Abstract
Purpose: To investigate the intrinsic brain connections at the time of interictal generalized spike‐wave discharges (GSWDs) to understand their mechanism of effect on brain function in untreated childhood absence epilepsy (CAE). Methods: The EEG‐functional MRI (fMRI) was used to measure the resting state functional connectivity during interictal GSWDs in drug‐naïve CAE, and three different brain networks—the default mode network (DMN), cognitive control network (CCN), and affective network (AN)—were investigated. Results: Cross‐correlation functional connectivity analysis with priori seed revealed decreased functional connectivity within each of these three networks in the CAE patients during interictal GSWDS. It included precuneus‐dorsolateral prefrontal cortex (DLPFC), dorsomedial prefrontal cortex (DMPFC), and inferior parietal lobule in the DMN; DLPFC‐inferior frontal junction (IFJ), and pre‐supplementary motor area (pre‐SMA) subregions connectivity disruption in CCN; ACC‐ventrolateral prefrontal cortex (VLPFC) and DMPFC in AN; There were also some regions, primarily the parahippcampus, paracentral in AN, and the left frontal mid orb in the CCN, which showed increased connectivity. Conclusions: The current findings demonstrate significant alterations of resting‐state networks in drug naïve CAE subjects during interictal GSWDs and interictal GSWDs can cause dysfunction in specific networks important for psychosocial function. Impairment of these networks may cause deficits both during and between seizures. Our study may contribute to the understanding of neuro‐pathophysiological mechanism of psychosocial function impairments in patients with CAE. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: EEG‐fMRI, CAE, functional connectivity, interictal GSWDs, resting state
INTRODUCTION
Multiple active subsystems exist in the brain at rest, resembling specific neuroanatomical networks [Biswal et al.,1995]. Thus, through examining the human brain as an integrative network of functionally interacting brain regions, we can obtain new insights about large‐scale neuronal communication in the brain. Resting state functional connectivity MRI (fcMRI) is widely used to investigate brain networks that exhibit correlated fluctuations. It has been used to examine how functional connectivity and information integration relates to human behavior and how this organization may be altered in brain diseases [Gotman et al.,2006]. Simultaneous recordings of the electroencephalogram (EEG) and functional MRI (EEG‐fMRI) can investigate different brain networks involved in metabolic changes resulting from the epileptic discharges seen in the scalp EEG.
The default mode network (DMN) is a medial cortical network, which has been found to be active at rest [Raichle et al.,2001], and to be deactivated when subjects perform attention‐demanding, goal‐oriented tasks [Shulman et al.,1997]. It takes an important part in self‐referential activities, including evaluating salience of internal and external cues, remembering the past, and planning the future [Buckner et al.,2008; Ralchle and Snyder,2007; Raichle et al.,2001; Spreng and Grady,2010]. In addition to the DMN, the other important system, the cognitive control network (CCN), serves attention demanding cognitive tasks and exhibits activity increases in frontal and parietal regions associated with top‐down modulation of attention and working memory tasks [Corbetta and Shulman,2002]. A third system, the affective network (AN) is involved in emotional processing [Bush et al.,2000; Mayberg et al.,1999; Ongur et al.,2003; Phillips et al.,2003] and, through its connections with other regions, regulates fear, vigilance, and autonomic and visceral function.
Childhood absence epilepsy (CAE) is the most common childhood epilepsy syndrome, accounting for 10–17% of all childhood onset epilepsy. It has a broad range of untreated cognitive, linguistic, and behavioral/emotional comorbidities [Caplan et al.,2008]. Unfortunately, the fundamental mechanisms of altered brain function in CAE are not yet known. It has the typical 3Hz generalized spike and wave discharges (GSWDs) in scalp EEG without clinical signs that is called interictal GSWDs. And the subclinical discharges may involve selective cortical and subcortical networks, while sparing other regions and contribute to the psychosocial problems in CAE.
Therefore, we hypothesized that interictal GSWDs can cause dysfunction in specific networks important for psychosocial function. Impairment of these networks may cause deficits both during and between seizures. In the present study, we used EEG‐fcMRI to investigate altered resting‐state functional connectivity in untreated CAE during interictal GSWDs. We examined the three different networks mentioned above: the CCN, the DMN, and the AN.
MATERIAL AND METHODS
Patients
Diagnosis was established according to the diagnostic scheme published by the International League Against Epilepsy in 2001 [Engel,2001]. All the patients were newly diagnosed and had not received epilepsy medications. The study was conducted in patients with CAE recruited from the epilepsy clinics at the West China Hospital for Neurology of Sichuan University. All patients underwent routine clinical neuroimaging and 24‐h video‐EEG monitoring to ensure that there were no structural abnormalities, but the frequent and stereotypical interictal GSWDs were confirmed. Patients were administered the Wechsler Intelligence Scale for Children China‐Revised (WISC‐CR) test [Gong and Cai,1993] and the tests were scored by a neuropsychologist and used along with age, gender and handedness to match cohorts of patients and controls. Patients with self‐reported mental disorders or cognitive handicaps were excluded. This study was approved by a responsible governmental agency at the Sichuan University. Informed consent for the study was obtained from each subject.
EEG Acquisition
The EEG was recorded using a 10/20 system with 21 Ag/AgCl electrodes soldered to 12 kV current‐limiting resistors applied on the scalp with conductive cream. The EEG device was an EBNeuro Mizar 40 (Florence, Italy), with 32 channels adapted for MR and a sampling rate of 4 kHz, which allowed suitable time resolution for detecting the switching effect of the readout gradient in the high slew rate condition. The EEG dynamic range was ±65.5 mV to prevent MRI artifact waveforms from saturating the EEG/ECG. The amplifier was connected to the recording computer outside the scanner room via a fiber‐optic cable. The MR artifact was filtered online [Garreffa et al.,2003]. The pulse artifact was minimized by using a locked arrangement of particular wires and an elastic cap. If no spikes were found in one session, that run's fMRI data was excluded in the future analysis.
fMRI Acquisition
A 3.0 T GE MRI scanner (EXCITE, Milwaukee) was used to acquire BOLD sensitive echo‐planar images (EPI) with continuous, simultaneous EEG. Foam padding was used to help secure the EEG leads, minimize motion, and improve patient comfort. At the same time, the patient's ears were packed with cotton balls and the patient assumed a resting state with their eyes closed. The gradient echo EPI sequence's parameters were as follows: 30 slices, 200 volumes, TR = 2,000ms, TE = 40 ms, FOV = 24 cm, matrix = 64 × 64, in‐plane resolution = 22 mm, and a flip angle = 90°. The structural image was also acquired during the interval of the first and second run (TR = 8.5 ms, TE = 3.4 ms, FOV = 24 cm × 24 cm, flip angle = 12°, matrix = 512 × 512, 156 slices). BOLD fMRI data were collected in successive runs of 6 min and 40 s. Additional scanning sessions were acquired in the same patient if no spikes were recorded during the real‐time EEG in one session.
Data Preprocess Analysis, Seed Defined, and Seed Correlation Analysis
Pre‐processing of fMRI data were conducted using the SPM8 software package [statistical parametric mapping http://www.fil.ion.ucl.ac.uk/spm]). The slice time correction, 3D motion detection and correction, spatial normalization to the Montreal Neurological Institute (MNI) template supplied by SPM, and spatial smoothing using an isotropic Gaussian kernel (8 mm full width at half maximum) were included. Several procedures were used to remove the possible variances from time course of each voxel. (i) Temporal band‐pass filtering (pass band 0.01–0.1 Hz) was conducted through a phase‐insensitive filtering (ii) Through linear regression, the time series was further corrected to eliminate the effect of six head motion parameters obtained in the realigning step and the effect of the signals from a CSF region, and a white matter (WM) region. The residuals of the regressions were linearly detrended, and then used for the next functional connectivity analysis.
On the basis of the literature [Sheline et al.,2010], three regions were selected as seeds to identify three different networks, the bilateral dorsolateral prefrontal cortex (DLPFC) (±36, 27, 29) in the CCN, the precuneus (±7, −60, 21) in the DMN, and the subgennual anterior cingulate cortex (ACC) (±10, 35, −2) in the affective ACC network. For each region, the time course form the bilateral spherical region‐of‐interest (radius 10 mm) was averaged. Subsequently, a seed correlation analysis was performed between the mean time course and data of all voxels in individual level. Individual correlation coefficients were normalized to Z‐scores by using Fisher's Z transformation. Finally, the individual Z scores were entered to random effect one‐sample t‐test in SPM8. Statistical map of significant functional connectivity for each seed was created. Significance level was set at P < 0.05, corrected for multiple comparisons using the FDR‐criterion [Genovese et al.,2002]. According to whether GSWDS s was detected during a particular session or not, the fMRI data was divided into interictal discharges group and non‐discharge group. To examine the difference between groups, two‐sample t‐test in SPM8 was used respectively in three networks. Significance level was set at P < 0.05, corrected for multiple comparisons using the false discovery rate (FDR)‐criterion [Genovese et al.,2002].
RESULTS
Clinical Features
Ten patients diagnosed with CAE were recruited (4 males, 6 females, mean age 7.65, range 5–11). None of them has received antiepileptic medications. Twenty‐eight sessions of EEG‐fMRI scanning in 10 patients were carried out. And interictal GSWDs were identified in twenty sessions in all patients. The interictal EEGs demonstrated generalized 3 Hz spike and waves, and the number of interictal GSWD epochs ranged from 3 to 11 (median, 6), and the duration of an epoch ranged from 0.6 to 3.8 s.
Functional Connectivity of the DMN
A connectivity map for each group was generated, and the connectivity patterns of positive and negative correlations appeared to be similar during visual inspection of the two groups (Fig. 1A,B). The DMN network was consistent with previous studies [Uddin et al.,2009]. The between‐group difference in DMN was obtained, which was defined by the cross‐correlation functional connectivity analysis with seed at precuneus. Compared with the control group, no increase in connectivity was found. However, decreased connectivity were detected at the bilateral DLPFC, dorsomedial prefrontal cortex (DMPFC) and inferior parietal lobule (Fig. 1C). Results revealed that DMN functional connectivity was reduced in the untreated CAE patients during interictal GSWDs.
Figure 1.
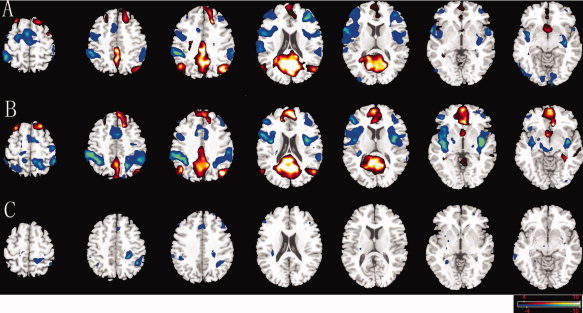
Comparison of connectivity maps in DMN. Connectivity of the DMN for interictal discharges group (A), non‐discharge group (B), and between‐group differences (C). Networks were identified by seed regions placed in the precuneus. No increased connection was found, and decreased connectivity was showed (blue) in DLPFC, DMPFC, and inferior parietal lobule in untreated CAE patients. The statistical threshold was P < 0.05 (FDR‐corrected, 20 adjacent voxels). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Functional Connectivity of the CCN
CCN was observed in brain regions previously defined as within the CCN for both groups [Cole and Schneider,2007]. Although the spatial maps look similar between the two groups (Fig. 2A,B), significant differences were observed in specific subregions of these areas (Fig. 2C). The between‐group difference in CCN was obtained, which was defined by the cross‐correlation functional connectivity analysis with seed at bilateral DLPFC. Compared with the control group, decreased connectivity was detected at the bilateral inferior frontal junction (IFJ), DLPFC, and pre‐supplementary motor area (pre‐SMA) subregions. And the increase in connectivity was found in the left frontal middle orbital gyrus (Fig. 2C). Results revealed that CCN functional connectivity was altered in the untreated CAE patients during interictal GSWDs.
Figure 2.
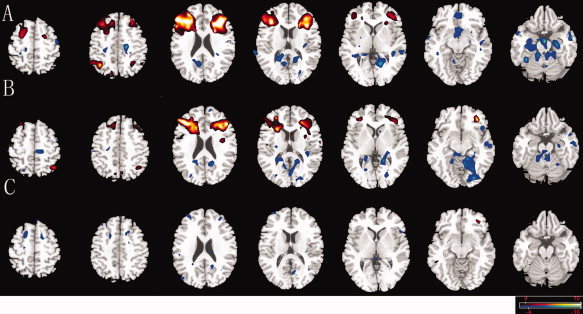
Comparison of connectivity maps in CCN. Connectivity of the CCN for interictal discharges group (A), non‐discharge group (B), and between‐group differences (C); Networks were identified by seed regions placed in the DLPFC. Decreased connectivity was showed (blue) in DLPFC, IFJ, and pre‐SMA subregions and increased connectivity was found in the left frontal middle orbital gyrus (red). The statistical threshold was P < 0.05 (FDR‐corrected, 20 adjacent voxels). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Functional Connectivity of the AN
AN (Fig. 3A,B) and between‐group differences (Fig. 3C) were also reported. The TRN areas are similar to previous reports [Bush et al.,2000; Modinos et al.,2010]. For the between‐group comparison, the bilateral ventrolateral prefrontal cortex (VLPFC), and DMPFC area are significantly more anti‐correlated with the ACC in trial group than in control group. There were also some regions, primarily the parahippcampus, paracentral in AN which showed increased connectivity (Fig. 3C). Results revealed that AN functional connectivity was altered in the CAE patients during interictal GSWDs.
Figure 3.
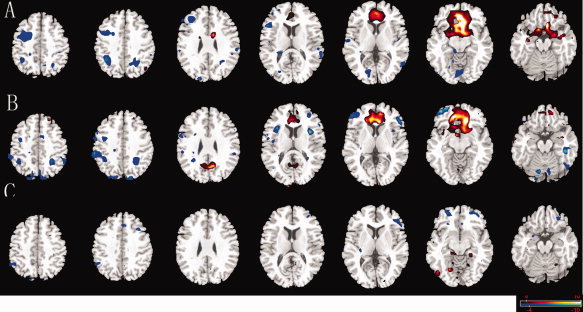
Comparison of connectivity maps in AN. Connectivity of the AN for interictal discharges group (A), non‐discharge group (B), and between‐group differences (C). Networks were identified by seed regions placed in the ACC. Decreased connectivity was showed (blue) in ACC, VLPFC, and DMPFC. Some regions, primarily the parahippcampus, paracentral area showed increased connectivity (red). The statistical threshold was P < 0.05 (FDR‐corrected, 20 adjacent voxels). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
To our knowledge, this is the first study directly investigating resting‐state functional connectivity of drug naïve CAE patients during interictal GSWDs, and three different networks including the CCN, the DMN, and the AN were examined. We found that during interictal GSWDs, CAE subjects mainly exhibited decreased connectivity within the three different networks. The findings indicate that interictal GSWDs can cause dysfunction in specific networks important for psychosocial function and impairment of these networks may cause deficits both during and between seizures.
Previous EEG‐fMRI studies in epilepsy patients with GSWDs revealed BOLD signal activation in the thalamus and deactivation in frontoparietal areas, the precuneus and the caudate nucleus [Aghakhani et al.,2004; Gotman et al.,2005; Hamandi et al.,2006; Laufs et al.,2006; Li et al.,2009; Moeller et al.,2008,2010]. And in drug naïve children with newly diagnosed absence epilepsy, this pattern was also found [Moeller et al.,2008]. These findings suggest that the brain regions involved in the pattern of deactivation are attributed to the DMN. Activity and connectivity of the DMN has been suggested to be involved in the integration of cognitive and emotional processing [Greicius et al.,2003] and monitoring the world around us [Gusnard and Raichle,2001]. Our previous work has found that DMN abnormalities in patients with absence epilepsy during resting interictal durations without interictal epileptic discharges [Luo et al.,2011]. In this study, we further found decreased connectivity in DMN during interictal GSWDs in drug naïve CAE patients. The decreased connectivity in DMN during interictal GSWDs may lead to an interruption of cognitive processes and the subclinical impairment of consciousness.
In our study, alterations of connectivity were detected at the subregions of CCN [Uddin et al.,2009]. In this network, DLPFC is for sustained attention and working memory [Curtis and D'Esposito,2003] and pre‐SMA plays an important role in cognitive motor control which involves sensory discrimination and decision making or motor selection for the action after stimuli [Ikeda et al.,1999]. IFJ is involved in the cognitive control and it is involved in the updating of task representations [Derrfuss et al.,2005]. Specifically, the right IFJ plays a crucial role in inhibitory control and suppression of inappropriate responses [Aron et al.,2004]. Our results revealed cognitive control network changed during the interictal epileptic discharges, and this may be responsible for the subclinical behavior changes. Another important network system, the AN, is important in fear, vigilance, and autonomic and visceral regulation. In our study, we found decreased functional connectivity of ACC, VLPFC, and DMPFC in AN network; These areas are within a predominantly prefrontal network, and has a consistent and prominent role in the down‐regulation of negative emotion [Ochsner and Gross,2005]. There were also some regions, primarily the parahippcampus, paracentral in AN network which showed increased connectivity. The AN has strong local connections to many limbic structures [Ongur et al.,2003; Pessoa,2008], and is particularly involved in the relationship of emotion/mood to visceral function [Phillips et al.,2003; Price and Drevets,2010]. Parahippocampal gyrus belongs to the ventromedial neural system, which is implicated in the early appraisal and encoding of emotional significance during regulation of behavioral responses to an emotional stimulus [Almeida et al.,2009]. Thus, our results indicate that AN network was altered during the GSWDs in CAE patients, and this may account for the behavioral/emotional comorbidities in CAE patients. On the other hand, from a network‐related point of view, our results found the DLPFC with decreased connectivity to the networks of DMN and CCN, the DMPFC with decreased connectivity to the networks of DMN and AN. This may indicate that DMN forms the core resting state network around which the other networks amalgamate [Deshpande et al.,2011] and specific networks are linked together. This may explain why the cognitive and behavioral/emotional comorbidities arising in distinct networks in CAE can occur concurrently and behave synergistically.
Interictal epileptic discharges (IED) can affect activity in brain regions [Barbarosie and Avoli,1997; Barbarosie et al.,2000]. In this study, our results revealed mainly decreased functional connectivity within each of these three networks in the CAE patients during interictal GSWDs. If the signals induced by IED were only the random noise, the correlation coefficient between signals should be decreased. However, the regions in these networks showed decreased activation responding to the IED in previous studies [Gotman et al.,2005; Li et al.,2009] and these results mean that the IED cause the inverse hemodynamic response function. Therefore, the change of network connections may be an inherent mechanism to weaken network connections and prevent overexcitability and the network connections attenuation seems to be negative feedback mechanisms that might prevent seizures in CAE. On the other hand, there is increasing evidence that IED can have a negative effect on cerebral function, which includes two types (transitory interruption of cognitive function and alteration of the physiological mechanisms involved in brain plasticity and memory) [Holmes and Lenck‐Santim,2006]. Recently, it has been found that hippocampal spikes that occurred during memory retrieval strongly impaired performance [Kleen et al.,2010]. However, there is no evidence yet to justify routine use of antiepileptic drugs to treat cognitive or behavioral problems in people with subclinical EEG discharges. Preliminary trials have confirmed the possibility of improving psychosocial function by suppressing IED with medication [Marston et al.,1993; Pressler et al.,2005]. Our results proved that resting‐state connectivity was altered and network‐specific decreases of synchronization were found in different networks during interictal GSWDs in drug‐naïve CAE without outspoken cognitive impairment. This may contribute to the cognitive and behavioral/emotional comorbidities in CAE. Our data indicate that suppression of discharges may be associated with significant improvement in psychosocial function and drug treatment is desirable or effective.
CONCLUSIONS
The current findings demonstrate significant alterations of resting‐state networks in drug naïve CAE subjects during interictal GSWDs and interictal GSWDs can cause dysfunction in specific networks important for psychosocial function. Impairment of these networks may cause deficits both during and between seizures. Our study may contribute to the understanding of neuro‐pathophysiological mechanism of psychosocial function impairments in patients with CAE. The application of connectivity analysis techniques based on EEG‐fMRI data may improve our understanding of the interactions between brain regions hemodynamically involved during GSWDs.
Acknowledgements
The authors acknowledge all participants in the study.
References
- Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, Gotman J ( 2004): fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain 127: 1127–1144. [DOI] [PubMed] [Google Scholar]
- Almeida JRC, Mechelli A, Hassel S, Versace A, Kupfer DJ, Phillips ML ( 2009): Abnormally increased effective connectivity between parahippocampal gyrus and ventromedial prefrontal regions during emotion labeling in bipolar disorder. Psychiatry Research‐Neuroimaging 174: 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA ( 2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8: 170–177. [DOI] [PubMed] [Google Scholar]
- Barbarosie M, Avoli M ( 1997): CA3‐driven hippocampal‐entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci 17: 9308–9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarosie M, Louvel J, Kurcewicz I, Avoli M ( 2000): CA3‐released entorhinal seizures disclose dentate gyrus epileptogenicity and unmask a temporoammonic pathway. J Neurophysiology 83: 1115–1124. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS ( 1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34: 537–541. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL ( 2008): The brain's default network ‐ Anatomy, function, and relevance to disease. Cogn Neurosci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Stahl L, Lanphier E, Vona P, Gurbani S, Koh S, Sankar R, Shields WD ( 2008): Childhood absence epilepsy: Behavioral, cognitive, and linguistic comorbidities. Epilepsia 49: 1838–1846. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W ( 2007): The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage 37: 343–360. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M ( 2003): Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7: 415–423. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY ( 2005): Involvement of the inferior frontal junction in cognitive control: Meta‐analyses of switching and stroop studies. Hum Brain Mapp 25: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Santhanam P, Hu X ( 2011): Instantaneous and causal connectivity in resting state brain networks derived from functional MRI data. Neuroimage 54: 1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J ( 2001): A proposed diagnostic scheme for people with epileptic seizures and with epilepsy. Report of the ILAE task force on classification and terminology. Aktuelle Neurologie 28: 305. [DOI] [PubMed] [Google Scholar]
- Garreffa G, Carni M, Gualniera G, Ricci GB, Bozzao L, De Carli D, Morasso P, Pantano P, Colonnese C, Roma V ( 2003): Real‐time MR artifacts filtering during continuous EEG/fMRI acquisition. Magn Reson Imag 21: 1175–1189. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Gong YX, Cai TS ( 1993): The Wechsler intelligence scale for children revised in china (C‐WISC).
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F ( 2005): Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA 102: 15236–15240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J, Kobayashi E, Bagshaw AP, Benar CG, Dubeau F ( 2006): Combining EEG and fMRI: A multimodal tool for epilepsy research. J Magn Reson Imag 23: 906–920. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V ( 2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME ( 2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Hamandi K, Salek‐Haddadi A, Laufs H, Liston A, Friston K, Fish DR, Duncan JS, Lemieux L ( 2006): EEG‐fMRI of idiopathic and secondarily generalized epilepsies. Neuroimage 31: 1700–1710. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Lenck‐Santim PP ( 2006): Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav 8: 504–515. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Yazawa S, Kunieda T, Ohara S, Terada K, Mikuni N, Nagamine T, Taki W, Kimura J, Shibasaki H ( 1999): Cognitive motor control in human pre‐supplementary motor area studied by subdural recording of discrimination/selection‐related potentials. Brain 122: 915–931. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Scott RC, Holmes GL, Lenck‐Santini PP ( 2010): Hippocampal interictal spikes disrupt cognition in rats. Ann Neurology 67: 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Lengler U, Hamandi K, Kleinschmidt A, Krakow K ( 2006): Linking generalized spike‐and‐wave discharges and resting state brain activity by using EEG/fMRI in a patient with absence seizures. Epilepsia 47: 444–448. [DOI] [PubMed] [Google Scholar]
- Li QF, Luo C, Yang TH, Yao ZP, He L, Liu L, Xu HR, Gong QY, Yao DZ, Zhou D ( 2009): EEG‐fMRI study on the interictal and ictal generalized spike‐wave discharges in patients with childhood absence epilepsy. Epilepsy Res 87: 160–168. [DOI] [PubMed] [Google Scholar]
- Luo C, Li QF, Lai YX, Xia Y, Qin Y, Liao W, Li SS, Zhou D, Yao DZ, Gong QY ( 2011): Altered Functional Connectivity in Default Mode Network in Absence Epilepsy: A Resting‐State fMRI Study. Hum Brain Map 32: 438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston D, Besag F, Binnie CD, Fowler M ( 1993): Effects of transitory cognitive impairment on psychosocial functioning of children with epilepsy: A therapeutic trial. Dev Med Child Neurology 35: 574–81. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL ( 1999): Reciprocal limbic‐cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry 156: 675–682. [DOI] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A ( 2010): Altered activation and functional connectivity of neural systems supporting cognitive control of emotion in psychosis proneness. Schizophrenia Res 118: 88–97. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Wolff S, Muhle H, Granert O, Jansen O, Stephani U, Siniatchkin M ( 2008): Simultaneous EEG‐fMRI in drug‐naive children with newly diagnosed absence epilepsy. Epilepsia 49: 1510–1519. [DOI] [PubMed] [Google Scholar]
- Moeller F, Muhle H, Wiegand G, Wolff S, Stephani U, Siniatchkin M ( 2010): EEG‐fMRI study of generalized spike and wave discharges without transitory cognitive impairment. Epilepsy Behav 18: 313–316. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ ( 2005): The cognitive control of emotion. Trends Cogn Sci 9: 242–249. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL ( 2003): Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 460: 425–449. [DOI] [PubMed] [Google Scholar]
- Pessoa L ( 2008): On the relationship between emotion and cognition. Nat Rev Neurosci 9: 148–158. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R ( 2003): Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry 54: 515–528. [DOI] [PubMed] [Google Scholar]
- Pressler RM, Robinson RO, Wilson GA, Binnie CD ( 2005): Treatment of interictal epileptiform discharges can improve behavior in children with behavioral problems and epilepsy. J Pediatr 146: 112–117. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC ( 2010): Neurocircuitry of mood disorders. Neuropsychopharmacology 35: 192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralchle ME, Snyder AZ ( 2007): A default mode of brain function: A brief history of an evolving idea. Neuroimage 37: 1083–1090. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan ZZ, Mintun MA ( 2010): Resting‐state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA 107: 11020–11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, Raichle ME, Fiez JA, Miezin FM, Petersen SE ( 1997): Top‐down modulation of early sensory cortex. Cereb Cortex 7: 193–206. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL ( 2010): Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci 22: 1112–1123. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AMC, Biswal BB, Castellanos FX, Milham MP ( 2009): Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp 30: 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]


