Abstract
Obesity is a key risk factor for the development of insulin resistance, Type 2 diabetes and associated diseases; thus, it has become a major public health concern. In this context, a detailed understanding of brain networks regulating food intake, including hormonal modulation, is crucial. At present, little is known about potential alterations of cerebral networks regulating ingestive behavior. We used “resting state” functional magnetic resonance imaging to investigate the functional connectivity integrity of resting state networks (RSNs) related to food intake in lean and obese subjects using independent component analysis. Our results showed altered functional connectivity strength in obese compared to lean subjects in the default mode network (DMN) and temporal lobe network. In the DMN, obese subjects showed in the precuneus bilaterally increased and in the right anterior cingulate decreased functional connectivity strength. Furthermore, in the temporal lobe network, obese subjects showed decreased functional connectivity strength in the left insular cortex. The functional connectivity magnitude significantly correlated with body mass index (BMI). Two further RSNs, including brain regions associated with food and reward processing, did not show BMI, but insulin associated functional connectivity strength. Here, the left orbitofrontal cortex and right putamen functional connectivity strength was positively correlated with fasting insulin levels and negatively correlated with insulin sensitivity index. Taken together, these results complement and expand previous functional neuroimaging findings by demonstrating that obesity and insulin levels influence brain function during rest in networks supporting reward and food regulation. Hum Brain Mapp, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: independent component analysis (ICA), obesity, fMRI, insulin, food, reward
INTRODUCTION
According to the World Health Organization, there are currently over 1.6 billion overweight and at least 400 million obese individuals worldwide (WHO 2010). Obesity is tightly linked to insulin resistance and is one of the major factors leading to chronic diseases like Type 2 diabetes. In this respect, obesity is associated with increased risk for numerous health conditions and heightened mortality [Flegal et al., 2002; Flegal et al. 2005].
Obesity is generated by an energy imbalance and associated with excessive food intake. Eating behavior itself is modulated by physiological, psychological, and cognitive factors. This clearly indicates that a basal brain control circuit like the hypothalamus, the major homeostatic control center, alone is not sufficient to explain eating behavior and weight control in general. Several brain imaging studies, investigating food related processing, discovered that obese individuals show altered brain activity in the gustatory and somatosensory regions, as well as reward related areas [Del Parigi et al., 2002; Del Parigi et al., 2005; Gautier et al., 2000; Gautier et al., 2001; Karhunen et al., 1997; Matsuda et al., 1999; Stice et al., 2008; Stice et al., 2009]. For instance, cortical areas associated with hunger and satiation are differentially activated in obese compared with lean individuals, which include the prefrontal cortex, hypothalamus, thalamus, several limbic/paralimbic areas, basal ganglia, temporal cortex, and the cerebellum [Del Parigi et al., 2002; Del Parigi et al., 2005; Gautier et al., 2000; Gautier et al., 2001; Karhunen et al., 1997; Matsuda et al., 1999]. Furthermore, brain regions related to motivational effects of food cues, especially the striatum, showed significantly reduced dopamine D2 receptor availability in obese individuals [Wang et al., 2001] and exhibited greater activation to high caloric food stimuli [Rothemund et al., 2007; Stoeckel et al., 2008], but less activation in response to food consumption in obese individuals [Stice et al., 2008]. Furthermore, hormonal signals, like insulin, act in the CNS as regulators of energy homeostasis through its receptors, expressed in the limbic forebrain [Marks et al., 1991; Unger et al., 1991]. Hereby it has been found in animal studies that insulin decreases the rewarding aspect of food through the “motivational circuitry,” such as by decreasing dopamine signaling [Figlewicz et al., 1994; Patterson et al., 1998]. Interestingly, in a recent magnetoencephalographic study we found that increasing insulin levels did not lead to increased neuronal activity in obese individuals in contrast to lean individuals. We called this effect cerebral insulin resistance [Tschritter et al., 2006]. This cerebral insulin resistance is also associated with peripheral insulin resistance [Tschritter et al., 2006; Tschritter et al., 2007a]. Further factors that relate to insulin resistance of the brain include elevated serum free fatty acid levels [Tschritter et al., 2009b], aging [Hennige et al., 2006; Tschritter et al., 2009a] and genetic background [Tschritter et al., 2007b].
However, no studies, so far, have evaluated the relationship between obesity and intrinsic brain function involved in the regulation of food and reward processing. We applied “resting state” functional magnetic resonance imaging (fMRI) to assess brain function in lean compared with obese subjects. By means of independent component analysis (ICA), we identified several temporally synchronous brain networks or “modes,” which are present in healthy subjects during rest to examine functional connectivity (FC) within these networks [Beckmann et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006]. We focused on five resting state networks (RSNs) that involve brain areas previously implicated in food and reward processing. Furthermore, these brain areas were observed to be differentially activated in obese compared with lean individuals [Del Parigi et al., 2002; Del Parigi et al., 2005; Gautier et al., 2000; Gautier et al., 2001; Karhunen et al., 1997; Matsuda et al., 1999; Stice et al., 2008; Stice et al., 2009]. The default mode network (DMN), includes regions which are active when subjects are in an awake, resting phase without any task, but whose activity diminishes during specific goal‐directed behaviors [Raichle et al., 2001]. The DMN includes the precuneus, posterior cingulate, medial prefrontal, and inferior parietal cortices [Correa et al., 2007]. There has been evidence implicating changes in parietal and cingulate regions in obese individuals [Rothemund et al., 2007; Volkow et al., 2009; Wang et al., 2002]. A second network, termed temporal lobe network, includes the primary and secondary auditory cortices and the insular cortex, which is part of the primary gustatory cortex [Beckmann et al., 2005; Damoiseaux et al., 2006]. A third network, termed basal ganglia network [Robinson et al., 2009], includes subcortical structures that are also involved in food and reward processing. A fourth network, termed prefrontal lobe network, includes the dorsolateral and ventrolateral prefrontal, orbitofrontal (OFC), and frontopolar prefrontal cortex. The prefrontal cortex is involved in inhibitory processes, like termination of feeding [Del Parigi et al., 2002]. A fifth network, termed motor sensory network, includes precentral and postcentral gyri [Beckmann et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006]. A Positron Emission Tomography (PET) study found that obese relative to lean adults showed greater resting metabolic activity in the oral somatosensory cortex, a region that encodes sensation in the mouth, lips, and tongue [Wang et al., 2002]. Extending these findings, an fMRI study found that obese versus lean adolescents showed greater activation in the oral somatosensory cortex in response to the anticipated intake of chocolate milkshake [Stice et al., 2008].
We hypothesized that obese subjects would show: (i) altered RSN connectivity, especially in brain regions known to be important in food and reward regulation; and (ii) that these alterations would be associated with body mass index (BMI), fasting insulin levels and insulin sensitivity index, which are directly correlated with obesity [Stephan et al., 1972; Woods et al., 1985].
MATERIALS AND METHODS
Subjects
Eleven lean (six women, BMI range 19.4–22.5 kg/m2, and age range 22–29 years) and 12 overweight or obese subjects (six women, BMI range 28.4–34.4 kg/m2, and age range 21–28 years) were recruited. Lean subjects were required to have a BMI from 18.5 to 24.0. Overweight and obese subjects were required to have a BMI >25. The overweight and obese subjects collectively will be referred to as “obese.” All subjects were healthy as ascertained by a physician; they did not suffer from psychiatric, neurological, or metabolic diseases. Any volunteer treated for chronic disease or taking any kind of medication other than oral contraceptives was excluded at screening. Impaired glucose metabolism was ruled out by standard 75 g oral glucose tolerance test (OGTT). All subjects were normal sighted or had corrected‐to‐normal vision. Informed written consent was obtained from all subjects and the local Ethics Committee approved the protocol. The demographic characteristics of the subjects are shown in Table I.
Table I.
Subjects' characteristics in lean vs. obese group
| Lean group | Obese group | P | |
|---|---|---|---|
| Gender (female/male) | 6/5 | 6/6 | – |
| Age (years) | 23.5 ± 2.1 | 24.7 ± 2.4 | 0.08 |
| BMI (kg/m2) | 20.9 ± 1.1 | 30.5 ± 1.8 | <0.001 |
| Fasting glucose (mmol/l) | 4.94 ± 0.34 | 4.96 ± 0.41 | 0.9 |
| Fasting insulin (pmol/l) | 37 ± 15 | 77 ± 33 | 0.0014 |
| OGTT‐derived insulin sensitivity index (AU) | 19.0 ± 7.1 | 11.5 ± 7.0 | 0.0067 |
| POMS‐scale: Depression (%) | 0.82 ± 1.45 | 2.84 ± 5.31 | 0.236 |
| POMS‐scale: Fatigue (%) | 21.53 ± 10.6 | 18.15 ± 14.1 | 0.526 |
| POMS‐scale: Anger (%) | 1.40 ± 1.8 | 3.76 ± 7.3 | 0.307 |
| POMS‐scale: Vigor (%) | 32.79 ± 17.29 | 42.33 ± 15.9 | 0.183 |
Data are presented as mean ± SD. P = P‐values for comparison of unadjusted loge‐transformed data by ANOVA. POMS = Profile of Mood States.
Study Design
After an overnight fast of at least 10 h, subjects completed two resting state functional imaging scanning visits separated on average by 4 weeks. To minimize circadian influence, subjects were scanned between 7 and 8 am. Before the experiment, subjects confirmed their fasting state. In addition, blood glucose and plasma insulin levels before each experiment were within the fasting range for all subjects. Before the experiment, subjects filled out a short German version of the “Profile of Mood States” (POMS; [McNair et al., 1981]), which is a 35 item questionnaire evaluate depression, fatigue, anger, and vigor (Table I). There was no statistical significant difference in mood states assessed by POMS between lean and obese subjects.
Data Acquisition
Whole‐brain functional magnetic resonance imaging (fMRI) data was obtained by using a 3.0 T scanner (Siemens Tim Trio, Erlangen, Germany). Functional data were collected by using echo‐planar imaging sequence: TR = 1.8s, TE = 30ms, FOV = 210 mm2, matrix 64 × 64, flip angle 90°, voxel size 3 × 3 × 4 mm3, slice thickness 3 mm, 1 mm gap and the images were acquired in ascending order. Each brain volume comprised 28 axial slices and each functional run contained 160 image volumes, resulting in a total scan time of 4.52 min. In addition, high‐resolution T1 weighted anatomical images (MPRage: 176 slices, matrix: 256 × 224, 1 × 1 x1 mm3) of the brain were obtained. All subjects were instructed not to focus their thoughts on anything in particular and to keep their eyes closed during the resting state MR acquisition.
Data Preprocessing
Functional image preprocessing and statistical analysis were carried out by using SPM5 (Wellcome Trust Centre for Neuroimaging, London, UK). Images were realigned to the first image. Unwarping of geometrically distorted EPIs was performed using the FieldMap Toolbox available for SPM5 to account for susceptibility by movement artifacts. A mean image was created and coregistered to the T1 structural image. The anatomical image was normalized to the Montreal Neurological Institute (MNI) template, and the resulting parameter file was used to normalize the functional images (voxel size: 3 × 3 × 3 mm3). Finally, the normalized images were smoothed with a three‐dimensional isotropic Gaussian kernel (FWHM: 10 mm). FMRI Data were high‐pass [cut off period 128 s) and low‐pass filtered (autoregression model AR(1)].
Independent Component Analysis (ICA)
Group spatial ICA [Calhoun et al., 2001] was used to decompose the smoothed, normalized fMRI images into 35 components using the GIFT software (http://icatb.sourceforge.net/) as follows. Dimension estimation, to determine the number of components, was performed using the minimum description length criteria, modified to account for spatial correlation [Li et al., 2007]. Data from all subjects were then concatenated and this aggregate data set reduced to 35 temporal dimensions using Principal Component Analysis, followed by an independent component estimation using infomax algorithm [Bell and Sejnowski, 1995].
Component Selection
For each subject, spatial maps were reconstructed and converted to z values. A two step process was used to select the components that most closely matched the default mode, temporal lobe, basal ganglia, prefrontal lobe, and motor sensory network. First, because functional connectivity networks have been detected in low‐frequency ranges [Cordes et al., 2001], a frequency filter was applied to remove any components in which high‐frequency signal (>0.1 Hz) constituted 50% or more of the total power in the Fourier spectrum. Next, the remaining low‐frequency components were spatially sorted in GIFT using masks derived from the wake forest university pick atlas (http://www.fmri.wfubmc.edu/download.htm) to identify the DMN, temporal lobe, basal ganglia, prefrontal lobe, and motor sensory network components. For the DMN mask we used precuneus, posterior cingulate, and Brodmann area (BA) 7, 10, and 39 [Correa et al., 2007], for the temporal lobe network mask we used the bilateral insular cortices, for the basal ganglia network we used bilaterally the pallidum, putamen, substantia nigra, subthalamic nucleus, and thalamus [Robinson et al., 2009], for the prefrontal lobe network mask we used BA 8, 9, 10, 11, 44, 45, 46, and 47 and for the motor sensory network mask we used BA 1,2,3, and 4. A spatial correlation of the masks with each of the components was performed and the component that had the best fit with each of the five masks was automatically selected for further analysis (Supporting Information Fig. 1). For each component, we created a sample‐specific component group map of all 23 subjects. These maps were used as a binary mask for statistical analyses within the corresponding component.
Figure 1.
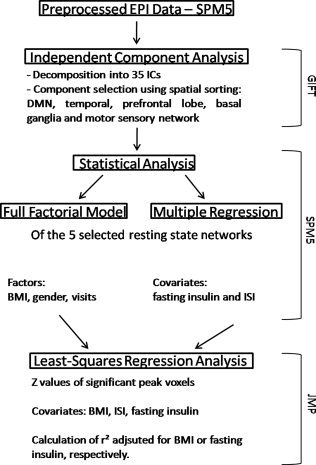
Data analysis overview. Summary sketch of the data analysis steps. Displayed on the right are the applied software packages. Abbreviations: ICs‐Independent Components, BMI‐body mass index, ISI‐insulin sensitivity index.
Statistical Analysis
Group comparison
The z values of the spatial maps represent the fit of a specific voxel BOLD timecourse to the group averaged component's timecourse. Hence, group analyses test the connectivity strength (i.e., signal synchronization) of each voxel to the whole spatial component. For group analyses, spatial maps of the DMN, temporal lobe, basal ganglia, prefrontal lobe, and motor sensory network were entered into SPM5 since the GIFT toolbox only allows one‐ or two‐sample t‐tests. For each component, a full factorial model (Factors: BMI, gender, and visits) was used to examine group differences. The factor “visit” was included to correct for repeated measurement. The resulting statistical maps were masked with the component‐specific group map to explore results within this network only. All group tests were thresholded at P<0.05, family‐wise error corrected (FWE).
Correlation of Component Maps with Anthropometric and Metabolic Parameters
For each of the five components, a multiple regression analysis was performed, in SPM5, using fasting insulin levels and insulin sensitivity index as covariates to quantify the association between network related functional connectivity and insulin levels. Non‐normally distributed variables were logarithmically transformed. Again the resulting statistical maps were masked with the component‐specific group map to explore results within this network only. All group tests were thresholded at P < 0.05 (FWE).
Since BMI, fasting insulin and insulin sensitivity index are highly related, further statistical analysis was applied to differentiate between BMI and insulin related effects (i.e., r 2 adjusted for BMI or fasting insulin). For this purpose the following procedure was used. Z values of individual GIFT maps were extracted for each peak voxel that showed significant group differences (Full factorial model, SPM5) or significant correlation with fasting insulin or insulin sensitivity index (Multiple Regression, SPM5). To determine BMI or insulin adjusted r 2, correlations for the extracted z values were calculated by using least‐squares regression analysis in JMP. P value of <0.05 was considered statistical significant (JMP Version 8.0.2, SAS Institute, Cary, NC). For a summary sketch for the functional data analysis, see Figure 1.
Blood Sampling and Analysis
Blood glucose was measured by a HemoCue blood glucose photometer using the glucose dehydrogenase method (HemoCueAB, Aengelholm, Sweden). Plasma insulin levels were determined with commercial chemiluminescence assays for ADVIA Centaur (Siemens Medical Solutions, Fernwald, Germany). In addition, all subjects underwent a standard 75g OGTT with determination of glucose and insulin every 30 min from which insulin sensitivity index was calculated as proposed by Matsuda and DeFronzo [Matsuda and DeFronzo, 1999].
Voxel Based Morphometry
The T1 weighted images were processed and examined using the VBM8 toolbox with default parameters (http://dbm.neuro.uni-jena.de/vbm.html) implemented in the SPM8 software (Wellcome Department of Imaging Neuroscience Group, London, UK; http://www.fil.ion.ucl.ac.uk./spm). Images were bias corrected, tissue classified and registered using linear and nonlinear transformations, within a unified model [Ashburner and Friston, 2005]. Gray matter (GM) segments were multiplied by the nonlinear components derived from the normalization matrix to preserve actual GM values locally (modulated GM volumes) and not multiplied by the linear components of the registration to account for individual differences in brain orientation, alignment, and size globally. Finally, the modulated volumes were smoothed with a Gaussian kernel of 10 mm full width at half maximum (FWHM).
Voxel‐wise GM differences between lean and obese subjects were examined by means of a two‐sample t‐test using age and gender as confounding covariates, which means that the effect of age and gender were removed from the data. To avoid possible edge effects between tissue types, we excluded all voxels with GM values of less than 0.1 (absolute threshold masking). Results were thresholded at P < 0.05 (FWE).
RESULTS
Component Identification
By means of Independent Component Analysis (ICA), 35 independent components (ICs) were extracted for the obese as well as for the lean group. Fourteen potentially functional relevant components were extracted for both groups. These components correspond to previously described RSNs, consisting of regions that are involved in working memory, the DMN, motor function, visual and auditory processing, and executive function [Beckmann et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006]. We additionally found a spatial pattern resembling the basal ganglia network, including the thalamus and supplementary motor areas [Damoiseaux et al., 2008; Di Martino et al., 2008; Robinson et al., 2009]. The 14 functionally relevant components were spatially sorted in GIFT (spatial correlation) using anatomical masks to identify the default mode, temporal lobe, basal ganglia, prefrontal lobe and motor sensory network components (r = 0.46, 0.34, 0.62, 0.28, and 0.30 respectively) (Supporting Information Fig. 1).
Group Comparison
We analyzed between group differences in corresponding RSNs, using a full factorial model (BMI, gender, visits), of the identified DMN, temporal lobe, basal ganglia, prefrontal lobe and motor sensory network contrasting the individual, back‐reconstructed IC patterns. No main effect for gender was observed in the five RSNs. A significant main effect for BMI was observed in the DMN and temporal lobe network (P <0.05, FWE). The precuneus/posterior cingulate cortex (PCC) (BA 7/23) showed increased FC strength bilaterally, whereas the right anterior cingulate cortex (ACC) (BA24) demonstrated reduced FC strength in the DMN in the obese group (Fig. 2 and Supporting Information Table I). The left insular cortex (BA13) revealed reduced FC strength in the temporal lobe network in the obese group (Fig. 3 and Supporting Information Table II). No group differences were observed in the prefrontal lobe, basal ganglia and motor sensory network component.
Figure 2.
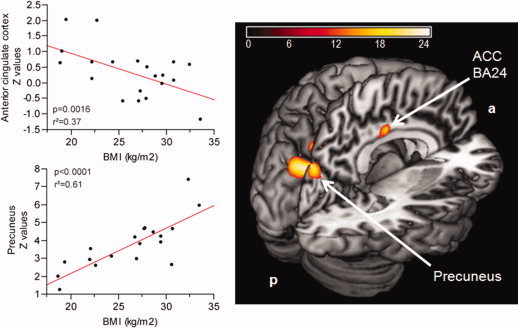
Contrast of DMN between lean and obese subjects. Color map represents significant (P <0.05, FWE) voxels of altered functional connectivity in obese compared to lean subjects. Color bar represents F‐values. The top scatter plot shows significant negative correlation between the right anterior cingulate cortex (BA24) (x = 3, y = −12, z = 36) and BMI, adjusted for fasting insulin levels. The bottom scatter plot shows significant positive correlation between the left precuneus (x = −3, y = −78, z = 33) and BMI, adjusted for fasting insulin levels.
Figure 3.
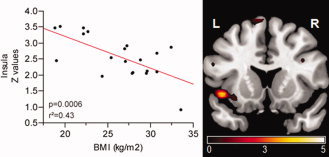
Contrast of temporal lobe network between lean and obese subjects. Color map represents significant (P < 0.05, FWE) voxels of decreased functional connectivity in obese compared to lean subjects. Color bar represents T‐values. Scatter plot shows significant negative correlation between the left insular cortex (x = −42, y = −6, z = 0) and BMI, adjusted for fasting insulin levels.
Correlation of RSN Maps with BMI, Fasting Insulin and Insulin Sensitivity Index
The multiple regression analysis (SPM5) revealed insulin associated functional connectivity in the prefrontal lobe and basal ganglia network (P < 0.05, FWE). No relationship was observed between insulin and the DMN, temporal lobe or motor sensory network. As expected, BMI, plasma insulin and insulin sensitivity index were highly correlated within this sample (BMI and insulin: P = 0.01, r 2 = 0.28; BMI and insulin sensitivity index: P = 0.005, r 2 = 0.33; insulin and Insulin sensitivity index: P < 0.0001, r 2 = 0.69).
Default mode network
A significant positive correlation was found between the left precuneus (x = −3/y = −78/z = 33) FC strength and BMI adjusted for fasting insulin (P < 0.0001, r 2adjusted = 0.61) (see Fig. 2), as well as a negative correlation was found between the right ACC (x = 3/y = −12/z = 36) functional connectivity and BMI adjusted for fasting insulin (P = 0.0016, r 2adjusted = 0.37) (see Fig. 2).
Temporal lobe network
A significant negative correlation was found between left insular cortex (x = −42/y = −6/z = 0) FC strength and BMI adjusted for fasting insulin (P = 0.0006, r2adjusted = 0.43) (see Fig. 3).
Prefrontal lobe network
A significant positive correlation was revealed between left orbitofrontal cortex (x = −30/y = 45/z = −12) FC strength and fasting insulin adjusted for BMI (P = 0.015, r2adjusted = 0.22) (see Fig. 4); a negative correlation was revealed between the left orbitofrontal cortex FC strength and insulin sensitivity index adjusted for BMI (P = 0.0012, r 2adjusted = 0.39) (see Fig. 4).
Figure 4.
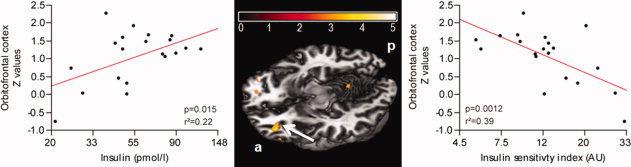
Relationship between prefrontal lobe network functional connectivity and fasting insulin levels (loge‐scaled) and insulin sensitivity index (loge‐scaled) in lean and obese subjects. Color map represents significant (P <0.05, FWE) voxels of insulin associated connectivity. Color bar represents T‐values. The left scatter plot shows a significant positive correlation between the left orbitofrontal cortex (x = −30, y = 45, z = −12) and fasting insulin, adjusted for BMI. The right scatter plot shows a significant negative correlation between left orbitofrontal cortex and insulin sensitivity index, adjusted for BMI.
Basal ganglia network
A significant positive correlation was revealed between right putamen (x = 33/y = 0/z = −9) FC strength and fasting insulin adjusted for BMI (P = 0.009, r2adjusted = 0.26) (see Fig. 5); a negative correlation was revealed between the right putamen FC strength and insulin sensitivity index adjusted for BMI (P <0.0001, r2adjusted = 0.65) (see Fig. 5).
Figure 5.
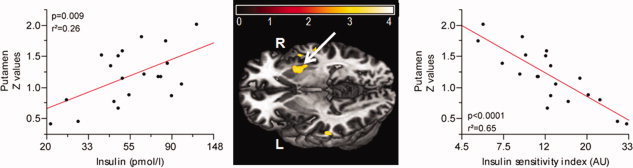
Relationship between basal ganglia network functional connectivity and fasting Insulin levels (loge‐scaled) and insulin sensitivity index (loge‐scaled) in lean and obese subjects. Color map represents significant (P <0.05, FWE) voxels of insulin associated activity. Color bar represents T‐ values. The left scatter plot shows a significant positive correlation between the right putamen (x = 33, y = 0, z = −9) and fasting insulin, adjusted for BMI. The right scatter plot shows a significant negative correlation between the right putamen and insulin sensitivity index, adjusted for BMI.
Motor sensory network
No correlations were found with fasting insulin and ISI
Structural differences between lean and obese subjects
To assess possible causes of altered functional connectivity, we analyzed our data for structural differences between both groups. The voxel‐wise analysis revealed no significant GM differences at a threshold of P < 0.05 (FWE).
DISCUSSION
The aims of the study were to explore the effects of obesity on the integrity of FC within the DMN, temporal lobe, basal ganglia, prefrontal lobe, and motor sensory network using resting state fMRI and investigate whether a relationship exists between FC strength and BMI, fasting insulin levels and insulin sensitivity of the subjects. We provide evidence that obese individuals differ substantially during rest in brain networks related to reward and food regulation compared with lean subjects.
Direct group comparison showed altered FC strength in the DMN and temporal lobe network in obese subjects. Regions of the affected RSNs did not differ significantly in volumetric aspects between the two groups, confirming the functional nature of the observed alterations. In the DMN, the PCC/precuneus expressed increased FC strength, whereas the ACC (BA24) expressed decreased FC strength in obese subjects. The PCC, part of the associative cortex, is involved in a wide range of higher order cognitive functions and is highly connected to higher cortical areas, including the prefrontal cortex [Cavanna and Trimble, 2006]. The ACC, on the other hand, is linked primarily to paralimbic and subcortical regions associated with affective and autonomic processes including OFC, nucleus accumbens and hypothalamus [Aharon et al., 2001; Damasio et al., 2000]. Recent evidence suggests that the PCC as well as the ACC activity can be influenced by BMI. PET studies revealed that the precuneus is associated with an enhanced metabolic activity during rest in obese individuals [Wang et al., 2002] and that baseline brain glucose metabolism in prefrontal regions and ACC is negatively correlated with BMI [Volkow et al., 2009]. A functional connectivity study demonstrated that ACC and PCC functional connectivity patterns show common regions implicated in the integration of cognitive and emotional stimuli [Greicius et al., 2003]. Thus, it has been postulated that functional connections between PCC and ACC form a critical link between higher cortical processing and basic processing necessary for calibrating affective and autonomic states [Greicius et al., 2003]. An intriguing speculation, based on our findings and previous results, is that an altered integration between PCC and ACC can contribute to an increased risk to overeat through an imbalance between cognitive and emotional processing. Using ICA, we were able to demonstrate that obese individuals show altered functional connectivity in the PCC and ACC within the DMN. The PCC was positively correlated with BMI, while the ACC (BA24) was negatively correlated with BMI. Nevertheless, additional investigations are needed to exemplify whether these changes are only within the DMN or if there is actually a change in functional connectivity between the PCC and ACC.
In the temporal lobe network, the insular cortex showed decreased FC strength in obese subjects. The insular cortex is considered to be the primary gustatory cortex [Kringelbach et al., 2004], representing an important relay of the neural circuitry connecting the hypothalamus, OFC, and the limbic system [Tataranni et al., 1999]. These areas, including the striatum, comprise the central orexigenic network, which is essential for regulating food intake. Although hunger has been associated with an increase in regional cerebral blood flow (rCBF), satiation has been associated with a decrease in rCBF in the insular cortex, suggesting that the insular cortex is affected by the state of hunger. However, this rCBF decrease was significantly greater in obese individuals [Gautier et al., 2000; Gautier et al., 2001]. Therefore, it has been proposed that obese individuals interpret the state of hunger as more threatening, which is additionally supported by our finding showing that FC strength in the insular cortex after an overnight fast was decreased in proportion to the BMI. Since the insular cortex is part of the central orexigenic network, it is tempting to speculate that overeating may compensate decreased FC strength in the orexigenic network. Interestingly, we did not find altered functional connectivity in the prefrontal lobe, basal ganglia, and motor sensory network in obese compared with lean individuals.
To understand the pathophysiology of excessive food intake in humans, it is crucial to further include hormonal modulation of eating behavior. Insulin is a prime candidate for feedback mechanisms to downregulate appetite and terminate food intake, as it acutely responds to food intake with a profound and transient increase [Marks et al., 1991; Unger et al., 1991; Zhao and Alkon, 2001]. Additionally, insulin crosses the blood‐brain barrier and binds to its widely expressed receptors in the brain [Marks et al., 1991; Unger et al., 1991]. Several studies have illustrated a negative relationship between changes in insulin and rCBF in the OFC and striatum following a meal [Del Parigi et al., 2002; Tataranni et al., 1999]. Moreover, animal studies have shown that insulin decreases the rewarding aspect of food, such as by decreasing dopamine signaling [Figlewicz et al., 1994; Patterson et al., 1998]. The aforementioned findings insinuate that an increase in insulin reduces the rewarding effect of food. Concurrently, our results revealed specific brain areas within the reward system that are sensitive to insulin. The correlation pattern indicates that subjects with higher fasting insulin levels were associated with stronger orbitofrontal and putamen functional connectivity independent of BMI. This implies that higher fasting insulin levels, which are correlated with lower insulin sensitivity indices, are associated with increased FC strength in reward related areas. Assuming that the enhanced FC of the reward system to elevated fasting insulin levels are an “over‐activation” of the reward related areas, further increased postprandial insulin levels would not be able to elicit an appropriate reward response to food intake.
Nevertheless, further studies are needed to elucidate the effect of insulin on the reward system
In conclusion, our findings suggest that obesity and insulin levels have substantial influence on brain function during rest in networks supporting reward and food regulation. We report BMI‐ and insulin‐associated FC strength within four RSNs. With an increasing number of individuals becoming obese, a detailed understanding of brain networks supporting food intake is vital. A unique advantage provided by the method we used (resting state fMRI) is that it allows one to examine task‐independent network functional connectivity. The absence of a task gets rid of issues as performance differences among groups and practice effects. The enhanced spatial resolution and reliance on endogenous signal rather than radionuclide tracers also make this approach preferable to PET. Increasing evidence suggests that intrinsic brain FC is important for healthy brain function. Changes in intrinsic brain FC have previously been related to several different medical conditions, such as Alzheimer's disease [Greicius et al., 2004], depression [Greicius et al., 2007], schizophrenia [Zhou et al., 2007], and autism [Di Martino et al., 2009]. Our data suggest that the analysis of intrinsic brain FC may enlarge the understanding of obesity and RSN connectivity may be a useful biological marker and treatment target for obesity.
We want to acknowledge some limitations of the this study. First, the number of subjects investigated was rather small; a larger sample size could improve the significance of the this study. Second, obesity has been associated with reduced cognitive abilities especially in elderly. However, we did not perform any cognitive tests.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1. Resting state networks. Group average default mode (A), prefrontal lobe (B), temporal lobe (C), basal ganglia (D), and motor sensory (E) features, extracted from fMRI data of lean and obese subjects, thresholded at P<0.05 FWE‐corrected.
Supporting Information Table 1.
Acknowledgements
The authors thank Maike Borutta and Anna Bury for excellent technical assistant.
REFERENCES
- Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC ( 2001): Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron 32: 537–551. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2005): Unified segmentation. Neuroimage 26: 839–851. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM ( 2005): Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ ( 1995): An information‐maximization approach to blind separation and blind deconvolution. Neural Comput 7: 1129–1159. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ ( 2001): A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14: 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR ( 2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129( Part 3): 564–583. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME ( 2001): Frequencies contributing to functional connectivity in the cerebral cortex in “resting‐state” data. AJNR Am J Neuroradiol 22: 1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Correa N, Adali T, Calhoun VD ( 2007): Performance of blind source separation algorithms for fMRI analysis using a group ICA method. Magn Reson Imaging 25: 684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD ( 2000): Subcortical and cortical brain activity during the feeling of self‐generated emotions. Nat Neurosci 3: 1049–1056. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA ( 2008): Reduced resting‐state brain activity in the “default network” in normal aging. Cereb Cortex 18: 1856–1864. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF ( 2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103: 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM ( 2006): fMRI resting state networks define distinct modes of long‐distance interactions in the human brain. Neuroimage 29: 1359–1367. [DOI] [PubMed] [Google Scholar]
- Del Parigi A, Gautier JF, Chen K, Salbe AD, Ravussin E, Reiman E, Tataranni PA ( 2002) Neuroimaging and obesity: Mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann NY Acad Sci 967: 389–397. [PubMed] [Google Scholar]
- Del Parigi A, Chen K, Salbe AD, Reiman EM, Tataranni PA ( 2005): Sensory experience of food and obesity: A positron emission tomography study of the brain regions affected by tasting a liquid meal after a prolonged fast. Neuroimage 24: 436–443. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP ( 2008): Functional connectivity of human striatum: A resting state FMRI study. Cereb Cortex 18: 2735–2747. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, Gotimer K, Klein DF, Castellanos FX, Milham MP ( 2009): Relationship between cingulo‐insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry 166: 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Szot P, Chavez M, Woods SC, Veith RC ( 1994): Intraventricular insulin increases dopamine transporter mRNA in rat VTA/substantia nigra. Brain Res 644: 331–334. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL ( 2002): Prevalence and trends in obesity among US adults, 1999‐2000. JAMA 288: 1723–1727. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail MH ( 2005): Excess deaths associated with underweight, overweight, and obesity. JAMA 293: 1861–1867. [DOI] [PubMed] [Google Scholar]
- Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M, Ravussin E, Reiman EM, Tataranni PA ( 2000): Differential brain responses to satiation in obese and lean men. Diabetes 49: 838–846. [DOI] [PubMed] [Google Scholar]
- Gautier JF, Del Parigi A, Chen K, Salbe AD, Bandy D, Pratley RE, Ravussin E, Reiman EM, Tataranni PA ( 2001): Effect of satiation on brain activity in obese and lean women. Obes Res 9: 676–684. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF ( 2007): Resting‐state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V ( 2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V ( 2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 101: 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennige AM, Sartorius T, Tschritter O, Preissl H, Fritsche A, Ruth P, Haring HU ( 2006): Tissue selectivity of insulin detemir action in vivo. Diabetologia 49: 1274–1282. [DOI] [PubMed] [Google Scholar]
- Karhunen LJ, Lappalainen RI, Vanninen EJ, Kuikka JT, Uusitupa MI ( 1997): Regional cerebral blood flow during food exposure in obese and normal‐weight women. Brain 120( Part 9): 1675–1684. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, de Araujo IE, Rolls ET. 2004. Taste‐related activity in the human dorsolateral prefrontal cortex. Neuroimage 21: 781–788. [DOI] [PubMed] [Google Scholar]
- Li YO, Adali T, Calhoun VD ( 2007): Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp 28: 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks JL, King MG, Baskin DG ( 1991): Localization of insulin and Type 1 IGF receptors in rat brain by in vitro autoradiography and in situ hybridization. Adv Exp Med Biol 293: 459–470. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA ( 1999): Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J, DeFronzo RA, Fox PT, Gao JH ( 1999): Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 48: 1801–1806. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppelmann LF ( 1981): Manual for the Profile of Mood states. San Diego: Educational and Industrial Testing Service. [Google Scholar]
- Patterson TA, Brot MD, Zavosh A, Schenk JO, Szot P, Figlewicz DP ( 1998): Food deprivation decreases mRNA and activity of the rat dopamine transporter. Neuroendocrinol 68: 11–20. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Basso G, Soldati N, Sailer U, Jovicich J, Bruzzone L, Kryspin‐Exner I, Bauer H, Moser E ( 2009): A resting state network in the motor control circuit of the basal ganglia. BMC Neurosci 10: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF ( 2007): Differential activation of the dorsal striatum by high‐calorie visual food stimuli in obese individuals. Neuroimage 37: 410–421. [DOI] [PubMed] [Google Scholar]
- Stephan F, Reville P, Thierry R, Schlienger JL ( 1972): Correlations between plasma insulin and body weight in obesity, anorexia nervosa and diabetes mellitus. Diabetologia 8: 196–201. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM ( 2008): Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. J Abnorm Psychol 117: 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Ng J, Zald DH ( 2009): Relation of obesity to consummatory and anticipatory food reward. Physiol Behav 97: 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW III, Twieg DB, Knowlton RC, Cox JE ( 2008): Widespread reward‐system activation in obese women in response to pictures of high‐calorie foods. Neuroimage 41: 636–647. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E ( 1999): Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 96: 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschritter O, Hennige AM, Preissl H, Grichisch Y, Kirchhoff K, Kantartzis K, Machicao F, Fritsche A, Haring HU ( 2009a): Insulin effects on beta and theta activity in the human brain are differentially affected by ageing. Diabetologia 52: 169–171. [DOI] [PubMed] [Google Scholar]
- Tschritter O, Hennige AM, Preissl H, Porubska K, Schafer SA, Lutzenberger W, Machicao F, Birbaumer N, Fritsche A, Haring HU. 2007a. Cerebrocortical beta activity in overweight humans responds to insulin detemir. PLoS One 2( 11): e1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschritter O, Preissl H, Hennige AM, Sartorius T, Grichisch Y, Stefan N, Guthoff M, Dusing S, Machann J, Schleicher E and others ( 2009b): The insulin effect on cerebrocortical theta activity is associated with serum concentrations of saturated nonesterified Fatty acids. J Clin Endocrinol Metab 94: 4600–4607. [DOI] [PubMed] [Google Scholar]
- Tschritter O, Preissl H, Hennige AM, Stumvoll M, Porubska K, Frost R, Marx H, Klosel B, Lutzenberger W, Birbaumer N, Häring HU, Fritsche A. ( 2006): The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: A magnetoencephalographic study. Proc Natl Acad Sci USA 103: 12103–12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschritter O, Preissl H, Yokoyama Y, Machicao F, Haring HU, Fritsche A ( 2007b): Variation in the FTO gene locus is associated with cerebrocortical insulin resistance in humans. Diabetologia 50: 2602–2603. [DOI] [PubMed] [Google Scholar]
- Unger JW, Livingston JN, Moss AM ( 1991): Insulin receptors in the central nervous system: Localization, signalling mechanisms and functional aspects. Prog Neurobiol 36: 343–362. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia‐Klein N, Logan J, Wong C, Thanos PK, Ma Y, Pradhan K. ( 2009): Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity (Silver Spring) 17: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Felder C, Fowler JS, Levy AV, Pappas NR, Wong CT, Zhu W, Netusil N ( 2002): Enhanced resting activity of the oral somatosensory cortex in obese subjects. Neuroreport 13: 1151–1155. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS ( 2001): Brain dopamine and obesity. Lancet 357: 354–357. [DOI] [PubMed] [Google Scholar]
- WHO 2010. Obesity and overweight. World Health Organization. Available at: http://www.who.int/dietphysicalactivity/publications/facts/obesity/en/. Accessed on March 25, 2010.
- Woods SC, Porte D, Jr. , Bobbioni E, Ionescu E, Sauter JF, Rohner‐Jeanrenaud F, Jeanrenaud B ( 1985): Insulin: Its relationship to the central nervous system and to the control of food intake and body weight. Am J Clin Nutr 42( 5 Suppl): 1063–1071. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Alkon DL ( 2001): Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol 177: 125–134. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, Liu H, Kuang F ( 2007): Functional dysconnectivity of the dorsolateral prefrontal cortex in first‐episode schizophrenia using resting‐state fMRI. Neurosci Lett 417: 297–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1. Resting state networks. Group average default mode (A), prefrontal lobe (B), temporal lobe (C), basal ganglia (D), and motor sensory (E) features, extracted from fMRI data of lean and obese subjects, thresholded at P<0.05 FWE‐corrected.
Supporting Information Table 1.


