Abstract
Motor symptoms of Parkinson's disease (PD) can be relieved by deep brain stimulation (DBS). The mechanism of action of DBS is largely unclear. Magnetoencephalography (MEG) studies on DBS patients have been unfeasible because of strong magnetic artifacts. An artifact suppression method known as spatiotemporal signal space separation (tSSS) has mainly overcome these difficulties. We wanted to clarify whether tSSS enables noninvasive measurement of the modulation of cortical activity caused by DBS. We have studied auditory and somatosensory‐evoked fields (AEFs and SEFs) of advanced PD patients with bilateral subthalamic nucleus (STN) DBS using MEG. AEFs were elicited by 1‐kHz tones and SEFs by electrical pulses to the median nerve with DBS on and off. Data could be successfully acquired and analyzed from 12 out of 16 measured patients. The motor symptoms were significantly relieved by DBS, which clearly enhanced the ipsilateral auditory N100m responses in the right hemisphere. Contralateral N100m responses and somatosensory P60m responses also had a tendency to increase when bilateral DBS was on. MEG with tSSS offers a novel and powerful tool to investigate DBS modulation of the evoked cortical activity in PD with high temporal and spatial resolution. The results suggest that STN‐DBS modulates auditory processing in advanced PD. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: magnetoencephalography, Parkinson's disease, deep brain stimulation, evoked potentials, auditory, evoked potentials, somatosensory, artifacts
INTRODUCTION
Parkinson's disease (PD) is a progressive extrapyramidal movement disorder with cardinal symptoms of rigidity, resting tremor, and hypokinesia. In PD, there is a deficiency of striatal dopamine caused by idiopathic degeneration of the dopaminergic neurons arising from the substantia nigra pars compacta. The prevalence of PD is estimated to be 9.5/1,000 for persons over 65 years of age [Hirtz et al.,2007].
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is known to be an effective treatment of disabling PD [Deuschl et al., German Parkinson Study Group, Neurostimulation Section,2006; Krack et al.,2003]. The mechanism of action of DBS is ambiguous. Currently, there is no consensus on whether DBS elicits inhibition or excitation of the target nuclei or whether the effect is local or system‐wide [Liu et al.,2008; McIntyre et al.,2004; Montgomery and Gale,2008]. The effects of DBS on human brain function are difficult to study. Positron‐emission tomography (PET) and single photon emission computed tomography (SPECT) reveal changes in blood flow or metabolic responses as indirect measures of neuronal activity [Perlmutter and Mink,2006] and their temporal resolution is therefore not high enough to reveal pathological oscillatory brain activity attributed to the pathophysiology of PD. Radiation exposure prevents frequent PET and SPECT scans. The deep brain stimulator, as an electrical device, precludes functional magnetic resonance imaging (fMRI), and transcranial magnetic stimulation (TMS) may be hazardous. DBS causes strong artifacts in electroencephalography (EEG) or magnetoencephalography (MEG) recordings.
In rodent models of PD, it is feasible to study the outcome of selective STN inhibition or excitation by employing optogenetics and solid‐state optics. No DBS‐like therapeutic action was obtained by optical inhibition targeted to excitatory glutamatergic STN neurons, local astroglia that inhibit neuronal firing in the STN, or by activating excitatory neurons in the STN. Instead, a selective high‐frequency optical stimulation in layer V of the primary motor cortex ameliorated PD symptoms in the manner of DBS [Gradinaru et al.,2009]. Moreover, epidural electrical stimulation of the dorsal columns in the spinal cord restored locomotion in Parkinsonian mice and rats. During dorsal column stimulation the spectral power of local field potentials in the primary motor cortex and in the striatum shifted from lower to higher frequencies [Fuentes et al.,2009]. Both experiments highlight the importance of cortical activity modifications by DBS in PD [Miller,2009].
DBS produces strong electromagnetic artifacts obscuring neural activity in MEG and EEG recordings. The recently introduced spatiotemporal signal space separation (tSSS) method [Taulu and Simola,2006] is effective in suppressing interference originating from distant or nearby sources with respect to the sensors as shown, for instance, by purposefully eliciting magnetic artifacts in healthy subjects [Taulu and Hari,2009], and it has been used to remove magnetic artifacts caused by DBS [Mäkelä et al.,2007; Park et al.,2009] and by vagus nerve stimulation in patients with epilepsy [Tanaka et al.,2009]. In the tSSS algorithm, the measured signals are first divided into two spatial parts by the signal space separation (SSS) method [Taulu and Kajola,2005]: one arising mainly from inside of the sensor helmet and the other mainly from outside, that is, about 50 cm or more from the sensor array. The problem of residual signals from sources not included in these two categories is addressed by the second step of the tSSS algorithm, in which the temporally correlated signal components between the inside and outside or residual parts of the signal are recognized and removed from the data [Taulu and Simola,2006]. As a result, the nearby artifacts not fully modeled by the basic SSS are compensated for by tSSS. Furthermore, signals from any magnetized artifact sources inside the brain that spatially resemble true brain sources, such as the deep brain stimulator, are typically also suppressed by tSSS. This is because such a device contains magnetized material, e.g., wires and a battery outside of the head but close to the sensors. Because of body motion, the magnetic parts cause artifacts that are removed by tSSS. The artifact signals originating from the device inside of the brain are mainly temporally correlated with the artifacts from the rest of the stimulator‐related instrumentation and thus removed by tSSS. This approach may enable noninvasive measurement of cortical activity modulations generated by DBS with high temporal and spatial resolution also in humans.
Existing MEG results suggest that there are changes in the cortical processing of auditory information in PD [Pekkonen et al.,1998], whereas SEFs appear to be normal [Mäkelä et al.,1993].
Our main focus was to study whether cortical activity could be measured from a group of advanced PD patients with DBS, using MEG and the novel tSSS method. In this study, we probed cortical activity by auditory and somatosensory stimuli, which produce well‐characterized evoked fields [Hari and Forss,1999; Mäkelä and Hari,1990]. The present findings indicate clearly that brain activity can be reliably measured from DBS patients and that cortical processing is modulated by DBS in patients with advanced PD.
MATERIALS AND METHODS
Sixteen advanced PD patients with bilateral STN‐DBS originally participated in the study. The study was approved by the Ethics Committee of Helsinki University Central Hospital and all patients gave informed written consent.
The data of four patients were rejected. In the first patient, dystonic movements when DBS was off produced such strong artifacts that MEG sensors were saturated, and tSSS filtering was unable to recover the brain signals. In the second patient, the head position could not be detected accurately enough to model the responses because of the disturbance that DBS caused to the head localization system. The third patient did not tolerate the DBS off condition even though the antiparkinsonian medication was continued during the measurements. In the fourth patient, DBS voltages were only 0.3 and 0.9 V. Her data were excluded from the analysis as DBS was ineffective.
The mean age of the remaining 12 patients (six females) was 62 years (49–75 years). They had received a diagnosis of Parkinson's disease on the average 13 years (range, 7–21 years) before the implantation of the bilateral STN DBS (Kinetra®, Medtronic, Minneapolis, MN). The MEG measurements were done 0.5 to 25 months (mean, 12 months) after the implantation. One patient had undergone thalamotomy of the right hemisphere 14 years before the DBS implantation. All patients used their normal antiparkinsonian medication during the measurements. The mean Hoehn and Yahr scores [Hoehn and Yahr,1967] were 2.5 (range, 2–4) when both medication and DBS on. The mean DBS voltage was 2.5 V (range, 2–3.9 on the right and 1.8–3.4 on the left side). The pulse width of the stimulation was 60 μs in 10 and 90 μs in 2 patients. The DBS frequency was adjusted to 130 Hz before MEG measurements to avoid interference with the head position indicator (HPI) coil signals. Bipolar stimulation was applied bilaterally in six patients, and monopolar bilaterally in one patient. Five patients had bipolar and monopolar stimulation on different hemispheres. The patients did not have clinical signs of dementia or depression. The average Mini‐Mental State Examination (MMSE) [Folstein et al.,1975] score of 10 patients was 28/30. The 30‐item Geriatric Depression Scale (GDS) [Yesavage et al.,1983] was performed in nine patients with the average GDS score 9/30 (SD 4.2, highest score 14). None of the patients had scores above the cutoff level recommended for the diagnosis of depression in Parkinsonian patients [Ertan et al.,2005] (Table I).
Table I.
Patient characteristics
| Patient | Sex | Age | Disease duration before operation (yr) | Time since STN stimulator implanted (mo) | UPDRS | MMSE | GDS | Hoehn and Yahr [1967] | LEDD (mg) | |
|---|---|---|---|---|---|---|---|---|---|---|
| DBS on | DBS off | |||||||||
| 1 | M | 59 | 14 | 25 | 23 | 31 | — | — | 2.5 | 2,160 |
| 2 | M | 49 | 9 | 25 | 15 | 25 | 30 | 13 | 2 | 1,555 |
| 3 | M | 68 | 21 | 17 | 30 | — | 26 | — | 4 | 1,645 |
| 4 | F | 67 | 11 | 2 | 12 | 14 | — | — | 2 | 1,115 |
| 5 | F | 55 | 19 | 1.5 | 22 | 60 | 30 | 14 | 2.5 | 1,170 |
| 6 | M | 58 | 12 | 24 | 33 | 38 | 27 | 12 | 2.5 | 1,200 |
| 7 | M | 59 | 11 | 24 | 17 | 20 | 29 | 1 | 2 | 1,290 |
| 8 | F | 65 | 10 | 1 | 16 | 23 | 30 | 6 | 2 | 1,060 |
| 9 | F | 68 | 14 | 0.6 | 44 | — | 30 | 7 | 3 | 480 |
| 10 | M | 60 | 16 | 0.5 | 34 | 46 | 24 | 11 | 2.5 | 1,500 |
| 11 | F | 75 | 13 | 1.6 | 50 | 50 | 27 | 6 | 4 | 510 |
| 12 | F | 60 | 7 | 25 | 39 | 49 | 29 | 9 | 3 | 620 |
To calculate the levodopa equivalent daily dose (LEDD), the following formula was used: 100 mg l‐dopa = 130 mg controlled‐release l‐dopa = 70 mg l ‐dopa + COMT inhibitor = 1 mg pramipexole = 5 mg ropinirole [Mamikonyan et al.,2008] = 4 mg rotigotine [Poewe et al.,2007].
DBS, deep brain stimulation; F, female; GDS, Geriatric Depression Scale; M, male; MMSE, Mini‐Mental State Examination; STN, subthalamic nucleus; UPDRS, Unified Parkinson's Rating Scale.
The measurements were performed with the 306‐channel Elekta Neuromag® MEG device (Elekta Oy, Helsinki, Finland) when the DBS was both on and off in a magnetically shielded room (Euroshield, Eura, Finland). A nurse remained with the patient in the shielded room to control the alertness of the patient. AEFs were elicited by 1‐kHz sinusoidal 50‐ms tone pips delivered to each ear separately through plastic tubes. Stimulus intensity was about 60 dB above the environmental noise, and we confirmed that the patients heard the tone pips clearly. SEFs were elicited by electrical 200‐μs square‐wave pulses, delivered to the median nerve at both wrists independently with an intensity producing a visible thumb twitch. Visual checkerboard stimuli were also presented to the patients: the visual‐evoked fields will be reported elsewhere. The time between sequential stimuli was 600 ms and different stimulus types were presented in random order. The minimum ISI was 0.6 seconds and the mean ISI 5.5 seconds for each stimulus type. The 600‐ms analysis period for evoked responses included a 100‐ms prestimulus baseline. About 100 averages of each stimulus type were collected. The recording passband was 0.03 to 330 Hz with a sampling rate of 1011 Hz. A vertical electro‐oculogram (EOG) was recorded simultaneously. The exact location of the head relative to the sensors was determined by indicator coils placed on the scalp. For alignment of the MEG and MRI coordinate system, the location of the coils with respect to head landmarks was determined with a 3D digitizer (Fastrak®, Polhemus, Colchester, VT).
The strong magnetic artifacts in the raw data caused by DBS were suppressed by the spatiotemporal signal space separation (tSSS) method [Taulu and Simola,2006] with an 8‐second time window and a subspace correlation limit of 0.9 [Medvedovsky et al.,2009] before data analysis. Subsequently, the responses were averaged and filtered with a 1 to 40 Hz passband for AEFs and 0.5 to 100 Hz for SEFs.
A single equivalent current dipole (ECD) with a spherical head model was used for the source analysis. MRI images were available for 10 patients; a sphere was matched to the inner surface of the skull in the area of interest. For the two patients without MRI, the x‐, y‐, and z‐coordinates of 0, 0, 40 mm were used for sphere origin, where the xy‐plane of the coordinate system is defined by the nasion and two preauricular points, and the z‐axis points up.
The sources of N100m auditory‐evoked responses were searched from contra‐ and ipsilateral hemispheres of each patient using a subset of 11 to 15 gradiometer pairs around the locus of the maximum response. N100m sources were estimated by sequential ECD fitting with a 1‐ms interval in the time period from 80 to 130 ms after the stimulus onset, with DBS both on and off.
A single dipole model was used to investigate the effect of DBS. Using the same model in each data set minimizes variation due to difference between source models; we postulated that the locations of cortical representations would not be changed by DBS. The ECD corresponding to the strongest dipole moment was chosen to represent the source when the three following requirements were fulfilled: (1) The dipole location must be stable during 10 ms around the maximum of chosen ECD: the variation of x‐, y‐, and z‐coordinates was required to be less than 5 mm in each direction. (2) The dipole explained more than 80% of the variance of the measured data (goodness of fit; g) among the selected channels. (3) The maximum of the ECD amplitude occurred within the time period defined previously for N100m. If two or more dipoles with same strengths fulfilled the criteria, the one with the best g value was chosen. The best dipole coordinates for auditory cortex source for each patient ipsi‐ and contralaterally for right and left ear stimulus were selected and used to calculate N100m source strengths with DBS on and off. The x‐coordinates varied between 38 and 67 mm (negative values for left hemisphere), the y‐coordinates between −7 and 21 mm in the right and −19 and 19 mm in the left hemisphere, and the z‐coordinates between 35 and 61 mm.
SEF sources were estimated by sequential ECD fitting with a 1‐ms interval and by searching for peaks of source amplitudes during the time period from 15 to 80 ms after the stimulus. SEFs were identified over the contralateral primary somatosensory cortex (SI) by selecting 12 to 14 gradiometer pairs around the locus of the maximum response. From the determined ECDs (with DBS on and off), the one best representing SI activity was chosen for each patient based on the g value and location. The x‐coordinates of these dipoles were between 26 and 45 mm (negative values for the left hemisphere), the y‐coordinates between −10 and 21 mm and the z‐coordinates between 76 and 95 mm. A single dipole model for SI activity of each patient and each hemisphere was constructed and the source strengths and latencies of N20m and P60m were calculated from dipole strength versus time curves with DBS on and off.
Comparisons between DBS on and off states were performed using the paired t test in SPSS (SPSS for Windows versions 13.0 or 17.0, SPSS, Chicago, IL). The results are reported as means ± standard deviation.
RESULTS
Motor symptoms were effectively relieved by DBS when on medication. Mean motor Unified Parkinson's Disease Rating Scale (UPDRS) scores were 28 ± 12 when DBS was on (n = 12) and 36 ± 15 when off (n = 10) (P < 0.05).
After artifact removal by tSSS, sources of evoked fields were analyzable in both hemispheres of all 12 patients. The effect of tSSS on the signal quality was already clear in the spontaneous MEG activity before and after tSSS (Fig. 1).
Figure 1.
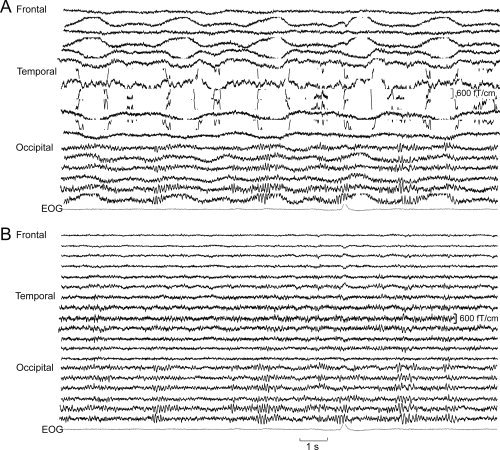
Spontaneous MEG activity over the left hemisphere of one patient with DBS on before (A) and after (B) applying tSSS. The DBS device and connecting wires are on the left side. Artifacts were strongest over the wires on the left temporal region.
tSSS effectively removed artifacts generated by DBS from auditory‐evoked fields in individual patients (Fig. 2). In both conditions, N100m responses to ipsi‐ and contralateral stimulation were found in the right hemisphere in 10 patients with g values over 75%. In the left hemisphere, ipsilateral responses were seen from seven and contralateral responses from eight patients. DBS significantly enhanced the N100m to ipsilateral stimulation in the right hemisphere (49 ± 17 nAm DBS on, 43 ± 16 nAm DBS off: P < 0.05). The mean source strengths of contralateral N100m increased nonsignificantly when the stimulator was on (Table II). Response latencies were not affected by DBS (Table II). Variation in the size of the effect was considerable, even between hemispheres of the same individual.
Figure 2.
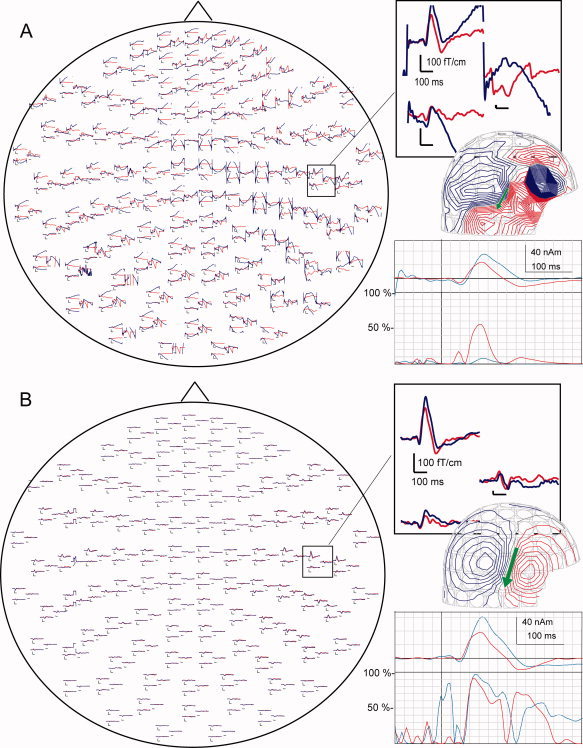
Auditory‐evoked fields (AEFs) to right‐ear stimuli in one patient before (A) and after (B) applying tSSS. The responses are viewed from above, with the nose pointing upwards. AEFs in the squares are shown in enlarged form in the inserts. DBS (on blue line, off red line) enhanced ipsilateral N100m. In magnetic field patterns, red lines indicate flux out and blue lines into the head. The contour step is 50 fT. The arrow indicates the equivalent current dipole, estimated from the corresponding field pattern. The corresponding dipole strength versus time curve and the goodness‐of‐fit of the model (g values) are shown under the magnetic field pattern.
Table II.
Source strengths and latencies of N100m (mean ± SD)
| Ear | DBS | Contralateral hemisphere | Ipsilateral hemisphere | ||
|---|---|---|---|---|---|
| Source strengths (nAm) | Latencies (ms) | Source strengths (nAm) | Latencies (ms) | ||
| Right | On | 47 ± 22 | 99 ± 10 | 49 ± 17* | 101 ± 15 |
| Off | 44 ± 19 | 98 ± 10 | 43 ± 16 | 100 ± 16 | |
| Left | On | 61 ± 26 | 96 ± 15 | 35 ± 20 | 110 ± 16 |
| Off | 58 ± 22 | 95 ± 9 | 33 ± 19 | 108 ± 13 | |
P < 0.05.
Somatosensory responses after tSSS when DBS was on and off could also be reliably scrutinized in individual patients (Fig. 3). SEF N20m responses to contralateral stimuli with DBS on and off were found from three and nine patients in the left and right hemisphere, respectively. P60m responses were detected from 10 left and 11 right hemispheres (Fig. 4). In the right hemisphere, the N20m source strength was nonsignificantly increased when DBS was on (27 ± 12 nAm vs. 24 ± 10 nAm). P60m source strengths were nonsignificantly stronger when the stimulator was on (left hemisphere 59 ± 21 nAm DBS on vs. 51 ± 21 nAm DBS off; right hemisphere 62 ± 18 nAm DBS on vs. 60 ± 24 nAm DBS off). Latencies had no significant difference between conditions (P60m in right hemisphere 58 ± 13 ms DBS on and 57 ± 14 ms DBS off and in left hemisphere 59 ± 9 ms DBS on and 61 ± 9 ms DBS off). Variation in the size of the effect was considerable even between hemispheres of the same individual.
Figure 3.
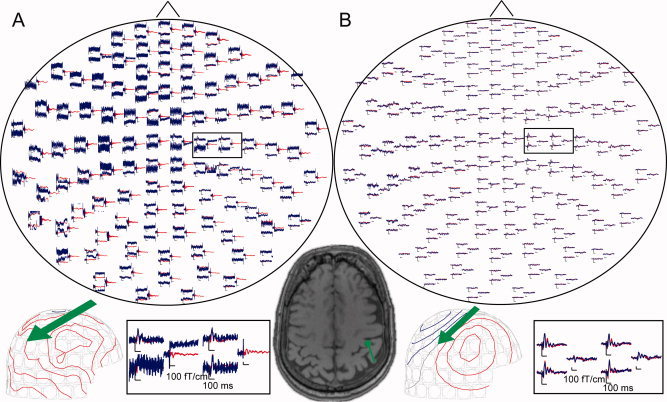
Somatosensory‐evoked fields (SEFs) to electrical pulses to left median nerve in one patient before (A) and after (B) applying tSSS. Blue lines indicate DBS on and red lines DBS off. The contour step is 100 fT. The arrows indicate the equivalent current dipoles, estimated from the corresponding field patterns. Source location and orientation are superimposed on the brain MRI of the patient. The signal exceeds the scale on some magnetometer channels when DBS is on.
Figure 4.
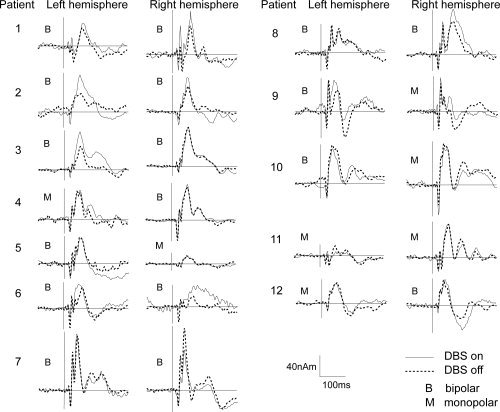
Dipole strengths versus time curves of SEFs from left and right hemispheres in all patients with DBS on and off. B indicates bipolar and M monopolar DBS stimulation. Bipolar stimulation seems to increase late responses in 11 hemispheres out of 17 and monopolar only in 2 cases out of 7.
DISCUSSION
This is the first MEG study of a large number of PD patients with DBS. Our results indicate that tSSS effectively removes strong magnetic artifacts generated by DBS from recorded MEG data in the majority of PD patients. Consequently, cortical activity can now be measured accurately with MEG in DBS patients. MEG with tSSS reveals the impact of STN‐DBS on auditory and somatosensory cortical processing. However, even with the new signal processing methods, the applicability of MEG is still limited to cases where the artifacts do not saturate the MEG sensors. Fortunately, this requirement is in most cases fulfilled and tSSS can be used to recover good quality data.
AEF N100m enhancement was significant on the group level in the right hemisphere for ipsilateral stimulation. Most patients had the strongest DBS artifacts in the left hemisphere, because the DBS battery and wires were installed in the left side. Despite tSSS, some artifacts may have remained in the processed data, increasing the variance of the evoked responses. Ipsilateral auditory pathways from the inner ear through the thalamus to the cortex are smaller than the contralateral ones. Hence the smaller ipsilateral pathways may be more sensitive to STN‐DBS than the more robust contralateral ones. Currently, there are no studies of the effect of DBS on human auditory‐evoked potentials.
Existing somatosensory‐evoked potential (SEP) results have presented both an increase and decrease in early cortical deflections in amplitude by DBS [Insola et al.,2005; Pierantozzi et al.,1999; Priori et al.,2001]. The present results display no significant difference between the DBS on/off conditions at the group level. That is at least partly explained by considerable inter‐ and intraindividual variation. Patients also received their normal antiparkinsonian medication during the MEG measurement to ensure that they could tolerate the entire recording session. The fact that the patients were on medication could partly explain the modest SEF findings.
Although the exact mechanism of DBS remains ambiguous, changes in AEFs and SEFs suggest that DBS modulates thalamocortical pathways, and/or cortical processing. The effect of DBS on late evoked potentials suggests that part of the effect of DBS occurs at the cortical level.
CONCLUSION
tSSS with MEG offers a novel and powerful tool to investigate the DBS modulation of evoked cortical activity in PD with a high temporal and spatial resolution. The present results suggest that STN‐DBS modulates auditory cortical processing in advanced PD. In addition to PD, DBS is considered useful in several other conditions, such as depression, obsessive‐compulsive disorder, Tourette's syndrome, chronic pain, and cluster headache [Kringelbach et al.,2007], and MEG with tSSS may provide an important insight into their pathophysiology.
Acknowledgements
The authors thank Jukka Lyytinen for UPDRS rating of some patients, Suvi Heikkilä and Jari Kainulainen for help in the measurements.
REFERENCES
- Deuschl G, Schade‐Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, Daniels C, Deutschlander A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J; German Parkinson Study Group, Neurostimulation Section ( 2006): A randomized trial of deep‐brain stimulation for Parkinson's disease. N Engl J Med 355: 896–908. [DOI] [PubMed] [Google Scholar]
- Ertan FS, Ertan T, Kzltan G, Uygucgil H ( 2005): Reliability and validity of the Geriatric Depression Scale in depression in Parkinson's disease. J Neurol Neurosurg Psychiatry 76: 1445–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR ( 1975): Mini‐mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Fuentes R, Petersson P, Siesser WB, Caron MG, Nicolelis MA ( 2009): Spinal cord stimulation restores locomotion in animal models of Parkinson's disease. Science 323: 1578–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K ( 2009): Optical deconstruction of parkinsonian neural circuitry. Science 324: 354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Forss N ( 1999): Magnetoencephalography in the study of human somatosensory cortical processing. Philos Trans R Soc Lond B Biol Sci 354: 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirtz D, Thurman DJ, Gwinn‐Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R ( 2007): How common are the “common” neurologic disorders?. Neurology 68: 326–337. [DOI] [PubMed] [Google Scholar]
- Hoehn MMMD, Yahr MDMD ( 1967): Parkinsonism: Onset, progression, and mortality. Neurology 17: 427–442. [DOI] [PubMed] [Google Scholar]
- Insola A, Mazzone P, Valeriani M ( 2005): Somatosensory evoked potential and clinical changes after electrode implant in basal ganglia of parkinsonian patients. Muscle Nerve 32: 791–797. [DOI] [PubMed] [Google Scholar]
- Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P ( 2003): Five‐year follow‐up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 349: 1925–1934. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ ( 2007): Translational principles of deep brain stimulation. Nat Rev Neurosci 8: 623–635. [DOI] [PubMed] [Google Scholar]
- Liu Y, Postupna N, Falkenberg J, Anderson ME ( 2008): High frequency deep brain stimulation: What are the therapeutic mechanisms? Neurosci Biobehav Rev 32: 343–351. [DOI] [PubMed] [Google Scholar]
- Mamikonyan E, Siderowf AD, Duda JE, Potenza MN, Horn S, Stern MB, Weintraub D ( 2008): Long‐term follow‐up of impulse control disorders in Parkinson's disease. Mov Disord 23: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian‐Le Goff L, Vitek JL ( 2004): Uncovering the mechanism(s) of action of deep brain stimulation: Activation, inhibition, or both. Clin Neurophysiol 115: 1239–1248. [DOI] [PubMed] [Google Scholar]
- Medvedovsky M, Taulu S, Bikmullina R, Ahonen A, Paetau R ( 2009): Fine tuning the correlation limit of spatio‐temporal signal space separation for magnetoencephalography. J Neurosci Methods 177: 203–211. [DOI] [PubMed] [Google Scholar]
- Miller G ( 2009): Neuropsychiatry. Rewiring faulty circuits in the brain. Science 323: 1554–1556. [DOI] [PubMed] [Google Scholar]
- Montgomery EB Jr, Gale JT ( 2008): Mechanisms of action of deep brain stimulation(DBS). Neurosci Biobehav Rev 32: 388–407. [DOI] [PubMed] [Google Scholar]
- Mäkelä JP, Hari R ( 1990): Long‐latency auditory evoked magnetic fields. Adv Neurol 54: 177–191. [PubMed] [Google Scholar]
- Mäkelä JP, Hari R, Karhu J, Salmelin R, Teräväinen H ( 1993): Suppression of magnetic mu rhythm during parkinsonian tremor. Brain Res 617: 189–193. [DOI] [PubMed] [Google Scholar]
- Mäkelä JP, Taulu S, Pohjola J, Ahonen A, Pekkonen E ( 2007): Effects of subthalamic nucleus stimulation on spontaneous sensorimotor MEG activity in a Parkinsonian patient. Int Congr Ser 1300: 345–348. [Google Scholar]
- Park H, Kim JS, Paek SH, Jeon BS, Lee JY, Chung CK ( 2009): Cortico‐muscular coherence increases with tremor improvement after deep brain stimulation in Parkinson's disease. Neuroreport 20: 1444–1449. [DOI] [PubMed] [Google Scholar]
- Pekkonen E, Ahveninen J, Virtanen J, Teräväinen H ( 1998): Parkinson's disease selectively impairs preattentive auditory processing: an MEG study. Neuroreport 9: 2949–2952. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW ( 2006): Deep brain stimulation. Annu Rev Neurosci 29: 229–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierantozzi M, Mazzone P, Bassi A, Rossini PM, Peppe A, Altibrandi MG, Stefani A, Bernardi G, Stanzione P ( 1999): The effect of deep brain stimulation on the frontal N30 component of somatosensory evoked potentials in advanced Parkinson's disease patients. Clin Neurophysiol 110: 1700–1707. [DOI] [PubMed] [Google Scholar]
- Poewe WH, Rascol O, Quinn N, Tolosa E, Oertel WH, Martignoni E, Rupp M, Boroojerdi B, SP 515 I ( 2007): Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson's disease: A double‐blind, double‐dummy, randomised controlled trial. Lancet Neurol 6: 513–520. [DOI] [PubMed] [Google Scholar]
- Priori A, Cinnante C, Genitrini S, Pesenti A, Tortora G, Bencini C, Barelli MV, Buonamici V, Carella F, Girotti F, Soliveri P, Magrini F, Morganti A, Albanese A, Broggi S, Scarlato G, Barbieri S ( 2001): Non‐motor effects of deep brain stimulation of the subthalamic nucleus in Parkinson's disease: Preliminary physiological results. Neurol Sci 22: 85–86. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Thiele EA, Madsen JR, Bourgeois BF, Stufflebeam SM ( 2009): Magnetoencephalographic analysis in patients with vagus nerve stimulator. Pediatr Neurol 41: 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Kajola MP ( 2005): Resentation of electromagnetic multichannel data: The signal space separation method. J Appl Phys 97: 124905–124910. [Google Scholar]
- Taulu S, Simola J ( 2006): Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51: 1759–1768. [DOI] [PubMed] [Google Scholar]
- Taulu S, Hari R ( 2009): Removal of magnetoencephalographic artifacts with temporal signal‐space separation: Demonstration with single‐trial auditory‐evoked responses. Hum Brain Mapp 30: 1524–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO ( 1983): Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17: 37–49. [DOI] [PubMed] [Google Scholar]


