Abstract
Most neuroimaging studies on planning report bilateral activations of the dorsolateral prefrontal cortex (dlPFC). Recently, these concurrent activations of left and right dlPFC have been shown to double dissociate with different cognitive demands imposed by the planning task: Higher demands on the extraction of task‐relevant information led to stronger activation in left dlPFC, whereas higher demands on the integration of interdependent information into a coherent action sequence entailed stronger activation of right dlPFC. Here, we used continuous theta‐burst stimulation (cTBS) to investigate the supposed causal structure‐function mapping underlying this double dissociation. Two groups of healthy subjects (left‐lateralized stimulation, n = 26; right‐lateralized stimulation, n = 26) were tested within‐subject on a variant of the Tower of London task following either real cTBS over dlPFC or sham stimulation over posterior parietal cortex. Results revealed that, irrespective of specific task demands, cTBS over left and right dlPFC was associated with a global decrease and increase, respectively, in initial planning times compared to sham stimulation. Moreover, no interaction between task demands and stimulation type (real vs. sham) and/or stimulation side (left vs. right hemisphere) were found. Together, against expectations from previous neuroimaging data, lateralized cTBS did not lead to planning‐parameter specific changes in performance, but instead revealed a global asymmetric pattern of faster versus slower task processing after left versus right cTBS. This global asymmetry in the absence of any task‐parameter specific impact of cTBS suggests that different levels of information processing may span colocalized, but independent axes of functional lateralization in the dlPFC. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: prefrontal cortex, lateralization, planning, TMS, cTBS, Tower of London
INTRODUCTION
Successful completion of purposive behavior beyond everyday routine relies on the ability to identify and select an appropriate sequence of actions before an actual execution. This ability—planning ahead future actions—comprises the mental conception and evaluation of several behavioral alternatives and their associated consequences [Goel, 2002; Ward and Morris, 2005]. It is one of the highest human cognitive abilities and, as such, it depends on the integrity of the prefrontal cortex [Owen, 2005]. In a recent study using functional magnetic resonance imaging (fMRI), Kaller et al. [ 2011a] have now shown that activations of left and right dorsolateral prefrontal cortex (dlPFC) double dissociate with regard to two different aspects of planning [see also Grafman et al., 2005]: Different cognitive demands on (i) processes of identifying and matching relevant information on differences between the current state and the intended goal state and (ii) processes of integrating interdependent information into a coherent action sequence were found to entail stronger involvement of left and right dlPFC, respectively [Kaller et al., 2011a]. In this and the present study, these two types of cognitive processes were separated experimentally in a variant of the Tower of London planning task [TOL; Berg and Byrd, 2002] using manipulations of Goal Configuration and Search Depth [Fig. 1; see also Kaller et al., 2011b].
Figure 1.

Experimental paradigm. In a set of three‐move Tower of London problems, the task parameters Search Depth and Goal Configuration were experimentally manipulated within a 2 × 2 factorial design. Search Depth involves the need to mentally accomplish intermediate moves and associated interdependencies. That is, even in relatively simple problems, it may be necessary to put a ball into position other than its goal position first in order to free the way for another ball to be placed into its respective final position (see the white ball in problems with an intermediate move). Goal Configuration concerns the obviousness of priorities for individual goal moves that can be deduced from the structure of the goal state. That is, Goal Configuration relates to the possible configurations of the goal state that differentially predispose the consecutive order of the final goal moves. For instance, if all three balls are stacked on a single rod, the ball at the bottom definitely has to be in its goal position before the ball that is second from the bottom, and so on [Adapted from Kaller et al., 2011a].
To illustrate the cognitive demands underlying these two parameters with a real‐life example, imagine you have invited some friends for dinner and you want to offer them a delicious three‐course meal [see also Penfield and Evans, 1935, for an early case report of a patient that, after frontal lobe resection, experienced excessive planning disturbances in a similar situation]. Even if you are highly proficient in preparing the individual courses separately, for arranging the whole meal on time you will have to mind your overall workflow. In some instances, the order in which you will work on your goals will be evident directly from the outset. For example, the order in which the courses be served is obvious, as it is defined conventionally. In contrast, the situation is less clear if you have to identify the spectrum of possible transformations and imposed restrictions by comparing and matching the present state with the overall goal. For example, the decision whether you begin with preparing the meat, the dessert, or the soup is not predefined by the final state but depends amongst others on the dishes' initial conditions and the estimated time taken for preparation. In the TOL task, demands arising from different levels of Goal Configuration1 are similar to these two example situations: A goal state consisting of a full tower with three balls stacked on a single rod allows to unambiguously identify the order in which the balls have to be placed into their goal positions (i.e., the bottom‐most ball has to be put there first, followed by the second and finally the top‐most ball). Instead, when balls are distributed across the goal state's pegs as a partial tower, this provides little direct information on the sequence of final goal moves but requires comparing and matching the identities and locations of balls across both the start state and the goal state (cf. Fig. 1; Goal Configuration).
To continue with an example on Search Depth, cooking a three‐course meal in a normally equipped kitchen inevitably entails high demands on taking into account the interdependencies between individual steps. For instance, one may have the choice between two alternative pots for preparing the soup that both will equivalently serve this purpose. However, given the limited facilities, choosing the large pot for the soup may hinder preparing subsequent dishes as one of them might not fit in the smaller pot. In the TOL task, similar demands are imposed by Search Depth. In the problems with an intermediate move illustrated in Figure 1, there are two equivalent alternatives for initially moving the white ball in order to release the gray ball. However, only one alternative of depositing the white ball leads to an optimal solution, whereas the other will block the subsequent goal move of the gray ball. Taking into account this interdependency during planning clearly requires mentally looking ahead, whereas problems without an intermediate move can be solved in a straightforward manner, placing each ball in its goal position directly [Kaller et al., 2011a; Owen, 2005].
The double dissociation between Goal Configuration and Search Depth and differential contributions of left and right dlPFC [Fig. 2A,B; Kaller et al., 2011a] is hence a strong indication that often reported bilateral dlPFC activation in complex tasks may indeed reflect concomitant operations of specific cognitive processes that show opposing lateralization. However, since fMRI methodology is purely correlative, it does not permit to infer causal relations between changes in the observed blood‐oxygen level dependent activations and the actual function of the underlying neural tissue [Sack, 2006]. Thus, with respect to the double dissociation between left and right dlPFC function and different aspects of planning, the fMRI results cannot be taken to imply that dlPFC activation is actually necessary for these cognitive operations.
Figure 2.
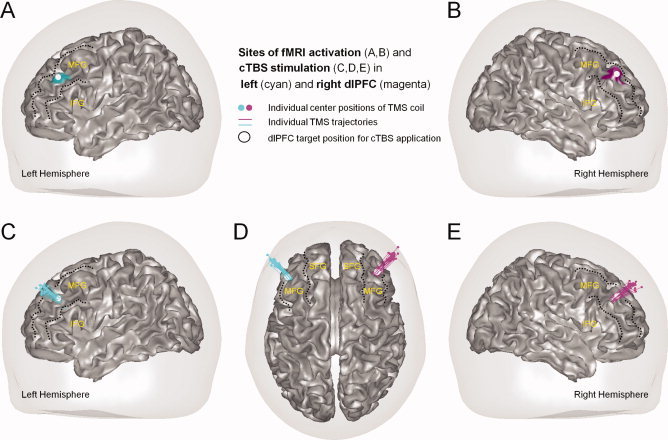
Dorsolateral prefrontal sites of (A,B) double dissociable fMRI activations in Kaller et al. [ 2011a] and (C–E) inhibitory TMS stimulation in the present study. Kaller et al. [ 2011a] recently showed that manipulations of Goal Configuration (cyan) and Search Depth (magenta) were associated with stronger activations of left (A) and right (B) dorsolateral prefrontal cortex (dlPFC), respectively. Based on these results, cTBS was applied in the present study separately over left and right dlPFC. Panels C–E display reconstructed coil positions (colored dots) and trajectories of cTBS pulses (vectors) of individual subjects in the experimental groups with stimulation of left (cyan) and right (magenta) dlPFC. Target positions for transcranial stimulation of left and right dlPFC are illustrated with a white spot (see A–E). Major prefrontal gyri are specified in yellow font (SFG, superior frontal gyrus; MFG, middle frontral gyrus; IFG, inferior frontal gyrus). All coordinates are normalized from individual into Montreal Neurological Institute (MNI) stereotactic standard space.
Here, to further elucidate the presumed differential contribution of left and right dlPFC to higher‐order cognition, we adopted an approach for causal structure‐function mapping using transcranial magnetic stimulation (TMS) in a sample of healthy subjects. Following transient lesions of left and right dlPFC induced by a continuous theta‐burst stimulation (cTBS) protocol, we expected to experimentally validate the double dissociation by observing dissociable stimulation effects on behavioral measures of planning accuracy and/or planning duration for different cognitive demands on Goal Configuration and Search Depth, respectively.
Surprisingly, however, we found an asymmetric effect of stimulation that, dependent on the side of the lesion, led to either global costs or benefits irrespective of the variations in task demands imposed by Goal Configuration and Search Depth: Disturbance of left dlPFC processing was associated with a decrease, whereas a disturbance of right dlPFC processing was followed by an increase in initial thinking time as a behavioral correlate of underlying planning processes [Kaller et al., 2004]. Thus, present neurophysiological and previous neuroimaging approaches to higher‐order cognition seem to unveil two qualitatively different kinds of functional lateralization for the dorsolateral part of the prefrontal cortex.
METHODS AND MATERIALS
Subjects
In total, 60 healthy volunteers aged between 20 and 32 years were recruited for participation. All subjects were right‐handed and had normal or corrected‐to‐normal vision (for sample description, see also paragraph on Experimental Groups). None of them was under medical treatment or reported a history of neurological or psychiatric disorders. Subjects were screened with respect to potential exclusion criteria for application of TMS and/or for prior acquisition of anatomical scans using magnetic resonance imaging (MRI). Written informed consent was obtained from all subjects in advance to the experiment. The study was conducted in accordance with the Declaration of Helsinki and the protocol had been approved by local ethics authorities. Subjects received a compensation of €50 for participation.
Before the TMS experiment, one subject had to be excluded due to an incidental finding in the anatomical MRI scan. Another subject was excluded based on extremely poor performance in the neuropsychological assessment preceding group assignment. Furthermore, one subject had to be excluded for safety reasons (acute head trauma between TMS Sessions 1 and 2), whereas another subject was suspended based on behavior noncompliant with instructions. The remaining 56 subjects were pseudo‐randomly assigned to four experimental groups (see below). Because of technical malfunction during TMS application, data of three subjects were not included in the analyses.
In sum, 7 out of the initially recruited 60 subjects were excluded before or during the experiment. In addition, data inspection for extreme values identified another subject whose average performance in the sham condition (62.5%) deviated more than the criterion of three times the interquartile range from the respective group mean accuracy (M = 92.9%, SD = 8.4%), and that subject was consequently excluded. Taken together, the reported analyses were hence based on a total of 52 included subjects (25 female).
Course of Experimental Sessions
Volunteers who were interested in participating were invited to a briefing session before they made their final decision on taking part in the experiment. In this session, written informed consent was obtained after screening for potential exclusion criteria and explanation of the TMS procedure and associated risks. Furthermore, subjects' resting motor threshold was measured to ensure that individual thresholds were below 60% of the maximum stimulator output in order to minimize stimulation‐induced muscle twitches.
The experiment comprised four separate sessions. In the first and second session, a neuropsychological assessment was administered and an anatomical MRI scan was acquired (see below). The TMS protocol was applied in the third and fourth sessions that were separated by 1 week. In one of the two stimulation sessions, real TMS was applied over the dlPFC, whereas sham stimulation was delivered over the posterior parietal cortex (PPC) in the other session (see below for further details on neuronavigation‐guided localization of stimulation sites). For female subjects, stimulation sessions were fixed to the follicular phase of the menstrual cycle in order to control for possible effects of ovarian hormones on cortical excitability [e.g., Smith et al., 2002] and neuropsychological functions [e.g., Krug et al., 2006].
Neuropsychological Assessment Before Group Assignment
To ensure sample homogeneity across experimental groups, several additional measures were acquired during the separate neuropsychological testing session. Subjects were screened extensively for handedness and degree of lateralization in order to preclude potential a priori group differences in hemispheric asymmetry. An in‐house handedness preference questionnaire based on the Edinburgh Handedness Inventory [Oldfield, 1971; Salmaso and Longoni, 1985] and the Hand Preference Test [Spreen and Strauss, 1998] was administered orally. Hand lateralization was also assessed behaviorally using the Dot Filling Task (DOT) that yields an index of left versus right hand accuracy [Tapley and Bryden, 1985]. Furthermore, a TOL standard set with more complex and demanding four‐, five‐, and six‐move‐problems was administered in order to assess general planning abilities [cf. Kaller et al., 2011b]. The Advanced Progressive Matrices [APM; Raven et al., 1998] were used in a speeded version (15‐min time limit) as a screening for fluid intelligence [cf. Hamel and Schmittmann, 2006] that was previously shown to be correlated with planning ability [cf. Unterrainer et al., 2004a]. In addition, as a measure of verbal crystallized intelligence, versions A and B of the Mehrfachwahl‐Wortschatz‐Test [MWT; Lehrl, 1999; Lehrl et al., 1991] were assessed immediately before the two TMS sessions as independent means to control group assignment.
Experimental Groups
Subjects were assigned pseudo‐randomly into four experimental groups counterbalanced with respect to side and order of stimulation: Two of the groups received a left‐hemispheric stimulation, with either real TMS in the first session and sham TMS in the second session (real‐sham) or the reversed order (sham‐real). The other two groups were stimulated in the right hemisphere, again with a succession of either real‐sham or sham‐real TMS across the two stimulation sessions.
Group assignment was balanced with respect to the following variables: age, sex, DOT, APM, and the TOL standard version (see above). Pseudo‐random allocation of subjects was specifically adjusted in order to minimize mean differences between groups and to maximize variance homogeneity across groups (Table I).
Table I.
Matching of the experimental groups with respect to age, sex, handedness, and several neuropsychological assessments
| Group factors | Group 1 | Group 2 | Group 3 | Group 4 | Sample differences | |
|---|---|---|---|---|---|---|
| Stimulation side | Left | Left | Right | Right | ||
| Stimulation order | Real‐sham | Sham‐real | Real‐sham | Sham‐real | F | P |
| Variables | ||||||
| Sample size (female) | 12 (7) | 14 (6) | 13 (6) | 13 (6) | n.a. | n.a. |
| Age M(SD) | 24.42 (3.14) | 24.43 (2.37) | 24.26 (2.52) | 24.56 (2.76) | 0.027 | 0.994 |
| Handedness M(SD) | 92.80 (11.54) | 96.75 (3.30) | 93.71 (10.09) | 91.26 (15.13) | 0.625 | 0.602 |
| DOT M(SD) | 0.31 (0.15) | 0.28 (0.12) | 0.32 (0.12) | 0.30 (0.13) | 0.176 | 0.912 |
| APM M(SD) | 21.50 (4.03) | 22.07 (4.63) | 21.69 (4.37) | 22.15 (3.85) | 0.068 | 0.977 |
| TOL Standard Set M(SD) | 17.92 (1.93) | 17.36 (2.24) | 17.23 (2.46) | 17.54 (2.70) | 0.201 | 0.895 |
| MWT‐A M(SD) | 29.42 (3.75) | 28.93 (2.62) | 30.23 (2.68) | 30.54 (3.48) | 0.735 | 0.536 |
| MWT‐B M(SD) | 27.33 (2.57) | 25.93 (4.09) | 28.62 (3.48) | 27.77 (3.68) | 1.376 | 0.261 |
M, mean; SD, standard deviation; n.a., not assessed; DOT, Dot Filling Task; APM, Advanced Progressive Matrices; TOL, Tower of London; MWT, Mehrfachwahl‐Wortschatz‐Test.
Experimental Paradigm: Tower of London Task (TOL)
The TOL task [Shallice, 1982] is a frequently used neuropsychological test instrument for assessing planning ability in various clinical and healthy populations [Kaller et al., 2011b]. In its original version, three balls of different colors are placed on three different rods of different lengths [Berg and Byrd, 2002]. Subjects are presented with a start state and are instructed to transform it into a given goal state. To solve the problem in the smallest possible number of moves, subjects are thus requested to plan ahead a solution before manually executing the moves. Three rules have to be followed: (i) only one ball can be moved at a time, (ii) balls must not be placed outside the tower, and (iii) if more than one ball is stacked on a rod, only the top‐most ball can be moved. Planning abilities are usually quantified by the number of correctly solved problems (i.e., within the minimum number of moves) and by the time taken to solve the problems, in particular the time elapsed between the presentation of the problem and the onset of the first move (initial thinking time) as an index of planning duration.
As in the previous fMRI study by Kaller et al. [ 2011a], participants were administered a computerized three‐ball variant of the TOL with equally sized rods [cf. Ward and Allport, 1997]. Because of the absence of height differences in rod sizes, the applied variant is more suitable for repeated presentation of structurally identical problems without subjects becoming aware of it [see also Kaller et al., 2009, 2011a; Unterrainer et al., 2005]. The computer program did not allow rule‐incongruent moves. The balls of the start state could be moved by a sequence of two button presses using a three‐button computer mouse [cf. Kaller et al., 2011a]: A ball was initially “picked up” with a press on the response button that corresponded one‐to‐one with the location of the rod on which the ball was actually placed (i.e., “left” if the ball was placed on the left rod, “middle” for the middle rod, and “right” for the right rod). Accordingly, to place the ball on a certain rod, again the appropriate button had to be pressed. Handling of the three‐button computer mouse was practiced during the neuropsychological testing session as well as in the two experimental sessions immediately before TMS application.
Subjects were instructed to complete the task as quickly and accurately as possible. Furthermore, written and verbal instructions placed strict emphasis on planning the solution in advance of actually moving the balls. During the experiment, instructions were repeatedly presented on screen between six individual blocks of eight successive problems. The task was presented on a 15″ portable notebook to permit administration in the TMS laboratory in order to provide a short time interval between stimulation and task completion. In both the real and the sham TMS session, planning in the TOL task was assessed immediately after stimulation to maximize chances that the effect of the applied TMS protocol would last for all trials of the session (however, see also analyses below and data provided in the Supporting Information, Fig. S4 and Table S2).
The following measures were recorded: Accuracy, initial thinking times, movement execution times, and overall solution times. Accuracy indicates whether a problem was solved correctly within the minimum number of moves. Initial thinking time refers to the time span from presentation onset of a problem to the first selection of a ball, whereas movement execution time is defined as the time between the first touch of the ball and the final solution. Consequently, the overall solution time results from summing up initial thinking and movement execution time.
Experimental Design and Problem Set
Since the primary intention of the present study was aimed at an experimental validation of the recently reported double dissociation between the activation of left and right dlPFC and two distinct cognitive processes during planning [Kaller et al., 2011a], the resulting experiment was designed to integrate the following two aspects: First, the design comprised a full replication of the factorial 2×2 within‐subjects manipulation of Search Depth (with vs. without an intermediate move) and Goal Configuration (partial tower vs. full tower) in a set of three‐move TOL problems as applied in the previous fMRI study [cf. Fig. 1; Kaller et al., 2011a]. Second, the design incorporated two additional factors associated with the applied transcranial stimulation, that is, Lesion Side (between‐subjects: left vs. right hemisphere) and Stimulation Type (within‐subjects: real vs. sham). As individual successions of Stimulation Type were counterbalanced across experimental groups (see above), the design also included an additional between‐subjects factor of Stimulation Order (between‐subjects: real‐sham vs. sham‐real).
As noted before, the factorial design of the applied TOL problem set (cf. Fig. 1) was identical to the study of Kaller et al. [ 2011a]. But whereas the previous fMRI experiment comprised a single session with 96 experimental trials in total, the present TMS study consisted of two separate stimulation sessions (within‐subjects factor Stimulation Type: real vs. sham) each comprising 48 trials (see also above). Besides this, the same set of eight structurally unique problems was used, each presented in six different isoforms (created by permutations of balls colors and peg positions), so that even repetitions of the same structural problem had an unique visual appearance. All other aspects concerning the pseudo‐random and counterbalanced selection of isoforms, presentation order, timing, and so forth were kept identical to the study of Kaller et al. [ 2011a]. Thus, in the present study, individual problem sets with counterbalanced presentation order and pseudo‐randomly varied permutations of ball colors and peg positions were used across subjects and sessions (real/sham stimulation) in order to keep potential carry‐over effects at a minimum. For more detailed information, please refer to the descriptions in Kaller et al. [ 2011a]. Also note that during analyses, the problem set was split into two halves each comprising 24 trials (i.e., six problems per cell of the 2 × 2 design). Primary results were based on the first half (see Results section below and also Tables S1, S2, and Fig. S4 in the Supporting Information).
MRI Acquisition, Preparation for Neuronavigation, and Stimulation Coordinates
High‐resolution 3D T1‐weighted anatomical brain images were acquired on a 3 Tesla whole‐body TIM Trio MR scanner (Siemens, Erlangen, Germany) using an 8‐channel head coil, with magnetization‐prepared rapid gradient echo images (MPRAGE; TR, 2200 ms; TE, 2.15 ms; FA, 12°; 160 sagittal slices; matrix size, 256 × 256; FOV, 256 mm; 1.0 mm3 cubic voxels).
Individual sites for TMS application were based on group activation foci reported in Kaller et al. [ 2011a] in the stereotactic Montreal Neurological Institute (MNI) space. That is, standardized coordinates for real TMS application over left (−45 33 31) and right dlPFC (40 38 34) were specified at the Euclidian center of the most significant mid‐dorsolateral fMRI activation peaks of the main effects of both Goal Configuration and Search Depth. Identical coordinates were used by Kaller et al. [ 2011a] for volumes‐of‐interest analyses and statistical testing of the presumed double dissociation (see also Fig. 2A,B). Likewise, standardized MNI coordinates for sham stimulation of the left (−26 −60 48) and right PPC (22 −70 52) were based on the posterior parietal activation peaks for the main effects of Goal Configuration and Search Depth in the previous fMRI study [Kaller et al., 2011a]. To determine these sites for each subject of the present study, the T1‐weighted anatomical scan was normalized into MNI space using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5). Target coordinates for TMS were defined in the normalized anatomical scan and backprojected from MNI into individual space by means of inverse normalization. Individual stimulation sites were accessed using a TMS‐specific neuronavigation device (LOCALITE TMS Navigator 1.6.5, LOCALITE GmbH, Sankt Augustin, Germany). Subjects' heads were captured by an optical tracking system before TMS application and coregistered with the individual anatomical scan based on a surface‐matching algorithm. Navigation to the individually specified stimulation coordinates (see above) was achieved by 3D entry‐target navigation. As can be seen in the reconstructions of recorded TMS coil positions and trajectories for the individual subjects (Fig. 2C–E), real stimulation was focused neatly on the dlPFC targets that were localized in left and right middle frontal gyrus (MFG). Despite some degree of interindividual variability in sulcal brain anatomy, Supporting Information Figures S1 and S2 further show that the group activation foci from Kaller et al. [ 2011a] used for TMS here in individual subjects were placed at comparable stereotactic locations and gross‐morphological structures.
Continuous Theta‐Burst Stimulation
Transcranial stimulation was accomplished with a Medtronic Mag Pro Stimulator (Dantec Medtronic, Skovlunde, Denmark) using a figure‐of‐eight coil (radius, 50 mm; Magnetic Coil Transducer MC‐B70, Dantec Medtronic) to generate biphasic repetitive magnetic pulses. The inhibitory stimulation protocol applied both in the sham and the real TMS condition was a cTBS protocol at 50 Hz, resulting in an interstimulus‐interval of 20 ms. Theta‐bursts consisting of trains with three pulses were repeated every 200 ms, giving a total of 600 pulses in 40 s. In previous studies, the same cTBS protocol has been shown to reduce excitability in the human motor cortex for at least 20 min [Huang et al., 2005; for an overview see Hoogendam et al., 2010]. In advance to the TMS, the actual level of the resting motor threshold was measured again. Subjects were reminded of possible adverse effects. Stimulation was given at 80% of the individual resting motor threshold in terms of the lowest possible stimulation intensity at which 5 out of 10 single pulses over the primary motor cortex elicited motor evoked potentials (MEPs) in the first dorsal interosseus muscle of the contralateral hand. As noted above, target coordinates for real stimulation of left and right dlPFC and sham stimulation of left and right PPC were based on the previously reported fMRI results [Kaller et al., 2011a]. For the sham stimulation, the coil was tilted away from the scalp in a 90° angle. This was already done during neuronavigation so that the sensation of the tilting could not be a hint for the kind of stimulation given. To further avoid an uncovering of the sham condition, subjects were blindfolded and wore earplugs during stimulation. To prevent head motion, subjects' heads were stabilized using an inflatable neck collar.
The position of the TMS coil in relation to the subject's head was continuously monitored by the neuronavigation system throughout the stimulation. For assessing stimulation acuity across groups, individual cTBS pulse trajectories were calculated (Fig. 2C–E). Neither the average distance between coil and target location nor the target mismatch nor the variability of the coil position during stimulation indicated a significant difference between groups (Table II). Experimental groups were also similar concerning subjects' resting motor thresholds that determined the individual stimulation intensity (see above). Inspection of the time intervals both between the two TMS sessions (real vs. sham) and within the TMS sessions (i.e., between offset of cTBS application and onset of TOL planning task) also did not reveal any group differences. Taken together, subsequent analyses confirmed that the four experimental groups did not differ with respect to any variables that might have influenced the effectiveness of the inhibitory transcranial stimulation (for an overview, see Table II).
Table II.
Matching of experimental groups with respect to TMS related variables
| Group factors | Group 1 | Group 2 | Group 3 | Group 4 | Sample differences | |
|---|---|---|---|---|---|---|
| Stimulation side | Left | Left | Right | Right | ||
| Stimulation order | Real‐sham | Sham‐real | Real‐sham | Sham‐real | F | P |
| Variables | ||||||
| Stim. intensity M(SD) | 33.92 (6.16) | 34.21 (4.95) | 32.92 (6.38) | 33.62 (5.77) | 0.120 | 0.948 |
| Coil‐target dist. M(SD) | 20.50 (2.07) | 20.07 (4.65) | 21.77 (2.05) | 22.69 (3.57) | 1.710 | 0.177 |
| Target mismatch M(SD) | 2.13 (1.00) | 1.86 (0.86) | 2.24 (0.94) | 2.30 (0.94) | 0.587 | 0.627 |
| Coil drift M(SD) | 0.37 (0.27) | 0.29 (0.21) | 0.25 (0.20) | 0.33 (0.28) | 0.589 | 0.625 |
| Int. b/w sessions M(SD) | 6.96 (1.65) | 7.22 (1.89) | 7.39 (1.35) | 6.76 (2.19) | 0.311 | 0.817 |
| Int. b/w TMS/plan. M(SD) | 2.33 (0.65) | 2.43 (0.51) | 2.00 (0.41) | 2.46 (0.52) | 2.102 | 0.112 |
Stim. intensity, stimulation intensity, set to 80% of the resting motor threshold (assessed before real cTBS application in percent of maximum stimulator output). Coil‐target dist., distance in mm between coil center and target in dlPFC. Coil drift indicates average shift in mm of the coil during stimulation. Int. b/w sessions indicates the time intervals between sessions in days, whereas Int. b/w TMS/plan. indicates the time interval between the end of real TMS application and beginning of the planning experiment in minutes.
RESULTS
Analyses were originally planned to be conducted on observations aggregated across all trials per session and subject. However, inspection of the data revealed that the effects of cTBS declined substantially during the session. Therefore, behavioral data were split into two halves before aggregation. Unless otherwise indicated, only data on accuracy and latency of the first half of trials per session will be reported in the following.
General Effects of cTBS Over Left and Right DLPFC
At first, a repeated measures multivariate analysis of variance (RM‐MANOVA) was conducted on accuracy in terms of the number of correctly solved problems and on latency in terms of the overall solution time in order to assess the global effects of cTBS application over left and right dlPFC. Between‐subject factors comprised Lesion Side (left vs. right dlPFC) and Stimulation Order (real‐sham vs. sham‐real), whereas Stimulation Type (real vs. sham) was entered as a within‐subject factor.
The multivariate analysis yielded significant interaction effects for Lesion Side × Stimulation Type (F (2,47) = 3.26, P = 0.047) and Stimulation Type × Stimulation Order (F (2,47) = 29.96, P < 0.001). Neither any of the main effects (Lesion Side, F (2,47) = 0.59, P = 0.559; Stimulation Order, F (2,47) = 0.08, P = 0.921; Stimulation Type, F (2,47) = 0.02, P = 0.983) nor the remaining two‐ and three‐way interactions (highest F = 0.57, lowest P = 0.571) reached significance. Subsequent univariate testing for the effect of Lesion Side × Stimulation Type revealed that the interaction originated from the overall solution time (F (1,48) = 4.84, P = 0.033), but not from accuracy in terms of correctly solved problems (F (1,48)< 0.01, P = 0.994). As the effect of a Stimulation Type × Stimulation Order interaction most likely reflects unspecific improvements of processing speed across sessions due to global retest effects, it was consequently not followed up (see also Fig. 3). Note that these unspecific improvements were also observed in previous studies and shown not to affect task‐demand specific effects of Goal Configuration and Search Depth on planning processes [for further details, see also Supporting Information of Kaller et al., 2011a].
Figure 3.

The Stimulation Type × Stimulation Order interaction in overall solution time can be attributed to an unspecific retest effect [see also Kaller et al., 2011a] between the two sessions irrespective of the application of real or sham stimulation. Error bars indicate standard error of mean (SEM). Symbols: ***, P < 0.001.
To further elucidate the source of the Lesion Side × Stimulation Type interaction, the RM‐MANOVA was repeated by substituting overall solution time by its two composing measures, that is, initial thinking time and movement execution time. Results revealed that the interaction effect can be attributed to the planning phase since it was present only for the initial thinking time (F (1,48) = 7.98, P = 0.007), but not during movement execution (F (1,48) = 0.83, P = 0.367).
In summary, assessing the general effects of cTBS yielded a significant interaction between the type (real vs. sham) and side (left vs. right dlPFC) of TMS application that was specific for planning latency, but not accuracy (see Fig. 4A,B). Moreover, the interaction was driven by an asymmetric cTBS effect: Compared to sham stimulation, disruption of left dlPFC processing was associated with a decrease in initial thinking time whereas, in contrast, impairment of right dlPFC function was followed by an increase of initial thinking time (Fig. 4B). Further posthoc analysis of the main effects of cTBS application carried out separately for the two levels of Lesion Side revealed strong trends toward a significant effect of Stimulation Type (real vs. sham) in both left (F (1,24) = 4.23, P = 0.051) and right dlPFC (F (1,24) = 3.75, P = 0.065).
Figure 4.

Global effects of cTBS over left and right dlPFC on (A) accuracy and (B) latency. Accuracy was close to ceiling and not affected by the stimulation in any way. Instead, initial thinking times showed asymmetric cTBS effect by means of a significant interaction between Stimulation Type and Lesion Side. Note that sham stimulation was applied over posterior parietal cortex. Error bars indicate standard error of mean (SEM). Symbols: **, P < 0.01.
Problem‐Structure Specific Effects of cTBS Over Left and Right DLPFC
To test for problem‐structure specific TMS effects, the above RM‐MANOVA on correctly solved problems, initial thinking and movement execution time was complemented by the two additional within‐subject factors Goal Configuration (full tower vs. partial tower) and Search Depth (with vs. without an intermediate move). As expected [cf. Kaller et al., 2011a], multivariate tests yielded—in addition to the so far reported results—highly significant main effects for Goal Configuration (F (3,46) = 49.57, P < 0.001) and Search Depth (F (3,46) = 41.81, P < 0.001) as well as a significant interaction between both factors (F (3,46) = 2.92, P = 0.044). However, neither the two‐way interactions with Lesion Side (Goal Configuration, F (3,46) = 0.73, P = 0.538; Search Depth, F (3,46) = 0.83, P = 0.467) and Stimulation Type (Goal Configuration, F (3,46) = 0.33, P = 0.802; Search Depth, F (3,46) = 0.89, P = 0.450) nor the three‐way interactions with Lesion Side and Stimulation Type (Goal Configuration, F (3,46) = 0.48, P = 0.701; Search Depth, F (3,46) = 0.79, P = 0.507) reached significance. Thus, no empirical evidence was found for the hypothesized hemispherical specificity of cTBS over left and right dlPFC with respect to different planning demands, that is, the experimental manipulations of Goal Configuration and Search Depth. A significant three‐way interaction could be observed for Goal Configuration × Stimulation Type × Stimulation Order (F (3,46) = 4.15, P = 0.011) while all other remaining two‐, three‐, four‐, and five‐way interactions including either Goal Configuration, Search Depth, or both also failed to reach significance.
Subsequent univariate testing revealed that the significant main effects for problem structure could be attributed mostly to all three dependent measures: correctly solved problems (Goal Configuration, F (1,48) = 19.03, P < 0.001; Search Depth, F (1,48) = 10.99, P < 0.001), initial thinking time (Goal Configuration, F (1,48) = 41.41, P < 0.001; Search Depth, F (1,48) = 116.25, P < 0.001), and movement execution time (Goal Configuration, F (1,48) = 17.59, P < 0.001; Search Depth, F (1,48) = 1.49, P = 0.229). Yet, univariate tests of the interaction between Goal Configuration × Search Depth failed to reach significance (correctly solved problems, F (1,48) = 0.49, P = 0.488; initial thinking time, F (1,48) = 1.10, P = 0.300) but revealed a trend for movement execution time (F (1,48) = 3.03, P = 0.088). Problem‐structure related effects on correctly solved problems and movement execution time are covered in Table III. Figure 5 illustrates the effects of Goal Configuration and Search Depth on initial thinking time with respect to the above reported asymmetric effect of cTBS application over left and right dlPFC.
Table III.
Overview on the main effects of Goal Configuration and Search Depth on correctly solved problems (ACC) and movement execution time (MET)
| Lesion Side | TMS | ACC | MET | |
|---|---|---|---|---|
| M (SEM) | M (SEM) | |||
| Goal Config | ||||
| Partial tower | Left dlPFC | Real | 96.47 % (1.24) | 2,330 ms (130) |
| Sham | 96.79 % (1.04) | 2,374 ms (131) | ||
| Right dlPFC | Real | 96.47 % (0.82) | 2,448 ms (142) | |
| Sham | 97.44 % (1.11) | 2,384 ms (104) | ||
| Full tower | Left dlPFC | Real | 92.95 % (1.77) | 2,066 ms (94) |
| Sham | 92.31 % (1.59) | 2,151 ms (106) | ||
| Right dlPFC | Real | 91.99 % (1.76) | 2,296 ms (144) | |
| Sham | 90.71 % (1.98) | 2,262 ms (107) | ||
| Search Depth | ||||
| High | Left dlPFC | Real | 90.71 % (2.28) | 2,272 ms (115) |
| Sham | 93.59 % (1.62) | 2,247 ms (126) | ||
| Right dlPFC | Real | 93.27 % (1.53) | 2,389 ms (139) | |
| Sham | 93.27 % (1.43) | 2,336 ms (110) | ||
| Low | Left dlPFC | Real | 98.72 % (0.60) | 2,124 ms (108) |
| Sham | 95.51 % (1.33) | 2,278 ms (119) | ||
| Right dlPFC | Real | 95.19 % (1.05) | 2,354 ms (146) | |
| Sham | 94.87 % (1.39) | 2,310 ms (99) |
Note that sham stimulation was applied over posterior parietal cortex.
Figure 5.

Effects of cTBS over left and right dlPFC with respect to experimental manipulations of (A,B) Goal Configuration and (C,D) Search Depth. In addition to the global asymmetric cTBS effect, significant main effects of the two structural problem parameters were evident in initial thinking times but no task‐demand specific influence of left or right‐hemispheric dlPFC stimulation. Note that sham stimulation was applied over posterior parietal cortex. Error bars indicate standard error of mean (SEM). Symbols: ***, P < 0.001.
Univariate testing of the significant three‐way interaction for Goal Configuration × Stimulation Type × Stimulation Order revealed that the effect was evident only for movement execution time (F (1,48) = 6.70, P = 0.013). Since an involvement of Stimulation Type × Stimulation Order in the three‐way interaction term implied a differential effect of Goal Configuration across the two experimental sessions (see above), data were restructured and aggregated according to the Experimental Session instead of Stimulation Type. As expected, the reanalysis revealed a significant two‐way interaction (F (1,53) = 7.73, P = 0.008) between Experimental Session (Session 1 vs. Session 2) and Goal Configuration (partial tower vs. full tower) without any additive or nonadditive involvements of Stimulation Order (real‐sham vs. sham‐real). That is, the effect of Goal Configuration on movement execution times decreased across sessions (see Fig. 6) but it was not susceptible to the transcranial stimulation per se.
Figure 6.
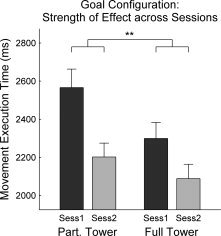
Decreasing effect of Goal Configuration on movement execution time across sessions. Error bars indicate standard error of mean (SEM). Symbols: **, P < 0.01.
Taken together, against expectations, none of the observed effects of Goal Configuration and/or Search Depth were found to be associated with the transcranial stimulation in general or with a particular side of applying the cTBS protocol. Instead, an asymmetric cTBS effect was observed independent of experimental manipulations of problem structure. That is, compared to sham stimulation, application of cTBS over left and right dlPFC was associated with global benefits and costs in initial thinking times, respectively (Fig. 4B), but not in accuracy or movement execution times.
Rerunning aforementioned analyses on data aggregated over the second halves of the experimental sessions (see also above) did not yield any cTBS related effects. An overview on these results is provided in the Supporting Information, Table S1. Further descriptive information on the effect of real cTBS across time (i.e., experimental blocks and sessions' first and second halves) can also be found in the Supporting Information, Figure S4 and Table S2.
As subjects are likely to show interindividual variability of TMS effects, further information on the effect of real cTBS on initial thinking time is provided in Supporting Information, Figure S3. Based on a standardization with respect to the latencies under sham stimulation, these data show that the distributions of initial thinking time following real stimulation of left and right dlPFC were shifted from each other by approximately a half standard deviation.
DISCUSSION
In the present study, continuous theta‐burst stimulation (cTBS) was used to modulate the functioning of left and right dlPFC during planning. We expected to corroborate the double dissociation of hemisphere and task parameter found in fMRI suggesting a predominantly left hemispheric dlPFC contribution to the extraction of information in Goal Configuration, and right hemispheric dlPFC dominance for mastering demands on Search Depth [cf. Kaller et al., 2011a]. In apparent contrast, however, we found that cTBS over left versus right dlPFC did differentially impact planning, but in a way that was independent of the specific task demands imposed by Goal Configuration and Search Depth. That is, compared to a sham condition, stimulation over left and right dlPFC led to global accelerations and decelerations of cognitive processing, respectively. Taken together, while recently reported fMRI data indicate a double dissociation between left and right dlPFC function and specific planning demands [Kaller et al., 2011a], here we provide clear‐cut evidence for another—qualitatively different—kind of functional lateralization between left and right prefrontal cortex in higher‐order cognitive processing.
To delineate potential ways of incorporating both the present asymmetric effect of cTBS and the previous fMRI double dissociation into a coherent account of left and right dlPFC function, an understanding of the cTBS effects is essential. To this aim, we begin with the putative local and remote effects of cTBS and the potential role of transcallosal inhibition for the observed asymmetry. Thereafter, we focus on the resulting implications for extant theories on the differential functions of left and right dlPFC. Finally, since the present data disclose a striking and so far unrecognized phenomenon that challenges previous conceptions of the mechanisms behind dlPFC functions, we close with discussing the future perspectives and, in particular, the potential of multimethod approaches to further elucidate the underlying neurophysiological and cognitive processes.
Putative Local and Remote cTBS Effects Over DLPFC
First and foremost, the unexpected result of an asymmetric global effect of stimulation leads to questions on the underlying neurophysiological mechanisms. In previous studies, the cTBS protocol applied here was repeatedly shown to produce a local inhibitory effect when utilized over primary motor cortex (M1) in terms of reduced MEP amplitudes and decreased short‐interval intracortical inhibition (SICI) measures [e.g., Huang et al., 2005, 2008; Suppa et al., 2008; for review see Hoogendam et al., 2010]. Yet, given that no objective physiological output measure comparable to MEP or SICI suppression is currently available for polymodal association areas such as the dlPFC, we can only assume that the cTBS protocol in the present study also exerted local inhibitory effects on the stimulated dlPFC circuitry [cf. Kähkönen et al., 2004].
Another factor that seriously limits interpretation of local cTBS effects is the putative degree of ongoing background activation. As was previously shown for M1, the brain state of the stimulated tissue before, during, and after cTBS application may strongly influence the nature of the observable after‐effects and can even completely invert their direction [cf. Gamboa et al., 2010; Gentner et al., 2008; Huang et al., 2008]. Given this state dependency of TMS, functionally distinct but spatially overlapping types and/or populations of neurons in the dlPFC might have been differentially modulated by the stimulation. In contrast to usual practice in experiments on primary motor cortex, the dlPFC was studied here under considerable cognitive load immediately following stimulation. Thus, it can neither be excluded that this might have additionally influenced cTBS after‐effects in general nor that this influence might even have affected stimulation effects differentially for left and right dlPFC.
Apart from these putative local effects, neural consequences of focal brain stimulation might not be restricted to the actual site of stimulation [for review, see Paus, 2005; Sack, 2006], but rather spread along anatomical connections affecting remote areas, for example, parietal cortex after dlPFC stimulation [Paus et al., 2001]. A specific remote effect, which is reliably observed in recent studies on the motor system, is a cTBS‐induced increase of activity in unstimulated contralateral homologue areas. In the motor system, these after‐effects are interpreted as net changes in the amount of ongoing transcallosal inhibition [e.g., Stefan et al., 2008; Stinear et al., 2009; Suppa et al., 2008]. Again assuming that the general principles observed in the motor system apply also on dlPFC function, and given the extensive connectivity of the prefrontal cortex [Fuster, 2008], it seems rather likely that the present results are also not merely due to local cTBS effects on the stimulated dlPFC but may also be mediated by its efferents to the nonstimulated contralateral dlPFC (as well as to other remote areas). Moreover, the observed asymmetric cTBS effect includes a paradoxical functional facilitation [cf. Kapur, 1996; Walsh and Pascual‐Leone, 2003] for left dlPFC stimulation that (i) is in opposite direction of the implied detrimental effects of the inhibitory stimulation protocol and that (ii) leads to an enhanced level of performance better than normal, or more exactly, than under sham stimulation (see Fig. 4B). Previous instances of paradoxical functional facilitation were commonly explained in the context of interhemispheric competition (or rivalry) and transcallosal inhibition [see Walsh and Pascual‐Leone, 2003]. Accordingly, a transient reduction in the excitability of mainly inhibitory transcallosal fibers was suggested as a neural mechanism behind paradoxical enhancements induced by repetitive TMS [cf. Fecteau et al., 2006; Kobayashi et al., 2004].
For the present asymmetry, decreases and increases in initial thinking time following stimulation of left and right dlPFC, respectively, might hence be due to a combination of both direct and indirect, that is, local and remote, cTBS after‐effects. That is, direct effects in terms of a local impairment in processing of the stimulated dlPFC may have been complemented by indirect effects via reduced transcallosal inhibition that led to enhanced excitability and facilitated processing of the contralateral, unstimulated dlPFC (see also below). This view is supported by a recent study on transcallosal inhibition between left and right inferior frontal gyrus (IFG) in language processing by Thiel et al. [ 2006], who combined an inhibitory repetitive TMS protocol with concurrent positron emission tomography. In line with the two regionally distinct kinds of after‐effects postulated here, stimulation of left IFG resulted in a local decrease of relative cerebral blood flow (rCBF) change during verb generation in the left IFG and a simultaneous remote increase of rCBF change in the contralateral homologue, that is right IFG [Thiel et al., 2006].
Functional Implications of the Asymmetric cTBS Effect
Present cTBS revealed a global asymmetric effect on planning processes with stimulation over left dlPFC entailing decreases and right dlPFC causing increases in initial thinking times. Consequently, the question remains how this phenomenon can be accommodated in functional terms. The putative interplay between locally circumscribed impairments after cTBS and a consequently increased activation of the contralateral homologue via reduced transcallosal inhibition suggests that observed behavioral effects stem from two tightly coupled sources, that is, local and remote effects, whose individual contribution cannot be resolved using a single behavioral measure alone. However, assuming that increases in prefrontal activation do not harm planning performance, we further suggest that right dlPFC might be more critical for planning than its left homologue [cf. Unterrainer et al., 2004b], at least for the simple three‐move problems applied here. That is, disruption of processing in right dlPFC led to observable behavioral costs that could not be compensated by presumed increased activation (via transcallosal disinhibition) of left dlPFC, whereas interfering with processing in left dlPFC led to speeded planning, and might reflect an overcompensation above normal levels by interhemispheric release of right dlPFC. Consequently, contributions of left dlPFC might hence be more dispensable for efficient planning performance in the present task than those of right dlPFC. Indirect support for an asymmetric stimulation effect comes from a recent study on the TOL using transcranial direct current stimulation (tDCS) by Dockery et al. [ 2009]. Similar to the present results, inspections of their reported average latencies imply a decrease and increase after inhibitory (cathodal) stimulation of left and right prefrontal cortex, respectively2.
The global cTBS effect evident in the present study was functionally independent of specific planning demands as varied by Goal Configuration and Search Depth. Therefore, we suggest that the TMS asymmetry most likely may be driven by either (i) a global task component implicated in all TOL problems independent of their parameter structure or (ii) by a global individual setting on a higher‐order level of behavioral control. Candidate task components that are crucially involved in each and every TOL trial would be the need to build up a representation of the visual task layout in working memory and/or subsequent spatial operations. Global individual settings may relate to individual emphasis on speed versus accuracy, meta‐strategic control such as the monitoring for requirements to switch strategies, or a preponderance of “habitual” versus controlled behavioral selection, amongst others. Evidence for a role of left dlPFC in the choice of strategy is provided by Jahanshahi et al. [ 1998; see also Jahanshahi and Dirnberger, 1999]. When applied over left dlPFC (but not over right dlPFC and medial PFC), TMS changed the strategies that participants adopted while performing a random number generation task. According to the authors, inhibition of the most habitual response was reversed by left‐hemispheric TMS (cf. transcallosal disinhibition), thus unleashing the most frequent response [Jahanshahi et al., 1998]. In a TMS study on planning, Basso et al. [ 2006] also found that functionally lesioning prefrontal cortices by bilateral stimulation did not only impair performance, but also reduced the switching between heuristics. Together, these studies implicate lateral prefrontal cortex in the selection of strategies and meta‐strategic flexibility. As flexibility usually is accomplished at the cost of prolonged processing times, variation of meta‐strategic control may account well for global, task‐parameter independent changes in processing time. Furthermore, in previous dual‐task studies on the TOL, the use of verbal versus visuo‐spatial strategies was investigated, with an apparent link to brain lateralization. Although interference induced by simple spatial pattern tapping impaired planning accuracy, articulatory suppression decreased response times, but accuracy remained unimpaired [Phillips et al., 1999]. Phillips et al.'s explanation for this effect draws on a suggestion by Brandimonte and Gerbino [ 1993] that articulatory suppression produces a shift from the use of an inefficient verbal strategy to a more appropriate visuo‐spatial strategy. For the present results, one could suggest that cTBS induced an inhibition of an inefficient verbal strategy leading to more efficient and faster processing. Correspondingly, interference of the more appropriate visuo‐spatial strategy with TMS over right dlPFC might have prolonged processing times. Nonetheless, despite an obvious parallel to the present decrease and increase in initial thinking times after cTBS over left and right dlPFC, respectively, a precise functional attribution of the present asymmetric results to distinct cognitive processes—be it planning‐genuine or other processes—remains to be resolved in future studies.
Discrepancies With Previous Imaging Results
Extant theories on prefrontal functions differ considerably with respect to the extent of functional specialization attributed to subregions within the prefrontal cortex. The most explicit assignment of specific cognitive functions to well‐defined regions within prefrontal cortex, including functional lateralization, is outlined in the model of structured event complexes [SECs; e.g., Grafman et al., 2005; Wood and Grafman, 2003]. Based on a functional parcellation of the prefrontal cortex, the SEC model predicts that left and right dlPFC are associated with different aspects of planning in tasks like the TOL. In detail, left dlPFC is thought to be focused on structurally analyzing propositional information that make up a plan, whereas right dlPFC is assumed to deal with the temporal and dynamic aspects by mediating the integration of information into a sequence [Grafman et al., 2005; Huey et al., 2006]. This assumption was substantiated by the recently shown double dissociation between differential fMRI activations of left and right dlPFC and different task demands imposed by experimental manipulations of Goal Configuration and Search Depth, respectively [Kaller et al., 2011a], which also formed the basis of the present study.
Yet, the present task‐demand independent cTBS results raise the question which factors might have produced the discrepancy with the previous fMRI dissociations. Conceivable explanations comprise several potential mechanisms that are not mutually exclusive. First of all, local functional impairments following cTBS might have been minimized by functional compensation via the contralateral homologue region. Despite clear emphasis on task‐dependent dlPFC lateralization, the nature of the double dissociation and the underlying hemispheric specializations reported by Kaller et al. [ 2011a] were not absolute but relative. That is, experimental manipulations of Goal Configuration and Search Depth resulted in significant functional changes on either side and dissociated with respect to the relative extents of the associated activation differences. Based on these bilateral but differential contributions, local perturbations might have hence been compensated by a takeover of function by the contralateral unstimulated dlPFC. In this respect, the state‐dependency of stimulation effects might have modulated spatially colocalized but functionally distinct neuronal populations to different extents potentially leading to the present coexistence between a global asymmetric TMS effect together with the absence of any task‐parameter specific stimulation effects.
Besides potential contra‐ (and/or ipsilateral) shifts of function, the applied three‐move TOL problems might have been too easy to capture any cTBS‐induced planning deficit at all, or the cTBS stimulation might have been to weak. Thus, successful performance of cognitive operations that are basic for visuo‐spatial planning, for instance, mental spatial transformations in PPC [cf. Champod and Petrides, 2007; Sack et al., 2002, 2005, 2007], might not fully depend on the control mechanisms exerted by left and right dlPFC—at least for these simple TOL problems, or these control mechanisms might not have been suppressed sufficiently to fully prevent their functioning. However, past evidence from lesion studies strongly supports both the crucial role of the lateral prefrontal cortex for planning as such [e.g., Goel and Grafman, 1995; Owen et al., 1990; Shallice, 1982; for an overview see Sullivan et al., 2009] as well as the proposed lateralization of two different aspects of planning [Morris et al., 1997a, b; see also Morris et al., 2005]. In studies using the Tower of Hanoi, a disc‐transfer task similar to the TOL [cf. Kaller et al., 2011b], patients with left frontal lesions were shown to be impaired particularly in problems that included perceptually mediated goal‐subgoal conflicts [Morris et al., 1997a]. Goal‐subgoal conflicts are defined as moves that are necessary steps on the solution path, but locally take the problem solver away from the global goal state by increasing the visual distance of the moved ball/disc to its goal peg [Kaller et al., 2011b]. Thus, patients with left frontal lesions seemed to lack a sufficient analysis of the structural features of the task and an abstraction of the problem beyond its perceptual characteristics, so that, in consequence, applications of simple heuristics such as “difference reduction” by perceptual matching would fail [but see also Goel and Grafman, 1995, for an alternative interpretation]. This would also be in accordance with the stronger left‐hemispheric dlPFC activation in association with Goal Configuration [Kaller et al., 2011a]. In contrast, patients with right frontal lesions were reported to be selectively impaired in problems with longer solution paths [Morris et al., 1997b] that, again in line with present conceptions of Search Depth, entail a larger number of intermediate moves and, consequently, increased demands on processes of integrating individual moves into a coherent sequence while taking into account their interdependencies [cf. Kaller et al., 2011a].
Together, in line with our previous fMRI results, a functional dissociation of left and right PFC in planning is well‐established theoretically and empirically, on grounds of cognitive neuroimaging and patient studies. In contrast, cTBS stimulation displayed a global effect on planning speed that was independent of specific planning demands. Although there are multiple potential explanations for the absence of specific impairments in planning with cTBS, of which we favor contralateral functional compensation—perhaps in combination with a too small impact of cTBS—these results nevertheless provide first evidence that global and task‐demand specific effects on planning latency may be subserved by dissociable neural bases. We suggest that these bases are colocalized in dlPFC, but from behavioral analyses and TMS alone, we cannot preclude the possibility that other regions may differentially influence planning speed, especially when PFC's processing is interrupted. The divergence between the present asymmetric cTBS effect and the expectations derived from the previous fMRI double dissociation might be reconciled by differential influences of the state dependency on stimulation effects for spatially overlapping but functionally distinct neuronal populations (see above). However, as state dependency was not addressed experimentally in this study, the present data cannot provide direct evidence for this explanation and further research is warranted.
Future Perspectives: Multimethodal Approaches
Further insight into the nature of the asymmetric cTBS effect and a better understanding of the differential contributions of left and right dlPFC in higher‐order cognition might be gained from a “perturb and measure” approach that combines transcranial stimulation with neuroimaging methods [Paus, 2005; see also Ruff et al., 2009]. Although not evident at the behavioral level, contralateral compensation might be revealed by task‐demand and stimulation‐side specific alterations of activation. On the other hand, the here applied cTBS protocol might not have been effective enough to induce reliable changes of the double dissociation pattern found in fMRI. In this case, task‐dependent dlPFC activation patterns under real stimulation should not be different from the sham condition. Thus, neuroimaging methods allowing to track the temporo‐spatial patterns of TMS‐induced reorganization might help to disclose potential compensatory mechanisms that may have prevented the detection of overtly observable task‐demand specific behavioral effects after cTBS [for a methodical overview, see also Siebner et al., 2009].
Concurrent recordings of eye movements might also prove fruitful for further enlightening the cognitive implications of the present results. Based on a manipulation of Goal Configuration and Search Depth similar to the present study, Kaller et al. [ 2009; see also Nitschke et al., in preparation] demonstrated using eye‐movement analyses that these two different cognitive aspects of planning are related to temporally dissociable phases during the initial thinking time. Given the present asymmetric cTBS effect, eye‐movement recordings could potentially elucidate the cognitive basis of stimulation‐induced increases and decreases in planning duration, that is, whether these costs and benefits are due to a temporally global acceleration/deceleration or may be temporally located to a specific time window or cognitive phase of the initial thinking time.
CONCLUSIONS
Taken together, interference in neural processing in left and right dlPFC induced by cTBS differentially impacted cognitive processing as shown here by an asymmetric stimulation effect on the behavioral outcomes in a planning task. However, the precise nature of the physiological mechanisms behind remains elusive since effects of cTBS are found to be modulated by a multitude of variables. Albeit speculative, a candidate mode of action might be that a local impairment of function by TMS stimulation displays remote effects in the unstimulated contralateral dlPFC via diminished transcallosal inhibition. Since behavioral effects of specific planning demands that previously revealed a double dissociation between left and right dlPFC in fMRI was unaffected by the present stimulation, both the present asymmetric cTBS effect and the previous double dissociation have to be conceived as two qualitatively different phenomena of functional lateralization between left and right prefrontal cortex in cognitive processing. Future studies should explicitly strive to disentangle the associated physiological and cognitive mechanisms.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
The authors thank Sonja Kappler and Hansjörg Mast for assistance in data acquisition. They further acknowledge Lena Köstering, Sandra Loosli, Kai Nitschke, and Nina Ruh for invaluable discussion of a previous version of this manuscript.
Footnotes
In the present manuscript, we use the term Goal Configuration instead of Goal Hierarchy previously used by Kaller et al. [ 2011a]. This is done in order to emphasize that, at least for simple three‐move problems with an intermediate move, it is not the ambiguity of the sequence per se that leads to latency effects on initial thinking times [cf. Kaller et al., 2004], but rather its identifiability and the related perceptual difficulty of matching and integrating information between start and goal states [Nitschke et al., in preparation]. Consequently, performance and/or latency effects following experimental manipulations of Goal Hierarchy in easier and more difficult TOL problems may not be attributable to the same underlying cognitive operations [Kaller et al., 2011b].
Note that this effect was not statistically tested for by the authors but can only be implied based on descriptive comparisons between mean planning durations for left, right, and sham stimulation of prefrontal cortex [see Fig. 3 in Dockery et al., 2009, p. 7274, reaction time data for first session]. Further, for cathodal stimulation of left prefrontal cortex, cathode and anode were placed over left dlPFC and right orbita, respectively, whereas for cathodal stimulation of right prefrontal cortex, cathode and anode were placed of right orbita and left dlPFC. Though stimulation with tDCS is less focused than with TMS, prefrontal sites for left and right cathodal stimulation nonetheless differed and present implications have to be treated with caution.
REFERENCES
- Basso D, Lotze M, Vitale L, Ferreri F, Bisiacchi P, Olivetti Belardinelli M, Rossini PM, Birbaumer N ( 2006): The role of prefrontal cortex in visuo‐spatial planning: A repetitive TMS study. Exp Brain Res 171: 411–415. [DOI] [PubMed] [Google Scholar]
- Berg WK, Byrd DL ( 2002): The Tower of London spatial problem‐solving task: Enhancing clinical and research implementation. J Clin Exp Neuropsychol 24: 586–604. [DOI] [PubMed] [Google Scholar]
- Brandimonte MA, Gerbino W ( 1993): When imagery fails: Effects of verbal recoding on accessibility of visual memory In: Cornoldi C, Brandimonte MA, Kaufman G, Riesberg D, editors. Stretching the Imagination. Oxford: University Press; pp 31–76. [Google Scholar]
- Champod AS, Petrides M ( 2007): Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proc Natl Acad Sci USA 104: 14837–14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery CA, Hueckel‐Weng R, Birbaumer N, Plewnia C ( 2009): Enhancement of planning ability by transcranial direct current stimulation. J Neurosci 29: 7271–7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Pascual‐Leone A, Théoret H ( 2006): Paradoxical facilitation of attention in healthy humans. Behav Neurol 17: 159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J ( 2008): The Prefrontal Cortex. London: Academic Press. [Google Scholar]
- Gamboa OL, Antal A, Moliadze V, Paulus W ( 2010): Simply longer is not better: Reversal of theta burst after‐effect with prolonged stimulation. Exp Brain Res 204: 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J ( 2008): Depression of human corticospinal excitability induced by magnetic theta‐burst stimulation: Evidence of rapid polarity‐reversing metaplasticity. Cereb Cortex 18: 2046–2053. [DOI] [PubMed] [Google Scholar]
- Goel V ( 2002): Planning: Neural and psychological In: Nadel L, editor. Encyclopedia of Cognitive Science. London: Nature Publishing Group; pp 697–703. [Google Scholar]
- Goel V, Grafman J ( 1995): Are the frontal lobes implicated in “planning” functions? Interpreting data from the Tower of Hanoi. Neuropsychologia 33: 623–642. [DOI] [PubMed] [Google Scholar]
- Grafman J, Spector L, Rattermann MJ ( 2005): Planning and the brain In: Morris R, Ward G, editors. The Cognitive Psychology of Planning. Hove: Psychology Press; pp 181–198. [Google Scholar]
- Hamel R, Schmittmann VD ( 2006): The 20‐minute version as a predictor of the Raven Advanced Progressive Matrices Test. Educ Psychol Meas 66: 1039–1046. [Google Scholar]
- Hoogendam JM, Ramakers GMJ, Di Lazzaro V ( 2010): Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 3: 95–118. [DOI] [PubMed] [Google Scholar]
- Huang Y, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC ( 2005): Theta burst stimulation of the human motor cortex. Neuron 45: 201–206. [DOI] [PubMed] [Google Scholar]
- Huang Y, Rothwell JC, Edwards MJ, Chen R ( 2008): Effect of physiological activity on an NMDA‐dependent form of cortical plasticity in human. Cereb Cortex 18: 563–570. [DOI] [PubMed] [Google Scholar]
- Huey ED, Krueger F, Grafman J ( 2006): Representations in the human prefrontal cortex. Curr Dir Psychol Sci 15: 167–171. [Google Scholar]
- Jahanshahi M, Dirnberger G ( 1999): The left dorsolateral prefrontal cortex and random generation of responses: Studies with transcranial magnetic stimulation. Neuropsychologia 37: 181–190. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Profice P, Brown RG, Ridding MC, Dirnberger G, Rothwell JC ( 1998): The effects of transcranial magnetic stimulation over the dorsolateral prefrontal cortex on suppression of habitual counting during random number generation. Brain 121: 1533–1544. [DOI] [PubMed] [Google Scholar]
- Kähkönen S, Wilenius J, Komssi S, Ilmoniemi RJ ( 2004): Distinct differences in cortical reactivity of motor and prefrontal cortices to magnetic stimulation. Clin Neurophysiol 115: 583–588. [DOI] [PubMed] [Google Scholar]
- Kaller CP, Unterrainer JM, Rahm B, Halsband U ( 2004): The impact of problem structure on planning: Insights from the Tower of London task. Brain Res Cogn Brain Res 20: 462–472. [DOI] [PubMed] [Google Scholar]
- Kaller CP, Rahm B, Bolkenius K, Unterrainer JM ( 2009): Eye movements and visuospatial problem solving: Identifying separable phases of complex cognition. Psychophysiology 46: 818–830. [DOI] [PubMed] [Google Scholar]
- Kaller CP, Rahm B, Spreer J, Weiller C, Unterrainer JM ( 2011a) Dissociable contributions of left and right dorsolateral prefrontal cortex in planning. Cereb Cortex 21: 307–317. [DOI] [PubMed] [Google Scholar]
- Kaller CP, Rahm B, Köstering L, Unterrainer JM ( 2011b) Reviewing the impact of problem structure on planning: A software tool for analyzing tower tasks. Behav Brain Res 216: 1–8. [DOI] [PubMed] [Google Scholar]
- Kapur N ( 1996): Paradoxical functional facilitation in brain‐behaviour research. A critical review. Brain 119: 1775–1790. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Théoret H, Schlaug G, Pascual‐Leone A ( 2004): Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology 62: 91–98. [DOI] [PubMed] [Google Scholar]
- Krug R, Born J, Rasch B ( 2006): A 3‐day estrogen treatment improves prefrontal cortex‐dependent cognitive function in postmenopausal women. Psychoneuroendocrinology 31: 965–975. [DOI] [PubMed] [Google Scholar]
- Lehrl S ( 1999): Mehrfachwahl‐Wortschatz‐Intelligenztest MWT‐B. Balingen: Spitta Verlag. [Google Scholar]
- Lehrl S, Merz J, Burkhard G, Fischer S ( 1991): Mehrfachwahl‐Wortschatz‐Intelligenztest (MWT‐A). Erlangen: Perimed. [Google Scholar]
- Morris RG, Miotto EC, Feigenbaum JD, Bullock P, Polkey CE ( 1997a) The effect of goal‐subgoal conflict on planning ability after frontal‐ and temporal‐lobe lesions in humans. Neuropsychologia 35: 1147–1157. [DOI] [PubMed] [Google Scholar]
- Morris RG, Miotto EC, Feigenbaum JD, Bullock P, Polkey CE ( 1997b) Planning ability after frontal and temporal lobe lesions in humans: The effects of selection equivocation and working memory load. Cogn Neuropsychol 14: 1007–1027. [DOI] [PubMed] [Google Scholar]
- Morris RG, Kotitsa M, Bramham J ( 2005): Planning in patients with focal brain damage: From simple to complex task performance In: Morris R, Ward G, editors. The Cognitive Psychology of Planning. Hove: Psychology Press; pp 153–179. [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Owen AM ( 2005): Cognitive planning in humans: New insights from the Tower of London task In: Morris R, Ward G, editors. The Cognitive Psychology of Planning. Hove: Psychology Press; pp 135–151. [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW ( 1990): Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 28: 1021–1034. [DOI] [PubMed] [Google Scholar]
- Paus T ( 2005): Inferring causality in brain images: A perturbation approach. Philos Trans R Soc Lond B Biol Sci 360: 1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Castro‐Alamancos MA, Petrides M ( 2001): Cortico‐cortical connectivity of the human mid‐dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci 14: 1405–1411. [DOI] [PubMed] [Google Scholar]
- Penfield W, Evans J ( 1935): The frontal lobe in man: A clinical study of maximum removals. Brain 58: 115–133. [Google Scholar]
- Phillips LH, Wynn V, Gilhooly KJ, Della Sala S, Logie RH ( 1999): The role of memory in the Tower of London task. Memory 7: 209–231. [DOI] [PubMed] [Google Scholar]
- Raven J, Raven JC, Court JH ( 1998): Manual for Raven's Advanced Progressive Matrices. Oxford: Oxford Psychologists Press Ltd. [Google Scholar]
- Ruff CC, Driver J, Bestmann S ( 2009): Combining TMS and fMRI: From ‘virtual lesions’ to functional‐network accounts of cognition. Cortex 45: 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack AT ( 2006): Transcranial magnetic stimulation, causal structure‐function mapping and networks of functional relevance. Curr Opin Neurobiol 16: 593–599. [DOI] [PubMed] [Google Scholar]
- Sack AT, Sperling JM, Prvulovic D, Formisano E, Goebel R, Di Salle F, Dierks T, Linden DEJ ( 2002): Tracking the mind's image in the brain. II. Transcranial magnetic stimulation reveals parietal asymmetry in visuospatial imagery. Neuron 35: 195–204. [DOI] [PubMed] [Google Scholar]
- Sack AT, Camprodon JA, Pascual‐Leone A, Goebel R ( 2005): The dynamics of interhemispheric compensatory processes in mental imagery. Science 308: 702–704. [DOI] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Bestmann S, Linden DEJ, Dechent P, Goebel R, Baudewig J ( 2007): Imaging the brain activity changes underlying impaired visuospatial judgments: Simultaneous FMRI, TMS, and behavioral studies Cereb Cortex 17: 2841–2852. [DOI] [PubMed] [Google Scholar]
- Salmaso D, Longoni AM ( 1985): Problems in the assessment of hand preference. Cortex 21: 533–549. [DOI] [PubMed] [Google Scholar]
- Shallice T ( 1982): Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci 298: 199–209. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Bergmann TO, Bestmann S, Massimini M, Johansen‐Berg H, Mochizuki H, Bohning DE, Boorman ED, Groppa S, Miniussi C, Pascual‐Leone A, Huber R, Taylor PCJ, Ilmoniemi RJ, Gennaro L de, Strafella AP, Kähkönen S, Klöppel S, Frisoni GB, George MS, Hallett M, Brandt SA, Rushworth MF, Ziemann U, Rothwell JC, Ward N, Cohen LG, Baudewig J, Paus T, Ugawa Y, Rossini PM ( 2009): Consensus paper: Combining transcranial stimulation with neuroimaging. Brain Stimul 2: 58–80. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM ( 2002): Effects of ovarian hormones on human cortical excitability. Ann Neurol 51: 599–603. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E ( 1998): A Compendium of Neuropsychological Tests. New York: Oxford University Press. [Google Scholar]
- Stefan K, Gentner R, Zeller D, Dang S, Classen J ( 2008): Theta‐burst stimulation: Remote physiological and local behavioral after‐effects. Neuroimage 40: 265–274. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Coxon JP, Verryt TS, Acharya PP, Byblow WD ( 2009): Repetitive stimulation of premotor cortex affects primary motor cortex excitability and movement preparation. Brain Stimul 2: 152–162. [DOI] [PubMed] [Google Scholar]
- Sullivan JR, Riccio CA, Castillo CL ( 2009): Concurrent validity of the tower tasks as measures of executive function in adults: A meta‐analysis. Appl Neuropsychol 16: 62–75. [DOI] [PubMed] [Google Scholar]
- Suppa A, Ortu E, Zafar N, Deriu F, Paulus W, Berardelli A, Rothwell JC ( 2008): Theta burst stimulation induces after‐effects on contralateral primary motor cortex excitability in humans. J Physiol (Lond) 586: 4489–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapley SM, Bryden MP ( 1985): A group test for the assessment of performance between the hands. Neuropsychologia 23: 215–221. [DOI] [PubMed] [Google Scholar]
- Thiel A, Schumacher B, Wienhard K, Gairing S, Kracht LW, Wagner R, Haupt WF, Heiss W ( 2006): Direct demonstration of transcallosal disinhibition in language networks. J Cereb Blood Flow Metab 26: 1122–1127. [DOI] [PubMed] [Google Scholar]
- Unterrainer JM, Rahm B, Kaller C, Quiske K, Hoppe‐Seyler K, Meier C, Müller C, Leonhart R, Halsband U ( 2004a): Planning abilities and the Tower of London: Is this task measuring a discrete cognitive function? J Clin Exp Neuropsychol 26: 846–856. [DOI] [PubMed] [Google Scholar]
- Unterrainer JM, Rahm B, Kaller CP, Ruff CC, Spreer J, Krause BJ, Schwarzwald R, Hautzel H, Halsband U ( 2004b) When planning fails: Individual differences and error‐related brain activity in problem solving. Cereb Cortex 14: 1390–1397. [DOI] [PubMed] [Google Scholar]
- Unterrainer JM, Rahm B, Halsband U, Kaller CP ( 2005): What is in a name: Comparing the Tower of London with one of its variants. Brain Res Cogn Brain Res 23: 418–428. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual‐Leone A ( 2003): Transcranial Magnetic Stimulation: A Neurochronometrics of Mind. Cambridge, Massachusetts: MIT Press. [Google Scholar]
- Ward G, Allport A ( 1997): Planning and problem solving using the five disc Tower of London task. Q J Exp Psychol A 50: 49–78. [Google Scholar]
- Ward G, Morris R ( 2005): Introduction to the psychology of planning In: Morris R, Ward G, editors. The Cognitive Psychology of Planning. Hove: Psychology Press; pp 1–34. [Google Scholar]
- Wood JN, Grafman J ( 2003): Human prefrontal cortex: Processing and representational perspectives. Nat Rev Neurosci 4: 139–147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


