Abstract
Recent studies suggest the existence of a visuo‐tactile mirror system, comprising the primary (SI) and secondary (SII) somatosensory cortices, which matches observed touch with felt touch. Here, repetitive transcranial magnetic stimulation (rTMS) was used to determine whether SI or SII play a functional role in the visual processing of tactile events. Healthy participants performed a visual discrimination task with tactile stimuli (a finger touching a hand) and a control task (a finger moving without touching). During both tasks, rTMS was applied over either SI or SII, and to the occipital cortex. rTMS over SI selectively reduced subject performance for interpreting whether a contralateral visual tactile stimulus contains a tactile event, whereas SII stimulation impaired visual processing regardless of the tactile component. These findings provide evidence for a multimodal sensory‐motor system with mirror properties, where somatic and visual properties of action converge. SI, a cortical area traditionally viewed as modality‐specific, is selectively implicated in the visual processing of touch. These results are in line with the existence of a sensory mirror system mediating the embodied simulation concept. Hum Brain Mapp, 2011. © 2011 Wiley Periodicals, Inc.
Keywords: crossmodal, mirror neuron system, rTMS, primary somatosensory cortex, visual processing, tactile observation, sensorimotor
INTRODUCTION
Animal and human studies have provided extensive evidence for the existence of neural circuits in the sensory‐motor system that are involved not only in the processing our own body‐related experiences but also in our observations of others' body‐related events. This has been widely demonstrated by seminal experiments in the motor domain showing that the observation of actions performed by other individuals activates frontal and parietal cortical areas that are typically involved in one's own action planning and execution, the so called “mirror neuron system” [Avenanti et al.,2007; Avikainen et al.,2002; Buccino et al.,2004; di Pellegrino et al.,1992; Fadiga et al.,1995,2005; Gallese et al.,1996; Gazzola et al.,2007a; Hari et al.,1998; Iacoboni et al.,2005; Raos et al.,2004; Rizzolatti and Craighero,2004; Rizzolatti et al.,1999,2001; Rossi et al.,2002]. These shared representations for action observation and execution seem to play a pivotal role in the understanding and imitation of action [Rizzolatti et al.,2001] as well as in understanding others' intentions [Iacoboni and Dapretto,2006; Iacoboni et al.,2005].
Beyond the domain of action, other brain systems with mirror properties have been described, including systems that are involved in both the observation as well as the experience of emotions [Carr et al.,2003; Wicker et al.,2003] and of pain [Avenanti et al.,2005; Botvinick et al.,2005; Jackson et al.,2005; Morrison et al.,2004; Singer et al.,2004; Valeriani et al.,2008; Wicker et al.,2003].
More recently, empirical evidence from functional imaging studies in humans has revealed the existence of a visuo‐tactile mirror system, which matches observed touch with felt touch. For instance, Schaefer and colleagues have demonstrated that viewing one's index finger being touched while receiving corresponding tactile stimulation results in a different activation of the primary somatosensory region that maps the index finger, as compared to conditions with no visual stimulation [Schaefer et al.,2005] or with asynchronous visuo‐tactile stimulation [Schaefer et al.,2006].
Even more intriguing is a report that the mere observation of touch automatically induces activation of the neural circuitry that is normally involved in our experience of touch. In other words, the observation of another person being touched activates both the primary (SI) and secondary somatosensory cortices (SII) [Blakemore et al.,2005; Schaefer et al.,2009]. These activations are somatotopically organised, following the so called sensory homunculus magnification in SI. As a result, observation of a face being touched activates the corresponding “face” area of SI, whereas observation of touch to the neck does not [Blakemore et al.,2005]. Moreover, the lateralisation of SI responses typically occurring when being touched on one side of the body is also present when observing a lateralised touch. Observation of touch to one side of another person's body produces contralateral SI activation [Blakemore et al.,2005; Schaefer et al.,2009]. In related work, another group [Keysers et al.,2004] used fMRI to show that seeing a touch activates SII, but not SI, with comparable neural responses when watching a human body part being touched (i.e., the legs) as well as touching an object. It seems that, for visually induced activity, SII does not differentiate what object is being touched (animate or inanimate). Conversely, some differentiation between seeing a human being touched versus an object being touched does emerge at the level of SI [Blakemore et al.,2005], where induced activity appears to be correlated with the degree of perceived intentionality of the observed touch [Ebisch et al.,2008]. Finally, the mirror activations of SI and SII seem unrelated to possible attribution of the observed touch to oneself (egocentric view) or to somebody else (allocentric view) [Keysers et al.,2004; Schaefer et al.,2009]. Activation in SI appears to be roughly independent of how easily the observed touch events can be integrated into one's own body scheme; however, slightly different patterns of responses in SI are associated with the two different viewpoints [Schaefer et al.,2009].
Overall, current evidence points to the existence of a visuo‐tactile mirroring system in the context of touch observation. On a broader behavioural level, this system may be involved in anticipating the effects of tactile stimulation on our body from a feed‐forward perspective, as well as in understanding the effects of tactile stimulation on other individuals as part of the broader neural circuitry for embodied simulation [Gallese,2005,2007; Grafton,2009].
To date, the significance of somatosensory activity during the observation of touch remains unclear, as it is not known whether SI or SII (or both) can process visual information related to tactile events in a functionally relevant way. In the case of neuroimaging studies, only correlations between brain and behaviour are indicated, but it is unknown whether those somatosensory areas are causally involved in the visual processing of touch events.
From a neurophysiological point of view, researchers have shown that the somatosensory cortices exhibit properties that are relevant for visual functions related to tactile events. First, some SI neurons code for arbitrary visual‐tactile associations. Animal studies have shown that SI neurons in monkeys may fire both in response to a tactile stimulus as well as in response to a visual stimulus that may have previously been associated with the tactile stimulus [Zhou and Fuster,1997,2000]. Therefore, it is probable that mirror activation of SI for touch events may involve a local SI mechanism. Additionally, the caudal part of SI features multimodal receptive fields and direct connections with the posterior parietal cortex (PPC), which contains bimodal visuo‐tactile neurons [Bremmer et al.,2001; Duhamel et al.,1998; Holmes and Spence,2004; Maravita et al.,2003; Nakashita et al.,2008; Rizzolatti et al.,1997]. In addition, SI also receives visual inputs from the more caudal parts of the PPC [Iwamura,2000]. Therefore, the connectivity between these regions may indeed be associated with the activation of SI when viewing touch events.
Furthermore SII, which receives inputs from the adjacent SI region and also directly from the thalamus, is responsible for tactile integration and is involved in higher‐level somatosensory processing [Haggard,2006; Iwamura,1998]. Therefore, SII has access to primary signal codes from the mechanoreceptors. Recent studies have also identified SII as a site of integration between somatosensory information and information from other sensory modalities, such as vision [Avikainen et al.,2002; Bremmer et al.,2001; Carlsson et al.,2000].
Taken together, this evidence suggests that both the SI and SII cortices possess mechanisms that could potentially code visually presented touches. Nevertheless, it remains to be established whether these areas are essential for the visual processing of touch in the human brain. In this study, we sought to address this issue by means of Transcranial Magnetic Stimulation (TMS). TMS allows researchers to investigate causality in the brain‐behaviour relationship, by temporarily interacting with the activity of neurons in brain areas that are underneath the magnetic field, which is administered by a coil positioned over the scalp [Miniussi et al.,2009; Pascual‐Leone et al.,2000]. One advantage of TMS over other neuroimaging methods is that TMS can be used to demonstrate that a brain region is causally essential for performing a given task.
In our study, high frequency repetitive TMS (rTMS) was applied over SI and SII during two tasks: a control visual discrimination task and a visual discrimination task involving tactile stimuli. As a control stimulation site, we also stimulated the occipital cortex (VI). We predicted that, if the somatosensory cortices selectively mediate visual functions in the tactile domain, rTMS over these areas would selectively interfere with the visual processing of tactile events, but it would have no effect on visual perception when the visual stimulus does not include a tactile component.
MATERIALS AND METHODS
Participants
Ten healthy participants took part in two tasks: Touch and No‐Touch (10 right‐handed subjects; 9 females and 1 male; mean age: 24 ±3). All participants gave written informed consent. They were naïve to the experimental procedure and to the purpose of the study. Neither of the participants had neurological, psychiatric, or other relevant medical problems, nor any contraindication to TMS [Rossi et al.,2009; Wassermann,1998]. The protocol was carried out in accordance with the ethical standards of the Declaration of Helsinki (BMJ 1991; 302: 1194) and was approved by the ethical committee at the University of Milano‐Bicocca.
The Touch and No‐Touch Tasks
During both tasks, participants sat at a distance of 45 cm in front of a PC monitor (Samsung SyncMaster 1200NF). Subjects were shown video‐clips of the experimental conditions, which were displayed on a dark screen background with a luminance of 0.01 cd/m2, exposure duration was 60 ms. All video clips depicted the index fingers of two hands, a left hand in the left hemifield and a right hand in the right hemifield from an egocentric view, at 15 degrees of eccentricity from the central fixation point (see Fig. 1). During the video clips, only one index finger moved downward along the vertical dimension for 0.9 cm in the target trials, and for 0.45 cm in the catch trials. In the Touch task, a left hand in the left side and a right hand on the right side were presented below the fingers (see Fig. 1A). Therefore, the target trials showed a hand being touched by the index finger, whereas in the catch trials the finger simply approached the hand, but did not actually touch it. In the No‐Touch task, no hands appeared below the index fingers, and thus the video clips showed only the left or right index finger moving downward to a different extent, making an identical motion to both the target trial and the catch trial of the Touch task (see Fig. 1B). Therefore, in both tasks, the moving visual stimulus (i.e., the index finger) exhibited the same direction and amount of motion (duration 60 ms) across the observed actions1.
Figure 1.
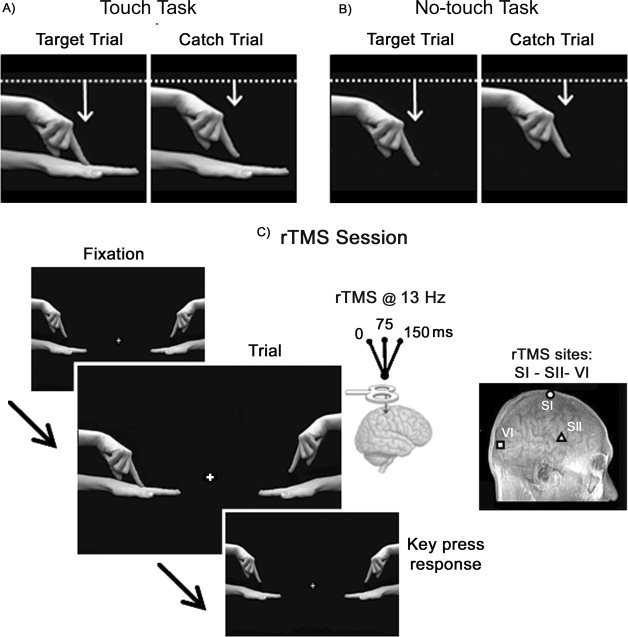
Schematic representation of the experimental tasks. Upper panels depict the types of stimuli used in the Touch Task (A) and in the No‐Touch Task (B). The target trial is shown on the left and the catch trial is shown on the right. The white lines represent the starting point of the moving hand and the white arrows indicate the direction of the movement and the different amplitudes in the target and catch trials. In both tasks, the visual stimuli (video clips of the moving index finger) exhibited identical motion duration (60 ms) and amplitude across the observed actions. C: Sequence of events in the rTMS session. Each trial started with the presentation of a central fixation cross. After a delay (>5 sec) the video clip started (60 ms of duration). At the onset of the clip, rTMS (13 Hz, three TMS pulses at 0, 75, 150 ms after stimulus onset) was delivered over one of the targeted areas (i.e., right hemisphere SI, SII, or VI). In the Touch Task, subjects were asked to indicate by pressing the response key whether the moving finger touched the hand below (target trial), while refraining from pressing if the finger approached the hand, but did not touch it (catch trial). In the No‐Touch Task, subjects were asked to discriminate the range of the finger's movement by pressing the response key to report the greater drop of the finger (target trial), and to refrain from responding to the smaller drop (catch trial).
During the tasks, subjects were required to look at a central fixation point. In the Touch task, subjects were asked to press the spacebar on the keyboard to report whether the visually presented moving finger had touched the hand below (target trial), regardless of the side on which the stimulus was presented, while refraining from pressing if the finger simply approached the hand without touching it (catch trial). During the No‐Touch task, the participants were asked to discriminate the distance of the finger's movement by pressing the response key (space bar) to report a greater shift of the finger (in the target trial this meant matching the extension of the movement of the touching finger of Touch task), and to refrain from responding to the smaller shift (in the catch trial this meant matching the extension of the movement of the catch trial from the Touch task). In both tasks, subjects were required to respond as quickly as possible to the target stimulus by pressing, with their right index finger, the space bar of the keyboard.
For both tasks, 48 trials were presented: 12 target trials and 12 target‐absent trials for each stimulus side (left vs. right hemifield). The inter‐trial interval varied between 5 and 7 s, to prevent any TMS carry‐over effects. The total duration of each task was 10 min.
The sequence and timing of the video clips as well as the TMS pulses were under computer control (E‐prime software, Psychology Software Tools, Inc., http://www.psychotoolbox.org) (see Fig. 1A).
TMS Protocol
During the TMS session for both tasks, TMS pulses were delivered using a Super Rapid Transcranial Magnetic Stimulator (Magstim, Whitland, UK http://www.magstim.com) connected with a figure‐of‐eight coil (double 70 mm diameter), which allowed focal cortical stimulation [Wassermann et al.,2008].
Before the experiment, the individual motor threshold (MT) at rest was determined for each participant by stimulating the hand area of the right motor cortex (MI). MT was defined as the minimum intensity that elicited detectable motor twitches in the digits of the resting left hand, i.e., a visible contraction during at least 3 of 6 consecutive single TMS pulses. The mean (± Standard Deviation, SD) MT was 55% (±10%) of the maximal output of the stimulator.
In both tasks, the cortical areas targeted for stimulation were SI, SII, and VI in the right hemisphere. The appropriate location for stimulating the SI hand area was identified for each subject as the site at which tactile extinction (i.e., failure to detect the contralateral stimulus when both hands are stimulated), or paraesthesia, could be most readily obtained (see below). Before beginning the experimental trials, the coil was moved approximately 2–4 cm posterior to the motor hotspot, until no detectable motor twitches occurred. Subsequent to confirming that this location was within the SI hand area, the subject performed a tactile detection task while single TMS pulses were delivered [Avenanti et al.,2007; Bolognini and Maravita,2007; Fiorio and Haggard,2005; Harris et al.,2002]. By moving the coil between trials, we were able to determine the position and orientation of the coil at which the TMS most reliably interfered with the tactile detection task.
Custom‐made electromagnetic solenoids (Heijo electronics, UK, http://www.heijo.com) attached to the participants' index fingers were used to deliver the tactile stimulations. Each subject was asked whether he or she felt a tactile stimulus on the left finger, right finger, or both fingers (10 trials for each stimulus location, plus 10 catch trials during which no tactile stimulus was presented). For each trial, a single TMS pulse, at 110% of MT intensity, was presented exactly 20 ms after the tactile stimulus, at which time we predicted it might disrupt tactile detection [Cohen et al.,1991; Harris et al.,2002]. During TMS stimulation, almost every subject reported paraesthesia to the contralateral (left) hand. Seven subjects out of ten reported a deficit in detecting the contralateral‐left tactile stimulus; the mean percentage of unfelt left stimuli during bilateral stimulation was 19%. These data provide independent confirmation that the scalp position chosen for the experiment was indeed over the somatosensory hand area.
The stimulation location corresponding to SII was localised 2.5 cm anterior and 6.5 cm superior to the preauricular point, in accord with previous TMS studies [Harris et al.,2002; Kanda et al.,2003]. Finally, the stimulation location corresponding to VI was 2 cm dorsal and 0.6 cm lateral to the calcarine sulcus, which corresponds to the primary visual cortex [Bolognini and Maravita,2007; Fernandez et al.,2002].
Additionally, to check for mislocalisation, we also localised the targeted areas using the SofTaxic Evolution Navigator system (E.M.S., Bologna, Italy, http://www.emsmedical.net). This system allows for the reconstruction of the cerebral cortex in Talairach coordinates, with a mean error of 2.11 mm, and a standard deviation of 2.04 mm, on the basis of digitised skull landmarks (nasion, inion, and two pre‐auricular points) as well as 50 additional, uniformly distributed points that are mapped on the scalp via a graphic user interface and a 3D optical digitiser (NDI, Polaris Vicra). An estimation of the single subject's cerebral volume is automatically calculated by means of a warping procedure, through the use of a generic MRI volume (template) on the basis of a set of points digitised from the subject's scalp. Following this procedure, we further checked, for each subject, the Talairach coordinates [Talairach and Tournoux,1988] of the targeted areas, previously localised through functional and/or anatomical procedures.
After localising each area over the scalp, the coil was positioned on the appropriate stimulation site during each experimental session and was supported as well as held in place by a mechanical device.
During the rTMS sessions, rTMS was delivered as a train of three pulses at a frequency of 13 Hz (i.e., at 0‐75‐150 ms with respect to the onset of the video clip, i.e., moving hand). Such time interval and stimulation parameters were chosen on the basis of previous experiments and ensured that the critical period of sensory processing was covered by TMS [Walsh and Rushworth,1999; Wassermann et al.,2008]. In particular, we take into account the chronometry of tactile processing in SI [Cohen et al.,1991] and that of visual processing in VI, as shown by previous TMS studies [Amassian et al.,1989; Breitmeyer et al.,2004; Kammer,2007].
The stimulus intensity used during all the experimental session was set at 110% of the individual MT. These parameters are consistent with safety recommendations for rTMS [Rossi et al.,2009] (see Fig. 1C). Participants tolerated the rTMS well and did not report any adverse effects.
Experimental Procedures
Throughout the experiment, subjects were comfortably seated in an armchair, in a quiet, dimly illuminated room. The experiment comprised five sessions for each of the two tasks: a training session and four experimental sessions. A training session always preceded the experimental protocol and allowed participants to become familiar with the task. Verbal feedback was given concerning the subject's performance after each trial during the training phase only.
The experimental sessions consisted of a baseline session, during which no rTMS stimuli were delivered, followed by the three rTMS sessions (i.e., SI‐rTMS, SII‐rTMS, and VI‐rTMS). The order of the two tasks and that of the four experimental sessions for each task was counterbalanced across participants, and performed in two different days, to avoid any carry over effects.
The duration of every session was approximately 10 min, and consequently the entire procedure for each task lasted about 2 h per subject. Between the sessions, subjects were allowed to rest and enjoy light refreshments.
Statistical Analysis
Statistical analysis was performed using Statistica for Windows, release 6.0 (StatSoft). A first analysis was conducted on the mean percentage of errors (target omissions plus incorrect responses to the catch trials, i.e., false alarms) through a repeated‐measures Analysis of Variance ANOVA with Task (Touch vs. No‐Touch), Session (Baseline, SI‐rTMS, SII‐rTMS, VI‐rTMS), and Side (Left‐ vs. Right‐sided stimuli, i.e., contralateral and ipsilateral to the TMS side) as the main factors (see Table I for the outcome of the main analysis).
Table 1.
F, P‐level, and pη2 values from ANOVAs of the main output (% of errors, d′, and c values)
| Factors (df) | F | P‐level | pη2 | |
|---|---|---|---|---|
| Errors (%) | Task (1,9) | 0.96 | 0.35 | 0.10 |
| Session (3,27) | 3.86 | 0.02a | 0.30 | |
| Side (1,9) | 0.73 | 0.42 | 0.08 | |
| Task × session (3,27) | 0.24 | 0.87 | 0.03 | |
| Task × side (1,9) | 0.21 | 0.65 | 0.02 | |
| Session × side (3,27) | 0.36 | 0.79 | 0.04 | |
| Task × session × side (3,27) | 0.43 | 0.74 | 0.05 | |
| Perceptual sensitivity (d′) | Task (1,9) | 0.46 | 0.51 | 0.05 |
| Session (3,27) | 3.48 | 0.03a | 0.28 | |
| Side (1,9) | 0.23 | 0.65 | 0.02 | |
| Task × session (3,27) | 0.66 | 0.58 | 0.07 | |
| Task × side (1,9) | 1.64 | 0.23 | 0.15 | |
| Session × side (3,27) | 0.23 | 0.88 | 0.02 | |
| Task × session × side (3,27) | 4.15 | 0.02a | 0.32 | |
| Response criterion (c) | Task (1,9) | 12.22 | 0.01a | 0.58 |
| Session (3,27) | 4.30 | 0.01a | 0.32 | |
| Side (1,9) | 0.09 | 0.77 | 0.01 | |
| Task × session (3,27) | 0.62 | 0.61 | 0.06 | |
| Task × side (1.9) | 0.04 | 0.85 | 0.00 | |
| Session × side (3,27) | 0.24 | 0.87 | 0.03 | |
| Task × session × side (3,27) | 0.11 | 0.95 | 0.01 |
Indicates a significant effect.
In addition, to separate genuine rTMS effects on perceptual sensitivity from changes in the response criterion, we then computed psychophysical indices derived from Signal Detection Theory [Green and Swets,1966]. This is a particularly relevant issue in the present context, given our concern that the online rTMS delivered during the tasks might have impacted the response accuracy by simply biasing the response criterion adopted by subjects. In this respect, the use of a rigorous psychophysical approach allows us to discern the stimulus‐related (i.e., perceptual sensitivity, d′) and subject‐related (i.e., response bias, c) rTMS influences on visual performance [Green and Swets,1966]. Here changes in sensitivity (d′ values) and in the response criterion (c values) were quantified for every experimental condition. Analyses of sensitivity and response bias were also performed via a two (Task) by four (Session) by two (Side) ANOVA. When appropriate, pairwise comparisons were calculated using the Newman‐Keuls test.
Finally, we measured the effect size in the ANOVAs, by calculating the partial Eta Squared (pη2), which measures the degree of association between an effect and the dependent variable, namely the proportion of the total variance that is attributable to a main factor or to an interaction [Cohen,1973].
RESULTS
Errors
The ANOVA results revealed a significant main effect only of the factor Session (F 3,27 = 3.86, P < 0.02, pη2 = 0.30), because errors increased when rTMS was delivered to SII (20%, P < 0.03) as compared to all the other experimental sessions: Baseline = 15%, SI‐rTMS = 14%, VI‐rTMS = 15% (see Fig. 2A). No other significant effects were found (see Table I).
Figure 2.
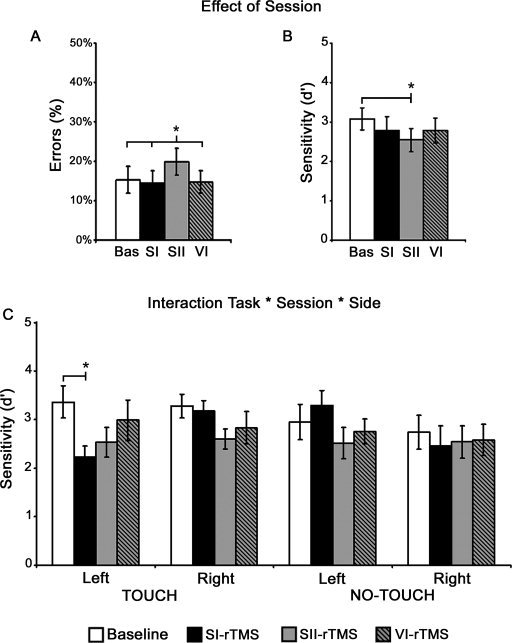
rTMS Effects on behaviour. A and B: Panels describe the main effect of Session, showing the mean values in each experimental session for the percentages of errors (F = 3.86, P < 0.02) (A), the perceptual sensitivity (F = 3.48, P < 0.03) (B). C: The graph describes the Task by Session by Side Interaction (F = 4.15, P < 0.02), showing the selective decrement of sensitivity induced by rTMS over SI for contralateral (left‐sided) visual stimuli depicting touch events. White bars represent the Baseline, blacks bars represent the effects of rTMS over SI (SI‐rTMS session), grey bars represent the effects of rTMS over SII (SII‐rTMS session), grey and black bars represent the effects of rTMS over VI (VI‐rTMS session). Asterisks indicate a significant rTMS effect. Error bars depict standard errors.
Signal Detection Measures
The analysis of sensitivity (d′) showed a significant main effect of Session (F 3,27 = 3.48, P < 0.03, pη2 = 0.28), with a significant difference only between the Baseline (3.08) and SII‐rTMS (2.54, P < 0.02) (SI‐rTMS = 2.78, P = 0.2; VI‐rTMS = 2.79, P = 0.09 as compared to the Baseline) (see Fig. 2B and Table I).
Of major interest was the significant interaction of Task by Session by Side (F 3,27 = 4.15, P < 0.02, pη2 = 0.32) which highlighted the highly specific effect of the rTMS interference2 (see Table I and Fig. 2C). To explore this interaction, we then conducted (i.e., SI, SII, VI) separate 3‐way ANOVAs for each stimulated area, with Task (Touch vs. No‐Touch), Session (Baseline vs. rTMS), and Side (Left‐ vs. Right‐sided stimuli) as the main factors. Only the ANOVA for SI‐rTMS showed again a significant triple interaction (F 1,9 = 4.88, P < 0.05), which highlighted a selective decrement of perceptual sensitivity in the Touch task; crucially, this disruptive effect was specific for the processing of contralateral, left‐sided visual stimuli depicting touches (2.23), as compared to left touching stimuli in the Baseline (3.36, P < 0.02). Only in the SI‐rTMS session, a significant difference between left and right stimuli in the Touch task (P < 0.05) and between left touching stimuli and left no‐touching stimuli (3.29, P < 0.03) emerged. Instead, sensitivity for left and right stimuli did not differ in the No‐Touch task in the SI‐rTMS session (P > 0.07) and between these two last conditions and those of the baseline (P > 0.07) (see Fig. 2C). For SII‐rTMS there was only a main effect of Session (F 1,9 = 5.38, P < 0.05), indicating a significant difference between SII condition and the Baseline irrespective of side or task, whereas for VI‐rTMS no effect reach significance (P > 0.2).
We further explore the effects of rTMS to SI via a 2 (Task) × 2 (Side) ANOVA, comparing only the sensitivity in the two SI‐rTMS sessions: the results showed only a significant Task by Side interaction (F 1,9 = 8.19, P < 0.01), showing again a decrement of sensitivity for left‐sided touching stimuli during SI stimulation, which was significantly different from the sensitivity for right‐sided touching stimuli and left‐sided no‐touching stimuli (P < 0.05); instead, sensitivity for left and right stimuli did not significantly change in the No‐Touch task during SI disruption (P = 0.1).
Finally, the analysis of response bias (c) showed a main effect of Task (F 1,9 = 12.22, P < 0.01, pη2 = 0.58), with significantly lower c values for the Touch Task (0.25) as compared to the No‐Touch task (0.60). This decrease in criterion value reflects a more liberal response criterion, with subjects more likely to report the target stimulus. A significant main effect of Session was also found (F 3,27 = 4.30, P < 0.01, pη2 = 0.32), again showing a significant decrease of the response criterion in the VI‐rTMS session (0.23), as compared only to the Baseline (0.68, P < 0.01). A marginally significant difference was found between the Baseline and SII‐rTMS (0.42, P = 0.056) and, to a lesser extent, with respect to SI‐rTMS (0.38, P = 0.07). Other effects did not reach significance (see Table I).
DISCUSSION
This study investigated the causal involvement of the human somatosensory cortices, SI and SII, in the visual processing of touch events. A growing body of brain imaging evidence supports the existence of a somatosensory mirror neuron system in the human brain that links observed and felt touch. Here we uncover the first evidence that SI and SII are activated as a result of visual input, namely during observation a human body being touched.
The more critical and original result of this study is the selective impairment of visual processing of touch due to the disruption of SI activity. Indeed, SI‐rTMS selectively reduced visual perceptual sensitivity (i.e., decreased d′ values) for detecting contralateral visual events comprising a tactile component. The interference of SI was selective in two ways: it was specific for the task, namely for discrimination of a hand being touched by an approaching finger (Touch Task), as well as for the side of the touching stimuli, as it affected only the perception of the contralateral (left‐sided) touch stimuli. Moreover, the rTMS effect was evident only at the perceptual level, as no significant change in the response criterion or in the error rate was apparent in the SI‐rTMS session.
The second finding was that rTMS over SII induced a nonspecific impairment in detecting visual stimuli depicting hand movements. This effect occurred regardless of the experimental task (Touch and No‐Touch) or the stimulated visual hemifield (i.e., ipsilateral or contralateral to the site of rTMS). More specifically, rTMS over SII significantly reduced the net accuracy when subjects had to detect whether a left‐ or right‐sided hand was touched by an approaching finger (Touch Task). In addition, rTMS over SII impaired participants' ability to discriminate the width of a finger's movement when touch was absent (No‐Touch Task). Across all conditions, this impairment of accuracy was accompanied by a significant decrement in perceptual sensitivity (i.e., lower d′ values) and by a nearly significant response bias (i.e., lower c values). This postperceptual effect reflects a change in the response criterion adopted by subjects during SII‐rTMS.
Finally, with respect to VI‐rTMS (i.e., the control stimulation site), this occipital stimulation affected a postperceptual level of visual processing, and induced a robust and reliable response bias in every experimental condition, regardless of experimental task or stimulus side. Although occipital TMS typically induces a visual suppression in perceptual tasks [Amassian et al.,1989], we did not identify any significant impairment of visual perception in the current VI‐rTMS session (although an overall decrement of perceptual sensitivity was highly consistent across all the VI‐rTMS sessions). It is noteworthy that in both of our tasks the visual stimuli depicted a moving hand; human actions with implied motion are preferentially processed by the visual motion area V5/MT, rather than by VI [Proverbio et al.,2009].
Returning to our key finding regarding the stimulation of SI, our results indicate that SI, but not SII, is causally and specifically related to the processing of observed touch. Indeed, SI‐rTMS selectively degrades visual processing of contralateral stimuli depicting touch at the perceptual level, an effect that was both task‐ and spatially specific. This effect is in line with most of the neuroimaging studies of the somatosensory mirror system, demonstrating a preferential activation of SI when viewing a human touch, as compared to a no‐touch condition [Schaefer et al.,2005] or to the view of an object being touched [Blakemore et al.,2005]. Moreover, the hemispheric lateralisation in SI that occurred when being touched on one side is also present when observing touch events involving the same side [Blakemore et al.,2005; Schaefer et al.,2009]; this is broadly consistent with our finding of a contralateral visual impairment for the sight of a hand being touched during unilateral SI‐rTMS.
Of particular interest, during the Touch Task, the touched hand was viewed from an egocentric perspective, while the touching finger was viewed from an allocentric view, as if reproducing a touch from another person to the observer's own hand. This egocentric perspective for the hand being touched should maximise the attribution of the observed touch to the observer's own body, allowing integration of the touch into the participant's own body schema. In other words, the egocentric view of the hand being touched may induce in the observer a self‐attribution of being touched. In this regard, it is interesting that only SI suggests a differential response to egocentric versus non‐egocentric body contact [Schaefer et al.,2009]. Ebisch et al. [2008] found a significant difference in SI, but not in SII, between the sight of intentional touch (by a human hand), compared to accidental touch involving an object (i.e., a tree branch). In particular, the activation in SI correlated positively with the degree of intentionality of the observed touch as rated by the observers. This finding led to the proposal that the mirror activity in SI might specifically reflect an automatic simulation of the proprioceptive aspects of the observed touch when intentionality is assumed by the observer [Ebisch et al.,2008]. Given this perspective, understanding an observed touch may be mediated by embodied simulation [Gallese2005,2006) through a visuo‐tactile mirroring system, where SI plays the pivotal role whenever the observed touch is perceived as intentional [Ebisch et al.,2008] or, with respect to our finding, when the observed touch is attributed to the observer's own body.
Direct evidence for a causal role of human SI in visuo‐tactile processing related to the body was recently provided by Fiorio and Haggard [2005] with respect to the “Visual Enhancement of Touch” (VET), i.e., enhancement of tactile processing induced by viewing the body [Kennett et al.,2001; Press et al.,2004; Taylor‐Clarke et al.,2002]. The authors showed that the VET can be reduced through the use of TMS over SI, but not SII. This suggests that observing the body may act at an early stage in stimulus elaboration and perception, allowing for an anticipatory tuning of the SI neural circuits that underlie tactile processing [Fiorio and Haggard,2005]. In addition to that study, we show here that SI can process visually presented touches even when concurrent tactile stimulation is not present, suggesting the existence of some form of purely visual processing in SI. In this context, our study provides novel evidence for a crossmodal role of SI in the visual perception of tactile events.
Beyond the evidence for a crossmodal role of SI, imaging studies have also showed that SI might have mirror proprieties. Action observation and execution increases neural activity in both motor and somatosensory areas [Rizzolatti and Craighero,2004]. Crucially, making the perceived action painful or more salient from a tactile or proprioceptive point of view causes an increase of the activity in SI [Avenanti et al.,2007; Betti et al.,2009; Bufalari et al.,2007; Costantini et al.,2005]. A recent study [Avenanti et al.,2007] demonstrated that TMS over SI selectively reduced the typical corticospinal mapping of biomechanically impossible movements [Fadiga et al.,1995,2005; Romani et al.,2005], which were associated with aversive somatic feelings, without impacting mirror responses to possible movements that did not evoke somatic feelings in the observer. Modulation of SI activity that is contingent upon observation of others' pain has also been described [Betti et al.,2009; Bufalari et al.,2007]. Therefore, it seems that the mirror function of SI is that of preferentially encoding the somatic component of action simulation.
Worth mentioning, a recent study showed that transcranial direct current stimulation (tDCS) delivered to the dorsolateral prefrontal cortex, but not to the sensori‐motor cortex, can modulate the feelings of unpleasantness and discomfort/pain during the observation of aversive images depicting humans in pain [Boggio et al.,2009]. However, that tDCS experiment does not distinguish the effect of viewing touching or no‐touching images and the authors used static images instead of dynamic actions; these differences may account for the lack of efficacy of tDCS over the sensorimotor cortex for the modulation of emotional processing.
Finally, the contralateral effect of SI‐rTMS during visual processing of touch events is also in line with the properties of the mirror neurons system, since each hemisphere is more strongly activated when viewing actions conducted by a model's contralateral hand than when viewing actions conducted by an ipsilateral hand [Aziz‐Zadeh et al.,2002]. Moreover, since the left hemifield stimuli depicted an egocentrically viewed left hand being touched, touching stimuli to a visually presented left hand would be the most adequate to induce a mirror activation of the contralateral right SI. In this view, our side‐specific TMS effect might suggest a “resonance” with the experience of the person being touched but also with the tactile sensation of the observer's own left hand being touched. Our setup does not allow to discern whether it is the occurrence of the contralateral left‐sided hand or the occurrence of a left hand in egocentric perspective that is related to the SI effect; additional experiments would be required to differentiate these two accounts.
With respect to SII‐rTMS effects, we found a significant perceptual impairment for detecting stimuli depicting a moving hand, regardless of the presence of a tactile component and the side (ipsilateral or contralateral) of the stimulus. Therefore, in contrast with SI activity, SII activity appears not to be specific for the visual perception of touches. This result is not entirely unexpected in the face of fMRI reports of SII recruitment during the observation of actions performed by other individuals. As mentioned above, a simultaneous motor as well as somatosensory mapping of actions takes place in the parietal node of the mirror motor system [Avikainen et al.,2002; Costantini et al.,2005; Gazzola and Keysers,2009; Grezes et al.,2003]. Most important for the present study, the somatic components of observed actions are encoded primarily in parietal areas, including SII, rather than in the frontal node of the mirror system [Avenanti et al.,2007; Avikainen et al.,2002; Gazzola and Keysers,2009; Gazzola et al.,2007b; Grezes et al.,2003; Hari et al.,1998]. In both of our tasks (i.e., Touch and No‐Touch), the video clips always depicted a moving hand. In this context, our nonspecific SII effect would reflect an associative visuo‐kinaesthetic function evoked by the perception of human hand movements in the video clips, regardless of the sight of touch. Finally, bilateral activation of SII occurred during both the sight of touch as well as during action observation [Avikainen et al.,2002; Blakemore et al.,2005; Ebisch et al.,2008; Keysers et al.,2004], which is consistent with our observation of a bilateral impairment induced by SII‐rTMS and with the existence of the bilateral receptive fields of SII cells [Iwamura,1998,2000]. Nevertheless a note of caution is needed in interpreting the SII results: since, in the human brain, SII is located deep in the lateral sulcus, its localization and the efficacy of TMS in reaching this area might be problematic.
Our findings provide further evidence for a mirror system for sensory observation, while clarifying the functional significance of the cortical somatosensory activations that subserve the ability to process the sight of touch. The primary and secondary sensory cortices appear to both be part of a multimodal sensory‐motor system with mirror properties, where somatic and visual properties of action converge. However, only SI, which is primarily involved in the experience of the sense of touch, appears to be selectively implicated in the visual processing of the human body part being touched. The visuo‐tactile mirroring mechanism of SI might reflect a specific mechanism for the simulation of somatosensory aspects related to the sight of touch delivered to a human body. By matching observed and felt touch, this area contributes to our capacity to understand the effect of tactile stimulation on another person, perhaps allowing us to more easily “resonate” with the body‐related experiences of the touched person.
Acknowledgements
The authors thank Carlo Toneatto and Katia Cividini for technical assistance and Emanuela Bricolo and Paola Ricciardelli for their insightful discussions of the preliminary results.
Footnotes
The parameter determination for the behavioural tasks was guided by a series of preliminary behavioural experiments, not described here, in which we systematically change the size of the hand, the duration of the visual stimulus, the amount of the index finger's movement, and the direction of the movement, in order to opportunely increase the difficulty of the task and to obtain a mean performance accuracy of at least 90% in both tasks. Specifically, ten different tasks were used, administered to a total of 28 subjects (mean age 25, ±1.5).
The pairwise test exploring directly the significant Task × Session × Side interaction highlighted the task‐specificity and lateralization of the effect of SI‐rTMS, showing only a significant difference between the Baseline and SI‐rTMS limited to the processing of contralateral, left‐sided visual stimuli depicting touches (P < 0.05). No other significant difference emerged (P > 0.1 for all comparisons).
REFERENCES
- Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell A, Eberle L ( 1989): Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr Clin Neurophysiol 74: 458–462. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM ( 2005): Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci 8: 955–960. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bolognini N, Maravita A, Aglioti SM ( 2007): Somatic and motor components of action simulation. Curr Biol 17: 2129–2135. [DOI] [PubMed] [Google Scholar]
- Avikainen S, Forss N, Hari R ( 2002): Modulated activation of the human SI and SII cortices during observation of hand actions. Neuroimage 15: 640–646. [DOI] [PubMed] [Google Scholar]
- Aziz‐Zadeh L, Maeda F, Zaidel E, Mazziotta J, Iacoboni M ( 2002): Lateralization in motor facilitation during action observation: A TMS study. Exp Brain Res 144: 127–131. [DOI] [PubMed] [Google Scholar]
- Betti V, Zappasodi F, Rossini PM, Aglioti SM, Tecchio F ( 2009): Synchronous with your feelings: Sensorimotor {gamma} band and empathy for pain. J Neurosci 29: 12384–12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Bristow D, Bird G, Frith C, Ward J ( 2005): Somatosensory activations during the observation of touch and a case of vision‐touch synaesthesia. Brain 128: 1571–1583. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Zaghi S, Fregni F ( 2009): Modulation of emotions associated with images of human pain using anodal transcranial direct current stimulation (tDCS). Neuropsychologia 47: 212–217. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Maravita A ( 2007): Proprioceptive alignment of visual and somatosensory maps in the posterior parietal cortex. Curr Biol 17: 1890–1895. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM ( 2005): Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage 25: 312–319. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG, Ro T, Ogmen H ( 2004): A comparison of masking by visual and transcranial magnetic stimulation: Implications for the study of conscious and unconscious visual processing. Conscious Cogn 13: 829–843. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, Zilles K, Fink GR ( 2001): Polymodal motion processing in posterior parietal and premotor cortex: A human fMRI study strongly implies equivalencies between humans and monkeys. Neuron 29: 287–296. [DOI] [PubMed] [Google Scholar]
- Buccino G, Lui F, Canessa N, Patteri I, Lagravinese G, Benuzzi F, Porro CA, Rizzolatti G ( 2004): Neural circuits involved in the recognition of actions performed by nonconspecifics: An FMRI study. J Cogn Neurosci 16: 114–126. [DOI] [PubMed] [Google Scholar]
- Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM ( 2007): Empathy for pain and touch in the human somatosensory cortex. Cereb Cortex 17: 2553–2561. [DOI] [PubMed] [Google Scholar]
- Carlsson K, Petrovic P, Skare S, Petersson KM, Ingvar M ( 2000): Tickling expectations: Neural processing in anticipation of a sensory stimulus. J Cogn Neurosci 12: 691–703. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL ( 2003): Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA 100: 5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J ( 1973): Eta‐squared and partial Eta‐squared in fixed factor anova designs. Educ Psychol Meas 33: 107–112. [Google Scholar]
- Cohen LG, Bandinelli S, Sato S, Kufta C, Hallett M ( 1991): Attenuation in detection of somatosensory stimuli by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 81: 366–376. [DOI] [PubMed] [Google Scholar]
- Costantini M, Galati G, Ferretti A, Caulo M, Tartaro A, Romani GL, Aglioti SM ( 2005): Neural systems underlying observation of humanly impossible movements: An FMRI study. Cereb Cortex 15: 1761–1767. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G ( 1992): Understanding motor events: A neurophysiological study. Exp Brain Res 91: 176–180. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME ( 1998): Ventral intraparietal area of the macaque: Congruent visual and somatic response properties. J Neurophysiol 79: 126–136. [DOI] [PubMed] [Google Scholar]
- Ebisch SJ, Perrucci MG, Ferretti A, Del Gratta C, Romani GL, Gallese V ( 2008): The sense of touch: Embodied simulation in a visuotactile mirroring mechanism for observed animate or inanimate touch. J Cogn Neurosci 20: 1611–1623. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G ( 1995): Motor facilitation during action observation: A magnetic stimulation study. J Neurophysiol 73: 2608–2611. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Craighero L, Olivier E ( 2005): Human motor cortex excitability during the perception of others' action. Curr Opin Neurobiol 15: 213–218. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Alfaro A, Tormos JM, Climent R, Martinez M, Vilanova H, Walsh V, Pascual‐Leone A ( 2002): Mapping of the human visual cortex using image‐guided transcranial magnetic stimulation. Brain Res Brain Res Protoc 10: 115–124. [DOI] [PubMed] [Google Scholar]
- Fiorio M, Haggard P. ( 2005): Viewing the body prepares the brain for touch: Effects of TMS over somatosensory cortex. Eur J Neurosci 22: 773–777. [DOI] [PubMed] [Google Scholar]
- Gallese V ( 2005): Embodied simulation: From neurons to phenomenal experience. Phenomenol Cogn Sci 4: 23–48. [Google Scholar]
- Gallese V ( 2006): Intentional attunement: A neurophysiological perspective on social cognition and its disruption in autism. Brain Res 107: 15–24. [DOI] [PubMed] [Google Scholar]
- Gallese V ( 2007): Embodied simulation: From mirror neuron systems to interpersonal relations. Novartis Found Symp 278: 3–12. [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G ( 1996): Action recognition in the premotor cortex. Brain 119: 593–609. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C ( 2009): The observation and execution of actions share motor and somatosensory voxels in all tested subjects: Single‐subject analyses of unsmoothed fMRI data. Cereb Cortex 19: 1239–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola V, Rizzolatti G, Wicker B, Keysers C ( 2007a) The anthropomorphic brain: The mirror neuron system responds to human and robotic actions. Neuroimage 35: 1674–1684. [DOI] [PubMed] [Google Scholar]
- Gazzola V, van der Worp H, Mulder T, Wicker B, Rizzolatti G, Keysers C ( 2007b) Aplasics born without hands mirror the goal of hand actions with their feet. Curr Biol 17: 1235–1240. [DOI] [PubMed] [Google Scholar]
- Grafton ST ( 2009): Embodied cognition and the simulation of action to understand others. Ann N Y Acad Sci 1156: 97–117. [DOI] [PubMed] [Google Scholar]
- Green D, Swets J ( 1966): Signal Detection Theory and PsychoPsychophysics. New York: Wiley. [Google Scholar]
- Grezes J, Armony JL, Rowe J, Passingham RE ( 2003): Activations related to “mirror” and “canonical” neurones in the human brain: An fMRI study. Neuroimage 18: 928–937. [DOI] [PubMed] [Google Scholar]
- Haggard P ( 2006): Sensory neuroscience: From skin to object in the somatosensory cortex. Curr Biol 16: R884–R886. [DOI] [PubMed] [Google Scholar]
- Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G ( 1998): Activation of human primary motor cortex during action observation: A neuromagnetic study. Proc Natl Acad Sci USA 95: 15061–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Miniussi C, Harris IM, Diamond ME ( 2002): Transient storage of a tactile memory trace in primary somatosensory cortex. J Neurosci 22: 8720–8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes NP, Spence C ( 2004): The body schema and the multisensory representation(s) of peripersonal space. Cogn Process 5: 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M ( 2006): The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci 7: 942–951. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar‐Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G ( 2005): Grasping the intentions of others with one's own mirror neuron system. PLoS Biol 3: e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura Y ( 1998): Hierarchical somatosensory processing. Curr Opin Neurobiol 8: 522–528. [DOI] [PubMed] [Google Scholar]
- Iwamura Y ( 2000): Bilateral receptive field neurons and callosal connections in the somatosensory cortex. Philos Trans R Soc Lond B Biol Sci 355: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J ( 2005): How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage 24: 771–779. [DOI] [PubMed] [Google Scholar]
- Kammer T ( 2007): Masking visual stimuli by transcranial magnetic stimulation. Psychol Res 71: 659–666. [DOI] [PubMed] [Google Scholar]
- Kanda M, Mima T, Oga T, Matsuhashi M, Toma K, Hara H, Satow T, Nagamine T, Rothwell JC, Shibasaki H ( 2003): Transcranial magnetic stimulation (TMS) of the sensorimotor cortex and medial frontal cortex modifies human pain perception. Clin Neurophysiol 114: 860–866. [DOI] [PubMed] [Google Scholar]
- Kennett S, Taylor‐Clarke M, Haggard P ( 2001): Noninformative vision improves the spatial resolution of touch in humans. Curr Biol 11: 1188–1191. [DOI] [PubMed] [Google Scholar]
- Keysers C, Wicker B, Gazzola V, Anton JL, Fogassi L, Gallese V ( 2004): A touching sight: SII/PV activation during the observation and experience of touch. Neuron 42: 335–346. [DOI] [PubMed] [Google Scholar]
- Maravita A, Spence C, Driver J ( 2003): Multisensory integration and the body schema: Close to hand and within reach. Curr Biol 13: R531–R539. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Ruzzoli M, Walsh V ( 2009): The mechanism of transcranial magnetic stimulation in cognition. Cortex 46: 128–130. [DOI] [PubMed] [Google Scholar]
- Morrison I, Lloyd D, di Pellegrino G, Roberts N ( 2004): Vicarious responses to pain in anterior cingulate cortex: Is empathy a multisensory issue? Cogn Affect Behav Neurosci 4: 270–278. [DOI] [PubMed] [Google Scholar]
- Nakashita S, Saito DN, Kochiyama T, Honda M, Tanabe HC, Sadato N ( 2008): Tactile‐visual integration in the posterior parietal cortex: A functional magnetic resonance imaging study. Brain Res Bull 75: 513–525. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A, Walsh V, Rothwell J ( 2000): Transcranial magnetic stimulation in cognitive neuroscience—Virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol 10: 232–237. [DOI] [PubMed] [Google Scholar]
- Press C, Taylor‐Clarke M, Kennett S, Haggard P ( 2004): Visual enhancement of touch in spatial body representation. Exp Brain Res 154: 238–245. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Riva F, Zani A ( 2009): Observation of static pictures of dynamic actions enhances the activity of movement‐related brain areas. PLoS One 4: e5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raos V, Evangeliou MN, Savaki HE ( 2004): Observation of action: Grasping with the mind's hand. Neuroimage 23: 193–201. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L ( 2004): The mirror‐neuron system. Annu Rev Neurosci 27: 169–192. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Fogassi L, Gallese V ( 1997): The space around us. Science 277: 190–191. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Fogassi L, Gallese V ( 1999): Resonance behaviors and mirror neurons. Arch Ital Biol 137: 85–100. [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V ( 2001): Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci 2: 661–670. [DOI] [PubMed] [Google Scholar]
- Romani M, Cesari P, Urgesi C, Facchini S, Aglioti SM ( 2005): Motor facilitation of the human cortico‐spinal system during observation of bio‐mechanically impossible movements. Neuroimage 26: 755–763. [DOI] [PubMed] [Google Scholar]
- Rossi S, Tecchio F, Pasqualetti P, Ulivelli M, Pizzella V, Romani GL, Passero S, Battistini N, Rossini PM ( 2002): Somatosensory processing during movement observation in humans. Clin Neurophysiol 113: 16–24. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual‐Leone A, Safety of TMS Consensus Group ( 2009): Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120: 2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Heinze HJ, Rotte M ( 2005): Seeing the hand being touched modulates the primary somatosensory cortex. Neuroreport 16: 1101–1105. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Flor H, Heinze HJ, Rotte M ( 2006): Dynamic modulation of the primary somatosensory cortex during seeing and feeling a touched hand. Neuroimage 29: 587–592. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Xu B, Flor H, Cohen LG ( 2009): Effects of different viewing perspectives on somatosensory activations during observation of touch. Hum Brain Mapp 30: 2722–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD ( 2004): Empathy for pain involves the affective but not sensory components of pain. Science 303: 1157–1162. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. ( 1988). Co‐planar stereotactic atlas of the human brain. Stuttgart: Thieme Verlag. [Google Scholar]
- Taylor‐Clarke M, Kennett S, Haggard P ( 2002): Vision modulates somatosensory cortical processing. Curr Biol 12: 233–236. [DOI] [PubMed] [Google Scholar]
- Valeriani M, Betti V, Le Pera D, De Armas L, Miliucci R, Restuccia D, Avenanti A, Aglioti SM ( 2008): Seeing the pain of others while being in pain: A laser‐evoked potentials study. Neuroimage 40: 1419–1428. [DOI] [PubMed] [Google Scholar]
- Walsh V, Rushworth M ( 1999): A primer of magnetic stimulation as a tool for neuropsychology. Neuropsychologia 37: 125–135. [PubMed] [Google Scholar]
- Wassermann EM ( 1998): Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol 108: 1–16. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby SH. ( 2008). The Oxford Handbook of Transcranial Stimulation. New York: Oxford University Press. [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G ( 2003): Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron 40: 655–664. [DOI] [PubMed] [Google Scholar]
- Zhou YD, Fuster JM ( 1997): Neuronal activity of somatosensory cortex in a cross‐modal (visuo‐haptic) memory task. Exp Brain Res 116: 551–555. [DOI] [PubMed] [Google Scholar]
- Zhou YD, Fuster JM ( 2000): Visuo‐tactile cross‐modal associations in cortical somatosensory cells. Proc Natl Acad Sci USA 97: 9777–9782. [DOI] [PMC free article] [PubMed] [Google Scholar]


