Abstract
Previous studies of the BOLD response in the injured brain have revealed neural recruitment relative to controls during working memory tasks in several brain regions, most consistently the right prefrontal cortex and anterior cingulate cortices. We previously proposed that the recruitment observed in this literature represents auxiliary support resources, and that recruitment of PFC is not abnormal or injury specific and should reduce as novelty and challenge decrease. The current study directly tests this hypothesis in the context of practice of a working memory task. It was hypothesized that individuals with brain injury would demonstrate recruitment of previously indicated regions, behavioral improvement following task practice, and a reduction in the BOLD signal in recruited regions after practice. Individuals with traumatic brain injury and healthy controls performed the n‐back during fMRI acquisition, practiced each task out of the scanner, and returned to the scanner for additional fMRI n‐back acquisition. Statistical parametric maps demonstrated a number of regions of recruitment in the 1‐back in individuals with brain injury and a number of corresponding regions of reduced activation in individuals with brain injury following practice in both the 1‐back and 2‐back. Regions of interest demonstrated reduced activation following practice, including the anterior cingulate and right prefrontal cortices. Individuals with brain injury demonstrated modest behavioral improvements following practice. These findings suggest that neural recruitment in brain injury does not represent reorganization but a natural extension of latent mechanisms that engage transiently and are contingent upon cerebral challenge. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: reorganization, traumatic brain injury, working memory, functional MRI, cognitive control
INTRODUCTION
As many as 2% of individuals living in the United States have a long‐term disability as a result of traumatic brain injury (TBI, Thurman et al., 1999), and more than 1 million individuals sustain TBI each year and survive (NCHS, 2010). Functional neuroimaging techniques now provide researchers with novel methods to investigate cognitive, motor, and emotional functioning consequences of neurological disruption. There is a growing literature examining basic information processing deficits following TBI in the areas of working memory (WM) (Christodoulou et al., 2001; Maruishi et al., 2007; McAllister et al., 1999, 2001; Newsome et al., 2007; Perlstein et al., 2004; Sanchez‐Carrion et al., 2008a, b), executive control (Scheibel et al., 2007, 2009; Turner and Levine, 2008), episodic memory (Ricker et al., 2001; Strangman et al., 2009), and processing speed (Hillary et al., 2010). The current study will focus on WM, which is defined as the capacity to maintain and manipulate a limited amount of information for a brief period of time (Baddeley, 1992), and is a fundamental cognitive ability that is requisite for complex cognitive tasks (Courtney, 2004; Goldman‐Rakic, 1995).
WM deficits are ubiquitous after neurological insult having been observed in TBI (McDowell et al., 1997; Stuss et al., 1985), multiple sclerosis (MS, Aubin‐Faubert, 1989; Demaree et al., 1999; Mostofsky et al., 2003; Rao et al., 1989a, b), schizophrenia (Cohen et al., 1997; Saykin et al., 1991, 1994), dementia (Bradley et al., 1989; Colette et al., 1999; Morris and Baddeley, 1988), and normal aging (Salthouse, 1992, 1996; Salthouse and Coon, 1993). Because WM deficits are nearly universal after neurological disruption, the functional imaging literature in the clinical neurosciences has focused on examining these deficits and others related to basic information processing. In functional imaging studies of TBI, specifically, there have been striking similarities in the findings, with most results pointing to increased neural activity in several brain regions, but most consistently in prefrontal cortex (PFC), and especially the dorsolateral PFC (DLPFC) (see Hillary, 2008). In the TBI literature, the most common finding discriminating individuals with moderate and severe TBI and control groups is recruitment of DLPFC (Hillary, 2008). What remains largely undetermined in this literature is the role of DLPFC recruitment in WM dysfunction and how the PFC modulates new learning in a disrupted neural system.
There are three presiding explanations that may account for the neural recruitment consistently observed in studies of WM dysfunction. The first, brain reorganization, supposes that additional DLPFC recruitment reflects underlying changes in the neural substrate and/or changes in the functional network associated with WM tasks (Sanchez‐Carrion et al., 2008a, b; see also Levin, 2003). This change is presumed to be permanent, and neural resources that are recruited are thought to maintain a positive relationship with performance (i.e., in the absence of DLPFC recruitment, performance decrements would appear). A second term, often used interchangeably with the first, is neural compensation. Neural compensation operates similarly to brain reorganization in that it implies a positive performance/activation relationship, but a critical difference from the former is that investigators using this term often make no inferences about alterations in the underlying neural substrate. That is, investigators describing neural recruitment as “compensatory” are often agnostic about its permanence, but take the very clear position that this neural recruitment (most often in DLPFC) is necessary for sustained task performance (Maruishi et al., 2007; McAllister et al., 1999, 2001; Scheibel et al., 2009; Turner and Levine, 2008). For the purposes of this paper, the term “compensation” will be used to describe this position. A third explanation can be dissociated from the former two explanations—it posits that neural recruitment in PFC largely represents a natural support mechanism operating in response to degraded performance. According to this hypothesis, which we propose to be formalized as the “latent support hypothesis,” neural recruitment is neither permanent, nor operating to bolster cognitive functioning (Hillary, 2008; Hillary et al., 2010). Rather, the PFC and occasional anterior cingulate cortex (ACC) recruitment observed in TBI and other neurological disorders represents the engagement of cognitive control and attentional resources (cf. Courtney, 2004; Weissman et al., 2006) to meet task demands in a “challenged” neural system (Hillary, 2008). This position argues that the PFC is subserving an identical support function as has been observed in healthy adults during task load manipulations (Braver, 1997; Jaeggi et al., 2003; Landau et al., 2004; Rypma and D'Esposito, 1999), but it is evident at lower thresholds after neurological disruption. The essential difference between the explanation offered here and the previous two is that it implies a negative performance/activation relationship and, therefore, has different implications for the role of neural recruitment in cognitive recovery (cf. Hillary et al., 2006). Therefore, the overarching goal of the current investigation is to test the viability of a latent support mechanism (i.e., cognitive and attentional control) by inducing plasticity in the networks through task practice. In doing so, we aim to observe changes in DLPFC and ACC involvement during repeated exposure to a WM task as performance is altered. Finally, a third region often shown to be recruited in these samples, the inferior parietal cortex, will be examined as an important additional region implicated in WM function, as revealed by fMRI (Owen, 2005).
Using Task Practice to Elicit Plasticity in PFC
A primary focus in the working memory literature has been to relate neural networks to observable deficit, with a focus on “deficit” and reduced capacity. Even after quite a significant disruption, however, frontal systems often remain largely intact, permitting a functional memory system, albeit at a reduced rate and capacity (DeLuca et al., 2000). In this context, the question then becomes: how does a disrupted neural system change during new learning and how does this change differ from that observed in an intact neural system? Previous research in healthy individuals has demonstrated that involvement of PFC diminishes following repeated practice of the task (Garavan et al., 2000; Jansma et al., 2001; Landau et al., 2004; Ramsey et al., 2004; for a review see Kelly and Garavan, 2005). While not universal, a finding supported by some replication is that right DLPFC may be differentially involved in handling task novelty (Alexander et al., 1994; Daffner et al., 2000; Knight and Scabini, 1998) and may show the greatest functional changes during acclimation to WM tasks (Milham et al., 2003). Interestingly, right DLPFC recruitment has been one of the most consistent findings in cross sectional work examining WM deficit in neurologically impaired samples such as TBI (for review see Hillary et al., 2006). Based upon the potential unique role of right DLPFC in managing task novelty and the common finding that in cases of TBI right DLPFC is often recruited, the primary goal of the current study is to examine maturational effects in WM networks, with specific interest in DLPFC, during repeated trials and after task practice of a well‐established WM task. Thus, it is a goal in this study to observe network changes associated with improving performance as new learning occurs. Ultimately, by examining short‐term changes in WM networks induced by practice, we aim to clarify the role of DLPFC in WM functioning after TBI, and examine whether the right DLPFC's recruitment in neurological samples supports a reorganization or compensation interpretation or, as we propose, a latent support hypothesis. In this study, we use a well‐established WM task (N‐back) and examine the influence of two scanning sessions separated by several trials of practice. With this manipulation, the goal is to test hypotheses regarding the nature of neural recruitment in TBI:
Hypothesis 1: Individuals sustaining TBI will improve on the n‐back task following task practice.
Hypothesis 2: Individuals sustaining TBI will demonstrate recruitment of additional resources in DLPFC and ACC compared to healthy adults at Time 1 (prior to task practice).
Hypothesis 3: Task practice will result in decreased involvement of DLPFC and resources originally recruited in individuals diagnosed with TBI.
METHOD
Subjects
Demographic and clinical characteristics of the sample are presented in Table I. TBI severity was defined using the Glasgow Coma Scale (GCS) in the first 24 hours after injury (Teasdale and Jennett, 1974), and GCS scores from 3–8 were considered “severe” and scores from 9–12, or individuals with significant neuroimaging findings, were considered “moderate.” All individuals with TBI had an initial GCS score between 3 and 12 or had at least one identifiable brain lesion site as confirmed by CT scans in their medical records, and were at least 1 year post injury. Candidates for the study were excluded if they had a history of previous neurological disorder such as seizure disorder, or significant neurodevelopmental psychiatric history (such as schizophrenia or bipolar disorder). These exclusions were covered in the IRB approved consent form and were discussed with the family members of each study participant. The final sample included 24 right‐handed participants aged 18–53. There were 12 healthy control participants (HCs) between the ages of 18 and 49 (M = 25.08, SD = 10.24) without any reported medical or psychiatric disabilities, and 12 participants diagnosed with moderate to severe TBI between the ages of 18 and 53 (M = 32.17, SD = 12.23). The mean education for HCs was 14.08 (SD = 1.93) and for TBI it was 13.75 (SD = 2.60).
Table I.
Demographic and cognitive testing results for TBI and HC samples
| Demographic variables | TBI (mean, SD) | HC (mean, SD) | Group comparison |
|---|---|---|---|
| Age | 32.2, 12.2 | 25.1, 10.2 | P = .13 |
| Education | 13.8, 2.60 | 14.1, 1.9 | P = .79 |
| Gender | 6 m, 6 f | 7 m, 5 f | P = .68 |
| Clinical variable | TBI sample | ||
| GCS score | Mean = 4.7; SD = 3.0; range = 3–12 | ||
| Acute hospital days | Mean = 21.4; SD = 16.3; range = 6–60 | ||
| Acute CT/MRI result (number of subjects) | DAI (4); Right frontal (4); Left frontal (2); parietal (6); PVA (4); Temporal (2) | ||
| Injury type | MVA = 9; MVA‐P = 1; Fall = 1; Skiing = 1 | ||
| Loss of consciousness | Mean = 9.2 days; SD = 2.0 | ||
Abbreviations: GCS, Glasgow coma scale; SD, standard deviation; DAI, diffuse axonal injury; PVA, peri‐ventricular area; MVA, motor vehicle accident; MVA‐P, motor vehicle accident against pedestrian.
Task in Scanner
The n‐back, a well established sequential letter task, was used to assess working memory (Chang et al., 2001; Kirchner, 1958; Speck et al., 2000). The task was presented in a boxcar (“block”) design. Each run of n‐back contained eight 20‐second task blocks, with 14‐second rest periods separating blocks. During each block, alphabetical letters were presented randomly at a rate of one every two seconds, totaling ten letters per block. The subjects were instructed to press a response button as quickly as possible to the target stimuli, where target stimuli were defined as whenever the current letter was the same as the letter immediately preceding it (1‐back task) or two letters prior (2‐back task). During each 10‐letter block, three or four target stimuli were presented at random time points that subjects should respond to. During each rest period, subjects were instructed to fixate on a small asterisk presented at the center of the display screen. Subjects performed one run each of the 1‐back and 2‐back in the scanner. Subjects then engaged in task practice outside of the scanner, during which they completed one novel run of 1‐back and one novel run of 2‐back that followed the same design described above. Subjects were then permitted to take a ten‐minute break. Finally, subjects re‐entered the scanner and performed one more run of 1‐back following by a run of 2‐back.
Neuropsychological Testing
On the same day as the MRI scanning, a battery of neuropsychological tests was administered to each participant to assess neuropsychological functioning. The battery assessed functions known to be impaired in individuals in TBI, such as processing speed, working memory, and verbal comprehension. Processing Speed tasks (which were always administered following the fMRI session) were the Trail‐Making Test (TMT) A and B (Army Individual Test Battery, 1990; Reitan and Wolfson, 1985). Working memory was assessed with the Digit Span subtest of the WAIS‐III, including simple attention/rehearsal (digits forward) and rehearsal/manipulation (digits backward) (Wechsler, 1997). The information subtest of the WAIS‐III was used to assess verbal comprehension (Wechsler, 1997).
Magnetic Resonance Imaging Procedure
MRI data were acquired using a Philips 3T system and a 6‐channel SENSE head coil (Philips Medical Systems, Best, The Netherlands) and a Siemens 3T Magnetom Trio (Siemens, New York City, USA). For all subjects, first, 3‐D high resolution T1‐weighted MPRAGE images (9.9 ms/4.6 ms/8° repetition time/echo time/flip angle (TR/TE/FA), 240 × 204 × 150 mm3 field of view (FOV), 256 × 205 × 150 acquisition matrix, two averages) were acquired to provide high resolution underlays for functional brain activation. Echo planar imaging (EPI) was used for functional imaging. Imaging parameters for EPI consisted of: 2,000 ms/30 ms/89°, TR/TE/FA, 230 × 230 mm2 FOV, 80 × 80 acquisition matrix, thirty‐four 4‐mm‐thick axial slices with no gap between slices.
Data Analysis
Preprocessing of the fMRI data was performed using SPM5 software (http://www.fil.ion.ucl.ac.uk/spm5). The first nine volumes were removed from analyses to control for initial signal instability. Preprocessing steps included realignment of functional data of each trial to the first functional image of that trial using affine transformation (Ashburner et al., 1997; Friston et al., 1995). Functional images were then coregistered to the individual's T1 MPRAGE, and all data were normalized using a standardized T1 template from the Montreal Neurological Institute, MNI, using a 12‐parameter affine approach and bilinear interpolation. Normalized time series data were smoothed with a Gaussian kernel of 8 × 8 × 10 mm3 to minimize anatomical differences and increase signal to noise ratio.
Functional Imaging Contrasts
First level analyses were conducted using the general linear model within SPM5 to produce intraindividual 1‐back activation relative to rest and 2‐back activation relative to contrasts. To test the hypotheses, we conducted a 2 × 2 × 2 mixed ANOVA including the between subjects effect of group, within subjects effect of load, and within subjects effect of practice. To isolate specific within‐load and directional effects, we conducted four specific group level analyses. Post‐hoc group activation maps were computed using random effects analysis (two‐sample t‐tests) to determine between group differences in activation for all 1‐back runs (two comparisons) and between group differences in activation for all 2‐back runs (two comparisons). Peaks from each contrast were converted to Talairach space via a linear conversion (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html) and submitted to the Talairach Daemon to approximate the locations of the regions most significantly indicated in each contrast. Of note, there were two reasons for not including a control task in addition to the task vs. baseline analysis conducted here. First, it was not a primary goal in this study to isolate specific cognitive mechanisms (e.g., separate WM encoding from rehearsal); the goal is to examine changes in neural networks after task practice. Second, there are a number of methodological pitfalls associated with using complex control tasks in clinical samples, including differential subtraction between subjects and even greater complications with the assumption of pure insertion (Price et al., 2006).
Region of Interest Analyses
To avoid selection bias of regions of interest (ROIs) and regression to the mean phenomena associated with improper selection (cf. Vul et al., 2009), standardized ROIs from the WFU PickAtlas were selected for the DLPFC (Brodmann's Areas [BA] 9 and 46) and ACC (BA32). To explore responses of other regions known to be important in WM, a priori ROIs from the PickAtlas were selected for Broca's area (BA 44/45) and the bilateral BA 39. See Figure 1 for a schematic representation of ROIs.
Figure 1.
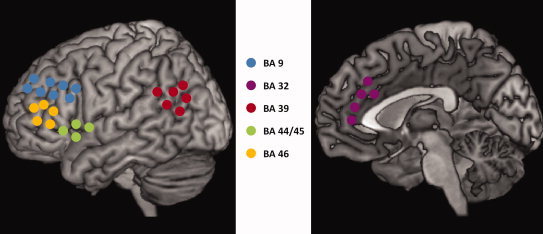
Schematic representation of ROIs.
The MarsBar toolbox was used to approximate the % signal change in each region during the n‐back for each individual. One‐sample within subject t‐tests were used to detect significant reductions in % signal change for each region for each load level of n‐back in both the TBI and HC samples. Additionally, the % signal change in the primary ROI, right BA46, for the HC and TBI samples was compared to determine if the TBI sample generally recruited BA46 more than HCs. Finally, within subject t‐tests were used to determine load effects on the % signal change in right BA46 within each sample.
RESULTS
Neuropsychological Findings
Individuals with TBI performed significantly more poorly than controls on both versions of the Trails task and the Stroop and showed trends toward statistical significance in Information and Digit span (Table II). Controls' z‐scores, relative to an age‐matched normative sample, ranged from 0.20 to 1.0, suggesting that they were slightly higher than but within 1 SD of the average matched individuals.
Table II.
Neuropsychological test results for the TBI and HC samples
| Neuropsychological results | TBI mean, SD | HC mean, SD | Group comparison |
|---|---|---|---|
| Trail making test A | 34, 8.98 | 21, 7.76 | P < 0.001 |
| Z‐scores | −1.28, 1.04 | 0.48, 1.10 | |
| Trail making test B | 83, 35.06 | 50, 13.6 | P = 0.007 |
| Z‐scores | −1.05, 1.41 | 0.20, 0.69 | |
| Stroop | 88, 20.34 | 105, 11.30 | P = 0.03 |
| Z‐scores | −0.61, 2.01 | 0.42, 1.86 | |
| Information | 15.67, 7.01 | 20.7, 5.52 | P = 0.08 |
| Z‐scores | 0.00, 1.28 | 1, 1.14 | |
| Digit span | 17.5, 11.36 | 19.27, 8.4 | P = 0.21 |
| Z‐scores for all digit spans | 0.11, 0.77 | 0.47, 0.74 |
In Scanner Performance
One 2 × 2 × 2 mixed ANOVA was conducted for reaction time with the following factors: group (between subjects), practice (within subjects), and load (within subjects). A main effect of task load was found, F(1, 23) = 39.558, P < 0.001. The main effects of group and practice as well as all interaction terms were not significant.
A 2 × 2 × 2 mixed ANOVA was conducted for accuracy with the following factors: group (between subjects), practice (within subjects), and load (within subjects). No significant effects were found.
Several post‐hoc t‐tests were conducted to isolate specific directional and within subject, within load effects. No significant differences in mean reaction times (RT) were found in n‐back runs before and after practice within the TBI sample. However, a within‐subjects t‐test revealed a significant reduction in RTs between the first and last three trials of 2‐back (P = 0.01). Additionally, to ensure a reliable average in RTs, values for each half‐run were aggregated for both loads and within both samples. A nearly significant reduction in RTs between the first half of the first run of 2‐back and the last half of the second run of 2‐back (from 821.89 to 736.522, P = 0.09, one‐tailed) in TBI. This suggests that individuals with TBI experienced learning during the task, but that learning effects may have been obscured by whole‐run comparisons of reaction times. In the HC sample, a main effect of practice on RT (P = 0.03) was found for only the 1‐back (Table III for RT data for all n‐back runs).
Table III.
TBI and HC reaction times for n‐back in scanner
| TBI mean, SD | HC mean, SD | Group comparison | |
|---|---|---|---|
| 1‐back pre | 669.97, 105.10 | 583.40, 93.04 | P = 0.02 |
| 1‐back post | 665.59, 176.60 | 544.52, 85.09 | P = 0.02 |
| 2‐back pre | 768.98, 177.60 | 709.58, 163.05 | P = 0.18 |
| 2‐back post | 739.72, 194.54 | 686.94, 142.20 | P = 0.21 |
| 1st three 1‐back trials | 655.75, 227.40 | 567.53, 117.10 | P = 0.14 |
| Last three 1‐back trials | 632.67, 249.96 | 547.76, 67.97 | P = 0.15 |
| 1st three 2‐back trials | 830.58, 197.06 | 713.14, 242.79 | P = 0.10 |
| Last three 2‐back trials | 653.83, 248.72 | 648.00, 158.86 | P = 0.47 |
Further, while no differences in mean run accuracy before and after practice were found during the 2‐back, a significant difference was found in 1‐back accuracy following task practice (P = 0.01) Among HCs, a nearly significant effect of practice on accuracy (P = 0.07) was found for the 2‐back (Table IV for accuracy data for all n‐back runs).
Table IV.
TBI and HC accuracy for n‐backs in scanner
| TBI mean, SD | HC mean, SD | Group comparison | |
|---|---|---|---|
| 1‐back pre | 80.56, 29.48 | 99.00, 1.81 | P = 0.007 |
| 1‐back post | 96.35, 5.43 | 98.75, 3.11 | P = 0.10 |
| 2‐back pre | 87.00, 13.11 | 84.42, 15.77 | P = 0.33 |
| 2‐back post | 82.33, 16.49 | 92.67, 7.40 | P = 0.03 |
The TBI sample was only significantly slower than controls during the 1‐back, and were significantly less accurate during the before practice run of 1‐back and after practice run of 2‐back (Tables III and IV).
BOLD Signal Change and Task Practice
BOLD signal main effects
F‐contrast SPM's were computed to depict the main effects of group, practice, and load as well as the interaction of group by practice, group by load, and practice by load. An inclusive threshold (P < 0.001 uncorrected) was used to capture those regions related to each effect of interest.
Group effects were found in the right cingulate gyrus (BA 24), right motor (BA 4) and motor planning (BA 6) regions, right precuneus (BA 7, 19), and right paracentral gyrus (BA 31); and in the left hemisphere, the right motor area (BA 4), right precuneus (BA 31), and right parietal lobe (Table V for an exhaustive list of regions different between groups). To isolate directional between groups, within load level effects, we conducted two post hoc t‐tests (see below).
Table V.
MNI coordinates and locations of peaks significantly predicted by the main effect of group
| Main effect: Group | Region | BA | X | Y | Z | Z‐value |
|---|---|---|---|---|---|---|
| Right hemisphere | Precentral gyrus | 4 | 60 | −4 | 22 | 3.18 |
| Middle frontal gyrus | 6 | 40 | 4 | 50 | 3.15 | |
| Medial frontal gyrus | 6 | 0 | −18 | 66 | 3.88 | |
| Precuneus | 7 | 2 | −76 | 40 | 3.24 | |
| Precuneus | 19 | 32 | −78 | 32 | 3.53 | |
| Cingulate gyrus | 24 | 8 | −8 | 44 | 3.23 | |
| Paracentral lobule | 31 | 2 | −34 | 48 | 3.3 | |
| Left hemisphere | Precentral gyrus | 4 | −18 | −30 | 70 | 3.44 |
| Precuneus | 31 | −22 | −84 | 28 | 3.62 | |
| Inferior parietal lobule | 40 | −48 | −32 | 34 | 3.38 |
A number of regions were affected by task practice: the right prefrontal cortex (BA 9, 10, 44, 45, 46, and 47), right parietal lobe (BA 40), right temporal lobe (BA 20, 38), right cingulate (BA 24, 31), right anterior cingulate (BA 32) right motor planning (BA 6), right insula (BA 13) and right caudate; and in the left hemisphere, the left prefrontal cortex (BA 10, 46, 47), left parietal lobe (BA 37, 40), left temporal lobe (BA 41), left sensory (BA 2), left occipital (BA 17), left motor planning (BA 6), and left claustrum, thalamus, putamen, and caudate (Fig. 2, Table VI for an exhaustive list of regions affected by task practice).
Figure 2.

Main effect of practice during the n‐back (P < 0.001).
Table VI.
MNI coordinates and locations of peaks significantly predicted by the main effect of practice
| Main effect: Practice | Region | BA | X | Y | Z | Z‐value |
|---|---|---|---|---|---|---|
| Right hemisphere | Postcentral gyrus | 2 | 62 | −20 | 30 | 3.53 |
| Middle frontal gyrus | 6 | 42 | 0 | 50 | 4.23 | |
| Superior frontal gyrus | 6 | 20 | 14 | 58 | 3.88 | |
| Inferior frontal gyrus | 9 | 54 | 10 | 34 | 4.49 | |
| Middle frontal gyrus | 10 | 38 | 58 | 2 | 3.46 | |
| Medial frontal gyrus | 10 | 20 | 64 | 8 | 4.22 | |
| Insula | 13 | 44 | 4 | −8 | 3.27 | |
| Middle temporal gyrus | 20 | 50 | −36 | −8 | 3.11 | |
| Cingulate gyrus | 24 | 2 | −2 | 36 | 3.52 | |
| Cingulate gyrus | 31 | 8 | −40 | 36 | 3.95 | |
| Anterior cingulate | 32 | 10 | 42 | 10 | 4.06 | |
| Superior temporal gyrus | 38 | 50 | 14 | −8 | 3.49 | |
| Inferior parietal lobule | 40 | 42 | −52 | 46 | 4.06 | |
| Inferior frontal gyrus | 44 | 54 | 10 | 16 | 3.31 | |
| Inferior frontal gyrus | 45 | 40 | 26 | 6 | 4.76 | |
| Middle frontal gyrus | 46 | 44 | 24 | 22 | 4.37 | |
| Inferior frontal gyrus | 47 | 36 | 34 | −2 | 4.86 | |
| Caudate | 18 | 22 | 2 | 4.88 | ||
| Left hemisphere | Postcentral gyrus | 2 | −54 | −26 | 38 | 3.24 |
| Sub‐gyral | 6 | −18 | 0 | 58 | 3.63 | |
| Middle frontal gyrus | 6 | −30 | −4 | 54 | 4.15 | |
| Precentral gyrus | 6 | −42 | −8 | 48 | 3.59 | |
| Precuneus | 7 | −20 | −80 | 38 | 3.1 | |
| Superior parietal lobule | 7 | −34 | −64 | 50 | 3.46 | |
| Middle frontal gyrus | 10 | −36 | 36 | 28 | 3.18 | |
| Insula | 13 | −52 | −38 | 22 | 3.36 | |
| Lingual gyrus | 18 | −34 | −74 | −14 | 3.4 | |
| Cingulate gyrus | 32 | −8 | 20 | 40 | 3.87 | |
| Fusiform gyrus | 37 | −46 | −52 | −22 | 3.24 | |
| Inferior parietal lobule | 40 | −42 | −50 | 42 | 3.59 | |
| Superior temporal gyrus | 41 | −44 | −28 | 14 | 3.2 | |
| Middle frontal gyrus | 46 | −44 | 24 | 22 | 3.19 | |
| Inferior frontal gyrus | 47 | −48 | 18 | −2 | 4.16 | |
| Claustrum | −22 | 18 | 14 | 3.41 | ||
| Thalamus | −24 | −20 | 18 | 3.1 | ||
| Putamen | −28 | −20 | 4 | 3.42 | ||
| Caudate | −4 | 0 | 0 | 3.19 |
Load effects were found in the right motor planning (BA 6), right precuneus (BA 7, 31), right occipital lobe (BA18), and right putamen, caudate, hypothalamus, and thalamus; and in the left hemisphere, the left prefrontal cortex (BA 9, 46), left cingulate (BA 32), left precuneus (BA 7), left temporal gyrus (BA 37, 39), left motor planning (BA 6), left medial globus pallidus, caudate, putamen, thalamus (Fig. 3, Table VII for an exhaustive list of regions affected by task load).
Figure 3.
Main effect of task load (P < 0.001).
Table VII.
MNI coordinates and locations of peaks significantly predicted by the main effect of load
| Main effect: Load | Region | BA | X | Y | Z | Z‐value |
|---|---|---|---|---|---|---|
| Right hemisphere | Precentral gyrus | 6 | 26 | 4 | 56 | 4.57 |
| Precuneus | 7 | 10 | −54 | 44 | 4.48 | |
| Lingual gyrus | 18 | 0 | −80 | 0 | 3.32 | |
| Precuneus | 31 | 20 | −58 | 30 | 3.13 | |
| Putamen | 20 | 12 | −10 | 4.77 | ||
| Caudate | 12 | 10 | −2 | 4.56 | ||
| Hypothalamus | 10 | −4 | −8 | 3.26 | ||
| Thalamus | 8 | −18 | 2 | 3.5 | ||
| Left hemisphere | Middle frontal gyrus | 6 | −30 | 6 | 56 | 3.46 |
| Precentral gyrus | 6 | −44 | −6 | 44 | 3.39 | |
| Precuneus | 7 | −12 | −62 | 46 | 4.47 | |
| Middle frontal gyrus | 9 | −42 | 24 | 32 | 3.74 | |
| Medial frontal gyrus | 32 | −8 | 10 | 52 | 3.52 | |
| Cingulate gyrus | 32 | −10 | 16 | 46 | 3.58 | |
| Fusiform gyrus | 37 | −48 | −54 | −12 | 3.45 | |
| Middle temporal gyrus | 39 | −36 | −76 | 26 | 4.72 | |
| Middle frontal gyrus | 46 | −44 | 20 | 22 | 4.76 | |
| Medial globus pallidus | −12 | 2 | 2 | 4.47 | ||
| Caudate | −16 | 16 | 2 | 5.7 | ||
| Putamen | −22 | 8 | −8 | 4.25 | ||
| Thalamus | −6 | −18 | 0 | 3.48 |
Table VIII.
Percent signal changes for ROIs for each run of n‐back
| Region | 1‐back pre | 1‐back post | Significance | 2‐back pre | 2‐back post | Significance |
|---|---|---|---|---|---|---|
| RBA46 (TBI) | 0.26 | 0.06 | 0.03* | 0.37 | 0.05 | 0.02* |
| (HC) | 0.03 | −0.15 | 0.07 | 0.47 | 0.07 | 0.008* |
| LBA46(TBI) | 0.38 | 0.08 | 0.0008* | 0.29 | 0.14 | 0.07 |
| (HC) | 0.05 | −0.09 | 0.14 | 0.35 | −0.02 | 0.006* |
| RBA9 (TBI) | 0.08 | 0.17 | 0.01* | 0.17 | 0.09 | 0.13 |
| (HC) | 0.04 | −0.12 | 0.04* | 0.34 | 0.07 | 0.002* |
| LBA9 (TBI) | 0.15 | 0.08 | 0.13 | 0.14 | 0.03 | 0.07 |
| (HC) | −0.01 | −0.09 | 0.28 | 0.26 | 0.09 | 0.005* |
| RACC (TBI) | 0.13 | 0.05 | 0.03* | 0.13 | 0.02 | 0.04* |
| (HC) | 0.01 | −0.05 | 0.13 | 0.24 | 0.00 | 0.006* |
| LACC (TBI) | 0.12 | 0.04 | 0.02* | 0.14 | 0.04 | 0.07 |
| (HC) | 0.00 | −0.05 | 0.17 | 0.23 | −0.02 | 0.008* |
| Broca's (TBI) | 0.22 | 0.11 | 0.11 | 0.24 | 0.10 | 0.05* |
| (HC) | 0.07 | −0.09 | 0.19 | 0.43 | 0.22 | 0.004* |
| R39 (TBI) | 0.06 | −0.09 | 0.02* | 0.03 | −0.04 | 0.21 |
| (HC) | −0.11 | −0.21 | 0.13 | 0.07 | −0.05 | 0.009* |
| L39 (TBI) | −0.03 | −0.04 | 0.39 | −0.05 | −0.04 | 0.23 |
| (HC) | −0.13 | −0.24 | 0.12 | 0.01 | −0.08 | 0.08 |
BOLD signal interaction effects
The interaction of group and practice demonstrated an effect in only the right claustrum. The interaction of group and load demonstrated effects in the right frontal lobe (BA 9, 47), the right motor planning region (BA 6), the right thalamus and lateral globus pallidus, left prefrontal lobe (BA 46), left anterior cingulate (BA 32), left parietal lobe (BA 7), left motor planning regions (BA 6, 8), and left putamen. The interaction of load and practice demonstrated effects in the right frontal lobe (BA 44, 9), right cingulate (32), right insula (BA 13), right sensory area (BA 3) right caudate, and the left anterior cingulate (BA 32) and insula (BA 13).
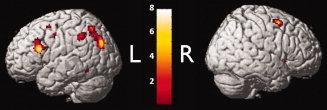
To examine Hypothesis 2, t‐contrasts were computed to depict regions of greater recruitment in TBI versus healthy controls and vice versa within each n‐back load level. To do so, an inclusive threshold (P < 0.001) was used to capture those regions demonstrating some between‐group difference. T‐contrasts comparing TBI activation to HC activation within each load level were implemented to isolate directional between group effects within the 1‐back and 2‐back. A number of regions were more active in TBI in the 1‐back: the right prefrontal cortex (BA 9, 10, 45, 46, 47), right cingulate (BA 24, 30, 31), right parietal lobe (BA 7, 19, 40), right motor (BA 4) motor planning regions (BA 6, 8), and right temporal gyrus (BA 22, 39), and BA 43; and in the left hemisphere, the left prefrontal cortex (BA 9, 10, 46), left parietal lobe (BA 19, 31, 39), left temporal lobe (BA 21, 38, 42), left insula (BA13), left cingulate gyrus (BA 29, 32), left occipital lobe (BA 18), and left parahippocampal gyrus (Fig. 4, see (Supporting Information Table I for an exhaustive list of regions of significant difference between groups).
Figure 4.

Regions more active in individuals with TBI than healthy controls during the 1‐back task. Note: Only three peaks (MNI coordinates: 56, −2, 24; −40, −40, 16; −34, 0, −32) were found for the contrast of regions greater in TBI than HCs during the 2‐back task. Only two peaks (MNI coordinates: −10, 20, 20; 24, −14, 34) were found for the contrast of regions greater in HCs than TBI during the 1‐back and only 2 peaks (MNI coordinates: −10, 20, 20; 34 8 30) were found for the contrast of regions greater in HCs than TBI during the 2‐back.
Only three regions (right BA6, left BA13, and left BA21) had significantly higher peaks of activation in TBI during the 2‐back compared to healthy controls, which were not visible on the cortical surface. Conversely, only two regions had peaks that were more active in HCs than TBI during the 1‐back (left BA24, right BA24), and three regions had peaks that were more active in HCs than TBI during the 2‐back (left BA7, left BA 9, right BA32).
ROI Analyses
To examine the role of specific regions in new learning and to examine within‐subject change, one‐tailed t‐tests in the TBI sample were conducted, revealing several practice effects on the BOLD signal in ROIs. The BOLD signal in the right DLPFC (BA46) during the 1‐back demonstrated a statistically significant reduction after practice and a significant reduction after practice during the 2‐back. The BOLD signal in right BA 9 decreased in the 1‐back and showed a trend toward reduction during the 2‐back. The BOLD signal decreased in the left DLPFC (BA46) in both the 1‐back and the 2‐back, and the left BA9 demonstrated a trend toward reduction after practice in both the 1‐back and the 2‐back. Other findings in regions demonstrated to be involved in WM included a reduction in BOLD signal in the RACC (BA 32) in the 1‐back and 2‐back, and in the LACC (BA32) in the 1‐back, and a reduction in BOLD signal in the right BA39 in the 1‐back; a trend toward reduction in Broca's area in both 1‐back and a significant reduction in Broca's in the 2‐back. A within subjects t‐test revealed no significant increase in BOLD activity from the 1‐back to the 2‐back within the TBI sample in RBA 46.
One‐tailed t‐tests in the HC sample revealed a trend toward reduction in RBA46 in the 1‐back and a significant reduction in RBA46 in the 2‐back. A significant reduction was found in LBA46 in the 2‐back, and a significant reduction of RBA9 in the 1‐back and 2‐back. A significant reduction was found in LBA9 in the 2‐back. Finally, a reduction in BOLD in the 2‐back was found for RACC, LACC, Broca's, and R39. A within subjects t‐test revealed a significant increase in BOLD activity from the 1‐back to the 2‐back within the HC sample in RBA46. A comparison of changes in % BOLD signal due to practice revealed no significant differences between groups at either load level, suggesting that the effects in the primary ROIs are equally affected by practice, irrespective of injury status. See Table VIII for a comprehensive overview of changes in the BOLD signal and accompanying significance values in ROIs.
Between group analyses of the primary ROI, RBA46, revealed greater BOLD signal in the TBI sample both before practice (P = 0.03) and after practice (P = 0.05). No significant differences between groups were found in RBA46 for the 2‐back (Figs. 5 and 6). An effect of task load (1‐back vs. 2‐back) was not found within individuals with TBI (P = 0.25) but was detected for healthy controls (P = 0.01).
Figure 5.

BOLD signal in RBA46 for HCs and TBI during the 1‐back (error bars represent 1 standard deviation; inset is a schematic of BA46).
Figure 6.

BOLD signal in RBA46 for HCs and TBI during the 2‐back (error bars indicate 1 standard deviation; inset is a schematic of BA46).
DISCUSSION
To the best of our knowledge, the current study represents the first effort in systems neuroscience to examine how practice of a cognitive task influences BOLD signal change after neurological compromise. These data offer insight into the nature of findings repeatedly demonstrated during cross‐sectional work, including the consistent observation that injury results in increased PFC involvement during WM tasks.
Of critical importance for determining the role of the PFC in recovery from TBI is to determine its relationship with task performance. In the cognitive neurosciences, one theorized role of right DLPFC during information processing is to support cognitive control, which is at the heart of all goal‐directed behavior. Cognitive control coordinates the cognitive activities that embody specific goals and the means of achieving those goals (Miller and Cohen, 2001). For example, when an individual encounters a new task, the DLPFC may modulate cognitive control by determining what level of control is necessary to develop task rules and until sufficient subroutines for task performance are established, increased “control” may be necessary. In the context of TBI, cognitive control may be consistently upregulated during WM tasks due to its high sensitivity to cerebral challenge. That is, as the challenged basal neural system engages to address a novel task, it will require cognitive control resources to establish task goals, procedures for execution of the task, and coordination of distributed resources. All individuals engage cognitive control resources to tolerate continually changing task demands; however, the elevated and constant degree of cerebral challenge represented by TBI likely increases network demands. In a scenario such as this, recruitment of cognitive control mechanisms would be markedly increased, which is represented in the nearly universal finding that DLPFC, and often right DLPFC is recruited after injury (Hillary et al., 2006).
Behavioral Improvement Following Practice
Behaviorally, individuals with TBI were significantly more accurate during the 1‐back following practice, and significantly faster during the 2‐back following practice. Behavioral improvements in each task may reflect increased attentiveness to task and adjustment following errors during the 1‐back and efficient acquisition of task strategies during the 2‐back. The lack of improvement in reaction times in the 1‐back may involve limits in basic processing speed in the TBI sample (as suggested by their slow speed on the Trails tasks), whereas limits to executive capacity may explain lack of increased accuracy during the 2‐back following practice in individuals to TBI. The n‐back was used in this study due to its extensive use in this literature, but this task likely has inherent ceiling effects with respect to new learning, likely attributable to its demand on immutable processes such as sustained attention and WM rehearsal.
Neural Recruitment in the Neurologically Compromised Sample
The goal of this study was to induce plasticity in PFC via task practice using a well‐established working memory task in individuals with TBI to test the hypothesis that right PFC recruitment represents a latent support system. A first step in doing so was to replicate previous findings that greater activation is often found in individuals with TBI relative to healthy individuals, especially in the right DLPFC and anterior cingulate cortex. We did not find greater activation in the TBI group for the 2‐back, but did find extensive recruitment of the bilateral DLPFC, bilateral parietal cortex, and cingulate cortex for the 1‐back. Importantly, individuals with TBI were significantly slower than controls only during the 1‐back, which suggests that the neural recruitment observed in TBI was in the presence of poorer performance and not facilitative of performance. The findings in the 2‐back here are similar to what has been observed with a task load of 3‐back (in TBI see McAllister et al., 1999, 2001 and in MS see Sweet et al., 2006) where neural resources recruited during the lower task load in the clinical sample are later recruited in the HC sample, thus diminishing between group differences. For the current data, this recruitment of relevant ROIs appears to occur in the HC sample from 1‐back to 2‐back. Of note, much of the current clinical sample sustained severe injuries, suggesting a greater level of cerebral challenge and lower threshold for recruitment of latent resources. The lack of change in TBI from 1‐back to the 2‐back and relatively slower RT in the 1‐back compared to controls supports the hypothesis that individuals with TBI were challenged continuously throughout the task and especially so during the 1‐back, whereas the HC sample were challenged primarily when task load was increased to 2‐back.
One unanticipated finding was that the bilateral ACC (BA24) revealed a peak of activity in the group SPM that was higher in HCs than TBI during the 1‐back, and a peak of activity in the ACC (BA32) that was higher in HCs than TBI in the 2‐back. Post hoc analyses in ROI data for BA32 in the 2‐back revealed no significant difference between groups during the 2‐back (P = 0.26), suggesting that this peak may be artifactual or resulting from a small region of neural tissue. However, there exists the possibility that BA 24 operates independently from BA32 during working memory performance and does not follow the pattern seen in other regions recruited in TBI, and that small regions within the relatively large anterior cingulate cortex contribute differentially to performance in TBI than in healthy individuals. Previous work has demonstrated that the relationship between accuracy and activity in the ACC is more tightly positively related to accuracy in healthy individuals than in brain injury (Scheibel et al., 2007), suggesting that at an equal level of accuracy, controls will demonstrate some increase in anterior cingulate activity, such as in the current findings. While it is not possible to determine with the current methods, the potentially distinct roles of BA 24 and BA 32 in modulating task acquisition after TBI warrants further investigation.
The BOLD Response and Task Practice
Our findings indicate that the BOLD activity in the TBI sample in bilateral DLPFC (Both BA 9 and 46), RACC, and Broca's area demonstrate some reduction following task practice. Additionally, the LACC and RBA39 demonstrate some reduced BOLD signal following practice during the 1‐back. Reductions in BOLD activity in these regions during the 1‐back may reflect early learning of basic task procedures that generalize to later runs of 1‐back and 2‐back, diminishing the extent to which the LACC and RBA39 demonstrated change during the 2‐back, though it is noteworthy that many of these regions also showed reductions in percent signal change during the 2‐back that did not reach significance. The ACC, which is responsive during tasks involving error monitoring and important in cognitive control (Kerns et al., 2004), demonstrated a bilaterally reduced BOLD signal following practice in the 1‐back as the incidence for errors to monitor decreased. Post‐hoc analyses regressing change in accuracy against change in the % signal change in ACC ROIs revealed no significant results, but this may be due to the relatively coarse nature of the BOLD signal during block designs and restricted range of accuracy change in the sample. Future investigations using event‐related designs to dissociate accurate from inaccurate trials may be relevant for examining this topic directly. Right ACC BOLD signal reductions during the 2‐back after practice may represent a general increase in ACC neural efficiency as part of the broader WM network, though the dissociability of right and left ACC function with respect to practice and online error monitoring should be further explored.
Most importantly, evidence of reduced BOLD signal after practice was found in all examined regions of the DLPFC, with a primary main effect in DLPFC, suggesting increased neural efficiency (Rypma et al., 2006) and consistent with prior work in TBI (Hillary et al., 2010). These findings are further consistent with the explanation that the marshalling of cognitive control resources is a critical component to right DLPFC recruitment; cognitive control functions to manage cortical resources, which would be especially relevant when engaging novel tasks. The healthy control sample demonstrated a similar effect of task practice on BOLD activity in the right DLPFC during the n‐back and an effect of practice on the RACC during the 2‐back, supporting the hypothesis that right DLPFC recruitment in TBI represents a natural engagement of support resources during cognitive challenge.
Specific Role of the Right PFC in Cognitive Challenge
In healthy adults, the right PFC serves an active role in facilitating the network's response to novel and challenging tasks as the network consolidates its activity to improve behavior or more efficiently engage in practiced behaviors; with increased task facility, information processing then shifts to greater left hemisphere involvement as one “interprets” the stimulus or action (Gazzaniga, 2000; Pardo et al., 1991). Because of reduced or disrupted cortical resources available to individuals with neurological injuries, we propose that it is the right PFC that plays a protracted role in accommodating novel task demands. This would be observed as increased resource allocation relative to healthy individuals, with gradual reductions in activity as familiarity with the task increases. In the current data, the right DLPFC, right ACC, and parietal regions in TBI show responsiveness to repeated exposures to cognitive tasks that approximate the changes observed in healthy individuals. Furthermore, reduced BOLD activation is observed in response to practice in the right DLPFC and ACC, despite relatively modest or non‐meaningful gains in performance, which may suggest that improved neural efficiency does not necessarily imply overtly improved behavioral efficiency. However, while the current findings do not directly dissociate a performance/learning contribution of the right DLPFC, this within‐subjects analysis demonstrates that higher BOLD signal does not facilitate better performance. It is possible that the PFC recruitment observed in previous WM studies examining a number of neurological disorders (for summary of these studies, see Hillary, 2008) would demonstrate similar responses to practice as those observed here. Thus, we suggest that support for the latent support hypothesis may not be isolated to TBI, but may also generalize to most conditions that result in acquired neurological challenge, such as multiple sclerosis, substance abuse, and HIV. Further research might conduct similar manipulations to examine this position.
Study Limitations
The current study has several limitations. Given the incredible time required to conduct this study (total MRI scanner and task practice time was greater than five hours), a block design was used. However, the block design used here convolves the BOLD response of any given trial with those of other trials, preventing the ability to discriminate specific BOLD responses from one another and reducing the power to detect detailed BOLD‐behavior relationships. Also, the nature of the n‐back task may prevent large behavioral changes in response to practice and result in insufficient behavioral variance to detect relationships to BOLD changes. While the current analyses suggest that the right PFC operates in the conjunction of a broader network during working memory and task acquisition, the current methods preclude detection of the relationships between task‐related regions and how these relationships may moderate performance. Additionally, no baseline performance or fMRI data were available for the TBI sample due to the difficulty in recruiting a priori cases likely to suffer injury and retaining such cases for later analyses. Thus, additional research may further elaborate the current findings. While within subjects analyses find support for latent support interpretations of BOLD recruitment, analyses of BOLD activity before and after injury during WM tasks could provide another critical test of the latent support hypothesis (that is, that performance decreases following injury at the same time that right PFC activity increases). Tasks that use event‐related designs or demonstrate more pronounced behavioral findings following practice may reveal more direct relationships between changes in neural recruitment and changes in performance and examine the extent to which neural efficiency may be linked to improvements or detriments (see Hillary et al., 2010 for one example). Functional connectivity analyses may reveal how the relationships between regions predict improvements in performance.
While the age of the control sample was nearly statistically significantly different between samples, the primary effects of interest (i.e., practice and the “latent support” provided by DLPFC and ACC) are investigated as within‐subject factors and theoretically are independent of age, except for early in life or in the case of normal senescence. Thus, the age ranges for both samples satisfy the age window during which these mechanisms are presumed to operate as hypothesized, and the effect is consistent despite any sample differences in age.
One relatively unexplored area of investigation in TBI is the influence of focal pathology on the BOLD signal, and this may have consequences for interpreting fMRI data (Hillary and Biswal, 2007). In the case of the current paper and specific hypotheses, we hoped to mitigate the influences of heterogeneity by focusing the analysis on a within‐subjects approach to testing the hypotheses. That is, if the BOLD signal is affected by injury (e.g., due to neural‐vascular decoupling), we anticipate that in any one subject, the influence of pathophysiology on the BOLD signal should be constant over the course of the experiment and therefore cannot change the BOLD signal in the hypothesized direction. The heterogeneity of the current sample and the observed findings elaborate on a general effect observed in the literature that the right DLPFC and ACC are often recruited despite vast heterogeneity in injury, including diffuse and focal pathology (Hillary, 2008; Hillary et al., 2006). Additionally, one previous study demonstrated no relationship between focal pathology and the BOLD signal either in the whole brain or in prefrontal areas (Scheibel et al., 2007). In sum, it appears that we are observing gross adaptive effects in neural systems in response to “neural disruption,” broadly defined.
A final consideration is the potential effect of fatigue on the current findings. Fatigue is common in TBI (Ziino and Ponsford, 2006); however, the relationship between fatigue and cognitive impairment and performance in TBI remains unclear, and possibly weak (Borgaro et al., 2005). However, the influence of fatigue on the current study is likely low for several reasons. All subjects were screened for accuracy and must have performed at 90% accuracy during at least one run of performance while in the scanner to ensure attention to and understanding of the task. Inattentiveness and fatigue are not implied by the current behavioral data, as neither accuracy nor reaction times decline following practice (i.e., over time) in either group. A recent fMRI study of fatigue in TBI defined fatigue during a cognitive task as a regional increase in BOLD activity (Kohl et al., 2009), which is not consistent with the current findings. Additionally, since the practice effects on the BOLD signal are virtually equivalent across groups, any fatigue effects not represented in the behavioral findings are constant in both groups and thus do not exclusively explain the effects in TBI. However, the possibility that fatigue has an influence on the BOLD signal during prolonged cognitive processing is an important topic, and may have a role in the current findings.
CONCLUSION
This is the first study to demonstrate responsiveness of regions recruited after neurological compromise to cognitive task practice. Our findings indicate that the BOLD signal in WM networks largely diminishes with repeated task exposure. Specifically, right DLPFC recruitment was particularly evident in the presence of impaired performance during the 1‐back in individuals with TBI. Finally, the right DLPFC responds similarly to practice in individuals with TBI and in HCs. Taken together, these findings suggest that the right DLPFC likely serves an identical role in neurological samples and controls, and recruitment in neurological samples represents the increased demand for attentional and cognitive control mechanisms to respond to increased task demands that result from damage to cortical resources. These findings do not support a functional reorganization or compensation explanation of right DLPFC recruitment, and instead support a latent support hypothesis of the function of the right DLPFC in the context of cerebral challenge.
Supporting information
Additional Supporting Information may be found in the online version of this article
Supporting Information Table I
REFERENCES
- Alexander JE, Porjesz B, Bauer LO, Kuperman S, Morzorati S, O'Connor SJ, Rohrbaugh J, Begleiter H, Polich J ( 1995): P300 hemispheric asymmetries from a visual oddball task. Psychophysiology 32: 467–475. [DOI] [PubMed] [Google Scholar]
- Army Individual Test Battery ( 1990): Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General's Office. [Google Scholar]
- Ashburner J, Neelin P, Collins DL, Evans A, Friston K ( 1997): Incorporating prior knowledge into image registration. Neuroimage 6: 344–352. [DOI] [PubMed] [Google Scholar]
- Baddeley A ( 1992): Working memory: The interface between memory and cognition. J Cogn Neurosci 4: 281–288. [DOI] [PubMed] [Google Scholar]
- Borgaro SR, Baker J, Wethe JV, Prigatano GP, Kwasnica C ( 2005): Subjective reports of fatigue during early recovery from traumatic brain injury. J Head Trauma Rehabil 20: 416–425. [DOI] [PubMed] [Google Scholar]
- Bradley VA, Welch JL, Dick DJ ( 1989): Visuospatial working memory in Parkinson's disease. J Neurol Neurosurg Psychiatr 52: 1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC ( 1997): A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5: 49–62. [DOI] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller E, Braun A, Jovicich J, Koch C, Itti L, Ernst T ( 2001): Neural correlates of attention and working memory deficits in HIV patients. Neurology 57: 1001–1007. [DOI] [PubMed] [Google Scholar]
- Christodoulou C, DeLuca J, Ricker JH, Madigan NK, Bly BM, Lange G, Kalnin AJ, Liu WC, Steffener J, Diamond BJ, Ni AC ( 2001): Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. J Neurol Neurosurg Psychiatr 71: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE ( 1997): Temporal dynamics of brain activation during a working memory task. Nature 386: 604–608. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Bechet S, Salmon E ( 1999): Phonological loop and central executive functioning in Alzheimer's disease. Neuropsychologia 37: 905–918. [DOI] [PubMed] [Google Scholar]
- Courtney SM ( 2004): Attention and cognitive control as emergent properties of information representation in working memory. Cogn Affect Behav Neurosci 4: 501–516. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LF, Acar D, Calvo V, Faust R, Chabrerie A, Kennedy B, Holcomb P ( 2000): The central role of the prefrontal cortex in directing attention to novel events. Brain 123: 927–939. [DOI] [PubMed] [Google Scholar]
- DeLuca J, Schultheis MT, Madigan NK, Christodoulou C, Averill A ( 2000): Acquisition versus retrieval deficits in traumatic brain injury: Implications for memory rehabilitation. Arch Phys Med Rehabil 81: 1327–1333. [DOI] [PubMed] [Google Scholar]
- Demaree HA, DeLuca J, Gaudino EA, Diamond BJ ( 1999): Speed of information processing as a key deficit in multiple sclerosis: Implications for rehabilitation. J Neurol Neurosurg Psychiatr 67: 661–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Poline JB, Frith CD, Heather JD, Frackowiak RSJ ( 1995): Spatial realignment and normalisation of images. Hum Brain Mapp 3: 165–189. [Google Scholar]
- Garavan H, Kelley D, Rosen A, Rao SM, Stein EA ( 2000): Practice‐related functional activation changes in a working memory task. Microsc Res Tech 51: 54–63. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS ( 2000): Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain 123: 1293–1326. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic PS ( 1995): Cellular basis of working memory. Neuron 14: 477–485. [DOI] [PubMed] [Google Scholar]
- Hillary FG ( 2008): Neuroimaging of working memory dysfunction and the dilemma with brain reorganization hypothesis. J Int Neuropsychol Soc 14: 526–534. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Biswal B ( 2007): The influence of neuropathology on the fMRI signal: A measurement of brain or vein? Clin Neuropsychol 21: 58–72. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Genova HM, Chiaravalloti, ND , Rypma B, DeLuca J ( 2006): Prefrontal modulation of working memory performance in brain injury and disease. Hum Brain Mapp 27: 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary FG, Genova HM, Medaglia JD, Fitzpatrick, NM , Chiou KS, Wardecker BM, Franklin RG, Wang J, DeLuca J ( 2010): The nature of processing speed deficits in traumatic brain injury: Is less brain more? Brain Imaging Behav 4: 141–154. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Seewer R, Nirkko AC, Eckstein D, Schroth G, Groner R, Gutbrod K ( 2003): Does excessive memory load attenuate activation in the prefrontal cortex? Load‐dependent processing in single and dual tasks: Functional magnetic resonance imaging study. Neuroimage 19: 210–225. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, Slagter HA, Kahn RS ( 2001): Functional anatomical correlates of controlled and automatic processing. J Cogn Neurosci 13: 730–743. [DOI] [PubMed] [Google Scholar]
- Kelly AMC, Garavan H ( 2005): Human functional neuroimaging of brain changes associated with practice. Cereb Cortex 15: 1089–1102. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS ( 2004): Anterior cingulate conflict monitoring and adjustments in control. Science 303: 1023–1026. [DOI] [PubMed] [Google Scholar]
- Kirchner WK ( 1958): Age differences in short‐term retention of rapidly changing information. J Exp Psychol 55: 352–358. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D ( 1998): Anatomic bases of event‐related potentials and their relationship to novelty detection in humans. J Clin Neurophysiol 15: 3–13. [DOI] [PubMed] [Google Scholar]
- Kohl AD, Wylie GR, Genova HM, Hillary FG, Deluca J ( 2009). The neural correlates of cognitive fatigue in traumatic brain injury using functional MRI. Brain Inj 23: 420–432. [DOI] [PubMed] [Google Scholar]
- Landau SM, Schumacher EH, Garavan H, Druzgal TJ, D'Esposito M ( 2004): A functional MRI study of the influence of practice on component processes of working memory. Neuroimage 22: 211–221. [DOI] [PubMed] [Google Scholar]
- Levin HS ( 2003): Neuroplasticity following non‐penetrating traumatic brain injury. Brain Inj 17: 665–674. [DOI] [PubMed] [Google Scholar]
- Maruishi M, Miyatani M, Nakao T, Muranaka H ( 2007): Compensatory cortical activation during performance of an attention task by patients with diffuse axonal injury: A functional magnetic resonance imaging study. J Neurol Neurosurg Psychiatry 78: 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, Mamourian MD, Weaver JB ( 1999): Brain activation during working memory 1 month after mild traumatic brain injury: A functional MRI study. Neurology 53: 1300–1308. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ ( 2001): Differential working memory load effects after mild traumatic brain injury. Neuroimage 14: 1004–1012. [DOI] [PubMed] [Google Scholar]
- McDowell S, Whyte J, D'Esposito M ( 1997): Working memory impairments in traumatic brain injury: Evidence from a dual‐task paradigm. Neuropsychologia 35: 1341–1353. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, Cohen NJ ( 2003): Practice‐related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage 18: 483–493. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD ( 2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Morris RG, Baddeley AD ( 1988): Primary and working memory functioning in Alzheimer‐type dementia. J Clin Exp Neuropsychol 10: 279–296. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JG, Abrams MT, Goldberg MC, Flower AA, Boyce A, Courtney SM, Calhoun VD, Kraut MA, Denckla MB, Pekar JJ ( 2003): fMRI evidence that the neural basis of response inhibition is task‐dependent. Brain Res Cogn Brain Res 17: 419–430. [DOI] [PubMed] [Google Scholar]
- Newsome MR, Scheibel RS, Steinberg JL, Troyanskaya, Sharma RG, Rauch RA, Li X, Levin HS ( 2007): Working memory brain activation following severe traumatic brain injury. Cortex 43: 95–111. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E ( 2005): N‐Back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Hum Brain Mapp 25: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME ( 1991): Localization of a human system for sustained attention by positron emission tomography. Nature 349: 61–64. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Cole MA, Demery JA, Seignourel PJ, Dixit NK, Larson MJ, Briggs RW ( 2004): Parametric manipulation of working memory load in traumatic brain injury: Behavioral and neural correlates. J Int Neuropsychol Soc 10: 724–741. [DOI] [PubMed] [Google Scholar]
- Price CJ, Crinion J, Friston KJ ( 2006): Design and analysis of fMRI studies with neurologically impaired patients. J Magn Reson Imaging 23: 816–826. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, Jansma JM, Jager G, Van Raalten T, Kahn RS ( 2004): Neurophysiological factors in human information processing capacity. Brain 127: 517–525. [DOI] [PubMed] [Google Scholar]
- Rao SM, Leo GJ, St Aubin‐Faubert P ( 1989a) On the nature of memory disturbance in multiple sclerosis. J Clin Exp Neuropsychol 11: 699–712. [DOI] [PubMed] [Google Scholar]
- Rao SM, St Aubin‐Faubert P, Leo GJ ( 1989b) Information processing speed in patients with multiple sclerosis. J Clin Exp Neuropsychol 11: 471–477. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D ( 1985): The halstead‐reitan neuropsychological test battery: Therapy and clinical interpretation. Tucson, AZ: Neuropsychological Press. [Google Scholar]
- Ricker JH, Hillary FG, DeLuca J ( 2001): Functionally activated brain imaging (O‐15 PET and fMRI) in the study of learning and memory after traumatic brain injury. J Head Trauma Rehabil 16: 191–205. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M ( 1999): The roles of prefrontal brain regions in components of working memory: Effects of memory load and individual differences. Proc Natl Acad Sci USA 96: 6558–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal B, D'Esposito M ( 2006): Neural correlates of cognitive efficiency. Neuroimage 33: 969–979. [DOI] [PubMed] [Google Scholar]
- Salthouse TA ( 1992): Working‐memory mediation of adult age differences in integrative reasoning. Mem Cognit 20: 413–423. [DOI] [PubMed] [Google Scholar]
- Salthouse TA ( 1996): The processing‐speed theory of adult age differences in cognition. Psychol Rev 03: 403–428. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Coon VE ( 1993): Influence of task‐specific processing speed on age differences in memory. J Gerontol 48: 245–255. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Carrion R, Fernandez‐Espejo D, Junque C, Falcon C, Bargallo N, Roig T, Bernabeu M, Tormos JM, Vendrell P ( 2008a): A longitudinal fMRI study of working memory in severe TBI patients with diffuse axonal injury. Neuroimage 43: 421–429. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Carrion R, Vendrell P, Junque C, Fernandez‐Espejo D, Falcon C, Bargallo N, Roig‐Rovira T, Ensenat‐Cantallops A, Bernabeu M ( 2008b): Frontal hypoactivation on functional magnetic resonance imaging in working memory after severe diffuse traumatic brain injury. J Neurotrauma 25: 479–494. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P ( 1991): Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry 48: 618–624. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC ( 1994): Neuropsychological deficits in neuroleptic naive patients with first‐episode schizophrenia. Arch Gen Psychiatry 51: 124–131. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Steinberg JL, Pearson DA, Rauch RA, Mao H, Troyanskaya M, Sharma RG, Levin HS ( 2007): Altered brain activation during cognitive control in patients with moderate to severe traumatic brain injury. Neurorehabil Neural Repair 21: 36–45. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Troyanskaya M, Steinberg JL, Goldstein FC, Mao H, Levin HS ( 2009): Effects of severity of traumatic brain injury and brain reserve on cognitive‐control related brain activation. J Neurotrauma 26: 1447–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck O, Ernst T, Braun J, Koch C, Miller E, Chang L ( 2000): Gender differences in the functional organization of the brain for working memory. Neuroreport 11: 2581–2585. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Hugenholtz H, Richard MT, LaRochelle S, Poirier CA, Bell IBA ( 1985): Subtle neuropsychological deficits in patients with good recovery after closed head injury. Neurosurgery 17: 41–47. [DOI] [PubMed] [Google Scholar]
- Strangmann GE, Goldstein R, O'Neil‐Pirozzi TM, Kelkar K, Supelana C, Burke D, Katz DI, Rauch SL, Savage CR, Glenn MB ( 2009): Neurophysiological Alterations During Strategy‐Based Verbal Learning in Traumatic Brain Injury. Neurorehab Neural Re 23: 226–236. [DOI] [PubMed] [Google Scholar]
- Sweet LH, Rao SM, Primeau M, Durgerian S, Cohen RA ( 2006): Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Hum Brain Mapp 27: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale G, Jennett B ( 1974): Assessment of coma and impaired consciousness. A practical scale. Lancet 13: 81–84. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE ( 1999): Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil 14: 602–615. [DOI] [PubMed] [Google Scholar]
- Turner GR, Levine B ( 2008): Augmented neural activity during executive control processing following diffuse axonal injury. Neurology 71: 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H ( 2009): Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci 4: 274–290. [DOI] [PubMed] [Google Scholar]
- Wechsler D ( 1997): Wechsler Adult Intelligence Scale‐III. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG ( 2006): The neural bases of momentary lapses in attention. Nat Neurosci 9: 971–978. [DOI] [PubMed] [Google Scholar]
- Ziino C, Ponsford J ( 2006): Selective attention deficits and subjective fatigue following traumatic brain injury. Neuropsychology 20: 383–390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article
Supporting Information Table I


