Abstract
Nocturnal enuresis is a common developmental disorder in children, and primary nocturnal enuresis (PNE) is the dominant subtype. The main purpose of this study was to investigate brain functional abnormalities specifically related to motor response inhibition in children with PNE using fMRI in combination with a Go/NoGo task. Twenty‐two children with PNE and 22 healthy children, group‐matched for age and sex, took part in this experiment. Although no significant between‐group differences in task performance accuracy were observed, PNE patients showed significantly longer response times on average. There were several brain regions with reduced activation during motor response inhibition in children with PNE: the bilateral inferior frontal gyri, right superior and middle frontal gyri, right inferior parietal lobe, bilateral cingulate gyri and insula. Our data indicate that response inhibition in children with PNE is associated with a relative lack of or delay in the maturation of prefrontal cortex circuitry that is known to suppress inappropriate responses. This result might give clues to understanding the pathophysiology of PNE. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: primary nocturnal enuresis, function MRI, inhibition, children, development
INTRODUCTION
Primary nocturnal enuresis (PNE) is a common finding in school‐age children, with a prevalence rate around 9% in children between 5 and 10 years of age [Lottmann and Alova,2007]. Children with enuresis often experience unnecessary emotional distress and have low self‐esteem [Garber,1996]. Although PNE is a common disorder with a large inpsychological impact on afflicted children and an economic impact on the children's families, the pathogenesis of enuresis is unclear. In the past, electroencephalograph (EEG) [Hallioglu et al.,2001], event‐related brain potential (ERP) [Karlidag et al.,2004], and startle blink [Freitag et al.,2006] have been used to study enuresis. It was found that maturational delay of the central nervous system is an important factor in the pathogenesis of PNE [Freitag et al.,2006; Hallioglu et al.,2001; Karlidag et al.,2004]. Children with PNE cannot prevent themselves from voiding during sleep, and that inability might be related to weak inhibition function. The literature shows that the prefrontal cortex (especially the inferior frontal gyrus) is the key area for inhibition function [Hampshire et al.,2010].
In fact, the prefrontal cortex (PFC) is important for various higher cognitive functions that have been shown to develop with age, even throughout adolescence [Rubia et al.,2006; Tsujimoto,2008]. The PFC is responsible for executive functions, which include mediating conflicting thoughts, making choices, predicting future events, and governing social control [Miller and Cohen,2001]. Previous research suggests that the PFC is important for bladder control; to maintain urine storage, the PFC inhibits the midbrain periaqueductal gray matter [Fowler and Griffiths,2010; Griffiths and Tadic,2008]. Children who have a poorly functioning PFC could have abnormal urine storage.
Therefore, we hypothesized that children with PNE have delayed development of the PFC and consequent response inhibition deficits. In this study, response inhibition function in children with PNE and in healthy children was investigated using the classic Go/NoGo task and functional magnetic resonance imaging (fMRI) analysis.
MATERIALS AND METHODS
Subjects
Forty‐four children between 8 and 15 years of age participated in the study, with the consent of children and their guardians. There were two groups of 22 children: the PNE group (14 males, 8 females) and the normal control group (13 males, 9 females). The children with PNE had a mean age of 10.5 (SD = 2.0) years; healthy controls were 11.5 (SD = 1.9) years old, on average. The mean duration of school education was 4.5 (SD = 2.0) years for children with PNE and 5.5 (SD = 1.9) years for healthy controls. All children were right‐handed and had an IQ >75, and all neurological and psychiatric diseases were excluded based on clinical examination and a structured interview. All of the 22 children with PNE were outpatients of the Shanghai Children's Medical Center. This study was approved by the IRB of Shanghai Children's Medical Center affiliated with Shanghai Jiao Tong University School of Medicine (No: SCMC‐201014). Additional clinical data on the patient group are given in Table I.
Table I.
Clinical data for the patient group
| Age | Gender | Weight (kg) | Bed‐wetting frequency (per day) | Bed‐wetting frequency (per week) | Bladder volume (ml) | Frequency with which patient wakes up for voluntary voiding | |
|---|---|---|---|---|---|---|---|
| 1 | 10 | M | 40 | 1 | 6 | 173 | Sometimes |
| 2a | 10 | M | 30 | 1 | 1–2 | 303 | Sometimes |
| 3a | 10 | M | 30 | 1 | 2 | 130 | Sometimes |
| 4 | 8 | M | 35 | 1 | 1 | 100 | Often |
| 5a | 11 | F | 35 | 1 | 4 | 132 | Sometimes |
| 6 | 8 | F | 25 | 2 | 7 | 140 | Sometimes |
| 7 | 9 | M | 48 | 0–1 | 1 | 130 | Sometimes |
| 8 | 10 | M | 29 | 2 | 7 | 100 | Sometimes |
| 9 | 10 | M | 49 | 1 | 2–3 | 155 | Sometimes |
| 10 | 15 | F | 50 | 2–3 | 7 | 510 | Never |
| 11a | 15 | F | 45 | 1 | 1 | 450 | Often |
| 12a | 8 | M | 22 | 1 | 1 | 235 | Sometimes |
| 13a | 11 | F | 25 | 1–2 | 1–2 | 251 | Often |
| 14 | 15 | M | 54 | 1–2 | 7 | 192 | Never |
| 15a | 8 | M | 34 | 2 | 5–6 | 260 | Sometimes |
| 16 | 8 | M | 26 | 2 | 7 | 190 | Sometimes |
| 17a | 12 | M | 32 | 2 | 5 | 350 | Sometimes |
| 18a | 11 | F | 34 | 1 | 7 | 165 | Sometimes |
| 19a | 13 | M | 41 | 1 | 2–3 | 245 | Often |
| 20a | 10 | F | 31 | 1 | 3–4 | 310 | Sometimes |
| 21a | 8 | F | 23 | 1–2 | 6 | 115 | Sometimes |
| 22a | 11 | M | 37 | 1 | 6 | 120 | Sometimes |
All patients wet the bed only at night. Age given in years; M = male; F = female.
These children can wake up after bed‐wetting.
fMRI Paradigm: Go/NoGo Task
The present study used the same version of the Go/NoGo task as that described by Menon et al. [2001]. In the task, the subjects were first induced to form a temporary response habit by following some repetitive stimulation‐response actions in the Go block. They were then asked to inhibit the habit in the Go/NoGo block, which consists of a random combination of stimulation‐response and stimulation‐no‐response actions. The Go/NoGo task requires motor response inhibition and selective attention without unduly loading working memory. The paradigm consists of a 30‐s rest epoch and 10 alternating 26‐s epochs of Go and Go/NoGo conditions, followed by a 30‐s rest epoch. All stimuli were shown in the IFIS‐SA system (Invivo Corporation, America), and the IFIS‐SA system recorded the key‐press responses of each subject. IFIS‐SA system is professional fMRI stimulation, using RF monitor, can realize the synchronization between the presentation and the scanner. In Go and Go/NoGo blocks, every presentation of letters was synchronized with the beginning of the scan. During the rest condition, subjects passively viewed a “cross” on a blank screen. There were two experimental conditions: Block A—the Go condition—had 26 targets and Block B—the Go/NoGo condition—had 13 targets and 13 non‐targets. During the experiment, subjects viewed a series of letters once every 2 s (1,000‐ms stimulus and 1,000 ms interstimulus interval) and responded with a key press to every letter except the letter “V.” All subjects responded using the forefinger of the right hand. In the Go (control) condition, subjects were presented a random sequence of letters other than the letter “V.” In the Go/NoGo (experimental) condition, subjects were presented with the letter “V” 50% of the time, thus requiring response to half the trials (Go trials) and response inhibition to the other half (NoGo trials).
Analysis of Performance Data
Response times (RT) and accuracy of performance of the task were recorded for patients and control subjects for both the Go and NoGo conditions. Average response time (RT) is the average value of response time in all blocks, including Go and Go/NoGo, but except the missing responses. Performance data (accuracy and RT) were compared using unpaired t tests to check for significant differences in performance between the two groups (P < 0.05).
fMRI Image Acquisition
All imaging procedures were conducted at the Shanghai Key Laboratory of Magnetic Resonance (East Normal University of China, Shanghai, China) on the Siemens 3.0 T Trio Tim MR system. Anatomical images covering the whole brain were collected using a T1‐weighted gradient echo pulse sequence (TR = 440 ms, TE = 2.46 ms, flip angle = 90°, 256 × 320 matrix, FOV = 220 mm, 32 slices), and subsequently used for exact anatomical localization. A total of 160 whole‐brain volumes were collected on 32 oblique slices (3‐mm thick, 33% Dist factor) using a T 2*‐weighted gradient echo spiral pulse sequence that was sensitive to blood oxygen level‐dependent (BOLD) contrast, with the following acquisition parameters: echo time (TE) = 30 ms, repetition time (TR) = 2,000 ms, flip angle = 90, FOV = 22 × 22 cm2, acquisition matrix = 64 × 64, voxel size = 3.4 × 3.4 × 3 mm3.
Individual‐Level fMRI Image Analysis
Functional images were analyzed with statistical parametric mapping software (SPM5; http://www.fil.ion.ucl.ac.uk./spm/spm5.html) and Matlab (Math‐Works, Natick, MA) software on a personal computer. Images from the first ten TRs at the beginning of each trial were discarded to enable the signal to achieve steady‐state equilibrium. Image preprocessing included motion correction and realignment of the images to each subject's first image. Sessions were then normalized using the mean functional volume resampled to 2 × 2 × 2 mm3 voxels in Montreal Neurological Institute (MNI) stereotaxic space. Spatial smoothing was performed on the functional images using a Gaussian filter (6‐mm full width half‐maximum, FWHM). All children with head movement exceeding 2 mm, regardless of rotation and translation, were excluded from further analysis.
In the first‐level analysis, we constructed for each individual subject a contrast between the Go/NoGo and Go conditions (experimental minus control) to evaluate the degree of brain activation specific for response inhibition. These contrast images were analyzed with SPM5 using a general linear model (GLM) to determine, voxel‐wise, t‐statistics for each subject. Height threshold was set at P < 0.001, uncorrected for multiple comparisons.
Group‐Level Analysis
The con or contrast (difference in β) images of the first‐level analysis were then used for the second‐level group statistics. For group analysis, a random effects model was used to determine, voxel‐wise, t‐statistics contrasting specific conditions of interest. These contrast images (Go/NoGo minus Go) of 22 PNE subjects and 22 control subjects were used to determine within‐group activation for the PNE and control groups by one‐sample t tests. Clusters of activation to display were defined as those surpassing a height threshold of P < 0.0001 (uncorrected at the voxel level) and an extent threshold of 10 voxels. However, only activation significant at the cluster level (P < 0.01) will be reported.
Group Difference Analysis
Two‐sample t tests were used to determine between‐group differences. A random effects model was used for between‐group analyses. Clusters of activation to display were defined as those surpassing a height threshold of P < 0.0001 and an extent threshold of 10 voxels for all within‐ and between‐group analyses. Only activation significant at the cluster level (P < 0.01) will be reported.
RESULTS
Task Performance
There were no significant differences between the two groups in either the average percentage of correct responses for the Go/NoGo task (95.2% ± 2.7% for children with PNE; 97.0% ± 1.7% for control children) or in the number of incorrect NoGo responses. But the average RT was longer in PNE children (P < 0.001): 522 ± 81 ms in children with PNE, and 487 ± 47 ms in control children.
Functional MRI Data
Within‐group activations
Within‐group activations for the contrast of motor response inhibition (Go/NoGo‐Go) are shown in Table II. Controls activated the bilateral inferior PFC, right superior and middle frontal gyri, bilateral supplementary motor areas (SMA), bilateral inferior parietal lobe, bilateral superior temporal gyri, right cingulate gyrus, bilateral insula, bilateral caudate nuclei, and right putamen. Children with PNE activated the bilateral inferior PFC, right superior and middle frontal gyri, bilateral SMA, bilateral inferior parietal lobe, bilateral superior temporal gyri, bilateral cingulate gyri, bilateral insula, bilateral caudate nuclei and right putamen. Thus, there was activation of similar brain networks in children with PNE and in healthy children during motor response inhibition. In both groups, the activated areas were distributed more in the right than in the left brain hemisphere.
Table II.
Significant within‐group activation during response inhibitiona in children with PNE and in healthy children and between‐group differences in activation
| Group and region | Z valueb | Number of voxels | Peak location | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Healthy children (HC) | |||||
| Right inferior frontal gyrus (BA47/45) | 5.46 | 680 | 52 | 16 | 2 |
| Left inferior frontal gyrus (BA47/45) | 5.12 | 162 | −36 | 10 | 4 |
| Right superior and middle frontal gyri (BA9/10) | 5.18 | 650 | 30 | 40 | 34 |
| Right SMA (BA6) | 4.72 | 225 | 6 | 16 | 62 |
| Left SMA (BA6) | 4.81 | 87 | −2 | 16 | 44 |
| Right inferior parietal lobe (BA40) | 4.94 | 1273 | 34 | −50 | 44 |
| Left inferior parietal lobe (BA40) | 4.84 | 76 | −66 | −38 | 20 |
| Right superior temporal gyrus (BA38) | 6.02 | 206 | 56 | −40 | 32 |
| Left superior temporal gyrus (BA38) | 4.84 | 104 | −66 | −38 | 20 |
| Middle cingulate gyrus (BA32/24) | 5.11 | 620 | 6 | 16 | 42 |
| Right insula (BA13) | 5.65 | 373 | 32 | 22 | 6 |
| Left insula (BA13) | 5.12 | 491 | −36 | 10 | 4 |
| Right caudate and putamen | 4.78 | 182 | 14 | −8 | 20 |
| Left caudate | 4.86 | 57 | −8 | 0 | 12 |
| PNE | |||||
| Right inferior frontal gyrus (BA47/45) | 5.87 | 249 | 30 | 20 | 8 |
| Left inferior frontal gyrus (BA47/45) | 5.12 | 132 | −32 | 16 | 16 |
| Right superior and middle frontal gyri (BA9/10) | 5.45 | 789 | 36 | 44 | 24 |
| Right SMA (BA6) | 5.89 | 471 | 14 | 2 | 66 |
| Left SMA (BA6) | 5.89 | 19 | 14 | 2 | 66 |
| Right inferior parietal lobe (BA40) | 4.53 | 178 | 50 | −38 | 48 |
| Left inferior parietal lobe (BA40) | 4.67 | 10 | −64 | −48 | 30 |
| Right superior temporal gyrus (BA38) | 5.04 | 105 | 48 | −18 | 8 |
| Left superior temporal gyrus (BA38) | 4.84 | 43 | −66 | −38 | 20 |
| Right and left cingulate gyri (BA32/24) | 5.40 | 410 | 10 | 32 | 2 |
| Right insula (BA13) | 5.56 | 480 | 34 | 10 | −4 |
| Left insula (BA13) | 5.45 | 304 | −38 | 10 | 0 |
| Right caudate and putamen | 4.72 | 267 | 20 | 4 | 8 |
| Left caudate | 4.71 | 24 | −10 | −4 | 18 |
| HC>PNE | |||||
| Right inferior frontal gyrus (BA47/45) | 6.07 | 740 | 32 | 22 | 8 |
| Left inferior frontal gyrus (BA47/45) | 4.74 | 124 | −40 | 20 | 0 |
| Right middle frontal gyrus (BA6) | 5.43 | 215 | 38 | 2 | 46 |
| Right superior and middle frontal gyri (BA9/10) | 5.45 | 478 | 30 | 40 | 34 |
| Right inferior parietal lobe (BA40) | 5.75 | 867 | 52 | −38 | 32 |
| Right and left cingulate gyri (BA32/24) | 4.97 | 376 | 12 | 14 | 42 |
| Right insula (BA13) | 5.46 | 287 | 28 | 18 | −10 |
| Left insula (BA13) | 5.45 | 299 | −38 | 14 | 8 |
| PNE>HC | |||||
| Left posterior cingulate cortex (BA28/10) | 5.86 | 405 | −10 | −54 | 22 |
| Right medial frontal gyrus (BA11/10) | 5.60 | 398 | 4 | 40 | −18 |
| Left anterior cingulate (BA32) | 5.43 | 65 | −14 | 34 | −4 |
| Left inferior and middle temporal gyri (BA21) | 5.10 | 117 | −56 | −8 | −22 |
| Left middle temporal gyrus (BA39) | 4.77 | 110 | −40 | −68 | 26 |
BA = Brodman's areas; X, Y, Z = MNI coordinates; SMA: supplementary motor area.
Assessed by the contrast Go/NoGo versus Go, P < 0.0001 uncorrected at the voxel level and cluster > 10 voxels and only activation significant at the cluster level (P < 0.01) will be reported.
For peak areas of activation.
Between‐group activations
Children with PNE showed significantly reduced activation compared to healthy children during response inhibition in the bilateral inferior frontal gyri, right superior and middle frontal gyri, right inferior parietal lobe, bilateral cingulate gyri and bilateral insula (see Table II and Fig. 1).
Figure 1.
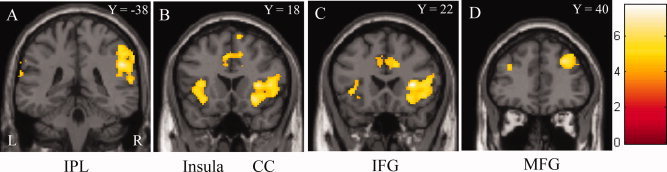
Children with PNE exhibited a reduction of brain activation in the bilateral IFG, right MFG, right IPL, bilateral insula and CC, when compared with healthy children. IFG: inferior frontal gyrus, MFG: middle frontal gyrus, IPL: inferior parietal lobe, CC: cingulate cortex. And L: left side, R: right side, Y = : Y coordinate in MNI space.
Children with PNE also showed some areas of overactivation during response inhibition compared to healthy children: the left posterior cingulate cortex, right medial frontal gyrus, left anterior cingulate, left inferior and middle temporal gyri and left middle temporal gyrus (see Table II).
DISCUSSION
Many reports in the literature describe the brain areas activated during the Go/NoGo task. The results of the present study, which showed in both the control and PNE groups that activation was most pronounced in the inferior frontal gyri, inferior parietal lobes, and anterior cingulate gyri during response inhibition, were consistent with prior reports [Aron et al.,2004; Mazzola‐Pomietto et al.,2009; Menon et al.,2001].
The main results of this study were that (1) similar brain regions were activated during motor response inhibition in healthy children and in those with PNE; (2) compared to healthy children, children with PNE exhibited a reduction in brain activation in the bilateral inferior frontal gyri, right superior and middle frontal gyri, right inferior parietal lobe, bilateral cingulate gyri and bilateral insula; (3) children with PNE showed increased activation in the left posterior cingulate, right medial frontal gyrus, left anterior cingulate, left inferior and middle temporal gyri and left middle temporal gyrus.
In general, our data show that the right hemisphere was underactivated, and the left hemisphere was overactivated, when children with PNE performed a motor response inhibition task. Garavan et al. [1999] reported that the dominant areas of activation during response inhibition are in the right hemisphere, including the middle and inferior frontal gyri, frontal limbic area, anterior insula, and inferior parietal lobe. Many other studies suggest that the right hemisphere is activated more than the left during response inhibition [Horn et al.,2003; Menon et al.,2001; Rubia et al.,2003]. Our finding of underactivation in the right hemisphere and overactivation in the left hemisphere indicates an abnormal network of brain areas involved in response inhibition in children with PNE.
Relative to healthy children, those with PNE exhibited a reduction of brain activation. We will discuss the functions of these areas and their potential roles in PNE pathology.
Children with PNE exhibited a reduction of brain activation in several PFC areas, including the bilateral inferior frontal gyri and the right superior and middle frontal gyri. The frontal lobes are necessary for inhibitory control. Previous results have shown that response inhibition leads to activation of the ventrolateral and dorsolateral PFC (VLPFC and DLPFC, respectively) bilaterally but with greater activation in the right hemisphere [Horn et al.,2003; Liddle et al.,2001; Menon et al.,2001; Rubia et al.,2003]. Previous research also suggests that the right inferior frontal gyrus is a key node in the brain network for response inhibition [Aron et al.,2004; Chamberlain and Sahakian,2007]. Furthermore, damage to the right superior medial frontal lobe in patients slows inhibitory control [Floden and Stuss,2006]. Thus, the PFC (especially the inferior frontal gyrus) plays an important role in response inhibition. Our results showed that children with PNE have an underactive right PFC (inferior frontal gyrus, superior, and middle frontal gyri) and left inferior frontal gyrus during response inhibition, which might be responsible for the dysfunction of response inhibition in PNE patients. It has been reported that the right inferior PFC is more strongly activated in adults compared with children/adolescents during successful inhibition [Rubia et al.,2007], which indicates that this structure is important in the developmental improvement observed in inhibitory abilities. Altogether, the underactivation of the PFC (especially the inferior frontal gyrus) in children with PNE suggests a developmental delay in the PFC and a response inhibition functional deficit.
In a series of positron emission tomography (PET) experiments by Blok et al. [1997], micturition was associated with increased blood flow in the right inferior frontal gyrus and other brain areas. Hruz et al. [2008] also reported that compared to passive filling of the bladder, emptying the bladder was associated with increased brain activity in the right inferior frontal gyrus. Similarly, we found one of the most significant reductions in activation in the right inferior frontal gyrus in children with PNE during response inhibition (see Table II and Fig. 1C). In the accepted model of bladder control, the PFC is associated with decision‐making in voiding [Fowler and Griffiths,2010; Griffiths and Tadic,2008]. In this model, during urine storage, the PFC prevents voiding by inhibiting the midbrain periaqueductal gray matter [Fowler and Griffiths,2010; Griffiths and Tadic,2008]. Dysfunction of the PFC during response inhibition might induce abnormal voiding behavior.
Compared to the Go condition, the right inferior parietal lobe was activated more during the Go/NoGo condition. The activation of parietal cortex might reflect a transient increase in attentional processes in the inhibition task (Go/NoGo condition) compared to the executive control task (Go condition). The PNE group had less activation of the right inferior parietal lobe during response inhibition, which suggests a decline in attention. A previous study reported that children with enuresis had a higher likelihood of inattentive symptoms than children without enuresis [Elia et al.,2009]. Moreover, children with attention deficit hyperactivity disorder (ADHD) are at risk for persistent enuresis [Baeyens et al.,2005]. Baeyens et al. [2004] reported that among 120 children, aged 6–12 years, with nocturnal enuresis, 15% were diagnosed with the full syndrome of ADHD and 22.5% met the criteria of the ADHD inattentive subtype. Thus, there in an association between enuresis and ADHD, and children with PNE might have attention problems. Although the patients in the present study were diagnosed with PNE but not ADHD, mild ADHD might not have been excluded.
The bilateral cingulate gyri and insula were also activated less during response inhibition in the PNE group relative to healthy subjects. Previous studies have indicated that the anterior cingulate cortex specifically monitors response conflict [Braver et al.,2001]. Ramautar et al. [2006] suggested that the insula in particular might be affected during a failure in inhibition. Menon et al. [2001] reported that the anterior‐ventral aspect of the cingulate cortex and the left and right insula are all activated during error processing (“incorrect NoGo” vs. “correct NoGo” events) in the Go/NoGo task. It has been found PNE has a large psychological impact on children, such as unnecessary emotional distress and low self‐esteem [Garber,1996]. It can be seen that the different level of activation in the cingulate gyri and bilateral insula in PNE groups might reflect different psychological processing during an error for a NoGo response.
There were some areas of overactivation in children with PNE compared to healthy children during response inhibition. The overactivated areas were mainly in the left hemisphere, which suggests that children with PNE might use the left hemisphere to compensate for the deficits in the right hemisphere during response inhibition.
In addition, our results show that children with PNE and healthy children had a similar percentage of correct responses on the Go/NoGo task, but the PNE group had a longer RT. Children with PNE have uncontrolled micturition during sleep, but they behave normally when awake, and our test required the two groups of children to be awake during the test. Thus, that the two groups performed the task with similar scores is not surprising. In fact, the extra time needed for the PNE group to perform the task is likely due to several factors, such as the task requiring more effort and the mood state of the children. The performance data was in line with the fMRI data.
A classical block design, as was used in the current study, has several advantages: it is simple and powerful, and it is not difficult to perform in children. In contrast, with a block design, the findings might not solely reflect response inhibition but also other cognitive functions, such as conflict detection and error monitoring. This is a limitation for our study. Since the prevalence rate of PNE is known to decrease with age, 2–3 years following brain imaging study would be useful to investigate the abnormal development in the brains of children with PNE. Further study of the structure, function and development process of the PFC in children with PNE is needed.
CONCLUSION
The present study showed that similar networks of brain regions were activated during motor response inhibition in the two groups. The magnitude of activation was weak in some brain areas in children with PNE compared to healthy children. Defective activation of the bilateral inferior frontal gyri and the right superior and middle frontal gyri in children with PNE indicates a relative weakness or delay in the maturation of response inhibition in the PFC. Furthermore, the decreased activation of the right inferior parietal lobe, bilateral cingulate gyri and insula might reflect inattention and different psychological processing when faced with an error for a NoGo response.
Contributor Information
Xiaoxia Du, Email: merryxiaoxia@163.com.
Gengying Li, Email: gyli@phy.ecnu.edu.cn.
REFERENCES
- Aron AR, Robbins TW, Poldrack RA ( 2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8: 170–177. [DOI] [PubMed] [Google Scholar]
- Baeyens D, Roeyers H, Hoebeke P, Verte S, Van Hoecke E, Walle JV ( 2004): Attention deficit/hyperactivity disorder in children with nocturnal enuresis. J Urol 171: 2576–2579. [DOI] [PubMed] [Google Scholar]
- Baeyens D, Roeyers H, Demeyere I, Verte S, Hoebeke P, Vande Walle J ( 2005): Attention‐deficit/hyperactivity disorder (ADHD) as a risk factor for persistent nocturnal enuresis in children: A two‐year follow‐up study. Acta Paediatr 94: 1619–1625. [DOI] [PubMed] [Google Scholar]
- Blok BF, Willemsen AT, Holstege G ( 1997): A PET study on brain control of micturition in humans. Brain 120 ( Part 1): 111–121. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A ( 2001): Anterior cingulate cortex and response conflict: Effects of frequency, inhibition and errors. Cereb Cortex 11: 825–836. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Sahakian BJ ( 2007): The neuropsychiatry of impulsivity. Curr Opin Psychiatry 20: 255–261. [DOI] [PubMed] [Google Scholar]
- Elia J, Takeda T, Deberardinis R, Burke J, Accardo J, Ambrosini PJ, Blum NJ, Brown LW, Lantieri F, Berrettini W, Devoto M, Hakonarson H. ( 2009): Nocturnal enuresis: A suggestive endophenotype marker for a subgroup of inattentive attention‐deficit/hyperactivity disorder. J Pediatr 155: 239–244.e5. [DOI] [PubMed] [Google Scholar]
- Floden D, Stuss DT ( 2006): Inhibitory control is slowed in patients with right superior medial frontal damage. J Cogn Neurosci 18: 1843–1849. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths DJ ( 2010): A decade of functional brain imaging applied to bladder control. Neurourol Urodyn 29: 49–55. [DOI] [PubMed] [Google Scholar]
- Freitag CM, Rohling D, Seifen S, Pukrop R, von Gontard A ( 2006): Neurophysiology of nocturnal enuresis: Evoked potentials and prepulse inhibition of the startle reflex. Dev Med Child Neurol 48: 278–284. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA ( 1999): Right hemispheric dominance of inhibitory control: An event‐related functional MRI study. Proc Natl Acad Sci USA 96: 8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber KM ( 1996): Enuresis: An update on diagnosis and management. J Pediatr Health Care 10: 202–208. [DOI] [PubMed] [Google Scholar]
- Griffiths D, Tadic SD ( 2008): Bladder control, urgency, and urge incontinence: Evidence from functional brain imaging. Neurourol Urodyn 27: 466–474. [DOI] [PubMed] [Google Scholar]
- Hallioglu O, Ozge A, Comelekoglu U, Topaloglu AK, Kanik A, Duzovali O, Yilgor E ( 2001): Evaluation of cerebral maturation by visual and quantitative analysis of resting electroencephalography in children with primary nocturnal enuresis. J Child Neurol 16: 714–718. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM ( 2010): The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage 50: 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW ( 2003): Response inhibition and impulsivity: An fMRI study. Neuropsychologia 41: 1959–1966. [DOI] [PubMed] [Google Scholar]
- Hruz P, Lovblad KO, Nirkko AC, Thoeny H, El‐Koussy M, Danuser H ( 2008): Identification of brain structures involved in micturition with functional magnetic resonance imaging (fMRI). J Neuroradiol 35: 144–149. [DOI] [PubMed] [Google Scholar]
- Karlidag R, Ozisik HI, Soylu A, Kizkin S, Sipahi B, Unal S, Ozcan C ( 2004): Topographic abnormalities in event‐related potentials in children with monosymptomatic nocturnal enuresis. Neurourol Urodyn 23: 237–240. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM ( 2001): Event‐related fMRI study of response inhibition. Hum Brain Mapp 12: 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottmann HB, Alova I ( 2007): Primary monosymptomatic nocturnal enuresis in children and adolescents. Int J Clin Pract 61 ( Suppl.155): 8–16. [DOI] [PubMed] [Google Scholar]
- Mazzola‐Pomietto P, Kaladjian A, Azorin JM, Anton JL, Jeanningros R ( 2009): Bilateral decrease in ventrolateral prefrontal cortex activation during motor response inhibition in mania. J Psychiatr Res 43: 432–441. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL ( 2001): Error‐related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 12: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD ( 2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Slagter HA, Kok A, Ridderinkhof KR ( 2006): Probability effects in the stop‐signal paradigm: The insula and the significance of failed inhibition. Brain Res 1105: 143–154. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E ( 2003): Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage 20: 351–358. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M ( 2006): Progressive increase of frontostriatal brain activation from childhood to adulthood during event‐related tasks of cognitive control. Hum Brain Mapp 27: 973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M ( 2007): Linear age‐correlated functional development of right inferior fronto‐striato‐cerebellar networks during response inhibition and anterior cingulate during error‐related processes. Hum Brain Mapp 28: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto S ( 2008): The prefrontal cortex: Functional neural development during early childhood. Neuroscientist 14: 345–358. [DOI] [PubMed] [Google Scholar]


