Abstract
In a temporal difference learning approach of classical conditioning, a theoretical error signal shifts from outcome deliverance to the onset of the conditioned stimulus. Omission of an expected outcome results in a negative prediction error signal, which is the initial step towards successful extinction and may therefore be relevant for fear extinction recall. As studies in rodents have observed a bidirectional relationship between fear extinction and rapid eye movement (REM) sleep, we aimed to test the hypothesis that REM sleep deprivation impairs recall of fear extinction through prediction error signaling in humans. In a three‐day design with polysomnographically controlled REM sleep deprivation, 18 young, healthy subjects performed a fear conditioning, extinction and recall of extinction task with visual stimuli, and mild electrical shocks during combined functional magnetic resonance imaging (fMRI) and skin conductance response (SCR) measurements. Compared to the control group, the REM sleep deprivation group had increased SCR scores to a previously extinguished stimulus at early recall of extinction trials, which was associated with an altered fMRI time‐course in the left middle temporal gyrus. Post‐hoc contrasts corrected for measures of NREM sleep variability also revealed between‐group differences primarily in the temporal lobe. Our results demonstrate altered prediction error signaling during recall of fear extinction after REM sleep deprivation, which may further our understanding of anxiety disorders in which disturbed sleep and impaired fear extinction learning coincide. Moreover, our findings are indicative of REM sleep related plasticity in regions that also show an increase in activity during REM sleep. Hum Brain Mapp 33:2362–2376, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: REM sleep, prediction error, fear extinction, fMRI, polysomnography, skin conductance
INTRODUCTION
In theoretical approaches of classical conditioning, learning is driven by prediction error signals that reflect the discrepancy between a prediction and its actual outcome [Rescorla and Wagner, 1972]. Temporal difference learning models propose that theoretical error signals shift from the onset of outcome deliverance to the onset of conditioned stimulus (CS) presentation during learning [Sutton and Barto, 1990], and neurophysiological studies have demonstrated that dopaminergic midbrain neurons signal in accordance with temporal difference models [Schultz, 1998; Schultz et al., 1997]. Prediction error signals have also been examined with functional magnetic resonance imaging (fMRI); prediction error signaling in appetitive conditioning is associated with increased activation in the ventral and dorsal striatum [Haruno and Kawato, 2006; Jensen et al., 2007; Knutson et al., 2001; O'Doherty et al., 2003]. Studies employing fear conditioning paradigms [Büchel and Dolan, 2000] to study prediction error signaling have also revealed increased activation in dopaminergic midbrain regions [Menon et al., 2007; Seymour et al., 2004] and the striatum and insula [Jensen et al., 2007; Schiller et al., 2008; Seymour et al., 2004]. The reported correlations reflect positive prediction updates that occur when a CS elicits a positive prediction about an outcome, or when an outcome is larger than anticipated. A negative prediction error occurs when an outcome is less than predicted, which was associated with increased activity in the ventromedial and dorsolateral prefrontal cortices, the left lateral orbitofrontal cortex and caudate, the middle temporal and angular gyri, and visual cortices [Spoormaker et al., 2011].
The relevance of negative prediction error signaling is that it is proposed to constitute the initial step to successful fear extinction [Spoormaker et al., 2011], which is a promising model for human anxiety disorders [Pape and Pare, 2010; Rauch et al., 2006]. Fear extinction and fear extinction consolidation are impaired in posttraumatic stress disorder (PTSD) patients [Milad et al., 2009; Wessa and Flor, 2007], which is mediated by altered activity in brain regions critical to extinction [Milad et al., 2009] such as the hippocampus and ventromedial prefrontal cortex [Kalisch et al., 2006; Milad et al., 2007, a, b; Phelps et al., 2004; Rauch et al., 2006]. Unclear is yet why fear extinction is impaired in PTSD patients, but a crucial role for disturbed sleep has been proposed [Germain et al., 2008; Levin and Nielsen, 2007]. This is in line with PTSD patients showing altered sleep parameters, such as increased rapid eye movement (REM) density [Kobayashi et al., 2007] and fragmented REM episodes [Mellman and Hipolito, 2006; Mellman et al., 2002; Spoormaker and Montgomery, 2008]. Studies in rodents have demonstrated that selective REM sleep deprivation impairs cued fear extinction [Silvestri, 2005] and consolidation of cued fear extinction [Fu et al., 2007]. Conversely, contextual extinction ameliorates sleep disturbances in rats [Wellman et al., 2008], and low frequency stimulation of the hippocampus after fear extinction impaired both REM sleep and recall of extinction [Deschaux et al., 2010]. A recent study in humans comparing a sleep group with a day group found that sleep generalized fear extinction [Pace‐Schott et al., 2009]. In a previous study employing afternoon sleep periods, we observed that subjects that slept well after the conditioning and extinction tasks (i.e., less interrupted sleep and more REM sleep) also had a reduced skin conductance response (SCR) to the extinguished stimulus, which was mediated by the ventromedial prefrontal cortex [Spoormaker et al., 2010].
The medial prefrontal cortex shows increased activity in REM sleep compared to nonREM (NREM) sleep [Braun et al., 1997; Maquet et al., 1996] that is temporally related to REMs in sleep [Hong et al., 1995, 2009; Peigneux et al., 2001], which is suggestive of REM sleep related plasticity. In addition, particularly the dorsal striatum shows a strong increase in activity in REM sleep [Braun et al., 1997], which is also temporally related with REMs [Hong et al., 2009; Miyauchi et al., 2009; Wehrle et al., 2005]. The question is whether these subcortical regions are involved in plasticity processes during REM sleep or simply reflect oculomotor control of REMs as in waking [Miyauchi et al., 2009]. Evidence of REM sleep related plasticity is the reported increased functional connectivity during REM sleep between the cuneus and the caudate after a visuomotor task with probabilistic sequences compared to random sequences [Peigneux et al., 2003]. Evidence suggestive of REM sleep related plasticity is our observation of increased functional connectivity between the amygdala and the caudate after a nap period with REM sleep [Spoormaker et al., 2010]. Observing altered activity in these regions after selective REM sleep deprivation would be an argument for REM sleep related plasticity.
As subcortical, paralimbic, and temporal lobe regions of interest are involved in both REM sleep and prediction error signaling during fear conditioning/extinction, we conducted a discriminatory fear conditioning, extinction, and recall of extinction procedure [Spoormaker et al., 2010], with simultaneous SCR and fMRI (SCR/fMRI) measurements. Fear conditioning occurred on day one, fear extinction on day two, and recall of fear extinction on day three. The first night (from day 1 to day 2) was a habituation night in the sleep laboratory that was used to exclude subjects with sleep disorders. At the start of the second night, subjects were randomized into a selective REM sleep deprivation group or a control group that received the same number of manual awakenings from NREM sleep stages in the sleep laboratory, to control for awakening arousal and stress [Horne and McGrath, 1984].
METHODS
Subjects
The study protocol was in line with the Declaration of Helsinki and was approved by the local ethical review committee. Subjects provided their written informed consent after the study protocol had been fully explained, and were reimbursed for participation. Eighteen nonsmoking, right‐handed male participants [mean age = 23.4 (± 2.8) years, range 18–30 years] underwent a general medical and structured psychiatric interview and clinical MRI to exclude present and past neurological, psychiatric, and sleep disorders. Participants were instructed to follow a regular sleep‐wake‐schedule with bedtimes between 23:00 and 08:00 hrs during the week prior to the experiment, documented by sleep protocols and wrist actigraphy. Furthermore, participants were asked to refrain from caffeine for at least 3 days prior and during the experiment. Standard exclusion criteria for MRI were used throughout the study. Because we observed significant between‐group differences in extinction in a 90‐min afternoon nap study in which subjects did or did not have REM sleep (N = 16) in our previous study [Spoormaker et al., 2010], we expected that examining a similar amount of subjects with a more robust overnight REM sleep deprivation approach would increase the statistical power. Our study sample had sufficient power (γ = 0.70) to detect large between‐group differences (Cohen's d = 1) with an alpha of 0.05 on the recall of extinction task, as determined with G*Power 3.0 [Faul et al., 2007].
Study Overview
Subjects had to maintain a regular sleep schedule in the 7 days prior to the experiment, which was verified by wrist actigraphy and sleep protocols. Day 1: Subjects performed the conditioning task at 6 p.m. to allow for sufficient consolidation of conditioning before extinction occurred 24 hrs later. (Fear extinction is typically performed immediately after conditioning but it is yet unclear whether conditioning is then fully consolidated, which is why we took a cautious approach.) The conditioning session was preceded by a brief habituation session in which all stimuli were presented three times. Night 1: Clinical and habituation night with full clinical polysomnography in the sleep laboratory to exclude subjects with a sleep disorders (no subject had a sleep disorder). Day 2: Subjects performed the extinction session at 6 p.m. After this, subjects were randomized into the experimental or control group based on a previously generated table with participant numbers and a randomized variable. Night 2: The experimental group was subjected to REM sleep deprivation (REMD, manually performed) and the control group received a matched amount of awakenings, however from NREM sleep stages. We used a manual REM sleep deprivation method, as pharmacological suppression of REM sleep would require agents that may affect subsequent fear extinction processes. Day 3: In the early morning (around 6–7 a.m.), subjects performed a brief reward learning task with monetary gains and a loss aversion task. In the late afternoon, subjects returned to the institute for the recall of extinction task (again at 6 p.m.). A brief interview about the subject's daily activities was conducted to ensure that subjects did not sleep during the day. After this, subjects performed a novel conditioning task; here we focus on the main tasks of conditioning, extinction, and recall of extinction.
Paradigms
The conditioning, extinction and recall of extinction tasks consisted of three basic geometrical figures that were presented 15 times each. Two were followed by shocks during conditioning, and one was followed by shocks during extinction (50% reinforcement schedule; safety stimulus: CS; extinguished stimulus: CSE; unextinguished stimulus: CSU). This is a minor adaptation from our previously used task [Spoormaker et al., 2010], which we employed to ensure that subjects would show stimulus‐specific extinction instead of context‐specific extinction. (If shocks are only administered in the first session, and no shocks are administered in the second session, then the initial nonshock trials in the third session could lead subjects to assume that this session is just like the second session a “nonshock” session, making between stimulus contrasts less informative.) No shocks were administered during recall of extinction. A partial reinforcement schedule was chosen because after continuous reinforcement, extinction may be successful in a few trials [Phelps et al., 2004].
EEG Acquisition in the Sleep Laboratory
For both nights, polysomnographic data were recorded and stored with a digital recorder (Comlab 32 Digital Sleep Lab, Brainlab V 3.3 Software, Schwarzer GmbH, Munich, Germany). Electrodes F3 and F4, C3 and C4, and O3 and O4 (referenced against the contralateral mastoid, filtered from 0.5 to 70 Hz), electrooculogram and mental/submental electromyogram (EMG) were measured at a sampling rate of 250 Hz. Sleep data analyses were performed by independent professional scorers blind to the study design using the criteria as described by Rechtschaffen and Kales (1968). The recordings in the clinical habituation night included additional measurements: nasal and oral thermistor channels, chest and abdominal respiratory movements, arterial oxygen saturation (finger oximetry), EMG of the legs, and an electrocardiogram. Clinical nights were evaluated by medical professionals to exclude subjects with sleep disorders.
REM sleep deprivation was performed manually and after each awakening (also in the control group), subjects were kept awake for three 30‐s epochs. Subjects in the experimental group were awoken at the first eye movement (deflection larger than 25% of the maximum deflection during calibration before sleep) if the EMG was flat. Subjects in the control group were awoken when they were not in REM sleep. This occurred at random times but with a similar distribution as the experimental group (e.g., more awakenings at the end of the sleep period).
Electrical Stimulation
Mild electrical shocks during the conditioning, extinction, and reversal learning paradigms were administered to the back of the right hand and acted as the unconditioned stimulus (US). Electrical shocks were pulses of 2 ms duration with intensities between 8 and 25 mA, generated by a Digitimer Stimulator (Model DS7, Digitimer, Hertfordshire, United Kingdom). Gold electrodes were custom made for electrical stimulation in the MR environment. Stimulation intensity was individually set before the scanning session. Subjects received the instruction: “Shocks should be uncomfortable but not painful.”
Physiological Data Recording and Statistical Analysis
SCR measurements were acquired at a sampling rate of 500 Hz from electrodes on the index and middle finger of the left hand using a BrainAmp ExG amplifier (Brain Products, Munich, Germany). Skin conductance data were baseline corrected and visually inspected for artifacts to discard bad intervals. The SCR was defined as the peak‐to‐peak amplitude difference in skin conductance of the largest positive deflection [Schiller et al., 2008] in a 0.5–4.5 s latency window [Milad et al., 2009] after stimulus‐onset, with a minimal response criterion of 0.02 μS [Phelps et al., 2004]. We further analyzed a second time‐window of interest that lasted from 4.5 to 7.5 s, because of the robust SCR around the timing of the omitted shock (around stimulus‐offset). This stimulus‐offset SCR and its neural correlates have been described in more detail [Spoormaker et al., 2010]. Previous experimental studies have conceptualized the stimulus‐onset SCR as an anticipatory response [Prokasy and Ebel, 1967], partly reflecting an orienting response, whereas the stimulus‐offset SCR was proposed to reflect a conditioned response to the US [Grings et al., 1962], in line with Pavlov's observation that the conditioned response is strongest at the time of expected outcome deliverance [Pavlov, 1927]. The occurrence of both responses is in line with a temporal difference learning approach, where the two responses would relate to a positive prediction error at stimulus‐onset and a negative prediction error after omission of the expected shock [Sutton and Barto, 1990]. The onset SCR is commonly analyzed in studies employing SCR, yet the offset SCR also appears to be increased after conditioning [Grings et al., 1962], something that we also observed in young healthy subjects [Spoormaker et al., 2011]. We therefore included both onset and offset SCR data in our analyses.
Raw skin conductance scores were square root transformed and scaled to each subject's maximal (square root transformed) US response to account for interindividual SCR variability [Delgado et al., 2008]. In the novel‐conditioning task, differential SCR scores were computed by subtracting the scaled SCR to the CS− from the scaled SCR to the CS+. In the extinction and recall of extinction task, the primary outcome variables comprised the change in mean SCR over all trials (trials 1–15) of a particular stimulus from extinction (day 2) to recall of extinction (day 3). We performed a MANOVA on the δ‐values from extinction (day 2) to recall of extinction (day 3) for all stimuli and time‐windows: this statistical test can be interpreted as a group × time interaction. As the mean SCR over 15 trials is a conservative outcome variable, we additionally computed five blocks of three consecutive trials (e.g., trials 1–3, 4–6, etc.) to test between‐group differences using one‐tailed independent samples t‐tests. All analyses were repeated with three covariates: sleep restriction (the difference between average total sleep time in the week preceding the experiment as measured by sleep diaries and total sleep time on the experimental night as measured by polysomnography), the amount of sleep stage 2 and the amount of deep sleep stage 4 in the experimental night (all in min). The latter two sleep stages were selected because the t‐value of the between‐group difference exceeded 1.0 (see Table I).
Table I.
Mean values (± SD) of sleep variables per group (in min)
| Control Group (n = 9) | REMD Group (n = 9) | T a | |
|---|---|---|---|
| Total sleep timeb | 347.3 (± 27.36) | 304.28 (± 34.01) | 2.96** |
| REM sleep amount | 52.3 (± 8.1) | 15.1 (± 9.2) | 9.14*** |
| NREM sleep amount | 292.9 (± 25.4) | 286.3 (± 34.6) | 0.46 |
| Stage 1 amount | 30.9 (± 19.6) | 37.8 (± 13.8) | 0.85 |
| Stage 2 amountc | 175.4 (± 26.5) | 151.4 (± 33.2) | 1.69 |
| Stage 3 amount | 39.9 (± 12.0) | 36.2 (± 13.4) | 0.61 |
| Stage 4 amountc | 46.7 (± 31.0) | 60.9 (± 24.9) | 1.08 |
| Latency wake to S1 | 10.8 (± 9.8) | 7.4 (± 5.3) | 0.90 |
| Latency S1 to S2 | 4.6 (± 2.7) | 11.0 (± 24.8) | 0.77 |
| Latency S2 to S3 | 10.1 (± 3.9) | 9.1 (± 3.8) | 0.56 |
| Latency S2 to S4 | 18.0 (± 8.7) | 13.9 (± 4.4) | 1.25 |
P < 0.01;
P < 0.001.
Undirected independent samples t‐tests (df = 16), with equal variances assumed, except for REM sleep amount (df = 8.5).
Total sleep time was subtracted from the average total sleep time in the week preceding the experiment (according to sleep diaries), to obtain a sleep restriction measure that was included as a covariate in the statistical SCR and fMRI analyses.
S2 and S4 amount were also included as a covariate in the statistical analyses.
Questionnaires
All participants filled out questionnaires regarding acute sleepiness [Akerstedt and Gillberg, 1990], attention, and vigilance (visual‐analogue scales) before fMRI measurements at day 2 and 3. Other questionnaires were filled before the start of the experiment and comprised the (State and) Trait Anxiety Inventory [Spielberger et al., 1983], the Beck Depression Inventory [Beck et al., 1996], and the big five inventory for personality factors [Lang et al., 2001].
fMRI Acquisition and Analysis
Whole brain fMRI was carried out at 1.5 Tesla (Signa Excite, GE, Milwaukee, WI) using an 8‐channel head coil and covering 25 slices [AC‐PC‐orientation, 64 × 64 matrix, 3 mm thickness, 1 mm gap; echo planar imaging (EPI), TR 2 s, TE 40 ms]. Postprocessing and statistical analyses were performed with SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Images were slice time corrected and realigned to the first volume using rigid body transformation. Functional data were normalized to the EPI template in Montreal Neurological Institute (MNI) space, resliced (voxel resolution 2 × 2 × 2 mm3), and spatially smoothed using a 6 × 6 × 6 mm full width at half maximum Gaussian kernel. After high‐pass filtering (128 s) fixed and random effects analyses were computed in SPM5.
All first level fMRI‐models were run with eight nuisance regressors: six affine motion correction regressors, one regressor reflecting global signal variations as derived from the cerebro‐spinal‐fluid mask and another derived from the white matter mask, both obtained during segmentation in native space. One first level analysis was performed with these eight nuisance regressors only, to generate residual images that were used for extraction of event‐related time‐courses. These time‐courses were demeaned and standardized to the temporal standard deviation.
The main first level analyses consisted of classical fMRI analyses and prediction error fMRI analyses, which were all run with the above described nuisance regressors. In the classical fMRI analysis, onsets and durations of stimuli were entered as the regressor of interest, together with first order time modulations. In the prediction error fMRI analysis, we used the analysis method from a previous study [Spoormaker et al., 2011] in which we demonstrated that the most optimal temporal difference model to examine brain activity in relation to positive and negative prediction errors (at stimulus‐onset and ‐offset, respectively) simply employed constant values for every stimulus‐onset (0–2 s) and ‐offset (3–5 s) period. The logic of using a prediction error signal window of 2 s was that the shock was timed at 3.1 s and offset of the visual stimulus was at 4 s. Omission of shock is more likely to be noticed after stimulus‐offset (second 4–5) than in the brief temporal window just before stimulus cessation (second 3.1–4), and a window duration of 2 s covers both. A constant value of 0.5 (at onset) and −0.5 (at offset) was used for all prediction errors. Therefore, this model does not constitute a temporal difference learning algorithm in the strict sense as no learning (increments or decrements in prediction error values) is assumed to take place due to shock occurrence or omission, yet it seems to elicit prediction error signal related brain activity most optimally [Spoormaker et al., 2011].
Second level random effects analyses were performed for statistical inference on the group level. For the classical analysis, we computed differential contrasts from extinction to recall of extinction for the three stimuli separately and entered these into a full factorial ANOVA. The group × stimulus interaction on these differential contrasts can be interpreted as a group × stimulus × time interaction, and we will use the latter term in the following. The full factorial ANOVA of the prediction error fMRI analysis was performed at recall of extinction only, because only the CS‐ was expected to show comparable prediction error time‐courses in both the extinction and recall of extinction tasks. Post‐hoc between‐group differences were calculated with independent samples t‐tests and were repeated with three covariates: sleep restriction, the amount of sleep stage 2 and the amount of deep sleep stage 4 in the experimental night (see section Physiological data recording and statistical analysis). Statistical maps of interest were sampled at a threshold of P < 0.001; a cluster based (whole brain) multiple test correction procedure was employed, with significance defined as cluster P‐values < 0.05 after correction for family wise error under consideration of nonstationary smoothness [Hayasaka et al., 2004]. All statistical parametric maps are in accordance with the neurological convention (left/right = left/right).
RESULTS
REM Sleep Intervention
There were no significant differences in the amount of times that subjects were awoken: 12.8 (± 2.1) times for the REM sleep deprivation (REMD) group and 11.4 (± 1.8) times for the control group [t (16) = 1.44, P = 0.17]. The experimental manipulation resulted in 14.2% (± 1.8%) REM sleep of the total sleep period in the control group and 4.2% (± 2.6%) in the REMD group, t (8.5) = 9.34, P < 0.001. Note that the Rechtschaffen and Kales criteria have a bias of epochs being coded as REM sleep, as epochs before a REM have to be classified retrospectively as REM sleep until the last preceding sleep spindle or K‐complex. These epochs primarily consist of “tonic REM sleep,” that is, epochs without eye movements. Regarding “phasic REM sleep,” which has been shown critical for plasticity processes [Datta et al., 2004] and which we defined as more than two eye movements in one epoch, subjects in the REMD group had on average 1.9 (± 1.8) epochs versus 32.6 (± 24.6) epochs in the control group [t (8.1) = 3.7; P < 0.01] reflecting 0.3% versus 5.0% of the total sleep period respectively (see Supporting Information Table S1 for an overview in the difference of amount of REM epochs according to various criteria). There were no significant differences for time spent in other sleep stages or in sleep latency to the various NREM stages (Table I).
Skin Conductance Responses (SCR)
A MANOVA on the SCR from the extinction task (day 2) to the recall of extinction task (day 3) revealed that the group × time interaction for the extinguished stimulus (CSE) was neither significant at stimulus‐onset nor at ‐offset [F (1,16) = 1.00 and 0.16; all P > 0.33], although there was trend for a significant difference at onset of the CSE in early recall trials [trials 4–6: t (16) = 1.54; P = 0.071] in the expected direction. However, when this analysis was re‐run with sleep restriction, S2 amount and S4 amount as covariates, this post‐hoc difference became significant [F (1,13) = t 2 (13) = 3.48; one‐sided P = 0.043), see Figure 1. Moreover, the group × time interaction on the average of all 15 stimulus‐onset CSE‐scores from extinction to recall of extinction showed a trend for significance [F (1,13) = 3.15; P = 0.099] when corrected for the influence of the covariates sleep restriction, S2 amount and S4 amount.
Figure 1.
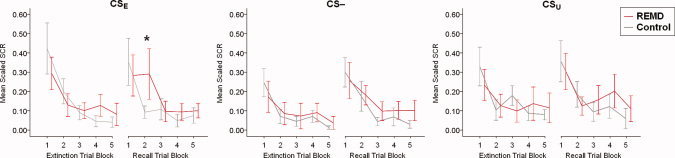
Skin conductance response (SCR) data at fear extinction and recall of fear extinction. The SCR at stimulus‐onset (time‐window 0.5–4.5 s) was summarized per trial block, with the first trial block representing stimulus presentations 1–3, the second trial block presentations 4–6, etc. Lines represent mean values of the scaled SCR (± standard error of the mean). The only significant between group difference controlled for the covariates sleep restriction, S2 amount and S4 amount occurred at the second trial block of the CSE at recall of extinction (* P < 0.05). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We further observed a trend for a significant group × time interaction for the safety stimulus (CS‐) at stimulus‐offset [F(1,16) = 4.13; P = 0.059], but not at stimulus‐onset [F (1,16) = 2.52; P = 0.62]. Post‐hoc tests on trial blocks of three consecutive trials revealed the most robust effects of REMD on stimulus‐offset of the CS‐ in late trial blocks 10–12 [t (9.8) = 1.65; P < 0.05] and 13–15 [t (9.2) = 2.42; P < 0.05]. The group × time interaction for the safety stimulus (CS‐) at stimulus‐offset was no longer significant [F (1,13) = 1.43; P = 0.254] when we controlled for the covariates sleep restriction, S2 amount and S4 amount, and only the last offset trial block (trials 13–15) remained significant, see Supporting Information Figure S1. There were neither significant group × time interactions nor significant post‐hoc differences for the unextinguished stimulus (CSU) at stimulus‐onset or ‐offset [F (1,16) = 0.39 and 0.64; all P > 0.44], and this was not altered by inclusion of the covariates sleep restriction, S2 and S4 amount.
The night sleep between the conditioning and extinction task appeared to have had a stabilizing effect on the crucial differential scores at onset CSE − CS‐ and CSU − CS‐, which are typically used as a measure of discriminatory fear conditioning. These differential scores were not yet significantly positive in the last conditioning trial block (trials 13–15; all P > 0.10) but were significant at the initial extinction trial block 24 hrs later (trials 1–3; all P < 0.05). See Supporting Information Figure S2 for the SCR data from conditioning to extinction in the whole group.
Classical fMRI Analysis
The regions involved in this task (relative to baseline) at extinction and recall of extinction consisted of the thalamus, middle cingulate cortex and supplementary motor area, insula, and visual cortices (see Supporting Information Fig. S3). The differential contrasts for each stimulus (from extinction to recall of extinction) were analyzed in a full factorial ANOVA, and the group × time × stimulus interaction was the contrast of interest. However, there were no significant clusters of activation in this contrast. The effect of time revealed clusters in the bilateral insula and putamen that reduced their activity in response to the stimuli over time; and a group × time interaction revealed a trend for a significant cluster in the left inferior temporal and fusiform gyrus, see Table II.
Table II.
Interaction and between‐group contrasts in the classical analysis on the differential contrasts from extinction to recall of extinction
| Cluster | Voxel | |||||
|---|---|---|---|---|---|---|
| P corr | k | F peak | X | Y | Z | |
| Average effect of condition (effect of time) | ||||||
| Insula and putamen (R) | 0.003 | 96 | 29.67 | 34 | −2 | 14 |
| Insula and putamen (L) | 0.018 | 76 | 23.96 | −32 | 2 | 0 |
| Cerebellum crus I and 6 (R) | 0.086 | 27 | 20.47 | 38 | −54 | −38 |
| Main effect of group (group × time interaction) | ||||||
| Inferior temporal and fusiform gyrus (L) | 0.070 | 22 | 29.70 | −42 | −12 | −36 |
| Post‐hoc test at recall of extinction: control > REMD (CSE) | ||||||
| Middle cingulate cortex (L,R) | 0.013 | 134 | t: 4.38 | 6 | 8 | 28 |
P corr stands for nonstationary, whole brain corrected cluster P‐values, k for the cluster size, F peak for the F‐value of the peak‐voxel, t for t‐value, [x, y, and z] coordinates are in MNI‐space. Note that there were no significant clusters of activation in the group × stimulus interaction. The cluster in the middle cingulate cortex at recall of extinction was not significant when controlled for the three covariates sleep restriction, S2 amount and S4 amount, due to trend‐wise correlations of this cluster with sleep restriction (P corr = 0.076, k = 56, t peak = 4.63) and with S2 amount (P corr = 0.057, k = 85, t peak = 6.26). A brainstem cluster at [4‐34‐28] was noted in the group × time interaction (P corr = 0.420, k = 14, F peak = 24.44).
An additional post‐hoc test at recall (control > REMD for all stimuli) revealed whole brain corrected cluster significance in a cluster in the dorsal anterior cingulate cortex (see Table II), but this cluster was not significant after correction for the covariates sleep restriction, S2 amount and S4 amount. This can be explained by trend‐wise correlations of this cluster with sleep restriction (P corr = 0.076, k = 56, and t peak = 4.63) and with S2 amount (P corr = 0.057, k = 85, and t peak = 6.26).
Prediction Error fMRI Analysis
As in our previous study [Spoormaker et al., 2011], the bilateral dorsolateral prefrontal cortex, the left middle temporal gyrus, and left putamen were involved in negative prediction error signaling, which can be observed in the negative contrast of the parametric modulation of prediction errors, see Figure 2 (cool colors) and Supporting Information Table S2.
Figure 2.
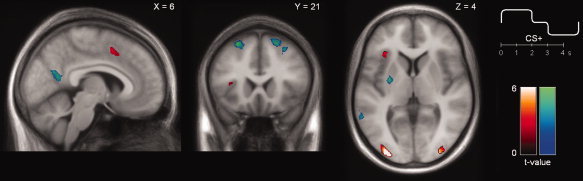
Main effects of the prediction error fMRI analysis. Regions activated in the positive (hot colors; positive PE) or negative contrast (cool colors; negative PE) of the parametric modulation of the stimuli during recall of extinction. Positive and negative PEs were entered into the same parametric modulation to a particular stimulus, with stimulus‐onsets (0–2 s) receiving a constant positive value and stimulus‐offsets (3–5 s) receiving a constant negative value, see upper right insert. The positive contrast of this parametric modulation reflects regions that are more active at stimulus‐onset compared to–offset, a negative contrast reflects the reverse. [X, Y, and Z] coordinates refer to the MNI coordinates of the respective slices. All depicted clusters had whole brain corrected significance (P corr < 0.05) under the assumption of nonstationary smoothness. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The group × stimulus interaction revealed a significant cluster in the left middle temporal gyrus (P corr < 0.05; k = 53), see Figure 3 and Table III. In addition, we computed stimulus‐specific interactions that revealed a trend for significance in a cluster in the right caudate and nucleus accumbens (P corr = 0.051; k = 20) for the group × stimulus interaction of the CSU and CS‐ (Fig. 2B and Table II). Moreover, we observed significant clusters in the right amygdala (P corr < 0.05; k = 40) and left temporal gyrus (P corr < 0.05; k = 152) for the group × stimulus interaction of the CSU and CSE (Fig. 2C and Table II). There were no significant clusters of activation for the group × stimulus interaction of the CSE and CS‐.
Figure 3.
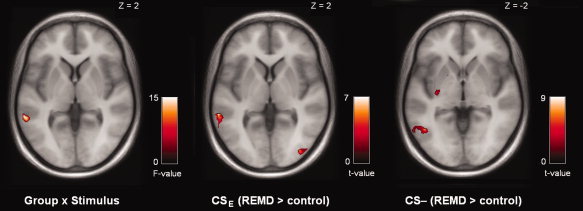
Interaction effects and between‐group comparisons of the prediction error fMRI analysis at recall of extinction. Panel A depicts the group × stimulus interaction of the prediction error signaling analysis at recall of extinction (P corr < 0.05, whole brain corrected). The other panels depict post‐hoc contrasts of between group differences for a specific stimulus controlled for the covariates sleep restriction, S2 amount and S4 amount, see Table III for an overview of significant clusters of activation. Note that there were no significant clusters of activation for the CSU. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table III.
Interaction and between‐group contrasts of the prediction error fMRI analysis at recall of extinction
| Cluster | Voxel | |||||
|---|---|---|---|---|---|---|
| P corr | k | t peak | X | Y | Z | |
| Group × stimulus interaction | ||||||
| Middle temporal gyrus (L) | 0.035 | 53 | F: 15.66 | −62 | −42 | 2 |
| Directed group × stimulus interaction (CS– and CSU) | ||||||
| Caudate, nucleus accumbens (R) | 0.051 | 20 | 5.00 | 6 | 4 | −6 |
| Directed group × stimulus interaction (CSE and CSU) | ||||||
| Middle temporal gyrus (L) | 0.002 | 152 | 4.80 | −56 | −40 | −2 |
| Amygdala, olfactory cortex (R) | 0.038 | 40 | 3.86 | 26 | 6 | −18 |
| Post‐hoc: REMD > control (CSE) | ||||||
| Middle temporal gyrus (L) | 0.005 | 94 | 7.79 | −62 | −40 | 2 |
| Middle occipital gyrus (R) | 0.010 | 111 | 7.69 | 48 | −80 | 6 |
| Sup/middle occipital gyrus (L) | 0.022 | 76 | 7.09 | −36 | −90 | 16 |
| Sup/middle occipital gyrus (L) | 0.011 | 80 | 6.82 | −26 | −76 | 34 |
| Lateral orbitofrontal cortex (L) | 0.058 | 10 | 5.42 | −36 | 30 | −12 |
| Post‐hoc: REMD > control (CS –) | ||||||
| Inf/middle temporal gyrus (L) | 0.004 | 169 | 9.46 | −46 | −60 | −2 |
| Sup/middle frontal gyrus (L) | 0.019 | 95 | 7.40 | −20 | 8 | 60 |
| Amygdala, hippocampus, pallidum (L) | 0.024 | 31 | 6.90 | −18 | −4 | −14 |
| Sup/middle frontal gyrus (R) | 0.003 | 130 | 6.52 | 30 | 0 | 60 |
| Putamen (L) | 0.093 | 19 | 5.65 | −28 | −12 | 0 |
P corr stands for nonstationary, whole brain corrected cluster P‐values, k for the cluster size, t peak for the t‐value of the peak‐voxel (F for F‐value), Inf for inferior, Sup for superior, [x, y, and z] coordinates are in MNI‐space. Post‐hoc contrasts were computed with sleep restriction, S2 amount and S4 amount as covariates. There were no significant clusters of activation at the employed threshold in other post‐hoc contrasts. The cluster in the amygdala and hippocampus was noted also for the CSE (left, P corr = 0.156, and right, P corr = 0.166) and for the CSU (left, P corr = 0.174).
Post‐hoc between‐group comparisons controlled for the covariates sleep restriction, S2 amount and S4 amount demonstrated most pronounced differences for the CSE and CS‐, see Table III. The REMD > control contrast of the CSE revealed significant clusters of activation in the left middle temporal gyrus and bilateral superior and middle occipital gyri, with a trend‐wise cluster in the left lateral orbitofrontal cortex. The REMD > control contrast of the CS‐ also revealed the left middle (and inferior) temporal gyrus, but in addition, clusters in the bilateral superior/middle frontal gyri, left putamen and left amygdala, and hippocampus. This amygdala and hippocampus cluster was also noted but not whole brain significant (both P = 0.16) for the CSE and CSU in the same REMD > control contrast.
To illustrate the direction of the differential activity in these clusters associated with prediction error signaling, event‐related time‐courses are provided in Figure 4. The time‐course in the middle temporal gyri for the CSE (upper two right panels) are flipped between groups. Further of interest are the three left panels of the CSU, which show that for the CSU between‐group differences were more pronounced in subcortical regions.
Figure 4.
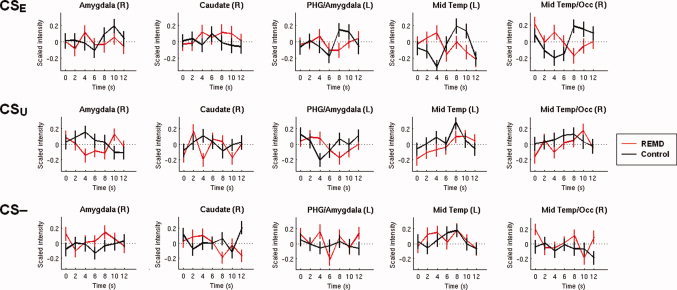
Event‐related time‐courses during recall of extinction. Event‐related time‐courses of regions showing significant between‐group differences of the parametric modulation of the stimuli at recall of extinction. Red lines reflect the mean standardized time‐courses of the REMD group and black lines reflect the mean standardized time‐courses of the control group to specific stimuli, averaged over all trials and subjects and extracted from the residual images. Vertical lines depict the standard error of the mean. Time‐courses were extracted from the peak voxel with a sphere with a radius of 5 mm; information on peak voxel coordinates can be found in Table III. PHG, parahippocampal gyrus; Mid Temp/Occ, middle temporal and occipital gyrus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Vigilance Control Measures
There were no between‐group differences on sleepiness or vigilance at any of the measurements (Supporting Information Table S3) and there were no between‐group differences on subjective sleep complaints, depression, anxiety complaints, or on personality factors (Supporting Information Table S4).
DISCUSSION
To date, fMRI studies on the effects of full and partial sleep deprivation have reported altered behavioral and blood‐oxygen‐level‐dependent (BOLD) signal responses in a multitude of tasks (for a review, see Chee and Chuah, 2008], including emotional tasks [Chuah and Chee, 2008; Sterpenich et al., 2007, 2009; Venkatraman et al., 2007; Yoo et al., 2007]. REM sleep has been proposed to be important for emotional processing [Walker and van der Helm, 2009], particularly for consolidation of emotional memories [Diekelmann and Born, 2010; Walker and Stickgold, 2004; Walker and van der Helm, 2009]. This first study on the fMRI effects of REM sleep deprivation observes robust between‐group differences in activity in temporal lobe regions, which show increased activity during REM sleep relative to slow wave sleep [Braun et al., 1997; Maquet et al., 1996] and are involved in a variety of memory processes [Eichenbaum et al., 2007], including emotional memory processes [Phelps, 2004].
fMRI Effects
The fMRI differences we observed were most robust in the so‐called prediction error fMRI analysis, in which we contrasted the onset of the stimuli with the offset – an analysis that supposedly highlights temporal difference error signals [Spoormaker et al., 2011]. These analyses revealed altered prediction error signaling in the middle temporal gyrus after REM sleep deprivation, with a reversed time‐course in response to the CSE in the REMD group relative to the control group. The REMD group demonstrated higher activity in the post‐hoc prediction error contrast of the CSE in the left middle temporal gyrus, bilateral middle occipital gyri, and trend‐wise in the left lateral orbitofrontal cortex. Moreover, the REMD group also demonstrated higher activity in response to the CS‐ in the left middle temporal gyrus, but additionally in the bilateral middle and superior frontal gyri, left putamen and left amygdala/hippocampus. This latter cluster was also noted in the between‐group comparison for the CSE and CSU, although it did not reach whole brain significance in these post‐hoc contrasts. Both the CSE and CS‐ signal safety, with the main difference being that the CS‐ was never paired with electrical shocks (unambiguous safety) and that the CSE is a conflicting stimulus that is thought to be associated both fear and extinction memories [Corcoran and Quirk, 2007a, b; Milad et al., 2009; Rauch et al., 2006]. Altered activity in the left middle temporal gyrus in response to the CSE (the stimulus for which the reversal of the time‐course was most pronounced) may be related to the role that this region has in signaling anticipation and omission of shocks [Spoormaker et al., 2011].
The (medial) orbitofrontal cortex is involved in fear extinction recall [Milad and Rauch, 2007, a, b; Rauch et al., 2005] and we noted a more lateral orbitofrontal cluster in the between‐group comparison of the CSE specifically. The orbitofrontal cortex has direct projections to the amygdala and interacts with temporal lobe regions, hypothalamus, and brainstem [Rempel‐Clower, 2007]. The involvement of the orbitofrontal cortex in both anxiety disorders [Milad and Rauch, 2007] and insomnia [Altena et al., 2010] suggests a similar neural substrate for some sleep and anxiety disorders; here it is noteworthy that the lateral orbitofrontal cortex has been repeatedly been observed in association with prediction error signaling in various aversive learning tasks [Seymour et al., 2005; Spoormaker et al., 2011].
Furthermore, the role of the superior frontal gyrus in response to the CS‐ could reflect compensatory recruitment in relation to conflict processing and to uncertainty evoked by intermittent stimulus presentations [Dunsmoor et al., 2007], and this appeared in concert with the left putamen for the CS‐ specifically. This is indicative of REM sleep related plasticity in a corticostriatal loop, and it is of note that the putamen shows increased activity in REM sleep relative to NREM sleep [Braun et al., 1997; Maquet et al., 1996] and activity in relation to REMs [Hong et al., 2009; Miyauchi et al., 2009; Wehrle et al., 2005].
A classical fMRI analysis that correlated the BOLD response with stimulus presentation did not reveal robust between‐group differences: the group × time × stimulus interaction was not significant and the group × time interaction yielded only one trend‐wise significant cluster. More notably, a post‐hoc between‐group comparison revealed one cluster in the middle cingulate cortex (corresponding to the dorsal anterior cingulate), but this cluster did not survive correction for the effects of covariates. In particular, sleep restriction—the amount of hours slept less than normal in the experimental night—and amount of light sleep stage 2 were correlated with activity in this cluster. Previous work has shown that the dorsal anterior cingulate is crucial for the expression of fear responses [Corcoran and Quirk, 2007a, b; Milad et al., 2007] and positively correlated with increasing SCRs to aversive stimuli [Milad et al., 2007]. The correlation with of this region with the covariate sleep restriction suggests that dorsal anterior cingulate activity could reflect compensatory activity due to sleep deprivation [Drummond et al., 2004, 2005], which may have been successfully executed in the control group only. Our data suggest that NREM sleep stages are involved in consolidation and recall of fear and safety, which is in line with the observation that interventions reducing slow wave activity (such mild acoustic sleep disruption resulting in shallow sleep) also blunt activation in temporal lobe regions [Van Der Werf et al., 2009].
Physiological Differences
The fMRI differences were paralleled by higher physiological SCR responses of the REMD group to the CSE in early recall of extinction trials, which reflects impaired consolidation of fear extinction. This is in the expected direction [Germain et al., 2008; Levin and Nielsen, 2007] and closely matches animal results reported by Fu et al. (2007) that also observed moderate effects in early recall of fear extinction trials in rodents. This difference occurred at early trials 4–6 and not at early trials 1–3, which could be related to the observation that the initial stimulus presentations elicited a strong SCR in all tasks. The time × group interaction for the physiological response to the CSE only showed a trend for significance, which may be due to the conservative primary outcome variable (average of all trials) that is less sensitive to temporary changes.
In a large behavioral study comparing a sleep group to wake group, Pace‐Schott et al. (2009) found no effect of sleep on recall of fear extinction (the effect size collapsed across trials was around 0.31), although they observed reduced SCR responses to the unextinguished stimulus in the sleep group. Our observed effect size for recall of fear extinction, collapsed across trials, was similar (Cohen's d = 0.30), but much larger at early recall trials (0.77). An initial and temporary between‐group difference is more likely to reflect a cognitive impairment (e.g., decreased recall) than a consistent difference across trials, which given the large variability in SCR scores could also reflect reduced physiological reactivity after sleep. This would be in line with findings from the same group that demonstrated that an afternoon nap (compared to resting waking) was associated with greater habituation of the physiological response to affective pictures [Pace‐Schott et al., 2010]. In this study, REM sleep occurrence in an afternoon nap was correlated with reduced physiological habituation, which contradicts our observation that the control group had a trend for lower physiological responses than the REMD group. This could be indicative of a dissociation between conditioned and intrinsically negative stimuli. It is of note that a full night of REM sleep versus REM sleep deprivation likely results in more robust effects than a brief REM sleep period in an afternoon nap, and an experimental REM sleep manipulation further overcomes the issue that individual differences in physiological reactivity and REM sleep occurrence in an afternoon nap are strongly correlated [Spoormaker et al., 2010].
In our data, controlling for the covariates S2 and S4 amount and sleep restriction diminished the effect on the CS‐, indicating that the effects of REM sleep on unambiguous safety may interact with other sleep stages. Larger behavioral studies employing REM and slow wave sleep deprivation [e.g., Genzel et al., 2009] would therefore be helpful to examine whether recall of safety versus physiological output is differently dependent on various sleep stages or the alternation of slow wave sleep and REM sleep [Diekelmann and Born, 2010; Walker and Stickgold, 2004]. Moreover, studies employing naps will be helpful to explore which NREM stages are correlated with recall of unambiguous safety.
Stimulus‐Onset Versus Stimulus‐Offset SCR
One open question is what the stimulus‐offset SCR actually reflects. The stimulus‐onset SCR is a measure with a long history in experimental psychology [Rodnick, 1937; Switzer, 1934] and has been continuously studied since the 1960s [Grings et al., 1962; Prokasy and Ebel, 1967]. This has allowed for a thorough understanding of the stimulus‐onset response and for instance, whether or not it is advisable to divide this interval in several windows of interest [Pineles et al., 2009]. Much less is known about the stimulus‐offset SCR, as the majority of behavioral studies use few trials with a 100% reinforcement schedule. Such a schedule is less suitable for fMRI, in which several repeated trials are needed to improve the signal‐to‐noise ratio, but also because electrical shocks cause artifacts in the echo‐planar images (therefore shock trials are typically discarded in fMRI studies on fear conditioning and extinction). In a previous study [Spoormaker et al., 2011], we noted that in the last conditioning trials, the differential score CS+ − CS‐ (reflecting discriminatory conditioning) was significantly positive at stimulus‐offset but not at stimulus‐onset. It may therefore be a more sensitive measure for discriminatory conditioning. However, this study showed that a 24‐hour interval in between fear conditioning and extinction stabilized this differential SCR at onset, which was significantly positive in the initial fear extinction trials. It cannot be excluded that the stimulus‐offset SCR reflects a physiological trait and that the trend for higher scores at stimulus‐offset in the REMD group indicates increased physiological responses after REM sleep deprivation. This would be first detected at the CS‐ as the variance of the SCR responses at CS‐ offset is minimal (most scores equal zero), because a SCR at CS‐ offset is rare compared to CS‐ onset [Spoormaker et al., 2011]. Cognitive interpretations of the between‐group difference at stimulus‐offset for the CS‐ are therefore preliminary, whereas the between‐group difference for the CSE occurred at stimulus‐onset only and is more likely to reflect a cognitive difference. To evaluate whether these effects reflect a physiological inhibition or a cognitive disruption, it would be interesting to assess predictions of fear stimuli in subjective ratings. We did not employ this in the current study because raising the awareness of stimulus‐contingencies may alter their sleep dependency akin to procedural skills [Robertson et al., 2004] and classical conditioning can occur independent from awareness [Knight et al., 2003]. Furthermore, SCR peak deflection approaches are sensitive and require large sample sizes, which is not realistic for fMRI studies. More sensitive approaches to examine the anticipatory SCR, such as dynamic causal modeling of the neuronal input [Bach et al., 2010], can be helpful in elucidating the SCR effects of sleep deprivation.
Methodological Considerations
One limitation is that on the basis of our data, we cannot conclude that the control group showed a “normal” pattern of responding. A next step would be to include a group that does not undergo any sleep deprivation to examine the normal temporal pattern of activity in the regions of interest. What we can conclude from our data is that REM sleep deprivation significantly alters prediction error signaling, and that the SCR responses to the CSE at recall of extinction were significantly higher after REM sleep deprivation.
Note that we did not find whole brain significant clusters of activity in the classical fMRI analysis, but this could have been a consequence of the relatively strict threshold employed in this study (whole brain corrected significance instead of small volume corrections). Striking is that even at the conservative threshold used, with N = 9 per group, we observed rather strong fMRI effects. This appears illustrative of the robustness of whole night REM sleep deprivation.
The fMRI effects we found in our study are unlikely to be caused by interference with other emotional memory demands, as the subjects performed only one overnight emotional memory task (consolidation of extinction learning). Moreover, we can exclude circadian rhythm fluctuations as a confounding factor since all sessions were at the same time of the day. However, it should be noted that our control group slept on average about 40 min longer than the REMD group. We corrected for the reduction of total sleep time in the experimental night relative to the week preceding the experiment by including this sleep restriction score as a covariate, as well as S2 and S4 amount. Moreover, the additional sleep time consisted almost exclusively of REM sleep, while the amount of NREM sleep did not differ between groups. We think this procedure has therefore limited confounding effects of prolonged NREM sleep. We found this manipulation to be preferable to keeping the total sleeping time constant (and therefore having different amounts of NREM sleep in both groups) or to performing no or a very different intervention (e.g., comparing REM deprivation with slow wave sleep deprivation).
Clinical Implications
Our findings may be relevant to understanding the role of REM sleep disturbances in the development of PTSD. Objective sleep disturbances as measured with polysomnography have been less pronounced than subjective sleep complaints in PTSD (for reviews see Harvey et al., 2003; Pillar et al., 2000], but this may have been due to confounding factors in the studied PTSD samples such as age, gender, co‐morbid depression, and substance abuse that all interact with sleep [Kobayashi et al., 2007]. In their meta‐analysis, Kobayashi et al. (2007) corrected for these confounding factors and demonstrated that PTSD patients show more stage 1 sleep, less slow wave sleep, and increased REM density. More specifically, fragmentation of REM sleep has been noted in PTSD patients [Mellman and Hipolito, 2006; Spoormaker and Montgomery, 2008] and this is ameliorated by the alpha1‐antagonist Prazosin [Taylor et al., 2008], which indicates increased noradrenalin output in PTSD patients due to locus coeruleus hyperactivity, a region that normally reaches its nadir in REM sleep [Pace‐Schott and Hobson, 2002]. Critically, REM fragmentation (shorter and more frequent REM sleep periods) as measured by polysomnography within 1 month after the traumatic event (traumatic injury) predicted PTSD symptom severity 6 weeks later [Mellman et al., 2002]. Moreover, REM duration correlates inversely with insomnia symptoms in PTSD [Mellman et al., 2007]. One recent study observed that REM sleep deprivation resulted in more disruptions in cardiac measures during rebound REM sleep in subjects with nightmares relative to subjects without nightmares, suggesting a dispositional sensitivity to REM sleep deprivation [Nielsen et al., 2010].
Fear extinction is a promising model for several human anxiety disorders, including PTSD [Pape and Pare, 2010; Rauch et al., 2006]. In PTSD patients, fear extinction and fear extinction recall is impaired [Milad et al., 2009; Wessa and Flor, 2007], which is associated with altered activity in the hippocampus and ventromedial prefrontal cortex [Milad et al., 2009]. Studies in rodents have demonstrated that a predatory encounter in rats curtails slow wave and REM sleep [Lesku et al., 2008], and that selective REM sleep deprivation impairs cued fear extinction [Silvestri, 2005] and consolidation of cued fear extinction [Fu et al., 2007]. Our results extend these findings by showing impaired recall of fear extinction after selective REM sleep deprivation in humans. Moreover, our findings elucidate the neural circuitry subserving this impairment, which was primarily located in a brain structure critical for a variety of memory processes: the temporal lobe (for a review see Eichenbaum et al., 2007]. This provides experimental evidence for the notion that REM sleep disturbances are central to the development of impaired recall of fear extinction and eventually, PTSD. Treating (REM) sleep disturbances and related complaints such as nightmares and insomnia in the wake of a traumatic event may therefore have an ameliorating effect on the development of PTSD and PTSD symptom severity (for a review of treatment efficacy, see Nappi et al., in press]. The role of prediction error signals—signals that are proposed to subserve fear conditioning and extinction—may be essential to this developmental model; more research is needed to study their relationship with sleep and their occurrence in patient samples.
CONCLUSIONS
In short, our results suggest that REM sleep deprivation affects a specific pattern of subcortical and temporal lobe regions, which are regions that show increased activity during REM sleep [Braun et al., 1997; Maquet et al., 1996]. The left middle temporal gyrus showed most robust between‐group differences in activity in association with prediction error signaling during recall of fear extinction and safety, paralleled by increased physiological responses to a previously extinguished stimulus after REM sleep deprivation. This may provide a greater understanding of dysfunctional fear processes in anxiety disorders such as PTSD, in which disturbed REM and impaired fear extinction learning coincide. Examining prediction error signaling appears a promising approach to studying the effects of REM sleep deprivation on the human brain.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
The authors would like to thank Karlheinz Honsberg, Armin Mann, Rheinhold Borschke, Elke Schreiter, Stephanie Alam, and Rosa Schirmer for their technical support; Birte Balzer, Luise Vogl, and Christine Zitzmann for their organizational support; and Dr. Pierre Beitinger and Dr. Petra Schüssler for the clinical evaluation of the polysomnographic recordings. VS acknowledges financial support from the Bavarian Academy of Sciences and Humanities. The authors thank the anonymous reviewers for their useful comments.
REFERENCES
- Akerstedt T, Gillberg, M ( 1990): Subjective and objective sleepiness in the active individual. Int J Neurosci 52: 29–37. [DOI] [PubMed] [Google Scholar]
- Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJ ( 2010): Reduced orbitofrontal and parietal gray matter in chronic insomnia: A voxel‐based morphometric study. Biol Psychiatry 67: 182–185. [DOI] [PubMed] [Google Scholar]
- Bach DR, Daunizeau J, Friston KJ, Dolan RJ ( 2010): Dynamic causal modelling of anticipatory skin conductance responses. Biol Psychol 85: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK ( 1996): Manual for the Beck Depression Inventory‐II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Selbie S, Belenky G, Herscovitch P ( 1997): Regional cerebral blood flow throughout the sleep‐wake cycle. An H2(15)O PET study. Brain 120: 1173–1197. [DOI] [PubMed] [Google Scholar]
- Büchel C, Dolan RJ ( 2000): Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol 10: 219–223. [DOI] [PubMed] [Google Scholar]
- Chee MW, Chuah LY ( 2008): Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Curr Opin Neurol 21: 417–423. [DOI] [PubMed] [Google Scholar]
- Chuah LY, Chee MW ( 2008): Functional neuroimaging of sleep deprived healthy volunteers and persons with sleep disorders: A brief review. Ann Acad Med Singapore 37: 689–694. [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ ( 2007a): Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci 27: 840–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ ( 2007b): Recalling safety: Cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr 12: 200–206. [DOI] [PubMed] [Google Scholar]
- Datta S, Mavanji V, Ulloor J, Patterson EH ( 2004): Activation of phasic pontine‐wave generator prevents rapid eye movement sleep deprivation‐induced learning impairment in the rat: A mechanism for sleep‐dependent plasticity. J Neurosci 24: 1416–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, Phelps EA ( 2008): Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 59: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschaux O, Thevenet A, Spennato G, Arnaud C, Moreau JL, Garcia R ( 2010): Low‐frequency stimulation of the hippocampus following fear extinction impairs both restoration of rapid eye movement sleep and retrieval of extinction memory. Neuroscience 170: 92–98. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J ( 2010): The memory function of sleep. Nat Rev Neurosci 11: 114–126. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Brown GG, Salamat JS, Gillin JC ( 2004): Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep 27: 445–451. [PubMed] [Google Scholar]
- Drummond SP, Meloy MJ, Yanagi MA, Orff HJ, Brown GG ( 2005): Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res 140: 211–223. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC ( 2007): Impact of continuous versus intermittent CS‐UCS pairing on human brain activation during Pavlovian fear conditioning. Behav Neurosci 121: 635–642. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C ( 2007): The medial temporal lobe and recognition memory. Annu Rev Neurosci 30: 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A ( 2007): G*Power 3: A flexible statistical power analysis for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191. [DOI] [PubMed] [Google Scholar]
- Fu J, Li P, Ouyang X, Gu C, Song Z, Gao J, Han L, Feng S, Tian S, Hu B ( 2007): Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus‐independent tasks in rats. Neurosci 144: 1186–1192. [DOI] [PubMed] [Google Scholar]
- Genzel L, Dresler M, Wehrle R, Grözinger M, Steiger A ( 2009): Slow wave sleep and REM sleep awakenings do not affect sleep dependent memory consolidation. Sleep 32: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Buysse DJ, Nofzinger E ( 2008): Sleep‐specific mechanisms underlying posttraumatic stress disorder: Integrative review and neurobiological hypotheses. Sleep Med Rev 12: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grings WW, Lockhart RA, Dameron LE ( 1962): Conditioning autonomic responses of mentally subnormal individuals. Psychol Monogr 76: 1–35. [Google Scholar]
- Haruno M, Kawato M ( 2006): Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus‐action‐reward association learning. J Neurophys 95: 948–959. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Jones C, Schmidt DA ( 2003): Sleep and posttraumatic stress disorder: A review. Clin Psych Rev 23: 377–407. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE ( 2004): Nonstationary cluster‐size inference with random field and permutation methods. Neuroimage 22: 676–687. [DOI] [PubMed] [Google Scholar]
- Hong CC, Gillin JC, Dow BM, Wu J, Buchsbaum MS ( 1995): Localized and lateralized cerebral glucose metabolism associated with eye movements during REM sleep and wakefulness: A positron emission tomography (PET) study. Sleep 18: 570–580. [DOI] [PubMed] [Google Scholar]
- Hong CC, Harris JC, Pearlson GD, Kim JS, Calhoun VD, Fallon JH, Golay X, Gillen JS, Simmonds DJ, van Zijl PC, Zee DS, Pekar JJ, ( 2009): fMRI evidence for multisensory recruitment associated with rapid eye movements during sleep. Hum Brain Mapp 30: 1705–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne J, McGrath MJ ( 1984): The consolidation hypothesis for REM sleep function: stress and other confounding factors‐a review. Biol Psychiatry 18: 165–184. [DOI] [PubMed] [Google Scholar]
- Jensen J, Smith AJ, Willeit M, Crawley AP, Mikulis DJ, Vitcu I, Kapur S ( 2007): Separate brain regions code for salience vs. valence during reward prediction in humans. Hum Brain Mapp 28: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ ( 2006): Context‐dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci 26: 9503–9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA ( 2003): Expression of conditional fear with and without awareness. Proc Natl Acad Sci U S A 100: 15280–15283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D ( 2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I, Boarts JM, Delahanty D ( 2007): Polysomnographically measured sleep abnormalities in PTSD: A meta‐analytic review. Psychophys 44: 660–669. [DOI] [PubMed] [Google Scholar]
- Lang FR, Lüdtke O, Asendorpf JB ( 2001): Testgüte und psychometrische Äquivalenz der deutschen Version des Big Five Inventory (BFI) bei jungen, mittelalten und alten Erwachsenen. Diagnostica 47: 111–121. [Google Scholar]
- Lesku JA, Bark RJ, Martinez‐Gonzalez D, Rattenborg NC, Amlaner CJ, Lima SL ( 2008): Predator‐induced plasticity in sleep architecture in wild‐caught Norway rats (Rattus norvegicus). Behav Brain Res 189: 298–305. [DOI] [PubMed] [Google Scholar]
- Levin R, Nielsen TA ( 2007): Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychol Bull 133: 482–528. [DOI] [PubMed] [Google Scholar]
- Maquet P, Péters J, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G ( 1996): Functional neuroanatomy of human rapid‐eye‐movement sleep and dreaming. Nature 383: 163–166. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Hipolito MM ( 2006): Sleep disturbances in the aftermath of trauma and posttraumatic stress disorder. CNS Spectr 11: 611–615. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B ( 2002): REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry 159: 1696–1701. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Pigeon WR, Nowell PD, Nolan B ( 2007): Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress 20: 893–901. [DOI] [PubMed] [Google Scholar]
- Menon M, Jensen J, Vitcu I, Graff‐Guerrero A, Crawley A, Smith MA, Kapur S ( 2007): Temporal difference modeling of the blood‐oxygen level dependent response during aversive conditioning in humans: Effects of dopaminergic modulation. Biol Psychiatry 62: 765–772. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL ( 2007): The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci 1121: 546–561. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL ( 2007a): A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry 62: 1191–1194. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL ( 2007b): Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 62: 446–454. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL ( 2009): Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi S, Misaki M, Kan S, Fukunaga T, Koike T ( 2009): Human brain activity time‐locked to rapid eye movements during REM sleep. Exp Brain Res 192: 657–667. [DOI] [PubMed] [Google Scholar]
- Nappi CM, Drummond SP, Hall JM: Treating nightmares and insomnia in posttraumatic stress disorder: A review of current evidence. Neuropharm (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen T, Paquette T, Solomonova E, Lara‐Carrasco J, Colombo R, Lanfranchi P ( 2010): Changes in cardiac variability after REM sleep deprivation in recurrent nightmares. Sleep 33: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ ( 2003): Temporal difference models and reward‐related learning in the human brain. Neuron 38: 329–337. [DOI] [PubMed] [Google Scholar]
- Pace‐Schott EF, Hobson JA. ( 2002). The neurobiology of sleep: Genetics, cellular physiology and subcortical networks. Nat Rev Neurosci 3: 591–605. [DOI] [PubMed] [Google Scholar]
- Pace‐Schott EF, Milad MR, Orr SP, Rauch SL, Stickgold R, Pitman RK ( 2009): Sleep promotes generalization of extinction of conditioned fear. Sleep 32: 19–26. [PMC free article] [PubMed] [Google Scholar]
- Pace‐Schott EF, Shepherd E, Spencer RM, Marcello M, Tucker M, Propper RE, Stickgold R. ( 2010). Napping promotes inter‐session habituation to emotional stimuli. Neurobiol Learn Mem 95: 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D ( 2010): Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90: 419–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP ( 1927): Conditioned reflexes. London: Oxford University Press. [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Delbeuck X, Degueldre C, Aerts J, Delfiore G, Luxen A, Maquet P ( 2001): Generation of rapid eye movements during paradoxical sleep in humans. Neuroimage 14: 701–708. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Destrebecqz A, Collette F, Delbeuck X, Phillips C, Aerts J, Del Fiore G, Degueldre C, Luxen A, Cleeremans A, Maquet P, ( 2003): Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid‐eye‐movements sleep. Neuroimage 20: 125–134. [DOI] [PubMed] [Google Scholar]
- Phelps EA ( 2004): Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol 14: 198–202. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE ( 2004): Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 43: 897–905. [DOI] [PubMed] [Google Scholar]
- Pillar G, Malhotra A, Lavie P ( 2000): Post‐traumatic stress disorder and sleep: What a nightmare! Sleep Med Rev 4: 183–200. [DOI] [PubMed] [Google Scholar]
- Pineles SL, Orr MR, Orr SP ( 2009): In alternative scoring method for skin conductance responding in a differential fear conditioning paradigm with a long‐duration conditioned stimulus. Psychophys 46: 984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokasy WF, Ebel HC ( 1967): Three components of the classically conditioned GSR in human subjects. J Exp Psychol 73: 247–256. [Google Scholar]
- Rauch SL, Milad MR, Orr SP, Quinn BT, Fischl B, Pitman RK ( 2005): Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport 16: 1909–1912. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA ( 2006): Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research–past, present, and future. Biol Psychiatry 60: 376–382. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A, editors ( 1968). A manual of standardized terminology, techniques and scoring system of sleep stages in human subjects. Los Angeles: Brain Information Service/Brain Research Institute, University of California. [Google Scholar]
- Rempel‐Clower NL ( 2007): Role of orbitofrontal cortex connections in emotion. Ann N Y Acad Sci 1121: 72–86. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR ( 1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement In Black WF, Prokasky WF, editors. Classical conditioning II: Current research and theory. New York: Appleton‐Century‐Crofts; pp 64–99. [Google Scholar]
- Robertson EM, Pascual‐Leone A, Press DZ ( 2004): Awareness modifies the skill‐learning benefits of sleep. Curr Biol 14: 208–212. [DOI] [PubMed] [Google Scholar]
- Rodnick EH ( 1937): Characteristics of delayed and trace conditioned responses. J Exp Psychol 20: 409–425. [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA ( 2008): From fear to safety and back: Reversal of fear in the human brain. J Neurosci 28: 11517–11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W ( 1998): Predictive reward signal of dopamine neurons. J Neurophys 80: 1–27. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR ( 1997): A neural substrate of prediction and reward. Science 275: 1593–1598. [DOI] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Dayan P, Koltzenburg M, Jones AK, Dolan RJ, Friston KJ, Frackowiak RS ( 2004): Temporal difference models describe higher‐order learning in humans. Nature 429: 664–667. [DOI] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, Dolan R ( 2005): Opponent appetitive‐aversive neural processes underlie predictive learning of pain relief. Nat Neurosci 8: 1234–1240. [DOI] [PubMed] [Google Scholar]
- Silvestri AJ ( 2005): REM sleep deprivation affects extinction of cued but not contextual fear conditioning. Phys Behav 84: 343–349. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. ( 1983). Manual for the State‐Trait Anxiety Inventory (Form Y). Palo Alto: Consulting Psychologists Press. [Google Scholar]
- Spoormaker VI, Montgomery P ( 2008): Disturbed sleep in post‐traumatic stress disorder: Secondary symptom or core feature? Sleep Med Rev 12: 169–184. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Sturm A, Andrade KC, Schröter MS, Goya‐Maldonado R, Holsboer F, Wetter TC, Sämann PG, Czisch M ( 2010): The neural correlates and temporal relationship between shock exposure, disturbed sleep and impaired consolidation of fear extinction. J Psychiatr Res 44: 1121–1128. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Andrade KC, Schröter MS, Sturm A, Goya‐Maldonado R, Sämann PG, Czisch M ( 2011): The neural correlates of negative prediction error signaling in human fear conditioning. Neuroimage 54: 2250–2256. [DOI] [PubMed] [Google Scholar]
- Sterpenich V, Albouy G, Boly M, Vandewalle G, Darsaud A, Balteau E, Dang‐Vu TT, Desseilles M, D'Argembeau A, Gais S, Rauchs G, Schabus M, Degueldre C, Luxen A, Collette F, Maquet P ( 2007): Sleep‐related hippocampo‐cortical interplay during emotional memory recollection. PLoS Biology 5, e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterpenich V, Albouy G, Darsaud A, Schmidt C, Vandewalle G, Dang Vu TT, Desseilles M, Phillips C, Degueldre C, Balteau E, Collette F, Luxen A, Maquet P ( 2009): Sleep promotes the neural reorganization of remote emotional memory. J Neurosci 29: 5143–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG ( 1990). Time‐Derivative Models of Pavlovian Reinforcement In Gabriel M, Moore J, editors. Learning and Computational Neuroscience: Foundations of Adaptive Networks. Cambridge MA: The MIT Press; pp. 497–537. [Google Scholar]
- Switzer SA ( 1934): Anticipatory and inhibitory characteristics of delayed conditioned reactions. J Exp Psychol 17: 603–620. [Google Scholar]
- Taylor FB, Martin P, Thompson C, Williams J, Mellman TA, Gross C, Peskind ER, Raskind MA ( 2008): Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: A placebo‐controlled study. Biol Psychiatry 63: 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf YD, Altena E, Schoonheim MM, Sanz‐Arigita EJ, Vis JC, De Rijke W, Van Someren EJ ( 2009): Sleep benefits subsequent hippocampal functioning. Nat Neurosci 12: 122–123. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Chuah YML, Huettel SA, Chee ML ( 2007): Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep 30: 603–609. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R ( 2004): Sleep‐dependent learning and memory consolidation. Neuron 44: 121–133. [DOI] [PubMed] [Google Scholar]
- Walker MP, van der Helm E ( 2009): Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull 135: 731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrle R, Czisch M, Kaufmann C, Wetter TC, Holsboer F, Auer DP, Pollmächer T ( 2005): Rapid eye movement related brain activation in human REM sleep using fMRI. Neuroreport 16: 853–857. [DOI] [PubMed] [Google Scholar]
- Wellman LL, Yang L, Tang X, Sanford LD ( 2008): Contextual fear extinction ameliorates sleep disturbances found following fear conditioning in rats. Sleep 31: 1035–1042. [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Flor H ( 2007): Failure of extinction of fear responses in posttraumatic stress disorder: Evidence from second‐order conditioning. Am J Psychiatry 164: 1684–1692. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP ( 2007): The human emotional brain without sleep–a prefrontal amygdala disconnect. Curr Biol 17: R877–878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


