Abstract
This study presents a meta‐analysis comparing hit and correct rejection (CR) conditions across 48 fMRI studies. Old/new (hit > CR) effects associated most consistently with (1) components of the default‐mode network, including the left angular gyrus, bilateral precuneus, and bilateral posterior cingulate regions, which may support the mental re‐experiencing of an old event, or ecphory; (2) components of the cognitive‐control network, involving the left dorsolateral and dorsomedial prefrontal cortex and bilateral intraparietal sulcus regions, which may mediate memory and non‐memory control functions; and (3) the caudate nucleus, a key part of the brain's reward system that may support the satisfaction tied to target‐detection. Direct comparisons of old/new effects between item versus source retrieval and “remember” versus “know” retrieval yielded three main sets of findings. First, default‐mode network regions showed greater old/new effects in conditions associated with richer ecphoric processing. Second, cognitive‐control network regions showed greater old/new effects in conditions associated with a greater demand for strategic‐retrieval processing. Third, the caudate nucleus showed greater old/new effects in conditions tied to greater confidence in target‐detection. New/old (CR > hit) effects most strongly associated with the bilateral medial temporal lobe, possibly reflecting greater encoding‐related activity for new than for old items, and the right posterior middle temporal regions, possibly reflecting repetition‐related neural priming for old items. In conclusion, neural activity distinguishing old from new events comprises an ensemble of multiple memory‐specific activities, including encoding, retrieval, and priming, as well as multiple types of more general cognitive activities, including default‐mode, cognitive‐control, and reward processing. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: fMRI, episodic memory, recognition memory, default‐mode network, hippocampus, meta‐analysis
INTRODUCTION
Aim of the Study
The ability to distinguish old from new events is fundamental to episodic memory retrieval. The advent of functional neuroimaging has enabled researchers to compare neural activity during the correct recognition of a studied item (a hit) versus the correct non‐recognition of a non‐studied, novel item (a correct rejection [CR]). Researchers have shown that these old/new effects (hit > CR), also called “retrieval success” effects, associate with an extensive network of frontal and parietal regions [Spaniol et al., 2009]. However, these effects have rarely implicated the medial temporal lobe [MTL; Henson, 2005], which plays a critical role in episodic memory. Researchers now generally accept that many retrieval‐related activations are not memory‐specific but rather mediate more general cognitive operations [Cabeza et al., 2003; Herron et al., 2004; Kim, 2010; O'Connor et al., 2010; Phillips et al., 2009; Sajonz et al., 2010; Vilberg and Rugg, 2009]. The present study aimed to provide a comprehensive meta‐analysis of previously reported old/new and new/old (CR > hit) effects. This meta‐analysis included only the data concerning visual information retrieval in healthy young adults. In addition to the generic purpose of integrating results across studies, this meta‐analysis examined several specific hypotheses regarding old/new and new/old effects.
Old/New Effects
Brain regions associated with old/new effects may be broadly divided into two types: components of the default‐mode network [e.g., Buckner et al., 2008; Raichle et al., 2001] and components of the cognitive‐control network [e.g., Miller and Cohen, 2001; Vincent et al., 2008]. Both the default‐mode and cognitive‐control networks are evolving constructs in need of stricter validation, and at best these two networks cover most, but not all, regions implicated in old/new effects. Thus, the dichotomy does not represent a strict categorization of retrieval‐related activity, but rather some useful heuristics that can guide further research. The author's motivation for proposing this dichotomy stemmed from the fact that some regions' old/new effects typically emerged as “less deactivations” (default‐mode network), whereas other regions' old/new effects typically emerged as “more activations” [cognitive‐control network; e.g., Buckner and Carroll, 2007; Fox et al., 2005; Golland et al., 2008; Kim et al., 2010]. This article's later sections review evidence for the two networks' involvement in old/new effects and also describe the author's hypothesis regarding the two networks' involvement in source versus item retrieval and “remember” versus “know” retrieval.
Default‐mode network
Originally, researchers defined the default‐mode network as the set of regions showing greater activity during the passive resting state than during attention‐demanding cognitive tasks [Raichle et al., 2001]. This network's main components comprise the anteromedial prefrontal cortex (PFC), posterior cingulate cortex, precuneus, angular gyrus, and MTL. Recent research [Fox et al., 2005; Fransson, 2005; Golland et al., 2008] on spontaneous fluctuations of low‐frequency (<0.1 Hz), blood oxygen level‐dependent signals has found intrinsic, correlated activity among these regions, suggesting a distributed functional network. Although old/new effects rarely associate with all members of the default‐mode network, the involvement of certain regions appears to be among the more robust findings. For example, old/new effects in posterior members of the default‐mode network (i.e., posterior cingulate cortex, precuneus, and angular gyrus) are typically strong, and they remain robust across manipulations of many experimental variables, including cue modality, motor intentions, and target/non‐target (old/new) ratio [Herron et al., 2004; Shannon and Buckner, 2004]. Furthermore, several recent studies [Addis et al., 2007; Hassabis and Maguire, 2007; Kim et al., 2010; Sajonz et al., 2010; Vannini et al., 2011; Vinogradov et al., 2006] have noted that old/new effects include components of the default‐mode network.
The functions of the default‐mode network are not yet fully understood. However, available evidence has indicated that, at its simplest, it supports internally oriented mentation, such as imagining the future (prospection), conceiving of others' viewpoints (theory of mind), social cognition, mental navigation, deductive and inductive reasoning, and mind‐wandering [Binder et al., 2009; Buckner and Carroll, 2007; Buckner et al., 2008; Gusnard, 2005; Hassabis et al., 2007; Legrand and Ruby, 2009; Spreng et al., 2009]. Neuroimaging studies of episodic retrieval have provided evidence that vivid memories, such as recollection [Montaldi et al., 2006; Vilberg and Rugg, 2007; Yonelinas et al., 2005], high‐confidence recognition [Daselaar et al., 2006; Kim and Cabeza, 2009], source memory [Lundstrom et al., 2005; Smith et al., 2005], and autobiographical remembering [Spreng et al., 2009; Svoboda et al., 2006] occur in association with components of the default‐mode network. For example, Yonelinas et al. [ 2005] showed that recollection activated the anteromedial PFC, posterior cingulate cortex, angular gyrus, and MTL. Combining these two lines of evidence, the author hypothesized that the default‐mode network's activity during episodic retrieval supports mental re‐experiencing of an old event, or ecphory [Tulving, 1983]. Ecphoric experience during a correct “old” decision (hit) and its absence during a correct “new” decision (CR), may directly account for the default‐mode network's involvement in old/new effects.
The present study addressed the following three issues regarding the default‐mode network's involvement in old/new effects. First, the author investigated which default‐mode network regions most consistently associated with old/new effects. Second, the author compared the default‐mode network regions' old/new effects in item versus source retrieval. In item‐retrieval tasks, participants try to remember items, with no other associated information; whereas, in source‐retrieval tasks, they must remember both the item and the context in which it appeared [e.g., color or position; Johnson et al., 1993]. Thus, a source‐retrieval task most likely involves richer ecphoric experience (objective recollection) than an item‐retrieval task does. Thus, the author predicted that default‐mode network regions' old/new effects would be stronger during a source‐ than during an item‐retrieval task. Third, either vividly remembered specific contextual details (recollection) or a feeling of oldness, in the absence of contextual details (familiarity), forms the basis of any memory [Yonelinas, 2002]. To distinguish between recollection and familiarity, studies use a Remember‐Know procedure, which discriminates between old items that are “remembered” (recollection) and old items that are “known” (familiarity). Since, by definition, recollection implicates a richer ecphoric experience than familiarity does, the author predicted that default‐mode network regions would associate more strongly with remember/new effects (remember‐hit > CR) than with know/new effects (know‐hit > CR). In sum, the author's hypotheses covered both objective recollection (source memory) and subjective recollection (“remember” memory) tasks.
Cognitive‐control network
Cognitive‐control demand is maximal when a task is novel, prone to interference, taxes working memory, involves conflict resolution, and/or needs strategic processing. Neuropsychological studies have indicated that the PFC is the most crucial site for cognitive control [Miller and Cummings, 1999]. In line with this evidence, functional neuroimaging studies [Badre and Wagner, 2004; Duncan and Owen, 2000; Koechlin et al., 1999; MacDonald et al., 2000; Miller and Cohen, 2001; Ridderinkhof et al., 2004] ascribed cognitive‐control functions primarily to several PFC regions, mainly the dorsolateral and dorsomedial PFC. These PFC regions routinely activate during control‐demanding cognitive tasks, including shifting attention [Derrfuss et al., 2005; Wager et al., 2004], working memory [Owen et al., 2005; Wager and Smith, 2003], and conflict resolution tasks [Laird et al., 2005b; Nee et al., 2007]. Neuroimaging studies have also provided evidence that cognitive control function is not unique to the PFC and, in fact, involves other regions in the brain, including the dorsal posterior parietal cortex [PPC; Brass et al., 2005; Corbetta and Shulman, 2002; Sohn et al., 2000]. Recent functional connectivity analysis studies [Cole and Schneider, 2007; Dosenbach et al., 2007; Seeley et al., 2007; Spreng et al., 2010; Vincent et al., 2008] have indicated that many cognitive‐control regions show correlated activity during task performance, suggesting a distributed control network. Based on broad, qualitative similarities among these studies' results, the author suggest that the cognitive‐control network mainly consists of the dorsolateral PFC, dorsomedial PFC, and dorsal PPC regions.
Although old/new effects rarely associate with all members of the cognitive‐control network, the involvement of specific regions have been among the more robust findings [Dobbins et al., 2002; Kahn et al., 2004; Lepage et al., 2000; Spaniol et al., 2009; Velanova et al., 2003; Wagner et al., 2005]. For example, old/new effects typically include activated regions in the dorsolateral PFC and/or intraparietal sulcus (IPS). Cognitive‐control network regions' old/new effects may involve two, broadly different, control functions. First, the regions' old/new effects may relate to memory control functions. Although basic retrieval operations, such as cue specification and retrieval attempts, are common to both hits and CRs, more strategic retrieval processes, such as iterative searches and verification of retrieved information, may engage more consistently during a hit than during a CR [Buckner, 2003; Moscovitch, 1992]. Second, the regions' old/new effects may also relate to non‐memory control functions. An influential study by Herron et al. [ 2004] showed many regions' old/new effects decreased or even reversed when old/new ratios increased [e.g., 25:75 to 75:25; see also Vilberg and Rugg, 2009]. Their results showed, with remarkably consistency, that cognitive‐control regions, but not default‐mode regions, were sensitive to old/new ratios. In an analogy to the “oddball paradigm,” O'Connor et al. [ 2010] supposed that an observer in a recognition memory test might expect to see new items (non‐targets) rather than old items (targets). Based on this supposition, they suggested that a correct “old” decision, but not a correct “new” decision, might demand control operations, to countermand a general expectation that items should be new.
The present meta‐analysis addressed the following three issues regarding the cognitive‐control network's involvement in old/new effects. First, the author investigated which cognitive‐control network regions most consistently associated with old/new effects. Second, source retrieval, which involves the search for experimenter‐specified contextual information, likely associates with a greater demand for controlled‐retrieval processing relative to item retrieval [Dobbins et al., 2002; Donaldson et al., 2009; Kahn et al., 2004]. Thus, the author predicted that cognitive‐control network regions' old/new effects would be stronger during a source‐ than during an item‐retrieval task. Consistent with this hypothesis, many prior studies [Dobbins and Han, 2006; Dobbins et al., 2003; Fan et al., 2003; Lundstrom et al., 2003] have reported that source retrieval activated components of the cognitive‐control network more strongly than item retrieval did. For example, Dobbins and Han [ 2006] showed that multiple left PFC and parietal regions showed greater activity during attempted context retrieval, as compared with during item retrieval. Third, controlled‐retrieval processing should engage strongly when memories are weak or familiarity‐based; whereas there should be little need for controlled retrieval when the memory signal is maximal or recollection‐based [Kim and Caneza, 2009; Moscovitch, 1992; Yonelinas et al., 2005]. Thus, the author predicted that cognitive‐control network regions would associate more strongly with know/new effects than with remember/new effects. Consistent with this hypothesis, a recent meta‐analysis of know/remember (know > remember) contrasts [Kim, 2010] found these associated mainly with the dorsal frontoparietal regions.
In sum, as with the author's hypotheses relating to the default‐mode network, the author's hypotheses for the cognitive‐control network covered both objective and subjective recollection tasks. Many prior investigations have explicitly or implicitly addressed hypotheses similar to the present ones relating to item versus source memory or “remember” versus “know” memory. However, these studies have rarely entertained such hypotheses in terms of network affiliations, instead focusing on a few specific regions of interest, such as the MTL [Daselaar et al., 2006; Eldridge et al., 2000], PFC [Achim and Lepage, 2005; Dobbins et al., 2002], and parietal regions [Ciaramelli et al., 2008; Lundstrom et al., 2005]. Thus, the present approach's main strength lies in its emphasis on a more global, system‐wide model, as well as on the meta‐analytic integration of convergent findings across studies.
Material‐type effects
The majority of prior studies on old/new effects [Chee et al., 2004; de Zubicaray et al., 2005; Henson et al., 2000] used verbal materials (i.e., words) as memoranda and found that old/new effects were predominantly left‐lateralized. The hemispheric specialization principle indicates the processing of verbal materials depends more on left‐ than on right‐hemispheric processing; whereas the processing of nonverbal, pictorial materials involves more right‐ than left‐hemispheric processing [Springer and Deutsch, 1997]. Thus, the predominant left‐lateralization of old/new effects found in prior studies may be due to the widespread use of verbal materials, rather than to retrieval‐related processing having any greater intrinsic association with the left‐hemisphere than with the right‐hemisphere. On the other hand, Guerin and Miller [ 2009; see also Leube et al., 2003; Leveroni et al., 2000] reported a predominance of left‐lateralization for parietal and other old/new effects, irrespective of stimulus type, suggesting that the left hemisphere plays a specialized role in retrieval processing. To address this issue, the author compared the old/new effects found by studies using verbal versus those using pictorial materials (e.g., pictures of common objects or faces).
New/Old Effects
A study by Tulving et al. [ 1996] was one of the first to describe brain regions that responded to new items to a greater degree than they did to old items. Their findings indicated that the “novelty” activations occurred in the right MTL region and bilaterally, in the temporal and parietal regions. Though relatively few neuroimaging studies have focused on new/old (CR > hit) effects, researchers have generally accepted these effects' relevance to understanding memory and other cognitive functions [Donaldson et al., 2001; Kahn et al., 2004]. However, two competing hypothesis for new/old effects exist [Habib, 2001]. First, Tulving et al. [ 1996] suggested the encoding system is biased to process novel, as opposed to familiar, information, because the system evolved to register information having high survival value. According to this “novelty‐encoding” hypothesis, new/old effects may reflect greater encoding‐related activity for new than for old items. Supporting the hypothesis, new items presented during a recognition memory test lead to “incidental” memory formation and subsequent memory effects [Buckner et al., 2001; Stark and Okado, 2003]. Second, studies of implicit memory tasks have shown repetition‐related “deactivations” for old items, or neural priming, which may support behavioral priming effects [Henson and Rugg, 2003]. Neural priming may also play a role in explicit memory tasks, by providing a familiarity signal [Daselaar et al., 2006; Gonsalves et al., 2005; Henson et al., 2003]. Most likely, new/old effects reflect both novelty‐encoding (activations for new items) and neural priming (deactivations for old items) effects, although one factor may be more important than the other is in a given region.
The present meta‐analysis addressed the following two issues regarding new/old effects. First, the author investigated which brain regions most consistently associated with new/old effects. This investigation was crucial, since studies have elucidated relatively little about such regions. The author was particularly interested in whether the effects included an MTL region. As noted before, old/new effects have rarely implicated an MTL region, despite its well‐established role in episodic memory [Squire et al., 2004]. Regarding this negative finding, certain studies [Buckner et al., 2001; Stark and Okado, 2003] proposed that retrieval‐related MTL activity (old > new) occurs, but encoding‐related MTL activity (new > old) offsets or even reverses it. A finding of MTL new/old effects in the current study would be consistent with this view and would also suggest that a low signal‐to‐noise ratio, susceptibility to MRI artifacts, or other nuisance factors play relatively minor roles in the MTL's lack of involvement in old/new effects. Finally, to address possible effects of material‐type on new/old effects, the author compared new/old effects in the studies using verbal versus those using pictorial materials. A limited number of available studies precluded meta‐analysis of other variables of potential interest (e.g., item vs. source retrieval).
Meta‐Analysis
The present study's principal methodology was a quantitative (i.e., statistical) meta‐analysis of the relevant literature. Meta‐analysis has played an increasingly important role in identifying significant concordances in brain activity patterns across many neuroimaging studies [Wager et al., 2007]. As the number of neuroimaging studies continues to grow, at a rapidly accelerating pace, discerning convergent and divergent findings among studies becomes increasingly important, as well as increasingly harder [Laird et al., 2009]. A quantitative meta‐analysis is a uniquely valuable tool for accomplishing this goal. The results of the present meta‐analyses identified the brain regions associating most reliably with old/new or new/old effects, as well as those most consistently exhibiting modulation of these effects by some commonly used experimental variables (source versus item retrieval, recollection vs. familiarity, and verbal vs. pictorial). Although several meta‐analysis studies on old/new effects emerged recently, most used a tabular method [Skinner and Fernandes, 2007], often focusing exclusively on specific regions of interest, such as the MTL [Diana et al., 2007; Henson, 2005] or PPC [Ciaramelli et al., 2008; Hutchinson et al., 2009; Vilberg and Rugg, 2008]. Those that used a quantitative as well as a whole‐brain approach [Kim, 2010; Spaniol et al., 2009] only partially addressed issues that overlap the current study. The author discusses some relevant findings from these studies later, in the context of the current study's findings.
MATERIALS AND METHODS
Study/Contrast Selection
To isolate all fMRI studies reporting an old/new (hit > CR), remember/new (remember‐hit > CR), know/new (know‐hit > CR), or new/old (CR > hit) contrast, the author completed multiple literature searches via PubMed. Additionally, the author performed a reference list check of recent neuroimaging memory study reviews [Ciaramelli et al., 2008; Diana et al., 2007; Spaniol et al., 2009; Uncapher and Wagner, 2009; Vilberg and Rugg, 2008], to identify relevant studies the online database search did not reveal. These search results were filtered to include only studies that (1) tested healthy, young participants; (2) presented retrieval cues via a visual modality; (3) performed whole‐brain analyses; and (4) reported coordinate‐based data analyses. Ultimately, the author selected a set of 48 independent studies, involving 767 participants, for inclusion in the meta‐analyses. The meta‐analyses examined 38 old/new, 8 remember/new, 5 know/new, and 19 new/old contrasts. For each contrast type, Appendix lists the selected studies, along with the number of participants, the retrieval‐cue material, the type of retrieval (item vs. source), the encoding task, the retention interval, and the number of reported foci for each study.
Additional criteria for selecting a study/contrast were as follows. First, in selecting subjective recollection studies, the author included studies using a standard remember‐know procedure as well as those using its slight variant, i.e., a response classification in terms of high‐confidence (HC) versus low‐confidence (LC). Although they lack a one‐to‐one correspondence, HC versus LC recognition, on average, aligns rather closely with “remember” versus “know” recognition, respectively [Dunn, 2004; Wixted and Stretch, 2004]. Second, in selecting contrasts from subjective recollection studies, the author included remember/new and know/new contrasts but excluded any remember/know or know/remember contrasts, both because the author's previous study [Kim, 2010] included a meta‐analysis of these contrasts and because the current study focused primarily on distinguishing “old” versus “new” recognition, rather than the two types of “old” recognition. Third, in selecting contrasts from objective recollection studies, the author included source‐correct (SC)‐hit/new contrasts but excluded source‐incorrect (SI)‐hit/new and (SC + SI)‐hit/new contrasts, both because relatively few studies reported them and because the current study focused on successful source retrieval (see below). Fourth, a minor subset of the selected studies included both young and old participants and reported common, but not separate, contrasts for the two age groups. In such cases, the author selected the common contrasts for meta‐analysis. Finally, another minor subset of the selected studies reported contrasts by using a regular statistical threshold, as well as a similar contrast using a more lenient threshold. The author excluded the activation foci from the latter contrasts, which usually targeted specific regions of interest. One example of such is the study by de Zubicaray et al. [ 2005], who used a statistical threshold of 0.001 for their whole‐brain analysis but used .005 for their a priori regions of interest.
Data Analyses
Old/new effects
The author performed the following three types of meta‐analyses on old/new (hit > CR) contrasts. The first analyzed all 38 included studies together. The second was a set of subgroup meta‐analyses, each involving a subset of included studies. Few dimensions by which one might distinguish the selected studies to ensure a meaningful analysis were available. One such was whether the retrieval cue was verbal (words) or pictorial (e.g., face photos). Another dimension was whether the retrieval task was an item‐ or a source‐memory task. Crossing these two dimensions classified the selected studies into four subgroups: verbal‐item (n = 22), pictorial‐item (n = 9), verbal‐source (n = 5), and pictorial‐source (n = 2). This portion of the study comprised three subgroup meta‐analyses, one each for the verbal‐item, pictorial‐item, and verbal‐source subgroups. The pictorial‐source subgroup could not accommodate this analysis, due to the limited number of available studies. The third meta‐analysis type, a subtraction meta‐analysis, directly compared pairs of study subgroups. Testing the effects of the material's nature (verbal vs. pictorial) required a subtraction meta‐analysis on the verbal‐item subgroup versus the pictorial‐item subgroup. Testing the effects of retrieval type (item vs. source) required a subtraction meta‐analysis on the verbal‐item subgroup versus the versus‐source subgroup.
Remember‐HC/new and know‐LC/new effects
In this category, the author also performed three meta‐analyses. The first meta‐analysis examined all eight remember‐HC/new (remember‐HC hit > CR) studies together. The second analyzed all five know‐LC/new (know‐LC hit > CR) studies together. The third, a subtraction meta‐analysis, directly compared remember‐HC/new and know‐LC/new effects. The remember‐HC/new or know‐LC/new effects could not accommodate a subgroup analysis, due the limited number of available studies.
New/old effects
Most of the selected studies reported a CR > hit contrast, but a minor subset reported a CR > remember‐hit contrast or CR > know‐hit contrast. A meta‐analysis of new/old effects examined the three types of contrasts together, because the limited number of available studies did not require separate analyses. The author performed three types of meta‐analyses on new/old contrasts. The first analyzed all 19 studies together. The second was a set of subgroup meta‐analyses, each involving a subset of the included studies. To perform these, the author divided the selected new/old studies into four subgroups: verbal‐item (n = 10), pictorial‐item (n = 6), verbal‐source (n = 1), and pictorial‐source (n = 2). This portion of the study comprised two subgroup meta‐analyses, one each for the verbal‐item and pictorial‐item subgroups. The third type of meta‐analysis was a subtraction meta‐analysis on the verbal‐item subgroup versus the pictorial‐item subgroup, to address how material‐type affected new/old effects.
A subtraction meta‐analysis between groups of unequal size could bias the results, because a larger group of studies would have greater statistical power to detect activation [Owen et al., 2005]. To address this issue, the author randomly selected a subsample of the larger group and thus made the comparison between two equal‐sized groups. For example, to compare old/new effects between the verbal‐item subgroup (n = 22) and the pictorial‐item subgroup (n = 9), the author randomly selected nine studies from the verbal‐item subgroup for the comparison. Random selection was applied in a flexible manner, so the selection would be unbiased with regard to the proportions of studies reporting relatively high versus relatively low numbers of activation foci. Random selection for the subtraction analysis between remember/new and know/new effects also balanced the proportions of studies using verbal versus pictorial materials.
Meta‐Analysis Techniques
In this study, the author performed activation likelihood estimation (ALE) meta‐analyses, as described by Laird et al. [ 2005a], performing all data processing via the GingerALE program, Version 1.1 (available at: http://www.brainmap.org). The author determined the spatial normalization space for each study and converted all activation foci reported in Montreal Neurological Institute (MNI) coordinates, into Talairach coordinates [Talairach and Tournoux, 1988]. Individual studies' activation foci were modeled as peaks of three‐dimensional Gaussian probability distributions, with a full‐width half‐maximum (FWHM) of 10 mm. Then, the author summed the three‐dimensional Gaussian distributions to create an ALE map estimating each voxel's activation likelihood across the entire set of studies. To determine the ALE map's statistical significance, the author employed a permutation test of randomly generated foci, computing 5,000 permutations using the same FWHM value and the same number of foci as in the ALE map. For each subtraction meta‐analysis, the author carried out a permutation test of the difference between the two subgroups. The author thresholded all permutation tests, with a false‐discovery rate (FDR) value of P < 0.05 [Genovese et al., 2002] and with clusters of suprathreshold voxels exceeding 400 mm3. For visualizing the meta‐analytic results, the author projected the thresholded ALE maps onto either an inflated population average landmark surface (PALS), via CARET software [Van Essen, 2005], or an International Consortium for Brain Mapping (ICBM) template, via MANGO software (available at: http://ric.uthscsa.edu/mango).
The present study includes the caveat that some subgroup meta‐analyses contained relatively few studies. A meta‐analysis based on a small number of studies can potentially yield unreliable results, reducing the meta‐analysis's statistical power to detect significant activation concordances while increasing the risk of outliers biasing the results. To reduce the risk of such, the author ensured that all meta‐analyses involved at least five independent studies (in the case of subtraction analyses, at least five studies in each subgroup) and had a relatively conservative spatial extent threshold (400 mm3). However, one should still take with caution the results of those subgroup meta‐analyses involving few studies; they need further confirmation in future meta‐analyses that include a larger number of studies.
RESULTS
Old/New Effects
All included studies
Table I and Figure 1 show the ALE meta‐analysis results for all included studies (n = 38). Old/new effects associated most consistently with seven neural regions: the left angular gyrus (Brodmann area [BA] 39, 40), bilateral precuneus (BA 7), bilateral posterior cingulate cortex (BA 23, 31), left dorsolateral PFC (BA 6, 8, 9, 46, 10), left dorsomedial PFC (BA 6, 8), bilateral dorsal PPC (BA 7, 19), and bilateral caudate nucleus. Most bilateral clusters were predominantly left‐lateralized. The left dorsolateral PFC cluster, which was the largest, included both the anterior and posterior extent of the middle frontal gyrus and extended into the inferior frontal gyrus's dorsal extent. The broad, qualitative similarity to prior descriptions of the default‐mode network indicated three of the seven clusters (left angular gyrus, bilateral precuneus, and bilateral posterior cingulate cortex) were components of the default‐mode network. Similarly, three other clusters (left dorsolateral PFC, left dorsomedial PFC, and bilateral dorsal PPC) were components of the cognitive‐control network. More minor clusters occurred in the left anterior insula, left middle temporal cortex (BA 21), and right anterior dorsolateral PFC (BA 10).
Table I.
Old/new effects: Results from an ALE meta‐analysis of all included studies
| Lobe | Region | H | BA | Talairach | Volume (mm3) | ALE (×103) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Frontal | Dorsolateral PFC, insula | L | 6, 8, 9, 46, 10 | −32 | 18 | 2 | 21,648 | 42.2 |
| Dorsolateral PFC | R | 10 | 28 | 52 | 12 | 504 | 18.3 | |
| Dorsomedial PFC | L | 6, 8 | −6 | 24 | 44 | 3,752 | 40.8 | |
| Insula | R | — | 28 | 18 | 8 | 496 | 19.3 | |
| Temporal | MTG | L | 21 | −60 | −40 | −8 | 936 | 22.3 |
| Parietal | Angular gyrus, dorsal PPC | L | 39, 40, 7, 19 | −36 | −64 | 42 | 13,640 | 57.3 |
| Dorsal PPC | R | 7, 19 | 32 | −70 | 34 | 5,256 | 43.5 | |
| Posterior cingulate cortex | B | 23, 31 | −4 | −40 | 32 | 5,288 | 37.5 | |
| Precuneus | B | 7, 31 | −6 | −70 | 30 | 10,152 | 66.5 | |
| Sublobar | Caudate nucleus | L | — | −10 | 2 | 10 | 4,992 | 31.7 |
| Caudate nucleus | R | — | 10 | 2 | 16 | 4,504 | 36.7 | |
ALE, activation likelihood estimation; BA, Brodmann area; H, hemisphere; MTG, middle temporal gyrus; PFC, prefrontal cortex; PPC, posterior parietal cortex.
Figure 1.

Brain regions associated with old/new effects in a meta‐analysis involving all included studies. AG, angular gyrus; CN, caudate nucleus; dlPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; dPPC, dorsal posterior parietal cortex; PCC, posterior cingulate cortex; PCU, precuneus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Item versus source retrieval
Table II and Figure 2 show the meta‐analysis results for the verbal‐item subgroup (n = 22), the verbal‐source subgroup (n = 5), and the subtraction analysis between the two subgroups. In both subgroups, major old/new effects occurred within a seven‐region network, identified via the meta‐analysis of the whole group. Each involved the left angular gyrus, left precuneus, bilateral posterior cingulate cortex, left dorsolateral PFC, left dorsomedial PFC, left IPS, and bilateral caudate nucleus (see Fig. 2A,B). Though the verbal‐item subgroup clusters were generally larger than the verbal‐source subgroup clusters were, interpretation of these differences requires caution, given that statistical power to detect activations was greater in the verbal‐item subgroup. The subtraction meta‐analyses below circumvented this problem by comparing two equal‐sized subgroups. The subtraction analyses showed that the default‐mode network regions (the left precuneus and bilateral posterior cingulate cortex), cognitive‐control network regions (left dorsolateral PFC, left dorsomedial PFC, and left IPS), and the caudate nucleus each associated with greater old/new effects during a source‐retrieval task than they did during an item‐retrieval task (see Fig. 2C). No regions significantly associated with the reverse effects: i.e., greater old/new effects during an item‐ than during a source‐retrieval task.
Table II.
Old/new effects in the verbal‐item and verbal‐source subgroups and variable old/new effects between the two subgroups
| Lobe | Region | H | BA | Talairach | Volume (mm3) | ALE (×103) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Verbal‐item: old > new | ||||||||
| Frontal | Dorsolateral PFC | L | 10, 46 | −38 | 44 | 12 | 3,984 | 22.0 |
| Dorsolateral PFC | L | 9, 6 | −42 | 10 | 40 | 4,512 | 24.2 | |
| Insula | L | — | −32 | 16 | 4 | 2,120 | 26.0 | |
| Insula | R | — | 28 | 18 | 6 | 480 | 14.4 | |
| Dorsomedial PFC | L | 8 | −6 | 20 | 46 | 1,296 | 20.3 | |
| Dorsomedial PFC | L | 9 | −6 | 32 | 30 | 776 | 16.8 | |
| Parietal | Angular gyrus, intraparietal sulcus | L | 39, 40, 7, 19 | −44 | −62 | 42 | 9,456 | 32.1 |
| Intraparietal sulcus | R | 19 | 32 | −70 | 34 | 5,360 | 33.2 | |
| Posterior cingulate cortex | B | 31, 23 | −4 | −36 | 32 | 3,960 | 23.8 | |
| Precuneus | L | 7, 31 | −6 | −70 | 30 | 4,944 | 40.7 | |
| Sublobar | Caudate nucleus | L | — | −10 | 0 | 10 | 7,360 | 21.1 |
| Caudate nucleus | R | — | 8 | 12 | 2 | 6,80 | 17.5 | |
| Thalamus | L | — | −10 | −36 | 4 | 1,144 | 14.0 | |
| Midbrain | L | — | −14 | −30 | −10 | 688 | 16.0 | |
| Verbal‐source: old > new | ||||||||
| Frontal | Dorsolateral PFC, insula | L | 9, 46 | −42 | 22 | 24 | 5,784 | 19.0 |
| Dorsolateral PFC | L | 6 | −32 | −2 | 58 | 488 | 10.0 | |
| Dorsomedial PFC | L | 6 | −6 | 16 | 48 | 1,992 | 15.9 | |
| Parietal | Angular gyrus, intraparietal sulcus | L | 39, 7 | −36 | −64 | 44 | 2,544 | 14.3 |
| Posterior cingulate cortex | B | 23 | 2 | −34 | 30 | 1,832 | 12.3 | |
| Precuneus | L | 7, 31 | −6 | −70 | 30 | 2,056 | 18.0 | |
| Sublobar | Caudate nucleus | R | — | 10 | 0 | 18 | 992 | 12.6 |
| Caudate nucleus | L | — | −12 | 2 | 10 | 592 | 12.0 | |
| Thalamus | L | — | −6 | −16 | 12 | 416 | 8.3 | |
| Cerebellum | R | — | 34 | −66 | −30 | 528 | 10.3 | |
| Verbal‐source old/new > verbal‐item old/new | ||||||||
| Frontal | Dorsolateral PFC | L | 46, 9 | −42 | 22 | 24 | 3,352 | 17.9 |
| Dorsomedial PFC | L | 8 | −8 | 16 | 46 | 1,072 | 12.3 | |
| Parietal | Intraparietal sulcus | L | 7 | −36 | −62 | 46 | 1,064 | 10.7 |
| Posterior cingulate cortex | B | 31 | 4 | −34 | 28 | 608 | 10.9 | |
| Precuneus | L | 7 | −8 | −68 | 28 | 808 | 11.8 | |
| Sublobar | Caudate nucleus | R | — | 10 | 0 | 20 | 456 | 10.8 |
| Cerebellum | R | — | 34 | −66 | −30 | 504 | 10.3 | |
| Verbal‐item old/new > verbal‐source old/new | ||||||||
| No significant activation | ||||||||
For abbreviations, see Table I.
Figure 2.

Brain regions associated with old/new effects in the verbal‐item (A) and verbal‐source (B) subgroups and with greater old/new effects for the verbal‐source subgroup than for the verbal‐item subgroup (C). IPS, intraparietal sulcus. For other abbreviations, see Figure 1. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Verbal versus pictorial material
Table III and Figure 3 show the meta‐analysis results for the pictorial‐item subgroup (n = 9) and the subtraction analysis between the verbal‐item and the pictorial‐item subgroups. The pictorial‐item subgroup also mainly showed old/new effects within a seven‐region network that involved the left angular gyrus, left posterior cingulate cortex, bilateral precuneus, bilateral dorsolateral PFC, left dorsomedial PFC, left IPS, and right caudate nucleus (see Fig. 3A). The subtraction analyses showed that the regions associated with greater old/new effects for verbal than for pictorial materials (the red regions in Fig. 3B), being the left dorsolateral PFC, left insula, left angular gyrus, and left precuneus regions, were larger than the regions associated with the reverse, i.e., greater old/new effects for pictorial than for verbal materials (the blue regions in Fig. 3B), which comprised a right dorsolateral PFC region.
Table III.
Old/new effects in the pictorial‐item subgroup and variable old/new effects between the verbal‐item and pictorial‐item subgroup
| Lobe | Region | H | BA | Talairach | Volume (mm3) | ALE (×103) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Pictorial‐item: old > new | ||||||||
| Frontal | Dorsolateral PFC | R | 10 | 26 | 52 | 12 | 1,136 | 15.3 |
| Dorsolateral PFC | L | 46 | −34 | 46 | 8 | 584 | 10.4 | |
| Dorsolateral PFC | L | 9 | −42 | 18 | 34 | 1,120 | 14.6 | |
| Dorsolateral PFC | R | 6 | 26 | 16 | 48 | 488 | 10.4 | |
| Dorsomedial PFC | L | 8 | −6 | 26 | 40 | 712 | 10.2 | |
| Temporal | MTG | L | 21 | −60 | −38 | −10 | 584 | 10.1 |
| Parietal | Angular gyrus, intraparietal sulcus | L | 39, 7, 19 | −40 | −60 | 38 | 3,600 | 15.6 |
| Intraparietal sulcus | L | 7 | −46 | −42 | 36 | 536 | 10.0 | |
| Posterior cingulate cortex | L | 31 | −8 | −44 | 28 | 456 | 8.4 | |
| Posterior cingulate cortex | L | 31 | −12 | −64 | 26 | 928 | 11.8 | |
| Precuneus | B | 7 | 2 | −74 | 38 | 1,432 | 14.0 | |
| Sublobar | Caudate nucleus | R | — | 10 | 10 | 12 | 480 | 10.0 |
| Putamen | L | — | −18 | 8 | −8 | 440 | 9.4 | |
| Verbal‐item old/new > pictorial‐item old/new | ||||||||
| Frontal | Dorsolateral PFC | L | 10 | −20 | 58 | 22 | 672 | 11.4 |
| Dorsolateral PFC | L | 46 | −34 | 42 | 16 | 800 | 13.8 | |
| Dorsolateral PFC | L | 46 | −46 | 40 | 4 | 880 | 12.9 | |
| Dorsolateral PFC | L | 6, 9 | −40 | 10 | 42 | 1,640 | 15.6 | |
| Insula | L | — | −44 | 18 | −4 | 472 | 11.3 | |
| Parietal | Angular gyrus, intraparietal sulcus | L | 39, 7 | −32 | −62 | 38 | 816 | 12.1 |
| Intraparietal sulcus | R | 7 | 30 | −70 | 34 | 1,208 | 17.4 | |
| Precuneus | L | 7 | −6 | −64 | 32 | 960 | 13.0 | |
| Pictorial‐item old/new > verbal‐item old/new | ||||||||
| Frontal | Dorsolateral PFC | R | 10 | 26 | 52 | 12 | 936 | −15.3 |
| Parietal | Precuneus | B | 7 | 2 | −72 | 40 | 480 | −10.3 |
For abbreviations, see Table I.
Figure 3.

Brain regions associated with old/new effects in the pictorial‐item subgroup (A), and variable old/new effects in a comparison of verbal‐item and pictorial‐item subgroups (B). IPS, intraparietal sulcus. For other abbreviations, see Figure 1. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Remember‐HC/New and Know‐LC/New Effects
Table IV and Figure 4 show the meta‐analysis results for remember‐HC/new effects (n = 8), know‐LC/new effects (n = 5), and the subtraction analysis between the two effects. First, remember‐HC/new effects associated most consistently with the left angular gyrus (BA 39), left precuneus (BA 7, 31), left posterior cingulate cortex (BA 23, 31), left posterior dorsolateral PFC (BA 6), left dorsomedial PFC (BA 6, 8), left IPS (BA 7), and right caudate nucleus (see Fig. 4A). Second, know‐LC/new effects associated most consistently with the left dorsolateral PFC (BA 9, 46, 10), left IPS (BA 7), and bilateral superior PPC/precuneus (BA 7; see Fig. 4B). Third, the subtraction analyses showed that default‐mode network regions (left angular gyrus, left precuneus, and left posterior cingulate cortex) showed a greater association with remember‐HC/new than with know‐LC/new effects; whereas cognitive‐control network regions (left anterior dorsolateral PFC and bilateral superior PPC/precuneus) associated with the reverse effects, i.e., greater know‐LC/new than remember‐HC/new effects (see Fig. 4C). Though the caudate nucleus associated with remember‐HC/new effects and not with know‐LC/new effects, the subtraction analysis did not detect this difference.
Table IV.
Remember‐HC/new and know‐LC/new effects and the subtraction results between them
| Lobe | Region | H | BA | Talairach | Volume (mm3) | ALE (×103) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Remember‐HC > new | ||||||||
| Frontal | Dorsolateral PFC | L | 6 | −40 | 4 | 44 | 2,800 | 13.2 |
| Dorsomedial PFC | L | 6 | −12 | 16 | 58 | 552 | 11.3 | |
| Dorsomedial PFC | L | 6, 8 | −6 | 30 | 36 | 568 | 12.1 | |
| Temporal | MTG | L | 21 | −58 | −24 | −4 | 808 | 10.3 |
| Parietal | Angular gyrus, intraparietal sulcus | L | 39, 7 | −48 | −60 | 24 | 2,944 | 14.5 |
| Posterior cingulate cortex | L | 23 | −6 | −26 | 30 | 680 | 10.2 | |
| Posterior cingulate cortex | L | 31 | −8 | −44 | 32 | 912 | 12.7 | |
| Precuneus | L | 31, 7 | −4 | −70 | 28 | 2,264 | 13.6 | |
| Sublobar | Caudate nucleus | R | — | 8 | 8 | −2 | 432 | 9.9 |
| Thalamus | L | — | −12 | −34 | 4 | 464 | 8.2 | |
| Know‐LC > new | ||||||||
| Frontal | Dorsolateral PFC | L | 10 | −32 | 48 | 18 | 744 | 11.0 |
| Dorsolateral PFC | L | 46 | −40 | 24 | 22 | 488 | 7.0 | |
| Dorsolateral PFC | R | 9 | 46 | 32 | 30 | 472 | 7.1 | |
| Insula | L | — | −52 | 16 | 8 | 448 | 6.9 | |
| Parietal | Superior PPC/precuneus | L | 7 | −12 | −70 | 46 | 632 | 8.5 |
| Superior PPC/precuneus | R | 7 | 6 | −74 | 46 | 728 | 10.7 | |
| Intraparietal sulcus | L | 7 | −22 | −68 | 34 | 576 | 7.5 | |
| Intraparietal sulcus | L | 7 | −36 | −58 | 42 | 456 | 7.1 | |
| Remember‐HC/new > know‐LC/new | ||||||||
| Parietal | Angular gyrus | L | 39 | −50 | −60 | 24 | 984 | 11.1 |
| Posterior cingulate cortex | L | 31 | −8 | −44 | 34 | 496 | 9.7 | |
| Posterior cingulate cortex | L | 23 | −8 | −28 | 30 | 536 | 9.9 | |
| Precuneus | B | 31 | 0 | −68 | 26 | 1,232 | 11.2 | |
| Sublobar | Thalamus | L | — | −12 | −34 | 4 | 664 | 8.2 |
| Know‐LC/new > remember‐HC/new | ||||||||
| Frontal | Dorsolateral PFC | L | 10 | −32 | 48 | 18 | 584 | −10.9 |
| Parietal | Superior PPC/precuneus | L | 7 | −12 | −70 | 46 | 448 | −8.5 |
| Superior PPC/precuneus | R | 7 | 6 | −74 | 46 | 528 | −10.6 | |
For abbreviations, see Table I.
Figure 4.

Brain regions associated with remember‐HC/new (A) and know‐LC/new effects (B), and the significant differences between the two effects (C). IPS, intraparietal sulcus; sPPC, superior posterior parietal cortex. For other abbreviations, see Figure 1. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
New/Old Effects
Table V and Figure 5A show the ALE meta‐analysis results for all included studies (n = 19). There were only two major clusters associated with new/old effects, one involving the MTL and the other involving a right posterior middle temporal gyrus (MTG) region and its transition to the occipital cortex (BA 37, 19). The MTL cluster bilaterally involved both the hippocampus and amygdala. Further minor clusters occurred in the right dorsomedial PFC (BA 9, 6) and right postcentral cortex (BA 2). Table V and Figure 5B–D show the meta‐analysis results for the verbal‐item subgroup (n = 10), the pictorial‐item subgroup (n = 6), and the subtraction analysis between the two subgroups. Focusing on the two major “new/old” clusters, identified by the meta‐analysis of the whole group, the author found new/old effects for the verbal‐item subgroup associated with the bilateral amygdala and right posterior MTG (see Fig. 5B) and those for the pictorial‐item subgroup associated with the bilateral hippocampus and right posterior MTG (see Fig. 5C). The subtraction analysis indicated that the bilateral hippocampus and right posterior MTL regions associated with greater new/old effects for pictorial than for verbal materials (see Fig. 5D).
Table V.
New/old effects in the whole group, verbal‐item and pictorial‐item subgroups, and variable new/old effects between the two subgroups
| Lobe | Region | H | BA | Talairach | Volume (mm3) | ALE (×103) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| All studies: new > old | ||||||||
| Frontal | Dorsomedial PFC | R | 9 | 6 | 48 | 16 | 648 | 9.9 |
| Dorsomedial PFC | R | 6 | 16 | −4 | 56 | 472 | 12.5 | |
| Temporal | Amygdala | L | — | −28 | 2 | −24 | 1,592 | 19 |
| Hippocampus | L | — | −28 | −18 | −18 | 912 | 14.8 | |
| Hippocampus, amygdala | R | — | 30 | −12 | −16 | 1,936 | 14.1 | |
| Posterior MTG | R | 37, 19 | 46 | −62 | 8 | 2,824 | 16 | |
| Parietal | Postcentral cortex | L | 2 | −44 | −30 | 54 | 440 | 11.6 |
| White matter | R | — | 22 | −48 | 48 | 768 | 11.5 | |
| Verbal‐item: new > old | ||||||||
| Frontal | Dorsomedial PFC | L | 6, 24 | −6 | −12 | 44 | 664 | 11.4 |
| Dorsomedial PFC | R | 6 | 6 | 6 | 52 | 504 | 8.7 | |
| Temporal | Amygdala | L | — | −28 | 2 | −24 | 1,136 | 12.6 |
| Amygdala | R | — | 36 | −4 | −26 | 808 | 10.1 | |
| Posterior MTG | R | 37, 19 | 50 | −64 | 10 | 536 | 7.9 | |
| Superior temporal gyrus | L | 41 | −48 | −18 | 12 | 448 | 7.7 | |
| Parietal | White matter | R | — | 20 | −50 | 48 | 568 | 9.7 |
| Pictorial‐item: new > old | ||||||||
| Frontal | Dorsomedial PFC | R | 9 | 8 | 44 | 18 | 424 | 7.3 |
| Temporal | Hippocampus | L | — | −30 | −18 | −18 | 608 | 10.2 |
| Hippocampus | R | — | 30 | −12 | −14 | 1,712 | 12.1 | |
| Posterior MTG | R | 37, 19 | 40 | −54 | −4 | 1,328 | 9.0 | |
| Sublobar | Putamen | R | — | 28 | −24 | 0 | 472 | 7.3 |
| Verbal‐item new/old > pictorial‐item new/old | ||||||||
| Frontal | Dorsomedial PFC | L | 31 | −6 | −14 | 44 | 536 | 11.2 |
| Temporal | White matter | R | — | 36 | −4 | −26 | 776 | 10.0 |
| Pictorial‐item new/old > verbal‐item new/old | ||||||||
| Temporal | Hippocampus | L | — | −30 | −18 | −18 | 536 | −10.2 |
| Hippocampus | R | — | 20 | 2 | 0 | 1,024 | −7.0 | |
| Posterior MTG | R | 37, 19 | 40 | −54 | −4 | 840 | −8.9 | |
For abbreviations, see Table I.
Figure 5.
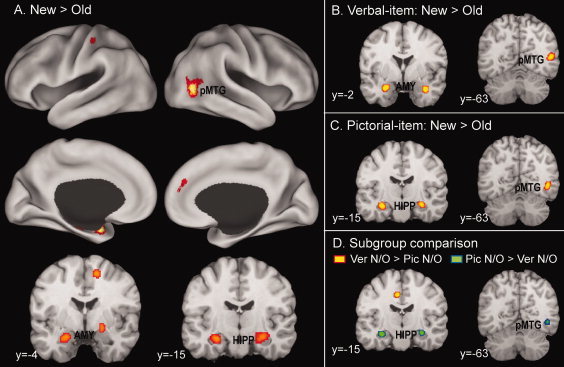
Brain regions associated with new/old effects in the whole group (A), in the verbal‐item subgroup (B), and in the pictorial‐item subgroup (C), as well as variable new/old effects in a comparison of the two subgroups (D). AMY, amygdala; HIPP, hippocampus; pMTG, posterior middle temporal gyrus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Additional Analyses
The subtraction meta‐analyses reported above considered a random selection of studies within a larger group of studies, to equalize the number of studies within each group. One limitation of this approach is that the results could be idiosyncratic to a particular random selection. To address this issue, for each subtraction meta‐analysis, the author performed two additional analyses, each using a different random selection of studies. The Supporting Information, available online, shows these results. The additional subtraction analyses between verbal‐source versus verbal‐item old/new effects (see Supporting Information Table S1), remember‐HC/new versus know‐LC/new effects (see Supporting Information Table S3), and verbal‐item versus pictorial‐item new/old effects (see Supporting Information Table S4) yielded results generally consistent with the original results, with a few minor exceptions. For example, both of the additional subtraction analyses involving verbal‐source and verbal‐item old/new effects indicated that default‐mode network regions (left precuneus and bilateral posterior cingulate cortex), cognitive‐control network regions (left dorsolateral PFC, left dorsomedial PFC, and left IPS), and the caudate nucleus each associated with greater old/new effects during source‐retrieval than they did during item‐retrieval tasks. However, the additional subtraction analyses involving verbal‐item versus pictorial‐item old/new effects yielded results showing greater variability (see Supporting Information Table S2), indicating a particular random selection drove certain effects. In this subtraction analysis, consistent findings across all three analyses were limited to the left dorsolateral PFC (BA 46), left insula, and left angular gyrus (BA 39), each associated with greater old/new effects for verbal than for pictorial materials, and to a right dorsolateral PFC region (BA 10), which associated with greater old/new effects for pictorial than for verbal materials.
DISCUSSION
Old/New Effects
These results reveal that old/new effects associated most consistently with seven neural regions: the left angular gyrus, bilateral precuneus, bilateral posterior cingulate cortex, left dorsolateral PFC, left dorsomedial PFC, bilateral IPS, and bilateral caudate nucleus. These regions largely show consistency with those regions one previous meta‐analysis of old/new effects found [Spaniol et al., 2009]. These regions may be broadly categorized into three types: (1) default‐mode network regions, involving the first three of the seven regions; (2) cognitive‐control network regions, involving the next three regions; and (3) the caudate nucleus. The author discusses each of these categories separately below.
Default‐mode network: Ecphory
The regions most consistently associating with old/new effects include three default‐mode network regions: the left angular gyrus, bilateral precuneus, and bilateral posterior cingulate cortex. Combining evidence showing the default‐mode network supports internally oriented mentation [Fox et al., 2005; Fransson, 2005; Golland et al., 2008] with evidence showing vivid memories, such as recollection [Montaldi et al., 2006; Vilberg and Rugg, 2007; Yonelinas et al., 2005], high‐confidence recognition [Daselaar et al., 2006; Kim and Cabeza, 2009], source memory [Lundstrom et al., 2005; Smith et al., 2005], and autobiographical remembering [Spreng et al., 2009; Svoboda et al., 2006], involve components of the default‐mode network, the author hypothesize that, during episodic retrieval, the network supports mental re‐experiencing of the old event, or ecphory. The current comparisons between old/new effects for item‐ versus source‐retrieval and for remember‐HC versus know‐LC provide supporting evidence for this hypothesis. First, default‐mode network regions, involving the left precuneus and bilateral posterior cingulate cortex, show greater old/new effects during a source‐retrieval task than they show during an item‐retrieval task (see Fig. 2C), likely reflecting richer ecphoric processing during source than item retrieval. Second, default‐mode network regions, including the left angular gyrus, left posterior cingulate cortex, and bilateral precuneus, associate more strongly with remember‐HC/new than with know‐LC/new effects (Fig. 4C), likely reflecting greater ecphoric processing during “remember” retrieval than during “know” retrieval.
Subcomponents of the default‐mode network most likely mediate different subcomponents of ecphoric processing. Researchers are currently debating the role of the ventral PPC (angular gyrus) in episodic memory retrieval [Hutchinson et al., 2009; Wagner et al., 2005]. Extending the attention model of Corbetta and Shulman [ 2002] to the domain of memory, the Attention to Memory (AtoM) model [Cabeza et al., 2008; Ciaramelli et al., 2010] indicates ventral PPC activity mediates the bottom‐up attentional processes captured by salient retrieval output. Alternatively, the output buffer hypothesis indicates the ventral PPC supports the maintenance of retrieved content in “something like the episodic buffer proposed by Baddeley [ 2000]” [Vilberg and Rugg, 2008; see also Guerin and Miller, 2011]. Both views are compatible with the ecphory hypothesis, and further studies are needed to adjudicate between these two views. The precuneus/posterior cingulate cortex's roles in episodic retrieval have received relatively little attention. Functional connectivity analysis studies [Andrews‐Hanna et al., 2010; Buckner et al., 2009; Cole et al., 2010; Fransson and Marrelec, 2008] indicate the precuneus/posterior cingulate cortex is among the more globally connected regions within the default‐mode network. Thus, these regions may act as a central, convergence node integrating retrieved information with other components of internally oriented mentation, such as self‐referential [Buckner and Carroll, 2007; Gusnard, 2005], semantic/conceptual [Binder et al., 1999, 2009], and social/emotional [Amodio and Frith, 2006; Saxe, 2006] processing.
Old/new effects do not associate significantly with other major components of the default‐mode network, such as the MTL and anteromedial PFC. This “null” finding requires comment, since it potentially goes against the ecphory hypothesis. First, the present meta‐analysis of new/old effects shows the MTL's strong involvement, likely reflecting greater encoding‐related activity for new than for old items (see below). Thus, the lack of MTL involvement in old/new effects may reflect, at least in part, the “masking” of retrieval‐related activity (old > new) by stronger encoding‐related activity (new > old), rather than a true absence of retrieval‐related activity in the MTL [Buckner et al., 2001; Stark and Okado, 2003], Second, researchers [D'Argembeau et al., 2007; Gusnard and Raichle, 2001; Johnson et al., 2006] had thought the anteromedial PFC played a pivotal role in self‐referential processing. Studies [Spreng et al., 2009; Svoboda et al., 2006] had shown that autobiographical memory retrieval typically involved strong self‐referential processing, as well as strong activation of the anteromedial PFC. However, laboratory‐based episodic retrieval, given its impoverished encoding environment [Cabeza and St Jacques, 2007], may typically involve weak self‐referential processing, as well as weak activation of the anteromedial PFC. Finally, the author's previous meta‐analysis study [Kim, 2010] shows that remember/know (remember > know) effects include both the MTL and anteromedial PFC regions, along with other default‐mode network regions, providing positive evidence for both regions' involvement in “remember” retrieval.
Cognitive‐control network: Memory and non‐memory control
The regions most consistently associating with old/new effects also include the left dorsolateral PFC, left dorsomedial PFC, and bilateral dorsal PPC. As mentioned previously, these three regions show correlated activity during control‐demanding cognitive tasks [Cole and Schneider, 2007; Dosenbach et al., 2007; Seeley et al., 2007; Vincent et al., 2008], suggesting a distributed control network. During episodic retrieval, these regions may support memory as well as non‐memory control functions. The current comparisons between old/new effects for item‐ versus source‐retrieval and for remember‐HC versus know‐LC retrieval provide evidence for the network's involvement in controlled‐retrieval functions. First, the left dorsolateral PFC, left dorsomedial PFC, and left IPS regions show greater old/new effects during a source‐retrieval task than they show during an item‐retrieval task (see Fig. 2C). As previous studies [Dobbins et al., 2006; Fan et al., 2003; Kahn et al., 2004; Lundstrom et al., 2003] that directly compare memory for item versus source reveal, these findings likely reflect greater demand for controlled‐retrieval processing during source than during item retrieval. Second, the left anterior dorsolateral PFC and bilateral superior PPC/precuneus regions associate more strongly with know‐LC/new than they do with remember‐HC/new effects (Fig. 4C), likely reflecting that “know” retrieval demands controlled‐retrieval processing to a greater degree than “remember” retrieval does.
Subcomponents of the cognitive‐control network most likely mediate differential subcomponents of controlled‐retrieval operations. A body of neuroimaging data on the PFC [MacDonald et al., 2000; Miller and Cohen, 2001; Ridderinkhof et al., 2004; Rushworth et al., 2007] indicates a dissociation between the dorsomedial PFC, which monitors performance and signals during any need for control adjustments, and the dorsolateral PFC, which implements control operations. Retrieval‐related activity in the PFC may dissociate similarly between the dorsomedial PFC, where activity may support the monitoring of controlled‐retrieval demand, and the dorsolateral PFC, where activity may support controlled‐retrieval operations [Badre and Wagner, 2004; Fleck et al., 2006; Velanova et al., 2003; Wheeler and Buckner, 2003]. One influential hypothesis regarding lateral PFC organization suggests a rostrocaudal control hierarchy, whereby the posterior‐to‐anterior lateral PFC supports progressively more abstract control‐operations [Badre, 2008; Christoff and Gabrieli, 2000; Koechlin et al., 1999]. Thus, the anterior dorsolateral PFC may engage in more abstract‐level control of retrieval operations, such as keeping a main goal in mind during iterative retrieval searches; whereas the more posterior dorsolateral PFC may more specifically reflect controlled search processes. In contrast, and based on distinct response patterns in the left PFC during source memory, Dobbins et al. [ 2002] propose a tripartite model of lateral PFC organization, in which the anterior ventrolateral PFC mediates semantic analysis/cue specification, the posterior ventrolateral PFC subserves phonological maintenance/rehearsal, and the posterior dorsolateral and frontopolar PFC support recollective monitoring.
With respect to the dorsal PPC, the present study indicates a dissociation between more inferior regions (the IPS), which are common to remember‐HC/new and know‐LC/new effects, and more superior regions, which are specific to know‐LC/new effects (see Fig. 4). The author's previous meta‐analysis study [Kim, 2010] report similar dissociations within the dorsal PPC. The aforementioned AtoM model [Cabeza et al., 2008] proposes an additional hypothesis, whereby dorsal PPC activity mediates top‐down attentional control, guided by retrieval goals. This hypothesis fits with data on the superior subregion of the dorsal PPC, which are specific to know‐LC/new effects, but not with data on the inferior subregion involving the IPS. The latter may mainly support non‐memory control functions (see below). The present data also indicate a functional dissociation within the posterior precuneus. While inferior portions of the posterior precuneus (xyz = 0, −68, 26) associate more strongly with remember‐HC/new effects than with know‐LC/new effects, suggesting a default‐mode network region, superior portions (xyz = −12, −70, 46) associate more strongly with know‐LC/new effects than with remember‐HC/new effects, suggesting a functional connection with the cognitive‐control network (see rightmost illustration in Fig. 4C). This functional dissociation between superior and inferior subregions is broadly consistent with recent evidence suggesting connectivity‐based subdivisions within the precuneus [Cauda et al., 2010; Marguiles et al., 2009; Vincent et al., 2008]. For example, a functional connectivity study by Vincent et al. [ 2008] indicates that the superior precuneus is part of the task‐positive/dorsal attention system. Thus, although researches have postulated that the precuneus is a core region in the default‐mode network, the present study (and recent connectivity studies) implicates distinct functional subdivisions.
An unresolved issue concerns whether, and to what extent, the cognitive‐control network regions' association with old/new effects may reflect non‐memory control operations. A promising approach to this issue is to subdivide the “old/new” regions into those that are sensitive to old/new ratios (i.e., the relative probability of old and new items during a recognition test) and others that are not sensitive to old/new ratios. Using this approach, Herron et al. [ 2004; see also Vilberg and Rugg, 2009] show that old/new effects in most cognitive‐control network regions decrease or even reverse when old/new ratios increase (e.g., from 25:75 to 75:25). Herron et al. [ 2004] propose that these regions' activities “reflect processes that are contingent upon, rather than in support of, successful recognition.” Related to this, Vilberg and Rugg [ 2008] suggest activity in the vicinity of the IPS reflects “something akin to the salience or ‘target‐value’ of the eliciting stimulus event.” Based on the hypothesis that an observer in a recognition memory test may expect to see new items (non‐targets) rather than old items (targets), O'Connor et al. [ 2010] suggest an “observer must countermand a general expectation that items are new to successfully execute a correct ‘old’ decision.” Using a memory analog of the Posner “attention‐cueing” paradigm, they also provide evidence that most retrieval‐success (old > new) regions also track what they call “expectancy violation” (invalid cueing > valid cueing). Thus, most cognitive‐control regions may support operations that countermand an expectation that items are new, as well as having memory‐specific control operations.
Caudate nucleus: Reward
Old/new effects also associate strongly with bilateral caudate nucleus regions. The caudate nucleus's involvement in old/new effects, despite occurring frequently in findings, has received little attention until quite recently. Animal studies provide a rich body of evidence that the caudate nucleus and adjacent striatal regions play a key role in the brain's reward system [Schultz, 2000]. In line with this evidence, functional neuroimaging studies in humans [de Greck et al., 2008; Delgado, 2007; Delgado et al., 2000] find that performance‐related positive and negative feedback (e.g., monetary rewards) modulates caudate activity. Recent studies [Han et al., 2010; Tricomi and Fiez, 2008] have proposed that human subjects in a recognition memory test may value detection of “old” items (targets) more than they value “new” items (non‐targets). Based on this supposition, these studies suggest that caudate nucleus activity during episodic retrieval may reflect the reward or satisfaction of successful target detection. Supporting this hypothesis, Han et al. [ 2010] report that the caudate nucleus shows old/new effects when the study paradigm provides incentive to hits, but it shows new/old effects when the paradigm provides incentive to CRs. In the present study, the caudate nucleus regions show greater old/new effects during a source‐retrieval task than they show during an item‐retrieval task, likely reflecting the greater reward or satisfaction associated with more confident target‐detection (objective recollection; see Fig. 2C). Though the caudate nucleus associates with remember‐HC/new effects but not with know‐LC/new effects, this difference is not significant in the subtraction analysis. However, the author's previous meta‐analysis study [Kim, 2010] shows that remember/know (remember > know) effects include a caudate nucleus region, also likely reflecting the greater satisfaction associated with more confident target‐detection (subjective recollection). Recent studies using resting‐state functional connectivity MRI [Barnes et al., 2010; Di Martino et al., 2008] find subregional differences within the striatum. For example, the dorsal caudate connects functionally to the lateral PFC, whereas the ventral striatum activity connects functionally to the anteromedial PFC. Thus, distinct caudate subregions may coactivate with either the default‐mode or cognitive‐control network, depending on task requirements.
Material‐type effects
The studies using verbal materials as well as pictorial materials report predominantly left‐lateralized old/new effects (see Figs. 2A and 3A). A direct comparison between the studies using verbal versus pictorial materials yields rather variable results, depending on the particular random selection of studies. This comparison shows greater reliability with regard to stronger old/new effects for verbal versus pictorial materials in the left dorsolateral PFC, left insula, and the left angular gyrus regions. Such a comparison also yields greater old/new effects for pictorial versus verbal materials in the right dorsolateral PFC region. Although future meta‐analysis studies, using a larger sample of studies, need to address the issue of reliable material‐type effects, the current results clearly show predominant left‐lateralization of old/new effects, even for pictorial materials. Thus, the widespread research using verbal materials is unlikely to adequately account for the common, predominant left‐lateralization of old/new effects. Rather, the left hemisphere appears dominant for recognition memory across stimulus domains [Guerin and Miller, 2009; Leube et al., 2003; Leveroni et al., 2000]. For example, upon directly comparing memory for words versus faces, Guerin and Miller [ 2009] report that the parietal old/new effects did not exhibit material‐specific lateralization but rather strong left hemisphere lateralization. Alternatively, the predominant left‐lateralization of old/new effects for pictorial materials in the current analysis may reflect, at least in part, the use of verbalizable pictorial materials (e.g., pictures of common objects) in many included studies. In line with this view, Klostermann et al. [ 2009] report that the parietal old/new effects for nonlinguistic auditory stimuli lateralized to the right hemisphere. Future studies might address whether certain classes of visual, nonverbal materials may yield predominantly right‐lateralized old/new effects in the parietal and other regions.
New/Old Effects
The meta‐analyses of new/old effects indicate only two major clusters, one involving the bilateral MTL and the other involving a right posterior MTG region and its transition to the occipital cortex (see Fig. 5A). The MTL cluster bilaterally involves the hippocampus and amygdala. According to one novelty‐encoding hypothesis [Tulving et al., 1996], the MTL regions' new/old effects may reflect greater encoding‐related activity for new than for old items [Kirchhoff et al., 2000; Law et al., 2005; Stark and Okado, 2003]. Though the effects may also reflect repetition‐related “deactivations” for old items, or neural priming [Daselaar et al., 2006; Gonsalves et al., 2005; Henson et al., 2003], a “pure” priming hypothesis does not account for “incidental” memory formation for new items and subsequent memory effects [Buckner et al., 2001; Stark and Okado, 2003]. Consistent with the novelty‐encoding hypothesis, Kumaran and Maguire [ 2006] show that hippocampal responses reflect mismatches between what is expected (based on past associations) and current sensory input. Poppenk et al. [ 2010] provide evidence of a functional dissociation along the hippocampus's longitudinal axis: the encoding of novel materials mainly involved the anterior hippocampus and amygdala, whereas the encoding of previously experienced materials mainly involved the posterior hippocampus. The current new/old effects fall mostly within the anterior half of the hippocampus and amygdala, consistent with these regions' specialization for encoding novel information.
As discussed, the current study shows the MTL's lack of involvement in old/new effects. The finding of strong MTL involvement in new/old effects has critical implications for interpreting the lack of MTL involvement in old/new effects. First, the MTLs non‐involvement in old/new effects may reflect, at least in part, a “masking” of retrieval‐related activity (old > new) by stronger, encoding‐related activity (new > old), rather than reflecting a true absence of retrieval‐related MTL activity. Second, a low signal‐to‐noise ratio, susceptibility to MRI artifacts, or other nuisance factors may play a rather minor role in the MTL's lack of involvement in old/new effects.
The analyses of material‐types indicate that new/old effects for verbal materials in MTL regions mainly involve the bilateral amygdala; whereas those for pictorial materials mainly involve the bilateral hippocampus. A direct comparison between new/old effects regarding studies that use verbal versus pictorial materials reveals greater new/old effects for pictorial than for verbal materials in MTL regions, mostly involving the bilateral hippocampus (see Fig. 5D). This material‐dependent MTL effect may relate to the widespread use of common words, which participants had encountered numerous times before the studies, as memoranda. Thus, participants have high pre‐experimental familiarity with the verbal materials, but not with the pictorial materials used in memory studies. Because of this, the greater MTL new/old effects for pictorial than for verbal materials may reflect greater encoding‐related activity for more novel than for familiar information. The author's previous meta‐analysis of subsequent memory effects [remembered > forgotten; Kim, 2011] indicates similar material‐dependent effects in MTL regions. Incidentally, this parallel finding with subsequent memory effects provides evidence that current new/old effects in the MTL reflect greater encoding‐related activity for new than for old items, rather than neural priming for old items.
The right posterior MTG cluster is not near any regions showing significant subsequent memory effects in the aforementioned meta‐analysis. Rather, this and adjacent regions frequently associate with repetition‐related priming effects [Henson and Rugg, 2003; Schacter and Buckner, 1998]. Thus, this region's new/old effects may mainly reflect priming‐related “deactivations” for old items. This priming effect may be perceptual in nature, given that (1) the cluster is in the visual association area (BA 19, 37); (2) it lateralizes to the right‐hemisphere, even for verbal materials; and (3) the effects are stronger for pictorial than for verbal materials (see Fig. 5D).
Conclusions
This meta‐analysis of old/new effects (hit > CR) indicated that such effects associated most strongly with: (1) components of the default‐mode network, including the left angular gyrus, bilateral precuneus, and bilateral posterior cingulate cortex regions, which may support mental re‐experiencing of an old event, or ecphory; (2) components of the cognitive‐control network, involving the left dorsolateral PFC, left dorsomedial PFC, and bilateral IPS regions, which may mediate memory and non‐memory control functions; and (3) the caudate nucleus, which is a key part of the brain's reward system and may support the reward or satisfaction tied to target‐detection. Direct comparisons of old/new effects between item versus source retrieval and “remember” versus “know” retrieval yielded three main sets of findings. First, default‐mode network regions exhibited greater activation in conditions associated with greater ecphoric processing, i.e., with source than with item retrieval and with “remember” than with “know” retrieval. Second, cognitive‐control network regions showed greater activation in conditions associated with greater demand for controlled‐retrieval processing, i.e., with source than with item retrieval and with “know” than with “remember” retrieval. Third, the caudate nucleus showed greater activation during source retrieval than it showed during item retrieval, likely reflecting the greater satisfaction associated with more confident target‐detection. The meta‐analysis of new/old effects (CR > hit) indicated that such effects associated most strongly with the bilateral MTL and right posterior MTG/occipital regions. The MTL's new/old effects may primarily reflect greater encoding‐related activity for new than for old items. This encoding‐related MTL activity (new > old) may mask retrieval‐related MTL activity (old > new), yielding an apparent lack of MTL involvement in old/new effects. The right posterior MTGs new/old effects may mainly reflect repetition‐related neural priming effects. Taken together, these findings suggest that differential neural activity for old versus new events is an ensemble of multiple memory‐specific activities, including encoding, retrieval, and priming, as well as of multiple types of more general cognitive activity, including default‐mode, cognitive‐control, and reward processing.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
DETAILS OF INDIVIDUAL STUDIES
Table .
APPENDIX: DETAILS OF INDIVIDUAL STUDIES
| Reference | Number of participants | Retrieval‐cue material | Type of retrieval | Encoding task | Retention intervala | Number of foci |
|---|---|---|---|---|---|---|
| Old > new | ||||||
| Achim and Lepage, 2005 | 18 | Clipart images | Item | Item/pair judgments | Short | 25 |
| Chee et al., 2004 | 16 | Words | Item | Living/non‐living judgments | Long | 8 |
| Chee et al., 2004 | 13 | Words | Item | Living/non‐living judgments | Long | 3 |
| Ciaramelli et al., 2010 | 14 | Words | Item | Forming a sentence including the item | Short | 10 |
| Daselaar et al., 2001 | 13 | Words | Item | Intentional encoding | Short | 4 |
| Daselaar et al., 2003 | 17 | Words | Item | Pleasant/unpleasant judgments | Short | 9 |
| de Zubicaray et al., 2005 | 14 | Words | Item | Intentional encoding | Short | 12 |
| Donaldson et al., 2001 | 22 | Words | Item | Abstract/concrete judgments | Short | 22 |
| Donaldson et al., 2009 | 26 | Words | Source | Forming a sentence including the item | Short | 28 |
| Han et al., 2010 | 19 | Words | Item | Syllable judgments | Short | 40 |
| Henson et al., 2000 | 12 | Words | Item | Pleasant/unpleasant judgments | Short | 4 |
| Henson et al., 2005 | 22 | Words | Item | Living/non‐living and alphabetical‐order judgments | Short | 24 |
| Herron et al., 2004 | 12 | Words | Item | Living/non‐living judgments | Short | 5 |
| Hornberger et al., 2006 | 17 | Words | Item | Size judgments | Short | 18 |
| Iidaka et al., 2006 | 16 | Line drawings | Item | Natural/man‐made and left/right judgments | Short | 20 |
| Jessen et al., 2001 | 17 | Words | Item | Continuous recognition | Short | 10 |
| Konishi et al., 2000 | 13 | Words | Item | Intentional encoding | Short | 30 |
| Lepage et al., 2010 | 18 | Clipart images | Item | Item/pair judgments | Short | 4 |
| Leube et al., 2003 | 12 | Face photos | Item | Male/female judgments | Short | 2 |
| Leveroni et al., 2000 | 11 | Face photos | Item | Pleasant/unpleasant judgments | Short | 3 |
| Lundstrom et al., 2003 | 21 | Words | Item | Emotion rating on a 3‐point scale | Short | 1 |
| Lundstrom et al., 2003 | 21 | Words | Source | Emotion rating on a 3‐point scale | Short | 12 |
| Lundstrom et al., 2005 | 16 | Words | Source | Emotion rating on a 3‐point scale | Long | 19 |
| Maratos et al., 2001 | 12 | Words | Item | Emotion rating on a 7‐point scale | Short | 32 |
| McDermott et al., 2000 | 24 | Words | Item | Intentional encoding | Short | 8 |
| Morcom et al., 2007 | 16 | Object photos | Source | Size and living/non‐living judgments | Short | 25 |
| O'Connor et al., 2010 | 19 | Words | Item | Syllable judgments | Short | 24 |
| Ragland et al., 2004 | 15 | Words | Item | Intentional encoding | Short | 5 |
| Ragland et al., 2006 | 13 | Words | Source | Uppercase/lowercase and concrete/abstract judgments | Short | 11 |
| Raposo et al., 2009 | 16 | Words | Source | Pleasant/unpleasant and concrete/abstract judgments | Short | 15 |
| Rombouts et al., 2001 | 9 | Color pictures | Item | Building‐presence judgments | Short | 1 |
| Sajonz et al., 2010 | 29 | Affective pictures | Item | Indoor/outdoor situation judgments | Long | 18 |
| Slotnick et al., 2003 | 8 | Abstract shapes | Item | Intentional encoding | Short | 6 |
| Smith et al., 2004 | 15 | Object pictures | Item | Emotion rating on a 3‐point scale | Short | 10 |
| Smith et al., 2005 | 18 | Object pictures | Source | Imagining a connection between item and background | Short | 16 |
| Tsukiura et al., 2005 | 18 | Words | Item | Forming a sentence, reading, and copying | Short | 10 |
| van der Veen et al., 2006 | 12 | Words | Item | Physical, phonological, and semantic judgments | Short | 5 |
| Zerseen et al., 2001 | 12 | Words | Item | Intentional encoding | Short | 16 |
| Remember‐HC > new | ||||||
| Fenker et al., 2005 | 20 | Words | Item | Intentional encoding | Short | 24 |
| Fliessbach et al., 2006 | 21 | Words | Item | Intentional encoding | Short | 11 |
| Henson et al., 1999 | 12 | Words | Item | Word/non‐word judgments | Short | 12 |
| Kim et al., 2010 | 12 | Words | Item | Semantic category judgments | Short | 6 |
| Sharot et al., 2004 | 13 | Affective pictures | Item | Visual complexity rating | Short | 15 |
| Trinkler et al., 2009 | 14 | Face photos | Item | Personally‐known/famous/unknown judgments | Short | 7 |
| Weis et al., 2004 | 16 | Scene photos | Item | Building/landscape judgments | Short | 17 |
| Woodruff et al., 2005 | 16 | Words | Item | Living/non‐living judgments | Short | 15 |
| Know‐LC > new | ||||||
| Duarte et al., 2010 | 13 | Line drawings | Item | Size judgments | Short | 11 |
| Fenker et al., 2005 | 20 | Words | Item | Intentional encoding | Short | 2 |
| Henson et al., 1999 | 12 | Words | Item | Word/non‐word judgments | Short | 7 |
| Kim et al., 2010 | 12 | Words | Item | Semantic category judgments | Short | 4b |
| Sharot et al., 2004 | 13 | Affective pictures | Item | Visual complexity rating | Short | 19 |
| New > old | ||||||
| Achim and Lepage, 2005 | 18 | Clipart images | Item | Item/pair judgments | Short | 3 |
| Daselaar et al., 2003 | 17 | Words | Item | Pleasant/unpleasant judgments | Short | 4 |
| de Zubicaray et al., 2005 | 14 | Words | Item | Intentional encoding | Short | 13 |
| Donaldson et al., 2001 | 22 | Words | Item | Abstract/concrete judgments | Short | 2 |
| Duarte et al., 2010 | 13 | Line drawings | Item | Size judgments | Short | 15 |
| Henson et al., 1999 | 12 | Words | Item | Word/non‐word judgments | Short | 24 |
| Henson et al., 2005 | 22 | Words | Item | Living/non‐living and alphabetical‐order judgments | Short | 1 |
| Hornberger et al., 2006 | 17 | Words | Item | Size judgments | Short | 6 |
| Jessen et al., 2001 | 17 | Words | Item | Continuous recognition | Short | 10 |
| Kahn et al., 2004 | 17 | Words | Source | Mental imagining and backward reading | Long | 15 |
| Kim et al., 2010 | 12 | Words | Item | Semantic category judgments | Short | 7 |
| Leveroni et al., 2000 | 11 | Face photos | Item | Pleasant/unpleasant judgments | Short | 7 |
| McDermott et al., 2000 | 24 | Words | Item | Intentional encoding | Short | 1 |
| Morcom et al., 2007 | 16 | Object photos | Source | Size and living/non‐living judgments | Short | 5 |
| Rombouts et al., 2001 | 9 | Color pictures | Item | Building‐presence judgments | Short | 35 |
| Sharot et al., 2004 | 13 | Affective pictures | Item | Visual complexity rating | Short | 1 |
| Slotnick et al., 2003 | 8 | Abstract shapes | Item | Intentional encoding | Short | 4 |
| van der Veen et al., 2006 | 12 | Words | Item | Physical, phonological, and semantic judgments | Short | 1 |
| Vilberg and Rugg, 2009 | 18 | Object photos | Source | Soft/hard and big/small judgments | Short | 17 |
Short = 1 h or less; long = 1 day or more.
Obtained from a re‐analysis of the original data.
REFERENCES
- Achim AM, Lepage M ( 2005): Dorsolateral prefrontal cortex involvement in memory post‐retrieval monitoring revealed in both item and associative recognition tests. Neuroimage 24: 1113–1121. [DOI] [PubMed] [Google Scholar]
- Addis D, Wong A, Schacter D ( 2007): Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia 45: 1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio D, Frith C ( 2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci 7: 268–277. [DOI] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL ( 2010): Functional‐anatomic fractionation of the brain's default network. Neuron 65: 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A ( 2000): The episodic buffer: A new component of working memory? Trends Cogn Sci 4: 417–423. [DOI] [PubMed] [Google Scholar]
- Badre D ( 2008): Cognitive control hierarchy and the rostro‐caudal organization of the frontal lobes. Trends Cogn Sci 12: 193–200. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner A ( 2004): Selection integration and conflict monitoring: Assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron 41: 473–487. [DOI] [PubMed] [Google Scholar]
- Barnes KA, Cohen AL, Power JD, Nelson SM, Dosenbach YBL, Miezin FM, Petersen SE, Schlaggar BL ( 2010): Identifying basal ganglia divisions in individuals using resting‐state functional connectivity MRI. Front Syst Neurosci, 4: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J, Frost J, Hammeke T, Bellgowan P, Rao S, Cox R ( 1999): Conceptual processing during the conscious resting state: a functional MRI study. J Cogn Neurosci 11: 80–93. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL ( 2009): Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cereb Cortex 19: 2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Ullsperger M, Knoesche T, Cramon D, Phillips N ( 2005): Who comes first? The role of the prefrontal and parietal cortex in cognitive control. J Cogn Neurosci 17: 1367–1375. [DOI] [PubMed] [Google Scholar]
- Buckner R ( 2003): Functional‐anatomic correlates of control processes in memory. J Neurosci 23: 3999–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R, Andrews‐Hanna J, Schacter D ( 2008): The brain's default network. Ann NY Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Buckner R, Carroll D ( 2007): Self‐projection and the brain. Trends Cogn Sci 11: 49–57. [DOI] [PubMed] [Google Scholar]
- Buckner R, Sepulcre J, Talukdar T, Krienen F, Liu H, Hedden T, Andrews‐Hanna J, Sperling R, Johnson K ( 2009): Cortical hubs revealed by intrinsic functional connectivity: Mapping assessment of stability and relation to Alzheimer's disease. J Neurosci 29: 1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME, Sheridan MA ( 2001): Encoding processes during retrieval tasks. J Cogn Neurosci 13: 406–415. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson I, Moscovitch M ( 2008): The parietal cortex and episodic memory: An attentional account. Nat Rev Neurosci 9: 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Prince S, Rice H, Weissman D, Nyberg L ( 2003): Attention‐related activity during episodic memory retrieval: A cross‐function fMRI study. Neuropsychologia 41: 390–399. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P ( 2007): Functional neuroimaging of autobiographical memory. Trends Cogn Sci 11: 219–227. [DOI] [PubMed] [Google Scholar]
- Cauda F, Geminiani G, D'Agata F, Sacco K, Duca S, Bagshaw AP, Cavanna AE ( 2010): Functional connectivity of the posteromedial cortex. PloS One 5: e13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Goh JOS, Lim Y, Graham S, Lee K ( 2004): Recognition memory for studied words is determined by cortical activation differences at encoding but not during retrieval. Neuroimage 22: 1456–1465. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gabrieli J ( 2000): The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology 28: 168–186. [Google Scholar]
- Ciaramelli E, Grady C, Levine B, Ween J, Moscovitch M ( 2010): Top‐down and bottom‐up attention to memory are dissociated in posterior parietal cortex: Neuroimaging and neuropsychological evidence. J Neurosci 30: 4943–4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E, Grady C, Moscovitch M ( 2008): Top‐down and bottom‐up attention to memory: A hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia 46: 1828–1851. [DOI] [PubMed] [Google Scholar]
- Cole M, Schneider W ( 2007): The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage 37: 343–360. [DOI] [PubMed] [Google Scholar]
- Cole MW, Pathak S, Schneider W ( 2010): Identifying the brain's most globally connected regions. Neuroimage 49: 3132–3148. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman G ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E ( 2007): Distinct regions of the medial prefrontal cortex are associated with self‐referential processing and perspective taking. J Cogn Neurosci 19: 935–944. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R ( 2006): Triple dissociation in the medial temporal lobes: Recollection familiarity and novelty. J Neurophysiol 96: 1902–1911. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rombouts SARB, Veltman DJ, Raaijmakers JGW, Lazeron RHC, Jonker C ( 2001): Parahippocampal activation during successful recognition of words: A self‐paced event‐related fMRI study. Neuroimage 13: 1113–1120. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SARB, Raaijmakers JGW, Jonker C ( 2003): Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain 126: 43–56. [DOI] [PubMed] [Google Scholar]
- de Greck M, Rotte M, Paus R, Moritz D, Thiemann R, Proesch U, Bruer U, Moerth S, Tempelmann C, Bogerts B ( 2008): Is our self based on reward? Self‐relatedness recruits neural activity in the reward system. Neuroimage 39: 2066–2075. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, McMahon KL, Eastburn MM, Finnigan S, Humphreys MS ( 2005): fMRI evidence of word frequency and strength effects in recognition memory. Cogn Brain Res 24: 587–598. [DOI] [PubMed] [Google Scholar]
- Delgado M ( 2007): Reward‐related responses in the human striatum. Ann NY Acad Sci 1104: 70–88. [DOI] [PubMed] [Google Scholar]
- Delgado M, Nystrom L, Fissell C, Noll D, Fiez J ( 2000): Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 84: 3072–3077. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, Von Cramon D ( 2005): Involvement of the inferior frontal junction in cognitive control: Meta‐analyses of switching and Stroop studies. Hum Brain Mapp 25: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP ( 2008). Functional connectivity of human striatum: A resting state fMRI study. Cereb Cortex 18: 2735–2747. [DOI] [PubMed] [Google Scholar]
- Diana R, Yonelinas A, Ranganath C ( 2007): Imaging recollection and familiarity in the medial temporal lobe: A three‐component model. Trends Cogn Sci 11: 379–386. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD ( 2002): Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron 35: 989–996. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Han S ( 2006): Cue‐ versus probe‐dependent prefrontal cortex activity during contextual remembering. J Cogn Neurosci 18: 1439–1452. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice H, Wagner A, Schacter D ( 2003): Memory orientation and success: Separable neurocognitive components underlying episodic recognition. Neuropsychologia 41: 318–333. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Buckner RL ( 2001): Dissociating memory retrieval processes using fMRI: Evidence that priming does not support recognition memory. Neuron 31: 1047–1059. [DOI] [PubMed] [Google Scholar]
- Donaldson D, Wheeler M, Petersen S ( 2009): Remember the source: Dissociating frontal and parietal contributions to episodic memory. J Cogn Neurosci 22: 377–391. [DOI] [PubMed] [Google Scholar]
- Dosenbach N, Fair D, Miezin F, Cohen A, Wenger K, Dosenbach R, Fox M, Snyder A, Vincent J, Raichle M ( 2007): Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104: 11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Graham KS, Henson RN ( 2010): Age‐related changes in neural activity associated with familiarity recollection and false recognition. Neurobiol Aging 31: 1814–1830. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen A ( 2000): Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23: 475–483 [DOI] [PubMed] [Google Scholar]
- Dunn J ( 2004): Remember‐know: A matter of confidence. Psychol Rev 111: 524–542. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA ( 2000): Remembering episodes: A selective role for the hippocampus during retrieval. Nat Neurosci 3: 1149–1152. [DOI] [PubMed] [Google Scholar]
- Fan J, Snodgrass JG, Bilder RM ( 2003): Functional magnetic resonance imaging of source versus item memory. Neuroreport 14: 2275–2281. [DOI] [PubMed] [Google Scholar]
- Fenker DB, Schott BH, Richardson‐Klavehn A, Heinze H‐J, Düzel E ( 2005): Recapitulating emotional context: Activity of amygdala hippocampus and fusiform cortex during recollection and familiarity. Eur J Neurosci 21: 1993–1999. [DOI] [PubMed] [Google Scholar]
- Fleck M, Daselaar S, Dobbins I, Cabeza R ( 2006): Role of prefrontal and anterior cingulate regions in decision‐making processes shared by memory and nonmemory tasks. Cereb Cortex 16: 1623–1630. [DOI] [PubMed] [Google Scholar]
- Fliessbach K, Weis S, Klaver P, Elger CE, Weber B ( 2006): The effect of word concreteness on recognition memory. Neuroimage 32: 1413–1421. [DOI] [PubMed] [Google Scholar]
- Fox M, Snyder A, Vincent J, Corbetta M, Van Essen D, Raichle M ( 2005): The human brain is intrinsically organized into dynamic anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P ( 2005): Spontaneous low‐frequency BOLD signal fluctuations: An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G ( 2008): The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage 42: 1178–1184. [DOI] [PubMed] [Google Scholar]
- Genovese C, Lazar N, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Golland Y, Golland P, Bentin S, Malach R ( 2008): Data‐driven clustering reveals a fundamental subdivision of the human cortex into two global systems. Neuropsychologia 46: 540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves B, Kahn I, Curran T, Norman K, Wagner A ( 2005): Memory strength and repetition suppression: Multimodal imaging of medial temporal cortical contributions to recognition. Neuron 47: 751–761. [DOI] [PubMed] [Google Scholar]
- Guerin SA, Miller MB ( 2011): Parietal cortex tracks the amount of information retrieved even when it is not the basis of a memory decision. Neuroimage 55: 801–807. [DOI] [PubMed] [Google Scholar]
- Guerin SA, Miller MB ( 2009): Lateralization of the parietal old/new effect: An event‐related fMRI study comparing recognition memory for words and faces. Neuroimage 44: 232–242. [DOI] [PubMed] [Google Scholar]
- Gusnard D ( 2005): Being a self: Considerations from functional imaging. Conscious Cogn 14: 679–697. [DOI] [PubMed] [Google Scholar]
- Gusnard D, Raichle M ( 2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Habib R ( 2001): On the relation between conceptual priming neural priming and novelty assessment. Scand J Psychol 42: 187–195. [DOI] [PubMed] [Google Scholar]
- Han S, Huettel S, Raposo A, Adcock R, Dobbins I ( 2010): Functional significance of striatal responses during episodic decisions: Recovery or goal attainment? J Neurosci 30: 4767–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire E ( 2007): Using imagination to understand the neural basis of episodic memory. J Neurosci 27: 14365–14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire E ( 2007): Deconstructing episodic memory with construction. Trends Cogn Sci 11: 299–306. [DOI] [PubMed] [Google Scholar]
- Henson R ( 2005): A mini‐review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Q J Exp Psychol B 58: 340–360. [DOI] [PubMed] [Google Scholar]
- Henson R, Cansino S, Herron J, Robb W, Rugg M ( 2003): A familiarity signal in human anterior medial temporal cortex? Hippocampus 13: 301–304. [DOI] [PubMed] [Google Scholar]
- Henson R, Hornberger M, Rugg MD ( 2005): Further dissociating the processes involved in recognition memory: An fMRI study. J Cogn Neurosci 17: 1058–1073. [DOI] [PubMed] [Google Scholar]
- Henson R, Rugg M ( 2003): Neural response suppression haemodynamic repetition effects and behavioural priming. Neuropsychologia 41: 263–270. [DOI] [PubMed] [Google Scholar]
- Henson R, Rugg M, Shallice T, Dolan R ( 2000): Confidence in recognition memory for words: dissociating right prefrontal roles in episodic retrieval. J Cogn Neurosci 12: 913–923. [DOI] [PubMed] [Google Scholar]
- Henson R, Shallice T, Dolan R ( 1999): Right prefrontal cortex and episodic memory retrieval: A functional MRI test of the monitoring hypothesis. Brain 122: 1367–1381. [DOI] [PubMed] [Google Scholar]
- Herron JE, Henson RNA, Rugg MD ( 2004): Probability effects on the neural correlates of retrieval success: An fMRI study. Neuroimage 21: 302–310. [DOI] [PubMed] [Google Scholar]
- Hornberger M, Rugg MD, Henson RNA ( 2006): fMRI correlates of retrieval orientation. Neuropsychologia 44: 1425–1436. [DOI] [PubMed] [Google Scholar]
- Hutchinson J, Uncapher M, Wagner A ( 2009): Posterior parietal cortex and episodic retrieval: Convergent and divergent effects of attention and memory. Learn Mem 16: 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Matsumoto A, Nogawa J, Yamamoto Y, Sadato N ( 2006): Frontoparietal network involved in successful retrieval from episodic memory spatial and temporal analyses using fMRI and ERP. Cereb Cortex 16: 1349–1360. [DOI] [PubMed] [Google Scholar]
- Jessen F, Flacke S, Granath D, Manka C, Scheef L, Papassotiropoulos A, Schild H, Heun R ( 2001): Encoding and retrieval related cerebral activation in continuous verbal recognition. Cogn Brain Res 12: 199–206. [DOI] [PubMed] [Google Scholar]
- Johnson M, Hashtroudi S, Lindsay D ( 1993): Source monitoring. Psychol Bull 114: 3–28. [DOI] [PubMed] [Google Scholar]
- Johnson M, Raye C, Mitchell K, Touryan S, Greene E, Nolen‐Hoeksema S ( 2006): Dissociating medial frontal and posterior cingulate activity during self‐reflection. Soc Cogn Affect Neurosci 1: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD ( 2004): Functional‐neuroanatomic correlates of recollection: Implications for models of recognition memory. J Neurosci 24: 4172–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H ( 2011): Neural activity that predicts subsequent memory and forgetting: A meta‐analysis of 74 fMRI studies. Neuroimage 54: 2446–2461. [DOI] [PubMed] [Google Scholar]
- Kim H ( 2010): Dissociating the roles of the default‐mode dorsal and ventral networks in episodic memory retrieval. Neuroimage 50: 1648–1657. [DOI] [PubMed] [Google Scholar]
- Kim H, Cabeza R ( 2009): Common and specific brain regions in high‐ versus low‐confidence recognition memory. Brain Res 1282: 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Daselaar S, Cabeza R ( 2010): Overlapping brain activity between episodic memory encoding and retrieval: Roles of the task‐positive and task‐negative networks. Neuroimage 49: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE ( 2000): Prefrontal‐temporal circuitry for episodic encoding and subsequent memory. J Neurosci 20: 6173–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klostermann EC, Loui P, Shimamura AP ( 2009): Activation of right parietal cortex during memory retrieval of nonlinguistic auditory stimuli. Cogn Affect Behav Neurosci 9: 242–248. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J ( 1999): The role of the anterior prefrontal cortex in human cognition. Nature 399: 148–151. [DOI] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL ( 2000): Neural correlates of episodic retrieval success. Neuroimage 12: 276–286. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA ( 2006): An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biol 4: 2372–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A, Fox P, Price C, Glahn D, Uecker A, Lancaster J, Turkeltaub P, Kochunov P, Fox P ( 2005a): ALE meta‐analysis: Controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A, McMillan K, Lancaster J, Kochunov P, Turkeltaub P, Pardo J, Fox P ( 2005b): A comparison of label‐based review and ALE meta‐analysis in the Stroop task. Hum Brain Mapp 25: 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Lancaster JL, Fox PT ( 2009): Lost in localization? The focus is meta‐analysis. Neuroimage 48: 18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law J, Flanery M, Wirth S, Yanike M, Smith A, Frank L, Suzuki W, Brown E, Stark C ( 2005): Functional magnetic resonance imaging activity during the gradual acquisition and expression of paired‐associate memory. J Neurosci 25: 5720–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand D, Ruby P ( 2009): What is self‐specific? Theoretical investigation and critical review of neuroimaging results. Psychol Rev 116: 252–282. [DOI] [PubMed] [Google Scholar]
- Lepage M, Ghaffar O, Nyberg L, Tulving E ( 2000): Prefrontal cortex and episodic memory retrieval mode. Proc Natl Acad Sci USA 97: 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Pelletier M, Achim A, Montoya A, Menear M, Lal S ( 2010): Parietal cortex and episodic memory retrieval in schizophrenia. Psychiatry Res 182: 191–199. [DOI] [PubMed] [Google Scholar]
- Leube D, Erb M, Grodd W, Bartels M, Kircher T ( 2003): Successful episodic memory retrieval of newly learned faces activates a left fronto‐parietal network. Cogn Brain Res 18: 97–101. [DOI] [PubMed] [Google Scholar]
- Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM ( 2000): Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci 20: 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM ( 2005): The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage 27: 824–834. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Petersson KM, Andersson J, Johansson M, Fransson P, Ingvar M ( 2003): Isolating the retrieval of imagined pictures during episodic memory: Activation of the left precuneus and left prefrontal cortex. Neuroimage 20: 1934–1943. [DOI] [PubMed] [Google Scholar]
- MacDonald A, Cohen J, Stenger V, Carter C ( 2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Maratos EJ, Dolan RJ, Morris JS, Henson RNA, Rugg MD ( 2001): Neural activity associated with episodic memory for emotional context. Neuropsychologia 39: 910–920. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M ( 2009): Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA 106: 20069–20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Jones TC, Petersen SE, Lageman SK, Roediger HL ( 2000): Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: An event‐related fMRI study. J Cogn Neurosci 12: 965–976. [DOI] [PubMed] [Google Scholar]
- Miller B, Cummings J ( 1999): The Human Frontal Lobes. New York: Guilford Press. [Google Scholar]
- Miller E, Cohen J ( 2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR ( 2006): The neural system that mediates familiarity memory. Hippocampus 16: 504–520. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD ( 2007): Age effects on the neural correlates of episodic retrieval: Increased cortical recruitment with matched performance. Cereb Cortex 17: 2491–2506. [DOI] [PubMed] [Google Scholar]
- Moscovitch M ( 1992): Memory and working‐with‐memory: A component process model based on modules and central systems. J Cogn Neurosci 4: 257–267. [DOI] [PubMed] [Google Scholar]
- Nee D, Wager T, Jonides J ( 2007): Interference resolution: Insights from a meta‐analysis of neuroimaging tasks. Cogn Affect Behav Neurosci 7: 1–17. [DOI] [PubMed] [Google Scholar]
- O'Connor A, Han S, Dobbins I ( 2010): The inferior parietal lobule and recognition memory: Expectancy violation or successful retrieval? J Neurosci 30: 2924–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A, McMillan K, Laird A, Bullmore E ( 2005): N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Hum Brain Mapp 25: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JS, Velanova K, Wolk DA, Wheeler ME ( 2009): Left posterior parietal cortex participates in both task preparation and episodic retrieval. Neuroimage 46: 1209–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, McIntosh AR, Craik FIM, Moscovitch M ( 2010): Past experience modulates the neural mechanisms of episodic memory formation. J Neurosci 30: 4707–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Siegel S, Kanes S, Gur RE ( 2004): Event‐related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am J Psychiatry 161: 1004–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Valdez JN, Loughead J, Gur RC, Gur RE ( 2006): Functional magnetic resonance imaging of internal source monitoring in schizophrenia: Recognition with and without recollection. Schizophr Res 87: 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M, MacLeod A, Snyder A, Powers W, Gusnard D, Shulman G ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo A, Han S, Dobbins IG ( 2009): Ventrolateral prefrontal cortex and self‐initiated semantic elaboration during memory retrieval. Neuropsychologia 47: 2261–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof K, Ullsperger M, Crone E, Nieuwenhuis S ( 2004): The role of the medial frontal cortex in cognitive control. Science 306: 443–447. [DOI] [PubMed] [Google Scholar]
- Rombouts S, Barkhof F, Witter M, Machielsen W, Scheltens P ( 2001): Anterior medial temporal lobe activation during attempted retrieval of encoded visuospatial scenes: An event‐related fMRI study. Neuroimage 14: 67–76. [DOI] [PubMed] [Google Scholar]
- Rushworth M, Buckley M, Behrens T, Walton M, Bannerman D ( 2007): Functional organization of the medial frontal cortex. Curr Opin Neurobiol 17: 220–227. [DOI] [PubMed] [Google Scholar]
- Sajonz B, Kahnt T, Margulies DS, Park SQ, Wittmann A, Stoy M, Ströhle A, Heinz A, Northoff G, Bermpohl F ( 2010): Delineating self‐referential processing from episodic memory retrieval: Common and dissociable networks. Neuroimage 50: 1606–1617. [DOI] [PubMed] [Google Scholar]
- Saxe R ( 2006): Uniquely human social cognition. Curr Opin Neurobiol 16: 235–239. [DOI] [PubMed] [Google Scholar]
- Schacter D, Buckner R ( 1998): Priming and the brain. Neuron 20: 185–195. [DOI] [PubMed] [Google Scholar]
- Schultz W ( 2000): Multiple reward signals in the brain. Nat Rev Neurosci 1: 199–207. [DOI] [PubMed] [Google Scholar]
- Seeley W, Menon V, Schatzberg A, Keller J, Glover G, Kenna H, Reiss A, Greicius M ( 2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL ( 2004): Functional‐anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci 24: 10084–10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Delgado MR, Phelps EA ( 2004): How emotion enhances the feeling of remembering. Nat Neurosci 7: 1376–1380. [DOI] [PubMed] [Google Scholar]
- Skinner E, Fernandes M ( 2007): Neural correlates of recollection and familiarity: A review of neuroimaging and patient data. Neuropsychologia 45: 2163–2179. [DOI] [PubMed] [Google Scholar]
- Slotnick S, Moo L, Segal J, Hart J ( 2003): Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cogn Brain Res 17: 75–82. [DOI] [PubMed] [Google Scholar]
- Smith APR, Henson RNA, Dolan RJ, Rugg MD ( 2004): fMRI correlates of the episodic retrieval of emotional contexts. Neuroimage 22: 868–878. [DOI] [PubMed] [Google Scholar]
- Smith APR, Henson RNA, Rugg MD, Dolan RJ ( 2005): Modulation of retrieval processing reflects accuracy of emotional source memory. Learn Mem 12: 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn M, Ursu S, Anderson J, Stenger V, Carter C ( 2000): The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA 97: 13448–13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson P, Kim A, Han H, Moscovitch M, Grady C ( 2009): Event‐related fMRI studies of episodic encoding and retrieval: meta‐analyses using activation likelihood estimation. Neuropsychologia 47: 1765–1779. [DOI] [PubMed] [Google Scholar]
- Spreng R, Mar R, Kim A ( 2009): The common neural basis of autobiographical memory prospection navigation theory of mind and the default mode: A quantitative meta‐analysis. J Cogn Neurosci 21: 489–510. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL ( 2010): Default network activity coupled with the frontoparietal control network supports goal‐directed cognition. Neuroimage 53: 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S, Deutsch G ( 1997): Left Brain Right Brain: Perspectives from Cognitive Neuroscience. New York: Freeman. [Google Scholar]
- Squire L, Stark C, Clark R ( 2004): The medial temporal lobe. Annu Rev Neurosci 27: 279–306. [DOI] [PubMed] [Google Scholar]
- Stark C, Okado Y ( 2003): Making memories without trying: Medial temporal lobe activity associated with incidental memory formation during recognition. J Neurosci 23: 6748–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon M, Levine B ( 2006): The functional neuroanatomy of autobiographical memory: a meta‐analysis. Neuropsychologia 44: 2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐planar Stereotaxic Atlas of the Human Brain. New York: Thieme. [Google Scholar]
- Tricomi E, Fiez J ( 2008): Feedback signals in the caudate reflect goal achievement on a declarative memory task. Neuroimage 41: 1154–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkler I, King JA, Doeller CF, Rugg MD, Burgess N ( 2009): Neural bases of autobiographical support for episodic recollection of faces. Hippocampus 19: 718–730. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Mochizuki‐Kawai H, Fujii T ( 2005): The effect of encoding strategies on medial temporal lobe activations during the recognition of words: An event‐related fMRI study. Neuroimage 25: 452–461. [DOI] [PubMed] [Google Scholar]
- Tulving E ( 1983): Elements of Episodic Memory. Oxford: Clarendon Press. [Google Scholar]
- Tulving E, Markowitsch H, Craik F, Habib R, Houle S ( 1996): Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex 6: 71–79. [DOI] [PubMed] [Google Scholar]
- Uncapher M, Wagner A ( 2009): Posterior parietal cortex and episodic encoding: Insights from fMRI subsequent memory effects and dual‐attention theory. Neurobiol Learn Mem 91: 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen FM, Nijhuis FAP, Tisserand DJ, Backes WH, Jolles J ( 2006): Effects of aging on recognition of intentionally and incidentally stored words: An fMRI study. Neuropsychologia 44: 2477–2486. [DOI] [PubMed] [Google Scholar]
- Van Essen D ( 2005): A population‐average landmark‐and surface‐based (PALS) atlas of human cerebral cortex. Neuroimage 28: 635–662. [DOI] [PubMed] [Google Scholar]
- Vannini P, O'Brien J, O'Keefe K, Pihlajamaki M, LaViolette P, Sperling RA ( 2011): What goes down must come up: Role of the posteromedial cortices in encoding and retrieval. Cereb Cortex 21: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Jacoby L, Wheeler M, McAvoy M, Petersen S, Buckner R ( 2003): Functional‐anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci 23: 8460–8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD ( 2007): Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia 45: 2216–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg K, Rugg M ( 2008): Memory retrieval and the parietal cortex: A review of evidence from a dual‐process perspective. Neuropsychologia 4: 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD ( 2009): An investigation of the effects of relative probability of old and new test items on the neural correlates of successful and unsuccessful source memory. Neuroimage 45: 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J, Kahn I, Snyder A, Raichle M, Buckner R ( 2008): Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100: 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Luks T, Simpson G, Schulman B, Glenn S, Wong A ( 2006): Brain activation patterns during memory of cognitive agency. Neuroimage 31: 896–905. [DOI] [PubMed] [Google Scholar]
- Wager T, Jonides J, Reading S ( 2004): Neuroimaging studies of shifting attention: A meta‐analysis. Neuroimage 22: 1679–1693. [DOI] [PubMed] [Google Scholar]
- Wager T, Lindquist M, Kaplan L ( 2007): Meta‐analysis of functional neuroimaging data: Current and future directions. Soc Cogn Affect Neurosci 2: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T, Smith E ( 2003): Neuroimaging studies of working memory: A meta‐analysis. Cogn Affect Behav Neurosci 3: 255–274. [DOI] [PubMed] [Google Scholar]
- Wagner A, Shannon B, Kahn I, Buckner R ( 2005): Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9: 445–453. [DOI] [PubMed] [Google Scholar]
- Weis S, Klaver P, Reul J, Elger CE, Fernandez G ( 2004): Temporal and cerebellar brain regions that support both declarative memory formation and retrieval. Cereb Cortex 14: 256–267. [DOI] [PubMed] [Google Scholar]
- Wheeler M, Buckner R ( 2003): Functional dissociation among components of remembering: control perceived oldness and content. J Neurosci 23: 3869–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted J, Stretch V ( 2004): In defense of the signal detection interpretation of remember/know judgments. Psychon Bull Rev 11: 616–641. [DOI] [PubMed] [Google Scholar]
- Woodruff C, Johnson J, Uncapher M, Rugg M ( 2005): Content‐specificity of the neural correlates of recollection. Neuropsychologia 43: 1022–1032. [DOI] [PubMed] [Google Scholar]
- Yonelinas A ( 2002): The nature of recollection and familiarity: A review of 30 years of research. J Mem Lang 46: 441–517. [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD ( 2005): Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci 25: 3002–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerssen GC, Mecklinger A, Opitz B, Cramon DY ( 2001): Conscious recollection and illusory recognition: An event‐related fMRI study. Eur J Neurosci 13: 2148–2156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


