Abstract
The concept of “social self” is often described as a representation of the self‐reflected in the eyes or minds of others. Although the appearance of one's own face has substantial social significance for humans, neuroimaging studies have failed to link self‐face recognition and the likely neural substrate of the social self, the medial prefrontal cortex (MPFC). We assumed that the social self is recruited during self‐face recognition under a rich social context where multiple other faces are available for comparison of social values. Using functional magnetic resonance imaging (fMRI), we examined the modulation of neural responses to the faces of the self and of a close friend in a social context. We identified an enhanced response in the ventral MPFC and right occipitoparietal sulcus in the social context specifically for the self‐face. Neural response in the right lateral parietal and inferior temporal cortices, previously claimed as self‐face‐specific, was unaffected for the self‐face but unexpectedly enhanced for the friend's face in the social context. Self‐face‐specific activation in the pars triangularis of the inferior frontal gyrus, and self‐face‐specific reduction of activation in the left middle temporal gyrus and the right supramarginal gyrus, replicating a previous finding, were not subject to such modulation. Our results thus demonstrated the recruitment of a social self during self‐face recognition in the social context. At least three brain networks for self‐face‐specific activation may be dissociated by different patterns of response‐modulation in the social context, suggesting multiple dynamic self‐other representations in the human brain. Hum Brain Mapp, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: self concept, social values, visual perception, social perception, magnetic resonance imaging, echo‐planar imaging, prefrontal cortex, parietal lobe, temporal lobe
INTRODUCTION
A distinction between the two concepts of self, namely, the “physical self” and the “social self,” is a common feature of recent multicomponent models of self in developmental and animal psychology [Bekoff and Sherman, 2004; Morin, 2006; Rochat, 2003]. Physical self is almost invariantly conceptualized as a visual‐kinesthetic representation of one's own body. Social self is conceptually dissociated from the physical self in that it is a representation of the self reflected in the eyes or minds of others, though the scope of the concept varies across models. Compared with the physical self, the social self is usually assumed to develop later in infants and is often considered to be associated with a large brain size or more sophisticated social behavior in animals [Gallup, 1982; Marino, 2002; Plotnik et al., 2006; Prior et al., 2008].
In humans, one's own face is assumed to represent both the physical self and social self. The association of one's face with the physical self is obvious since the face is a part of the body. The engagement of the social self, at least in its minimal sense of being relevant to the perceptions of others, has several lines of empirical and experimental support. It is widely held that we direct attention toward publicly observable aspects of the self (i.e., public self‐consciousness) [Fenigstein et al., 1975]. Although the degree of explicit public self‐consciousness varies across individuals [Fenigstein et al., 1975], some extent of interest in the appearance of one's own face seems to be common on an implicit level. This has been supported by the effect a recognized face's resemblance to the self‐face has on social behavior [DeBruine et al., 2008; Goodman et al., 2007]. Cognitive processes of the social self underlying such a behavioral manifestation may be in part driven by the social value of the self‐face. Given the social value of the face in social survival [Grammer et al., 2003; Reis et al., 1982], a concern for the appearance of one's own face has ecological and evolutionary advantages. Improved facial appearance due to orthodontic surgery is shown to affect the self‐esteem and mood of patients [Nicodemo et al., 2008].
The enigma in neuroimaging studies of self‐face recognition is, however, scant evidence for the involvement of the known neural correlates of the social self. The processes relevant to social self typically involve the medial prefrontal cortex (MPFC) [Amodio and Frith 2006; Krueger et al., 2009; Van Overwalle, 2009]. This region responds to self‐directed eye gaze and to hearing one's own name called [Kampe et al., 2003; Schilbach et al., 2006]. It shows higher activation during a personality‐trait judgment of the self than during that of the other [Craik et al., 1999; Kelley et al., 2002; Ochsner et al., 2005], and during the perception of one's own reputation than during that of others [Izuma et al., 2008]. Self‐conscious emotions, such as guilt and embarrassment, also activate this region [Takahashi et al., 2004; Zahn et al., 2009]. On the other hand, self‐face recognition rarely activates the MPFC. In recent studies that adopted a sufficiently stringent statistical threshold, neural response specific to the self‐face was often found in the lateral cortical regions predominantly in the right hemisphere [Morita et al., 2008; Sugiura et al., 2008; Sugiura et al., 2006; Sugiura et al., 2005; Uddin et al., 2005]. Specifically, the regions included the right lateral frontal cortex (in the inferior frontal gyrus and around the precentral sulcus), the parietal cortex (around the intraparietal and postcentral sulci), and the inferior occipitotemporal cortex. Activation in these regions has often been attributed to processes relevant to the physical self [Sugiura et al., 2008; Sugiura et al., 2006; Uddin et al., 2007]. Although a single previous study reported a small cluster of self‐face‐specific activation in the MPFC using a liberal threshold [Platek et al., 2006], the finding has not been replicated [Platek and Kemp, 2009]. The discrepancy between the implicated neural substrates of self‐face recognition and those of the social self is not a new concept. Indeed, it has been pointed out before [Gillihan and Farah, 2005; Uddin et al., 2007].
We hypothesized that involvement of the cognitive process relevant to the social self, or activation of the MPFC during self‐face recognition, are conditional to the social context. Here the social context implies an environment where one automatically takes a socially adaptive stance. We contrived the social context simply by exposing subjects to other people, who served as references for the evaluation of one's own social value. This idea is based on a social comparison theory [Festinger, 1954; Morse and Gergen, 1970], in which people are assumed to automatically evaluate their own social value by comparing themselves with others when comparable others are available. Mere exposure to others is classically known to affect human behavior presumably via a change in one's recognition of themselves in the social context [Darley and Latane, 1968; Hunt and Hillery, 1973]. It has been demonstrated that when subjects are incidentally exposed to a face picture or a description of comparable others, their self‐evaluation is modulated by the facial attractiveness or perceived dominance of the presented others [Cash et al., 1983; Gutierres et al., 1999].
In a series of face recognition task trials, we manipulated the richness of the social context by changing the number of unfamiliar faces used as distracters. We assumed that self‐face recognition in a context where a large number of distracter faces are presented induces the social self (high social context; Fig. 1a, right). On the contrary, when the self‐face is presented in a task setting where a small number of distracter faces are presented (low social context; Fig. 1a, left), as in previous studies (e.g., Morita et al., 2008; Sugiura et al., 2006; Uddin et al., 2005], the degree to which the social self is involved should be minimal.
Figure 1.
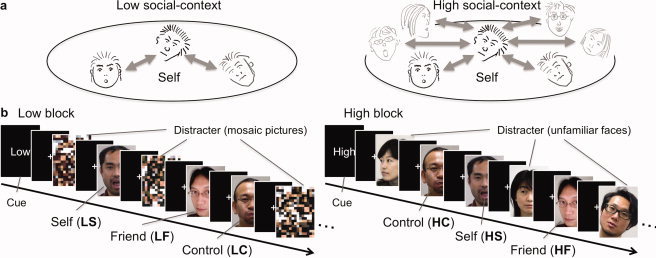
Experimental design. (a) Concept of social context in this study. In the low and high (rich) social contexts, the numbers of comparable others are small and large, respectively. People are assumed to automatically evaluate their own social value by comparing themselves with others, to a larger degree in the latter than in the former context. The faces in lighter gray can be considered to represent the unfamiliar distracter faces in the high social‐context block (Fig. 1b). (b) Schema of stimulus presentation. Three target faces (self, friend, and control) and distracters were presented in a random order in both types of blocks. Distracters were mosaic pictures and unfamiliar faces in the low and high social‐context blocks, respectively. Each block started from the presentation of the block‐type cue (2,400 ms) followed by 1,200‐ms presentations of pictures with an inter‐stimulus interval of 2,400 ms. A central fixation cross was presented throughout the block. Event‐related responses to the self, friend, and control faces were separately modeled for the low (LS, LF, and LC, respectively) and high (HS, HF, and HC, respectively) blocks.
We searched for a cortical response to the self‐face modulated by the social context, particularly expecting an enhanced MPFC response as a result of the enriched social context (i.e., high vs. low). We also predicted that this modulation would not be observed in the cortical regions where self‐face‐specific involvement had been suggested in previous studies (i.e., equivalent to the low social context). Such regions include the right lateral parietal and temporal cortices, where self‐face‐specific activation has been established, and the bilateral temporoparietal regions, where self‐face‐specific deactivation has been reported [Sugiura et al., 2008].
MATERIALS AND METHODS
Participants
Twenty‐six healthy undergraduate students (18 males, eight females; age, 18–24 years) were recruited from Tohoku University. They responded to our advertisement in pairs or groups of three as close friends of the same gender. Written informed consent was obtained from each subject. No subject had any history of neurological or psychiatric illness. All were right‐handed, as measured by the Edinburgh Handedness Inventory [Oldfield, 1971]. The experimental protocol was approved by the ethical committee of Tohoku University.
Because three subjects who produced an excessive amount of head motion during the fMRI measurement were excluded, data from 23 subjects (16 males, seven females; age, 18–24 years) were analyzed.
Stimuli and Tasks
Three types of target faces, a subject's own face (self, S), the face of a close friend (friend, F), and a specific unfamiliar face (control, C), were presented intermixed with either random mosaic pictures (low social‐context block, L), or with various other unfamiliar faces (high social‐context block, H). The design was intended to model event‐related responses to the self, friend, and control faces separately for the low (LS, LF, and LC, respectively) and high (HS, HF, and HC, respectively) blocks. (Fig. 1b). The friend was included as a high‐level control to exclude the effect of person familiarity, as in previous studies (e.g., Platek et al., 2006; Sugiura et al., 2005]. Each subject performed a familiar/unfamiliar judgment for each presented face to maintain and assure the subject's attention.
The target faces were prepared in the following procedures. A set of 25 pictures of the face in different non‐emotional expressions with directed or diverted eye‐gaze (see Sugiura et al., 2006 for details) was prepared for each subject, using a digital camera under the same lighting conditions, one to three weeks prior to the experiment. For each subject, a set of 24 pictures of the subject's own face and a set of 24 pictures of the face of his/her friend recruited at the same time were used for the self and friend stimuli, respectively. Note that the processes for visual face perception were controlled because the same face was presented once as a self stimulus and once as a friend stimulus to two friends. A set of 24 face pictures from an unfamiliar subject were selected for the control stimuli; the face did not have features (e.g., glasses, dyed hair, facial hair) distinct from those of the self and friend faces to prevent such features from enabling the subjects to perform the familiar/unfamiliar judgment task without identifying the presented face. The pictures of expressionless faces in the frontal view were not included in each set, and were spared for only the practice session.
The 72 distracter faces used in the high blocks for all the subjects were composed of two different face pictures of 36 people (18 males, 18 females), which were selected from a collection of similar pictures used in a previous self‐face study [Sugiura et al., 2006] prepared using the same procedures and subject source (i.e., same age range). Six mosaic pictures were prepared for the low blocks.
In each block, six images of the target faces, namely, two self‐face, two friend face, and two control face, were presented. In the low block, these six target faces as well as six mosaic pictures were presented in a randomized order. In the high block, the six target faces and six distracter faces were presented in a random order. Each face was presented for 1200ms followed by an eye‐fixated rest of 2400ms. The inter‐stimulus interval was not jittered, which we do not consider problematic because we were interested in the neural response to a specific target‐face (primarily the self, and later the friend's face as well), rather than a pure event‐related response (see Methodological considerations in the Discussion for our detailed rationale). Each block started with the presentation of a 2400‐ms cue indicating a block type (i.e., high, low), followed by an eye‐fixated rest of 2400ms, and then presentation of 12 stimuli (Fig. 1b). No additional resting state was inserted between the blocks. The block‐type cue was necessary to make the subjects aware of the nature of the upcoming block. Without the cue, subjects would not be aware of the block's context until the presentation of the first distracter. The high and low blocks were each alternated 12 times. Each picture of the target‐face appeared once in the low block, and once in the high block. The main part of the experiment session (1152s) was preceded by a practice session (60s), which included one miniature version of both the high and low blocks (one self, one friend, one control, and three distracter‐face/mosaic stimuli). A 12‐s rest followed the practice session to allow a major part of the hemodynamic response from the practice session to disappear. The visual stimulus was projected onto a semi‐lucent screen attached to the head‐coil of the MRI scanner and was viewed via a mirror.
Each subject was asked to judge the familiarity of the presented face and to press one of two buttons as quickly as possible; the right index and middle fingers were assigned to familiar (the self and friend) and unfamiliar faces, respectively. No response was required for the mosaic stimuli. In the instructions of the task, each subject was informed that the block‐type cue indicates only the difference in the number of presented faces in the block and has nothing to do with the task they should perform (i.e., familiarity judgment).
fMRI Data Acquisition
Thirty‐four transaxial gradient‐echo images (echo time = 50 ms, flip angle = 90°, slice thickness = 3 mm, slice gap = 0.99 mm, FOV = 192 mm, matrix = 64 × 64, voxel size = 3 × 3 × 3.99 mm3) covering the whole cerebrum were acquired at a repetition time of 3000 ms, using an echo planar sequence and a Siemens Symphony (1.5 T; Siemens, Erlangen, Germany) MR scanner. While 408 scans were acquired in total (1,224s), the first 24 scans acquired during the practice session and the 12‐s rest period were excluded from the analysis.
fMRI Image Preprocessing
The following preprocessing procedures were performed using Statistical Parametric Mapping (SPM5) software (Wellcome Department of Imaging Neuroscience, London, UK) and MATLAB (Mathworks, Natick, MA): the adjustment of acquisition timing across slices, correction for head motion, spatial normalization using the EPI‐MNI template, and smoothing, using a Gaussian kernel with a full‐width at half‐maximum of 8 mm.
fMRI Data Analysis
A conventional two‐level approach for event‐related fMRI data was adopted, using SPM5. A voxel‐by‐voxel multiple regression analysis was conducted in the first‐level within‐subject (fixed effects) model. Expected signal changes were modeled for nine conditions, including the six conditions of interest (i.e., HS, HF, HC, LS, LF, LC), the conditions for the distracter faces (in the high block), the mosaic pictures (in the low block), and the error trials. Any trials with an incorrect response were categorized as error trials. Although both the HC condition and the condition for the distracter faces were characterized by unfamiliar‐face presentations in the high block, only the HC condition was comparable to other conditions of interest in that the same face was repeatedly presented. A model of the expected signal change was constructed using the hemodynamic response function provided by SPM5. A high‐pass filter with a cutoff period of 192 s, twice the period spanning a set of high and low blocks [Henson, 2003], was used to eliminate the artifactual low‐frequency trend.
Voxel‐by‐voxel statistical inference on contrasts of parameter estimates was then performed on the second‐level between‐subject (random effects) model, using one‐sample t‐tests. In some analyses, to identify a specific activation pattern, analysis of the main contrast was confined to areas that showed significant activation in one or two specific contrasts [i.e., mask (s)]. The statistical threshold was set to P < 0.001 for height and corrected to P < 0.05 for multiple comparisons using cluster size; assuming the whole brain as a search volume, irrespective of the application of the mask. The images for the masks were generated using a threshold of P < 0.05 (uncorrected).
Finally, for each identified activated area, we examined ad hoc the full activation profile. The analyses were conducted on parameter estimates at each activation peak using SPSS Statistics 17.0 (SPSS Inc., Chicago, IL). First the modulation effect (high vs. low blocks) was assessed for each target face using a paired t‐test (two tailed; P < 0.05, uncorrected). Second, specificity of the modulation effect of a particular target‐face was inferred using a two‐way (two block type × three face type) repeated‐measure ANOVA. Self‐face‐specific modulation was expected to produce a significant modulation effect only for the self‐face (not for the friend's face or the control face), and a significant interaction between the block type and target‐face type in the ANOVA.
In the second‐level voxel‐by‐voxel analyses, the following contrasts were tested
-
1
The modulation of activation for the self‐face in the social context, which is our major interest, was identified using the contrast HS − LS.
-
2
To examine whether self‐face‐specific activation previously reported was modulated by the social context, regions showing higher activation for the self than for the friend face were identified as they were in previous studies [e.g., Platek et al., 2006; Sugiura et al., 2005]. Analyses for the high and low blocks used contrasts HS − HF and LS − LF, respectively. The analyses were performed separately with consideration for the possibility that the activation would not be replicable in the high block due to the modulation.
-
3
Similarly, to examine whether self‐specific deactivation previously reported [Sugiura et al., 2008] was modulated by the social context, regions where activation was higher for both friend and control stimuli relative to self stimuli were identified separately for the two block types. For the high block, the contrast (HF + HC) ‐ 2HS was masked by contrasts HF − HS and HC − HS (to assure that activation was higher for both friend and control stimuli than for self stimuli). For the low block, (LF + LC) ‐ 2LS was masked by LF − LS and LC − LS.
Behavioral Data Analysis
The percentages of correct responses and the mean reaction times during the familiar/unfamiliar judgment were analyzed using SPSS Statistics. Analyses focused on the modulation effect induced by the social context (effect of the block type) and the difference between the three target‐face types among the six conditions of interest, as they conform to the analyses of the fMRI data.
RESULTS
Behavioral Data
The behavioral data are summarized in Table I. No significant difference between the high and low blocks (P ≥ 0.05; paired t‐test, two tailed) was found for any face type in either behavioral‐data set. A two‐way (two block type x three face type) repeated‐measure ANOVA also revealed no significant main effect or interaction (P ≥ 0.05; F test) in either behavioral‐data set.
Table I.
Behavioral data
| Self | Friend | Control | Distracter | ||||
|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | High | |
| Correct response (%) | 99.8 ± 1.1 | 99.2 ± 3.0 | 98.7 ± 3.0 | 97.7 ± 3.0 | 98.2 ± 3.3 | 98.5 ± 3.3 | 99.1 ± 1.4 |
| Reaction time (ms) | 721 ± 89 | 722 ± 86 | 729 ± 81 | 734 ± 89 | 722 ± 92 | 705 ± 93 | 724 ± 82 |
The percentage of correct responses (%) and mean reaction times (ms) are shown for each condition. Values are the mean ± standard deviation.
fMRI Data
-
1
The contrast HS − LS, intended for the modulation of activation for the self‐face, identified activation in the ventral part of the MPFC and in the posterior‐medial region with peaks located at the posterior cingulate cortex and the bilateral occipitoparietal sulci (Fig. 2; Table II). As a result of the ad hoc analysis of the activation profile (Table II), modulation was specific to the self‐face in the MPFC (Fig. 2a) and the right occipitoparietal sulcus (Fig. 2b), in that the modulation was significant (P < 0.05; paired t‐test, two tailed) only for the self‐face and the block type × face type interaction (P < 0.05; two‐way repeated‐measure ANOVA).
-
2
The contrast LS − LF, intended for the replication of previously reported self‐face‐specific activation, identified activation in the right inferior temporal gyrus (posterior part), several regions in the right parietal cortices, and the inferior frontal gyrus (Fig. 3; Table III). The parietal activation cluster included the peaks at the intraparietal sulcus (posteromedial part), the junction of the intraparietal sulcus and postcentral suclus, and the supramarginal gyrus (the anterior bank of the postcentral sucus). Activation peaks in the inferior frontal gyrus were located at the pars opercularis and pars triangularis. Enhancement of neural response as a result of the social context was not observed in these regions for the self‐face nor the control face (Table III). However, significant enhancement was observed for the friend's face in all regions but the pars triangularis of the inferior frontal gyrus. In the inferior temporal and supramarginal gyri, the interaction of the block type and face type was significant (P < 0.05; two‐way repeated‐measure ANOVA). The contrast HS − HF replicated the activation in the pars triangularis of the right inferior frontal gyrus only (Table III).
-
3
The contrasts (HF + HC) ‐ 2 × HS, intended for the replication of previously reported self‐face‐specific deactivation, identified activation in the left middle temporal gyrus and the right supramarginal gyrus (Fig. 4; Table IV). In these regions, no significant modulation (P < 0.05; paired t‐test, two tailed) or interaction (P < 0.05; two‐way repeated‐measure ANOVA) was observed (Table IV). The contrast (LF + LC) ‐ 2 × LS gave no significant activation.
Figure 2.
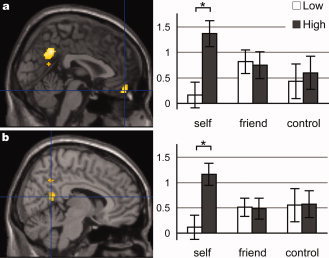
Self‐face‐specific response enhancement in an enriched social context. (a) the ventral MPFC [2 50−20]. (b) the right occipitoparietal sulcus [8 −54 14]. Activation is superimposed onto the sagittal section (at the peak voxel) of the standard anatomical image of SPM5 (left panel). Activation profiles (right panel) show the parameter estimate (partial regression coefficient or beta value) at each peak voxel. *P < 0.05. Significant interaction of block type and face type in both peaks was given by ANOVA.
Table II.
Enhanced response to self‐face in the enriched social context
| Structure | Peak | Cluster | Modulation (P) | ANOVA (P) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | T | Size | P | Self | Friend | Control | Context | Face | Interaction | |
| MPFC (ventral) | 2 | 50 | −20 | 4.57 | 110 | 0.045 | <0.001 | 0.024 | 0.011 | |||
| Posterior cingulate cortex | 0 | −54 | 36 | 5.63 | 397a | <0.001 | <0.001 | 0.031 | ||||
| Occipitoparietal sulcus | −6 | −62 | 14 | 4.98 | a | <0.001 | <0.001 | |||||
| 8 | −54 | 14 | 4.30 | a | <0.001 | 0.018 | 0.022 | |||||
Significant activation in the contrast HS – LS. Coordinates (x, y, z) and the t‐value of the activation peak, the size of the activation cluster (number of voxels; 2 × 2 × 2 mm3 / voxel; lowercase letters indicate that the peak is in the same cluster as other peaks with the same letter) are shown. For each peak voxel, P‐values of the modulation effects for the three face types (paired t‐test, two‐tailed), and P‐values of the ANOVA (F‐test) for the main effect of Context (high and low), main effect of Face (self, friend, and control), and Context × Face interaction, are presented. L, left; R, right; M, para‐midplane.
Figure 3.
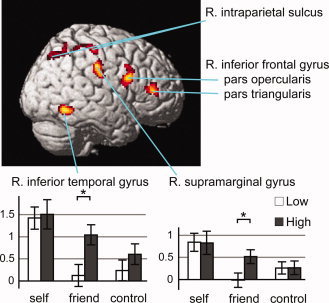
Self‐face‐specific activation (low block) and self‐friend assimilation (high block). Activation in the contrast LS − LF is surface‐rendered onto the standard anatomical image of SPM5: the right inferior temporal gyrus [54 −56 −12], intraparietal sulcus (posteromedial [24 −70 48], junction with the postcentral sulcus [54 −26 54]), supramarginal gyrus [56 −22 38], and inferior frontal gyrus (pars opercularis [48 10 22], pars triangularis [44 38 10]). All regions but the pars triangularis of the inferior frontal gyrus showed social‐context‐dependent modulation for the friend face. Interaction of the block type and face type was significant in the inferior temporal and supramarginal gyri. Other details are the same as for Figure 2.
Table III.
Self‐face‐specific activation
| Structure | Peak | Cluster | Modulation (P) | ANOVA (P) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X | y | z | T | Size | P | Self | Friend | Control | Context | Face | Interaction | ||
| Inferior temporal gyrus (posterior) | R | 54 | −56 | −12 | 5.53 | 197 | 0.002 | <0.001 | 0.001 | <0.001 | 0.010 | ||
| Intraparietal sulcus (posteromedial) | R | 24 | −70 | 48 | 4.62 | 197 | 0.002 | 0.030 | <0.001 | ||||
| Intraparietal sulcus /Postcentral sulcus | R | 54 | −26 | 54 | 4.63 | 113 | 0.036 | 0.044 | 0.001 | ||||
| Supramarginal gyrus | R | 56 | −22 | 38 | 5.67 | 221 | 0.001 | <0.001 | 0.001 | 0.041 | |||
| Inferior frontal gyrus (pars opercularis) | R | 48 | 10 | 22 | 5.90 | 250 | <0.001 | 0.003 | <0.001 | <0.001 | |||
| Inferior frontal gyrus (pars triangularis) | R | 44 | 38 | 10 | 4.99 | 164 | 0.006 | <0.001 | |||||
Significant activation in the contrast LS – LF. The contrast HS – HF replicated the activation in the pars triangularis of the right inferior frontal gyrus only (Peak [x, y, z, T] = [48, 38, 8, 5.10]; Cluster [Size, P] = [174, 0.009]). Details of the presentation are the same as those for Table II.
Figure 4.
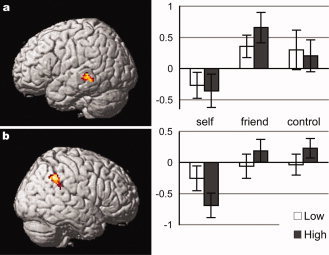
Self‐face‐specific deactivation. Activation in the contrast HF + HC − 2 × HS is surface‐rendered onto the standard anatomical image of SPM5. (a) the left middle temporal gyrus [‐64 −34 −2]. (b) the right supramarginal gyrus [56 −46 38]. Other details are the same as for Fig. 2.
Table IV.
Self‐face‐specific inactivation
| Structure | Peak | Cluster | Modulation (P) | ANOVA (P) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | T | Size | P | Self | Friend | Control | Context | Face | Interaction | |
| Middle temporal gyrus | −64 | −34 | −2 | 4.58 | 132 | 0.036 | <0.001 | |||||
| Supramarginal gyrus | 56 | −46 | 38 | 4.78 | 189 | 0.006 | 0.003 | |||||
Significant activation in the contrast HF + HC – 2 × HS. The contrast LF + LC – 2 × LS gave no significant activation. Details of the presentation are the same as those for Table II.
DISCUSSION
The results are the first to provide neuroscientific evidence that the social self is involved in self‐face recognition and the involvement is conditional based on the social context. As expected, enhanced neural response to the self‐face in the enriched social context, specifically the increased number of other faces for reference, was observed in the MPFC. On the contrary, the right lateral cortical and bilateral temporoparietal regions, which have been previously reported to show self‐face‐specific activation and deactivation, respectively, are shown to be irrelevant to the social self. These regions did not show response modulation induced by the social context.
Unexpectedly, some of the right lateral cortical regions, including the inferior temporal and supramarginal gyri, previously assumed to be self‐face‐specific, presented an enhanced response to the friend's face in the high block. That is, the response of these regions to the friend's face was similar to that to the self‐face when the social context was enriched. This assimilation of the friend with the self, in terms of the neural response in these regions, may suggest a dynamic modulation of the meaning of personal familiarity in the social context.
Enhanced Medial‐Cortical Response to the Self‐Face by the Social Context
Self‐face‐specific enhancement of activation was observed in the ventral, rather than the dorsal, part of the MPFC. This appears to support our assumption that the modulation effect induced by the social context in this study was relevant to the social evaluation process. Evidence from neuropsychological and functional imaging studies suggests the ventral part of the MPFC plays a critical role in representing the values of competing options, including both eventually chosen and unchosen options, during the choice decision [Arana et al., 2003; Camille et al., 2004; Coricelli et al., 2005]. The effect a lesion has on this region or activation of this region is sensitive to future abstract values, that is, prospective monetary or social reward, rather than immediate monetary rewards [Bechara et al., 2000; Moll et al., 2006; Moretti et al., 2009]. The role of this region seems to be particularly important when the values are presented in a social context [Moretti et al., 2009; Walton et al., 2004]. These characteristics fit well with the social value of one's own appearance. One's appearance carries various abstract social values, such as health, personality traits, socioeconomical state, and reproductive values. These values are future values since we don't know when these perceived values may affect our survival.
Although the specific cognitive process underlying the social self in this region is unknown, it is interesting to speculate that enhanced self‐face‐specific activation of this region reflects the recalibration of the social values of the self relative to comparable others. This interpretation is congruent with the social comparison theory [Festinger 1954; Morse and Gergen, 1970] and the suggested role of this region in representing the values of competing options [Arana et al., 2003; Camille et al., 2004; Coricelli et al., 2005]. When human adults see their own faces with comparable other faces available, this brain system may automatically update their social values by referring to the values of available others, as was behaviorally demonstrated for physical attractiveness previously [Cash et al., 1983; Gutierres et al., 1999].
The social self relevant to the ventral part of the MPFC, implicated in the social evaluation process, likely comprises only a part of the complex cognitive construct of the social self housed in the MPFC. The locations of MPFC activation claimed to reflect the social self varied considerably across studies from the level of the frontal pole [Craik et al., 1999; Izuma et al., 2008; Kampe et al., 2003; Kelley et al., 2002; Ochsner et al., 2005; Schilbach et al., 2006; Takahashi et al., 2004] to the orbitofrontal cortex [Zahn et al., 2009], although they have all typically been assigned to the ventral part of MPFC [Amodio and Frith, 2006; Krueger et al., 2009; Van Overwalle, 2009]. The polar part may be, for example, involved when one is aware that others notice or are paying attention to them [Kampe et al., 2003; Schilbach et al., 2006]; such awareness is closely related to, but distinct from, the social evaluation process.
Although the enhanced response caused by the enriched social context was also self‐face‐specific in the right occipitoparietal sulcus, its interpretation will be largely left to future research. The posterior medial cortex is sometimes implicated in self‐relevant processes together with the MPFC [Northoff et al., 2006; Uddin et al., 2007], occasionally with reference to the “default mode network” [Raichle et al., 2001]. However, self‐specificity in this region is typically manifested as a response to self‐relevance, which does not dissociate the self from the friend [Sugiura et al., 2005]. In addition, we have no explanation for why response enhancement was self‐face‐specific only in this region and not in the right counterpart or posterior cingulate cortex (significant interaction in the ANOVA).
One may suspect that enhanced self‐face‐specific activation in the high block is sufficiently explained by the higher saliency of the familiar face due to the larger number of distracter faces in the high than low block. We do not accept this alternative account because (i) the enhancement of saliency should affect activation not only for the self‐face but also for the friend's face, and (ii) the effect of saliency should also be evident in the differential activation between target faces in the low block, in contrast to the results.
Assimilation of Friend With Self in Right Lateral Cortical Activation
The observed right lateral parietal and temporal activation for the friend's face in the high block suggests that the simple concept of the self‐specific recruitment of these regions is no longer valid. This finding, however, does not devalue the role of these regions in visual self‐recognition. Evidence for the involvement of these regions in the physical self, or the visual‐kinesthetic representation of one's own body, is robust [Ehrsson et al., 2004; Fink et al., 1999; Tsakiris et al., 2008; Yomogida et al., 2010], and this system no doubt plays a critical role in visual self‐recognition.
The finding suggests that the right lateral cortical system supporting the physical self also underlies the representations of socioemotional relationships with personally familiar people. This idea is congruent with the concept of viewing a close relationship with a friend or family member as an inclusion of others in the self [Aron et al., 1991]. Recent neuroimaging studies demonstrated the activation of these or adjacent regions during the recognition of faces of family members [Platek and Kemp, 2009] and friends, but not famous people [Sugiura et al., 2011]. Enhanced access to the representation of a socioemotional relationship during the high block is explained by the demand of the familiar/unfamiliar task; access to the representation was essential in the high block, but dispensable in the low block where only a single unfamiliar face (i.e., LC) was repeatedly presented.
A clear explanation for the common involvement of the right lateral cortical system, both in the physical self and in the representations of a socioemotional relationship, is so far absent. One possible explanation for this is the potential role of visual‐kinesthetic integration in the development of socioemotional interaction [Gergely 2001]. In this explanation, the system first detects a perfect visual‐kinesthetic matching to establish the physical self in early infancy. Later, the same system acquires sensitivity to a loose contingency between one's own motion and stimuli from socially reactive objects. An example of such a loose contingency is a mother's imitated reaction to her baby's vocalizations and emotional expressions. The importance of such contingency detection in socioemotional development is widely accepted [Tarabulsy et al., 1996].
Further functional segregation of the right lateral cortical regions may be suggested by the inhomogeneous patterns in the observed friend‐self assimilation across the regions. A pure friend‐self assimilation, as shown by significant friend‐specific modulation and significant interaction, was observed in the inferior temporal and supramarginal gyri only. On the contrary, the pars triangularis of the inferior frontal gyrus did not show the same assimilation. This region showed clear self‐face‐specific activation exhibiting no social‐context‐dependent modulation, suggesting the unique self‐specific role of this region. This unique role may be congruent with a previous report that only this region presented a negative correlation between neural response and degree of embarrassment during self‐face recognition [Morita et al., 2008].
Self‐Specific Deactivation of Temporoparietal Regions
A lower activation of the posterior part of the left middle temporal gyrus and the right supramarginal gyrus for the self‐face than for the friend's or the control faces is consistent with our previous observation [Sugiura et al., 2008]. Based on the suggested involvement of these regions in the processing of social information, we have speculated that observed deactivation reflects the suppression of such a process for self‐related stimuli in terms of parsimony. The current results do not contradict this speculation.
Conceptual Implication for Self‐Models
Taking these considerations together, our results demonstrate the dynamic nature of multiple self‐other representations in the human brain. Brain regions showing self‐face‐specific activation can be divided into at least three networks exhibiting different patterns of modulation in the social context. The medial cortical structures, including the ventral MPFC, show response‐enhancement in the social context. The right lateral parietal and temporal cortices, responsive specifically to the self‐face, become responsive to the friend's face in the enriched social context. No modulation was observed for self‐face‐specific activation in the pars triangularis of the inferior frontal gyrus, and for self‐face‐specific deactivation in the left middle temporal gyrus and the right supramarginal gyrus. These observations lend neuroscientific support to multi‐component models of the self [Bekoff and Sherman, 2004; Morin, 2006; Rochat, 2003].
Inclusion of the self‐evaluation process in the explanation of the self‐face recognition mechanism may conclude controversy on the criteria of mirrored self‐recognition in comparative psychology. Since Gallup [Gallup, 1970], self‐directed behavior in front of the mirror, usually probed by a mark attached to unseen parts of an animal's or an infant's body (i.e., mark test), was assumed to be evidence of visual self‐recognition. A report of pigeons' self‐directed behavior in front of a mirror after training [Epstein et al., 1981] has brought furious controversy on the criteria of self‐recognition. Many researchers thereafter only accepted spontaneous, rather than trained, self‐directed behavior as a critical condition of “genuine” self recognition [Schilhab, 2004]. We consider the spontaneity of self‐directed behavior to be closely associated with self‐evaluation during self‐face recognition, as the behavior is triggered by one's interest in one's own appearance which stems from the social values one sees in one's own appearance. The ventral MPFC may underpin the “genuine” self‐recognition that comparative psychologists are unwilling to accept for pigeons.
Methodological Considerations
We used face stimuli with a variety of expressions and eye‐gaze directions. This variety may make some contribution to the contexts where self‐face‐specific response is induced. This may be true for the context of the physical self, given that self‐specific right parietofrontal activation has typically been reported in studies where faces with expressions were used as stimuli [Morita et al., 2008; Sugiura et al., 2008; Sugiura et al., 2006; Sugiura et al., 2005; but see Uddin et al., 2005]. A contribution to the social context is also possible given the social connotation carried by those facial expressions and eye‐gaze directions.
One may have concerns about the validity of our fMRI experimental design in that the inter‐stimulus interval was not jittered and the resting periods between blocks were very short. We do not consider these to be problematic because we focused our interest on the neural responses to a specific target‐face or its modulation in the social context. The omission of jittering may cause contamination of the effect of preceding or subsequent trials to the estimation, which may result in artifactual activation or deactivation in a pure event‐related analysis. However, this possible artifact is not likely to show target‐face‐specificity because this confounding effect should exist equally across the target faces. That is, the artifactual activation or deactivation, if any, should be cancelled out in the tests of target‐face‐specificity. The short length of the between‐block resting period is also not problematic because we were interested in the differential event‐related response between target faces, rather than activation against the resting baseline.
CONCLUSIONS
The assumed neural correlate of the social self, the MPFC, was activated during self‐face recognition in the enriched social context. Given the ventral activation focus in the MPFC, and knowing the involvement of this region in the social evaluation process, this activation may reflect a part of the functioning of the social self that is relevant to the social value of the self‐face. Although activation in the right lateral parietal and temporal regions, previously implicated in the physical self, was not modulated for the self‐face in the social context, it was enhanced for the friend's face in the enriched social context. This may suggest that this system, the physical self, may also underlie representations of socioemotional relationships with personally familiar people. No such social‐context‐induced modulation was observed for self‐face‐specific activation in the pars triangularis of the inferior frontal gyrus, nor for self‐face‐specific deactivation in the left middle temporal gyrus and the right supramarginal gyrus. Taking these observations together, self‐face‐specific activation may be designated to at least three brain networks exhibiting different modulation‐patterns in the social context, suggesting a dynamic nature of multiple self‐other representations in the human brain.
REFERENCES
- Amodio DM, Frith CD ( 2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci 7: 268–77. [DOI] [PubMed] [Google Scholar]
- Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC ( 2003): Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci 23: 9632–9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A, Aron EN, Tudor M, Nelson G ( 1991): Close relationships as including other in the self. J Pers Soc Psychol 60: 241–253. [Google Scholar]
- Bechara A, Tranel D, Damasio H ( 2000): Characterization of the decision‐making deficit of patients with ventromedial prefrontal cortex lesions. Brain 123: 2189–2202. [DOI] [PubMed] [Google Scholar]
- Bekoff M, Sherman PW ( 2004): Reflections on animal selves. Trends Ecol Evol 19: 176–180. [DOI] [PubMed] [Google Scholar]
- Camille N, Coricelli G, Sallet J, Pradat‐Diehl P, Duhamel JR, Sirigu A ( 2004): The involvement of the orbitofrontal cortex in the experience of regret. Science 304: 1167–1170. [DOI] [PubMed] [Google Scholar]
- Cash TF, Cash DW, Butters JW ( 1983): Mirror, mirror, on the wall contrast effects and self‐evaluations of physical attractiveness. Pers Soc Psychol Bull 9: 351–358. [Google Scholar]
- Coricelli G, Critchley HD, Joffily M, O'Doherty JP, Sirigu A, Dolan RJ ( 2005): Regret and its avoidance: A neuroimaging study of choice behavior. Nat Neurosci 8: 1255–1262. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, Kapur S ( 1999): In search of the self: A positron emossion tomography study. Psychol Sci 10: 26–34. [Google Scholar]
- Darley JM, Latane B ( 1968): Bystander intervention in emergencies—Diffusion of responsibility. J Pers Soc Psychol 8: 377–383. [DOI] [PubMed] [Google Scholar]
- DeBruine LM, Jones BC, Little AC, Perrett DI ( 2008): Social perception of facial resemblance in humans. Arch Sex Behav 37: 64–77. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Spence C, Passingham RE ( 2004): That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science 305: 875–877. [DOI] [PubMed] [Google Scholar]
- Epstein R, Lanza RP, Skinner BF ( 1981): Self‐awareness in the pigeon. Science 212: 695–696. [DOI] [PubMed] [Google Scholar]
- Fenigstein A, Scheier MF, Buss AH ( 1975): Public and private self‐consciousness—Assessment and theory. J Consult Clin Psychol 43: 522–527. [Google Scholar]
- Festinger L ( 1954): A theory of social comparison processes. Hum Relat 7: 117–140. [Google Scholar]
- Fink GR, Marshall JC, Halligan PW, Frith CD, Driver J, Frackowiak RSJ, Dolan RJ ( 1999): The neural consequences of conflict between intention and the senses. Brain 122: 497–512. [DOI] [PubMed] [Google Scholar]
- Gallup GG ( 1970): Chimpanzees. Self‐recognition. Science 167: 86–87. [DOI] [PubMed] [Google Scholar]
- Gallup GG ( 1982): Self‐awareness and the emergence of mind in primates. Am J Primatol 2: 237–248. [DOI] [PubMed] [Google Scholar]
- Gergely G ( 2001): The obscure object of desire: ‘Nearly, but clearly not, like me’: Contingency preference in normal children versus children with autism. Bull Menninger Clin 65: 411–426. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, Farah MJ ( 2005): As self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychol Bull 131: 76–97. [DOI] [PubMed] [Google Scholar]
- Goodman GS, Sayfan L, Lee JS, Sandhei M, Walle‐Olsen A, Magnussen S, Pezdek K, Arredondo P ( 2007): The development of memory for own‐ and other‐race faces. J Exp Child Psychol 98: 233–242. [DOI] [PubMed] [Google Scholar]
- Grammer K, Fink B, Moller AP, Thornhill R ( 2003): Darwinian aesthetics: Sexual selection and the biology of beauty. Biol Rev Camb Philos Soc 78: 385–407. [DOI] [PubMed] [Google Scholar]
- Gutierres SE, Kenrick DT, Partch JJ ( 1999): Beauty, dominance, and the mating game: Contrast effects in self‐assessment reflect gender differences in mate selection. Pers Soc Psychol Bull 25: 1126–1134. [Google Scholar]
- Henson RNA. 2003. Analysis of fMRI time series In: Frackowiak RSJ, Friston KJ, Frith C, Dolan R, Friston KJ, Price CJ, Zeki S, Ashburner J, Penny WD, editors. Human Brain Function, 2nd ed Maryland Heights: Academic Press. [Google Scholar]
- Hunt PJ, Hillery JM ( 1973): Social facilitation in a coaction setting—Examination of effects over learning trials. J Exp Psychol 9: 563–571. [Google Scholar]
- Izuma K, Saito DN, Sadato N ( 2008): Processing of social and monetary rewards in the human striatum. Neuron 58: 284–294. [DOI] [PubMed] [Google Scholar]
- Kampe KKW, Frith CD, Frith U ( 2003): “Hey John”: Signals conveying communicative intention toward the self activate brain regions associated with “mentalizing,” regardless of modality. J Neurosci 23: 5258–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF ( 2002): Pinding the self? An event‐related fMRI study. J Cogn Neurosci 14: 785–794. [DOI] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, Grafman J ( 2009): The medial prefrontal cortex mediates social event knowledge. Trends Cogn Sci 13: 103–109. [DOI] [PubMed] [Google Scholar]
- Marino L ( 2002): Convergence of complex cognitive abilities in cetaceans and primates. Brain Behav Evol 59: 21–32. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira‐Souzat R, Grafman J ( 2006): Human fronto‐mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci USA 103: 15623–15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti L, Dragone D, di Pellegrino G ( 2009): Reward and social valuation deficits following ventromedial prefrontal damage. J Cogn Neurosci 21: 128–140. [DOI] [PubMed] [Google Scholar]
- Morin A ( 2006): Levels of consciousness and self‐awareness: A comparison and integration of various neurocognitive views. Conscious Cogn 15: 358–371. [DOI] [PubMed] [Google Scholar]
- Morita T, Itakura S, Saito DN, Nakashita S, Harada T, Kochiyama T, Sadato N ( 2008): The role of the right prefrontal cortex in self‐evaluation of the face: A functional magnetic resonance imaging study. J Cogn Neurosci 20: 342–355. [DOI] [PubMed] [Google Scholar]
- Morse S, Gergen KJ ( 1970): Social comparison, self‐consistency, and concept of self. J Pers Soc Psychol 16: 148–156. [DOI] [PubMed] [Google Scholar]
- Nicodemo D, Pereira MD, Ferreira LM ( 2008): Self‐esteem and depression in patients presenting Angle Class III malocclusion submitted for orthognathic surgery. Med Oral Patol Oral Cir Bucal 13: E48–E51. [PubMed] [Google Scholar]
- Northoff G, Heinzel A, Greck M, Bennpohl F, Dobrowolny H, Panksepp J ( 2006): Self‐referential processing in our brain—A meta‐analysis of imaging studies on the self. Neuroimage 31: 440–457. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JDE, Kihsltrom JF, D'Esposito M ( 2005): The neural correlates of direct and reflected self‐knowledge. Neuroimage 28: 797–814. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Platek SM, Kemp SM ( 2009): Is family special to the brain? An event‐related fMRI study of familiar, familial, and self‐face recognition. Neuropsychologia 47: 849–858. [DOI] [PubMed] [Google Scholar]
- Platek SM, Loughead JW, Gur RC, Busch S, Ruparel K, Phend N, Panyavin IS, Langleben DD ( 2006): Neural substrates for functionally discriminating self‐face from personally familiar faces. Hum Brain Mapp 27: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnik JM, de Waal FBM, Reiss D ( 2006): Self‐recognition in an Asian elephant. Proc Natl Acad Sci USA 103: 17053–17057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior H, Schwarz A, Gunturkun O ( 2008): Mirror‐induced behavior in the magpie (Pica pica): Evidence of self‐recognition. PLoS Biol 6: 1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis HT, Wheeler L, Spiegel N, Kernis MH, Nezlek J, Perri M ( 1982): Physical attractiveness in social‐interaction. II. Why does appearance affect social experience. J Pers Soc Psychol 43: 979–996. [Google Scholar]
- Rochat P ( 2003): Five levels of self‐awareness as they unfold early in life. Conscious Cogn 12: 717–731. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wohlschlaeger AM, Kraemer NC, Newen A, Shah NJ, Fink GR, Vogeley K ( 2006): Being with virtual others: Neural correlates of social interaction. Neuropsychologia 44: 718–730. [DOI] [PubMed] [Google Scholar]
- Schilhab TSS ( 2004): What mirror self‐recognition in nonhumans can tell us about aspects of self. Biol Philos 19: 111–126. [Google Scholar]
- Sugiura M, Mano Y, Sasaki A, Sadato N ( 2011): Beyond the memory mechanism: Person‐selective and nonselective processes in recognition of personally familiar faces. J Cogn Neurosci 23: 699–715. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Sassa Y, Jeong H, Horie K, Sato S, Kawashima R ( 2008): Face‐specific and domain‐general characteristics of cortical responses during self‐recognition. Neuroimage 42: 414–422. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Sassa Y, Jeong H, Miura N, Akitsuki Y, Horie K, Sato S, Kawashima R ( 2006): Multiple brain networks for visual self‐recognition with different sensitivity for motion and body part. Neuroimage 32: 1905–1917. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R ( 2005): Cortical mechanisms of visual self‐recognition. Neuroimage 24: 143–149. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y ( 2004): Brain activation associated with evaluative processes of guilt and embarrassment: An fMRI study. Neuroimage 23: 967–974. [DOI] [PubMed] [Google Scholar]
- Tarabulsy GM, Tessier R, Kappas A ( 1996): Contingency detection and the contingent organization of behavior in interactions: Implications for socioemotional development in infancy. Psychol Bull 120: 25–41. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Costantini M, Haggard P ( 2008): The role of the right temporo‐parietal junction in maintaining a coherent sense of one's body. Neuropsychologia 46: 3014–3018. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP ( 2007): The self and social cognition: The role of cortical midline structures and mirror neurons. Trends Cogn Sci 11: 153–157. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kaplan JT, Molnar‐Szakacs I, Zaidel E, Iacoboni M ( 2005): Self‐face recognition activates a frontoparietal “mirror” network in the right hemisphere: An event‐related fMRI study. Neuroimage 25: 926–935. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F ( 2009): Social cognition and the brain: A meta‐analysis. Hum Brain Mapp 30: 829–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MFS ( 2004): Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci 7: 1259–1265. [DOI] [PubMed] [Google Scholar]
- Yomogida Y, Sugiura M, Sassa Y, Wakusawa K, Sekiguchi A, Fukushima A, Takeuchi H, Horie K, Sato S, Kawashima R ( 2010): The neural basis of agency: An fMRI study. Neuroimage 50: 198–207. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Paiva M, Garrido G, Krueger F, Huey ED, Grafman J ( 2009): The neural basis of human social values: Evidence from functional MRI. Cereb Cortex 19: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]


