Abstract
We used coordinate‐based meta‐analysis in order to objectively quantify gray matter abnormalities reported in nine Voxel‐Based Morphometry studies of developmental dyslexia. Consistently across studies, reduced gray matter volume in dyslexic readers was found in the right superior temporal gyrus and left superior temporal sulcus. These results were related to findings from previous meta‐analyses on functional brain abnormalities in dyslexic readers. Convergence of gray matter reduction and reading‐related underactivation was found for the left superior temporal sulcus. Recent studies point to the presence of both functional and structural abnormalities in left temporal and occipito‐temporal brain regions before reading onset. Hum Brain Mapp 34:3055–3065, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: dyslexia, reading, magnetic resonance imaging, meta‐analysis, cerebral cortex
INTRODUCTION
The underlying neural dysfunctions in developmental dyslexia were the focus of review articles published on both functional and structural brain abnormalities in dyslexic readers [Démonet et al., 2004; Eckert, 2004; Heim and Keil, 2004; McCandliss and Noble, 2003; Pugh et al., 2000; Sandak et al., 2004; Schlaggar and McCandliss, 2007; Shaywitz and Shaywitz 2005]. In the case of functional abnormalities, three quantitative, coordinate‐based meta‐analyses summed up the large body of existing literature [Maisog et al., 2008; Richlan et al., 2009, 2011]. Functional abnormalities were mainly found in left hemisphere occipito‐temporal, temporo‐parietal, and inferior frontal language regions [for a review see Richlan, in press]. In the case of structural abnormalities such an objective quantification of abnormalities is missing. Therefore, this study provides a meta‐analysis of Voxel‐Based Morphometry studies of gray matter abnormalities in developmental dyslexia.
Starting with 19th century neurological examinations [Dejerine, 1891, 1892], there is a long history of studying neuroanatomical abnormalities in acquired dyslexic readers. With respect to developmental dyslexia, significant progress was made in the 70s and 80s of the last century with the histological postmortem brain examinations by Galaburda et al. Specifically, Galaburda and Kemper 1979 found reduced left‐right asymmetry of the planum temporale—localized on the dorsal bank of the superior temporal gyrus, posterior to Heschl's gyrus—in a postmortem examination of the brain of a developmental dyslexia case. In addition, Galaburda et al. 1985 and Humphreys et al. 1990 reported findings of neuronal ectopias and architectonic dysplasias in left perisylvian regions of several additional dyslexia cases. These cortical anomalies were assumed to develop prenatally during neuronal migration. However, some of the eight cases examined by the Galaburda group may have suffered from comorbid problems which may have been reflected in the reported brain abnormalities. It was also suggested that the dyslexic brains had been stored for a longer period of time than those of the control subjects and therefore were more prone to suffering from cell shrinkage and other postmortem alterations [Heim and Keil, 2004].
With computed tomography (CT) and magnetic resonance imaging (MRI), it became possible to study the structure of a larger number of brains in vivo. Still, analysis of these brain images was difficult and required manual tracing of specific regions of interest based on expert neuroanatomical knowledge. Statistical comparisons were mainly limited to these subjectively defined regions (e.g., the planum temporale) and to rather coarse anatomical measures such as global cerebral volume. This unsatisfactory state of affairs was overcome by statistical methods capable of examining differences between brain images in an unbiased and objective way such as Statistical Parametric Mapping [Friston et al., 1990]. A major advance in the analysis of structural T1‐weighted MR scans was the introduction of Voxel‐Based Morphometry (VBM) by Ashburner and Friston 2000, and the subsequent optimization of this method [Mechelli et al., 2005]. VBM is an objective and powerful tool to study local tissue concentrations on the voxel‐level together with automatic segmentation of gray matter (GM), white matter (WM), and cerebro‐spinal fluid (CSF). The basic idea behind VBM is to identify a particular tissue type — usually gray matter — in the scan of each subject (segmentation) and to warp these tissue maps to a common anatomical space (normalization). The normalized tissue maps are then spatially blurred (smoothing) and a voxel‐by‐voxel statistical analysis of this preprocessed data is performed. VBM provides a general measure of local GM volume or density which is the product of several aspects of cortical architecture such as surface area, folding complexity, and thickness [Hutton et al., 2009]. VBM has been used to investigate both normal brain development [e.g., Good et al., 2001] and pathological alterations [e.g., Mummery et al., 2000]. A PubMed search with the keyword “voxel‐based morphometry” identified over 1700 studies and a number of meta‐analyses were performed on these studies [e.g., Honea et al., 2005]. However, the VBM method was shown to suffer from potential limitations, which will be considered in the Discussion.
With respect to developmental dyslexia, a number of VBM studies of gray matter (GM) abnormalities were published [Black et al., 2012; Brambati et al., 2004; Brown et al., 2001; Eckert et al., 2005; Hoeft et al., 2007; Kronbichler et al., 2008; Menghini et al., 2008; Pernet et al., 2009a, 2009b; Raschle et al., 2011; Silani et al., 2005; Steinbrink et al., 2008; Vinckenbosch et al., 2005]. Inspection of the results of these studies, at first sight, revealed only limited convergence. By meta‐analyzing the original studies, this work aimed at quantifying objectively on a voxel‐by‐voxel basis which regions were consistently reported with GM abnormalities. In the Discussion we relate the present meta‐analytic results of structural abnormalities to our previous meta‐analyses on functional abnormalities [Richlan et al., 2009, 2011].
Two of the VBM studies—in addition to GM abnormalities—investigated white matter (WM) abnormalities in dyslexic readers [Eckert et al., 2005; Silani et al., 2005]. Specifically, Eckert et al. 2005 reported WM reduction in a right temporo‐parietal region, whereas Silani et al. 2005 reported WM reduction in three left hemisphere regions (underneath inferior frontal, postcentral, and supramarginal gyri, respectively). Because of the small number of peaks and their obvious inconsistency with respect to localization, meta‐analytic quantification of these WM abnormalities was omitted.
Another method in the study of WM abnormalities became available with diffusion tensor imaging (DTI) techniques allowing examination of the integrity of fiber tracts [e.g., Basser et al., 1994]. The emergence of this technique was paralleled by a conceptual focus on functional integration among different brain regions in contrast to a focus on functional specialization of discrete brain regions. Several neuroimaging studies investigated dyslexic abnormalities in structural connectivity [Beaulieu et al., 2005; Carter et al., 2009; Deutsch et al., 2005; Dougherty et al., 2007; Frye et al., 2008, 2011; Jäncke et al., 2007; Keller and Just, 2009; Klingberg et al., 2000; Nagy et al., 2004; Niogi and McCandliss, 2006; Odegard et al., 2009; Qiu et al., 2008; Richards et al., 2008; Rimrodt et al., 2010; Rollins et al., 2009; Steinbrink et al., 2008]. However, these abnormalities in structural connectivity are frequently not reported in terms of 3D coordinates in standard stereotactic space, and therefore cannot be included in the present coordinate‐based meta‐analytic approach (see Material and Methods).
MATERIALS AND METHODS
We performed several PubMed searches with the Keywords “dyslexia” and “imaging” to identify relevant structural studies. For meta‐analytic quantification of GM abnormalities, only VBM studies reporting direct group comparisons between nonimpaired and dyslexic readers of an alphabetic script in a standardized stereotactic space (Talairach or MNI) were used. On the basis of these criteria we identified nine studies: Brambati et al. 2004, Brown et al. 2001, Eckert et al. 2005, Hoeft et al. 2007, Kronbichler et al. 2008, Menghini et al. 2008, Silani et al. 2005, Steinbrink et al. 2008, and Vinckenbosch et al. 2005. For homogeneity we did not include a VBM study with Chinese dyslexic readers [Siok et al., 2008]. Two VBM studies were not eligible for inclusion in the meta‐analysis because they studied prereading kindergarteners with a family history of developmental dyslexia rather than diagnosed dyslexics [Black et al., 2012; Raschle et al., 2011]. However, a substantial proportion of these children can be expected to experience major difficulties in the course of learning to read [Scerri and Schulte‐Körne, 2010], and therefore these studies are very interesting and useful for discussion. Another VBM study examined changes in GM volume following an eight week reading instruction in dyslexic children, but was not eligible for inclusion in the meta‐analysis because it did not include a nonimpaired sample [Krafnick et al., 2011]. Also not included were the VBM studies by Pernet et al. [2009a, 2009b]. Specifically, Pernet et al. 2009a failed to find direct group differences in GM volume, and instead reported group differences in correlations between GM volume and behavioral measures. Likewise, Pernet et al. 2009b did not report direct group comparisons but rather used a classification approach to search for voxels in which GM volume of dyslexic readers was found to lie outside the normal range.
A total number of 266 participants (134 dyslexic and 132 nonimpaired readers) were included in the 9 selected studies. These studies and their main characteristics are listed in Table 1. Three of the studies were done with English participants, two each with German and Italian participants, and one with French participants. One study [Silani et al., 2005] included English, French, and Italian dyslexic readers. With respect to age, the participants were mainly adolescents and young adults; that is, they were not in early stages of their reading career. All of the nine studies reported peaks of dyslexic GM reductions which survived statistical thresholds corrected for multiple comparisons. In contrast, peaks of GM increases surviving a corrected threshold were only reported in two studies [Silani et al., 2005; Vinckenbosch et al., 2005]. These peaks were localized in the left posterior middle temporal gyrus [Silani et al., 2005] and in the right precentral gyrus [Vinckenbosch et al., 2005] respectively. A total number of 45 peaks (43 for GM reduction and 2 for GM increase) entered the present meta‐analysis.
Table 1.
Main characteristics of the included studies and number of peaks used in the meta‐analysis
| Year | First author | N | Dys | Con | Native language | Age mean (SD) | Modulated VBM (absolute volumes preserved) | ROI analysis | Threshold | No. of foci (reduced/increased GM) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel‐level (height) P < | Cluster‐level (extent) P < or no. of voxels | ||||||||||
| 2008 | Kronbichler | 28 | 13 | 15 | German | 15.7 (0.7) | X | X | 0.05 corr. | 100 | 9/0 |
| 2008 | Menghini | 20 | 10 | 10 | Italian | 40.8 (6.9) | X | X | 0.005 unc. | 0.05 corr. | 2/0 |
| 2008 | Steinbrink | 16 | 8 | 8 | German | 21.9 (4.1) | X | — | 0.05 corr. | 650 | 2/0 |
| 2007 | Hoeft | 38 | 19 | 19 | English | 14.4 (2.2) | X | — | 0.01 corr. | 0.01 corr. | 6/0 |
| 2005 | Eckert | 26 | 13 | 13 | English | 11.4 (8.2) | X | — | 0.00001 unc. | 0.001 corr. | 5/0 |
| 2005 | Silani | 64 | 32 | 32 | English, French, Italian | 25.3 (5.0) | X | X | 0.05 corr. | — | 1/1 |
| 2005 | Vinckenbosch | 23 | 13 | 10 | French | range 17–30 | — | — | 0.01 corr. | 0.05 corr. | 1/1 |
| 2004 | Brambati | 21 | 10 | 11 | Italian | 29.5 (range 13–57) | X | — | 0.05 corr. | 25 | 9/0 |
| 2001 | Brown | 30 | 16 | 14 | English | 24.0 (5.0) | — | — | 0.05 unc. | 0.05 corr. | 8/0 |
For meta‐analysis, Effect‐Size Signed Differential Mapping (ES‐SDM; http://www.sdmproject.com) software, version 2.14 was used [Radua and Mataix‐Cols, 2009; Radua et al., in press]. Signed Differential Mapping combines positive features from other methods such as Multilevel Kernel Density Analysis (MKDA) and Activation Likelihood Estimation (ALE), and was used in one of our previous meta‐analyses on functional dyslexic abnormalities [Richlan et al., 2011]. All peaks were transformed to Talairach space and meta‐analysis was restricted to a specific GM template provided by the software. For each study, effect‐size maps (Hedge's g) and variance maps were created. For peak voxels, effect sizes were calculated from their respective t values and the number of included participants per group (dyslexic vs. nonimpaired). For the rest of the voxels, effect sizes were estimated by their respective distance to peak voxels by means of an unnormalized Gaussian kernel (FWHM = 20 mm). A random effects model was used to combine the data from the nine studies. To examine statistical significance, the location of the voxels was randomized within the GM template (500 permutations). Finally, the meta‐analytic map was thresholded using a voxel‐level (height) threshold of P < 0.005 (uncorrected) and a cluster‐level (extent) threshold of 10 voxels. For ES‐SDM, this uncorrected threshold was found to optimally balance sensitivity and specificity, and to be an approximate equivalent to a corrected threshold of P < 0.05 in original neuroimaging studies [Radua et al., in press]. However, we also applied a false discovery rate (FDR) threshold of q < 0.05 but none of the results survived this correction. To evaluate the robustness of the findings, we relied on inspecting how many of the original studies contributed to the identification of the meta‐analytic clusters (see below)—a method already applied in our previous meta‐analyses [Richlan et al., 2009, 2011].
RESULTS
Table 2 shows the brain regions identified with GM reduction in the meta‐analytic map. No brain regions were identified with GM increase. The clusters are characterized by the Talairach coordinates and the ES‐SDM z‐values of the maxima and submaxima of the reduction, as well as by the spatial extent. In Figure 1A the clusters with GM reduction are rendered on a template brain. Figure 1B shows coronal slices at y = −50 and y = −34, respectively.
Table 2.
Gray matter reductions in developmental dyslexia identified in the present meta‐analysis
| Region | Talairach‐coordinates | ES‐SDM z‐value | Voxels | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R superior temporal gyrus | 50 | −36 | 18 | 1.93 | 86 |
| 52 | −42 | 18 | 1.89 | ||
| L superior temporal sulcus | −54 | −50 | 10 | 1.70 | 24 |
| −48 | −52 | 12 | 1.55 | ||
| −56 | −46 | 6 | 1.48 | ||
Figure 1.
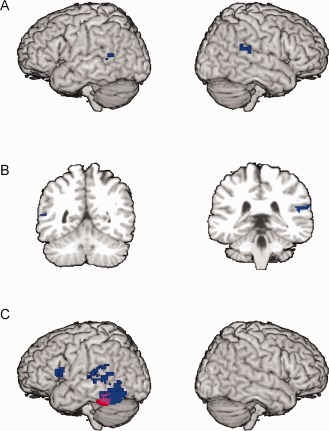
A: Surface rendering of gray matter reductions identified in the present meta‐analysis of structural brain abnormalities. B: Coronal slices at (Talairach coordinates) y = −50 and y = −34, respectively. C: Underactivation in dyslexic children (red) and dyslexic adults (blue) identified in our previous meta‐analysis of functional brain abnormalities [Richlan et al., 2011].
Figure 1A,B and Table 2 show convergent GM reduction in dyslexic compared to nonimpaired readers in the right superior temporal gyrus (STG) and in the left superior temporal sulcus (STS). Specifically, the cluster in the right hemisphere was localized in the posterior dorsal bank of the STG near the temporo‐parietal junction, while the cluster in the left hemisphere was localized in the posterior STS between the superior and middle temporal gyri (MTG). As the right STG cluster was localized more anterior and superior than the left STS cluster, these regions may not be treated as homologous. With respect to spatial extent, the right STG cluster was more than three times bigger than the left STS cluster.
For further evaluation of convergence of GM reductions, Table 3 shows which of the original VBM studies reported peaks of GM reductions contributing to the identification of the present meta‐analytic clusters. Substantial convergence was found for both clusters with five (of nine) studies contributing to each cluster. Furthermore, Table 3 reports additional findings of the original studies which found no support in the present meta‐analysis. Notably, four studies found GM reduction in left ventral occipito‐temporal (OT) regions including inferior temporal and fusiform gyri, but these peaks were too scattered for reliable meta‐analytic clustering. Similarly, the left cerebellum was reported with GM reduction in four original VBM studies, but again, this region failed to survive the meta‐analytic threshold. Further peaks of GM reduction were identified in regions typically associated with phonological or articulatory output processes such as the inferior frontal gyrus, precentral gyrus, supplementary motor area, insula, and basal ganglia, as well as in regions associated with visual processing such as the lingual and medial occipital gyri.
Table 3.
Convergence of gray matter reductions
| Year | First author | R STG | L STS | Additional regions |
|---|---|---|---|---|
| 2008 | Kronbichler | X | L/R fusiform, L/R cerebellum | |
| 2008 | Menghini | R supplementary motor, R superior parietal | ||
| 2008 | Steinbrink | X | X | — |
| 2007 | Hoeft | X | X | L inferior frontal, L/R insula, L anterior cingulate, L lentiform, L/R pre‐/postcentral, L/R inferior parietal |
| 2005 | Eckert | L lentiform, L supramarginal, L/R lingual, L cerebellum | ||
| 2005 | Silani | X | — | |
| 2005 | Vinckenbosch | L inferior temporal | ||
| 2004 | Brambati | X | X | L/R planum temporale, L inferior temporal, L/R fusiform, L/R cerebellum |
| 2001 | Brown | X | X | L/R frontal pole, L/R inferior frontal, L/R caudate, R precentral, L inferior temporal, R medial occipital, L/R cerebellum |
VBM studies reporting peaks of gray matter reduction contributing to the identification of the present meta‐analytic clusters are marked with an X. Furthermore, additional findings of the original studies which found no support in the present meta‐analysis are reported.
DISCUSSION
This article provides an objective summary of studies reporting gray matter (GM) abnormalities in samples of dyslexic readers by quantitatively meta‐analyzing nine original Voxel‐Based Morphometry (VBM) studies. The meta‐analysis, based on 45 peaks, identified GM reduction in the right superior temporal gyrus (STG) and in the left superior temporal sulcus (STS). In the following, we discuss the evidence for these structural abnormalities and relate them to functional abnormalities.
Right Superior Temporal Gyrus
Peaks contributing to the cluster of GM reduction in the posterior dorsal bank of the right STG near the temporo‐parietal junction were reported in five of the nine original VBM studies. This right hemisphere GM reduction was rather unexpected as previous studies identified structural abnormalities primarily in the left temporal lobe. As noted in the Introduction, the seminal histological postmortem brain examinations of Galaburda and colleagues found perisylvian anomalies (i.e., ectopias and dysplasias) primarily—although not exclusively—in the left hemisphere [e.g., Galaburda et al., 1985; Humphreys et al., 1990]. A brain imaging study by Eliez et al. 2000, not included in the present meta‐analysis, measured GM volume in the major lobes of the brain and reported reduced GM volume only for the left but not for the right temporal lobe of dyslexic adults. The present finding of GM reduction in the right STG is also difficult to reconcile with early reports which measured extents of the planum temporale, a region in the dorsal bank of the STG, posterior to Heschl's gyrus. These studies were based on early findings that nonimpaired readers typically exhibit planum temporale asymmetry (i.e., larger left than right) and that dyslexic readers fail to exhibit this asymmetry due to abnormally large extent of the right planum temporale [e.g., Galaburda and Kemper, 1979; Geschwind and Levitsky, 1968]. From larger extent of the right planum temporale, one would have expected dyslexic readers to exhibit increased GM in the right STG, but this was not the case. However, the finding of abnormal planum temporale symmetry in developmental dyslexia found no support in newer studies [e.g., Leonard et al., 2006].
A recent finding by Carreiras et al. [2009] is of interest for interpretation of the present results. This VBM study compared ex‐guerillas who did or did not learn to read as adults and found increased GM volume in bilateral temporo‐parietal (TP) and dorsal occipital regions to accompany learning to read. This finding points to the possibility that the right STG GM reduction found in our meta‐analysis reflects the reduced reading experience of dyslexic readers. Against this interpretation stand the results of Raschle et al. 2011, who found pre‐readers with a high genetic risk for dyslexia to exhibit reduced GM in both left and right TP regions. In a similar study, Black et al. 2012 found maternal history of reading disability to be associated with reduced bilateral TP GM volume in a sample of 5 to 6 year old beginning readers. For these young children the reduction in GM volume cannot be attributed to a reduced amount of reading experience. In summary, the GM reduction in the right STG was an unexpected finding which in earlier work was not paid as much attention as left hemisphere abnormalities. However, as evidenced by two recent studies with young children, right STG GM reduction—together with left temporal GM reduction—may be an early neuroanatomical signature for later reading problems.
Left Superior Temporal Sulcus
Similar to the cluster in the right STG, peaks contributing to the cluster in the left posterior STS (between superior and middle temporal gyri) were reported in five of the nine original VBM studies. As mentioned in the previous section, this finding is in line with evidence for left perisylvian cortical anomalies identified in post‐mortem brain examinations [e.g., Galaburda et al., 1985; Humphreys et al., 1990], as well as with evidence from early neuroimaging studies [Eliez et al., 2000]. Damage to the left STS was classically associated with a disruption in auditory speech comprehension (Wernicke's aphasia). In newer conceptions [e.g., Hickok and Poeppel, 2007], the left STS—as opposed to the bilateral STG, which is associated with auditory spectrotemporal analysis—is thought to be an important region for the representation and/or processing of phonological information. Thus, it is activated during both the perception and production of speech, as well as during active maintenance of phonemic information.
In functional neuroimaging studies of developmental dyslexia, the left STS frequently exhibits underactivation during reading or reading‐related tasks [e.g., Blau et al., 2010; Meyler et al., 2007; Paulesu et al., 2001]. In the dominant version of the phonological deficit explanation, a language‐phonological deficit localized in left STG/STS regions is assumed to affect the emergence of phoneme awareness at the beginning of learning to read, which constitutes the proximal cause for developmental dyslexia [e.g., Shaywitz and Shaywitz, 2005; Snowling, 2000; Vellutino and Fletcher, 2005]. However, other studies suggest that the left STS plays a central role in the integration of auditory and visual information [e.g., van Atteveldt et al., 2004]. Therefore, during reading, its main function may be more directly related to serial grapheme‐phoneme conversion. Dyslexic underactivation of this region in response to demands on letter‐speech sound integration was interpreted as resulting from a failure to develop neural systems specialized for efficient interactive processing of auditory and visual linguistic inputs [Blau et al., 2010]. The convergence between studies of structural brain abnormalities and studies of functional brain abnormalities in dyslexic readers will be discussed in more detail in the following section.
Convergence of Structural and Functional Brain Abnormalities
The right STG and left STS regions with convergent GM reduction only partially overlap with regions identified in the meta‐analyses of regions exhibiting reduced activation in response to reading or reading‐related tasks [Maisog et al., 2008; Richlan et al., 2009, 2011; for a review see Richlan, in press]. Figure 1C presents the regions with underactivation in dyslexic children (red) and underactivation in dyslexic adults (blue) identified by Richlan et al. 2011. The comparison with Figure 1A shows structure–function convergence for the left STS abnormalities, although the extent of the functional abnormalities appears enlarged compared with the structural ones. In contrast, the GM reduction in the right STG found no functional correspondence. Additionally, we did not identify structural equivalents to the underactivation in the left ventral occipito‐temporal (OT) regions and in the left inferior frontal gyrus (IFG) shown in Figure 1C.
The convergence between functional and structural abnormalities in the left STS and the nonconvergence in the right STG (i.e., reduced GM, no reduced activation) is of interest. One may speculate that the reduced GM in both the left and the right hemisphere is caused by abnormalities in prenatal brain development, but only the reduced GM in the left STS affects learning to read, with the consequence of reduced functional engagement. The left STS dysfunction is commonly interpreted as reflecting impaired phonological reading of unfamiliar letter strings in the early stage of learning to read, which secondarily affects the build‐up of orthographic word memories for efficient word recognition in the left ventral OT cortex [e.g., Pugh et al., 2000].
The results of our functional meta‐analysis in Figure 1C are difficult to reconcile with this developmental hypothesis, as the studies with dyslexic children provided convergent evidence for left ventral OT underactivation (marked in red), whereas the studies with adults exhibited underactivation in an extended left OT and in left superior temporal areas (marked in blue). Consistent with the early emergence of left OT underactivation in dyslexic children are studies showing early engagement of left OT regions by nonimpaired reading development. Brem et al. 2010 found prereaders in kindergarten—after a few weeks of letter‐sound training—to exhibit an increased left OT response in reading‐related tasks. Correspondingly, Maurer et al. 2007 found that second graders with a familial risk for dyslexia and poor progress in learning to read exhibited reduced tuning of the electrophysiological response in the left OT. In addition, even before learning to read, the at‐risk children exhibited a reduced bilateral OT response to symbols. Recently, Bach et al. [in press] found that prediction of reading skills in 2nd grade based on behavioral measures was significantly improved by adding ERP and fMRI responses of the left OT region before learning to read to the prediction model. A further recent study with prereaders found underactivation in bilateral OT and left superior and middle temporal regions in children with a family history of developmental dyslexia in response to a phonological matching task [Raschle et al., 2012].
With respect to GM abnormalities, the present meta‐analysis failed to identify left ventral OT regions. However, as already noted, four of the nine original VBM studies (see Table 3) reported reduced GM in this region (including inferior temporal and fusiform gyri). A possible explanation for the absence of left ventral OT GM reduction in the meta‐analytic results is that the peaks of the original studies were too scattered for reliable clustering. In particular, the location of the peaks varied most along the posterior‐anterior direction with some peaks located in posterior fusiform regions (at around y = −60) and others in anterior inferior temporal regions (at around y = −10). The location of the former corresponds to the typical left ventral OT region with dyslexic underactivation identified in functional studies [Richlan, in press; Richlan et al., 2009, 2011] while the location of the latter corresponds to a region associated with heteromodal semantic memory [Binder and Desai, 2011] typically not identified with functional abnormalities in dyslexic readers.
Although the studies in the present meta‐analysis failed to identify convergent structural abnormalities in left ventral OT regions, other studies are suggestive of such abnormalities. Specifically, Frye et al. 2010 provided evidence for a left OT abnormality by reporting reduction of GM volume and of cortical surface area in dyslexic adults. This study was not included in the present meta‐analysis as no coordinates were reported for group differences. For dyslexic children, a recent VBM study by Krafnick et al. 2011 showed that behavioral gains following an eight week reading training were accompanied by GM increases in the left fusiform gyrus, left precuneus, right hippocampus, and right cerebellum. For interpretation of possible left OT abnormalities the VBM study by Raschle et al. 2011 is of specific importance. This study found reduced GM volume of prereaders with a family history of developmental dyslexia not only in bilateral TP regions but also in a left OT region. Furthermore, across children with and without a risk for dyslexia, GM volume in left OT and left TP regions correlated positively with rapid automatized naming performance, which is an important predictor for later reading skills [e.g., Landerl and Wimmer, 2008]. These findings suggest that GM reduction in the left OT—similar to GM reduction in the left STS and in the right STG—may not arise secondarily but may be present even before learning to read.
To draw accurate conclusions on the structure–function relationship, longitudinal studies with measurement of both structural abnormalities and functional abnormalities in response to reading or reading‐related tasks would be important. Apparently, such studies do not exist yet. To our knowledge there are only three studies which investigated both structural and functional abnormalities. Hoeft et al. 2007 found GM reduction and reading‐related underactivation in a left TP region, whereas Silani et al. 2005 found reduced GM volume in a left posterior MTG region that was previously identified with underactivation in a PET study of the same adult participants [Paulesu et al., 2001]. Investigating Chinese dyslexic children, Siok et al. 2008 found co‐occurrence of GM reduction and fMRI underactivation and in the left middle frontal gyrus, which is assumed to be engaged by memorizing the stroke patterns of the Chinese words.
As evident from Table 3, 4 original VBM studies reported GM reduction in the cerebellum. Although the present meta‐analysis failed to identify convergent GM abnormalities in cerebellar regions, there are other findings suggestive of such abnormalities. Specifically, Pernet et al. 2009b found cerebellar GM volumes of dyslexic readers to be either above or below the normal range, and reported volume of the right cerebellum (together with the right lentiform nucleus of the basal ganglia) to most reliably separate dyslexic and nonimpaired readers. In addition, Leonard et al. 2001 reported increased leftward asymmetry of the cerebellar lobes of dyslexic readers. The possible cerebellar abnormalities are of potential interest, as both the skill automatization deficit hypothesis of Nicolson et al. 2001 and the magnocellular deficit hypothesis of Stein and Walsh 1997 posit a dysfunction of the cerebellum in developmental dyslexia.
White Matter Abnormalities in Developmental Dyslexia
As mentioned in the Introduction, a number of recent studies focused on dyslexic abnormalities of white matter (WM) tracts. These diffusion tensor imaging (DTI) studies frequently found reduced organization of fiber tracts in left temporal and left temporo‐parietal regions [Beaulieu et al., 2005; Carter et al., 2009; Deutsch et al., 2005; Klingberg et al., 2000; Nagy et al., 2004; Niogi and McCandliss, 2006; Richards et al., 2008; Rimrodt et al., 2010; Rollins et al., 2009; Steinbrink et al., 2008]. However, there is still little agreement on which fiber tracts within the WM are specifically affected [Ben‐Shachar et al., 2007]. Possible candidates include the corpus callosum (left‐right direction, connecting the cerebral hemispheres), the corona radiata (inferior–superior direction, connecting the cerebellum, thalamus, and brain stem with cortical motor and somatosensory regions), and the main fiber tracts running in the posterior–anterior direction. These are the superior longitudinal fasciculus (SLF, connecting occipito‐temporal, temporo‐parietal, and inferior frontal language regions), the inferior longitudinal fasciculus (ILF, connecting occipital and temporal regions), and the inferior fronto‐occipital fasciculus (IFOF, connecting occipital, parietal and prefrontal regions).
The SLF is of specific interest because it connects the occipito‐temporal, temporo‐parietal, and inferior frontal regions identified as major components of the left hemisphere reading network in numerous functional neuroimaging studies. Further fMRI studies found evidence for functional coupling of these regions in response to reading demands in nonimpaired readers and functional disruption in dyslexic readers [Cao et al., 2008; Richlan et al., 2010; Shaywitz et al., 2003; van der Mark et al., 2011]. Two of the 9 original VBM studies reported left inferior frontal GM reduction but this was not sufficient to reach statistical significance [Brown et al., 2001; Hoeft et al., 2007]. Interestingly, one of the VBM studies which—in addition to GM—investigated WM abnormalities in dyslexic readers reported reduced WM in the depth of the left inferior frontal and left temporo‐parietal cortex, respectively [Silani et al., 2005]. However, the relationship between WM, GM, and functional activation as measured by fMRI is still very unclear. Even more difficult to answer are the questions about how abnormalities in these domains might be related to each other, and how they might exert influence over each other during development. Certainly, more basic research on these issues is required before comprehensive models integrating the various findings on brain abnormalities in developmental dyslexia can be put forward.
Voxel‐Based Morphometry: Methodological Concerns
Although Voxel‐Based Morphometry (VBM) has been extensively used in order to study structural brain abnormalities in various diseases, several potential limitations exist when comparing patients to control subjects. Specifically, the normalization step during the VBM procedure was the subject of much debate [Ashburner and Friston, 2001; Bookstein, 2001; Mechelli et al., 2005]. It was put forward that abnormalities in the brain scans of patients may lead to systematic group‐specific misregistration when trying to match these images to an average brain template. As a result, the VBM method would be sensitive to this registration bias rather than to structural abnormalities per se. On the contrary, it was argued that the normalization step relies on a relatively simple warping method, which attempts to find a global match between brain images (i.e., matching overall size and shape). Therefore, only severe pathologies on the macroscopic level (i.e., tumors and artero‐venous malformations) would lead to misregistration. The presence of such atypical tissue types would additionally lead to misclassification during the segmentation step, which relies on tissue probability maps for GM, WM, and cerebro‐spinal fluid obtained from healthy brains. With respect to developmental dyslexia, no evidence for macroscopic brain abnormalities exists, thus rendering the possibility of misregistration or misclassification unlikely.
However, even in the absence of severe pathologies, one may use an additional processing step in order to compensate for subtle volumetric changes introduced by the normalization step (e.g., artificial enlargement of smaller regions). This optional step is referred to as “modulation,” and is basically a multiplication of the spatially normalized image by its relative volume before and after normalization. Thus, the absolute volume of the normalized image is preserved. As evident from Table 3, seven of the nine VBM studies of the present meta‐analysis included this step. Inclusion of the modulation step also has an effect on the interpretation of VBM results: modulated VBM can be thought of as a measure of absolute volume of a tissue class in a region, whereas unmodulated VBM can be thought of as a measure of relative concentration of a tissue class (in relation to the other classes) in a region.
A further critique of the VBM method concerns its volume‐based registration approach. As mentioned previously, it uses a relatively simple warping method attempting to match the overall size and shape of brains. It was shown that a surface‐based registration approach, which attempts to match cortical (gyral/sulcal) folding patterns, can lead to improved intersubject registration [e.g., Fischl et al., 2008]. Another advantage of the surface‐based approach is that it allows the measurement of more specific properties of GM architecture such as cortical thickness, as opposed to VBM which provides a more general measure of GM volume. The presently described limitations of the VBM method, together with the well‐known problems of classical significance testing, may have led to false positive results or misses. However, this makes the attempt to objectively synthesize original findings through meta‐analysis even more essential.
CONCLUSIONS
The present quantitative, coordinate‐based meta‐analysis of nine Voxel‐Based Morphometry studies of gray matter abnormalities associated with developmental dyslexia found converging evidence for reduced gray matter in the right superior temporal gyrus and left superior temporal sulcus. Reports of gray matter reduction in left occipito‐temporal and cerebellar regions failed to reach statistical significance. The structural abnormalities were related to functional abnormalities identified in previous meta‐analyses. Structure–function convergence was found for the left superior temporal sulcus, which is consistently reported with underactivation in dyslexic readers during reading or reading‐related tasks. Recent evidence from prereaders with a family history of developmental dyslexia suggests early presence of both functional and structural abnormalities in left temporal and occipito‐temporal brain regions.
ACKNOWLEDGMENTS
The authors would like to thank Joaquim Radua for helpful comments and Anna Martin, Joe Miller, and Philipp Schwartenbeck for proofreading the manuscript.
REFERENCES
- Ashburner J, Friston KJ (2000): Voxel‐based morphometry—The methods. Neuroimage 11:805–821. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2001): Why voxel‐based morphometry should be used. Neuroimage 14:1238–1243. [DOI] [PubMed] [Google Scholar]
- Bach S, Richardson U, Brandeis D, Martin E, Brem S: Print‐specific multimodal brain activation in kindergarten improves prediction of reading skills in second grade (in press). [DOI] [PubMed]
- Basser PJ, Mattiello J, Lebihan D (1994): MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, Phillips L (2005): Imaging brain connectivity in children with diverse reading ability. Neuroimage 25:1266–1271. [DOI] [PubMed] [Google Scholar]
- Ben‐Shachar M, Dougherty RF, Wandell BA (2007): White matter pathways in reading. Curr Opin Neurobiol 17:258–270. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH (2011): The neurobiology of semantic memory. Trends Cogn Sci 15:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JM, Tanaka H, Stanley L, Nagamine M, Zakerani N, Thurston A, Kesler S, Hulme C, Lyytinen H, Glover GH, Serrone C, Raman MM, Reiss AL, Hoeft F (2012): Maternal history of reading difficulty is associated with reduced language‐related gray matter in beginning readers. Neuroimage 59:3021–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau V, Reithler J, van Atteveldt N, Seitz J, Gerretsen P, Goebel R, Blomert L (2010): Deviant processing of letters and speech sounds as proximate cause of reading failure: A functional magnetic resonance imaging study of dyslexic children. Brain 133:868–879. [DOI] [PubMed] [Google Scholar]
- Bookstein FL (2001): “Voxel‐based morphometry” should not be used with imperfectly registered images. Neuroimage 14:1454–1462. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, Perani D (2004): Regional reductions of gray matter volume in familial dyslexia. Neurology 63:742–745. [DOI] [PubMed] [Google Scholar]
- Brem S, Bach S, Kucian K, Guttorm TK, Martin E, Lyytinen H, Brandeis D, Richardson U (2010): Brain sensitivity to print emerges when children learn letter‐speech sound correspondences. Proc Natl Acad Sci USA 107:7939–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WE, Eliez S, Menon V, Rumsey JM, White CD, Reiss AL (2001): Preliminary evidence of widespread morphological variations of the brain in dyslexia. Neurology 56:781–783. [DOI] [PubMed] [Google Scholar]
- Cao F, Bitan T, Booth JR (2008): Effective brain connectivity in children with reading difficulties during phonological processing. Brain Lang 107:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JC, Lanham DC, Cutting LE, Clements‐Stephens AM, Chen X, Hadzipasic M, Kim J, Denckla MB, Kaufmann WE (2009): A dual DTI approach to analyzing white matter in children with dyslexia. Psychiatry Res 172:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejerine J (1891): Sur un cas de cecité verbale avec agraphie, suivi d'autopsie. Memoires Societé Biologique 3:197–201. [Google Scholar]
- Dejerine, J (1892): Contribution of l'étude anatomo‐pathologique et clinique des differentes varietiés de cecité verbale. Memoires Societé Biologique 4:61–90. [Google Scholar]
- Démonet JF, Taylor MJ, Chaix Y (2004): Developmental dyslexia. Lancet 363:1451–1460. [DOI] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JD, Wandell B (2005): Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex 41:354–363. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Ben‐Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA (2007): Temporal‐callosal pathway diffusivity predicts phonological skills in children. Proc Natl Acad Sci USA 104:8556–8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert M (2004): Neuroanatomical markers for dyslexia: A review of dyslexia structural imaging studies. Neuroscientist 10:362–371. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, Berninger V (2005): Anatomical signatures of dyslexia in children: Unique information from manual and voxel based morphometry brain measures. Cortex 41:304–315. [DOI] [PubMed] [Google Scholar]
- Eliez S, Rumsey JM, Giedd JN, Schmitt JE, Patwardhan AJ, Reiss AL (2000): Morphological alteration of temporal lobe gray matter in dyslexia: An MRI study. J Child Psychol Psychiatry 41:637–644. [DOI] [PubMed] [Google Scholar]
- Fischl B, Rajendran N, Busa E, Augustinack J, Hinds O, Yeo BT, Mohlberg H, Amunts K, Zilles K (2008): Cortical folding patterns and predicting cytoarchitecture. Cereb Cortex 18:1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Dolan RJ, Lammertsma AA, Frackowiak RSJ (1990): The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab 10:458–466. [DOI] [PubMed] [Google Scholar]
- Frye RE, Hasan K, Xue L, Strickland D, Malmberg B, Liederman J, Papanicolaou A (2008): Splenium microstructure is related to two dimensions of reading skill. Neuroreport 19:1627–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Liederman J, Hasan KM, Lincoln A, Malmberg B, McLean J 3rd, Papanicolaou A (2011): Diffusion tensor quantification of the relations between microstructural and macrostructural indices of white matter and reading. Hum Brain Mapp 32:1220–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Liederman J, Malmberg B, McLean J, Strickland D, Beauchamp MS (2010): Surface area accounts for the relation of gray matter volume to reading‐related skills and history of dyslexia. Cereb Cortex 20:2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM, Kemper TL (1979): Cytoarchitectonic abnormalities in developmental dyslexia: A case study. Ann Neurol 6:94–100. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N (1985): Developmental dyslexia: Four consecutive patients with cortical anomalies. Ann Neurol 18:222–233. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W (1968): Human brain: Left‐right asymmetries in temporal speech region. Science 161:186–187. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14:21–36. [DOI] [PubMed] [Google Scholar]
- Heim S, Keil A (2004): Large‐scale neural correlates of developmental dyslexia. Eur Child Adolesc Psychiatry 13:125–140. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D (2007): The cortical organization of speech processing. Nat Rev Neurosci 8:393–402. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor‐Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, Deutsch GK, Siok WT, Reiss AL, Whitfield‐Gabrieli S, Gabrieli JD (2007): Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci USA 104:4234–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE (2005): Regional deficits in brain volume in schizophrenia: A meta‐analysis of voxel‐based morphometry studies. Am J Psychiatry 162:2233–2245. [DOI] [PubMed] [Google Scholar]
- Humphreys P, Kaufmann WE, Galaburda AM (1990): Developmental dyslexia in women: Neuropathological findings in three patients. Ann Neurol 28:727–738. [DOI] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N (2009): A comparison between voxel‐based cortical thickness and voxel‐based morphometry in normal aging. Neuroimage 48:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L, Siegenthaler T, Preis S, Steinmetz H (2007): Decreased white‐matter density in a left‐sided fronto‐temporal network in children with developmental language disorder: Evidence for anatomical anomalies in a motor‐language network. Brain Lang 102:91–98. [DOI] [PubMed] [Google Scholar]
- Keller TA, Just MA (2009): Altering cortical connectivity: Remediation‐induced changes in the white matter of poor readers. Neuron 64:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA (2000): Microstructure of temporo‐parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging. Neuron 25:493–500. [DOI] [PubMed] [Google Scholar]
- Krafnick AJ, Flowers DL, Napoliello EM, Eden GF (2011): Gray matter volume changes following reading intervention in dyslexic children. Neuroimage 57:733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Wimmer H, Staffen W, Hutzler F, Mair A, Ladurner G (2008): Developmental dyslexia: Gray matter abnormalities in the occipitotemporal cortex. Hum Brain Mapp 29:613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landerl K, Wimmer H (2008): Development of word reading fluency and spelling in a consistent orthography: An 8‐year follow‐up. J Educ Psychol 100:150–161. [Google Scholar]
- Leonard CM, Eckert MA, Lombardino LJ, Oakland T, Kranzler J, Mohr CM, King WM, Freeman A (2001): Anatomical risk factors for phonological dyslexia. Cereb Cortex 11:148–157. [DOI] [PubMed] [Google Scholar]
- Leonard C, Eckert M, Given B, Virginia B, Eden G (2006): Individual differences in anatomy predict reading and oral language impairments in children. Brain 129:3329–3342. [DOI] [PubMed] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF (2008): A meta‐analysis of functional neuroimaging studies of dyslexia. Ann NY Acad Sci 1145:237–259. [DOI] [PubMed] [Google Scholar]
- Maurer U, Brem S, Bucher K, Kranz F, Benz R, Steinhausen HC, Brandeis D (2007): Impaired tuning of a fast occipito‐temporal response for print in dyslexic children learning to read. Brain 130:3200–3210. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Noble KG (2003): The development of reading impairment: A cognitive neuroscience model. Ment Retard Dev Disabil Res Rev 9:196–204. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J (2005): Voxel‐based morphometry of the human brain: Methods and applications. Curr Med Imaging Rev 1:105–113. [Google Scholar]
- Menghini D, Hagberg GE, Petrosini L, Bozzali M, Macaluso E, Caltagirone C, Vicari S (2008): Structural correlates of implicit learning deficits in subjects with developmental dyslexia. Ann NY Acad Sci 1145:212–221. [DOI] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Lee D, Hoeft F, Whitfield‐Gabrieli S, Gabrieli JD, Just MA (2007): Brain activation during sentence comprehension among good and poor readers. Cereb Cortex 17:2780–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR (2000): A voxel‐based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Ann Neurol 47:36–45. [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T (2004): Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci 16:1227–1233. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Dean P (2001): Developmental dyslexia: The cerebellar deficit hypothesis. Trends Neurosci 24:508–511. [DOI] [PubMed] [Google Scholar]
- Niogi SN, McCandliss BD (2006): Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia 44:2178–2188. [DOI] [PubMed] [Google Scholar]
- Odegard TN, Farris EA, Ring J, McColl R, Black J (2009): Brain connectivity in non‐reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia 47:1972–1977. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Démonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N (2001): Dyslexia: cultural diversity and biological unity. Science 291:2165–2167. [DOI] [PubMed] [Google Scholar]
- Pernet C, Andersson J, Paulesu E, Demonet JF (2009a): When all hypotheses are right: a multifocal account of dyslexia. Hum Brain Mapp 30:2278–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet CR, Poline JB, Demonet JF, Rousselet GA (2009b): Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neurosci 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA (2000): Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment Retard Dev Disabil Res Rev 6:207–213. [DOI] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Zhou K, Khong PL (2008): Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: Voxel‐wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage 41:223–232. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix‐Cols D (2009): Voxel‐wise meta‐analysis of grey matter changes in obsessive‐compulsive disorder. Br J Psychiatry 195:393–402. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix‐Cols D, Phillips ML, El‐Hage W, Kronhaus DM, Cardoner N, Surguladze S: A new meta‐analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps (in press). [DOI] [PubMed]
- Raschle NM, Chang M, Gaab N (2011): Structural brain alterations associated with dyslexia predate reading onset. Neuroimage 57:742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Zuk J, Gaab N (2012): Functional characteristics of developmental dyslexia in left‐hemispheric posterior brain regions predate reading onset. Proc Natl Acad Sci USA 109:2156–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards T, Stevenson J, Crouch J, Johnson LC, Maravilla K, Stock P, Abbott R, Berninger V (2008): Tract‐based spatial statistics of diffusion tensor imaging in adults with dyslexia. AJNR Am J Neuroradiol 29:1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F (2012): Developmental dyslexia: dysfunction of a left hemisphere reading network. Front Hum Neurosci 6:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H (2009): Functional abnormalities in the dyslexic brain: A quantitative meta‐analysis of neuroimaging studies. Hum Brain Mapp 30:3299–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H (2011): Meta‐analyzing brain dysfunctions in dyslexic children and adults. Neuroimage 56:1735–1742. [DOI] [PubMed] [Google Scholar]
- Richlan F, Sturm D, Schurz M, Kronbichler M, Ladurner G, Wimmer H (2010): A common left occipito‐temporal dysfunction in developmental dyslexia and acquired letter‐by‐letter reading? PLoS One 5:e12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimrodt SL, Peterson DJ, Denckla MB, Kaufmann WE, Cutting LE (2010): White matter microstructural differences linked to left perisylvian language network in children with dyslexia. Cortex 46:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins NK, Vachha B, Srinivasan P, Chia J, Pickering J, Hughes CW, Gimi B (2009): Simple developmental dyslexia in children: Alterations in diffusion‐tensor metrics of white matter tracts at 3 T. Radiology 251:882–891. [DOI] [PubMed] [Google Scholar]
- Sandak R, Mencl WE, Frost SJ, Pugh KR (2004): The neurobiological basis of skilled and impaired reading: Recent findings and new directions. Sci Stud Read 8:273–292. [Google Scholar]
- Scerri TS, Schulte‐Körne G (2010): Genetics of developmental dyslexia. Eur Child Adolesc Psychiatry 19:179–197. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD (2007): Development of neural systems for reading. Annu Rev Neurosci 30:475–503. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA (2005): Dyslexia (specific reading disability). Biol Psychiatry 57:1301–1309. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Pugh KR, Holahan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC (2003): Neural systems for compensation and persistence: Young adult outcome of childhood reading disability. Biol Psychiatry 54:25–33. [DOI] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet JF, Fazio F, Perani D, Price C, Frith CD, Paulesu E (2005): Brain abnormalities underlying altered activation in dyslexia: A voxel based morphometry study. Brain 128:2453–2461. [DOI] [PubMed] [Google Scholar]
- Siok WT, Niu Z, Jin Z, Perfetti CA, Tan LH (2008): A structural‐functional basis for dyslexia in the cortex of Chinese readers. Proc Natl Acad Sci USA 105:5561–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowling MJ (2000): Dyslexia. Oxford, UK:Blackwell. [Google Scholar]
- Stein J, Walsh V (1997): To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci 20:147–152. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Müller HP, Juengling FD, Kassubek J, Riecker A (2008): The contribution of white and gray matter differences to developmental dyslexia: Insights from DTI and VBM at 3.0 T. Neuropsychologia 46:3170–3178. [DOI] [PubMed] [Google Scholar]
- Van der Mark S, Klaver P, Bucher K, Maurer U, Schulz E, Brem S, Martin E, Brandeis D (2011): The left occipitotemporal system in reading: disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. Neuroimage 54:2426–2436. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM (2005): Developmental dyslexia In: Snowling MJ, Hulme CJ, editors.The Science of Reading: A Handbook. Oxford, UK:Blackwell; pp362–378. [Google Scholar]
- Vinckenbosch E, Robichon F, Eliez S (2005): Gray matter alteration in dyslexia: Converging evidence from volumetric and voxel‐by‐voxel MRI analyses. Neuropsychologia 43:324–331. [DOI] [PubMed] [Google Scholar]


