Abstract
The aim of this event‐related fMRI study was to investigate the cortical networks involved in case processing, an operation that is crucial to language comprehension yet whose neural underpinnings are not well‐understood. What is the relationship of these networks to those that serve other aspects of syntactic and semantic processing? Participants read Basque sentences that contained case violations, number agreement violations or semantic anomalies, or that were both syntactically and semantically correct. Case violations elicited activity increases, compared to correct control sentences, in a set of parietal regions including the posterior cingulate, the precuneus, and the left and right inferior parietal lobules. Number agreement violations also elicited activity increases in left and right inferior parietal regions, and additional activations in the left and right middle frontal gyrus. Regions‐of‐interest analyses showed that almost all of the clusters that were responsive to case or number agreement violations did not differentiate between these two. In contrast, the left and right anterior inferior frontal gyrus and the dorsomedial prefrontal cortex were only sensitive to semantic violations. Our results suggest that whereas syntactic and semantic anomalies clearly recruit distinct neural circuits, case, and number violations recruit largely overlapping neural circuits and that the distinction between the two rests on the relative contributions of parietal and prefrontal regions, respectively. Furthermore, our results are consistent with recently reported contributions of bilateral parietal and dorsolateral brain regions to syntactic processing, pointing towards potential extensions of current neurocognitive theories of language. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: language comprehension, fMRI, syntax, case processing, Basque
INTRODUCTION
From a noisy and dynamic unfolding linguistic signal, people generally compute meaning relatively effortlessly and effectively. The relative ease with which language is used in everyday life belies the highly complex computational and neural infrastructure of the language faculty. Amongst many other things, language users must apply the particular rules of a language to combine word‐elicited information into multiword representations, such as phrases or sentences. Understanding these processes, and their implementation in the brain, has traditionally been of central importance in neurobiological theories of language [e.g., Bornkessel‐Schlesewsky and Schlesewsky,2009a; Friederici,1998; Hagoort,2009; Hagoort et al.,1999; Petersson et al.,2010, for reviews].
A crucial aspect of sentence comprehension is to distinguish sentential arguments and to interpret their respective thematic roles [e.g., Bornkessel‐Schlesewsky and Schlesewsky,2009a,b; Dowty,1991; Jackendoff,2002]. As a simple demonstration, the rather crucial difference between “the dog bit the man” and “the dog was bitten by the man” lies in who is the agent and who is the patient of the sentence (i.e., who is biting whom). In many languages of the world, in particularly those with relatively free word order, this process is guided by a case system that marks the grammatical functions of arguments, apart from order or structural information [e.g., Fillmore,1968]. Case marking can be related to thematic roles because, for example, sentential subjects and objects are usually (but not necessarily) associated with thematic roles of agent and patient, respectively [see Laka,2006; Primus,2002]. In languages without a case system, thematic roles are more strongly determined by argument prominence [Van Valin,2005], which in turn relies on factors such as word order, animacy and definiteness. According to the distinctness principle [Bornkessel‐Schlesewsky and Schlesewsky,2009b], thematic role identification is facilitated when all arguments in a described event are as distinct as possible from one another in terms of all available dimensions of prominence. Importantly, languages with case marking require that the language system process case‐related morphosyntactic information alongside other types of syntactic and semantic information. The implementation of these processes in the brain, however, is yet unknown.
In this study, we used event‐related functional magnetic resonance imaging (fMRI) to examine the functional neuroanatomical correlates of case processing in Basque (Euskara), the last remaining pre‐Indo‐European language in Western Europe [e.g., Trask,1997], which is spoken predominantly in the Basque Country, located in northeastern Spain and southwestern France. Basque is an ergative–absolutive language that uses absolutive case‐marking for higher arguments of intransitive verbs, often described as subjects, but requires ergative case‐marking for higher arguments of transitive verbs, with absolutive case for transitive lower arguments or objects [see Bossong,1984; De Rijk,2007; Holmer,2001; Laka,1996; Ortiz de Urbina,1989]. In addition, Basque has main and auxiliary verbal agreement: the auxiliary verb that accompanies most main verbs agrees not only with the subject, but also with any direct object and the indirect object present.
In the transitive Basque sentence “Gizonak lehiatilan jaso ditu sarrerak goizeak” (approximate translation: “The man at the box office has received the tickets in the morning”) the singular subject “Gizonak” has ergative case marking, but the plural object “sarrerak” has absolutive case marking. Although different cases that are both marked with ‐ak can in principle lead to ambiguity, this is not the case when, as in this example sentence, the animate “Gizonak” appears first as a readily available subject, and, moreover, when the inflected auxiliary verb “ditu” reinforces the subject being singular while also heralding a plural object (N.B., this latter argument only holds in a Subject‐Verb‐Object sentence). Therefore, when readers encounter the object “sarrerak,” they need to use information from different syntactic constraints, that is, case morphology and number agreement, as well as semantic information, to arrive at the meaning of the sentence.
Our motivation to study the cortical networks for case processing is to gain insights into how the brain accomplishes the structure building and thematic assignment operations that are coextended with case information, and to uncover whether these operations rely on the same brain regions that process other types of syntactic and semantic information. In our experimental design, we therefore compared the processing consequences of a thematic integration problem due to case conflict, with those of a morphosyntactic problem due to a number agreement mismatch, and to those of a semantic problem in which an argument due to semantic constraints cannot bear the thematic role it is assigned [see Kuperberg et al.,2008, for a related study that does not involve case morphology]. We examined the cortical systems that deal with the interpretation problems that arise due to double–ergative case conflict, incorrect verb‐noun object number agreement, and semantic anomaly (see Table I, for example sentences). In ergative languages, the ergative case marking is reserved for sentential agents [e.g., Bossong,1984; Holmer,2001; Laka,1996,2006], and ergative case marking for a patient is considered to be ungrammatical. Similarly, an object noun with a number inflection that does not match the number as heralded by a preceding verb auxiliary is considered ungrammatical [e.g., Arregi,2001]. However, whereas it stands to argue that both errors ultimately require the language system to engage in repair or reanalysis processes during sentence comprehension [e.g., Friederici,2002], they may rely on different types of conceptual representations in doing so (e.g., thematic roles versus quantity information), and therefore be associated with qualitatively different processing consequences, as described below.
Table I.
Example sentences and approximate translation for each condition
| a. Case violation: | |||||
| Gizon‐a‐k | lehiatil‐a‐n | jaso | dit‐u | sarrer‐ek | goiz‐ean |
| Man‐the‐[erg.sg.] | box office‐the‐loc | received | them‐root‐he | ticket‐the‐[erg.pl.] | morning‐loc |
| The man at the box office has received the tickets in the morning | |||||
| b. Number agreement violation: | |||||
| Gizon‐a‐k | lehiatil‐a‐n | jaso | dit‐u | sarrer‐a | goiz‐ean |
| Man‐the‐[erg.sg.] | box office‐the‐loc | received | them‐root‐he | ticket‐the‐[abs.sg.] | morning‐loc |
| The man at the box office has received the ticket in the morning | |||||
| c. Semantic anomaly: | |||||
| Gizon‐a‐k | lehiatil‐a‐n | jaso | dit‐u | begi‐a‐k | goiz‐ean. |
| Man‐the‐[erg.sg.] | box office‐the‐loc | received | them‐root‐he | eye‐the‐[abs.pl.] | morning‐loc |
| The man at the box office has received the eyes in the morning | |||||
| d. Correct control: | |||||
| Gizon‐a‐k | lehiatil‐a‐n | jaso | dit‐u | sarrer‐ak | goiz‐ean |
| Man‐the‐[erg.sg.] | box office‐the‐loc | received | them‐root‐he | ticket‐the‐[abs.pl.] | morning‐loc |
| The man at the box office has received the tickets in the morning | |||||
Critical words are underlined for expository purposes.
One analysis of the processing challenge faced when encountering an incorrectly marked ergative object is that now two arguments are competing for the same structural position, the subject position. When the subject and object are both animate this may lead to problems with thematic integration, as two identically case‐marked arguments cannot be thematically hierarchized [Bornkessel‐Schlesewsky and Schlesewsky,2009b]. However, when subject is animate and the object is inanimate, the difference in animacy may facilitate the hierarchization because people can use their knowledge that inanimate arguments are less agentive or less likely agents. This idea has received support from event‐related potential studies on German sentence comprehension [Frisch and Schlesewsky,2001,2005]. A comparable case conflict in German (a nominative case‐marked argument following a nominative case‐marked argument) has been reported to elicit a biphasic N400‐P600 response if the second argument is animate, but only a P600 effect if the second argument is inanimate. The N400 results have been taken to reflect problems with thematic integration that could be avoided or overcome by the use of knowledge that inanimate arguments are less agentive. In contrast, the P600 results in both comparisons have been taken to reflect more on general processing consequences of two arguments competing for a single position. In a related study on ergative case agreement in Basque, the absence of ergative case marking on a pronoun where it was required also elicited a biphasic N400‐P600 response [Zawiszewski et al.,2010], possibly reflecting similar problems with thematic hierarchizing [Frisch and Schlesewsky,2001,2005]. Therefore, we hypothesized that the ergative case conflict in this study will elicit enhanced activity in the brain regions that are associated with syntactic repair and reanalysis and that are thought to underlie P600 effects. In neurocognitive accounts of syntactic processing, these regions include the posterior superior temporal gyrus [e.g., Bornkessel‐Schlesewsky and Schlesewsky,2009a,b; Friederici and Kotz,2003; Grodzinsky and Friederici2006; Kotz et al.,2003] and possibly the basal ganglia [e.g., Kotz et al.,2003]. According to the model of syntactic processing as formulated by Friederici and Kotz [2003] and the eADM model [Bornkessel and Schlesewsky,2006; Bornkessel‐Schlesewsky and Schlesewsky,2008,2009a,b], case conflict may elicit additional activation in posterior regions of the left inferior frontal gyrus (LIFG) in as far thematic role assignment is perturbed [but see Bornkessel‐Schlesewsky and Schlesewsky,2009b; Bornkessel et al.,2005; Frisch and Schlesewsky,2001,2005; Grewe et al.,2006,2007, for arguments that this will not be the case when subject and object differ in animacy). If this were true, case conflict may draw upon activity in brain regions that are adjacent to or partly overlapping with anterior regions of the LIFG sensitive to a semantic manipulation [e.g., Baumgaertner et al.,2002; Kiehl et al.,2002; Kuperberg et al.,2003,2008; Nieuwland et al.,2007]. Similarly, although the MUC model [e.g., Hagoort,2005] underspecifies particular syntactic operations, it could be taken to predict that resolving case conflict requires intensified syntactic unification processes, as also subserved by posterior parts of the LIFG and the left premotor cortex.
To investigate the specificity of the neural processing consequences of case conflict, we included the number agreement violation condition as a morphosyntactic control. Sentences with an object number that does not agree with the inflected verb auxiliary can be assumed to elicit similar syntactic repair or reanalysis [e.g., Friederici,2002]. Indeed, number violations are also associated with P600 effects [e.g., Barber and Carreiras,2005; Davidson and Indefrey,2007]. Number agreement violations may thus elicit activity increases in the left posterior superior temporal gyrus and the basal ganglia [Friederici and Kotz,2003] and show considerable overlap with increases seen to case violations. However, recent fMRI results suggest that number agreement violations could be associated with a different pattern of results. Carreiras et al. [2010] reported that determiner‐noun number agreement violations in Spanish word pairs (e.g., “Los‐singular piano‐plural”) elicited activation increases in the left inferior frontal gyrus, the right intraparietal sulcus and the superior parietal gyrus. These results were taken as evidence for the involvement of quantity processing mechanisms beyond the standard language mechanisms. It must be noted, however, that these regions did not show similar effects to agreement errors in noun–adjective word pairs (e.g., “Faro‐singular altos‐plural”), and that in other studies these regions have shown activity increases to other types of manipulations as well [e.g., Folia et al.,2009; Kuperberg et al.,2003,2008; Nieuwland et al.,2007]. Nevertheless, one possible prediction for our study is thus that the number agreement violations elicit activity increases in inferior and superior parietal regions, perhaps in addition to the brain regions often associated with syntactic repair and reanalysis.
To investigate whether the neural processing consequences of a thematic problem induced by case conflict are similar to those induced by a thematic problem in which an argument cannot bear the thematic role it is assigned (i.e., a lexical‐semantic violation), we included a semantic anomaly as a semantic control [see also Kuperberg et al.,2008]. For semantic anomalies, we predicted enhanced activity particularly in the left and right anterior inferior frontal gyrus (BA 45/47), as has been reported by numerous fMRI studies [e.g., Baumgaertner et al.,2002; Kiehl et al.,2002; Kuperberg et al.,2003,2008; Nieuwland et al.,2007], possibly reflecting the increased amount of semantic retrieval and selection [e.g., Badre and Wagner,2002; Bookheimer,2002] needed to build a situation model from semantically unexpected or implausible input. As mentioned above, some neurocognitive accounts thus predict that semantic anomalies elicit activations that partly overlap with or are adjacent to the more posterior regions of the left inferior frontal gyrus that are possibly recruited by case and number violations [e.g., Friederici,2002; Hagoort,2005].
In summary, the aim of this study was to investigate the cortical networks involved in case processing, a crucial facet of language comprehension, and their relationship to networks that serve other aspects of syntactic and semantic processing. While in the scanner, participants read sentences one word at a time that contained case violations, number agreement violations, or semantic anomalies, or correct control sentences, and evaluated the sentences on whether they were acceptable or unacceptable. We predicted that case violations would elicit enhanced activity in the left superior temporal gyrus, and possibly in the basal ganglia and posterior regions of the left inferior frontal gyrus. Further, we predicted that, instead of or in addition to these regions, number agreement violations would elicit enhanced activity in the right intraparietal sulcus and the superior parietal gyrus. Finally, the inclusion of a semantic anomaly in our design allowed us to address whether the pattern of brain activity for these syntactic aspects of language comprehension would be dissociable from patterns elicited by problems with semantic processing.
MATERIALS AND METHODS
Participants
Twenty‐four right‐handed college students (12 males, mean age = 23.1 years) participated in this study for monetary reimbursement. Four participants were excluded from the final analysis due to excessive movement during the experiment (three participants) or to poor behavioral performance (one participant; average performance across condition <50%). All participants were native speakers of Basque, and had normal or corrected‐to‐normal vision. None of them used medication or had a history of drug abuse, head trauma, neurological, or psychiatric illness. The experiment was approved by the institutional ethical committee, and informed consent was obtained from all subjects.
Construction of Stimuli
We created 120 Basque transitive sentences with a length between six and eight words, according to the SVO template <Animate Subject> <Verb + Auxiliary> <Inanimate Object>. Animate subjects involved proper names or definite noun phrases and always occurred in sentence‐initial position, whereas inanimate objects always involved definite noun phrases and never occurred in sentence‐final position. Across conditions, the sentences only differed in the inanimate object that could be semantically correct or incorrect and grammatical or ungrammatical (see example items in Table I). The semantically correct or anomalous nouns were matched for log frequency in both the E‐hitz corpus (M = 0.99/1.09; P > 0.10; Perea et al.,2006) and the Elebilab/Ametzagaiña database (M = 2.28/2.31; P > 0.10; Landa Ijurko,2009). Correct control sentences contained semantically correct and syntactically correct plural objects, corresponding to the preceding verb auxiliaries that always heralded a plural object. Case violations contained semantically correct but incorrectly case‐marked plural objects, as a second ergative case marking indicates an ill‐formed construction. Number agreement violations contained semantically correct but incorrectly marked singular objects, due to a mismatch with the auxiliary that heralds a plural object. Semantic anomalies contained syntactically correct plural objects that did not match the semantic constraints of the preceding verb. In addition, we included 60‐filler sentences that were syntactically and semantically correct and that had a length between four and nine words.
EXPERIMENTAL PROCEDURES
Before entering the scanner, participants were informed that they would be reading sentences one word at a time, presented via back‐projection onto the middle of the screen, and would view the stimuli via a mirror attached to the head coil. They were instructed to minimize movement and read the sentences attentively, and to judge the sentence for acceptability with a left‐ or right‐hand button‐press (left‐ or right‐hand assignment for acceptable or unacceptable was counterbalanced across participants). It was explained to them that the sentences could contain the different type of semantic and syntactic violations and that they should judge the sentences as acceptable when they were both semantically and syntactically correct and judge them as unacceptable when they were either semantically or syntactically unacceptable.
Four trial lists were used (each subject was randomly assigned to one of the four trial lists, so that the lists were equally distributed across subjects). For the first list, 30 items from each condition were pseudo‐randomly mixed with the filler sentences such that no trial type occurred more than three times consecutively and trials of each type were matched on average list position. The other lists were derived from the first by rotating the trial types. The 180 sentences were divided in 3 runs (presented in fixed‐order across trial lists) of ∼11 min each. Subjects were in the scanner for a total time of about 45 min.
Each sentence was presented word by word with a word duration of 300 ms (but 600 ms for sentence‐final words) and SOA of 300 ms, with black letters on an almost‐white background. Following every final word, a blank (bright) screen was presented for 500 ms, followed by a response‐screen that automatically disappeared after 2000 ms. The response‐screen presented the Basque equivalent of “Acceptable?” (“Onargarria?”) in the middle of the screen with “yes” and “no” below it on the left or right side. Participants were instructed to judge the sentences as quickly as possible upon seeing the response‐screen and were told that the response‐screen would disappear automatically after 2,000 ms to proceed with the next trial. If participants responded in time, the screen would remain blank for the remainder of the 2,000 ms, after which a fixation mark was presented for 5, 6, 7, or 8 s, followed by the presentation of a blank screen for 500 ms before the first word of the following sentence was presented. Participants were instructed to fixate on the middle of the screen for the duration of the fixation period and simply await the start of the next trial.
fMRI Data Acquisition, Preprocessing, and Statistical Analysis
Imaging took place on a 3‐T MR scanner (Siemens TrioTim) with echoplanar imaging capability. Head motion was minimized using pillows and cushions around the head. Each subject then viewed one of the four counterbalanced sentence lists with the sentence trials and fixation trials, divided by three functional runs. Each functional run lasted around 670 s during which whole head T2*‐weighted EPI‐BOLD fMRI data were acquired using an interleaved even acquisition EPI sequence (volume TR = 2 s; TE = 30 ms; flip angle = 90°; 32 axial slices; matrix size = 64 × 64; slice thickness = 3 mm; slice gap = 0.75 mm; transverse orientation acquisition; isotropic voxel‐size = 3 × 3 × 3 mm3). After three functional runs, subjects underwent one conventional high‐resolution 3D structural scan, using a T1‐weighted MPRAGE sequence (176 transverse slices; volume TR = 2,530 ms; TE = 2.97 ms; TI = 1,100 ms; transverse orientation acquisition; flip angle = 7°; slice matrix = 256 × 256; slice thickness = 1 mm, slice gap = 0.5 mm).
Image preprocessing and statistical analysis was performed using the SPM5 and SPM8 software (http://www.fil.ion.ucl.ac.uk). The functional EPI‐BOLD contrast images were realigned, and the subject mean was coregistered with the corresponding averaged structural MRI by using mutual information optimization. These images were subsequently slice‐time corrected, spatially normalized (images were resampled with a 2 × 2 × 2 mm3 resolution), transformed into a common space (MNI‐T1 template), and spatially filtered with an isotropic 3D Gaussian kernel (10 mm FWHM). The fMRI data were analyzed statistically by using the general linear model and statistical parametric mapping. We included the following explanatory variables: onset of the critical word up to the offset of the sentence‐final word for each condition separately, and the fixation period. Importantly, only correctly evaluated sentences were included in the model. Effects of no‐interest included one regressor that pooled sentence windows up to the onset of the critical word for all conditions with all filler sentence time windows, and additional regressors for session and subject effects. The explanatory variables in each model were temporally convolved with the canonical hemodynamic response function along with its temporal derivative [Friston et al.,1998], while controlling for serial correlations with an autoregressive AR(1) model, as provided by SPM8. Low‐frequency noise was removed with a high‐pass filter (time constant 128 s). For the statistical analysis, parameter estimates for the explanatory variables were generated for each subject. Subsequently, only the parameters involving the critical sentence parts and the parameter for the fixation period were subjected to a second‐level random effects analysis with nonsphericity correction for correlated repeated measures. The following linear contrasts (and their reverse counterparts) were specified: case violation > correct control (ERG > CON), number agreement violation > correct control (NUM > CON), semantic anomaly > correct control (SEM > CON), and case violation > number agreement violation (ERG > NUM).
In the whole brain analysis, the results of the random effects analyses were thresholded at P = 0.001 (uncorrected) and voxel extent k = 50, and the cluster‐size statistics were used as the test statistic. All clusters that include more than 100 voxels are reported, but only clusters at P ≤ 0.05 corrected for multiple comparisons using the false discovery rate [FDR; Genovese et al.,2002] were considered significant. In the following, we use the terms activation and deactivation as synonyms for a relative increase and decrease in BOLD signal, respectively. All local maxima are reported as MNI coordinates [Evans et al.,1993]. Anatomical location and approximate Brodmann areas and were determined using the AAL toolbox for SPM8 [Tzourio‐Mazoyer et al.,2002] and with the xjView toolbox (http://www.alivelearn.net/xjview8).
In addition, we performed region‐of‐Interest (ROI) analyses using the Marsbar toolbox [Brett et al.,2002] to examine activation patterns across conditions for peak voxels of the clusters that showed activity increases compared to correct control sentences in the pairwise comparisons. Using average parameter estimates per condition for each subject and for each ROI, we performed a four level (condition: case violation, number agreement violation, semantic anomaly, and correct control) repeated measures analysis of variance (ANOVA) with follow‐up pairwise comparisons (LSD).
RESULTS
Behavioral Results
Participants responded more accurately and had faster reaction times to case violations compared to the other conditions (see Fig. 1a,b). We compared behavioral response accuracy and reaction time (for correct responses) for the four conditions using a 4‐level repeated measures analysis of variance (ANOVA). Behavioral performance was significantly different between conditions in terms of accuracy (F(3, 19) = 6.42, P = 0.001) and reaction time (F(3, 19) = 9.42, P < 0.001). Pairwise comparisons (LSD) revealed that participants responded more accurately to case violations than all other conditions (P < 0.01 for each comparison) but responded equally accurate to number agreement violations, semantic anomalies and correct sentences. This pattern of results was identical for reaction time to ergative violations (P < 0.01 for each comparison), but participants also gave marginally faster responses to number agreement violations (P = 0.051) and semantic anomalies (P = 0.067) than to correct sentences.
Figure 1.
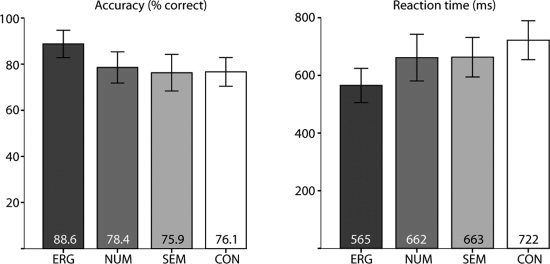
Average response accuracy (percentage correct) and reaction time in (ms) for each of the conditions (ERG = case violation, NUM = number agreement violation, SEM = semantic anomaly, CON = correct control, with error bars indicating 95% confidence intervals.
fMRI Results
We first investigated the contrasts that involved the case violations, number agreement violations and semantic anomalies, each compared to the correct control sentences. The corresponding statistical results are presented in Table II. As visible from Figure 2a, case violations (ERG > CON) were associated with activation increases in several large parietal regions including the posterior cingulate gyrus, precuneus as well as the left and right inferior parietal lobules (encompassing the left and right supramarginal gyri). Number agreement violations (NUM > CON) were associated with similar though smaller activation increases in the left and right inferior parietal lobules, but, in contrast to the case violations, also evoked significant activation increases in the left middle frontal gyrus. A direct comparison of case violations and number agreement violations (ERG > NUM) did not evoke significant activation clusters in either direction [although using a more liberal P = 0.01 (uncorrected) threshold revealed clearly visible parietal clusters for ERG > NUM and prefrontal clusters for NUM > ERG]. Finally, semantic anomalies (SEM > CON) led to enhanced activity in left and right inferior frontal gyrus (extending into the superior temporal gyrus) and the bilateral insula, and in a large medial region encompassing the middle frontal and superior frontal gyrus.
Table II.
Brain regions with peak voxel MNI‐coordinates and approximate Brodmann′s area (BA) that showed significant differential effects in the three pair‐wise comparisons to the correct control sentences
| BA | p | k | Z | Coordinates | |||
|---|---|---|---|---|---|---|---|
| x | Y | z | |||||
| (a) Case violations > Correct control | |||||||
| 1. Cingulate gyrus, posterior cingulated | 23/31 | <0.001 | 1,457 | 5.21 | 0 | −30 | 28 |
| 3.45 | −8 | 0 | 36 | ||||
| 3.44 | −4 | −28 | 46 | ||||
| 2. Left and right precuneus | 7/31 | <0.001 | 2,088 | 5.02 | −18 | −68 | 32 |
| 4.56 | 4 | −70 | 40 | ||||
| 4.51 | −4 | −70 | 44 | ||||
| 3. Right inferior parietal lobule | 40 | <0.001 | 2,279 | 4.81 | 50 | −44 | 40 |
| 4.57 | 60 | −38 | 32 | ||||
| 4.21 | 46 | −58 | 46 | ||||
| 4. Left inferior parietal lobule | 40 | <0.001 | 1,825 | 4.65 | −42 | −46 | 42 |
| 4.31 | −52 | −46 | 44 | ||||
| 3.77 | −60 | −34 | 36 | ||||
| (b) Number agreement violations > Correct control | |||||||
| 5. Right inferior parietal lobule | 40 | 0.04 | 634 | 4.18 | 36 | −52 | 42 |
| 4.10 | 42 | −44 | 38 | ||||
| 6. Left middle frontal gyrus | 9 | 0.04 | 778 | 4.11 | −44 | 32 | 34 |
| 10/46 | 3.79 | −44 | 54 | 8 | |||
| 10 | 3.73 | −44 | 48 | 24 | |||
| 7. Left inferior parietal lobule | 0.49 | 223 | 4.09 | −42 | −46 | 42 | |
| 3.40 | −46 | −36 | 36 | ||||
| 8. Right middle frontal gyrus | 0.93 | 100 | 3.41 | 44 | 46 | 16 | |
| 3.25 | 50 | 40 | 20 | ||||
| 3.19 | 42 | 50 | 6 | ||||
| (c) Semantic anomalies > Correct control | |||||||
| 9. Left inferior frontal gyrus | 47 | <0.005 | 999 | 5.01 | −50 | 34 | −16 |
| Left superior temporal gyrus | 38 | 4.35 | −36 | 26 | −24 | ||
| Left inferior frontal gyrus, insula | 47/13 | 4.10 | −32 | 20 | −20 | ||
| 10. Right inferior frontal gyrus | 47 | 0.01 | 771 | 4.82 | 46 | 38 | −18 |
| 4.67 | 44 | 28 | −14 | ||||
| Right inferior frontal gyrus, insula | 47/13 | 4.55 | 28 | 16 | −18 | ||
| 11. Middle frontal gyrus | 8 | <0.005 | 1,161 | 4.51 | −4 | 42 | 44 |
| 12. Middle frontal gyrus | 9 | 3.79 | −6 | 48 | 22 | ||
| Superior frontal gyrus | 8 | 3.65 | 6 | 38 | 56 | ||
P‐values correspond to cluster‐level statistical tests with FDR‐correction at P ≤ 0.05. Z‐values correspond to the local maxima in the relevant cluster (multiple local maxima are reported when they are more than 8 mm apart). Only clusters of k > 100 are included.
Figure 2.
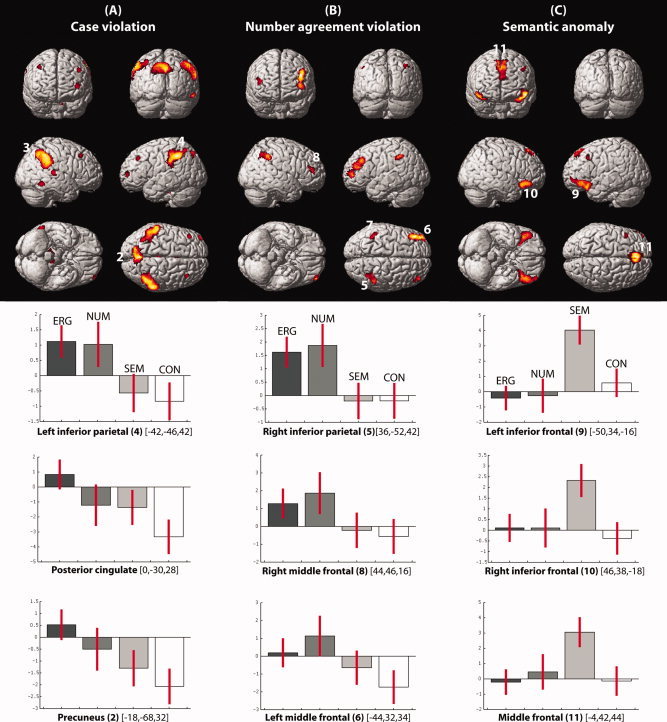
Upper graphs present the results of the pair‐wise comparisons across all subjects (thresholded at P ≤ 0.001 uncorrected, voxel extent threshold = 50, presented according to neurological convention). (A) Case violations > correct control, (B) number agreement violations > correct control, and (C) semantic anomalies > correct control. Lower graphs show the contrast estimates for all four conditions (compared to the fixation baseline) with 90% confidence intervals at nine of the peak voxels that showed significant activation increases for the pairwise comparisons (ERG = case violations, NUM = number agreement violations, SEM = semantic anomalies, CON = correct control sentences). Note that we do not wish to make claims about any of the conditions compared to the fixation condition: the sole purpose of these graphs is to assist the reader in surveying the patterns of relative activations across conditions and brain regions.
The above pairwise comparisons suggest a general pattern wherein case violations and number agreement violations elicit activation increases in overlapping parietal and prefrontal regions, although with differential contributions (predominantly parietal increases for case violations, strongest prefrontal increases for number agreement violations. In contrast, semantic anomalies elicited activation increases in brain regions that did not differentiate between the other conditions. This pattern is also visible from the lower graphs in Figure 2, which shows the average parameter estimates for each sentence condition compared to fixation for nine peak‐voxels within the clusters as reported in Table II (N.B., we do not wish to make claims about any of the conditions compared to the fixation condition: the sole purpose of these graphs is to assist the reader in surveying the patterns of relative activations across conditions and brain regions), and it was also borne out in the subsequent ROI analyses. Note that while all ROIs showed a main effect of condition (F > 4 for each ROI), out of parsimony we will only report the results of the pairwise comparisons. ROI 1–4: In the posterior cingulate (ROI 1), case violations elicited activation increases compared to number agreement violations (P < 0.05), semantic anomalies (P < 0.001) and correct control sentences (P < 0.001). Number agreement violations and semantic anomalies also elicited increases compared to correct control sentences (P < 0.05), but did not differ from one another. In the precuneus (ROI 2), case violations elicited activation increases compared to semantic anomalies (P < 0.005) and correction control sentences (P < 0.005), but not to number agreement violations. No significant differences were found for number agreement violations compared neither with semantic anomalies nor for semantic anomalies and correct control sentences. In both right and left inferior parietal regions (ROI 3 and 4), case violations and number agreement violations each elicited activation increases to correct control sentences (P < 0.002 for both comparisons) and semantic anomalies (P < 0.001 and P < 0.05, respectively), but did not differ from each other and neither did the correct control sentences and semantic anomalies. ROIs 5–8: In the right inferior parietal region (ROI 5), number agreement violations and case violations did not differ from one another but each elicited activation increases compared to correct control sentences and semantic anomalies (P < 0.01 for each comparison). Correct control sentences and semantic anomalies did not show significant activation differences. The left middle frontal gyrus (ROI 6) showed a similar pattern of results, with the exception that number agreement violations and case violations elicited activation increases compared to semantic anomalies that were only marginally significant (P < 0.1 for each comparison). The left inferior parietal region (ROI 7) showed the exact same pattern of results as ROI 5. In the right middle frontal gyrus (ROI 8), number agreement violations and case violations both elicited activation increases only compared to the correct control sentences (P < 0.01 for each comparison), but only number agreement violations elicited increases compared to semantic anomalies (P < 0.05). ROIs 9–11: These ROIs all showed the same pattern of results, with semantic anomalies eliciting activation increases compared to case violations (P < 0.01 for each comparison), number agreement violations (P < 0.05 for each comparison), and correct control sentences (P < 0.01 for each comparison), but no differences between the other thee conditions (P > 0.1 for each comparison).
These ROI analyses did not reveal statistically significant differences between case violations and number agreement violations in prefrontal and parietal ROIs (except for the posterior cingulate). However, a follow‐up 2 (ROI‐type: prefrontal and parietal) by 2 (condition: case violation and number agreement violation) repeated measures ANOVA that used the average values across prefrontal ROIs (6 and 8) versus parietal ROIs (1–4) for case violations and number agreement violations separately, show a strongly significant ROI‐type by condition interaction effect (F(1, 19) = 11.66, P < 0.005). This interaction effect is consistent with the patterns of results as observed in the whole‐brain analysis, that the distinction between case violations and number agreement violations rests on the relative contributions of parietal and prefrontal regions respectively.
DISCUSSION
The objective of this study was to investigate the functional neuroanatomical correlates of case agreement processing. We compared event‐related BOLD‐fMRI responses to Basque sentences containing case violations, number agreement violations, and semantic anomalies, and to sentences that were both syntactically and semantically correct. This allowed us to compare the processing consequence of a thematic integration problem due to case conflict, with those of a morphosyntactic problem due to a number agreement mismatch, and to those of a semantic problem in which an argument due to semantic constraints cannot bear the thematic role it is assigned. Our results can be summarized as follows. Compared to correct control sentences, case violations elicited activation increases in a number of large parietal regions including the posterior cingulate, the precuneus and the left and right inferior parietal gyri. Number violations also elicited activations in the left and right inferior parietal gyri, but elicited additional activations in the left middle frontal gyri and, to a lesser extent, its right homologue. In contrast, semantic anomalies only elicited activations in the left and right anterior prefrontal gyri and a dorsomedial prefrontal region. Follow‐up ROI analyses showed that in all of these activated clusters, except for the posterior cingulate, case violations, and number agreement violations showed rather similar responses. However, these violation types elicited differential responses in prefrontal versus parietal ROIs, as shown in a significant ROI by condition interaction. The distinction between case and number violations therefore seemed to rely on the relative contributions from parietal versus prefrontal regions respectively. The overall distinction between the syntactic violations and semantic anomaly was clear‐cut: the regions that showed activation increases to the syntactic violations (the left and right inferior parietal regions and the precuneus) did not differentiate semantic anomaly and correct control, whereas the regions that showed activation increases to semantic anomaly did not distinguish case violations, number violations and correct control sentences from each other.
Results of the acceptability judgment task showed that participants responded faster and more accurately to case violations than to all other conditions. Better performance for syntactic violations versus semantic violations or correct control sentences has often been reported [e.g., Kuperberg et al.,2003,2008; McElree and Griffith,1995] and may reflect the delay of the output of conceptual processing relative to that of the finite rule system for syntactic processing. Performance may have been better for case violations than for number agreement violations due to larger salience of a double ergative case marking, which poses clear problems for constructing sentence meaning (while number agreement violation does not necessarily, e.g., without further context buying one or multiple tickets is similarly plausible), and perhaps due to relative infrequent ergative case marking for inanimate nouns. Interestingly, reaction time in the behavioral task was mirrored by the BOLD responses in posterior cingulate (faster responses were associated with more activity in this region), which was the only brain region that differentiated case violations from all other conditions. This finding is consistent with the results of Kuperberg et al. [2003], who reported that only activity in the posterior cingulate exactly mirrored the reaction time patterns for syntactic and semantic violations. These patterns of results are suggestive of a more general role of this region during violation detection, consistent with its assumed role in allocating attentional resources during task‐performance [e.g., Hayden et al.,2010].
Contributions of Parietal and Prefrontal Regions to Processing Case and Number
Perhaps the most salient finding of this study was that processing case violations and number agreement violations drew upon largely overlapping neural circuits, albeit with subtle differences in the contributions from parietal and prefrontal regions respectively. The absence of strong neuroanatomical differences for these two types of violations may be exacerbated by the fact that a double ergative case violation in Basque may also constitute an agreement problem. This is because although the first ergative noun phrase can be processed without any problems, the second ergative noun phrase is blocked from concording with the auxiliary, resulting in an agreement violation between argument and verb, similar as in the number agreement violation1.
The hypothesis that the right intraparietal sulcus subserves quantity‐related processing evoked by number agreement violations [Carreiras et al.,2010] seems difficult to uphold, given that in this study the largest effects in this region were evoked by case violations. A direct comparison of case and number agreement violations did not generate any significant clusters using the standard voxel‐level threshold, but whereas case violations only evoked significant clusters compared to correct control sentences in parietal regions, the largest cluster for number agreement violations was in the dorsolateral prefrontal cortex (middle frontal gyrus, BA 9/46). The current contributions of medial and bilateral inferior parietal and of dorsolateral prefrontal cortex to processing syntactic violations are consistent with results from related studies that used gender‐mismatching pronouns or article‐noun gender agreement violations in Dutch [Folia et al.2009; Nieuwland et al.,2007], or verb inflection violations in English [Kuperberg et al.,2003,2008; see also Ni et al.,2000]. Our results, however, do not straightforwardly map onto extant neurocognitive models of syntactic processing [Bornkessel‐Schlesewsky and Schlesewsky,2009a,b; Friederici and Kotz,2003]. These models predict that number and case violations elicit activations in brain regions that are assumed to play a role in generating P600 effects [see Díaz et al.,2011, who report P600 effects for similar case and number agreement violations in Basque], in particular the left posterior superior temporal gyrus and possibly the basal ganglia. It is therefore possible that medial and bilateral parietal regions and perhaps dorsolateral regions also contribute to P600 effects [Folia et al.2009; Kuperberg et al.,2003,2008; Nieuwland et al.,2007].
The functional significance of activations in these areas in response to the two types of syntactic violations remains tentative. The available studies that report such effects have in common that they require participants to detect morphosyntactic errors [Folia et al.,2009; Kuperberg et al.,2003,2008; Nieuwland et al.,2007], rather than, for example, phrase structure errors [e.g., Friederici et al.,2006]. To the extent that morphosyntactic error detection is contingent on knowledge of what the correct expression would be, detection might invoke repair or correction processes. To engage in such processes while continuing to read might incur increased verbal working memory load, a function that has often been ascribed to the inferior parietal cortex [e.g., Ravizza et al.,2004]. However, under this general account one would expect to observe a similar effect in this region for semantic anomalies. An alternative possibility, as suggested by Kuperberg et al. [2003], is that the bilateral and medial parietal activations are actually reduced deactivations, reflecting the fact that more difficult tasks generally lead to stronger deactivations in these “resting‐state” regions. However, this explanation does not readily explain why we did not see similar effects in these regions for number agreement violations and semantic anomalies, which were approximately similarly easy to detect. One perhaps clear difference between, on one hand, the syntactic anomalies and, on the other hand, the semantic anomalies, is that whereas the detection of syntactic anomaly may be relatively straightforward (i.e., based on a finite rule system, one considers what the correct case ending or number agreement inflection is), this may not be the case for semantic violations. Although speculative, the effects in medial and bilateral inferior parietal may thus reflect the successful outcome of a morphosyntactic repair or correction process. A related explanation can be considered for the dorsolateral prefrontal activations to syntactic anomalies. As reported by Indefrey et al. [2001], left dorsolateral prefrontal cortex may be specifically activated when participants are required to correct syntactic violations (as compared with other types of violations) during sentence comprehension. However, why the contribution of this prefrontal region was slightly larger for number agreement violations than for case violations remains unknown. Moreover, there is no strong argument to assume that participants were actually correcting or repairing syntactic violations, which was not required of them to perform the detection task at hand.
We note that a caveat to the current findings is that our violation paradigm can only shed light on a limited aspect of case processing, the situation where the reader encounters an ungrammatical case‐marking. Investigations of other aspects of case processing, for example, its role in argument hierarchy resolution for different word‐order constructions [Bornkessel et al.,2005], can shed light on the neural mechanisms for processing grammatically licensed and unambiguous case constructions.
Distinct Neural Circuits for Syntactic and Semantic Processing
Processing syntactic violations clearly drew upon qualitatively different brain circuits than processing semantic anomalies. This is consistent with a large body of neuroimaging studies has investigated whether and which brain regions are differentially sensitive to syntactic and semantic aspects of language [e.g., for review, see Bookheimer,2002; Bornkessel and Friederici,2007; Kaan and Swaab, 2002; Kuperberg et al.,2003,2008; Luke et al.,2002; Newman et al.,2001; Ni et al.,2000; Osterhout et al., 2002]. Consistent with this body of literature, semantic anomalies selectively activated the bilateral anterior inferior prefrontal gyrus [e.g., Bookheimer,2002]. In contrast to other studies, however, semantic anomalies also evoked activation increases in dorsomedial prefrontal cortex. One tentative interpretation of this finding is that participants engaged in additional inferencing to try to generate a semantically plausible alternative to the semantically anomalous sentences. Although not all studies that have used an acceptability task have reported dorsomedial effects for semantic anomalies [e.g., Kuperberg et al.,2003], this type inferencing could have been encouraged by the semantic acceptability task in which participants may have assumed that for each semantic anomaly there may have been a correct or at least more plausible answer, or perhaps were inclined to enrich the implausible sentences to make sense of them. Such inferences have been associated with activations in dorsomedial prefrontal cortex [e.g., Kuperberg et al.,2006; Nieuwland et al.,2007].
CONCLUSIONS
The aim of this event‐related fMRI study was to investigate the cortical networks involved in case processing, a crucial aspect of language comprehension whose neural underpinnings are not well‐understood. Our results suggest that whereas syntactic and semantic anomalies clearly recruit distinct neural circuits, case, number violations recruit largely overlapping neural circuits, and that the distinction between the two rests on the relative contributions of parietal and prefrontal regions, respectively. Our results are consistent with recently reported contributions of bilateral parietal and dorsolateral brain regions to syntactic processing, pointing towards potential extensions of current neurocognitive theories of language.
Acknowledgements
The authors are grateful to Javi Miqueleiz and Saioa Larraza for help with stimulus construction, to Adam Zawiszewski for providing us with a small set of sample stimuli, and to two anonymous reviewers for helpful comments on an earlier version of this manuscript. This research has been partially supported by Grant CONSOLIDER‐INGENIO2010 CSD2008‐00048 from the Spanish Ministry of Education. MSN is supported by a Plan Nacional research grant from the Spanish Ministry of Science and Innovation (PSI2010‐18087)
Footnotes
We thank an anonymous reviewer for this observation.
REFERENCES
- Arregi K ( 2001): Person and number inflection in Basque In: Albizu P, Fernández B, editors. On Case and Agreement. Kasu eta Komunztaduraren gainean. Bilbo: Euskal Herriko Unibertsitatea. [Google Scholar]
- Badre D, Wagner, AD ( 2002): Semantic retrieval, mnemonic control, and prefrontal cortex. Behav Cogn Neurosci Rev 3: 206–218. [DOI] [PubMed] [Google Scholar]
- Barber H, Carreiras M ( 2005): Grammatical Gender and Number Agreement in Spanish: An ERP Comparison. J Cogn Neurosci 17: 137–153. [DOI] [PubMed] [Google Scholar]
- Baumgaertner A, Weiller C, Buchel C ( 2002): Event‐related fMRI reveals cortical sites involved in contextual sentence integration. Neuroimage 16: 736–745. [DOI] [PubMed] [Google Scholar]
- Bookheimer S ( 2002): Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci 25: 151–188. [DOI] [PubMed] [Google Scholar]
- Bornkessel I, Schlesewsky M ( 2006): The extended argument dependency model: A neurocognitive approach to sentence comprehension across languages. Psychol Rev 113: 787–821. [DOI] [PubMed] [Google Scholar]
- Bornkessel‐Schlesewsky I D, Friederici AD ( 2007): Neuroimaging studies of sentence and discourse comprehension In: Gaskell MG, editor. The Oxford handbook of psycholinguistics. Oxford: Oxford University Press; pp 407–424. [Google Scholar]
- Bornkessel‐Schlesewsky I, Schlesewsky M ( 2008): An alternative perspective on ‘semantic P600’ effects in language comprehension. Brain Res Rev 59: 55–73. [DOI] [PubMed] [Google Scholar]
- Bornkessel‐Schlesewsky I, Schlesewsky M ( 2009a): Processing syntax and morphology: A neurocognitive perspective. Oxford: Oxford University Press. [Google Scholar]
- Bornkessel‐Schlesewsky I, Schlesewsky M ( 2009b) The role of prominence information in the real‐time comprehension of transitive constructions: A cross‐linguistic approach. Lang Linguist Compass 3: 19–58. [Google Scholar]
- Bornkessel I, Zysset S, Friederici AD, von Cramon DY, Schlesewsky M ( 2005): Who did what to whom? The neural basis of argument hierarchies during language comprehension. Neuroimage 26: 221–233. [DOI] [PubMed] [Google Scholar]
- Bossong G ( 1984): Ergativity in Basque. Linguistics 22: 341–392. [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J ( 2002): Region of interest analysis using the MarsBar toolbox for SPM 99. Poster presented at the 8th International Conference on Functional Mapping of the Human Brain. Sendai, Japan.
- Carreiras M, Carr L, Barber H, Hernández A ( 2010): Where syntax meets math: Right Intraparietal Sulcus activation in response to grammatical number agreement violations. Neuroimage 49: 1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D J, Indefrey P ( 2007): Inverse relation between event‐related and time‐frequency violation responses in sentence processing. Brain Res 1158: 81–92. [DOI] [PubMed] [Google Scholar]
- De Rijk R ( 2007): Standard Basque, a progressive grammar. Cambridge MA: MIT Press. [Google Scholar]
- Díaz B, Sebastián‐Gallés N, Erdocia K, Mueller J L, Laka I ( 2011): On the cross‐linguistic validity of electrophysiological correlates of morphosyntactic processing: A study of case and agreement violations in Basque. J Neurolinguistics 24: 357–373. [Google Scholar]
- Dowty D ( 1991): Thematic proto‐roles and argument selection. Language 67: 547–619. [Google Scholar]
- Evans A C, Collins D L, Mills S R, Brown ED., Kelly RL, Peters TM ( 1993): IEEE Conference Record, Nuclear Science Symposium, and Medical Imaging Conference, San Francisco. pp 1813–1817.
- Indefrey P, Hagoort P, Herzog H, Seitz R, Brown C ( 2001): Syntactic processing in left prefrontal cortex is independent of lexical meaning. Neuroimage 14: 546–555. [DOI] [PubMed] [Google Scholar]
- Fillmore C ( 1968): The case for case. In Universals in Linguistic Theory In: Bach E, Harms R, editors. New York: Holt. [Google Scholar]
- Folia V, Forkstam C, Hagoort P, Petersson KM ( 2009): Language comprehension: The interplay between form and content In: Taatgen NA, van Rijn H, editors. Proceedings of the 31st annual conference of the Cognitive Science Society. Cognitive Science Society, Austin, TX: pp 1686–1691. [Google Scholar]
- Friederici AD ( 1998): The neurobiology of language comprehension In: Friederici AD, editor. Language comprehension: A biological perspective. Berlin: Springer; pp 263–301. [Google Scholar]
- Friederici AD ( 2002): Towards a neural basis of auditory sentence processing. Trends Cogn Sci 6: 78–84. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA ( 2003): The brain basis of syntactic processes: Functional imaging and lesion studies. Neuroimage 20: S8–S17. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Fiebach CJ, Schlesewsky M, Bornkessel I, von Cramon DY ( 2006): Processing linguistic complexity and grammaticality in the left frontal cortex. Cereb Cortex 16: 1709–1717. [DOI] [PubMed] [Google Scholar]
- Frisch S, Schlesewsky M ( 2001): The N400 reflects problems of thematic hierarchizing. Neuroreport 12: 3391–3394. [DOI] [PubMed] [Google Scholar]
- Frisch S, Schlesewsky M ( 2005): The resolution of case conflicts from a neurophysiological perspective. Brain Res Cogn Brain Res 25: 484–498. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R ( 1998): Event‐related fMRI: characterizing differential responses. Neuroimage 7: 30–40. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Grewe T, Bornkessel I, Zysset S, Wiese R, von Cramon DY, Schlesewsky M ( 2006): Linguistic prominence and Broca's area: The influence of animacy as a linearization principle. Neuroimage 32: 1395–1402. [DOI] [PubMed] [Google Scholar]
- Grewe T, Bornkessel‐Schlesewsky I, Zysset S, Wiese R, von Cramon DY, Schlesewsky M ( 2007): The role of the posterior superior temporal sulcus in the processing of unmarked transitivity. Neuroimage 35: 343–352. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y, Friederici AD ( 2006): Neuroimaging of syntax and syntactic processing. Curr Opin Neurobiol 16: 240–246. [DOI] [PubMed] [Google Scholar]
- Hagoort P ( 2005): On broca, brain, and binding: A new framework. Trends Cogn Sci 9: 416–423. [DOI] [PubMed] [Google Scholar]
- Hagoort P ( 2009): Reflections on the neurobiology of syntax In: Bickerton D, Szathmáry E, editors. Biological foundations and origin of syntax. Cambridge, MA: MIT Press; pp 279–296. [Google Scholar]
- Hagoort P, Brown CM, Osterhout L ( 1999): The neurocognition of syntactic processing In: Brown CM, Hagoort P, editors. The Neurocognition of Language. Oxford: University Press; pp 273–316. [Google Scholar]
- Hayden BY, Smith DV, Platt ML ( 2010): Cognitive control signals in posterior cingulate cortex. Front Hum Neurosci 4: 223. Published online 2010 December 6. doi:10.3389/fnhum.2010.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmer A ( 2001): The ergativity parameter. Working Papers 48, Lund University.
- Jackendoff R ( 2002): Foundations of language: Brain, meaning, grammar, evolution. Oxford: University Press. [DOI] [PubMed] [Google Scholar]
- Kaan E, Swaab TY ( 2002): The brain circuitry of syntactic comprehension. Trends Cogn Sci 6: 350–356. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Liddle PF ( 2002): Reading anomalous sentences: An event‐related fMRI study of semantic processing. Neuroimage 17: 842–850. [PubMed] [Google Scholar]
- Kotz SA, Frisch S, von Cramon DY, Friederici AD ( 2003): Syntactic language processing: ERP lesion data on the role of the basal ganglia. J Int Neuropsychol Soc 9: 1053–1060. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Holcomb PJ, Sitnikova T, Greve D, Dale AM, Caplan D ( 2003): Distinct patterns of neural modulation during the processing of conceptual and syntactic anomalies. J Cogn Neurosci 15: 272–293. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Lakshmanan BM, Caplan DN, Holcomb PJ ( 2006): Making sense of discourse: An fMRI study of causal inferenceing across sentences. Neuroimage 33: 343–361. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Sitnikova T, Lakshmanan BM ( 2008): Neuroanatomical distinctions within the semantic system during sentence comprehension: Evidence from functional magnetic resonance imaging. Neuroimage 40: 367–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laka I ( 1996): A Brief Grammar of Euskara, the Basque Language. University of the Basque Country. <http://www.ehu.es/grammar>.
- Laka, I ( 2006): On the nature of case in Basque: structural or inherent? In: Broekhuis H, Corver N, Koster J, Huybregts R, Kleinhenz U, editors. Organizing Grammar: Linguistic Studies in Honor of Henk van Riemsdijk. Berlin, New York: Mouton de Gruyter; 374–382. [Google Scholar]
- Landa I jurko J ( 2009): Elebilab/Amarauna, http://amarauna.org/elebilab/, Ametzagaiña I+D.
- Luke K, Liu H, Wai Y, Wan Y, Tan L ( 2002): Functional anatomy of syntactic and semantic processing in language comprehension. Hum Brain Mapp 16: 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElree B, Griffith T ( 1995): Syntactic and thematic processing in sentence comprehension: Evidence for a temporal dissociation. J Exp Psychol Learn Mem Cogn 21: 134–157. [Google Scholar]
- Newman AJ, Pancheva R, Ozawa K, Neville HJ, Ullman MT ( 2001): An event‐related fMRI study of syntactic and semantic violations. J Psycholinguist Res 30: 339–364. [DOI] [PubMed] [Google Scholar]
- Ni W, Constable RT, Mencl WE, Pugh KR, Fulbright RK, Shaywitz SE, Shaywitz BA, Gore J ( 2000): An event‐related neuroimaging study distinguishing form and content in sentence processing. J Cogn Neurosci 12: 120–133. [DOI] [PubMed] [Google Scholar]
- Nieuwland MS, Petersson KM, Van Berkum JJA ( 2007): On sense and reference: Examining the functional neuroanatomy of referential processing. Neuroimage 37: 993–1004. [DOI] [PubMed] [Google Scholar]
- Ortiz de Urbina, J ( 1989): Parameters in the grammar of Basque. Foris: Dordrecht. [Google Scholar]
- Osterhout L, Kim A, Kuperberg G (2002): The Neurobiology of Sentence Comprehension To appear in Spivey M, Joanaisse M, McRae K, editors. The Cambridge Handbook of Psycholinguistics. Cambridge: Cambridge University Press. [Google Scholar]
- Perea M, Urkia M, Davis CJ, Agirre A, Laseka E, Carreiras M ( 2006): E‐Hitz: A word‐frequency list and a program for deriving psycholinguistic statistics in a agglutinative language (Basque). Behav Res Methods 38: 610–615. [DOI] [PubMed] [Google Scholar]
- Petersson KM, Folia V, Hagoort P ( 2010): What artificial grammar learning reveals about the neurobiology of syntax. Brain Lang (in press). doi:10.1016/j.bandl.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Primus B ( 2002): Proto‐Roles and Case Selection in Optimality Theory. Theorie des Lexikons, 122. Arbeiten des Sonderforschungsbereichs 282. Düsseldorf: Universität. [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA ( 2004): Functional dissociations within the inferior prefrontal cortex in verbal working memory. Neuroimage 22: 562–573. [DOI] [PubMed] [Google Scholar]
- Trask L ( 1997): The History of Basque. Routledge, London. [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Van Valin RD ( 2005): Exploring the syntax‐semantics interface. Cambridge: Cambridge University Press. [Google Scholar]
- Zawiszewski A, Gutierrez E, Fernández B, Laka I ( 2010):. Language distance and non‐native syntactic processing: evidence from event‐related potentials. Bilingualism: Lang Cogn. doi: 10.1017/S1366728910000350. [Google Scholar]


