietal OFC) than odorless air [(congruent odor + STlow) − (odorless air + STlow)] (Figure 3). When presented with high‐concentrated NaCl solution, the congruent odor significantly increased brain activations in the right medial OFC, medial frontal gyrus, and inferior frontal gyri than the odorless air (Table I).
Figure 3.
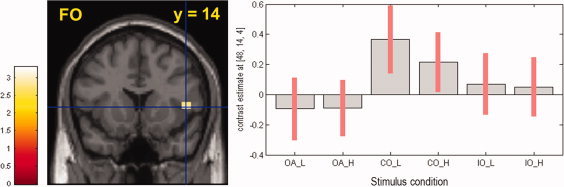
Brain regions activated by t‐contrast [(congruent odor + salty taste) − (odorless air + salty taste)]. In comparison with odorless air combination, a salty‐congruent combination of odor and taste activated inferior FO area (MNI coordinates: x = +48, y = +14, z = +4). Right‐sided figure presents contrast estimates of all stimuli conditions: OA = odorless air, CO = congruent odor, IO = incongruent odor, L and H = low and high concentrations, respectively. Reported activation was significant at P uncorrected < 0.005 (≥3 voxels). For details, see Table I. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In a reverse contrast condition [(odorless air + STlow + high) − (congruent odor + STlow + high)], in comparison with the salty‐congruent combination of odor and taste, the odorless air with salty taste produced significantly higher brain activations in the right posterior cingulum [MNI coordinates x, y, z = +3, −43, +13, Z score = 3.46, P uncorrected < 0.001] and left thalamus [MNI −9, −13, +7, Z score = 3.22, P uncorrected = 0.001; MNI −9, −19, +13, Z score = 2.99, P uncorrected < 0.001].
Incongruent odor‐induced brain activation in comparison with odorless air
As opposed to a salty‐congruent odor, an incongruent odor (i.e., strawberry odor) did not show significantly brain activations in the presence of NaCl solution than odorless air [(incongruent odor + STlow + high) − (odorless air + STlow + high)].
Congruent odor‐induced brain activation in comparison with incongruent odor
As seen in Table II, when compared with an incongruent combination, a salty‐congruent combination of odor and taste significantly enhanced brain activations in multiple brain regions [(congruent odor + STlow + high) − (incongruent odor + STlow + high)]. Specifically, in the presence of NaCl solutions, the salty‐congruent odor produced significantly higher neural activations in the brain areas related to olfactory and/or gustatory processing (e.g., bilateral anterior ventral insular, right inferior FO, right inferior parietal lobule, right piriform cortex, left caudomedial OFC, and ACC) than the incongruent odor.
Table II.
Brain regions where salty‐congruent combination of odor and taste induced significantly higher neural activations than incongruent combination
| Brain region | Voxel size | x | Y | z | T value | Z score | P value |
|---|---|---|---|---|---|---|---|
| (Congruent odor + STlow+high) − (incongruent odor + STlow+high) | |||||||
| Inferior FO | 4 | 63 | 11 | 19 | 3.12 | 3.07 | 0.001 |
| Anterior cingulate cortex | 8 | 9 | 41 | 4 | 2.92 | 2.87 | 0.002 |
| Middle cingulate cortex | 9 | 9 | 2 | 34 | 2.96 | 2.91 | 0.002 |
| 12 | 2 | 25 | 2.73 | 2.69 | 0.004 | ||
| Insula/anterior | 8 | 45 | 17 | −2 | 3.19 | 3.13 | 0.001 |
| Insula/anterior | 3 | −36 | 14 | −8 | 2.74 | 2.70 | 0.003 |
| Insula/posterior | 82 | 33 | −40 | 19 | 4.44 | 4.29 | 0.000 |
| Inferior parietal lobule | 51 | −34 | 22 | 3.59 | 3.51 | 0.000 | |
| Piriform cortex | 22 | 27 | 11 | −14 | 3.19 | 3.13 | 0.001 |
| Putamen | 3 | 27 | 5 | 13 | 2.73 | 2.69 | 0.004 |
| Inferior parietal OFC | 12 | −42 | 35 | −14 | 3.40 | 3.32 | 0.000 |
| Caudomedial OFC | 5 | −18 | 32 | −17 | 3.16 | 3.10 | 0.001 |
| Superior frontal gyrus | 113 | 21 | 47 | 19 | 3.47 | 3.40 | 0.000 |
| 24 | 59 | 22 | 3.20 | 3.14 | 0.001 | ||
| 30 | 47 | 28 | 3.11 | 3.06 | 0.001 | ||
| Superior frontal gyrus | 13 | 9 | 59 | 25 | 3.10 | 3.04 | 0.001 |
| Middle frontal gyrus | 3 | −27 | 50 | 25 | 2.91 | 2.86 | 0.002 |
| Middle frontal gyrus | 3 | 45 | 29 | 34 | 2.92 | 2.87 | 0.002 |
| Inferior frontal gyrus | 25 | −51 | 26 | 10 | 3.44 | 3.36 | 0.000 |
| Superior temporal gyrus | 6 | −45 | 2 | −14 | 3.12 | 3.07 | 0.001 |
| Superior temporal gyrus | 6 | −60 | −64 | 16 | 3.20 | 3.14 | 0.001 |
| Middle temporal gyrus | 5 | 54 | −25 | −8 | 2.96 | 2.91 | 0.002 |
| Postcentral gyrus | 3 | −66 | −25 | 19 | 2.90 | 2.85 | 0.002 |
| Vermis | 4 | 0 | −49 | 1 | 2.87 | 2.82 | 0.002 |
| Extranuclear | 28 | 6 | 20 | 16 | 3.83 | 3.73 | 0.000 |
| −3 | 23 | 13 | 2.74 | 2.70 | 0.003 | ||
| 6 | 8 | 22 | 2.67 | 2.63 | 0.004 | ||
STlow and SThigh: low and high concentrations of salty taste solution, respectively. x, y, z indicates MNI coordinates. OFC, orbitofrontal cortex. All reported activations were significant at P uncorrected < 0.005 (≥3 voxels).
In addition, the contrast effect between congruent and incongruent combinations of odor and taste was different in relation to tastant concentrations. Specifically, the congruent odor‐induced brain activation was more pronounced in the presence of low‐concentrated NaCl solution than in that of high‐concentrated NaCl solution (Table III and Fig. 4]. For example, when compared with an incongruent odor, the salty‐congruent odor produced significantly higher activations in multiple brain regions (e.g., bilateral anterior ventral insular, right inferior FO, right rolandic operculum, right ACC, superior frontal gyrus, angular gyrus, and claustrum) when presented with low‐concentrated NaCl solution but not with high‐concentrated NaCl solution. In contrast, such brain areas of left hippocampus, left caudoparietal OFC, left posterior insula, and right amygdala were significantly more activated only when presented with congruent combination of odor and high‐concentrated NaCl solution (Table III). No significant difference in neural activation was observed in the brain areas resulted from subtraction of congruent combination from incongruent combination of odor and taste [(incongruent odor + STlow + high) − (congruent odor + STlow + high )].
Table III.
Brain regions where salty‐congruent odor relative to incongruent odor induced significantly higher neural activations in relation to tastant concentration
| Brain region | Voxel size | x | y | z | T value | Z score | P value |
|---|---|---|---|---|---|---|---|
| (Congruent odor + STlow) − (incongruent odor + STlow) | |||||||
| Inferior FO | 3 | 60 | 11 | 19 | 3.06 | 3.01 | 0.001 |
| Anterior cingulate cortex | 8 | 6 | 20 | 16 | 2.84 | 2.80 | 0.003 |
| 6 | 29 | 16 | 2.74 | 2.70 | 0.003 | ||
| Cingulate gyrus | 12 | −12 | −10 | 31 | 3.59 | 3.51 | 0.000 |
| Insula/anterior | 8 | 42 | 11 | −5 | 2.93 | 2.88 | 0.002 |
| Insula/anterior | 7 | −39 | 11 | −8 | 2.82 | 2.77 | 0.003 |
| Insula/middle | 4 | −39 | −22 | 19 | 2.95 | 2,90 | 0.002 |
| Superior frontal gyrus | 12 | 21 | 41 | 22 | 3.07 | 3.01 | 0.001 |
| Medial frontal gyrus | 10 | 18 | 50 | 7 | 2.98 | 2.93 | 0.002 |
| Middle frontal gyrus | 3 | 39 | 41 | 13 | 3.17 | 3.11 | 0.001 |
| Middle frontal gyrus | 3 | −30 | 59 | 7 | 2.83 | 2.79 | 0.003 |
| Inferior frontal gyrus | 4 | −51 | 26 | 10 | 2.98 | 2.93 | 0.002 |
| Precentral gyrus | 6 | 39 | −13 | 28 | 2.92 | 2.88 | 0.002 |
| Superior temporal gyrus | 98 | 36 | −40 | 16 | 4.35 | 4.21 | 0.000 |
| 57 | −40 | 10 | 3.32 | 3.26 | 0.001 | ||
| Rolandic operculum | 51 | −31 | 22 | 3.29 | 3.23 | 0.001 | |
| Superior temporal gyrus | 8 | −60 | −64 | 16 | 3.53 | 3.45 | 0.000 |
| Superior temporal gyrus | 18 | −42 | 2 | −14 | 3.26 | 3.20 | 0.001 |
| Superior temporal gyrus | 13 | 42 | 2 | −29 | 3.26 | 3.19 | 0.001 |
| 36 | 2 | −20 | 2.97 | 2.92 | 0.002 | ||
| Superior temporal gyrus | 8 | 54 | −25 | −5 | 3.09 | 3.03 | 0.001 |
| Superior temporal gyrus | 4 | 48 | −10 | −5 | 2.72 | 2.68 | 0.004 |
| Middle temporal gyrus | 15 | 48 | −61 | 4 | 3.49 | 3.41 | 0.000 |
| Middle temporal gyrus | 25 | 48 | −70 | 19 | 3.07 | 3.02 | 0.001 |
| 54 | −76 | 10 | 2.98 | 2.93 | 0.002 | ||
| 57 | −70 | 19 | 2.94 | 2.89 | 0.002 | ||
| Angular gyrus | 28 | 57 | −55 | 25 | 3.38 | 3.31 | 0.000 |
| Claustrum | 30 | 30 | −1 | 16 | 3.24 | 3.18 | 0.001 |
| (Congruent odor + SThigh) − (incongruent odor + SThigh) | |||||||
| Insula/posterior | 3 | −33 | −34 | 19 | 2.90 | 2.86 | 0.002 |
| Cingulate gyrus | 21 | 12 | −1 | 31 | 3.61 | 3.53 | 0.000 |
| Hippocampus | 7 | −24 | −25 | −8 | 3.18 | 3.12 | 0.001 |
| Amygdala | 4 | 21 | 5 | −17 | 2.69 | 2.65 | 0.004 |
| Caudoparietal OFC | 4 | −45 | 35 | −17 | 2.81 | 2.76 | 0.003 |
| Middle frontal gyrus | 6 | 27 | 32 | 31 | 2.83 | 2.79 | 0.003 |
| Inferior frontal gyrus | 3 | 45 | 26 | 13 | 2.71 | 2.67 | 0.004 |
| Superior temporal gyrus | 12 | 57 | −28 | 7 | 3.48 | 3.41 | 0.000 |
| Inferior temporal gyrus | 3 | 57 | −22 | −20 | 2.95 | 2.90 | 0.002 |
| Extranuclear | 3 | −18 | −13 | 13 | 2.78 | 2.74 | 0.003 |
STlow and SThigh: low and high concentrations of salty taste solution, respectively. x, y, z indicates MNI coordinates. OFC: orbitofrotnal cortex. All reported activations were significant at P uncorrected < 0.005 (≥3 voxels).
Figure 4.
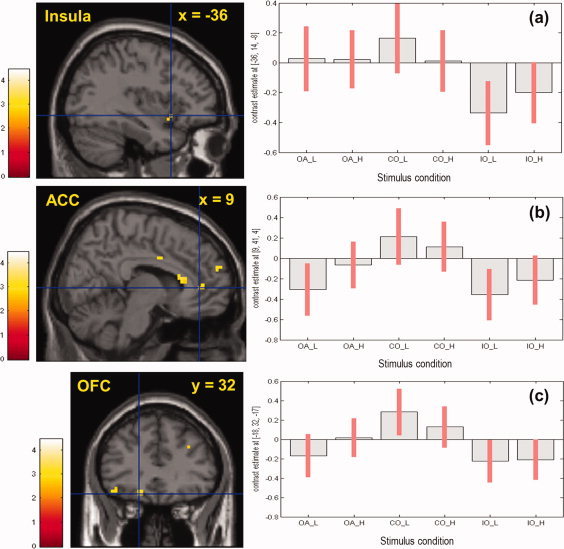
Brain regions activated by t‐contrast [(congruent odor + salty taste) − (incongruent odor + salty taste)]. In comparison with incongruent combination, a salty‐congruent combination of odor and taste activated anterior insula ((a), MNI coordinates: x = −36, y = +14, z = −8), ACC ((b) MNI +9, +44, +4), and caudomedial OFC ((c) MNI −18, −32, −17). Right‐sided figures present contrast estimates of all stimuli conditions: OA = odorless air, CO = congruent odor, IO = incongruent odor, L and H = low and high concentrations, respectively. Reported activation was significant at P uncorrected < 0.005 (≥3 voxels). For details, see Tables II and III. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
It is well documented that an excessive intake of dietary salt increases the risk of chronic diseases. Accordingly, food industries have been attempting various strategies to decrease sodium contents in the processed food products [for a review, see Doyle and Glass, 2010]. This study aimed to investigate whether a salty‐congruent odor can enhance saltiness and/or taste pleasantness on the basis of psychophysical and neuroanatomical results. The main findings of this study were as follows.
-
1
Compared with incongruent odors and/or odorless air, congruent odors increased intensity ratings of salty or sweet taste solution; however, the congruent odor‐induced saltiness enhancement was not obtained in the behavioral data of Experiment 2.
-
2
Compared with an incongruent odor and/or odorless air, a salty‐congruent odor produced significantly higher neural activations in multiple brain regions associated with odor–taste integration (e.g., insular, FO, ACC, and OFC); however, the neuroanatomical results was not correlated with the behavioral data of Experiment 2.
-
3
The congruent odor‐induced taste enhancement appears to be more pronounced in the low‐concentrated taste solution than in the high‐concentrated one.
-
4
Compared with an incongruent odor and/or odorless air, a congruent odor increased either unpleasantness of saltiness or pleasantness of sweetness in the aqueous solution.
This study supports the previous notion that congruent odors could amplify taste intensity [Bingham et al., 1990; Burseg et al., 2010; Busch et al., 2009; Clark and Lawless, 1994; de Araujo et al., 2003; Djordjevic et al., 2004; Frank and Byram, 1988; Frank et al., 1989; Frank et al., 1993; Lawrence et al., 2009; Lawrence et al., 2011; Sakai et al., 2001; Schifferstein and Verlegh, 1996] based on psychophysical (Experiment 1) and neuroimaging results (Experiment 2). In particular, our study adds new evidence to a growing list of odor–taste integration. That is, a salty‐congruent odor (i.e., bacon odor), but not an incongruent odor (i.e., strawberry odor), significantly magnified perceived saltiness, which is in accordance with the earlier studies [Busch et al., 2009; Djordjevic et al., 2004; Lawrence et al., 2009; Lawrence et al., 2011].
One might argue that the congruent odor‐induced saltiness enhancement appeared not to be consistent due to a lack of significance in the behavioral result of Experiment 2. One plausible explanation for this discrepancy between Experiments 1 and 2 relates to differences in the experimental design. That is, although odor and taste stimuli used in Experiments 1 and 2 were identical, the experimental set‐up to present these stimuli was slightly different. In particular, in Experiment 1, participants rated taste intensity and pleasantness immediately after receiving taste stimuli; whereas in Experiment 2, they estimated these ratings ∼20 s after the onset of taste stimulation. The delayed judgment condition (e.g., 20 s after the onset of taste stimulation) might lead participants to be in more ambiguous when they performed intensity and pleasantness ratings of taste stimulus. In contrast, fMRI measures the hemodynamic response that follows about 6 s after the onset of neuronal response [Bandettini et al., 1993; Liao et al., 2001], which might explain the lack of significance in the association between the behavioral and neuroanatomical results in Experiment 2. In addition, it should be noticed that the behavioral data in Experiment 2 was separately obtained after completion of each brain scanning session.
Another explanation for the discrepancy of behavioral results between Experiments 1 and 2 is the absence of replication of ratings. Specifically, while the psychophysical ratings were repeated 12 times during the session in Experiment 1, no replicated ratings of taste intensity and pleasantness was acquired in Experiment 2. This might increase a possibility to produce unstable behavioral data in Experiment 2. For example, in contrast to Experiment 1, the behavioral data in Experiment 2 demonstrated that participants could not discriminate the saltiness intensity of low‐ and high‐concentrated NaCl solutions in the presence of odorless air.
The congruent odor‐induced saltiness seems to take place at a central level of processing [Djordjevic et al., 2004]. Because olfactory stimuli were administered via an orthonasal route (i.e., nose), the possibility of odor–taste interaction in the mouth was very low. That is, the saltiness enhancement by congruent odor seems not to be obtained at a peripheral level but at a central level of processing. As addressed in the Introduction section, participants can estimate the saltiness of foods by the names of the foods [Lawrence et al., 2009]. Furthermore, participants perceived increased saltiness of weak sodium chloride when they imagined a salty odor such as soy sauce [Djordjevic et al., 2004]. The neuroimaging result of our study supported the central processing of odor–taste integration. That is, when compared with an incongruent combination of odor and taste, a salty‐congruent combination yielded significantly higher neural activations in the brain areas associated with higher‐order olfactory and/or gustatory processing: for example, anterior insula and OFC [Gottfried, 2006; Small and Prescott, 2005; Small, 2006].
To date, a series of neuroimaging studies investigating odor and/or taste processing have revealed that both stimuli converge on specific brain regions: insula [Cerf‐Ducastel and Murphy, 2001; de Araujo et al., 2003; Hummel et al., 2007; O'Doherty et al., 2001; Rolls et al., 2003; Savic et al., 2000; Small et al., 1999; Spetter et al., 2010; Veldhuizen et al., 2010], operculum [Cerf‐Ducastel and Murphy, 2001; Cerf‐Ducastel and Murphy, 2001; de Araujo et al., 2003; de Araujo et al., 2003; Hummel et al., 2007; Hummel et al., 2007; O'Doherty et al., 2001; O'Doherty et al., 2001; Rolls et al., 2003; Savic et al., 2000; Savic et al., 2000; Small et al., 1999; Small et al., 1999; Veldhuizen et al., 2010; Veldhuizen et al., 2010], OFC [Sobel et al., 1998], and ACC [de Araujo et al., 2003; O'Doherty et al., 2001; Rolls et al., 2003; Savic et al., 2000; Veldhuizen et al., 2010]. A fMRI study by Small et al. [ 2004] showed that a congruent mixture of sweet taste and retronasal vanilla odor induced higher neural activation in multiple brain regions including anterior dorsal insula, anterior ventral insula/caudal OFC, FO, ACC, and posterior parietal cortex, compared with an incongruent mixture of salty taste and retronasal vanilla odor. Another fMRI study by de Araujo et al. [ 2003] found that subjective ratings of consonance between tastes and retronasal odors were significantly correlated with activations of medial anterior part of OFC. In their review, Small and Prescott [ 2005] argued that insula, operculum, OFC, and ACC are suggestive areas responding to multimodal integration, as well as unimodal olfactory and gustatory stimuli. In addition, de Araujo et al. [ 2003] suggested that agranular part of insula and adjoining the caudal OFC are potential areas activated by both unimodal gustatory and orthonasal/retronasal olfactory stimuli. Our neuroimaging results support the previous studies. That is, the salty‐congruent combination of odor and taste activated brain areas such as anterior insula, FO, caudomedial and parietal OFC, and ACC significantly more than the incongruent combination.
As addressed earlier, in most neuroimaging studies reporting the odor–taste integration, odors were presented via a retronasal route (i.e., mouth); little is known about the association between orthonasal odor and taste. In the PET study by Small et al. [ 1997], they presented either unimodal or bimodal stimuli of taste and orthonasal odors during the scanning. Congruent combinations of odor and taste stimuli produced no significant increase of regional cerebral blood flow in the brain regions associated with odor or taste processing than unimodal condition of either odor or taste stimulus. On the contrary, the congruent combinations relative to unimodal condition showed neural deactivations in the bilateral anterior insula/FO and right caudolateral OFC. Our results were partly in line with this deactivation of bimodal combination in comparison with unimodal presentation. Specifically, compared with the salty‐congruent combination, odorless air condition with high‐concentrated salty solution yielded higher activation in right anterior insula [MNI coordinates x, y, z = +33, −1, +16, Z score = 2.86, P uncorrected = 0.002], left thalamus [MNI −12, −13, +4, Z score = 2.80, P uncorrected = 0.003], and right postcentral gyrus [MNI +36, −31, +37, Z‐score = 2.74, P uncorrected = 0.003]. Of interest, the right anterior insula [MNI +33, −1, +16] is close to the part of gustatory cortex responding to either gustatory or olfactory stimulus delivered via retronasal route (e.g., in aqueous solution) but not by orthonasal administration [de Araujo et al., 2003; Rolls et al., 2003]. However, our results also show that the salty‐congruent combination of odor and taste increases neural activations in multiple brain regions than odorless air combination with salty taste. One of the plausible explanations for this contrast result can be found in a different method of odor presentation. Specifically, in the study by Small et al. [ 1997], participants were asked to sniff an odor‐saturated Q‐tip waved under their nose and to indicate if an olfactory stimulus was present by pressing a key, which may induce a spatial disparity‐induced selective attention [Small et al., 1997; Small, 2006]. As opposed to their presentation, in our study, participants were presented with a constant airstream (7 L/min) through whole experimental scanning even in the odorless air condition and between stimuli (i.e., OFF‐blocks). No additional task requiring attention was allowed to them during the experimental scans. This experimental design could minimize a somatosensory‐related effect between presence and absence of airstream, apart from olfactory stimuli [Rolls et al., 2003] and/or a deactivation resulted from spatial disparity‐induced selective attention [Small, 2006]. In addition, no attention task given during the scan might reduce the selective attention‐induced neural deactivation [Mozolic et al., 2008].
In addition, compared with a retronasal odor, a preceding orthonasal odor may induce an odor‐induced taste expectation. That is, after smelling certain food aroma via an orthonasal route, subjects may anticipate the immediate receipt of its associated taste. Several brain imaging studies found that brain regions activated by anticipatory cues (e.g., visual or olfactory stimulus) are locally separable from the regions recruited during consummatory reward: that is, the receipt of chemosensation [O'Doherty et al. 2002; Small et al., 2008]. Small et al. [ 2008] demonstrated that food odors that anticipate the immediate arrival of their associated drink produced greater neural activations in the amygdala and mediodorsal thalamus compared with food odors that predict the immediate receipt of a tasteless solution, compared with the receipt of the drink. In addition, the right anterior insula and left OFC responded to both the anticipatory food odor and its associated drink, reflecting the integration of the anticipatory and consummatory rewards may take place in these areas. However, because we did not separate the anticipatory and consummatory phases in Experiment 2, further studies investigating the role of odor‐induced taste expectation on the odor–taste integration would be very interesting.
It is worth noting that the congruent odor‐induced taste enhancement was more obvious in the low‐concentrated tastant than high‐concentrated one in the psychophysical (Experiment 1) and neuroimaging (Experiment 2) results. These findings support previous psychophysical study demonstrating a congruent odor‐induced saltiness in low‐concentrated salty solution but not in high‐concentrated one [Djordjevic et al., 2004]. In addition, Schifferstein and Verlegh [ 1996] demonstrated that the strawberry odor‐induced sweetness increased with the odor concentration, but it decreased with the taste concentration. In fact, it seems that cross‐modal enhancement is more robust when unimodal stimulus is relatively weaker or more ambiguous [Calvert, 2001]. For example, let us suppose that we are watching certain speech of interviewee with a subtitle on TV news. When the speech of interviewee is clear and easily understandable, we do not look at the subtitle. However, when the vocal output of interviewee is not enough clear to understand, we tend to rely on the subtitle for better understanding. Similarly, participants appeared to rely on the congruent odor when they were presented with relatively low‐concentrated taste solution more strongly. In addition, it can be assumed that compared with in the presence of low‐concentrated taste solution, a congruent odor may have less space to amplify the taste intensity when presented with high‐concentrated taste solution because the taste solution itself is already strong enough to be perceived (i.e., a ceiling effect may occur). This might explain the non‐significant influence of odor congruency on intensity ratings in the high‐concentrated sucrose solution. In fact, early studies have reported that the odor congruency has little or no influence on the sweetness enhancement in the high‐concentrated sucrose solution than in the low‐concentrated one [Djordjevic et al., 2004; Frank et al., 1989; Schifferstein and Verlegh, 1996].
Our findings herein demonstrate that presenting a salty‐congruent odor can be alternative strategy to reduce salt intake in daily life. However, two issues still remain before applying this strategy to food products or daily cuisine. The first issue arising is how to optimize the effect of salty‐congruent odors on saltiness enhancement without increasing unpleasantness to food products. Specifically, Experiment 1 demonstrated that participants rated the salty solution as significantly more unpleasant when they were presented with a congruent odor than when presented with either an incongruent odor or odorless air. That is, even though a salty‐congruent odor can enhance saltiness in salty food products, the salty odor may produce more unpleasantness to the applied products, which leads to rejection of consumers. Schifferstein and Verlegh [ 1996] argued that congruency is necessary to produce a congruent odor‐induced taste enhancement but the degree of congruency is not directly related to the degree of taste enhancement. Furthermore, Lawrence et al. [ 2009, 2011] insisted that odor quality and its intensity contribute to determine to the salty odor‐induced saltiness, compared with the degree of odor congruency. With this background, it can be hypothesized that another salty‐congruent odors having less unpleasant tone (e.g., cheese, delicatessen, or soy sauce) may not only increase a perceived saltiness but also decrease the salty odor‐induced unpleasantness.
The influence of salty‐congruent odor on pleasantness ratings of salty foods may be different depending on the food product type. That is, watery and salted solution is generally unpleasant [Spetter et al., 2010]. It is only pleasant in real foods such as sparkling water; the presence of bubbles in addition to its trigeminal component (e.g., CO2) highly contributes to liking for such salted solution. Therefore, it is conceivable that in salty (pleasant) food products a decrease of saltiness can be linked to a decrease in food liking; in contrast, an increase of saltiness can increase the food liking. In this study, NaCl solution itself was not pleasant. Accordingly, it is not surprising that participants showed more disliking to the congruent odor‐induced saltiness enhancement in the NaCl solution. Therefore, further study is warranted to find appropriate odors with both saltiness and less unpleasantness. In addition, the food product type should be considered to elucidate the influence of salty‐congruent odor on pleasantness ratings of salty foods.
As mentioned in the Introduction section, as an odor perception through the orthonasal route often precedes the experience of retronasal odor and taste during food intake, we focused on the association between orthonasal odor and taste in this study. Therefore, the second issue is that the odor–taste integration is likely to be different in relation to spatial delivery route of odors [de Araujo et al., 2003; Small and Prescott, 2005; Small et al., 1997; Small et al., 2004; Welge‐Lüssen et al., 2009]. For example, using olfactory event‐related potentials, Welge‐Lüssen et al. [ 2009] reported that the P2‐peak latency in response of vanillin odor administered via a retronasal route was relatively shorter in the presence of congruent sweet taste than in the presence of incongruent sour taste, whereas shorter peak latencies were observed in the orthonasal presentation condition, regardless of congruency. However, intensity ratings of sweet taste seemed to be identical between orthonasal and retronasal presentations of vanillin odor. In addition, many psychophysical studies have demonstrated that the odor‐induced taste enhancement was present in both orthonasal [Djordjevic et al., 2004; Sakai et al., 2001] and retronasal odor conditions [Bingham et al., 1990; Clark and Lawless, 1994; Frank and Byram, 1988; Frank et al., 1989; Frank et al., 1993; Lawrence et al., 2009; Sakai et al., 2001; Schifferstein and Verlegh, 1996]. For example, Sakai et al. [ 2001] demonstrated that participants judged aspartame solution as more intense when they were presented with vanilla odor via either orthonasal or retronasal route.
In summary, this study supports the idea that congruent odors enhance taste intensity in both salty and sweet taste solution; however, the congruent odor‐induced saltiness enhancement was not consistently observed in the behavioral results of this study (i.e., Experiment 2). In particular, it is worth noting that our findings demonstrate the congruent odor‐induced saltiness enhancement at the neuroanatomical level. The salty‐congruent odor increased unpleasantness in the salted solution, which reflects that salt reduction in practice cannot be simply established by adding a salty‐congruent odor to the food products. Having said that, this study presents some evidence that the addition of a salty‐congruent odor can be an alternative way to reduce excessive salt intake. Nevertheless, many questions still remain concerning the salty‐congruent odor‐induced taste unpleasantness.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1.
REFERENCES
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS ( 1993): Processing strategies for time‐course data sets in functional MRI of the human brain. Magn Reson Med 30: 161–173. [DOI] [PubMed] [Google Scholar]
- Barylko‐Pikielna N, Kostyra E ( 2007): Sensory interaction of umami substances with model food matrices and its hedonic effect. Food Qual Pref 18: 751–758. [Google Scholar]
- Bingham AF, Birch GG, de Graaf C, Behan JM, Perring KD ( 1990): Sensory studies with sucrose‐maltol mixtures. Chem Senses 15: 447–456. [Google Scholar]
- Breslin PAS, Beauchamp GK ( 1997): Salt enhances flavour by suppressing bitterness. Nature 387: 563. [DOI] [PubMed] [Google Scholar]
- Brown IJ, Tzoulaki I, Candeias V, Elliott P ( 2009): Salt intakes around the world: Implications for public health. Int J Epidemiol 38: 791–813. [DOI] [PubMed] [Google Scholar]
- Burseg KMM, Camacho S, Knoop J, Bult JHF ( 2010): Sweet taste intensity is enhanced by temporal fluctuation of aroma and taste, and depends on phase shift. Physiol Behav 101: 726–730. [DOI] [PubMed] [Google Scholar]
- Busch JLHC, Batenburg M, van der Velden R, Smit G ( 2009): Reviewing progress towards finding an acceptable natural flavor alternative to salt. Agro Food Ind Hi‐Tech 20: 66–68. [Google Scholar]
- Calvert GA ( 2001): Crossmodal processing in the human brain: Insights from functional neuroimaging studies. Cereb Cortex 11: 111–123. [DOI] [PubMed] [Google Scholar]
- Cerf‐Ducastel B, Murphy C ( 2001): fMRI activation in response to odorants orally delivered in aqueous solutions. Chem Senses 26: 625–637. [DOI] [PubMed] [Google Scholar]
- Cerf‐Ducastel B, Murphy C ( 2004): Validation of a stimulation protocol suited to the investigation of odor‐taste integrations with fMRI. Physiol Behav 81: 389–396. [DOI] [PubMed] [Google Scholar]
- Clark CC, Lawless HT ( 1994): Limiting response alternatives in time‐intensity scaling: An examination of the halo‐dumping effect. Chem Senses 19: 583–594. [DOI] [PubMed] [Google Scholar]
- Cutler JA, Follmann D, ( 1997); Allender PSRandomized trials of sodium reduction: An overview. Am J Clin Nutr 65: 643S–651S. [DOI] [PubMed] [Google Scholar]
- de Araujo IET, Rolls ET, Kringelbach ML, McGlone F, Phillips N ( 2003): Taste‐olfactory convergence, and the representation of the pleasantness of flavor, in the human brain. Eur J Neurosci 18: 2059–2068. [DOI] [PubMed] [Google Scholar]
- Desmond E ( 2006): Reducing salt: A challenge for the meat industry. Meat Sci 74: 188–196. [DOI] [PubMed] [Google Scholar]
- Djordjevic J, Zatorre RJ, Jones‐Gotman M ( 2004): Odor‐induced changes in taste perception. Exp Brain Res 159: 405–408. [DOI] [PubMed] [Google Scholar]
- Doyle ME, Glass KA ( 2010): Sodium reduction and its effect on food safety, food quality, and human health. Comp Rev Food Sci Food Safety 9: 44–56. [DOI] [PubMed] [Google Scholar]
- Evans AD, Collins DL, Millst SR, Brown ED, Kelly RL, Peters TM ( 1993): 3D statistical neuroanatomical models from 305 MRI volumes. Proc IEEE Nuci Sci Symp Med Imaging 3: 1813–1817. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR ( 1975): Mini‐mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Frank RA, Byram J ( 1988): Taste‐smell interactions are tastant and odorant dependent. Chem Senses 13: 445–455. [Google Scholar]
- Frank RA, Ducheny K, Mize SJS ( 1989): Strawberry odor, but not red color, enhances the sweetness of sucrose solutions. Chem Senses 14: 371–377. [Google Scholar]
- Frank RA, van der Klaauw NJ, Schifferstein HN ( 1993): Both perceptual and conceptual factors influence taste‐odor and taste‐taste interactions. Percept Psychophys 54: 343–354. [DOI] [PubMed] [Google Scholar]
- FSAI Scientific Committee ( 2005): Salt and Health: Review of the Scientific Evidence and Recommendations for Public Policy in Ireland. Food Safety of Authority of Ireland (FSAI): Dublin, Ireland. [Google Scholar]
- Gottfried JA ( 2006): Smell: Central nervous processing. Adv Otorhinolaryngol 63: 44–69. [DOI] [PubMed] [Google Scholar]
- He FJ, MacGregor GA ( 2009): A comprehensive review on salt and health and current experience of worldwide salt reduction programs. J Hum Hypertens 23: 363–384. [DOI] [PubMed] [Google Scholar]
- He FJ, Jenner KH, MacGregor GA ( 2010): WASH‐World Action on Salt and Health. Kidney Int 78: 745–753. [DOI] [PubMed] [Google Scholar]
- Hummel T, Konnerth OG, Rosenheim K, Kobal G ( 2001): Screening of olfactory function with a four‐minute odor identification test: Reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol 110: 976–981. [DOI] [PubMed] [Google Scholar]
- Hummel C, Frasnelli J, Gerber J, Hummel T ( 2007): Cerebral processing of gustatory stimuli in patients with taste loss. Behav Brain Res 185: 59–64. [DOI] [PubMed] [Google Scholar]
- Katsiari MC, Voutsinas LP, Alichanidis E, Roussis IG ( 1998): Manufacture of Kefalograviera cheese with less sodium by partial replacement of NaCl with KCl. Food Chem 61: 63–70. [Google Scholar]
- Kobal G, Klimek L, Wolfensberger M, Gudziol H, Temmel A, Owen CM, Seeber H, Pauli E, Hummel T ( 2000): Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combing tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol 257: 205–211. [DOI] [PubMed] [Google Scholar]
- Kremer S, Mojet J, Shimojo R ( 2009): Salt reduction in foods using naturally brewed soy sauce. J Food Sci 74: 255–262. [DOI] [PubMed] [Google Scholar]
- Landis BN, Welge‐Luessen A, Brämerson A, Bende M, Mueller CA, Nordin S, Hummel T ( 2009): “Taste strips”—A rapid, lateralized, gustatory beside identification test based on impregnated filter papers. J Neurol 256: 242–248. [DOI] [PubMed] [Google Scholar]
- Lawrence G, Salles C, Septier C, Busch J, Thomas‐Danguin T ( 2009): Odour‐taste interactions: A way to enhance saltiness in low‐salt content solutions. Food Qual Pref 20: 241–248. [Google Scholar]
- Lawrence G, Salles C, Palicki O, Septier C, Busch J, Thomas‐Danguin T ( 2011): Using cross‐modal interactions to counterbalance salt reduction in solid foods. Int Dairy J 21: 103–110. [Google Scholar]
- Liao CH, Worsley KJ, Poline J‐B, Aston JAD, Duncan GH, Evans AC ( 2001): Estimating the delay of the fMRI response. Neuroimage 16: 593–606. [DOI] [PubMed] [Google Scholar]
- MacGregor GA, Sever PS ( 1996): Salt‐overwhelming evidence but still no action: Can a consensus be reached with the food industry? CASH (Consensus Action on Salt and Hypertension). BMJ 312: 1287–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Mattes RD, Donnelly D ( 1991): Relative contributions of dietary sodium sources. J Am Coll Nutr 10: 383–393. [DOI] [PubMed] [Google Scholar]
- Mohan S, Campbell NRC, Willis K ( 2009): Effective population‐wide public health interventions to promote sodium reduction. CMAJ 181: 605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojet J, Heidema J, Christ‐Hazelhof E ( 2004): Effect of concentration on taste‐taste interactions in foods for elderly and young subjects. Chem Senses 29: 671–681. [DOI] [PubMed] [Google Scholar]
- Mozolic J, Joyner D, Hugenschmidt CE, Peiffer AM, Kraft RA, Maldjian JA, Laurienti PJ ( 2008): Cross‐modal deactivations during modality‐specific selective attention. BMC Neurol 8: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F ( 2001): Representation of pleasant and aversive taste in the human brain. J Neurophysiol 85: 1315–1321. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ ( 2002): Neural responses during anticipation of a primary taste reward. Neuron 33: 815–826. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, de Araujo ( 2003): Different representations of pleasant and unpleasant odours in the human brain. Eur J Neurosci 18: 695–703. [DOI] [PubMed] [Google Scholar]
- Sakai N, Kobayakawa T, Gotow N, Saito S, Imada S ( 2001): Enhancement of sweetness ratings of aspartame by a vanilla odor presented either by orthonasal or retronasal routes. Percept Mot Skills 92: 1002–1008. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyas B, Larsson M, Roland P ( 2000): Olfactory functions are mediated by parallel and hierarchical processing. Neuron 26: 735–745. [DOI] [PubMed] [Google Scholar]
- Schifferstein HNJ, Verlegh PWJ ( 1996): The role of congruency and pleasantness in odor‐induced taste enhancement. Acta Psychol (Amst) 94: 87–105. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones‐Gotman M, Zatorre RJ, Petrides M, Evans AC ( 1997): Flavor processing: More than the sum of its parts. Neuroreport 8: 3913–3917. [DOI] [PubMed] [Google Scholar]
- Small DM, Zald DH, Jones‐Gotman M, Zatorre RJ, Pardo JV, Frey S, Petrides M ( 1999): Human cortical gustatory areas: A review of functional neuroimaging data. Neuroreport 10: 7–14. [DOI] [PubMed] [Google Scholar]
- Small DM, Voss J, Mak YE, Simmons KB, Parrish T, Gitelman D ( 2004): Experience‐dependent neural integration of taste and smell in the human brain. J Neurophysiol 92: 1892–1903. [DOI] [PubMed] [Google Scholar]
- Small DM, Prescott J ( 2005): Odor/taste integration and the perception of flavor. Exp Brain Res 166: 345–357. [DOI] [PubMed] [Google Scholar]
- Small DM ( 2006): Central gustatory processing in humans. Adv Otorhinolaryngol 63: 191–220. [DOI] [PubMed] [Google Scholar]
- Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F ( 2008): Separable substrates for anticipatory and consummatory food chemosensation. Neuron 57: 786–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PB, Iannilli E, Hummel T ( 2011): Segregation of gustatory cortex in response to salt and umami taste studied through event‐related potentials. Neuroreport 22: 299–303. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JD ( 1998): Sniffing and smelling: Separate subsystems in the human olfactory cortex. Nature 392: 282–286. [DOI] [PubMed] [Google Scholar]
- Spetter MS, Smeets PA, de Graaf C, Viergever MA ( 2010): Representation of sweet and salty taste intensity in the brain. Chem Senses 35: 831–840. [DOI] [PubMed] [Google Scholar]
- Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP ( 2009): Salt intake, stroke, and cardiovascular disease: Meta‐analysis of prospective studies. BMJ 339: b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Nachtigal D, Teulings L, Gitelman DR, Small DM ( 2010): The insular taste cortex contributes to odor quality coding. Front Hum Neurosci 4: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welge‐Lüssen A, Husner A, Wolfensberger M, Hummel T ( 2009): Influence of simultaneous gustatory stimuli on orthonasal and retronasal olfaction. Neurosci Lett 454: 124–128. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Takahashi C ( 1984): Interactions of monosodium glutamate and sodium chloride on saltiness and palatability of a clear soup. J Food Sci 49: 82–85. [Google Scholar]
- Zatorre RJ, Jones‐Gotman M ( 1990): Right‐nostril advantage for discrimination of odors. Percept Psychophys, 47: 526–531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1.


