Abstract
Previous studies have shown that people who develop psychopathology such as posttraumatic stress disorder (PTSD) following stress exposure are characterized by reduced hippocampal (HC) volume and impaired HC functional connectivity with the ventromedial prefrontal cortex (vmPFC). Nevertheless, the exact interrelationship between reduced HC volume and HC‐vmPFC connectivity deficits in the context of stress has yet to be established. Furthermore, it is still not clear whether such neural abnormalities are stress induced or precursors for vulnerability. In this study, we combined measurements of MRI, functional MRI (fMRI), and diffusion tensor imaging (DTI) to prospectively study 33 a priori healthy Israeli soldiers both pre‐ and post‐exposure to stress during their military service. Thus, we were able to assess the contributions of structural and functional features of the HC and its connectivity to the onset and progression of maladaptive response to stress (i.e., increased PTSD symptoms post‐exposure). We found that soldiers with decreased HC volume following military service (i.e., post‐exposure) displayed more PTSD‐related symptoms post‐exposure as well as reduced HC‐vmPFC functional and structural connectivity post‐exposure, compared to soldiers with increased HC volume following military service. In contrast, initial smaller HC volume pre‐exposure did not have an effect on any of these factors. Our results therefore suggest that reduction in HC volume and connectivity with the vmPFC together mark a maladaptive response to stressful military service. As stress‐induced HC volume reductions were previously shown to be reversible, these localized biological markers may carry valuable therapeutic potential. Hum Brain Mapp 34:2808–2816, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: functional connectivity, structural connectivity, volumetry, uncinate fasciculus, PTSD, DTI, MRI, fMRI
INTRODUCTION
Psychological stress affects both the structure and function of the brain [Sapolsky, 1992]. Stress‐related structural impairments have been especially documented in the hippocampus (HC) perhaps because of its important role in stress regulation, as reflected, for example, in its high number of cortisol receptors [McEwen, 1999]. A large crop of animal studies has shown how exposure to stress can damage the HC structure by atrophying its dendritic processes, causing neuronal loss and suppressing its synaptic plasticity and neurogenesis [Diamond et al., 1994; Gould et al., 1997; Joels et al., 2004; Magarinos et al., 1996; Pavlides et al., 2002; Sapolsky et al., 1990; Shors and Thompson, 1992; Uno et al., 1989]. In humans, reduced HC volume has been the most commonly found neural structural abnormality in people who were exposed to traumatic stress and consequently develop an anxiety disorder such as posttraumatic stress disorder (PTSD) [Brambilla et al., 2002; Smith, 2005].
Considering the critical role that the HC plays in learning and memory [Zola‐Morgan and Squire, 1990), its volume reduction was associated with the various memory deficits that PTSD patients suffer from [Bremner et al., 1993; Yehuda et al., 1995]. One specific memory process in which a deficit seems highly relevant to the development and preservation of stress‐related anxiety is fear extinction [Charney and Bremner, 1983; Shekhar et al., 2001]. Extinction of conditioned fear is an essential need for adaptive recovery from traumatic experience and involves the unlearning of fear reaction to situations that were previously associated with negative outcome but currently can be considered as safe [Rothbaum and Davis, 2003]. Indeed, PTSD patients are known to suffer from impaired ability to extinguish fear [Blechert et al., 2007; Peri et al., 2000], and furthermore, exaggerated and persistent fear responses to reminders of the traumatic event is one of the main clinical characteristics of PTSD [DSM‐IV‐time repetition (TR), 2000].
The HC is but one structure involved in complex memory processes as fear extinction, interacting with a cluster of different structures of the limbic system, notably the amygdala and the ventromedial prefrontal cortex (vmPFC) [LaBar et al., 1998; Phelps et al., 2004]. Specifically, a recent functional MRI (fMRI) study suggested that the involvement of both the HC and vmPFC is important for adequate fear extinction [Milad et al., 2007]. This is also supported by animal findings showing that low frequency stimulation of the HC immediately after extinction training suppresses long‐term potentiation induction in the HC‐vmPFC pathways and disrupts extinction [Farinelli et al., 2006]. Stress was also shown to have an effect on structural connectivity within this network when the integrity of the uncinate fasciculus (UF) fiber, which is the white matter fiber tract that anatomically connects the HC to the vmPFC [Carpenter, 1983], was reduced in a group of children with early deprivation stress [Eluvathingal et al., 2006].
Taken together, these studies suggest that reduced HC volume and insufficient HC‐vmPFC connectivity may be involved in stress vulnerability; yet, to the best of our knowledge, no study has addressed the nature of the interrelationship between HC volume and properties of its functional and structural connectivity with the vmPFC in the context of stress in humans. Furthermore, because of conflicting evidence, it is not clear whether reduced HC volume is stress induced or a precursor for vulnerability [Gilbertson et al., 2002; Vermetten et al., 2003]. To address those issues, we prospectively followed a group of 33 a priori healthy soldiers throughout their combative military service in the Israeli Defense Forces (IDF). Specifically, the study's first time point was immediately before the beginning of the military draft while participants were still civilians but already recruited to the military, and the second time point was 18 months later while participants were serving in front line combative IDF units. In previous fMRI studies using the same study population, we found that such combative military service in the IDF includes exposure to multiple real‐life stressful events [Admon et al., 2009; Admon et al., 2012]. In this study, however, we combined various MRI measurements [structural MRI, fMRI, and diffusion tensor imaging (DTI)] to assess the soldiers' HC volumes as well as the strength of their HC‐vmPFC functional and structural connectivity at each time point. We hypothesized that reduced HC volume would be related to weaker functional and structural HC‐vmPFC connectivity and, furthermore, that this relation would constitute a neural marker for either induced or predisposing maladaptive response to stress.
MATERIALS AND METHODS
Participants
Of the initial 37 participants in Admon et al. 2012, four participants had to be excluded in this study because of coregistration artifacts. Thus, this study group comprised 33 healthy 18‐year‐old soldiers (18 male), recently drafted to mandatory military service to serve as combat paramedics in the IDF. To be drafted for an elite IDF unit as combat paramedics, a recruit must first pass a series of mental and physical tests as well as an examination by both a physician and a psychiatrist. These processes are meant to rule‐out, among other things, any history of psychiatric or neurological disorders, past or present use of psychoactive drugs, or exposure to trauma. Since at the first time point of this study our participants were already recruited for the combat paramedics unit and had passed all of the above selection criteria, we can rule‐out the existence of any of these factors in our sample before entering the study. Finally, all participants provided written informed consent approved by both the Tel Aviv Sourasky Medical Center and the IDF ethics committees.
PTSD‐Related Symptom Evaluation
We used the Posttraumatic Stress Diagnostic Scale (PDS) questionnaire to yield quantified severity measures of PTSD‐related symptoms at each time point [Foa et al., 1997]. This formal self‐reported questionnaire of stress‐related experience and symptoms also contains an open question regarding the type and frequency of stressful experiences. We instructed the participants to complete the questionnaire in regard to the past 18 months, and thus we were able to evaluate post‐exposure both the type and frequency of individual experiences endured during their military service as well as to quantify the severity of symptoms that were developed following such experiences (Supporting Information Table 1). Indeed, during the 18 months of their military service, all of the soldiers were exposed to multiple stressful experiences that, as a direct outcome of their shared role as combat paramedics, were highly comparable and included mostly repeated events of treating a fellow soldier with severe injury sustained during combat. Because our definition of maladaptive response to stress was based on self‐reports following the stress exposure, nevertheless, a complete clinical assessment would be necessary to validate the PDS score.
MRI Data Acquisition
At both time points MRI scans were performed on a 3.0‐T MRI scanner (GE Signa EXCITE, Milwaukee, WI) with a standard eight‐channel head coil. Identical anatomical 3D spoiled gradient‐echo sequences for the whole brain were obtained with high‐resolution isotropic 1‐mm slice thickness field of view (FOV): 25 × 18; matrix: 256 × 256; and TR/time echo (TE): 7.3/3.3). fMRI was performed with gradient echo‐planar imaging (EPI) sequence of functional T 1*‐weighted images (TR/TE/flip angle = 2,500 ms/35 ms/90°, with FOV 20 × 20 cm and matrix size 64 × 64) divided into 44 axial slices (thickness: 3 mm with no gap) covering the entire brain. DTI was performed with a spin‐echo diffusion‐weighted EPI sequence (FOV: 20 × 20; matrix: 128 × 128; and TR/TE = 12,000/97) divided into 48 axial slices of 3‐mm thickness with no gap covering the entire brain. Images were obtained with both 19 directional diffusion encoding (b = 1,000 s/mm2 for each direction) and no diffusion encoding (b = 0 s/mm2). To ensure high data reliability, a high signal‐to‐noise ratio was achieved by repeating the DTI scan twice and averaging the images.
HC Volume Estimation
HC volume segmentation was performed using a standardized and validated manual segmentation protocol [Pruessner et al., 2000]. Before segmentation, several algorithms were used to prepare the images for manual segmentation. This included correction for magnetic field nonuniformities [Sled et al., 1998], linear stereotaxic transformation [Collins et al., 1994] into coordinates based on the Talairach atlas [Talairach and Tournoux, 1988], and resampling onto a 1‐mm rectangular voxel grid using a linear interpolation kernel. To minimize variation induced by the repeated scanning, we performed intraindividual coregistration between the two scans of each subject, after linear registration of the first time point. Volumetric analysis was then performed with the interactive software package DISPLAY developed at the Montreal Neurological Institute. This program allows visualization of MR images in all orientations and rotations. Volumes of the HC were then measured using manual segmentation guidelines as previously described [Pruessner et al., 2000]. HC estimation included the HC proper (dentate gyrus and the corpus ammoni substructures), alveus/fimbria region, the subiculum, and the uncus. Segmentation was performed on coronal images and was cross checked in the horizontal and sagittal orientation except for the head of the HC, where the horizontal orientation was primarily used. All segmentations were performed three times, each time with a separate and independent rater who was blind to the time of testing and subject characteristics. Although three raters performed the initial segmentation, all labels were validated and if necessary revised by the original author of the segmentation protocol (JCP) before statistical analysis. Intrarater and inter‐rater reliability for this protocol has been established previously [Pruessner et al., 2000].
HC‐vmPFC Functional Connectivity
In a previous study, we used HC time course as a regressor in a whole‐brain voxel‐based correlation analysis to show that the HC‐vmPFC functional connectivity increases post‐exposure to stress relative to pre‐exposure in response to reminders of the stress. We then calculated the strength (i.e., correlation coefficient) of this functional connectivity between the HC and the vmPFC for each soldier at each time point of pre‐exposure and post‐exposure to stress [Admon et al., 2009]. Because this study group was already involved in our previous study, we were able to use in here those previously reported individual measures of HC‐vmPFC functional connectivity strengths of pre‐exposure and post‐exposure.
HC‐vmPFC Structural Connectivity
We focused our analyses on average fractional anisotropy (FA) value of the entire UF as it is considered the most reliable measure of anatomical fiber tract integrity, thus indicating on the strength of structural connectivity [Lim and Helpern, 2002]. Tractography analysis was performed using MRIStudio software (John Hopkins University, Baltimore, MD). Compact white matter fiber tracking was performed based on the fiber assignment by continuous tracking algorithm. Diffusion tensor tractography of our predefined fiber of interest, the UF, was preformed using anatomical landmarks as previously reported [Kier et al., 2004]. Specifically, the UF tracking used an FA threshold of 0.25 and angle threshold of 70°. The anterior commissure as identified on a coronal DTI color‐coded plane was used as an anatomical landmark to identify the two regions where the UF superior and inferior segments traverse the coronal plane. A Boolean “OR” operation on region 1 (lower portion of external capsule) combined with an “AND” operation on region 2 (temporal lobe) were sufficient to construct the UF while avoiding any mixing with other tracts [Kier et al., 2004]. UF identification was performed by an independent rater who was blind to the time of testing and subject characteristics. Once the UF fiber tract was reconstructed, its volume was recorded and visualized in 3D and its mean diffusivity, radial diffusivity, axial diffusivity, and FA values were extracted.
General Analysis Approach
As can be seen in Supporting Information Table 1, we had insufficient variability in the level of PTSD symptoms developed post‐exposure, which severely hampers the detection of a reliable relation to HC volume at the individual level (i.e., a third of our sample exhibited no symptoms whatsoever following exposure). Therefore, to compare different contributions of the HC to the severity of PTSD‐related symptoms post‐exposure to stress, we operationalized discrete measures for the effect of stress exposure on HC volume and for the predisposing effect of HC volume. Specifically, increased or decreased volume post‐exposure vs. pre‐exposure was operationalized as a categorical measure for the effect of stress exposure on HC volume, whereas below or above the group median standardized HC volume pre‐exposure constituted a measure for the predisposing effect of HC volume. No direct relation was found between these two categorical factors (Fisher's exact; P = 0.14). In both categorical factors, however, there was a direct relation between belonging to a specific category in the left HC and belonging to the same category in the right HC (Fisher's exact; P < 0.001 for the effect of stress exposure on HC volume and P < 0.005 for the predisposing effects of HC volume). This, along with the fact that our previous functional connectivity finding was related solely to the left HC side [Admon et al., 2009], led us to focus all our analyses in this study on the measurements taken from the left HC side.
Statistical Analysis
To associate the change in HC volume with the severity of PTSD‐related symptoms pre‐exposure and post‐exposure to stress, we used a repeated‐measure analysis of variance (ANOVA) in which HC increase or decrease in volume post‐exposure vs. pre‐exposure constituted the categorical factor and the severity of PTSD‐related symptoms pre‐exposure and post‐exposure was the dependent factor. Similar ANOVAs were performed with the use of either the functional or structural HC‐vmPFC connectivity pre‐exposure and post‐exposure as dependent factors as well as with the initial standardized HC volume pre‐exposure as a categorical factor. In all analyses throughout the study, we controlled for both the effects of gender and of the individual frequency of stressful encounters (Supporting Information Table 1).
RESULTS
Maladaptive Behavioral Effects of Stress Exposure
post‐exposure to multiple stressful encounters throughout their military service in the IDF, 20 soldiers (≈60% of the sample size) displayed PTSD‐related symptoms to some extent. For 19 of them, the severity of symptoms increased compared with pre‐exposure (Supporting Information Table 1). Symptoms included nightmares of the events, re‐experiencing the events, trying to avoid thinking/talking of the events, high arousal, decreased concentration, and others. Nevertheless, all soldiers stayed on‐duty and fully functional in their original role as combat paramedics throughout the study.
Effects of Stress Exposure on HC Volume
We divided the sample into two groups based on the effects of stress exposure on left HC volume, which was operationalized as the direction of the change in HC volume post relative to pre‐exposure to stress. The individual value of HC volume change was then normalized to percent change relative to initial individual HC volume, thus avoiding any effects of differences in initial HC volume. This yielded a group of soldiers whose HC volume decreased (n = 22; average change in HC volume = −1.8%) and a group whose HC volume increased (n = 11; average change in HC volume = +1.2%) following exposure to stress (see group distribution in Fig. 1A). Repeated‐measures ANOVA of this categorical factor (i.e., increase or decrease) revealed a significant interaction with the severity of PTSD‐related symptoms pre‐exposure vs. post‐exposure (F = 3.7; P < 0.05). Post hoc tests assigned this effect to the increase in PTSD‐related symptoms post‐exposure that was evident only in soldiers with decreased HC volume (P < 0.005; Fig. 1B). When evaluating the effect of change in HC volume on the strength of HC‐vmPFC functional connectivity pre‐exposure‐ vs. post‐exposure, we first found a main effect of increased HC‐vmPFC functional connectivity post‐exposure (F = 51; P < 0.001), on which we reported previously [Admon et al., 2009]. However, the change in HC volume also interacted with the strength of HC‐vmPFC functional connectivity pre‐exposure vs. post‐exposure (F = 4.9; P < 0.05), as more prominent connectivity post‐exposure was found in soldiers with increased HC volume (P < 0.01; Fig. 1C). Finally, Figure 1D shows that the same analysis for the FA value of the UF (i.e., structural integrity) also revealed a significant interaction (F = 9; P < 0.005), stemming from the increase in UF integrity post‐exposure vs. pre‐exposure in the group of soldiers with increased HC volume (P < 0.05) compared with the decrease in UF integrity in the group with decreased HC volume (P < 0.05).
Figure 1.
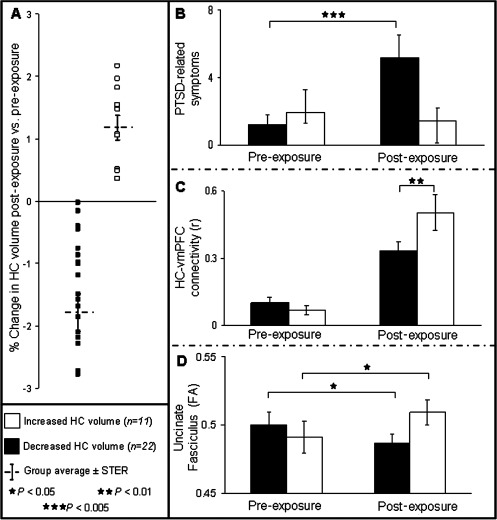
Effects of stress exposure on HC volume. (A) The plot shows the change in HC volume of each soldier postexposure vs. pre‐exposure to stress. Soldiers were divided into two groups based on the direction of change (increased HC volume, white squares and decreased HC volume, black squares). Group averages are shown by gray cross. The individual value of HC volume change was normalized to percent change by dividing it by the individual initial HC volume, thus avoiding any effects of differences in initial HC volume. (B) Bar graphs representing the groups' average severity of PTSD‐related symptom at each time point. Note that the increase in symptom severity postexposure to stress was evident only for the soldiers with decreased HC volume (P < 0.005). (C) Bar graphs representing the groups' average strength of HC‐vmPFC functional connectivity at each time point. Note the overall increase in HC‐vmPFC functional connectivity postexposure to stress, which was more prominent for the soldiers with increased HC volume (P < 0.01). (D) Bar graphs representing the groups' average FA value of the UF (i.e., structural integrity) at each time point. Note that soldiers with increased HC volume had increased UF integrity postexposure relative to pre‐exposure (P < 0.05), whereas soldiers with decreased HC volume had decreased UF integrity postexposure relative to pre‐exposure (P < 0.05). (HC, hippocampus; UF, uncinate fasciculus; FA, fractional anisotropy; N = 33; error bars ± SEM).
Predisposing Effects of HC Volume
By dividing the soldiers according to the predisposing measure of below or above the group median left standardized HC volume pre‐exposure, we defined two new groups of soldiers: below the median (n = 17; standardized HC volume pre‐exposure = 4,494 mm3) and above the median (n = 16; standardized HC volume pre‐exposure = 5,526 mm3; see group distribution in Fig. 2A). Repeated measures ANOVA of this categorical factor revealed no significant interaction with either the severity of PTSD‐related symptoms (F = 0.9; p = 0.76), the strength of HC‐vmPFC functional connectivity (F = 2.1; p = 0.15), or the integrity of the UF (F = 0.01; p = 0.91) (Figures 2B‐D, respectively). Taken together, it suggests that inter‐individual variations in HC volume pre‐exposure did not possess predictive value in our study. There were only two main effects in this analysis, the main effect of total increase in PTSD‐related symptoms (F = 4.8; P < 0.05) and in HC‐vmPFC functional connectivity (F = 43; P < 0.001) post‐exposure, findings on which we already reported [Admon et al., 2009].
Figure 2.
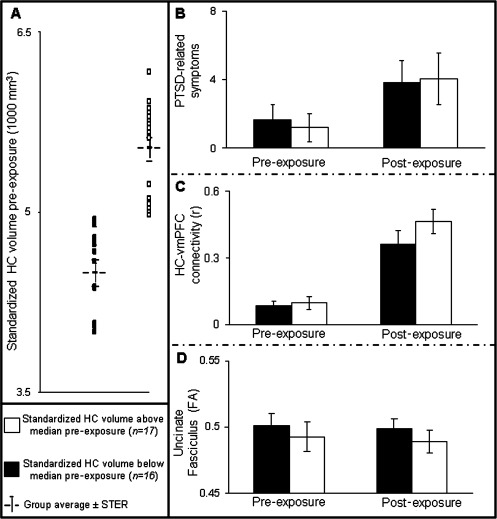
Predisposing effects of HC volume. (A) The plot shows the initial standardized HC volume pre‐exposure of each soldier. Soldiers were divided into two groups based on this measure (above the median, white squares and below the median, black squares). Group averages are shown by gray cross. Note that this measure interacted neither with the severity of PTSD‐related symptoms (B), the strength of the HC‐vmPFC functional connectivity (C) nor with the average FA value of the UF (D). There were only two main effects in that analysis, the main effect of total increase in PTSD‐related symptoms (P < 0.05) and in HC‐vmPFC functional connectivity (P < 0.001) postexposure to stress. (HC, hippocampus; UF, uncinate fasciculus; FA, fractional anisotropy; N = 33; error bars ± SEM).
DISCUSSION
In this study, we used a multiparametric prospective MRI approach that combines measurements of structural volume via MRI, functional connectivity via fMRI, and structural connectivity via DTI to study a group of 33 soldiers over the course of their stressful military service in the IDF. Thus, we were able to investigate structural and functional contributions of the HC and its connectivity with the vmPFC to the onset and progression of PTSD‐related symptoms post‐exposure to stress. We found that soldiers with more PTSD‐related symptoms post‐exposure exhibited a decrease in HC volume as well as a reduction in HC‐vmPFC functional and structural connectivity post‐exposure vs. pre‐exposure. Our results therefore suggest that reduction in HC volume and connectivity with the vmPFC following exposure to repeated military stress are related to increased PTSD related symptoms, thus might indicate a maladaptive response to such real life stressor.
Extensive animal literature has linked exposure to excessive stress with HC volume reduction [McEwen, 1999]. In humans, there have been several cross‐sectional and longitudinal studies probing changes in HC volume throughout normal life development [Giedd et al., 1996; Gogtay et al., 2006; Suzuki et al., 2005]; however, none measured the effect of stress exposure on HC plasticity. Other human studies were able to associate maladaptive reaction to stressful events with reduced HC volume by examining patients with stress‐related psychiatric disorders [Brambilla et al., 2002; Smith, 2005], but the retrospective nature of these studies did not allow them to attribute such abnormalities to either the effect of the stress or predisposition. As far as we know, our study is the first to directly relate HC volume reduction in humans following exposure to stress with a tendency to develop more PTSD‐related symptoms. This finding may be regarded as contradicting prior evidence from homozygotic twins suggesting that smaller HC volume is a predisposing risk factor [Gilbertson et al., 2002]. Nevertheless, the lack of evidence for a predisposing effect of HC volume on the severity of PTSD‐related symptoms developed following stress in this study could stem from the significant interindividual variability that exists in the range of HC volumes in the normal population [Lupien et al., 2007], interfering with the ability to detect individuals at risk based on this measure. It is also possible, however, that the lack of evidence for a predisposing effect of HC volume in this study is due to specific factors related to our sample; for example, the age range of our sample was small, participants were all rather young, and PTSD symptoms at the end of the military service were in the moderate range.
The relevance of HC volume reduction following stress to a maladaptive response to stress is supported here not only by its association with more PTSD‐related symptoms post‐exposure to stress; but also by the association that HC volume reduction seems to have with diminished connectivity of the HC with the vmPFC following stress, both at the structural and functional levels. Such disruptions in structural and/or functional connectivity were interpreted as implying poor communication between brain regions, resulting in deficient information exchange [Kim and Whalen, 2009]. Reduced functional connectivity strength specifically between the HC and the vmPFC was suggested as a neural marker for deficient fear extinction ability, which may lead to pathologically elevated and persistent conditioned fear [Milad et al., 2009], a major clinical characteristic of PTSD [DSM‐IV‐TR, 2000]. Recently, we added further support for this model by showing that following stressful military service in the IDF healthy soldiers exhibit increased functional connectivity of the HC with the vmPFC in response to exposure to stress reminders. Importantly, such connectivity capability indeed seems to have an adaptive value, as a stronger increase in functional connectivity following military service was associated with fewer PTSD‐related symptoms [Admon et al., 2009]. The association between reduction in HC volume and insufficient ability to extinguish fear is further supported by a previous finding that another structural feature in this network, vmPFC gray matter thickness, was also found to be related to extinction ability [Milad et al., 2005]. Finally, in support of the acquired nature of deficient fear extinction in PTSD, a unique behavioral study in a twin cohort showed that such deficiency was not evident in the healthy co‐twins of PTSD combat veterans [Milad et al., 2008].
Several limitations should be considered in the conjunction of this study. First, in the absence of histological data and analysis, we can only speculate whether the observed reduction in HC volume is related to neuronal loss, thus representing real atrophy, or to other more transient mechanisms. This is especially relevant as the observed change in HC volume was small, in the order of few percents. Indeed, there is now an increasing body of evidence suggesting that disease‐related changes in HC volume might be transient, thus not representing an irreversible neuronal loss [Swaab et al., 2005]. Second, different reasons for the increase of HC volume in the apparently more resilient group should also be considered. Once again, our data do not allow speculation regarding the neuronal and molecular mechanisms that may be involved in such phenomena. Nevertheless, a previous study showed an increase in HC volume throughout adolescence in healthy normal civilian participants (i.e., relatively stress‐free environment) [Suzuki et al., 2005]. Considering that our participants were also in their adolescence, this may suggest that the demonstrated increase in HC volume displayed here in a third of the sample is part of a normal maturation process, whose continuation despite exposure to stress may indicate resiliency.
Perhaps, the most interesting question then is what kind of physiological or psychological mechanisms might have contributed to these opposite changes in HC volume, which are then further related to connectivity and symptom changes in these individuals? Here, a variety of factors could play a role, from lifestyle to nutrition to social support or coping mechanisms; unfortunately, these factors were not assessed in this study and, thus, it will be the goal of future studies to identify these exact mechanisms.
In summary, in spite of certain limitations, our results uniquely suggest that reduction in HC volume and connectivity with the vmPFC following exposure to repeated military stress might indicate a maladaptive response to such real life stressor. Notably, successful treatment with antidepressants has been shown to reverse the already established stress‐induced HC atrophy in animals [Magarinos et al., 1999] and to increase HC volume in human PTSD patients [Vermetten et al., 2003]. Our identification of HC volume reduction as a localized biological marker of stress‐induced maladaptive response to stress may therefore carry therapeutic potential.
Supporting information
Supporting Information Table 1. Stressful encounters during military service and consequent rise in PTSD‐related symptoms
REFERENCES
- Admon R, Lubin G, Rosenblatt JD, Stern O, Kahn I, Assaf M, Hendler T (2012): Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cereb Cortex. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben‐Ami H, Hendler T (2009): Human vulnerability to stress depends on amygdala's predisposition and hippocampal plasticity. Proc Natl Acad Sci USA 106:14120–14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH (2007): Fear conditioning in posttraumatic stress disorder: Evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther 45:2019–2033. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Barale F, Caverzasi E, Soares JC (2002): Anatomical MRI findings in mood and anxiety disorders. Epidemiol Psichiatr Soc 11:88–99. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Innis RB, McCarthy G, Charney DS (1993): Deficits in short‐term memory in posttraumatic stress disorder. Am J Psychiatry 150:1015–1019. [DOI] [PubMed] [Google Scholar]
- Carpenter M (1983): Human Neuroanatomy. Baltimore, MD:Williams & Wilkins. [Google Scholar]
- Charney D, Bremner J (1999): The neurobiology of anxiety disorders In: Charney D, Nestler E, Bunney B, editors.Neurobiology of Mental Illness. Oxford:Oxford University Press; pp494–517. [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC (1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18:192–205. [PubMed] [Google Scholar]
- Diamond DM, Fleshner M, Rose GM (1994): Psychological stress repeatedly blocks hippocampal primed burst potentiation in behaving rats. Behav Brain Res 62:1–9. [DOI] [PubMed] [Google Scholar]
- DSM‐IV‐TR (2000): American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. Washington, DC:American Psychiatric Association; 4 p. [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M, Chugani DC, Makki M (2006): Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics 117:2093–2100. [DOI] [PubMed] [Google Scholar]
- Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R (2006): Hippocampal train stimulation modulates recall of fear extinction independently of prefrontal cortex synaptic plasticity and lesions. Learn Mem 13:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E, Cashman L, Jaycox L, Perry K (1997): The validation of a self‐report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. Psychol Assess 9:445–451. [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL (1996): Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4‐18 years. J Comp Neurol 366:223–230. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK (2002): Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 5:1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF III, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Clasen L, Toga AW, Giedd JN, Rapoport JL, Thompson PM (2006): Dynamic mapping of normal human hippocampal development. Hippocampus 16:664–672. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E (1997): Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 17:2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, Verkuyl M, Lucassen PJ, Krugers HJ (2004): Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress 7:221–231. [DOI] [PubMed] [Google Scholar]
- Kier EL, Staib LH, Davis LM, Bronen RA (2004): Anatomic dissection tractography: A new method for precise MR localization of white matter tracts. AJNR Am J Neuroradiol 25:670–676. [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ (2009): The structural integrity of an amygdala‐prefrontal pathway predicts trait anxiety. J Neurosci 29:11614–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA (1998): Human amygdala activation during conditioned fear acquisition and extinction: A mixed‐trial fMRI study. Neuron 20:937–945. [DOI] [PubMed] [Google Scholar]
- Lim KO, Helpern JA (2002): Neuropsychiatric applications of DTI—A review. NMR Biomed 15:587–593. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Evans A, Lord C, Miles J, Pruessner M, Pike B, Pruessner JC (2007): Hippocampal volume is as variable in young as in older adults: Implications for the notion of hippocampal atrophy in humans. Neuroimage 34:479–485. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Flugge G, Fuchs E (1996): Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci 16:3534–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, Deslandes A, McEwen BS (1999): Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol 371:113–122. [DOI] [PubMed] [Google Scholar]
- McEwen BS (1999): Stress and hippocampal plasticity. Annu Rev Neurosci 22:105–122. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL (2005): Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci USA 102:10706–10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL (2007): Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 62:446–454. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK (2008): Presence and acquired origin of reduced recall for fear extinction in PTSD: Results of a twin study. J Psychiatr Res 42:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL (2009): Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66:1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Nivon LG, McEwen BS (2002): Effects of chronic stress on hippocampal long‐term potentiation. Hippocampus 12:245–257. [DOI] [PubMed] [Google Scholar]
- Peri T, Ben‐Shakhar G, Orr SP, Shalev AY (2000): Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol Psychiatry 47:512–519. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004): Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 43:897–905. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC (2000): Volumetry of hippocampus and amygdala with high‐resolution MRI and three‐dimensional analysis software: Minimizing the discrepancies between laboratories. Cereb Cortex 10:433–442. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M (2003): Applying learning principles to the treatment of post‐trauma reactions. Ann N Y Acad Sci 1008:112–121. [DOI] [PubMed] [Google Scholar]
- Sapolsky R (1992): Stress, the Aging Brain and the Mechanisms of Neuron Death. Cambridge, MA:Massachusetts Institute of Technology. [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE (1990): Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci 10:2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, McCann UD, Meaney MJ, Blanchard DC, Davis M, Frey KA, Liberzon I, Overall KL, Shear MK, Tecott LH, Winsky L (2001): Summary of a National Institute of Mental Health workshop: Developing animal models of anxiety disorders. Psychopharmacology (Berl) 157:327–339. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Thompson RF (1992): Acute stress impairs (or induces) synaptic long‐term potentiation (LTP) but does not affect paired‐pulse facilitation in the stratum radiatum of rat hippocampus. Synapse 11:262–265. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC (1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97. [DOI] [PubMed] [Google Scholar]
- Smith ME (2005): Bilateral hippocampal volume reduction in adults with post‐traumatic stress disorder: A meta‐analysis of structural MRI studies. Hippocampus 15:798–807. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hagino H, Nohara S, Zhou SY, Kawasaki Y, Takahashi T, Matsui M, Seto H, Ono T, Kurachi M (2005): Male‐specific volume expansion of the human hippocampus during adolescence. Cereb Cortex 15:187–193. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ (2005): The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev 4:141–194. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar Stereotaxic Atlas of the Human Brain; 3‐Dimensional Proportional System: An Approach to Cerebral Imaging. New York:Thieme. [Google Scholar]
- Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM (1989): Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci 9:1705–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD (2003): Long‐term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry 54:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Keefe RS, Harvey PD, Levengood RA, Gerber DK, Geni J, Siever LJ (1995): Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry 152:137–139. [DOI] [PubMed] [Google Scholar]
- Zola‐Morgan SM, Squire LR (1990): The primate hippocampal formation: Evidence for a time‐limited role in memory storage. Science 250:288–290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table 1. Stressful encounters during military service and consequent rise in PTSD‐related symptoms


