Abstract
As Chinese reading engages a different neural network from alphabetic language reading, we investigate whether leftward lateralization of the arcuate fasciculus (AF), as observed in the Western population, is also present in the Chinese population and if it does, whether it is associated with better reading ability. Diffusion tensor tractography analysis on 75 Chinese subjects of three age groups (first graders, fourth graders, and college students) showed that 70–83% of them had leftward lateralization of the AF. The pattern of lateralization did not differ significantly among the three groups, suggesting that lateralization of the AF is formed at an early age and before one enters first grade. Among the first graders, who had just started to learn to read, subjects with strongly leftward lateralized AF scored significantly higher than those with other defined lateralization patterns in Chinese (P = 0.001) and English (P = 0.036) reading tasks. This association was not observed among the fourth graders and college students who were experienced Chinese readers. Among the fourth graders, females were found to obtain significantly higher Chinese (P = 0.033) and English reading scores than males (P = 0.002). Our study suggests a differential effect of leftward lateralization of the AF on reading ability at different stages of reading development in the Chinese population. Hum Brain Mapp, 2011. © 2011 Wiley Periodicals, Inc.
Keywords: language, DTI, tractography, reading, arcuate fasciculus, superior longitudinal fasciculus, MRI, gender
INTRODUCTION
Language is a unique capability of human beings that distinguishes us from other species, and therefore it has been the subject of research for many centuries. In the 19th century, lesion studies have associated speech production with Broca's area [Broca,1861] and speech comprehension with Wernicke's area [Wernicke,1874]. Modern studies using advanced neuroimaging techniques including PET and fMRI have identified a more complex network of segregated brain regions involved in language processing, which included the above two classical regions for alphabetic languages [Poldrack et al.,2001; Price,2000; Turkeltaub et al.,2002]. For harmonic functioning of the segregated brain regions, efficient information exchange among these regions through white‐matter fiber systems is vital. Thus, neuroanatomical studies of these fiber systems have been performed to improve the understanding of language functioning in the brain [Catani and Mesulam,2008]. Postmortem studies in the 19th century have identified the arcuate fasciculus (AF), which connects the Broca's and Wernicke's areas, and plays an important role in language functioning. Damage to the fasciculus has been linked to language deficits, including conduction aphasia.
Diffusion tensor imaging (DTI) is an MR imaging technique that measures the diffusion coefficient in many directions and characterizes the orientational variation of diffusivity using a tensor model [Basser et al.,1994]. By sequentially following the direction with the largest diffusivity, which approximates the traveling direction of a bundle of parallel fibers, it is possible to trace white‐matter tracts in vivo and noninvasively. This technique, diffusion tensor tractography (DTT) [Basser et al.,2000; Mori et al.,1999], is a unique technique for tracking white‐matter tracts in the living brain. It has been used to illustrate perisylvian fiber systems, presumably important for language functioning, and has been feasible in demonstrating the AF connecting Broca's and Wernicke's areas [Catani et al.,2005; Glasser and Rilling,2008; Mori et al.,2002; Wakana et al.,2007]. An anthropological comparative study using DTT has shown that the AF is prominent in human beings but small or nonobservable in non‐human primates [Rilling et al.,2008], further supporting the evolutionary significance of the AF in developing advanced brain functions such as language (see also Zhang et al. [2007]). A study showed that “fiber density” of AF was highly leftward lateralized [Nucifora et al.,2005] in the human brain, and, therefore, it was postulated to be an anatomical substrate for the leftward lateralization of language function. This finding was later replicated by multiple groups [Catani et al.,2007; Eluvathingal et al.,2007; Lebel and Beaulieu,2009; Vernooij et al.,2007]. A combined DTI and fMRI study further showed that the lateralization of AF highly correlated with functional lateralization of language processing, with higher degree of AF leftward asymmetry associated with stronger activation in the left hemisphere during language tasks [Vernooij et al.,2007]. Furthermore, there is some evidence that lateralization pattern of the AF is correlated with language ability measures in the alphabetic language population [Catani et al.,2007; Lebel and Beaulieu,2009].
All previous studies investigating AF asymmetry were on alphabetic language speakers/readers. Chinese language, however, differs dramatically from alphabetic languages in the writing system. On the other hand, a phonetic system, pinyin, is taught to new primary‐school students to facilitate the denotation of Chinese characters and has clear letter‐sound conversion rules as in alphabetic languages. Previous studies have shown that Chinese readers engage in different brain network for language processing [Siok et al.,2004,2008; Tan et al.,2005]. Particularly, the left posterior superior temporal area, including the Wernicke's area, was found to be inactive during phonological processing of the Chinese characters, which is in marked contrast to brain activity in alphabetic language readers. Therefore, previous findings on lateralization of the AF from Western populations cannot be readily generalized to Chinese. On the other hand, studying neural processing in Chinese readers could provide further insights to the general theory of language [Perfetti et al.,2005]. The finding of inactive left posterior superior temporal system during phonological processing of Chinese characters poses an intriguing question as to whether the AF is also leftward lateralized in the Chinese population. Furthermore, if lateralization does exist, does it change over age in degree, and how does it correlate with language ability measures such as reading scores? We attempt to answer these questions by performing DTT and assessing reading scores in three age groups of school going students and young adults, who were native Chinese (Mandarin) speakers and learned English as a second language.
METHODS
Subjects
Seventy‐five normal healthy ethnic Chinese right‐handed school‐going students and young adults were recruited, which comprised three age groups. Informed consent was obtained from the subjects and/or their parent as appropriate, and the study was approved by the institutional review board. Data from these subjects were used in a previous study focusing on the development of the brain from childhood to young adulthood [Qiu et al.,2008]. All subjects were native Chinese (Mandarin) speakers who learned English as a second language. Group 1 subjects in late childhood (n = 24, male = 13, age range = 6.8–7.9 years, mean ± SD = 7.4 ± 0.3 years) were first grade students, typically having just started to learn to read. Group 2 subjects in early adolescence (n = 27, male = 16, age range = 9.4–11.5 years, mean ± SD = 10.3 ± 0.5 years) were fourth grade students and had acquired substantial reading skills. Group 3 young adults (n = 24, male = 11, age range = 18.6–26.1 years, mean ± SD = 22.8 ± 2.3 years) were undergraduate students in a Beijing university and were highly fluent readers.
Reading Scores
We evaluated the subjects' reading ability in Chinese and English by asking them to read aloud 200 Chinese characters and 200 English words (giving a maximum possible score of 200 each for Chinese reading and English reading). These characters/words were selected from textbooks used in primary schools in Beijing for first to sixth graders, 20 from each. The remaining 160 were selected from low‐frequency characters/words in a linguistic corpus, which were not covered in primary school textbooks. Characters and words were arranged in a sequence of increasing difficulty (as determined by grade level and visual complexity or stroke number). Subjects were asked to read the characters/words aloud as quickly and accurately as possible within 3 min. Among the 75 subjects, Chinese and English reading scores were obtained from 23, 26, and 17 subjects from Groups 1, 2, and 3, respectively. The mean ± SD of Chinese reading scores was 56.6 ± 26.6, 97.38 ± 13.8, and 113.5 ± 16.4 for the three groups, respectively, and the mean±SD of English reading score was 8 ± 9.6, 43.7 ± 35.9, and 94.5 ± 13.4 for the three groups, respectively (see also Tables III and IV).
Table III.
In each group, mean, and standard deviation of Chinese and English reading scores in subjects with strongly leftward (SL) lateralized arcuate fasciculus and subjects with other lateralization patterns including bilateral but leftward lateralized, bilateral but rightward lateralized and strongly rightward lateralized arcuate fasciculus
| Chinese reading score mean (SD) | |||||
|---|---|---|---|---|---|
| SL | Others | P value (lateralization pattern) | P value (gender) | P value (interaction) | |
| Group 1 | |||||
| Female | 70.1 (12.5) | 46.0 (2.8) | 0.004 | 0.207 | 0.770 |
| Male | 62.1 (18.9) | 33.3 (10.0) | |||
| Total | 66.1 (15.9) | 38.4 (10.0) | |||
| Group 2 | |||||
| Female | 103.25 (6.2) | 109.0 (5.0) | 0.798 | 0.033 | 0.249 |
| Male | 96.4 (10.5) | 87.4 (19.5) | |||
| Total | 97.7 (9.5) | 95.3 (18.7) | |||
| Group 3 | |||||
| Female | 108.2 (16.6) | 119.5 (19.1) | 0.856 | 0.779 | 0.198 |
| Male | 124.0 (16.1) | 109.3 (18.4) | |||
| Total | 113.0 (17.3) | 112.7 (17.5) | |||
Note subjects with undefined lateralization pattern was not included in the “Others” category.
Table IV.
In each group, mean and standard deviation of English reading scores in subjects with strongly leftward (SL) lateralized arcuate fasciculus and subjects with other lateralization patterns including bilateral but leftward lateralized, bilateral but rightward lateralized, and strongly rightward lateralized arcuate fasciculus
| English reading score mean (SD) | |||||
|---|---|---|---|---|---|
| SL | Others | P value (lateralization pattern) | P value (gender) | P value (interaction) | |
| Group 1 | |||||
| Female | 7.6 (12.5) | 5.0 (2.8) | 0.208 | 0.776 | 0.413 |
| Male | 13.7 (15.4) | 2.0 (1.7) | |||
| Total | 10.6 (11.4) | 3.2 (2.5) | |||
| Group 2 | |||||
| Female | 57.0 (44.4) | 94.3 (17.6) | 0.061 | 0.002 | 0.426 |
| Male | 20.0 (27.0) | 35.7 (23.2) | |||
| Total | 28.0 (34.9) | 55.7 (34.2) | |||
| Group 3 | |||||
| Female | 96.9 (9.0) | 83.5 (24.7) | 0.336 | 0.547 | 0.532 |
| Male | 96.7 (20.3) | 93.8 (14.8) | |||
| Total | 96.8 (12.1) | 90.3 (16.8) | |||
Note subjects with undefined lateralization pattern was not included in the “Others” category.
Data Acquisition
MRI was performed at the Beijing MRI Center for Brain Research of the Chinese Academy of Sciences using a 3‐T imager (Siemens, Erlangen, Germany) with a standard head coil. DTI data were acquired using single‐shot spin‐echo echo‐planar imaging with TR = 6,000 ms, TE = 84 ms, acquisition matrix = 64 × 64, field of view = 192 mm (in‐plane resolution = 3 × 3 mm), and slice thickness = 3 mm with no gap. Diffusion‐sensitizing gradient encoding was applied in six directions with a diffusion‐weighted factor b = 1,000 s/mm2, and one image (b0 image) was acquired without use of a diffusion gradient, that is, b = 0 s/mm2. For each encoding direction, around 48 axial images parallel to the anterior commisure–posterior commisure plane were acquired to cover the entire brain. DTI sequence was repeated four times to improve signal‐to‐noise ratio (SNR).
Image Preprocessing
Images were processed using the FSL (FMRIB Software Library, FMRIB, Oxford, UK) [Smith et al.,2004] software package. DTI images were resampled to 1 × 1 × 1 mm to aid in visual identification of anatomical regions. For each subject, all images including diffusion weighted and b0 images were affinely coregistered to the b0 image of the first repetition using FMRIB's Linear Image Registration Tool [Jenkinson and Smith,2001] to correct for Eddy current‐induced distortion and subject‐motion effects. Brain mask was created from the first b0 image using Brain extraction Tool [Smith,2002], and FMRIB's Diffusion Toolbox [Behrens et al.,2003] was used to compute the diffusion tensor map, which was then decomposed to calculate the FA, eigen values, and eigen vectors maps.
Tractography on Group‐Averaged DTI Datasets
To perform tractography in group‐averaged data, we used the nonlinear registration tool FNIRT in FSL to normalize all the FA images to the FA template in FSL. The derived transformation parameters were applied to the diffusion tensor image using the “Vecreg” tool, which properly reorients the diffusion tensor to accounts for the effect caused by spatial transformation using Preservation of Principal Direction method [Alexander et al.,2001]. The resolution for the transformed image was chosen to be 1 × 1 × 1 mm. The diffusion tensor images from all the subjects from each group were then averaged and decomposed to produce group averaged FA, eigen value, and eigen vector maps. These images were then converted to a format compatible with DTIStudio (John Hopkins University), which was then used to perform the tractography of the AF. Two ROI approaches were used for the tractography, and the ROIs were placed on color‐coded FA map with reference to a white‐matter atlas (see Fig. 1). The first ROI was placed in the coronal plane over a bottleneck region through which all components of superior longitudinal fasciculus including the AF pass, whereas the second ROI was placed in an axial plane in posterior–superior temporal region to isolate the AF. Spurious tracts, mostly those that pass through the external or internal capsule and are not part of AF, were rejected. A FA threshold of 0.2 and an angle threshold of 45° were used. The tractography analysis was performed separately in each hemisphere and repeated for all three group‐averaged images.
Figure 1.
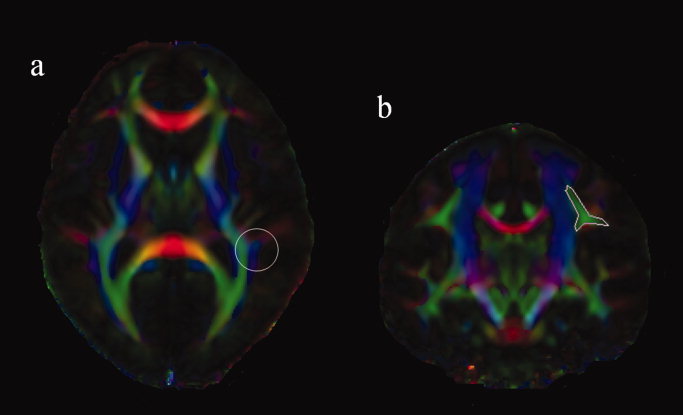
Figure shows the placement the two region‐of‐interest (ROI) at an axial plane (a) and a coronal plane (b), respectively, for the tractography of the arcuate fasciculus.
Individual Subject Tractography
Tractography of AF was also performed for each individual subject in the native image space using the same ROIs as in the group tractography. The number of tracts (NT) traced for both the left and right AF was calculated by DTIStudio. Lateralization Index (LI) was calculated using the following formula:
For the case of extreme leftward asymmetry of AF where the left AF can be traced while no right AF tracts can be defined, LI equals 1. Conversely, for the case of extreme rightward asymmetry of AF where the right AF can be traced while no left AF tracts can be defined, LI equals −1. In some cases, no tracts can be traced for both left and right AF, and the LI was undefined for these cases. We, hence, divided the subjects into five categories according to their LI: strong leftward‐lateralized (SL) for subjects with LI > 0.9, leftward lateralized bilateral representation (BL) with 0 < LI < 0.9, rightward lateralized bilateral representation (BR) with −0.9 < LI < 0, strong rightward‐lateralized (SR) for subjects with LI < −0.9 and undefined for subjects in which no tracts can be traced for both left and right AF. For the following analyses that involved LI, only subjects with defined LI were considered.
Statistical Analysis
Analysis of variance (ANOVA) was performed to test for difference in the NT of both left and right AF among the three groups. Chi‐square test was first performed to examine association of lateralization pattern with age group and gender. Two‐way ANOVA analysis was also performed on LI with age group and gender as independent variables. To examine the relationship between lateralization pattern of AF and reading ability, we performed univariate general linear model analysis on Chinese reading score with independent variables including age group and gender as factors, LI as a covariate, and the interaction terms between them. Additional follow‐up analyses were performed to further evaluate the relationship. These included separate ANOVA analysis for each age group on reading score with lateralization pattern, gender, and the interaction term as independent variables as well as Welch's t test. Welch's t test was used instead of Student's t test to be immune to the effects of unbalanced sample size and unequal variable between the two test groups. Similar analyses were performed for English reading score. All the analyses were performed using SPSS (Version 11.0, SPSS, Chicago, IL), and a P value of <0.05 was considered statistically significant.
RESULTS
Tractography on Group‐Averaged Datasets
For all three age groups, highly leftward lateralized AF was found in all three group‐averaged DTI dataset, such that only the left AF could be robustly reconstructed whilst no pathways of the right AF could be reconstructed (see Fig. 2). It showed a strikingly consistent pattern in the configuration of the AF among the three age groups.
Figure 2.
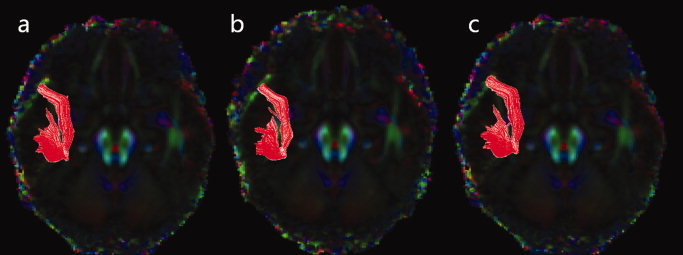
Figure shows tractography results of the arcuate fasciculus in the Group 1 (a), Group 2 (b), and Group 3 (c). Only the left AF can be reconstructed in all three groups, and the shape and the pattern of the AF are nearly identical among the three groups.
Individual Lateralization Pattern
More variations were found for the results of tractography of individual subjects. Figure 3 shows representative cases with lateralization patterns of SL, BL, BR, and SR for the AF. Table I shows the distribution of subjects in the five categories of patterns of lateralization of AF. Leftward lateralization of the AF was found in 75% of the first graders, 70.3% of the fourth graders, and 83.3% of college students; and strongly leftward lateralized AF was found in 62.5%, 48.1%, and 45.8% of the first graders, the fourth graders, and the college students, respectively. Pair‐wise Chi‐square test showed no significant association of the distribution of lateralization pattern of the AF with age group (P = 0.470) or gender (P = 0.574). Among cases in which LI were defined, ANOVA showed no significant difference in LI between the three age groups (P = 0.622) or between males and females (P = 0.599) or their interaction (P = 0.509) (Table II). However, ANOVA analysis showed significant effect of age group (P = 0.017) on the NT for the left AF, whereas no significant effect was found for gender (P = 0.4440) or the interaction between gender and age group (P = 0.535). Similar analysis on the NT for the right AF showed no significant effects of age group, gender, or the interaction between them.
Figure 3.
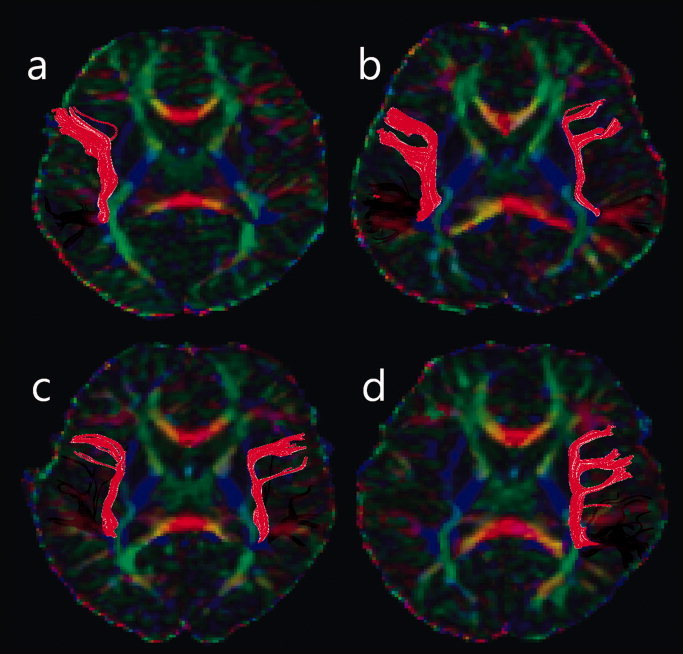
Figure shows representative cases of lateralization patterns of strongly leftward lateralized (a), bilateral but left lateralized (b), bilateral but rightward lateralized (c), and strongly rightward lateralized (d) arcuate fasciculus.
Table I.
Distribution of subjects in the four categories of lateralization patterns for male and female among the three age groups
| Number of subjects with different lateralization pattern (percentage within age group) | ||||||
|---|---|---|---|---|---|---|
| SL | BL | BR | SR | UD | Total | |
| Group 1 | ||||||
| Female | 7 (29.2%) | 1 (4.2%) | 0 (0%) | 1 (4.2%) | 2 (8.3%) | 11 (45.8%) |
| Male | 8 (33.3%) | 2 (8.3%) | 1 (4.2%) | 0 (0%) | 2 (8.3%) | 13 (54.2%) |
| Total | 15 (62.5%) | 3 (12.5%) | 1 (4.2%) | 1 (4.2%) | 4 (16.7%) | 24 (100.0%) |
| Group 2 | ||||||
| Female | 5 (18.5%) | 1 (3.7%) | 2 (7.4%) | 0 (.0%) | 3 (11.1%) | 11 (40.7%) |
| Male | 8 (29.6%) | 5 (18.5%) | 1 (3.7%) | 1 (3.7%) | 1 (3.7%) | 16 (59.3%) |
| Total | 13 (48.1%) | 6 (22.2%) | 3 (11.1%) | 1 (3.7%) | 4 (14.8%) | 27 (100.0%) |
| Group 3 | ||||||
| Female | 7 (29.2%) | 4 (16.7%) | 0 (0%) | 1 (4.2%) | 1 (4.2%) | 13 (54.2%) |
| Male | 4 (16.7%) | 5 (20.8%) | 1 (4.2%) | 1 (4.2%) | 0 (0%) | 11 (45.8%) |
| Total | 11 (45.8%) | 9 (37.5%) | 1 (4.2%) | 2 (8.3%) | 1 (4.2%) | 24 (100.0%) |
Chi‐square test showed no significant difference in the distribution of the lateralization patterns between the three age groups (P = 0.470). Abbreviations: SL, strongly leftward lateralized; BL, bilateral but leftward lateralized bilateral representation; BR, bilateral but rightward lateralized representation; SR, strongly rightward lateralized; UD, undefined for subjects in which no tracts can be traced for both left and right arcuate fasciculus.
Table II.
The mean and standard deviation (SD) of number of tract (NT) for the left and right arcuate fasciculus and Lateralization Index (LI) for females and males among three age groups
| LI | Left NT | Right NT | |
|---|---|---|---|
| Group 1 | |||
| Female | 0.725 (0.665) | 473.0 (465.7) | 158.4 (460.9) |
| Male | 0.793 (0.437) | 452.4 (329.3) | 73.5 (163.4) |
| Group 2 | |||
| Female | 0.588 (0.587) | 705.4 (597.4) | 125.0 (170.9) |
| Male | 0.601 (0.585) | 722.9 (657.7) | 137.7 (202.6) |
| Group 3 | |||
| Female | 0.775 (0.571) | 948.0 (802.5) | 125.3 (223.4) |
| Male | 0.464 (0.618) | 1429.3 (1489.6) | 544.0 (686.0) |
Lateralization of the AF and Reading SCORES
Univariate general linear model analysis was performed on Chinese reading score with the following independent variables including age group and gender as factors, LI as a covariate, and the pair‐wise interaction terms between age group, gender, and LI. This analysis showed significant effects of age group (P < 0.001) and its interaction with LI (P = 0.038), whereas no significant effects were found for other terms. Follow‐up ANOVA analysis was then performed in each age group. Considering the unbalanced sample size among different lateralization patterns with the majority of the subjects having SL lateralization pattern, we grouped together BL, BR, and SR lateralization patterns. The ANOVA analysis hence included the binary lateralization pattern and gender as well as their interaction term as independent variables. The results showed that, in Group 1, the mean Chinese reading score for subjects with SL lateralization pattern was greater than those with BL, BR, or SR lateralization pattern (P = 0.004) (Table III). And post hoc Welch's t test showed that subjects with SL lateralization pattern scored significantly higher than those with other lateralization pattern with a mean difference of 27.7 points (P = 0.001). Meanwhile, in Group 2, females scored significantly higher than males (P = 0.033). No significant effect of gender, lateralization pattern, or the interaction between them was found for Group 3.
Univariate general linear model analysis on English reading score showed significant effect of age group (P <0.001), gender (P = 0.014), and the interaction between them (P = 0.003), and no significant effect was found for the other terms. A similar follow‐up analysis as used for Chinese reading score showed that, in Group 2, females scored significantly higher than males (P = 0.002) (Table IV). There was no significant effect of gender, lateralization pattern, or the interaction between them in Groups 1 and 3. However, given the finding that Group 1 subjects with SL lateralization pattern had a significantly higher mean Chinese reading score than other lateralization patterns, we performed a two‐sample Welch's t test on English reading score comparing subjects with SL to those with other lateralization patterns. The results showed significantly higher English reading score in subjects with SL lateralization pattern (P = 0.036).
DISCUSSION
In this study, we have evaluated the lateralization pattern of the AF using DTT among three age groups from late childhood to young adulthood. Different patterns of lateralization were found among the three age groups, with 70–83% of the population showing leftward lateralization, of which about 50% were strongly leftward lateralized. The Chinese reading score of subjects with strong leftward lateralization in the AF was in average 27.7 higher than those with bilateral or rightward representation. Among the first graders, significantly higher English reading score was also found in students with strongly leftward lateralized AF compared to those with other defined lateralization patterns. Such correlation was not observed for the other two older groups. Among the fourth graders, females were found to obtain significantly higher Chinese and reading scores than males, which is consistent with previous findings [Catani et al.,2007].
Before we begin further discussions, it is important to recognize that the NT traced using tractography is by no means directly correlated to the number of underlying neuronal fibers. However, the NT obtained from tractography does reflect brain connectivity at a macroscopic scale [Basser et al.,2000; Catani et al.,2007; Mori et al.,1999]. In a large proportion of our cohort, no tracts could be reconstructed for the right AF. There were also some subjects in which no tracts could be traced for the AF in both hemispheres. This however does not suggest that the AF is missing in these subjects but rather that the connection may be not the dominant population throughout the entire trajectory to be detectable at the resolution currently possible.
Leftward lateralization of the AF has been reported in adults using DTT [Catani et al.,2007; Eluvathingal et al.,2007; Lebel and Beaulieu,2009; Nucifora et al.,2005; Parker et al.,2005; Vernooij et al.,2007; Zhang et al.,2007]. Our study found leftward lateralization to already be present at an early age, before ∼7 years of age, in keeping with findings in the Western population [Lebel and Beaulieu,2009]. Also, there was no significant difference in the degree of leftward lateralization with increasing age groups. However, it is important to note that the NT of the left AF changed significantly with increasing age. Together, these observations suggest that the lateralization of the AF is already developed at a young age, and thereafter the AF undergoes proportional development bilaterally with no further change in the lateralization pattern. Lateralization of the AF has been found to be related to functional lateralization of brain activation during fMRI tasks [Vernooij et al.,2007], lending support to the notion that differences in brain activation have anatomical substrates. Our findings of highly leftward lateralized AF also in a group of Chinese readers suggest that the AF participates in aspects of language processing that are universal to language/writing systems.
Our study is the first to investigate the relationship between lateralization of the AF and language ability spanning a period from childhood to adulthood and allows the comparison of the relationship at different stages of language development. An interesting finding of this study is that strong leftward lateralization of the AF is associated with superior reading abilities at a young age, and this advantage is no longer evident at adulthood. This may be due to different reading strategies adopted by children learning to read and experienced adult readers. There is evidence suggesting that two routes are involved in single‐word reading, a direct route with addressed phonology by which the pronunciation of a word is directly accessed from visual‐orthography of a word as a whole, and an indirect route with assembled phonology by which subcomponents of a word are mapped to sound units, or phonemes, and assembled to form the pronunciation of a word [Price,2000; Turkeltaub et al.,2002]. Alphabetic languages are believed to heavily engage assembled phonology; and previous studies have identified the left inferior frontal gyrus, the left posterior superior temporal region extending to the supramarginal gyrus, and the left ventral–temporal occipital region to be three segregated systems for phonological processing in alphabetic languages [Price,2000; Tan et al.,2005]. The posterior–superior temporal system was found to be engaged in assembled phonology while the left inferior frontal gyrus was found to be engaged in subvocal rehearsal and assembled phonology [Tan et al.,2005]. The AF is a major white‐matter tract that connects the posterior–superior temporal region to the ventral frontal system. Indeed, a recent study [Lebel and Beaulieu,2009] has shown that the leftward asymmetry of the AF was associated with higher scores in Peabody Picture Vocabulary Test and a Phonological Processing task in a group of presumably native English reading children. Although there is no similar data in adults, a similar advantage is expected.
Compared to alphabetic words, Chinese characters are made up of strokes that are packed into square configuration and the characters map onto phonology at syllable level [Tan et al.,2005]. There is no way to read a Chinese character by recoursing to letter‐to‐sound conversion, that is, through the assembled route, as is possible with alphabetic languages; but rather addressed phonology is predominantly adopted through rote memory of mapping between the visual form and the pronunciation of characters. Meta‐analysis has also shown that phonological processing for Chinese characters involves distinct brain regions compared to alphabetic languages [Tan et al.,2005]. A striking difference is that the left posterior–superior temporal system, which is central to alphabetic language processing, is nearly silent during Chinese character reading. Phonological processing of Chinese characters recruits the left middle frontal gyrus for addressed phonology by performing visual–spatial analysis and mapping of orthography to phonology, the left superior parietal region for phonological store, and the left inferior frontal region for subvocal rehearsal. The left AF is not situated in a position to facilitate communication in this network. We believe it is this fact that explains our findings of essentially identical Chinese reading score between adult subjects with strongly leftward lateralized AF and other lateralization patterns. Given this explanation, it may seem puzzling that strong leftward lateralization of the AF was associated with highly significant better Chinese reading performance among the first graders. However, it is important to note that before students are taught to read Chinese characters, they are taught a phonetic system, pinyin, which is designed to facilitate the denotation of Chinese characters and has clear letter‐sound conversion rules as in alphabetic languages. Pinyin phonetic system, introduced in early 1970s, comprises of 26 English letters and 13 letter groups as its sound units [Chen et al.,2002; Fu et al.,2002]. Unlike Chinese characters, the pronunciation of a pinyin can be readily derived from its visual form by any person who masters a set of conversion rules. Pinyin therefore bears more resemblance to alphabetic language and, arguably, predominantly engages the assembled phonology route. Better pinyin ability is expected to be associated with better Chinese reading. Among the first graders, we found significantly higher Chinese and English reading scores in students with strongly leftward lateralized AF compared to students with other lateralization patterns, similar to the studies in native English readers. It is possible that such an advantage is realized through better assembled phonological ability for both pinyin reading and English reading. By the fourth grade, when the students become experienced in reading Chinese characters, the brain has been “shaped” and now uses a reading strategy, which does not rely on pinyin or other assembled phonological processes. The advantage of strongly leftward lateralization of the AF therefore diminishes among the fourth graders and becomes no longer evident in highly experienced adult readers. Thus, strong lateralization of the AF was not associated with higher English reading scores in adults. Indeed, previous studies have shown adult Chinese–English bilinguals adopt the same neural network used in their first language (Chinese) for their second language (English) reading [Chee et al.,1999; Tan et al.,2003], but this network is different from the one adopted by native English readers [Tan et al.,2003]. Studies comparing Chinese character and pinyin reading have also shown largely overlapping neural network in adult readers. It would be interesting to compare neural networks engaged in pinyin reading between children who have finished pinyin study but are yet to learn to read Chinese characters and adults who are experienced Chinese readers, to understand this transition. We postulate that there would be differences in the neural network between the two groups, and this difference would be the substrate of the differential relationship between lateralization pattern of the AF and Chinese reading ability between the first graders and the adults.
It is noteworthy that a similar study [Catani et al.,2007] found that more symmetric AF correlated with better verbal recall in adults reading the alphabetical language. It is likely that verbal recall recruits a different brain network than word reading for which a more symmetric AF represent an advantage. Further studies are required to fully elucidate the whole picture of fiber systems involved in language processing.
A limitation of our study is that we used a DTI encoding scheme with only six directions and four repetitions. It is suggested that it is advantageous to acquire diffusion‐weighted images with more directions than with making repetitions over fewer directions [Jones,2004]. However, a study also showed that a six‐direction encoding scheme with SNR higher than 30 provided comparable quality in FA estimate for detection of group difference [Landman et al.,2007; Lebel and Beaulieu,2009], and six‐direction encoding scheme was used successfully in a previous study for tractography [Lebel and Beaulieu,2009]. In our study, the SNR is about 64 for the nondiffusion‐weighted image without averaging over repetitions. We used the same imaging protocol for all the subjects; therefore, while an encoding scheme with larger number of directions may provide higher sensitivity, our results are not biased.
In conclusion, using DTT, we have found highly leftward lateralized AF in a large proportion of Chinese subjects ranging from first grader who just started to learn to read to adult college students who were experienced readers. Our results suggest that lateralization pattern of the AF has been formed early at age before one enters primary school and undergoes no significant changes up to young adulthood. Strongly, leftward lateralization of the AF is associated with better Chinese and English reading ability at an early stage of reading acquisition, possibly through better assembled phonological ability. This advantage disappears as one becomes an experienced Chinese reader, adopting an addressed phonology approach, and we postulate that a different neural network is recruited in which the AF plays an insignificant role is recruited.
REFERENCES
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC ( 2001): Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans Med Imaging 20: 1131–1139. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D ( 1994): Estimation of the effective self‐diffusion tensor from the NMR spin echo. J Magn Reson B 103: 247–254. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A ( 2000): In vivo fiber tractography using DT‐MRI data. Magn Reson Med 44: 625–632. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen‐Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM ( 2003): Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magn Reson Med 50: 1077–1088. [DOI] [PubMed] [Google Scholar]
- Broca P ( 1861): Remarques sur le siege de la faculte du langage articule, suivies d'une observation d'aphemie (perte de la parole). Bull Soc Anthropol 6: 330–357. [Google Scholar]
- Catani M, Mesulam M ( 2008): The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex 44: 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH ( 2005): Perisylvian language networks of the human brain. Ann Neurol 57: 8–16. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK ( 2007): Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA 104: 17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Tan EW, Thiel T ( 1999): Mandarin and English single word processing studied with functional magnetic resonance imaging. J Neurosci 19: 3050–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Fu S, Iversen SD, Smith SM, Matthews PM ( 2002): Testing for dual brain processing routes in reading: A direct contrast of chinese character and pinyin reading using FMRI. J Cogn Neurosci 14: 1088–1098. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing‐Cobbs L ( 2007): Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex 17: 2760–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Chen Y, Smith S, Iversen S, Matthews PM ( 2002): Effects of word form on brain processing of written Chinese. Neuroimage 17: 1538–1548. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Rilling JK ( 2008): DTI tractography of the human brain's language pathways. Cereb Cortex 18: 2471–2482. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S ( 2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5: 143–156. [DOI] [PubMed] [Google Scholar]
- Jones DK ( 2004): The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: A Monte Carlo study. Magn Reson Med 51: 807–815. [DOI] [PubMed] [Google Scholar]
- Landman BA, Farrell JA, Jones CK, Smith SA, Prince JL, Mori S ( 2007): Effects of diffusion weighting schemes on the reproducibility of DTI‐derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. Neuroimage 36: 1123–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C ( 2009): Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp 30: 3563–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC ( 1999): Three‐dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45: 265–269. [DOI] [PubMed] [Google Scholar]
- Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, Pearlson GD, Melhem ER, Solaiyappan M, Raymond GV, Moser HW, van Zijl PC ( 2002): Imaging cortical association tracts in the human brain using diffusion‐tensor‐based axonal tracking. Magn Reson Med 47: 215–223. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Melhem ER, Gur RE, Gur RC ( 2005): Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport 16: 791–794. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Luzzi S, Alexander DC, Wheeler‐Kingshott CA, Ciccarelli O, Lambon Ralph MA ( 2005): Lateralization of ventral and dorsal auditory‐language pathways in the human brain. Neuroimage 24: 656–666. [DOI] [PubMed] [Google Scholar]
- Perfetti CA, Liu Y, Tan LH ( 2005): The lexical constituency model: Some implications of research on Chinese for general theories of reading. Psychol Rev 112: 43–59. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich M, Gabrieli JD ( 2001): Relations between the neural bases of dynamic auditory processing and phonological processing: Evidence from fMRI. J Cogn Neurosci 13: 687–697. [DOI] [PubMed] [Google Scholar]
- Price CJ ( 2000): The anatomy of language: Contributions from functional neuroimaging. J Anat 197 ( Pt 3): 335–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Zhou K, Khong PL ( 2008): Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: Voxel‐wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage 41: 223–232. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, Behrens TE ( 2008): The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci 11: 426–428. [DOI] [PubMed] [Google Scholar]
- Siok WT, Perfetti CA, Jin Z, Tan LH ( 2004): Biological abnormality of impaired reading is constrained by culture. Nature 431: 71–76. [DOI] [PubMed] [Google Scholar]
- Siok WT, Niu Z, Jin Z, Perfetti CA, Tan LH ( 2008): A structural‐functional basis for dyslexia in the cortex of Chinese readers. Proc Natl Acad Sci USA 105: 5561–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM ( 2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De LM, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De SN, Brady JM, Matthews PM ( 2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 ( Suppl 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Feng CM, Siok WT, Perfetti CA, Xiong J, Fox PT, Gao JH ( 2003): Neural systems of second language reading are shaped by native language. Hum Brain Mapp 18: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Laird AR, Li K, Fox PT ( 2005): Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta‐analysis. Hum Brain Mapp 25: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA ( 2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. Neuroimage 16: 765–780. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der LA ( 2007): Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right‐ and left‐handed healthy subjects: A combined fMRI and DTI study. Neuroimage 35: 1064–1076. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van ZP, Mori S ( 2007): Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36: 630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke C ( 1874): Der Aphasische Symptomenkomplex. Eine Psychologische Studie auf Anatomischer Basis. Breslau: Cohn und Welgert. [Google Scholar]
- Zhang J, Evans A, Hermoye L, Lee SK, Wakana S, Zhang W, Donohue P, Miller MI, Huang H, Wang X, van Zijl PC, Mori S ( 2007): Evidence of slow maturation of the superior longitudinal fasciculus in early childhood by diffusion tensor imaging. Neuroimage 38: 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]


