Abstract
Introduction: Lack of illness awareness or anosognosia occurs in both schizophrenia and right hemisphere lesions due to stroke, dementia, and traumatic brain injury. In the latter conditions, anosognosia is thought to arise from unilateral hemispheric dysfunction or interhemispheric disequilibrium, which provides an anatomical model for exploring illness unawareness in other neuropsychiatric disorders, such as schizophrenia. Methods: Both voxel‐based morphometry using Diffeomorphic Anatomical Registration through Exponentiated Lie Algebra (DARTEL) and a deformation‐based morphology analysis of hemispheric asymmetry were performed on 52 treated schizophrenia subjects, exploring the relationship between illness awareness and gray matter volume. Analyses included age, gender, and total intracranial volume as covariates. Results: Hemispheric asymmetry analyses revealed illness unawareness was significantly associated with right < left hemisphere volumes in the anteroinferior temporal lobe (t = 4.83, P = 0.051) using DARTEL, and the dorsolateral prefrontal cortex (t = 5.80, P = 0.003) and parietal lobe (t = 4.3, P = 0.050) using the deformation‐based approach. Trend level associations were identified in the right medial prefrontal cortex (t = 4.49, P = 0.127) using DARTEL. Lack of illness awareness was also strongly associated with reduced total white matter volume (r = 0.401, P < 0.01) and illness severity (r = 0.559, P < 0.01). Conclusion: These results suggest a relationship between anosognosia and hemispheric asymmetry in schizophrenia, supporting previous volume‐based MRI studies in schizophrenia that found a relationship between illness unawareness and reduced right hemisphere gray matter volume. Functional imaging studies are required to examine the neural mechanisms contributing to these structural observations. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: schizophrenia, insight, anosognosia, illness awareness, VBM, deformation‐based analysis
INTRODUCTION
Lack of illness awareness or anosognosia is common among individuals with schizophrenia [Buckley2007; Jablensky et al.,1992; Olfson et al.,2006]. Lack of illness awareness contributes to medication nonadherence, poor treatment outcomes, higher rates of relapse, and rehospitalization [Amadoret al.,1994; Drake et al.,2007; Kemp and David,1996; Olfson et al.,2006]. Lack of illness awareness is also unresponsive to commonly used psychological interventions [Olfson et al.,2006].
The neural mechanisms of illness awareness in schizophrenia are not understood [David,1990]. Anosognosia is also observed in right hemisphere lesions due to stroke, traumatic brain injury, frontotemporal, and Alzheimer's dementia [Orfei et al.,2008]. Anosognosia associated with right hemispheric lesions or interhemispheric dysfunction may provide an anatomical model for exploring the neural correlates in other neuropsychiatric conditions with illness unawareness, such as schizophrenia [Shad et al.,2007].
To date, a number of studies have used volume‐based analyses to study the structural correlates of illness awareness and schizophrenia. Investigations that used a region of interest approach demonstrated volumetric reductions within right frontal regions, specifically the orbitofrontal (OFC), dorsolateral prefrontal (DLPFC), anterior cingulate cortices [Flashman et al.,2001; Sapara et al.,2007; Shad et al.,2006,2004], and also the right parietal lobe [Shad et al.,2007]. The few studies that used voxel‐based morphometry (VBM), which allows for the investigation of focal differences in neuroanatomy across the whole brain, however, have produced mixed results [Bassitt et al.,2007; Berge et al.,2010; Cooke et al.,2008; Morgan et al.,2010; Parellada et al.,2011]. One study of schizophrenia outpatients found no association between illness awareness and brain volume [Bassitt et al.,2007]. Another study of subjects with chronic schizophrenia and schizoaffective disorder found contributions from different regions within the temporal and parietal lobes associated with different aspects of illness awareness [Cooke et al.,2008]. Similarly inconsistent, studies of first episode patients have implicated a variety of brain regions in association with poor illness awareness, including decreased cingulate and superior temporal lobe volume bilaterally, and decreased left insula and right cuneus volumes [Morgan et al.,2010]; decreased gray matter volume in the cerebellum, inferior temporal gyrus, and inferior and medial frontal gyri bilaterally [Berge et al.,2010]; and reduced frontal and parietal volumes [Parellada et al.,2011].
The major limitation to compare volume‐based studies of illness awareness is the methodological differences between them. Some investigations have included all persons with psychosis (schizophrenia and schizoaffective disorder) versus others that were limited to first episode psychosis. Studies also differed in terms of the MR image preprocessing algorithms and illness awareness measurements that were used. Measures of illness awareness were devised by combining different aspects of scales, performing factor analyses, or using different scales entirely.
Because of the discrepancies within the literature among volume based studies, the aim of this work was to test the hypothesis of an association between reduced right hemisphere brain volume (relative to the left hemisphere) and illness awareness in schizophrenia. To accomplish this, an analysis of hemisphere asymmetry was performed, which may be more sensitive to these differences than a standard VBM approach. The findings were hypothesized to be lateralized to the right hemisphere with reduced brain volume in the prefrontal cortex, anterior temporal lobe, insula, and parietal regions based on the neurological literature and the studies in schizophrenia that used a region of interest approach.
METHODOLOGY
Subjects
This study is a cross‐sectional analysis that included 52 treated voluntary schizophrenia subjects recruited by advertisement/flyers posted across the hospital's campus and/or referral by the psychiatrist of record. The sample analyzed for the current study was collected by our laboratory from various PET studies that were approved by the Research Ethics Board of the Centre for Addictions and Mental Health, Toronto. Written informed consent was obtained after full explanation of the study procedures and risks. An assessment of psychiatric disorders was performed using the MINI‐Plus structured interview [Sheehan et al.,1998]. Symptoms of schizophrenia were assessed using the Positive and Negative Symptom Scale (PANSS) by experienced psychiatrists trained in administering the PANSS instrument [Kay et al.,1987]. Subjects were excluded if they had a history of head trauma resulting in loss of consciousness that required medical intervention; positive screen for pregnancy or were breast feeding at the time of the study; serious or unstable medical or neurological condition; met DSM‐IV TR [APM,2000] criteria for current substance abuse (except nicotine and caffeine) or dependence; serious suicidal ideation or had a likelihood of a serious suicide or homicide attempt; schizoaffective disorder, schizophreniform disorder or any affective disorder; or metal implants that would preclude the MRI scan. Urine toxicology screens were done as part of the initial assessment. Standard urine pregnancy tests were performed to exclude pregnancy.
Statistical Analysis
Statistical analyses of clinical and demographic variables were carried out with SPSS software (version 13.0). Anosognosia was measured using the PANSS insight and judgment item (G12), which is the most widely used and validated measure of illness awareness; highly correlated (r > 0.88) with more complex illness awareness or insight scales [Sanz et al.,1998]. Means and standard deviations were calculated for the demographic and clinical data, gray, white, CSF, and total intracranial volume. Spearman rho (r) correlations were performed between these variables and illness awareness scores. Mann‐Whitney U tests were used to compare illness awareness scores among males and females. The significance level for tests was established at P < 0.05.
MR Imaging Parameters
Participants underwent high‐resolution MRI fast spin echo T 1‐weighted imaging (fast spoiled gradient echo, TE = 5.3–15 ms, TR = 8.9–12 ms, FOV = 20 cm 3D, 256 × 256, voxel 0.8 × 0.8 × 1.5 mm3 isotropic, NEX = 1) acquired on a 1.5‐T Sigma‐GE scanner (General Electric Medical Systems, Milwaukee, WI).
MRI Data Analysis
MRI data were analyzed using SPM8 software package (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) running on MATLAB (version R2010a). Each T 1 structural MRI was preprocessed using the Diffeomorphic Anatomical Registration through Exponentiated Lie Algebra (DARTEL) method outlined in the SPM8 manual (see SPM website). The voxel size for all images after normalization was 2 × 2 × 2 mm3. The segmented normalized images were smoothed to a full width half maximum Gaussian kernel of 8 mm [Honea et al.,2005; White et al.,2001; Wilke et al.,2003]. A visual check was performed to ensure the quality, and origin of each image was oriented to the anterior commissure‐posterior commissure line using the SPM check registration function.
In order to perform a hemispheric analysis, each native image was flipped along the mid‐sagittal plane to create a mirror coronal image volume. Both the native and flipped images were preprocessed using the same DARTEL method described above. Following normalization, the flipped image was subtracted from the native image using the ImCalc function on SPM8 to create a delta image volume, capturing the voxel‐by‐voxel differences between each hemisphere.
To lend support to the initial analysis of hemispheric asymmetry performed with DARTEL, a second deformation‐based morphology strategy that does not involve tissue segmentation was used [Chung et al.,2001]. First, each native MRI volume was corrected for intensity nonuniformities due to MRI gradient inhomogeneities [Sled et al.,1998] and then transformed to MNI space using a linear transformation with 9 degrees of freedom (three scales, rotations, and translations). The transformed volume was then flipped along the mid‐sagittal plane to create a mirror coronal image of it. All volumes were transformed such that the mid‐sagittal plane was parallel to the Y–Z plane by applying half the rigid‐boy transformation (three translations and rotations). Each MRI volume was matched and nonlinearly registered to its respective flipped coronal image volume. The generated transformed image volume was used for the asymmetry analysis. All linear [Collins et al.,1994] and nonlinear [Collins et al.,1995] transformations were estimated using the mni_autoreg package, which is part of the MINC toolbox (http://www.bic.mni.mcgill.ca/ServicesSoftware/MINC). Intrasubject nonlinear transformations were estimated using an optimized version of the ANIMAL algorithm [Robbins et al.,2004]. The final transformation is represented by a set of local translations defined on grid nodes with 2 mm spacing. Each nonlinear transformation was then blurred with an 8 mm Gaussian kernel in each dimension and the Jacobian determinant [Chung et al.,2001] (a measure of local expansion and contraction) of each transformation was calculated. In order to perform a group analysis, Jacobian determinants were nonlinearly transformed to MNI using the nonlinear transformation [Collins et al.,1995] that matches each flipped input volume to the ICBM 152 template [Grabner et al.,2006].
Global Gray and White Matter Volume Analyses
In order to obtain volume measurements of the brain lobes of interest, the ANIMAL algorithm [Collin et al.,1995] was used. This technique uses spatial and intensity priors defined on a template to estimate a GM/WM classification. A lobe‐wise atlas defined on the template is then nonlinearly transformed using the inverse of the subject‐to‐atlas nonlinear transformation [Collin et al.,1995]. The regions of interest included were the gray and white matter volumes of the frontal, parietal, and temporal lobes. Partial correlations were performed with illness awareness, controlling for age, gender, and total intracranial volume (given by the sum of all the voxels within the gray matter, white matter, and cerebrospinal fluid compartments of each subject). A Bonferroni correction was calculated to control for multiple comparisons, setting the significance level at P < 0.01.
VBM and Brain Hemisphere Asymmetry Analyses
To examine the relationship between regional gray matter volume and illness awareness using VBM, a regression analysis was carried out with SPM8. Similarly, to investigate the relationship between regional gray matter hemispheric asymmetry (delta images) and illness awareness, a separate regression analysis was performed with SPM8. In both the analyses, age, gender, and total intracranial volume (given by the sum of all the voxels within the gray matter, white matter, and cerebrospinal fluid compartments of each subject) were entered as covariates.
Each within‐group analysis of normalized smoothed gray matter images comprised two contrasts, one to test for positive associations between regional gray matter volume and illness awareness scores, and the other to test for negative associations. Correlations were investigated on a voxel‐by‐voxel basis. As the a priori hypothesis was specific to the right frontal, parietal, and temporal lobes a mask was used to confine the statistical search to these regions. The threshold for these a priori regions was set at P < 0.001 level of significance (t > 3.25). A cluster was reported as significant if it was ≥ 20 voxels in size and the peak survived a family‐wise error (FWE) correction for multiple comparisons of P < 0.05 [Friston et al.,1996].
For our second deformation‐based morphology strategy, voxel‐wise statistics were estimated using the multistat function in the fmristat package [Worsley et al.,2002] (http://www.math.mcgill.ca/keith/fmristat/). Correction for multiple comparisons using a FWE of P < 0.05 was estimated with the stat_summary function.
RESULTS
Clinical and Demographic Variables
The demographic and clinical data are presented in Table I. The associations between lack of illness awareness scores and demographic and clinical variables are also presented in Table I. Illness severity (total PANSS, positive, negative, and general scores) was strongly associated with lack of illness awareness. No other significant associations were identified.
Table I.
Subject demographic and clinical data, and Spearman correlations (r) with lack of illness awareness (PANSS G12 item)
| Mean (SD) | r | |
|---|---|---|
| N | 52 | |
| Age, range | 41.5 (14.5), 23–77 | 0.121, P = 0.395 |
| Gender (M:F) | 33:19 | z = −1.051, P = 0.293a |
| Age of illness onset | 24.5 (5.7) | 0.088, P = 0.537 |
| Duration of Illness | 17.0 (14.1) | 0.018, P = 0.898 |
| Total PANSS scoreb | 43.0 (11.6) | 0.559* |
| PANSS positive score | 10.8 (3.6) | 0.397* |
| PANSS negative score | 12.0 (6.4) | 0.503* |
| PANSS general scoreb | 20.3 (5.6) | 0.498* |
| Total intracranial volume | 1655 cm3 (215.8) | −0.150, P = 0.288 |
| Antipsychotic agent | X 2 = 1.88, df = 3, P = 0.597c |
Mann‐Whitney U‐test.
Excluding insight and judgment item G12.
Kruskal‐Wallis H‐test.
P < 0.01.
Global Brain Volume
In the analysis investigating the relationship between lack of illness awareness and total gray and white matter volumes in the frontal, parietal, and temporal lobes, controlling for age, gender, and total intracranial volume, significant correlations were found between lack of illness awareness and reduced global white matter volume and reduced parietal lobe white matter volume (Table II). There were no significant associations found between gray matter volume and illness awareness scores.
Table II.
Partial correlations (r) between lack of illness awareness (PANSS G12 item) and regional brain volumes obtained with the ANIMAL algorithm (Collins et al.,1995)
| Region | Mean (SD) cm3 | r |
|---|---|---|
| Gray matter | ||
| Left frontal | 111.8 (25.7) | 0.186 |
| Right frontal | 112.4 (27.3) | 0.193 |
| Left parietal | 54.3 (10.9) | 0.174 |
| Right parietal | 53.8 (10.8) | 0.213 |
| Left temporal | 76.4 (13.6) | 0.177 |
| Right temporal | 75.2 (13.4) | 0.221 |
| White matter | ||
| Left frontal | 83.8 (15.0) | −0.342 |
| Right frontal | 85.5 (15.4) | −0.321 |
| Left parietal | 48.0 (11.4) | −0.378* |
| Right parietal | 46.6 (11.6) | −0.376* |
| Left temporal | 42.7 (8.6) | −0.318 |
| Right temporal | 41.6 (8.7) | −0.280 |
| Left total gray matter | 333.8 (63.4) | 0.193 |
| Right total gray matter | 332.3 (65.0) | 0.204 |
| Left total white matter | 207.5 (40.7) | −0.401* |
| Right total white matter | 204.8 (41.6) | −0.393* |
| Total intracranial gray matter | 666.1 (128.2) | 0.199 |
| Total intracranial white matter | 412.2 (82.0) | −0.401* |
P < 0.01
VBM: Gray Matter Volume and Illness Awareness
In the analysis exploring the relationship between gray matter volume using DARTEL and illness unawareness, controlling for age, gender, and total intracranial volume, no significant positive or negative relationships were identified.
Brain Hemisphere Asymmetry and Illness Awareness
The analysis of hemisphere asymmetry using DARTEL revealed associations between lack of illness awareness and right < left hemisphere volumes in the anteroinferior temporal lobe, OFC, and medial prefrontal cortex (Table III and Fig. 1). Only the finding in the anteroinferior temporal lobe survived correction for multiple comparisons. Trend level significance was obtained for the right medial prefrontal cortex (Table III and Fig. 1).
Table III.
Relationship between gray matter hemisphere asymmetry and illness awareness, controlling for age, gender, and total intracranial volume
| Cluster Maxima | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brain Region | BA | Voxels per clustera | P value Uncorrected | P value FWE corrected | x | y | z | T value | P value Uncorrected | P value FWE corrected |
| VBM | ||||||||||
| Right ant. temporal lobe | 20 | 165 | 0.049 | 0.130 | 51 | 0 | −50 | 4.83 | <0.001 | 0.051 |
| Right medial PFC | 10 | 29 | 0.389 | 0.664 | 1 | 50 | 0 | 4.49 | <0.001 | 0.127 |
| Right lateral OFC | 47 | 172 | 0.045 | 0.119 | 48 | 48 | −12 | 4.04 | <0.001 | 0.363 |
| Deformation‐based | ||||||||||
| Right dorsolateral PFC | 46 | 1457 | <0.001 | 0.023 | 27 | 49 | 24 | 5.80 | <0.001 | 0.003 |
| Right angular gyrus | 39 | 751 | <0.001 | 0.345 | 44 | −65 | 44 | 4.30 | <0.001 | 0.050 |
| Right insula | 47 | 341 | <0.001 | 0.300 | 43 | 23 | 2 | 3.57 | <0.001 | 0.350 |
PFC, prefrontal cortex; OFC, orbitofrontal cortex; FEW, family wise error.
VBM = 1.5 mm3 and deformation‐based = 1 mm3.
Figure 1.
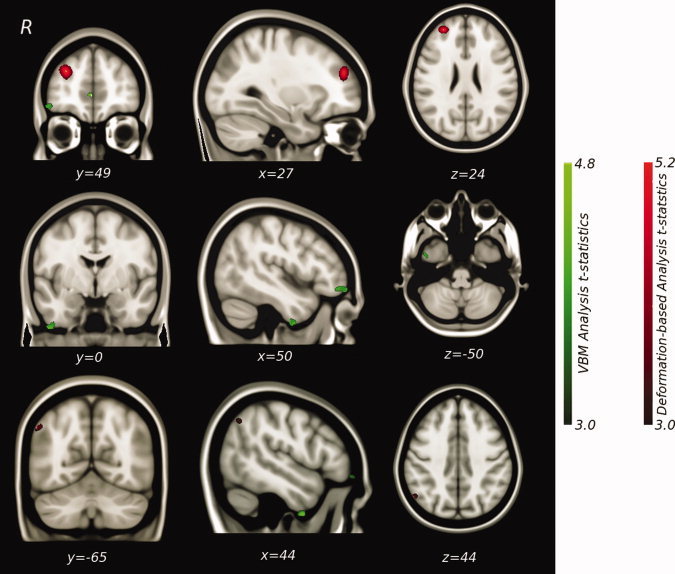
Regional gray matter hemisphere asymmetry correlations (right < left) with lack of illness awareness.
The second analysis of hemisphere asymmetry using the deformation‐based morphology strategy that does not involve tissue segmentation showed associations between lack of illness awareness and right < left hemisphere volumes in the DLPFC, angular gyrus/parietal lobe, and insula (Table III and Fig. 1). The associations in the DLPFC and parietal lobe survived correction for multiple comparisons.
DISCUSSION
Anosognosia, was first coined by Babinski [1914], and literally means lacking knowledge (gnosis) of disease (nosos). It is commonly associated with right hemispheric damage secondary to stroke, neurodegeneration, or brain injury [Orfei et al.,2008]. Ansogonosia in these contexts provides a neuroanatomical prototype for exploring lack of illness awareness in other neuropsychiatric conditions, such as schizophrenia [Shad et al.,2007]. An intriguing aspect of schizophrenia is that persons with the disorder vary in their degree of anosognosia. They can have equally bizarre delusions or perceptual disturbances but can be quite dissimilar in their ability to recognize that these experiences arise from their mind rather than a part of objective reality.
This study is the first to perform a hemispheric asymmetry analysis exploring the relationship between gray matter volume and illness unawareness in patients with schizophrenia. The results revealed an association between anosognosia in schziophrenia and relatively reduced gray matter volume in right hemisphere frontotemporoparietal regions, namely the right anteroinferior temporal lobe, DLPFC and right parietal lobe (i.e., angular gyrus). These findings support the results of other volume‐based imaging studies that used a region of interest approach, which have collectively identified an association between reduced right hemisphere frontotemporoparietal gray matter volume and anosognosia in schizophrenia [Flashman et al.,2001; Sapara et al.,2007; Shad et al.,2004,2006,2007].
For most healthy individuals, anatomical and functional asymmetry is the norm with respect to language ability and handedness, which are typically lateralized to the left hemisphere in association with greater left planum temporale volume. The right hemisphere (in particular, the right parietal lobe), to a lesser degree, also appears dominant for certain visuospatial abilities [Hugdahl,2000; Toga and Thompson,2003]. In contrast to healthy individuals, there is some, although conflicting evidence that persons with schizophrenia have reduced temporal lobe asymmetry, which is proposed to contribute to the experience of auditory hallucinations and other positive symptoms [Oertel‐Knochel and Linden, 2011].
To explain the role of the right hemisphere in illness awareness, the cerebral hemispheres may serve distinct functions when confronted with discrepant cognitive or sensory stimuli [Ramachandran et al.,2007]. The left hemisphere may be responsible for various classical psychodynamic defense mechanisms, such as denial, rationalization, confabulation, and repression, to reconcile conflicting or threatening information in order to maintain mental stability and coherence [Ramachandran,1995]. The right hemisphere, on the other hand, is hypervigilant to sensory inconsistencies serving to orient and reality test in response to divergent or unexpected stimuli [Posner and Petersen,1990; Ramachandran,1995; Ramachandran et al.,2007]. Together, the hemispheres provide a system of “checks” (right hemisphere) and “balances” (left hemisphere). The right hemisphere brings to consciousness threats to self (one's ingrain biopsychosocial patterns of expectations [Basch,1988]), whereas the left hemisphere attempts to reconcile and integrate conflicting or objectionable stimuli, such as the illness itself. This theory gains some support from the neurochemical literature [Toga and Thompson,2003; Tucker and Williamson,1984], which shows a leftward asymmetry of dopamine levels in the globus pallidus and basal ganglia [Glick et al.,1982; Wagner et al.,1983], and a rightward lateralization of norepinephrine neurons in the ventrolateral nuclei of the thalamus [Oke et al.,1978]. The above notion of hemispheric specialization remains highly debated and requires further study [Toga and Thompson,2003].
There are a number of methodological issues with voxel‐based MRI data analysis that can contribute to the inconsistency and reliability of findings, including differing tissue segmentation and normalization algorithms, and smoothing filter size [Henley et al.,2010; Honea et al.,2005; Tsang et al.,2008; White et al.,2001]. In the present study, VBM was performed using DARTEL, which achieves more accurate intersubject registration of brain images, providing better localization and sensitivity than the older unified segmentation method [Ashburner,2007; Ashburner and Friston,2005]. To ensure the reliability of the findings from the initial asymmetry analysis, a separate investigator carried out a second deformation‐based algorithm that uses the Jacobian of the deformation field to detect volumetric changes and does not involve tissue segmentation [Chung et al.,2001]. Deformation‐based procedures are limited by the accuracy of the nonlinear transformation and the heterogeneity of sulcal patterns in both hemispheres (see for example [Van Essen2005]). In this case, however, the accuracy of the ANIMAL algorithm has been verified in several recent studies [Chakravarty et al.,2009; Robbins et al.,2004] and compares favorably to other nonlinear registration algorithms [Klein et al.,2009]. Although the results of the hemisphere asymmetry analyses were not precise in terms of the clusters identified (i.e., reduced right anteroinferior temporal and medial prefrontal vs. right dorsolateral PFC and angular gyrus) the main hypothesis of relatively reduced frontotemporoparietal volume was confirmed.
In addition to the main finding, we also found an association between lack of illness awareness and reduced white matter volume (Table II). Diverse regional deficits in white (and gray) mater volume are commonly reported in schizophrenia [Honea et al.,2005]. The effects of these volumetric changes are unclear but may represent a cortical‐subcortical or interhemispheric “misconnection syndrome” [Spalletta et al.,2003] that permits left hemisphere dominance over the right. These findings require replication. Similar to a prior VBM study [Bassitt et al.,2007], this study did not identify any significant associations between regional gray matter volume and a lack of illness awareness. Surprisingly, no associations were identified between regional gray matter volumes and anosognosia using a region of interest approach (Table II).
The findings of this study are limited by a few factors. First, we used only one measure of insight. Although lack of insight is measured by a single item on the PANSS, it has been strongly correlated with other more elaborate standardized measures of insight (Pearson's r > 0.88) [Sanz et al.,1998]. Moreover, factor analyses of the various components of insight (i.e., general illness awareness, symptom awareness, awareness of the consequences of the disorder, and awareness of need for treatment) have produced single factor solutions, suggesting that these components may represent a single construct [Birchwood,1994; Cuesta et al.,2000; McEvoy et al.,1989; Mohamed et al.,2009]. Second, the illness unawareness scores were highly correlated with illness severity (Table I). To address the issue of colinearity, subsequent analyses were performed to assess the relationship between illness severity (PANSS total scores, positive symptom, negative symptom and general symptom subscale scores, and delusion item P1 scores) and regional hemisphere asymmetry using VBM. No significant associations were identified in any of these subsequent analyses (Supporting Information Tables A–E), suggesting the study's findings are specific to anosognosia. Third, our sample consisted of treated schizophrenia subjects (n = 52) receiving different antipsychotics, which may have differential effects on illness awareness. That being said, we did not see a significant effect of either illness duration or antipsychotic agent on anosognosia (Table I). Moreover, there is little‐to‐no evidence within the literature to support the possibility of an effect of antipsychotic medication type on illness awareness [Pallanti et al.,1999].
In summary, this is the first study to perform an analysis of hemisphere asymmetry for anosognosia in schizophrenia. The findings of an association between relatively reduced right regional frontotemporoparietal brain volume and lack of illness awareness are in support of prior volume‐based studies that used a region of interest approach, however, require replication.
The study authors are unaware of any functional imaging studies that have directly explored the relationship between illness awareness and schizophrenia. To determine the degree to which the right hemisphere and its associated regions are involved in insight in schizophrenia future functional imaging research is essential. By identifying the brain correlates of anosognosia, common to multiple psychiatric and neurological conditions, the possibility of direct intervention will emerge, including the use of novel pharmacological compounds and transcranial magnetic stimulation to target the neurocircuit of illness awareness.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supplemental Tables A‐E
Acknowledgements
Penny Barsoum and Wanna Mar for their research coordination activities.
REFERENCES
- Amador XF, Flaum M, Andreasen NC, Strauss DH, Yale SA, Clark SC, Gorman JM ( 1994): Awareness of illness in schizophrenia and schizoaffective and mood disorders. Arch Gen Psychiatry 51: 826–36. [DOI] [PubMed] [Google Scholar]
- APM ( 2000): The Diagnostic and Statistical Manual of Mental Disorders: American Psychiatric Pub Group. [Google Scholar]
- Ashburner J ( 2007): A fast diffeomorphic image registration algorithm. Neuroimage 38: 95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2005): Unified segmentation. Neuroimage 26: 839–851. [DOI] [PubMed] [Google Scholar]
- Babinski J (1914): Contribution à l'étude des troubles mentaux dans l'hémiplégie organique cérébrale (anosognosie). Revue Neurologique 27:845–848. [Google Scholar]
- Basch MF ( 1988): Understanding Psychotherapy: The Science Behind the Art. New York: Basic Books. [Google Scholar]
- Bassitt DP, Neto MR, de Castro CC, Busatto GF ( 2007): Insight and regional brain volumes in schizophrenia. Eur Arch Psychiatry Clin Neurosci 257: 58–62. [DOI] [PubMed] [Google Scholar]
- Berge D, Carmona S, Rovira M, Bulbena A, Salgado P, Vilarroya O ( 2010): Gray matter volume deficits and correlation with insight and negative symptoms in first‐psychotic‐episode subjects. Acta Psychiatr Scand 123: 431–439. [DOI] [PubMed] [Google Scholar]
- Birchwood M, Smith J, Drury V, Healy J, Macmillan F, Slade M ( 1994): A self‐report Insight Scale for psychosis: reliability, validity and sensitivity to change. Acta Psychiatr Scand 89: 62–67. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Wirshing DA, Bhushan P, Pierre JM, Resnick SA, Wirshing WC ( 2007): Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs 21: 129–141. [DOI] [PubMed] [Google Scholar]
- Chakravarty MM, Sadikot AF, Germann J, Hellier P, Bertrand G, Collins DL ( 2009): Comparison of piece‐wise linear, linear, and nonlinear atlas‐to‐patient warping techniques: analysis of the labeling of subcortical nuclei for functional neurosurgical applications. Hum Brain Mapp 30: 3574–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Worsley KJ, Paus T, Cherif C, Collins DL, Giedd JN, Rapoport JL, Evans AC ( 2001): A unified statistical approach to deformation‐based morphometry. Neuroimage 14: 595–606. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC ( 1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Collins DL, Holmes CJ, Peters TM, Evans AC ( 1995): Automatic 3‐D model‐based neuroanatomical segmentation. Human Brain Mapping 3: 190–208. [Google Scholar]
- Cooke MA, Fannon D, Kuipers E, Peters E, Williams SC, Kumari V ( 2008): Neurological basis of poor insight in psychosis: a voxel‐based MRI study. Schizophr Res 103: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta MJ, Peralta V, Zarzuela A ( 2000): Reappraising insight in psychosis. Multi‐scale longitudinal study. Br J Psychiatry 177: 233–240. [DOI] [PubMed] [Google Scholar]
- David AS ( 1990): Insight and psychosis. Br J Psychiatry 156: 798–808. [DOI] [PubMed] [Google Scholar]
- Drake RJ, Dunn G, Tarrier N, Bentall RP, Haddock G, Lewis SW ( 2007): Insight as a predictor of the outcome of first‐episode nonaffective psychosis in a prospective cohort study in England. J Clin Psychiatry 68: 81–86. [DOI] [PubMed] [Google Scholar]
- Flashman LA, McAllister TW, Johnson SC, Rick JH, Green RL, Saykin AJ ( 2001): Specific frontal lobe subregions correlated with unawareness of illness in schizophrenia: a preliminary study. J Neuropsychiatry Clin Neurosci 13: 255–257. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD ( 1996): Detecting activations in PET and fMRI: levels of inference and power. Neuroimage 4( 3 Pt 1): 223–235. [DOI] [PubMed] [Google Scholar]
- Glick SD, Ross DA, Hough LB ( 1982): Lateral asymmetry of neurotransmitters in human brain. Brain Res 234: 53–63. [DOI] [PubMed] [Google Scholar]
- Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL ( 2006): Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv 9( Pt 2): 58–66. [DOI] [PubMed] [Google Scholar]
- Henley SM, Ridgway GR, Scahill RI, Kloppel S, Tabrizi SJ, Fox NC, Kassubek J ( 2010): Pitfalls in the use of voxel‐based morphometry as a biomarker: examples from huntington disease. AJNR Am J Neuroradiol 31: 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE ( 2005): Regional deficits in brain volume in schizophrenia: a meta‐analysis of voxel‐based morphometry studies. Am J Psychiatry 162: 2233–2345. [DOI] [PubMed] [Google Scholar]
- Hugdahl K ( 2000): Lateralization of cognitive processes in the brain. Acta Psychol (Amst) 105: 211–235. [DOI] [PubMed] [Google Scholar]
- Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper JE, Day R, Bertelsen A ( 1992): Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten‐country study. Psychol Med Monogr Suppl 22: 1–97. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA ( 1987): The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276. [DOI] [PubMed] [Google Scholar]
- Kemp R, David A ( 1996): Psychological predictors of insight and compliance in psychotic patients. Br J Psychiatry 169: 444–450. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV ( 2009): Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 46: 786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy JP, Apperson LJ, Appelbaum PS, Ortlip P, Brecosky J, Hammill K, Geller JL, Roth L ( 1989): Insight in schizophrenia. Its relationship to acute psychopathology. J Nerv Ment Dis 177: 43–47. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Rosenheck R, McEvoy J, Swartz M, Stroup S, Lieberman JA ( 2009): Cross‐sectional and longitudinal relationships between insight and attitudes toward medication and clinical outcomes in chronic schizophrenia. Schizophr Bull 35: 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan KD, Dazzan P, Morgan C, Lappin J, Hutchinson G, Suckling J, Fearon P, Jones PB, Leff J, Murray RM, et al. ( 2010): Insight, grey matter and cognitive function in first‐onset psychosis. Br J Psychiatry 197: 141–148. [DOI] [PubMed] [Google Scholar]
- Oertel‐Knochel V, Linden DE: 0000 Cerebral Asymmetry in Schizophrenia. Neuroscientist 2011. Apr 25 [Epub ahead of print] PMID: 21518811. [DOI] [PubMed] [Google Scholar]
- Oke A, Keller R, Mefford I, Adams RN ( 1978): Lateralization of norepinephrine in human thalamus. Science 200: 1411–1413. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Wilk J, West JC ( 2006): Awareness of illness and nonadherence to antipsychotic medications among persons with schizophrenia. Psychiatr Serv 57: 205–211. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Robinson RG, Bria P, Caltagirone C, Spalletta G ( 2008): Unawareness of illness in neuropsychiatric disorders: phenomenological certainty versus etiopathogenic vagueness. Neuroscientist 14: 203–222. [DOI] [PubMed] [Google Scholar]
- Pallanti S, Quercioli L, Pazzagli A ( 1999): Effects of clozapine on awareness of illness and cognition in schizophrenia. Psychiatry Res 86: 239–249. [DOI] [PubMed] [Google Scholar]
- Parellada M, Boada L, Fraguas D, Reig S, Castro‐Fornieles J, Moreno D, Gonzalez‐Pinto A, Otero S, Rapado‐Castro M, Graell M, et al. ( 2011): Trait and state attributes of insight in first episodes of early‐onset schizophrenia and other psychoses: a 2‐year longitudinal study. Schizophr Bull 37: 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE ( 1990): The attention system of the human brain. Ann Rev Neurosci 13: 13–25. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS ( 1995): Anosognosia in parietal lobe syndrome. Conscious Cogn 4: 22–51. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, McGeoch PD, Williams L ( 2007): Can vestibular caloric stimulation be used to treat Dejerine‐Roussy Syndrome? Med Hypotheses 69: 486–488. [DOI] [PubMed] [Google Scholar]
- Robbins S, Evans AC, Collins DL, Whitesides S ( 2004): Tuning and comparing spatial normalization methods. Med Image Anal 8: 311–323. [DOI] [PubMed] [Google Scholar]
- Sanz M, Constable G, Lopez‐Ibor I, Kemp R, David AS ( 1998): A comparative study of insight scales and their relationship to psychopathological and clinical variables. Psychol Med 28: 437–446. [DOI] [PubMed] [Google Scholar]
- Sapara A, Cooke M, Fannon D, Francis A, Buchanan RW, Anilkumar AP, Barkataki I, Aasen I, Kuipers E, Kumari V ( 2007): Prefrontal cortex and insight in schizophrenia: a volumetric MRI study. Schizophr Res 89: 22–34. [DOI] [PubMed] [Google Scholar]
- Shad MU, Muddasani S, Prasad K, Sweeney JA, Keshavan MS ( 2004) Insight and prefrontal cortex in first‐episode schizophrenia. Neuroimage 22: 1315–1320. [DOI] [PubMed] [Google Scholar]
- Shad MU, Muddasani S, Keshavan MS ( 2006): Prefrontal subregions and dimensions of insight in first‐episode schizophrenia—a pilot study. Psychiatry Res 146: 35–42. [DOI] [PubMed] [Google Scholar]
- Shad MU, Keshavan MS, Tamminga CA, Cullum CM, David A ( 2007): Neurobiological underpinnings of insight deficits in schizophrenia. Int Rev Psychiatry 19: 437–446. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC ( 1998): The Mini‐International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 59( Suppl 20): 22–33;quiz 34–57. [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC ( 1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17: 87–97. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Tomaiuolo F, Marino V, Bonaviri G, Trequattrini A, Caltagirone C ( 2003): Chronic schizophrenia as a brain misconnection syndrome: a white matter voxel‐based morphometry study. Schizophr Res 64: 15–23. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM ( 2003): Mapping brain asymmetry. Nat Rev Neurosci 4: 37–48. [DOI] [PubMed] [Google Scholar]
- Tsang O, Gholipour A, Kehtarnavaz N, Gopinath K, Briggs R, Panahi I ( 2008): Comparison of tissue segmentation algorithms in neuroimage analysis software tools. Conf Proc IEEE Eng Med Biol Soc 2008: 3924–3928. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Williamson PA ( 1984): Asymmetric neural control systems in human self‐regulation. Psychol Rev 91: 185–215. [PubMed] [Google Scholar]
- Van Essen DC ( 2005): A Population‐Average, Landmark‐ and Surface‐based (PALS) atlas of human cerebral cortex. Neuroimage 28: 635–662. [DOI] [PubMed] [Google Scholar]
- Wagner HN, Jr. , Burns HD, Dannals RF, Wong DF, Langstrom B, Duelfer T, Frost JJ, Ravert HT, Links JM, Rosenbloom SB, et al. ( 1983): Imaging dopamine receptors in the human brain by positron tomography. Science 221: 1264–1266. [DOI] [PubMed] [Google Scholar]
- White T, O'Leary D, Magnotta V, Arndt S, Flaum M, Andreasen NC ( 2001): Anatomic and functional variability: the effects of filter size in group fMRI data analysis. Neuroimage 13: 577–588. [DOI] [PubMed] [Google Scholar]
- Wilke M, Kassubek J, Ziyeh S, Schulze‐Bonhage A, Huppertz HJ ( 2003): Automated detection of gray matter malformations using optimized voxel‐based morphometry: a systematic approach. Neuroimage 20: 330–343. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC ( 2002): A general statistical analysis for fMRI data. Neuroimage 15: 1–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supplemental Tables A‐E


