Abstract
Laterality of human brain varies under healthy aging and diseased conditions. The alterations in hemispheric asymmetry may embed distinct biomarkers linked to the disease dynamics. Statistical parametric mapping based on high‐resolution magnetic resonance imaging (MRI) and image processing techniques have allowed automated characterization of morphological features across the entire brain. In this study, 149 subjects grouped in healthy young, healthy elderly, mild cognitive impairment (MCI), and Alzheimer's disease (AD) were investigated using multivariate analysis for regional cerebral laterality indexed by surface area, curvature index, cortical thickness, and subjacent white matter volume measured on high‐resolution MR images. Asymmetry alteration of MCI and AD were characterized by marked region‐specific reduction, while healthy elderly featured a distinct laterality shift in the limbic system in addition to regional asymmetry loss. Lack of the laterality shift in limbic system and early loss of asymmetry in entorhinal cortex may be biomarkers to identify preclinical AD among other dementia. Multivariate analysis of hemispheric asymmetry may provide information helpful for monitoring the disease progress and improving the management of MCI and AD. Hum Brain Mapp 34:3400–3410, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: high‐resolution MRI, hemispheric asymmetry, healthy aging, mild cognitive impairment, Alzheimer's disease, statistical parametric mapping, MANCOVA, voxel‐based morphometry, human brain mapping
INTRODUCTION
Structural and functional asymmetries are believed to be beneficial features of the human brain and well confirmed by postmortem neuroanatomy and in vivo neuroimaging (Davidson and Hugdahl, 1995; Eberstaller, 1884; Geshwind and Lacobini, 1999; Hellige, 1993; Price, 2000). Substantial improvement in magnetic resonance imaging (MRI) and image processing techniques has allowed automated characterization of morphological features across the entire brain. Based on the MR images with higher resolution and contrast, region‐of‐interest (ROI) and whole‐brain analyses have revealed anatomic findings that correspond well with functional lateralization. The most consistent observation was the leftward asymmetries in regions involved in language processing that agrees with the reported left‐hemispheric dominance for language processing (Broca, 1861; Good et al., 2001; Price, 2000; Watkins et al., 2001; Wernicke, 1874). The anterior cingulate cortex (ACC), which is associated with a wide variety of autonomic as well as rational cognitive functions, was reported to be larger on the right side (Pujol et al., 2002). Studies on the depth asymmetry of central sulcus (CS) have shown the handedness effect that it is deeper in the dominant hemisphere with respect to the nondominant side (Amunts et al., 2000; Annett, 1985; Beaton, 1997; Davatzikos and Bryan, 2002).
Alteration in hemispheric asymmetry has been referenced in studies of healthy aging, brain injury, or neurological disorders in which the anatomical and functional integrity of the brain were impaired (Albert and Moss, 1988; Brown and Jaffe, 1975; Cabeza, 2002; Cherbuin et al., 2010; Derflinger et al., 2011). Activity in the frontal lobes was revealed to be less lateralized during cognitive performance in healthy elderly than in the young (Cabeza, 2002; Cabeza et al., 2004; Dennis et al., 2007), known as the HAROLD model. Right hemi‐aging model (Albert and Moss, 1988; Brown and Jaffe, 1975) proposes that the right hemisphere presents greater age‐related decline than its left counterpart. Thompson et al. (2007) demonstrated that cortical atrophy occurred earlier and progressed faster in the left hemisphere than in the right in patients with Alzheimer's disease (AD). Hippocampal asymmetry was found to significantly reduce in subjects with dementia (Barnes et al., 2005a; Shi et al., 2009). However, the cerebral atrophy and asymmetry changes in cognitively impaired patients are more complicated and thus less well‐established in comparison with healthy aging. Previous investigations mostly focused on the individual variables including structural volume, cortical surface area, cortical thickness, sulcal depth, gyral length, and vertex position in the exploration of cerebral asymmetries under normal or diseased conditions, and discrepancies were shown across a variety of independent studies (Crespo‐Facorro et al., 2011; Good et al., 2001; Hutsler et al., 1998; Luders et al., 2005; Lyttelton et al., 2009; Posthuma et al., 2002). Regions of significant asymmetries observed by supramarginal gyral (SMG) length and surface area (Gannon et al., 2005; Lyttelton et al., 2009) appeared not to be lateralized when indexed by cortical thickness (Luders et al., 2005). As the aforementioned variables are intercorrelated such that changes in one would alter the others (Im et al., 2008; Kochunov et al., 2005, 2008), we applied a general method in this study that collectively explores the relevant indices for a more comprehensive assessment on the hemispheric asymmetry and investigated its usefulness in differentiating groups of mild cognitive impairment (MCI) converter and AD patient from healthy elderly.
MATERIALS AND METHODS
Subjects and MR Imaging
This study was approved by the local institutional review board, and informed consent was obtained from each subject on participating in the study. A total of 149 right‐handed subjects were recruited and categorized into groups of healthy young (n = 33, male/female = 16/17, mean age 28.94 ± 5.70 years), healthy elderly (n = 44, male/female = 23/21, mean age 75.48 ± 6.34 years), MCI converters who had developed to AD later (n = 39, male/female = 27/12, mean age 75.44 ± 7.31 years), and AD patients (n = 33, male/female = 17/16, mean age 77.88 ± 7.51 years) (Table 1). Medical history was reviewed carefully for each subject to rule out endocrinal, neurological, or psychiatric illnesses before qualifying for the study. Cognitive impairment of MCI and AD were scaled based on the Clinical Dementia Rating (CDR) (Morris, 1993) and Mini‐Mental State Examination (MMSE) (Folstein et al., 1975), by which CDR = 0 and 24 ≤ MMSE ≤ 30 is categorized as normal, CDR = 0.5 and 20 ≤ MMSE ≤ 26 as very mild dementia or mild cognitive impairment, CDR = 1 and 20 ≤ MMSE ≤ 26 as mild dementia, CDR = 2 and 10 ≤ MMSE ≤ 22 as moderate dementia, CDR = 3 and MMSE ≤ 12 as severe dementia.
Table 1.
Summarized demographic information of the subjects in four groups
| Groups | Number of subjects (male/female) | Mean age (years) (range) | Mean MMSE score (range) | CDR scale |
|---|---|---|---|---|
| Healthy young | 33 (16/17) | 28.94 (22 ∼ 55) | N/A | N/A |
| Healthy elderly | 44 (23/21) | 76.06 (59.98 ∼ 89.67) | 28.07 (24 ∼ 30) | 0 |
| MCI | 39 (27/12) | 75.48 (56.25 ∼ 87.81) | 20.97 (5 ∼ 30) | 0.5 |
| AD | 33 (17/16) | 77.66 (58.75 ∼ 88.84) | 15.64 (2 ∼ 26) | 1 ∼ 3 |
CDR: clinical dementia rating; MMSE: Mini‐mental state examination.
MPRAGE sequence was applied for the healthy young group to obtain T1‐weighted MR images (T1WI) on a 3T scanner (MAGNETOM Trio A Tim System, Siemens, Erlangen, Germany) with a high‐resolution 32‐channel head coil. The typical imaging parameters were TR/TE 1900/2.53 ms, FA 9°, FOV 250 × 256 mm2, in plane resolution of 1 × 1 × 1 mm3. T1WI with the same resolution for the groups of healthy elderly, MCI converter, and AD were retrieved from the Alzheimer's disease Neuroimaging Initiative (ADNI) database (http://www.loni.ucla.edu/ADNI) in which subjects have been recruited from over 50 sites across the U.S. and Canada.
Image Processing
Image processing and variable calculation were conducted using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/). The T1WI was intensity normalized, skull stripped, and aligned to a standard space from which gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) were segmented. The cortical surface along with the GM/WM interface was reconstructed followed by the inflation of the folded surfaces, automatic topology correction, and mapping to the standard spherical coordinate system defined by FreeSurfer brain atlas, which enables automated anatomical parcellation of cerebral cortex into 34 gyral (Dale et al., 1999; Dale and Sereno, 1993; Fischl et al., 1999) and subjacent white matter ROIs for both hemispheres of each subject (Fig. 1) (Desikan et al., 2006; Fischl et al., 2004). Surface area, mean curvature index, cortical thickness, and the subjacent WM volume were calculated as morphological variables from the constructed surface on each ROI for both hemispheres (Fischl and Dale, 2000; Han et al., 2006; Schaer et al., 2008).
Figure 1.
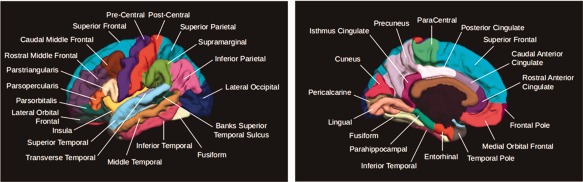
Thirty‐four parcellated and annoted cortical ROIs by FreeSurfer. Left: Lateral view of cortical parcellation on left hemisphere. Right: Medial view of cortical parcellation on left hemisphere.
Lateralization Definition and Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS 17.0, SPSS Corp., Chicago, IL, USA). Analysis of variance (ANOVA) was employed to assess the hemispheric morphological differences for each group. For each ROI, surface area, curvature index, cortical thickness, and subjacent WM volume were used as individual response variables respectively. Subject gender and age were introduced as covariates. Significantly asymmetric regions were defined where P < 0.05 for each variable. ROI‐based multivariate analysis of covariance (MANCOVA) was performed to test the composite morphological differences between left and right hemispheres for each group, in which surface area, curvature index, cortical thickness, and subjacent WM volume were introduced as collective response variables with gender and age as covariates. The lateralization index of significantly asymmetric regions with P < 0.05 was defined as LI = Σi (Li − Ri)/(Li + Ri), where L i and R i were the mean value of the ith variable for the left and right hemisphere, respectively. LI > 0 indicates a leftward asymmetry and LI < 0 a rightward asymmetry. Multiple‐comparison correction was performed across ROIs in all analyses to control false discovery rate (FDR) at a significance level of 0.05. Statistical parameters were mapped to the cortical surfaces to facilitate visual inspection.
RESULTS
There was no age difference among groups of healthy elderly, MCI, and AD (P > 0.05). The mean and standard deviation of cortical surface area, mean curvature index, cortical thickness, and subjacent WM volume for each group were summarized in Table 2. From healthy young to AD, surface area, cortical thickness, and WM volume gradually decreased at a commeasurable rate for both hemispheres by 10%, 22%, and 19.7% on the average, respectively; while mean curvature index increased by 18.4% in the left hemisphere versus 11.9% in the right.
Table 2.
Summary of the measures of individual variables for both hemispheres of each group (mean ± standard deviation)
| Groups | Surface area (×104 mm2) | Curvature index (1/mm) | Cortical thickness (mm) | WM volume (×105 mm3) | ||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | |
| i | 8.50 ± 0.61 | 8.56 ± 0.66 | 9.56 ± 2.46 | 10.05 ± 2.52 | 2.68 ± 0.08 | 2.68 ± 0.07 | 2.03 ± 0.19 | 2.04 ± 0.20 |
| ii | 7.73 ± 0.71 | 7.76 ± 0.72 | 9.00 ± 1.45 | 9.56 ± 3.83 | 2.40 ± 0.10 | 2.42 ± 0.11 | 1.81 ± 0.25 | 1.82 ± 2.47 |
| iii | 7.98 ± 0.76 | 8.03 ± 0.77 | 10.46 ± 3.14 | 10.60 ± 3.06 | 2.30 ± 0.12 | 2.29 ± 0.14 | 1.78 ± 0.21 | 1.77 ± 0.22 |
| iv | 7.65 ± 0.98 | 7.71 ± 0.99 | 11.32 ± 3.98 | 11.19 ± 3.62a | 2.09 ± 0.21 | 2.10 ± 0.22 | 1.63 ± 0.22 | 1.65 ± 0.23 |
Group i: healthy young; Group ii: healthy elderly; Group iii: mild cognitive impairment; Group iv: Alzheimer's disease.
An outlier with a mean curvature index of 77.29 was excluded.
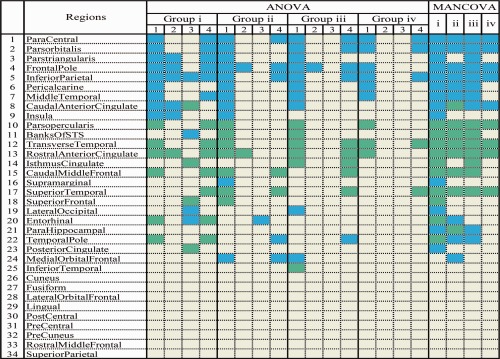
Parcellated ROIs of significant asymmetry by ANOVA and MANCOVA are summarized in Table 3. Surface area and subjacent WM volume identified more ROIs of lateralization in comparison with mean curvature index and cortical thickness, especially in groups of healthy elderly, MCI, and AD (Fig. 2). An overall gradual reduction with regional heterogeneity in hemispheric asymmetry was observed from healthy young to healthy elderly, MCI, and AD sequentially.
Table 3.
Distribution of regional asymmetries in the 34 gyral based ROIs by ANOVA and MANCOVAa
|
Figure 2.
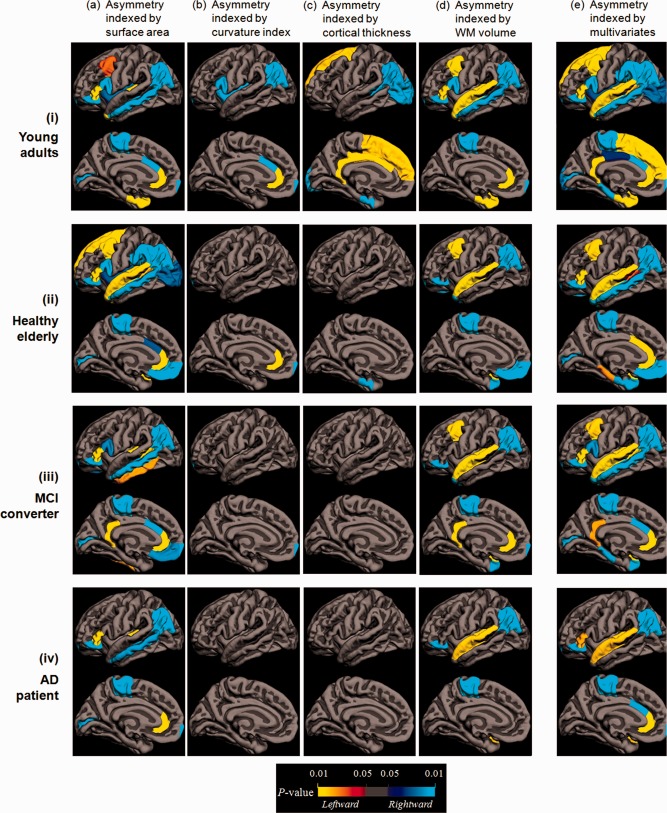
Regional asymmetries by ANOVA indexed by surface area (column a), curvature index (column b), cortical thickness (column c), subjacent white matter volume (column d), and MANCOVA (column e) in groups of healthy young (row i), healthy elderly (row ii), MCI (row iii), and AD (row iv). Heterogeneity and inconsistency in hemispheric asymmetry was observed by ANOVA. MANCOVA proved to be more sensitive to detect more regions with asymmetry. Red‐yellow represents leftward lateralization and blue‐cyan represents rightward lateralization. All P values were corrected to control false discovery rate at a significance level of 0.05.
Hemispheric Asymmetry by ANOVA
For the healthy young group, the inferior parietal lobule was the only common ROI of significant lateralization by all variables among the 34 parcellated regions (Fig. 2i, P < 0.05 corrected). The notable rightward asymmetry in caudal anterior cingulate cortex indexed by surface area and mean curvature reversed to leftward determined by cortical thickness. The entorhinal cortex was observed lateralizing to the left hemisphere with respect to surface area and subjacent WM volume, yet contrarily to the right by cortical thickness. No lateralization was identified by any individual index in 13 of the 34 ROIs including lateral and medial‐orbito frontal gyri, rostral middle frontal gyrus, precentral gyrus, postcentral gyrus, precuneus, superior parietal lobule, supramarginal gyrus, cuneus, lingual gyrus, fusiform gyrus, inferior temporal gyrus, and parahippocampal gyrus.
In healthy elderly, MCI, and AD, no common ROI of significant asymmetry was found by all variables. Asymmetries identified in the healthy young group in caudal middle frontal gyrus, medial‐orbito frontal gyrus, parsorbitalis, paracentral lobule, inferior parietal lobule, superior temporal gyrus, and supramarginal gyrus were mostly preserved in healthy elderly when indexed by surface area and subjacent WM volume (Fig. 2ii, panels a and d) but barely retained when determined by mean curvature and cortical thickness (Fig. 2ii, panels b and c), followed by a further reduction in MCI and AD groups (Fig. 2iii and 2iv, panels b and c).
In comparison to healthy young, 16, 16, and 24 of the 34 parcellated regions manifested a lack of lateralization by individual index in the groups of healthy elderly, MCI, and AD, respectively.
Substantial intra‐group heterogeneity in hemispheric asymmetry resulted from ANOVA with a relative consistency in ROIs with rightward lateralization in parsorbitalis, paracentral lobule, supramarginal gyrus, and leftward lateralization in transverse temporal gyrus, indexed by surface area and subjacent WM volume in all groups.
Hemispheric Asymmetry by MANCOVA
A wider range of lateralization was identified in all groups by MANCOVA (Table 3). In the healthy young group, 15 regions showed hemispheric asymmetry including caudal middle frontal gyrus, parsorbitalis, Broca's area (parsopercularis and parstriangularis), superior frontal gyrus, paracentral lobule, inferior parietal lobule, supramarginal gyrus, calcarine sulcus, superior and middle temporal gyri, transverse temporal gyrus (Heschl's gyrus), entorhinal cortex, parahippocampal gyrus, cingulate gyrus, and insula cortex, among which lateralization of supramarginal and parahippocampal gyri were not picked up by any individual index in ANOVA.
Compared with the healthy young group, lateralization of healthy elderly shifted in regions of caudal anterior cingulate, temporal pole, entorhinal and parahippocampal gyri, in addition to reduction or loss of asymmetry in regions of superior frontal gyrus, parstriangularis, supramarginal gyrus, lateral occipital gyrus, insula, and posterior cingulate gyrus, while MCI and AD groups featured a marked overall reduced lateralization in entorhinal cortex, caudal middle frontal gyrus, parstriangularis, middle temporal gyrus, calcarine sulcus, parahippocampal gyrus, temporal pole, and isthmus cingulate. Additionally, asymmetry increased in isthmus cingulate gyrus, while it diminished in entorhinal cortex and medial orbital frontal gyrus in MCI.
A further loss of asymmetry from MCI to AD was observed in regions of caudal middle frontal gyrus, banks of superior temporal sulcus, calcarine sulcus, and temporal pole, in addition to parahippocampal gyrus and isthmus cingulate. Across‐group preservation of lateralization was seen in regions of superior temporal gyrus, transverse temporal gyrus, parstriangularis, parsorbitalis, inferior parietal lobule, frontal pole, rostral anterior cingulate, and paracentral lobule. Both ANOVA and MANCOVA revealed nine identical parcellated regions that were nonlateralized in all groups (lateral orbital frontal, precentral, postcentral, rostral middle frontal, precuneus, cuneus, superior parietal, lingual, and fusiform) (Table 3, Fig. 2).
DISCUSSION
In this work, we investigated the alteration in regional hemispheric asymmetry in groups of healthy young, healthy elderly, MCI, and AD by quantifying the morphological variables of human brain individually and collectively.
Regional Laterality by ANOVA Versus MANCOVA
Hemispheric asymmetry based on the individual structural index manifested substantial intra‐group discrepancy in terms of the number and location of the identified lateralization, especially in areas of limbic system. For instance, in the healthy young group sixteen regions were revealed to be lateralized indexed by surface area, whereas the count were 6, 8, and 13 by curvature index, cortical thickness and subjacent WM volume, respectively. The calcarine sulcus was detected as a significantly asymmetric region by surface area but appeared nonlateralized by other variables; similarly inconsistency was observed in the superior frontal gyrus by cortical thickness. Lack of consistency in lateralization by a single morphological variable may largely be attributed to an overall disproportionate interaction among the indices during their alteration (Im et al., 2008), by which change of one would alter others to an indeterminate extent. The intercorrelation among variables may be overlooked in univariate studies. Thus, conflicting conclusions on the lateralization could be drawn for the same cerebral region by different index. The underlying rationale of the discrepancies reported in previous studies may lie in the morphological variable used besides the variety of image processing methodology or index calculation algorithms (Gannon et al., 2005; Luders et al., 2005; Lyttelton et al., 2009). Alterations in neuron count, size, or density over the process of healthy aging and diseased conditions would modify a number of shape features collectively rather than individually, although the whole bundle of relevant features may not be significantly associated with the undermined physical and mental abilities (Bonte et al., 2006; Jack et al., 2002; Schroeter et al., 2009).
The multivariate analysis proved to be more sensitive in picking up subtle alterations of hemispheric asymmetries in this study (for example, the lateralization identification in the supramarginal and parahippocampal gyri in groups of healthy young and elderly, respectively). This was considered to be improved sensitivity with the concern of overestimation about multivariate analysis allayed by the detection of the identical nine nonlateralized regions in all groups by ANOVA and MANCOVA. The laterality of the somatosensory region (Coghill et al., 2001), temporal gyri, and anterior cingulate gyri (Albanese et al., 1995) in this study are consistent with previous reports (Penhune et al., 1996; Steinmetz, 1996).
Healthy Aging, MCI and AD
Decline of hemispheric asymmetry during healthy aging and disease progress have been consistently reported in studies of electroencephalography (EEG) (Bellis et al., 2000; Deslandes et al., 2008), near‐infrared spectroscopy (Fallgatter et al., 1997; Herrmann et al., 2006), behavioral (Reuter‐Lorenz et al., 1999), and structural and functional MRI (Cabeza, 2002; Cabeza et al., 2004; Dennis et al., 2007; Li et al., 2009). Such an observation was also confirmed by the multiple shape variables analysis of this study (as shown in Fig. 3). Reduced asymmetry impedes compensation and interaction between the hemispheres, which impairs the working efficiency of the brain (Oertel et al., 2010). Consequently, a wide range of neurofunctional decline would become noticeable or even interfere with daily life.
Figure 3.
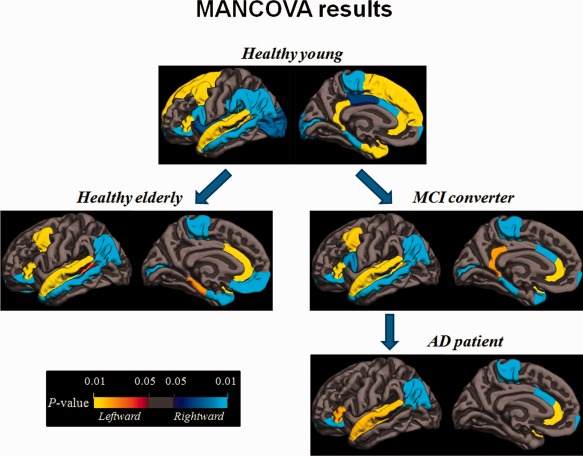
MANCOVA results on the four groups. Compared with the young, the healthy elderly lost asymmetries on the superior frontal gyrus, the supramarginal gyrus, and the insula cortex. The remarkably asymmetric regions were almost matched between the healthy elderly and the MCI group, except for the entorhinal cortex with early laterality loss. As MCI progresses to AD, further loss of asymmetry was identified in caudal middle frontal gyrus, calcarine sulcus, middle temporal gyrus, parahippocampal gyrus, and the temporal pole. Distinctively, healthy elderly featured laterality shift in caudal anterior cingulate, temporal pole, entorhinal and parahippocampal cortices, which is lacked in MCI and AD. Red‐yellow represents leftward lateralization and blue‐cyan represents rightward lateralization. All P values were corrected to control false discovery rate at a significance level of 0.05.
In this study, healthy elderly lost asymmetries mainly in the superior frontal, supramarginal gyri, and insula cortex, which are believed to be involved in the higher cognitive functions. Loss of laterality in these regions may dampen the coordination of brain function resulting in common aging problems such as forgetfulness, slowed reaction time, and difficulty in learning new tasks.
The intriguing divergence in the evolution of brain laterality was observed in healthy elderly as compared with MCI and AD in this study, that is, lateralization in healthy elderly featured shifted in the caudal anterior cingulate cortex, parahippocampal gyrus, and entorhinal cortex, while the age matched groups of MCI and AD featured a progressive loss of asymmetry to a greater extent without the aforementioned laterality shift. This may suggest that MCI and AD are ruled by mechanisms distinct from healthy aging in the process of structural alteration and cognitive decline, although their clinical manifestations may overlap at certain time window.
As brain asymmetry is believed to be favorable for efficient multitask information processing and survival (Rogers, 2002), the shifted lateralization of healthy elderly in caudal anterior cingulate, parahippocampal, and entorhinal gyri may be more protective and compensatory than destructive in the maintenance of the cognitive and emotional homeostasis (Lütcke and Frahm, 2008), probably in response to the regional atrophy and decreased laterality during aging (Liu et al., 2010, 2011; Sowell et al., 2003). Lack of the reversed laterality in these areas may leave the hemispheres less coordinated in the process of loss of tissue volume and laterality in late life, as presented in the age‐matched MCI and AD in this study.
The underlying rationale of the observed divergence in the laterality evolution in the limbic system remained puzzling to us, but it may be associated with the genetically regulated tissue atrophy of the brain in which peri‐sylvian temporal areas and limbic cortices were reported to be under greater genetic control (Posthuma et al., 2002; Thompson et al., 2001). Failure in the proper genetic regulation may predispose disturbance in laterality modification adaptive to a healthy late life in MCI and AD patients. Lack of shift in laterality in the limbic system during aging may be a biomarker relating with a distinct phenotype of AD with specific patterns of brain tissue atrophy (van der Flier et al., 2011) and could potentially be used for differentiating healthy aging from preclinical AD.
Entorhinal cortex was found to first lose asymmetry in MCI of this study, along with progressive reduction of laterality starting in the temporal region and limbic cortices, which was consistent with previous findings of the disease dynamic of AD (Thompson et al., 2003). The early onset of asymmetry loss in entorhinal cortex may reflect the genetic vulnerability (Dickerson, 2007) and could be another biomarker potentially helpful in early detection of MCI from healthy aging, as it was identified to first undergo tissue loss and neurofibrillary tangle histologically (van der Flier et al., 2011), along with functional deterioration in MCI converters and AD patients (Devanand et al., 2007; Sw et al., in press).
The MCI cases recruited in this study were all converters who developed AD eventually. This may partially explain that the pattern of laterality evolution between the two groups were similar with more extensive loss of asymmetry in AD in areas of caudal middle frontal gyrus, calcarine sulcus, middle temporal gyrus, parahippocampal gyrus, and temporal pole. These regions are functionally associated with writing, visual processing, accessing word meaning while reading, contemplating distance, recognizing known face, memory encoding, and retrieval, as well as social and emotional processing. Asymmetry loss in these areas may further disturb the performance of cognitive and emotional processing, which may partially explain the associated worsened clinical manifestations of AD patient, such as disorientation to time and places, failure to recognize close family members and withdrawal from usual interests and activities.
Limitations and Future Work
Gender effect, a critical and controversial issue for research of AD, was not explored in this study. A variety of structural (Coffey et al., 1998; Derflinger et al., 2011), functional (Hugdahl et al., 2006; Tomasi and Volkow, in press; Voyer, 1996), metabolic (Colla et al., 2003; Ott et al., 2000), and clinical (Barnes et al., 2005b; Jorm and Jolley, 1998; Letenneur et al., 1999) studies on sex difference in laterality of AD subjects have yielded contradicting results. Multiple factors such as sample size and level of sex hormone (Hausmann, 2005) were believed to play a role in the laterality variation between genders. Heterogeneity in the neuropsychological (Ripich et al., 1995), behavioral (Ott et al., 1996), and structural manifestations may suggest that laterality alterations be rather regionally specific than sexually dimorphic in the progression of MCI and AD, and gender may be of more importance as a factor diversifying disease expression.
In addition, cortical variable measurement in this study was automatically processed using FreeSurfer without manual intervention, by which accuracy in tissue segmentation may be affected due to the regionally varied contrast of the gray/white matter border. Automatic segmentation is sensitive to the signal‐to‐noise ratio (SNR) of the MR images and the software performance. MPRAGE protocol used to acquire the T1WI in ADNI is a well‐established protocol with standard imaging parameters. The MRI quality control center of ADNI at the Mayo Clinic selected the MPRAGE series with better images at each time point based on the centralized and standardized criteria. If both scans were of equivalent high quality, the magnitude images were combined to produce a single image with an improved SNR by approximately . The improved SNR and contrast to noise ratio with high resolution of MPRAGE images would benefit the segmentation and parcellation of brain regions with minimized partial volume effect, which results in realistic and accurate depictions of anatomic details as evaluated in previous studies (Fischl, 2012; Zhong et al., 2010).
The segmentation software we adopted, FreeSurfer, has been validated and widely used in computational neuroimaging analysis. Each post‐processed image generated by FreeSurfer is quality checked for global success or failure (https://surfer.nmr.mgh.harvard.edu/). However, systematic error of automatic segmentation is ineliminable during image processing. Standard procedures of FreeSurfer were followed in this study to minimize any fault other than systematic error. The segmentation and/or parcellation difference induced in image processing may not overwhelm the real hemispheric differences with ROI‐based hemispheric asymmetry determined within the individual group.
Also, only structural MRI was involved in this work. Combination with functional and quantitative MR techniques is expected to be more capable in differentiating MCI and AD from other types of dementia with additional information provided for microstructure integrity, energy metabolism, and tissue characterization.
CONCLUSIONS
Hemispheric asymmetry alterations in MCI and AD were region‐specific and evolved in a manner distinct from that of healthy elderly, investigated with MANCOVA. Laterality shift in the limbic system may be part of the compensatory and protective mechanism counteracting the age‐related atrophy as well as the overall laterality reduction during normal aging. Lack of the shift and early loss of asymmetry in entorhinal cortex could be biomarkers to differentiate preclinical AD from healthy aging other types of dementia. Hemispheric asymmetry determined with multivariate analysis and voxel‐based morphometry can provide information helpful for monitoring disease dynamic and improving the management of MCI and AD.
ACKNOWLEDGMENTS
Data collection for this project was partially funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI). ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles.
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.ucla.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
REFERENCES
- Albanese A, Merlo A, Mascitti T, Tornese E, Gomez E, Konopka V, Albanese E (1995): Inversion of the hemispheric laterality of the anterior cingulate gyrus in schizophrenics. Biol Psychiat 38:13–21. [DOI] [PubMed] [Google Scholar]
- Albert M, Moss M (1988):Geriatric Neuropsychology.New York:Guilford Press. [Google Scholar]
- Amunts K, Jancke L, Mohlberg H, Steinmetz H, Zilles K (2000): Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia 38:304–312. [DOI] [PubMed] [Google Scholar]
- Annett M (1985):Left, Right, Hand and Brain: The Right Shift Theory.London:Lawrence Erlbaum. [Google Scholar]
- Barnes J, Scahill R, Schott J, Frost C, Rossor M, Fox N (2005a): Does Alzheimer's disease affect hippocampal asymmetry? Evidence from a cross‐sectional and longitudinal volumetric MRI study. Dement Geriatr Cogn Disord 19:338–344. [DOI] [PubMed] [Google Scholar]
- Barnes L, Wilson R, Bienias J, Schneider J, Evans D, Bennett D (2005b): Sex differences in the clinical manifestations of Alzheimer Disease Pathology. Arch Gen Psychiatry 62:685–691. [DOI] [PubMed] [Google Scholar]
- Beaton A (1997): The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender and dyslexia: a review of the evidence. Brain Lang 60:255–322. [DOI] [PubMed] [Google Scholar]
- Bellis T, Nocol T, Kraus N (2000): Aging affects hemispheric asymmetry in the neural representation of speech sounds. J Neurosci 20:791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonte F, Harris T, Hynan L, Bigio E, White C (2006): Tc‐99m HMPAO SPECT in the differential diagnosis of the dementias with histopathologic confirmation. Clin Nucl Med 31( 7):376–378. [DOI] [PubMed] [Google Scholar]
- Broca P (1861): Remarques sur le siège de la faculté du langage articulé, suivies d'une observation d'aphémie (perte de la parole) Bull Soc Anthropol 6:330–357. [Google Scholar]
- Brown J, Jaffe J (1975): Hypothesis on cerebral dominance. Neuropsychologia 13:107–110. [DOI] [PubMed] [Google Scholar]
- Cabeza R (2002): Hemispheric asymmetry reduction in old adults: The HAROLD model. Psychol Aging 17:85–100. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar S, Dolcos F, Prince S, Budde M, Nyberg L (2004): Task‐independent and task‐specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex 14:364–375. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Reglade‐Meslin C, Kumar R, Sachdev P, Anstey K (2010): Mild cognitive disorders are associated with different patterns of brain asymmetry than normal aging: the PATH through life study. Frontiers in psychiatry 1. [DOI] [PMC free article] [PubMed]
- Coffey C, Lucke J, Saxton J, Ratcliff G, Unitas L, Billig B, Bryan R (1998): Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol 55(2):169–179. [DOI] [PubMed] [Google Scholar]
- Coghill R, Gilron I, Iadarola M (2001): Hemispheric lateralization of somatosensory processing. J Neurophysiology 85:2602–2612. [DOI] [PubMed] [Google Scholar]
- Colla M, Ende G, Bohrer M, Deuschle M, Kronenberg G, Henn F, Heuser L (2003): MR spectroscopy in Alzheimer's disease: gender differences in probabilistic learning capacity. Neurobio Aging 24:545–552. [DOI] [PubMed] [Google Scholar]
- Crespo‐Facorro B, Roiz‐Santianez R, Perez‐Iglesias R, Mata I, Rodriguez‐Sanchez J, Tordesillas‐Gutierrez D, de la Foz V, Tabares‐Seisdedos R, Sanchez E, Andreasen N, Magnotta V, Vazquez‐Barquero JL (2011): Sex‐specific variation of MRI‐based cortical morphometry in adult healthy volunteers: The effect on cognitive functioning. Prog Neuropsychopharmacol Biol Psychiatry 35:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A, Sereno M (1993): Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci 5:162–176. [DOI] [PubMed] [Google Scholar]
- Dale A, Fischl B, Sereno M (1999): Cortical surface‐based analysis i: Segmentation and surface reconstruction. NeuroImage 9( 2):179–194. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Bryan R (2002): Morphometric analysis of cortical sulci using parametric ribbons: A study of the central sulcus. J Comput Assist Tomogr 26:298–307. [DOI] [PubMed] [Google Scholar]
- Davidson R, Hugdahl K (1995):Brain asymmetry.Cambridge, MA:MIT Press. [Google Scholar]
- Dennis N, Kim H, Cabeza R (2007): Effects of aging on true and false memory formation: An fMRI study. Neuropsychologia 45:3157–3166. [DOI] [PubMed] [Google Scholar]
- Derflinger S, Sorg C, Gaser C, Myers N, Arsic M, Kurz A, Zimmer C, Wohlschlager A, Muhlau M (2011): Grey‐matter atrophy in Alzheimer's disease is asymmetric but not lateralized. J Alzheimer Dis 25:347–357. [DOI] [PubMed] [Google Scholar]
- Desikan R, Segonne F, Fischl B, Quinn B, Dickerson B, Blacker D, Buckner R, Dale A, Maguire R, Hyman B, Albert MS, Killiany RJ (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Deslandes A, de Moraes H, Pompeu F, Ribeiro P, Cagy M, Capitao C, Alves H, Piedade R, Laks J. (2008): Electroencephalographic frontal asymmetry and depressive symptoms in the elderly. Biol Psychol 79:317–322. [DOI] [PubMed] [Google Scholar]
- Devanand D, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, Rusinek H, Pelton G, Honig L, Mayeux R, Stern Y, Tabert MH, de Leon MJ (2007): Hippocampal and entorhinal atrophy in mild cognitive impairment: Prediction of Alzheimer disease. Neurology 68:828–836. [DOI] [PubMed] [Google Scholar]
- Dickerson B (2007): The entorhinal cortex: An anatomical mediator of genetic vulnerability to Alzheimer's disease. Lancet Neurol 6:471–473. [DOI] [PubMed] [Google Scholar]
- Eberstaller O (1884): Zür Oberflachen Anatomie der Grosshirn Hemisphaeren. Wien Med 479:642–644. [Google Scholar]
- Fallgatter A, Roesler M, Sitzmann L, Heidrich A, Mueller T, Strik W (1997): Loss of functional hemispheric asymmetry in Alzheimer's dementia assessed with near‐infrared spectroscopy. Cogn Brain Res 6:67–72. [DOI] [PubMed] [Google Scholar]
- Fischl B (2012): FreeSurfer. NeuroImage 62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale A (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno M, Dale A (1999): Cortical surface‐based analysis‐ii: Inflation, flatting, and a surface‐based coordinate system. Neuroimage 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat D, Busa E, Seidman L, Goldstein J, Kennedy D, Carviness V, Makris N, Rosen B, Dale A (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P (1975): Mini‐mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Gannon P, Kheck N, Braun A, Ralph L, Holloway R (2005): Planum parietale of chimpanzees and orangutans: A comparative resonance of human‐like planum temporale asymmetry. Anat Rec A Discov Mol Cell Evol Biol 287:1128–1141. [DOI] [PubMed] [Google Scholar]
- Geshwind D, Lacobini M (1999):Structural and functional asymmetries of the frontal lobes.New York:Guilford Publications. [Google Scholar]
- Good C, Johnsrude I, Ashburner J, Henson R, Friston K, Frackowiak R (2001): Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel‐based morphometric analysis of 465 normal adult human brains. Neuroimage 14:685–700. [DOI] [PubMed] [Google Scholar]
- Han X, Jovichich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A (2006): Reliability of MRI‐derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage 32:180–194. [DOI] [PubMed] [Google Scholar]
- Hausmann M (2005): Hemispheric asymmetry in spatial attention across the menstrual cycle. Neuropsychologia 43:1559–1567. [DOI] [PubMed] [Google Scholar]
- Hellige J (1993):Hemispheric Asymmetry: What's Right and what's Left.Cambridge, MA:Harvard University Press. [Google Scholar]
- Herrmann M, Walter A, Ehlis A‐C, Fallgatter A (2006): Cerebral oxygenation changes in the prefrontal cortex: Effects of age and gender. Neurobiol Aging 27:888–894. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Thomsen T, Ersland L (2006): Sex differences in visuo‐spatial processing: An fMRI study of mental rotation. Neuropsychologia 44:1575–1583. [DOI] [PubMed] [Google Scholar]
- Hutsler J, Loftus W, Gazzaniga M (1998): Individual variation of cortical surface area asymmetry. Cereb Cortex 8:11–17. [DOI] [PubMed] [Google Scholar]
- Im K, Lee J, Seo S, Kim S, Na D (2008): Sulcal morphology changes and their relationship with cortical thickness and gyral white matter volume in mild cognitive impairment and Alzheimer's disease. Neuroimage 43:103–113. [DOI] [PubMed] [Google Scholar]
- Jack C Jr., Dickson D, Parisi J, Xu Y, Cha R, O'Brien P, Edland S, Smith G, Boeve B, Tangalos E, Kokmen E, Petersen RC (2002): Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 58:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm A, Jolley D. (1998): The incidence of dementia: A meta‐analysis. Neurology 51:728–733. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Mangin J, Coyle T, Lancaster J, Thompson P, Rivière D, Cointepas Y, Régis J, Schlosser A, Royall D, Zilles K, Mazziotta J, Toga A, Fox PT (2005): Age‐related morphology trends of cortical sulci. Hum Brain Mapp 26:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Thompson P, Coyle T, Lancaster J, Kochunov V, Royall D, Mangin J, Rivière D, Fox P (2008): Relationship among neuroimaging indices of cerebral healthy during normal aging. Hum Brain Mapp 29:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütcke H, Frahm J (2008): Lateralized anterior cingulate function during error processing and conflict monitoring as revealed by high‐resolution fMRI. Cereb Cortex 18:508–515. [DOI] [PubMed] [Google Scholar]
- Letenneur L, Gilleron V, Commenges D, Helmer C, Orgogozo J, Dartigues J. (1999): Are sex and educational level independent predictors of dementia and Alzheimer's disease? Incidence data from the PAQUID project. J Neurol Neurosurg Psychiatry 66:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Moore A, Tyner C, Hu X. (2009): Asymmetric connectivity reduction and its relationship to HAROLD in aging brain. Brain Res 1295:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Wen W, Zhu W, Trollor J, Reppermund S, Crawford J, Jin J, Luo S, Brodaty H, Sachdev P (2010): The effects of age and sex on cortical sulci in the elderly. Neuroimage 51:19–27. [DOI] [PubMed] [Google Scholar]
- Liu T, Wen W, Zhu W, Kochan N, Trollor J, Reppermund S, Jin J, Luo S, Brodaty H, Sachdev P (2011): The relationship between cortical sulcal variability and cognitive performance in the elderly. Neuroimage 56:865–873. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr K, Thompson P, Rex D, Jancke L, Toga A (2005): Hemispheric asymmetries in cortical thickness. Cereb Cortex 16:1232–1238. [DOI] [PubMed] [Google Scholar]
- Lyttelton O, Karama S, Ad‐Dab'bagh Y, Zatorre R, Carbonell F, Worsley K, Evans A (2009): Positional and surface area asymmetry of the human cerebral cortex. Neuroimage 46:895–903. [DOI] [PubMed] [Google Scholar]
- Morris J (1993): Clinical dementia rating (CDR): Current version and scoring rules. Neurology 43:2412–2414. [DOI] [PubMed] [Google Scholar]
- Oertel V, Knöchel C, Rotarska‐Jagiela A, Schönmeyer R, Lindner M, van de Ven V, Haenschel C, Uhlhaas P, Maurer K, Linden D (2010): Reduced laterality as a trait marker of schizophrenia—Evidence from structural and functional neuroimaging. J Neurosci 30:2289–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott B, Tate C, Gordon N, Heindel W (1996): Gender differences in the behavioral manifestations of Alzheimer's disease. J Am Geriatr Soc 44:583–587. [DOI] [PubMed] [Google Scholar]
- Ott B, Heindel W, Tan Z, Noto R (2000): Lateralized cortical perfusion in women with Alzheimer's disease. J Gend Specif Med 3:29–35. [PubMed] [Google Scholar]
- Penhune V, Zatorre R, MacDonald J, Evans A (1996): Interhemispheric anatomical differences in human primary auditory cortex: Probabilistic mapping and volume measurement from magnetic resonance scans. Cereb Cortex 6:661–672. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus E, Baaré W, Hulshoff Pol H, Kahn R, Boomsma D (2002): The association between brain volume and intelligence is of genetic origin. Nat Neurosci 5:83–84. [DOI] [PubMed] [Google Scholar]
- Price C (2000): The anatomy of language: Contributions from functional neuroimaging. J Anat 197:335–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Lopez A, Deus J, Cardoner N, Vallejo J (2002): Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. Neuroimage 15:847–855. [DOI] [PubMed] [Google Scholar]
- Reuter‐Lorenz P, Stanczak L, Miller A (1999): Neural recruitment and cognitive aging: Two hemispheres are better than one, especially as you age. Psychol Sci 10:494–500. [Google Scholar]
- Ripich D, Petrill S, Whitehouse P, Ziol E (1995): Gender differences in language of AD patients: A longitudinal study. Neurology 45:299–302. [DOI] [PubMed] [Google Scholar]
- Rogers L (2002):Advantages and Disadvantages of Lateralization.Cambridge:Cambridge University Press. [Google Scholar]
- Schaer M, Bach Cuadra M, Tamarit L, Lazeyras F, Eliez S, Thiran J (2008): A surface‐based approach to quantify local cortical gyrification. IEEE Trans Med Imaging 27:161–170. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Stein T, Maslowski N, Neumann J (2009): Neural correlates of Alzheimer's disease and mild cognitive impairment: A systematic and quantitative meta‐analysis involving 1,351 patients. Neuroimage 47:1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Liu B, Zhou Y, Yu C, Jiang T (2009): Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: Metaanalyses of MRI studies. Hippocampus 19:1055–1064. [DOI] [PubMed] [Google Scholar]
- Sowell E, Peterson B, Thompson P, Welcome S, Henkenius A, Toga A (2003): Mapping cortical change across the human life span. Nat Neurosci 6:309–315. [DOI] [PubMed] [Google Scholar]
- Steinmetz H (1996): Structure, functional and cerebral asymmetry: In vivo morphometry of the planum temporale. Neurosci Biobehav Rev 20:587–591. [DOI] [PubMed] [Google Scholar]
- Seo SW, Lee JH, Jang SM, Kim ST, Chin J, Kim GH, Kim JH, Roh JH, Kim MJ, Kim SH, Na DL (2012): Neurochemical alterations of the entorhinal cortex in amnestic mild cognitive impairment (aMCI): a three‐year follow‐up study. Arch Gerontol Geriatr 54:192–196. [DOI] [PubMed] [Google Scholar]
- Thompson P, Cannon T, Narr K, van Erp T, Poutanen V, Huttunen M, Lönnqvist J, Standertskjöld‐Nordenstam C, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW (2001): Genetic influences on brain structure. Nat Neurosci 4:1253–1258. [DOI] [PubMed] [Google Scholar]
- Thompson P, Hayashi K, de Zubicaray G, Janke A, Rose S, Semple J, Herman D, Hong M, Dittmer S, Doddrell D, Toga AW (2003): Dynamics of gray matter loss in Alzheimer's disease. J Neurosci 23:994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P, Hayashi K, Dutton R, Chiang M, Leow A, Sowell E, De Zubicaray G, Becker J, Lopez O, Aizenstein H, Toga AW (2007): Tracking Alzheimer's disease. Ann NY Acad Sci 1097:183–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND (2012): Laterality patterns of brain functional connectivity: gender effects. Cereb Cortex 22:1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier W, Pijenburg Y, Fox N, Scheltens P. (2011): Early‐onset versus later onset Alzheimer's disease: The case of the missing APOE 4 allele. Lancet Neurol 3:280–288. [DOI] [PubMed] [Google Scholar]
- Voyer D. (1996): On the magnitude of laterality effects and sex differences in functional lateralities. Laterality 1:51–83. [DOI] [PubMed] [Google Scholar]
- Watkins K, Paus T, Lerch J, Zijdenbos A, Collins D, Neelin P, Taylor J, Worsley K, Evans A. (2001): Structural asymmetries in the human brain: A voxel‐based statistical analysis of 142 MRI scans. Cereb Cortex 11:868–877. [DOI] [PubMed] [Google Scholar]
- Wernicke C (1874):Der aphasische Symptomencomplex: eine psychologische Studie auf anatomischer Basis.Breslau:Cohn und Welgert. [Google Scholar]
- Zhong J, Phua D, Qiu A (2010): Quantitative evaluation of LDDMM, FreeSurfer and CARET for cortical surface mapping. Neuroimage 52:131–141. [DOI] [PubMed] [Google Scholar]


