Abstract
Intra‐active touch (IAT) is a process that involves a body part doing the touching (active touch [AT]) and another body part being touched (passive touch [PT]) simultaneously. The brain representation related to IAT is still unclear. A total of 23 subjects carried out angle discrimination under PT, AT and IAT conditions with functional magnetic resonance imaging. All of the tasks were strictly dependent on cutaneous feedback from the finger(s). As the subjects were able to perceive the angle stimuli from the right (touching) and left (touched) sides during the IAT condition, we expected there would be greater brain activation with the IAT condition than for the AT or PT condition. Therefore, we hypothesized that the region within and/or around the intraparietal sulcus (IPS) and the part of the primary somatosensory cortex (SI) that is associated with high‐level tactile spatial processing would be more active during the IAT task than during the AT and PT tasks. Compared with the areas activated by the motor somatosensory control task, the most prominent activation areas evoked by the three‐angle discrimination tasks were in the SI and secondary somatosensory cortex areas in the bilateral parietal operculum, IPS, lateral occipital complex, insula and cerebellum. Finally, we directly compared IAT with AT and PT, and the results suggest that the contralateral part of IPS and part of the SI are more active under IAT conditions than under either AT or PT conditions. These results suggest that both hemispheres contribute to angle discrimination during IAT. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: functional magnetic resonance imaging, intraparietal sulcus, somatosensory cortex, active touch, passive touch, index finger
INTRODUCTION
Katz [ 1989] pioneered the discussion of the concepts of passive touch (PT), active touch (AT) and dual touch. To date, there has been a substantial amount of research devoted to studying the differences in shape perception and discrimination between AT and PT [Gibson, 1962; Heller, 1984; Klatzky et al., 1985]. However, Katz defined dual touch as the sensation that is produced when individuals touch themselves, and he introduced the issue of dual touch from a phenomenological standpoint, relying mostly on introspection. Recently, Bolanowski et al. [Bolanowski et al., 1999; Bolanowski et al., 2004; Verrillo et al., 2003] defined intra‐active touch (IAT) (i.e., a case of dual touch) as a movement that involves actively moving a touching surface over another surface of the body that is passively being touched. For example, this sensation will be perceived when human manipulates an object cooperatively with both hands (e.g., take a tennis ball and roll it between your hands).
In the past decade, many neuroimaging studies [Bodegård et al., 2001; Harada et al., 2004; Hlushchuk et al., 2006; Pleger et al., 2006; Reed et al., 2004] have explored the human ability for tactile object recognition and the processing of tactile object recognition by AT and PT in the brain. The results suggested that shape perception by both AT and PT involves a widely distributed cerebral network including the primary somatosensory cortex (SI), secondary somatosensory cortex (SII) [Blatow et al., 2007; Gardner et al., 2000; Jones, 1986; Roland et al., 1998], part of the intraparietal sulcus (IPS) [Bodegård et al., 2001; Miquée et al., 2008; Saito et al., 2003; Stilla et al., 2007] and a region within the lateral occipital complex (LOC) [Reed et al., 2004; Zhang et al., 2005].
In contrast, Bolanowski et al. [ 1999] made the first attempts since Katz's [ 1989] earlier studies to address the matter of IAT psychophysically. In this study [Bolanowski et al., 1999], the subjects were asked to estimate the perceived size of nine steel balls during AT (the balls were actively rolled between the fingertip and a thick piece of paper), PT (the balls were rolled on the fingertip) and IAT (the balls were actively rolled between the fingertip and several other body sites such as the thumb, thenar eminence and forearm) conditions. The principal finding of their study was that both the actively touching and the passively stimulated skin region contribute to the perceptual experience of IAT but only when the intra‐active touching involved the glabrous skin of the hands. However, it is still unclear which neural mechanisms are crucial for processing shape discrimination by IAT. The lack of scientific attention on IAT might be due to the methodological problems that are associated with stimulus presentation and response recording during functional neuroimaging in humans. However, IAT processing is considered to play a key role in skilful and sensitive object manipulation during our daily activities. Therefore, the aims of this study are to investigate the neural correlates of tactile shape discrimination processing under IAT condition and to identify the differences in cortex activity among IAT, AT and PT.
A recent review article [Iwamura, 1998] indicates that the postcentral and additional somatosensory cortices support a hierarchical scheme of information processing. Specifically, the findings indicated that the hierarchical scheme involved in information processing within the SI (areas 3a, 3b, 1 and 2) in the postcentral gyrus (poCG) and area 5 in the parietal cortex also involved the IPS. A more recent human positron emission tomography (PET) study [Bodegård et al., 2001] indicated that the initial processing of skin contact takes place in the low‐level areas (i.e., areas 3b and 1) and that the computation of shapes and the elaborate reconstruction of shapes takes place in the high‐level areas (i.e., area 2 of the SI and a portion of the IPS).
The SI is located in the poCG and contains a somatotopic organization of body representations. This area was the first cortical region to be shown and to be involved in the perception of touch [Bodegård et al., 2001; Iwamura, 1998; Roland et al., 1998]. These previous studies indicated that areas 3b and 1 of the SI are activated by all types of touch signals from the mechanoreceptors of skin, such as the indentation of the object–skin contact. In contrast, area 2 of the SI was significantly activated more by shape and surface curvature than roughness and object–skin contact. In addition, IPS is known as a multisensory area that engages tactile, visual and auditory spatial processing (for review, see Stein and Stanford, 2008]. For instance, when active and passive tactile discrimination of the shape of three‐dimensional ellipsoids were contrasted to roughness and brush velocity discrimination, the anterior part of the IPS was more activated by both shape discrimination conditions in a PET study [Bodegård et al., 2001]. Moreover, functional magnetic resonance imaging (fMRI) studies have shown this area to be more active during tactile two‐dimensional grating orientation discrimination than during the process of judging the spacing between gratings [Kitada et al., 2006; Zhang et al., 2005]. Therefore, we hypothesized that the region within and/or around the IPS and part of the SI are more active in IAT conditions than in AT and PT conditions, because the tactile shape information from both the left and right sides in intra‐active conditions was considered to activate more tactile‐related, high‐level regions for shape computation and reconstruction.
In this study, we used block‐designed fMRI to test our hypothesis. Here, to assess the crucial IAT mechanisms, we used a restricted working definition of shape that can be applied to any object with angles, as was used in our previous study [Wu et al., 2010]. Before the fMRI experiment, the psychophysical experiment was designed to investigate the difference in the accuracy of angle discrimination using the intra‐active, active and passive modes of touch. The results indicate that the mean accuracy of the intra‐active condition was significantly higher than for the AT and PT conditions. The fMRI results demonstrated that the activation of both the hemispheres including bilateral IPS and SI contribute to the performance of the intra‐active task.
MATERIALS AND METHODS
Subjects
To confirm the difference for each mode of touch and to select the tactile stimuli for the fMRI experiment, we conducted a psychophysical pilot experiment outside of the MR scanner. A total of 10 right‐handed male volunteers aged 20–26 years are consented to participate in the psychophysical pilot experiment.
In the fMRI experiment, 23 healthy, right‐handed male volunteers ranging in age from 20 to 29 years (mean age of 23.6 years) participated. Handedness was confirmed with the Edinburgh Handedness Inventory [Oldfield, 1971] and an average index of 89% indicated strong right‐handedness in our subjects. All subjects were naive with respect to the purpose of the fMRI experiment and had not participated in the previous psychophysical pilot experiment. In addition, all subjects reported no loss of tactile sensation or any unusual experiences with haptic input. Before the start of the fMRI experiment, all subjects participated in a training session outside of the MR scanner in which they were instructed about the protocol and had to perform all procedures. The ethical committee of the Peking University Third Hospital approved the research procedures.
Tactile Stimuli
As discussed in our previous study [Wu et al., 2010], the shape of the objects can be specified as a series of edges that are spatially related to one another. However, in the current experiment, we used a restricted working definition of shape that can be applied to any object with angles. As shown in Figure 1a, the raised angles consisted of custom‐built plastic shapes that were raised 5.0 mm from a 16.0‐mm square base. The angles varied in two spatial dimensions and were formed by two convex lines at the centre of the 16.0‐mm square base with an accuracy of ±0.1°; the lines were 8.0‐mm long and 1.5‐mm wide. In the pilot experiment, one standard angle (60°) and 10 comparison angles (61°, 62°, 63°, 64°, 65°, 66°, 67°, 68°, 69° and 70°) were used to examine differences in the accuracy of angle discrimination during AT, PT and IAT.
Figure 1.
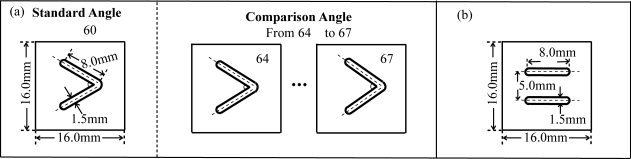
(a) The size and configuration of the raised angle patterns (SA = 60°, CA = 64°, 65°, 66° and 67°) used in the fMRI experiment. (b) The size and configuration of the somatosensory control pattern. SA, Standard angle; CA, Comparison angle.
In the fMRI experiment, we selected the comparison angles from those used in the psychophysical pilot experiment. As shown in Figure 5a, the mean discrimination thresholds (75% correct) for the IAT, AT and PT conditions were 2.1°, 3.6° and 3.9°, respectively. Therefore, we selected four comparison angles that differed from the standard angle (60°) by +4°, 5°, 6° and 7° for the fMRI experiment (Fig. 1a). The final set of comparison angles could be discriminated by the subject using a prescribed motor sequence (i.e., press, release, press and release) within 10 sec, with a mean accuracy rate of 75% or above.
Figure 5.
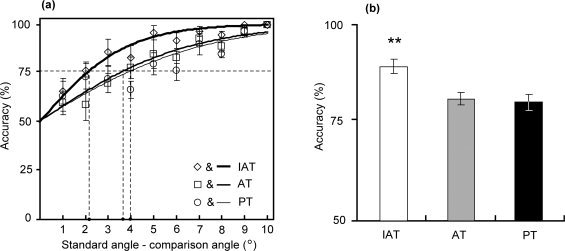
(a) Performance of 10 subjects in the pilot angle discrimination experiment for the IAT (empty diamonds), AT (empty squares) and PT (empty circles) tasks. Accuracy is plotted as a function of the angular difference between the comparative angle (61°‐70°) and the standard angle (60°). The logistic curves (solid lines) fit all of the data. The horizontal dashed line indicates an accuracy of 75%. The value on the horizontal axis at the intersection between the 75% line and each logistic curve is defined as the angle discrimination threshold. The discrimination thresholds are 2.1° for the IAT task, 3.6° for the AT task and 3.9° for the PT task. (b) Mean accuracy values of the IAT, AT and PT tasks in the pilot angle discrimination experiment. The vertical error bars represent the SEM. **, P < 0.01.
Furthermore, under the IAT condition, the subjects were required to touch the angle with their right index finger, and the same angle pattern that was presented on the right side was touched to their left index finger synchronously (see details in fMRI Procedures section). In contrast, subjects were only required to touch the angle using their right index finger under the AT condition, and one angle was touched to their left index finger under the PT condition. Because the increased tactile input of the IAT condition activates unwanted areas during the fMRI experiment, we needed to control the sensory input under the active and passive conditions but needed to omit any angle components. Here, a simple tactile pattern was used as a motor somatosensory control (MSC) pattern, which consisted of two parallel convex lines as shown in Figure 1b. The contact area and the height of the MSC pattern were the same for each angle.
Apparatus
In this study, we used the same angle delivery device for both the psychophysical pilot and the fMRI experiment (Fig. 2) [Yang and Wu, 2010]. As shown in Figure 2a, the hand fix blocks, index finger holder and index finger platelet were present in the device to keep the palms of subjects closed comfortably in the hand platelets and to keep the bilateral index fingers of subjects in the correct location during the experiment. Two tactile stimuli were affixed on the left and right sides of a plastic stick as shown on the right side of Figure 2a. When the subjects pushed the stimulus on the right side of the stick, the stimulus on the left pressed on the left fingertip of subjects with approximately 90 ms delay. The position of the hands and the tactile pattern contact location on the finger pad are shown in Figure 2b. The angle delivery device was composed of nonferrous plastic. One of the ultrasonic motors for the tactile patterns consisted of nonferrous metals, and no magnetic fields were produced when they were rotated. The control and signal cables and the drive motor of the angle delivery device were contained within the plastic frame, eliminating any possible contact with the subject.
Figure 2.
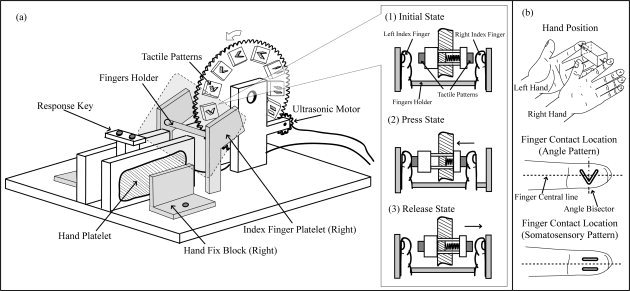
(a) The tactile pattern delivery device used in the fMRI experiment. During the experiment, the hands of subject were fixed on the device by a hand fix block, and the index fingers of subject were fixed by the index finger holder and index finger platelet. In addition, the subjects were able to do the “Press, Release” procedure smoothly due to a plastic spring as shown in (1) (2) and (3). (b) The hand position and tactile pattern contact location on the finger tap. For the angle pattern: the imaginary bisector of angle is perpendicular to the centre line of finger. For the MSC pattern: the raised lines were parallel to the long axis to the finger.
Psychophysical Pilot Experiment
The subjects were asked to place their right index finger at the initial placement point on the device. The subjects wore an eye mask during the task to prevent them from receiving visual feedback. Then, the subjects were asked to perceive the size of a pair of angles (i.e., each pair consisted of one standard angle and one comparison angle) using their right index fingertip, their left index fingertip or both index fingertips and were asked to identify the larger angle in each pair. The experimental procedure and the data acquisition were controlled by a personal computer to ensure accuracy. The responses of subjects were stored along with information about the trial, including the values of all of the angles and the presentation order of all of the angles.
fMRI Task Design
We used a block‐design fMRI paradigm to assess task‐related neuronal activity during the angle discrimination tasks by PT, AT and IAT. Six 360‐s sessions were designed and executed using a 3.0‐Tesla Siemens Magnetom MR‐Scanner (Peking University Third Hospital, Beijing, China). Each session consisted of six alternating ON blocks (i.e., three angle discrimination blocks and three MSC blocks) interspersed by six Rest blocks, each 30 s in duration. The experimental paradigm is illustrated in Figure 3.
Figure 3.
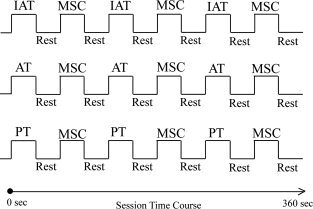
Diagram of the experimental paradigm. Each subject participated in six 360‐s sessions. Two sessions used an on–off block design for IAT, MSC and Rest tasks; two sessions used an on–off block design for AT, MSC and Rest tasks; two sessions used an on–off block design for PT, MSC and Rest tasks. Each block was 30 s long and included six stimulus presentations.
The subject lay supine in the MRI tunnel with earplugs and was instructed to relax. The subject was asked to fixate on a white cross (viewing angle, 1.5 × 1.5 degrees) projected from a liquid crystal display (LCD) projector through a mirror onto a semitransparent screen that was hung 3.0 m from the eyes of the subject. The arms of the subject were extended to the device as shown in Figure 2a and comfortably supported by cushions. The left index finger of the subject was fixed with tape to an immovable plastic platelet on the left side of the device. The right index finger was put on the platelet symmetrically to the left index finger on the right side of the device and could freely move horizontally. The response keys, which the subject could press with his right thumb, were set in the centre of the device. For each angle discrimination trial, the subjects were instructed to press two angle patterns using the glabrous skin of the right, left or both index fingers with a 2‐s delay. One standard angle and one comparison angle were used in each trial. The subject was then asked to identify the larger angle of each pair (two‐alternative forced choice). The subject provided the answer by pressing left key if the first angle was larger or by pressing right key if the second angle was larger. This experiment used a pseudorandom order to present the standard and comparison angles. The standard angle was either the first or the second angle in each pair presented to subjects. Each subject performed all six sessions on the same day. Including the break time between each session, the total scanning time was 60–70 min for each subject.
fMRI Procedures
IAT Task
An IAT session consisted of three IAT blocks of IAT, three MSC blocks and six Rest blocks (Fig. 3). To ensure that all subjects could perform each session accurately, a short set of instructions for each session was given using the microphone system in the MR control room before each session started. As shown in Figure 4, for each IAT block, the trial onset was cued by a green visual cue (a small filled circle; viewing angle, 4.0 × 4.0 deg) on the screen for 0.2 s. The subject was instructed to press the right angle first with his right index finger, and the left angle (i.e., the same size as touched by the right index finger) was pressed passively onto his left index finger at almost the same time (with approximately 90 ms delay). The duration of the first angle pattern presentation was 3 s, and the subject was instructed to return his right index finger back to the initial position when a red visual cue was presented. After a 2‐s inter‐stimulus interval, the same green visual cue was presented, and the subject performed the same procedures as above to touch the second angle in 3 s. A blue visual cue was then presented after the second angle pattern was presented, and the subject was asked to return his right index finger back to the initial position and press the response key for no more than 2 s. The total duration of one angle‐pair discrimination trial was 10 s, and there were three trials in each IAT block. In an IAT session, the subject was instructed to recognize the angle size by both of the angles pressing actively with the right index finger and passively pressed on the left index finger.
Figure 4.
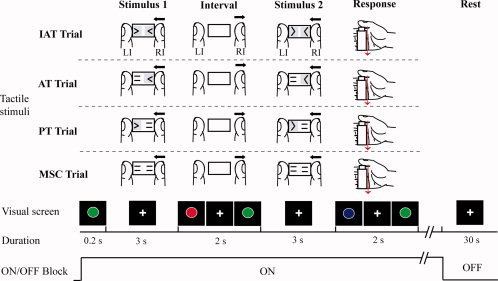
The diagram illustrates one trial paradigm for the IAT, AT, PT and MSC trials. During the experiment, subjects were asked to keep their left index finger motionless and use the right thumb to press the response key upon presentation of the response cue. For Rest trials, subjects were instructed to focus their attention on the screen; no stimulus was presented and no movement was required. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For each MSC block, the subject was instructed to perform the same procedures as in the IAT blocks following the visual cues. However, instead of the angle pattern, the somatosensory control pattern was presented on both sides. The subject was instructed to alternate pressing the response keys without any tactile discrimination in the response period. The MSC block was designed to control for the sensory input, finger motion and task demands of the angle discrimination task. One 30‐s Rest block followed each ON block; however, no stimuli were presented. The participant was instructed to fixate on the white cross and keep his head as still as possible.
AT and PT tasks
The procedures described for the IAT session were also applied during the AT and PT sessions (Fig. 3). However, in the AT session, the angles were presented to the right index finger of subject, and the somatosensory control pattern was presented to the left index finger of subject (Fig. 4). In the PT session, the somatosensory control pattern was presented to the right index finger of subject, and the angle patterns were presented to the left index finger of subject (Fig. 4). The subject was instructed to recognize the unilateral angle pattern and to ignore the somatosensory control pattern on the opposite side. The premise was that the processing of angle discrimination relies on the tactile spatial reference system, whereas the somatosensory control pattern does not require this stage of processing. This somatosensory control pattern was designed to control for the sensory input factors, as well as the task demands of the angle discrimination task. The IAT, AT and PT sessions were performed two times by each subject in pseudorandom order (e.g., session sequence: IAT, AT, PT, IAT, AT, PT).
MR Scanning and Data Processing
Blood oxygenation level‐dependent (BOLD) functional MRI signal data were collected from each subject using a 3.0‐Tesla Siemens Magnetom MR‐Scanner with echo planner imaging (EPI) capability. Standard sequence parameters were used to obtain the functional images as follows: T 2*‐weighted echo planner imaging; repetition time, 3,000 ms; echo time, 35 ms; flip angle, 90°; matrix, 64 × 64; 35 axial slices, 3.5 mm in thickness with a 0.5‐mm interslice gap covering the whole brain; and in‐plane resolution, 3.0 × 3.0 mm. Before the acquisition of functional images, T 1‐weighted high‐resolution anatomical images were obtained (voxel size, 1.0 × 1.0 × 1.2 mm3).
We used SPM5 (Statistical Parametric Mapping 5, Wellcome Department of Imaging Neuroscience, University College London, London, UK) implemented in MATLAB 7.0 (MathWorks, Natick, MA) to process and analyze the fMRI data. The first four volumes of each fMRI run were discarded due to unsteady magnetization. First, the functional images from each run were realigned. The T 1‐weighted anatomical images were then coregistered to the first scan in the functional image, and the resulting coregistered T 1‐weighted anatomical images were normalized to standard T 1 template images as defined by the Montreal Neurological Institute (MNI). Finally, these spatially normalized functional images were smoothed with a full width at half maximum 8‐mm Gaussian kernel.
Behavioural data were collected with Presentation software (version 0.61, Neurobehavioural Systems, Inc., http://www.neurobs.com/) and statistically evaluated with SPSS software (version 12.0J; SPSS Japan, Tokyo, Japan). Statistical analyses of fMRI data were conducted at two levels using the general linear model framework. The task‐related neural activities under each condition were modelled with a boxcar function convoluted with a canonical hemodynamic response function. The time series for each voxel was high‐pass and low‐pass filtered by a canonical hemodynamic response function. We also added motion parameters estimated in the realignment process as confounding effects in the design matrix. Activation maps were generated for the IAT, AT, PT and MSC tasks by contrasting the activity during each of the three tasks with the Rest task: IAT–Rest, AT–Rest, PT–Rest and MSC–Rest (Table I). Activation maps were corrected (P < 0.001) by the false discovery rate approach implemented in SPM5 [Friston et al., 1994, 1995]. To reveal activation maps of regions specifically involved in the angle discrimination component during IAT, AT and PT tasks, we also compared the three tasks with the MSC task: IAT–MSC, AT–MSC and PT–MSC (Table II, P < 0.05, corrected). In addition, we directly contrasted the IAT task activity pattern to those of the AT and PT tasks: IAT–AT and IAT–PT (Table III). Because we had an a priori anatomical hypothesis that the IPS and the SI associated with the performance of angle discrimination by IAT would be active (see Introduction section), we restricted the search space and used a small volume correction (P < 0.05).
Table I.
Main Foci of Task‐Related Activity (n=23)
| Anatomical region/ Functional region | Brodmann area | Side | IAT‐Rest | AT‐Rest | PT‐Rest | MSC‐Rest | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | z value | x | y | z | z value | x | y | z | z value | x | y | z | z value | |||
| Frontal‐parietal areas | ||||||||||||||||||
| CS, poCG/SI,SII | 1/2/3/5/7 | L | −40 | −28 | 50 | 7.36 | −46 | −28 | 46 | 7.31 | −44 | −30 | 40 | 6.98 | 46 | −30 | 50 | 7.35 |
| R | 52 | −28 | 40 | 7.33 | 44 | −40 | 52 | 7.55 | 58 | −24 | 42 | 6.99 | 52 | −28 | 48 | 5.82 | ||
| SMG/SII | 39/40 | L | −54 | −26 | 30 | 7.04 | −44 | −38 | 34 | 5.85 | −54 | −24 | 28 | 6.76 | ||||
| R | 56 | −26 | 32 | 6.95 | 46 | −32 | 34 | 6.04 | ||||||||||
| preCG, iFG/MI | 4 | L | −16 | −2 | 58 | 6.44 | −18 | −8 | 54 | 5.62 | −16 | −4 | 58 | 5.65 | −34 | −18 | 56 | 6.53 |
| R | ||||||||||||||||||
| preCG/ dPM,SMA | 6/8 | L | −22 | 16 | 42 | 6.28 | −26 | 22 | 50 | 5.36 | −26 | 10 | 52 | 5.88 | ||||
| R | 8 | 26 | 42 | 5.61 | 8 | 28 | 52 | 5.60 | 32 | 2 | 54 | 6.70 | ||||||
| SMG, IPS/SII | 7/40 | L | −32 | −52 | 52 | 5.95 | −38 | −40 | 40 | 6.17 | −54 | −24 | 28 | 6.76 | −56 | −24 | 22 | 6.55 |
| R | 32 | −56 | 44 | 6.24 | 36 | −48 | 46 | 7.11 | 46 | −32 | 34 | 6.04 | 38 | −38 | 52 | 5.65 | ||
| iFG,preCG/v,dPM | 6/44 | L | −48 | 10 | 14 | 5.61 | −46 | 6 | 20 | 6.83 | −36 | 26 | 28 | 5.40 | −56 | 6 | 26 | 5.56 |
| R | 50 | 32 | 22 | 6.12 | 56 | 8 | 22 | 6.45 | 44 | 6 | 26 | 6.03 | 52 | 10 | 36 | 5.72 | ||
| iFG, DLPFC | 45/46/47 | L | −46 | 32 | 10 | 5.49 | −32 | 30 | 22 | 5.71 | −32 | 38 | 28 | 5.15 | ||||
| R | 38 | 42 | 30 | 5.93 | 36 | 38 | 2 | 6.12 | 32 | 22 | 12 | 5.71 | 34 | 46 | 30 | 5.25 | ||
| Occipital‐temporal areas | ||||||||||||||||||
| mTG, iOG, LOC | 19/37 | L | −44 | −60 | 6 | 5.22 | −44 | −64 | −12 | 4.87 | −42 | −60 | −4 | 5.84 | ||||
| R | 48 | −58 | −2 | 5.31 | 44 | −54 | −2 | 5.29 | 48 | −52 | 6 | 5.37 | ||||||
| Cerebellum | ||||||||||||||||||
| Cerebellum | L | −30 | −66 | −28 | 5.62 | −34 | −54 | −34 | 5.50 | −26 | −58 | −34 | 5.78 | −32 | −56 | −30 | 4.83 | |
| R | 22 | −46 | −26 | 7.19 | 22 | −48 | −28 | 6.37 | 24 | −46 | −26 | 6.56 | 20 | −66 | −22 | 3.80 | ||
| Other areas | ||||||||||||||||||
| Anterior Insula | L | −30 | 22 | 0 | 5.57 | −30 | 18 | 0 | 5.67 | −26 | 18 | 6 | 5.66 | |||||
| R | 28 | 24 | 6 | 5.59 | 30 | 24 | 8 | 5.61 | 24 | 16 | 4 | 5.65 | ||||||
| Thalamus | L | −18 | −20 | 12 | 5.81 | −14 | −22 | 12 | 6.36 | −18 | −12 | 16 | 5.37 | |||||
| R | 18 | −6 | 12 | 5.71 | 12 | −8 | 8 | 5.86 | 18 | −8 | 10 | 5.41 | ||||||
The MNI coordinates (x, y, z) and z‐values (P<0.001, corrected) are given for the activated clusters. CS, central sulcus; DLPFC, dorsolateral prefrontal cortex; dPM, dorsal premotor cortex; iFG, inferior frontal gyrus; iOG, inferior occipital gyrus; IPS, intraparietal sulcus; LOC, lateral occipital complex; MI, primary motor cortex; mTG, middle temporal gyrus; poCG, postcentral gyrus; preCG, precentral gyrus; SI, primary somatosensory cortex; SII, secondary somatosensory cortex; SMA, supplementary motor area; SMG, supramarginal gyrus; vPM, ventral premotor cortex.
Table II.
Main foci of angle discrimination‐related Activity (n=23)
| Anatomical region/ B Functional region | Brodmann area | Side | IAT‐MSC | AT‐MSC | PT‐MSC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | z value | x | y | z | z value | x | y | z | z value | |||
| Frontal‐parietal areas | ||||||||||||||
| iFG, DLPFC | 45/46/47 | L | −38 | 36 | 2 | 4.03 | −28 | 40 | 2 | 4.10 | −42 | 38 | 0 | 3.18 |
| R | 40 | 40 | −8 | 3.88 | 28 | 36 | 2 | 5.51 | 28 | 26 | −4 | 3.85 | ||
| iFG | 6/44 | L | −28 | 8 | 24 | 3.66 | ||||||||
| R | 54 | 20 | 24 | 3.53 | 56 | 10 | 24 | 3.32 | 52 | 22 | 22 | 3.49 | ||
| sFG / dPM, SMA | 6/8 | L | −4 | 36 | 38 | 3.43 | −8 | 30 | 38 | 3.51 | −8 | 26 | 38 | 3.45 |
| R | 6 | 34 | 40 | 3.08 | 8 | 28 | 48 | 3.49 | 14 | 34 | 48 | 3.15 | ||
| IPS/SII | 7 | L | −36 | −54 | 48 | 2.81 | −46 | −54 | 46 | 2.16 | ||||
| R | 26 | −34 | 36 | 3.36 | 36 | −54 | 42 | 3.49 | 38 | −52 | 42 | 2.15 | ||
| Occipital‐temporal areas | ||||||||||||||
| LOC | 19/37 | L | −46 | −62 | −8 | 2.40 | −48 | −62 | −8 | 2.73 | ||||
| R | 48 | −62 | −6 | 2.28 | 46 | −64 | 6 | 2.66 | 42 | −50 | −8 | 3.08 | ||
| Other areas | ||||||||||||||
| Anterior Insula | L | −30 | 22 | −4 | 3.73 | −30 | 18 | −4 | 3.57 | −28 | 22 | −4 | 3.15 | |
| R | 26 | 30 | 4 | 3.83 | 26 | 24 | 16 | 3.99 | 26 | 22 | 6 | 3.14 | ||
| Thalamus | L | −10 | −14 | 16 | 2.97 | −12 | −8 | 6 | 3.22 | |||||
| R | 10 | −16 | 12 | 2.96 | 14 | −8 | 4 | 2.74 | ||||||
The MNI coordinates (x, y, z) and z‐values are given for the activated clusters.
Table III.
Differences in angle discrimination‐related activity between IAT and AT, IAT and PT (n=23)
| Anatomical region/ Functional region | Brodmann area | Side | IAT‐AT | IAT‐PT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | z value | x | y | z | z value | |||
| CS, poCG/SI, SII | 1/2/3/7 | R | 38 | −26 | 48 | 3.47 | ||||
| SPL /SII | 5/7 | R | 30 | −46 | 58 | 3.31 | ||||
| CS, poCG, SMG/SI, SII | 1/2/3/5/7/40 | L | −42 | −32 | 42 | 3.13 | ||||
| IPS /SII | 7/40 | L | −42 | −44 | 48 | 3.34 | ||||
The MNI coordinates (x, y, z) and z‐values are given for the activated clusters.
RESULTS
Result of the Psychophysical Pilot Experiment
The mean accuracy for each of the comparison angles was computed and plotted as a function of the angular difference between the comparison angle and the standard angle (Fig. 5a). The accuracies were fitted to a logistic function, and the discrimination threshold (75% correct) was computed from the logistic function in a similar manner to our previous study [see Wu et al. 2010]. As shown in Figure 5a, the discrimination threshold was represented by the intersection of the accuracy line (solid line) and the 75% line (dashed line). The angle discrimination threshold was 2.1° for the IAT condition, 3.6° for the AT condition and 3.9° for the PT condition. Moreover, the mean accuracy was 89.6% (standard error of the mean [SEM] = ±1.9) for the IAT condition, 81.3% (SEM = ±1.6) for the AT condition and 80.4% (SEM = ±2.1) for the PT condition (Fig. 5b). A one‐way analysis of variance revealed that accuracy was highest for the IAT condition (F(2,29) = 3.35; P = 0.007). This result indicated that the accuracy of angle discrimination using IAT was significantly higher than when using AT or PT.
Task Performance in the Scanner
An analysis of the behavioural data collected during MRI scanning indicated that the IAT, AT and PT tasks were challenging and of comparable difficulty. The mean accuracies were 82.3% (SEM = ±3.5) for the IAT task, 81.2% (SEM = ±2.5) for the AT task and 80.6% (SEM = ±2.4) for the PT task. A one‐way analysis of variance showed that there was no significant difference among the IAT, AT and PT tasks (F (2,66) = 0.15; P = 0.86).
fMRI Results
As Figure 6 shows, all of the angle discrimination tasks activated a widespread set of brain regions (relative to the Rest baseline used in this study) when both the index fingers were stimulated. To reveal areas showing significantly increased BOLD signals under conditions in which subjects were required to discriminate angles by touch, these signals were compared with signals obtained during the Rest condition. To determine the basic activation patterns during tactile angle discrimination by IAT, AT and PT, we used the IAT–Rest, AT–Rest and PT–Rest contrasts. In addition, to confirm the activation pattern of the MSC task, we also compared MSC with Rest: MSC–Rest. The MNI coordinates of the activated clusters and their significant z‐values (P < 0.001, corrected) are listed in Table I.
Figure 6.
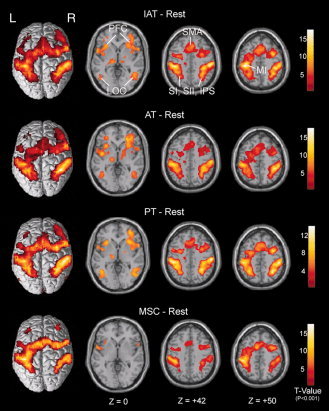
Across‐subject (n = 23) conjunction activation for IAT–Rest, AT–Rest, PT–Rest and MSC–Rest. Areas of significant activation are overlaid on normalized brain slices based on the anatomic T 1 slices. The main areas of activation for the IAT, AT and PT tasks are seen bilaterally in the SI, SII, LOC, SMA, PFC, IPS and left MI regions, but no activation is seen in right MI regions. In contrast, the main areas of activation for the MSC task are seen bilaterally in the SI, SII, PFC and left MI regions, but not in the LOC, SMA and IPS regions, which are associated with higher‐level tactile angle discrimination. Note the absence of primary visual cortex activation. L, left side; R, right side. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 6 shows clusters of activation as labelled in colour overlays on normalized anatomic T 1 slices. As expected for movement of the right hand, the primary motor cortex (MI) adjacent to the central sulcus (CS) in the left hemisphere (i.e., contralateral to the hand used for palpation) was activated strongly in these contrasts. As bilateral tactile stimulation was presented, bilateral activation of the SI in the poCG was observed, and this extended bilaterally into the SII in the poCG and superior parietal lobe, premotor areas, the supplementary motor area (SMA) and the prefrontal cortex (PFC). However, the activation adjacent to and in the IPS and PFC was strongest in the IAT–Rest, AT–Rest and PT–Rest contrasts. In particular, activation of the bilateral LOC was observed in the IAT–Rest, AT–Rest and PT–Rest contrasts but not in the MSC–Rest contrast. In addition, these contrasts also displayed activation of the bilateral anterior insula, thalamus and cerebellum.
To determine the areas specifically activated during angle discrimination without finger (i.e., right index finger and thumb) movement and simple somatosensory input during the IAT, AT and PT tasks, we compared the three angle discrimination tasks with the MSC task: IAT–MSC, AT–MSC and PT–MSC. The MNI coordinates of the activated clusters and their significant z‐values (P < 0.05, corrected) are listed in Table II. As shown in Figure 7, the areas showing significantly increased BOLD signals during the IAT, AT and PT tasks when compared with those of the MSC task. When the angle discrimination tasks were contrasted with the MSC task, the regions associated with the angle discrimination process included the bilateral inferior parietal somatosensory association areas (a part of the IPS) and the SII. Activation was also observed bilaterally in the SMA, LOC and PFC. Finally, the anterior insula was activated bilaterally in all contrasts, and with the exception of the PT–MSC contrast, the thalamus was also activated bilaterally.
Figure 7.
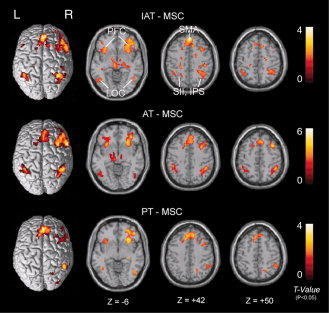
Across‐subject (n = 23) conjunction activation for IAT–MSC, AT–MSC and PT–MSC. Areas of significant activation are overlaid on normalized brain slices based on the anatomic T 1 slices. The main areas of activation for tactile angle discrimination are seen bilaterally in the LOC, SMA and PFC regions. The IPS region was activated bilaterally in both IAT–MSC and AT–MSC contrasts, but activation was only observed in the right IPS in PT–MSC contrasts. L, left side; R, right side. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To determine the cortex regions activated specifically by angle discrimination during IAT, we used direct IAT–AT and IAT–PT contrasts. The MNI coordinates of the activated clusters and their significant z‐values (P < 0.05, corrected) are listed in Table III. As shown in Figure 8a,b, the areas showing significantly increased BOLD signals during the IAT task compared with the AT and PT tasks were the right CS/poCG and IPS for IAT–AT and the left CS/poCG and IPS for IAT–PT. To investigate the differences in brain activation during angle discrimination by IAT, AT and PT, regions of interest (ROIs) with spheres 8 mm in diameter in the CS/poCG and IPS were identified for all subjects using the IAT–AT and IAT–PT contrasts. In addition, we plotted the histograms using percentage signal change between stimulation and MSC periods in Figure 8a,b. A t‐test showed that the percentage signal changes of both the right CS/poCG and IPS were significant for the IAT and AT tasks (P = 0.009; P = 0.032, respectively). The percentage signal changes of both the left CS/poCG and IPS were also significant for the IAT and PT tasks (P = 0.017; P = 0.021, respectively).
Figure 8.
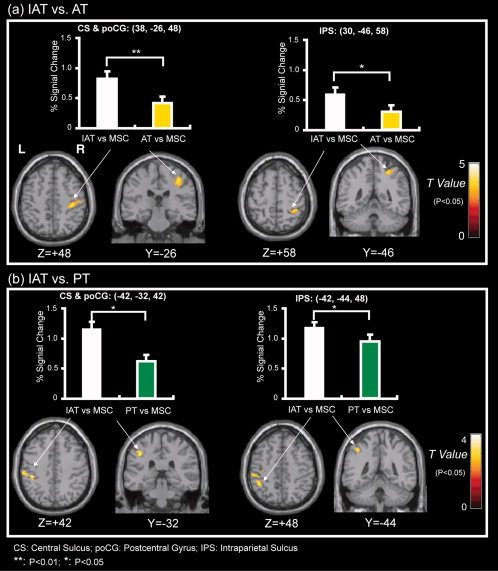
Across‐subject (n = 23) conjunction activation for IAT–AT and IAT–PT. Areas of significant activation are overlaid on normalized brain slices based on the anatomic T 1 slices. The ROIs in the CS, poCG and IPS were identified for all subjects using the IAT–AT and IAT–PT contrasts. The bar graph above shows the percentage change in BOLD signals between angle stimulation and MSC periods. (a) The main area of activation for IAT–AT. The results show that the right SI and IPS were activated more extensively by IAT than AT tasks. (b) The main activation area for IAT–PT. The results show that the left SI and IPS were activated more extensively by IAT than PT tasks. L, left side; R, right side. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
This is the first study to describe neural activation associated with tactile angle discrimination by IAT. The tactile angle discrimination task in this study involved the activation of a widely distributed cerebral network that includes somatosensory, premotor, supplementary motor, PFC and multisensory regions (Table I and Fig. 6). The main finding of this study suggests that although the areas showing activity during the IAT, AT and PT tasks compared with the MSC task revealed a similar cerebral network, unilateral activation of the IPS and SI was significantly higher during the IAT task than during the AT and PT tasks (Table III and Fig. 8). As hypothesized, a part of the IPS and the SI was activated strongly by IAT; in contrast, no increased activation in other low‐level tactile related regions was observed.
Behavioural Performance and Task Design
In the pilot experiment, we found that the accuracy of angle discrimination by IAT was significantly higher than the accuracies of AT and PT. Because these angles could be felt with the fingertip, the critical feedback was entirely due to cutaneous responses. This result suggests that the cutaneous input from both the right and left index fingers contributes to angle discrimination during IAT.
The present fMRI experiment was designed to examine the neural correlates of tactile angle discrimination processing under AT, PT and IAT conditions. The MSC pattern consisted of double parallel convex lines on the planar surface, and there was no change in orientation or spatial translation during the whole experiment. Comparison of the angle discrimination tasks with the MSC task revealed that multiple brain areas were activated. Because the MSC task was easier than the other conditions, the activation might reflect differences in attentional demands as well as in the cognitive and discriminative processing between the task conditions.
Moreover, to ensure the comparison angles used in the fMRI experiment could be discriminated by the subject using the prescribed motor sequence (Fig. 4), we selected four comparison angles which were suprathreshold for the IAT task from the psychophysical pilot experiment. Accordingly, the angle discrimination was easier in the IAT task compared with the AT and PT tasks in the fMRI experiment. In all of the tactile tasks, the sequential angle discrimination and responses were easy to perform, as suggested by the observation that all of the tasks were performed with high accuracy (around 80%). Although there was a trend for the accuracy of IAT task in the MRI scanner to be higher than that of AT and PT tasks, these did not reach significance (as described in the Results section). Hence, contrasting the angle discrimination tasks with the MSC tasks should highlight the angle discrimination processing, and contrasting the IAT task with the AT and PT tasks should highlight the specific processing for IAT.
Activation Common to All Tactile Tasks
The tactile procedures activated a widespread set of brain regions when both index fingers were stimulated relative to the Rest baseline (Fig. 6). Because each subject was instructed to touch the tactile patterns and press the response keys with their right index finger and thumb, the left MI adjacent to the CS was activated strongly in these contrasts. The contralateral activation in this area corresponded with the results of Weiller et al. [ 1996] and Mima et al. [ 1999], which showed strong activation in the contralateral MI during a lateral simple motor task. In addition, similar activation was not apparent in the right MI because all of the subjects were instructed to keep their left hands motionless during these experiments.
As bilateral tactile stimulation was presented in the IAT, AT and PT tasks, bilateral activation in the SI was observed, and this extended bilaterally into the SII and superior parietal lobe, premotor areas, SMA, LOC, IPS and PFC (Figs. 6, 7). These results are consistent with the results of previous human neuroimaging studies with PET and fMRI [Blatow et al., 2007; Bodegård et al., 2001; Newmana et al., 2005; Peltier et al., 2007; Reed et al., 2004; Saito et al., 2003; Van Boven et al., 2005]. In particular, partial activation of the IPS and LOC was not apparent in the MSC–Rest contrasts. These areas are known to compute both of two‐ and three‐dimensional shape representations during tactile shape discrimination processing [Bodegård et al., 2001; Kitada et al., 2006; Miquée et al., 2008; Stilla et al., 2007]. Subjects were asked to discriminate the size of the angles that they were presented with in the IAT, AT and PT tasks; however, no tactile discrimination processes were required in the MSC task. In addition, the visual cues presented in this study activated the primary visual area, which was not apparent in the above contrasts. This is because the subjects were asked to open their eyes and fixate on the white cross on the screen during the Rest blocks. Activation in the primary visual area was cancelled when the tactile tasks were compared with Rest.
Activation of Angle Discrimination Processing
The areas specifically involved in the angle discrimination component without finger movement and simple somatosensory input appeared by contrasting IAT, AT and PT to MSC (Fig. 7). We found a relative preference for tactile angle stimuli discrimination processing in the SI, SII, SMA, LOC and PFC regions in both hemispheres. However, bilateral activation of the IPS was observed in the IAT–MSC and AT–MSC contrasts, but the PT–MSC contrast only led to significant activation of the IPS in the right hemisphere (Table II and Fig. 7). The pattern of activation of all of these areas is in agreement with earlier published reports of cortical activation during tactile shape and grating discrimination procedures [Burton and Sinclair, 2000; Fabri et al., 2005; Kitada et al., 2006; Zhang et al., 2005]. The cortex around the IPS has been previously reported to be active during tactile shape and grating recognition [Amedi et al., 2001; Bodegård et al., 2001; Newmana et al., 2005; Van Boven et al., 2005]. In addition, a previous fMRI study [Kitada et al., 2006] reported that right hand tactile orientation stimulation activated the bilateral IPS; however, the same orientation stimulation on the left hand only produced a greater activation relative to the sensorimotor control task in the right IPS. LOC activity in this study was also selective for object geometry and was relative to the angle when stimuli were presented haptically, as was described previously [Amedi et al., 2001, 2002; Newmana et al., 2005]. Previous studies have shown that parts of the LOC in both the hemispheres are active during both visual and haptic object explorations [Amedi et al., 2001, 2002; James et al., 2002]. A portion of the bilateral prefrontal cortical area (PFC) was more active in the angle discrimination tasks than during MSC. This finding is consistent with previous studies that demonstrated that the PFC is a part of a network of areas involved in tactile working memory [Kitada et al., 2005; Kostopoulos et al., 2007; Reed et al., 2005; Romo et al., 1999; Van Boven et al., 2005]. The bilateral activation of PFC areas found in this study was interpreted as angle discrimination processing during the IAT, AT and PT tasks.
Neural Activation Specific for IAT
In this study, we found that the tactile angle discrimination procedures activated similar brain regions under intra‐active, AT and PT conditions. In contrast, as we hypothesized, strong activation in the contralateral part of the IPS and SI areas extending posteriorly into the SII was observed in the IAT–AT and IAT–PT contrasts (Fig. 8 a,b).
Neurons in the SI are activated when the skin is touched, and the human SI cortex is activated, for the most part, by contralateral tactile stimuli [Iwamura, 1998]. The authors of a previous PET study [Bodegård et al., 2001] suggested that areas 3b and 1 are activated by the basic signals from skin mechanoreceptors and that this information is further computed into spatial shape representations in area 2. In this study, the subjects were able to discriminate the size of the angle pattern from the right (touching) and left (touched) sides during the IAT task. In contrast, the subjects were only exposed to the unilateral angle pattern during the AT and PT tasks. Even when the somatosensory control pattern was available to be touched (i.e., AT tasks), or to touch the subject (i.e., PT task), on the opposite side, the subjects can not obtain any useful information related to angle discrimination. Therefore, our results suggest that the tactile angle information processing that occurred during the IAT task caused a strong activation of the contralateral SI area when the two‐dimensional tactile angles were used in our study.
The IPS has been demonstrated to be important for visual orientation judgment [Eacott and Gaffan, 1991]. However, this area is also known to be recruited during two‐ and three‐dimensional shape discrimination [Bodegård et al., 2001; Iwamura, 1998; Newmana et al., 2005] Iwamura [ 1998] and Bodegård et al. [ 2001] have indicated that the IPS is a high‐level area and that it plays an important role in the computation and elaborate reconstruction of tactile shapes. Recent fMRI studies [Kitada et al., 2006; Van Boven et al., 2005] have also shown that the bilateral IPS subregions were activated by tactile grating orientation (two‐dimensional) discrimination tasks compared with relative sensorimotor control tasks. For example, Kitada et al. [ 2006] demonstrated that the contrast of a tactile orientation task versus a sensorimotor control task significantly activated the bilateral areas around the IPS when the tactile grating orientation stimulus was presented to the right fingertip. However, contrast of the stimulation of the left finger (like the right orientation stimulation) versus the sensorimotor control task only activated the right IPS. In this study, we also observed similar patterns of activation around the IPS when the AT (right angle stimulation only) or PT (left angle stimulation only) task was contrasted with the MSC task, as described in the Kitada et al. [ 2006] study (Table II and middle bottom panel of Fig. 7). In contrast, the subjects in the IAT task likely obtained more information about the angle from both the right and left fingers. Therefore, the strong activation in the contralateral part of the IPS, as shown in Table III and Figure 8, might be crucial in increasing tactile angle information during an IAT task.
Based on our results, we conclude that strong activation of the contralateral part of the IPS and SI areas may be related to processes that link the presented angle patterns to the right and left index fingers during the IAT task. In other words, the subjects perceived more tactile spatial information in the IAT task, which activated the contralateral IPS and SI significantly, even though the angle patterns were presented to the contralateral side in the AT and PT tasks. In conclusion, these results provide evidence that both hemispheres contribute to the performance of the intra‐active task.
Acknowledgements
We thank Dr. Takanori Kochiyama with the Brain Activity Imaging Center, Advanced Telecommunications Research Institute International, Kyoto, Japan, for experimental task design and fMRI data analysis and the students Qingyuan He of Peking University Third Hospital and Liang Zhou of Neuroscience Research Institute in Peking University Health Science Center who helped with the data acquisition.
REFERENCES
- Amedi A, Malach R, Hendler T, Peled S, Zohary E ( 2001): Visuo‐haptic object‐related activation in the ventral visual pathway. Nat Neurosci 4: 324–330. [DOI] [PubMed] [Google Scholar]
- Amedi A, Jacobson G, Hendler T, Malach R, Zohary E ( 2002): Convergence of visual and tactile shape processing in the human lateral occipital complex. Cereb Cortex 12: 1202–1212. [DOI] [PubMed] [Google Scholar]
- Blatow M, Nennig E, Durst A, Sartor K, Stippich C ( 2007): fMRI reflects functional connectivity of human somatosensory cortex. NeuroImage 37: 927–936. [DOI] [PubMed] [Google Scholar]
- Bodegård A, Geyer S, Grefkes C, Zilles K, Roland PE ( 2001): Hierarchical processing of tactile shape in the human brain. Neuron 31: 317–328. [DOI] [PubMed] [Google Scholar]
- Bolanowski SJ, Verrillo RI, McGlone F ( 1999): Passive, active and intra‐active (self) touch. Somatosens Motor Res 16: 304–311. [DOI] [PubMed] [Google Scholar]
- Bolanowski SJ, Verrillo RI, McGlone F ( 2004): Passive, active and intra‐active (self) touch. Behav Brain Res 148: 41–45. [DOI] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ ( 2000): Attending to and remembering tactile stimuli: A review of brain imaging data and single‐neuron responses. J Clin Neurophysiol 17: 575–591. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D ( 1991): The role of monkey inferior parietal cortex in visual discrimination of identity and orientation of shapes. Behav Brain Res 46: 95–98. [DOI] [PubMed] [Google Scholar]
- Fabri M, Polonara G, Salvolini U, Manzoni T ( 2005): Bilateral cortical representation of the trunk midline in human first somatic sensory area. Hum Brain Mapp 25: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Jezzard P, Turner R ( 1994): Analysis of functional MRI time‐series. Hum Brain Mapp 1: 153–171. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J‐B, Frith CD, Frackowiak RSJ ( 1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Gardner EP, Kandel ER ( 2000): Touch In: Kandel ER, Schwartz TM, Jessell TM, editors. Principles of Neural Science. New York: McGraw‐Hill; pp 451–471. [Google Scholar]
- Gibson JJ ( 1962): Observation on active touch. Psychol Rev 69: 477–491. [DOI] [PubMed] [Google Scholar]
- Harada T, Saito DN, Kashikura K, Sato T, Yonekura Y, Honda M, Sadato N ( 2004): Asymmetrical neural substrates of tactile discrimination in humans: A functional magnetic resonance imaging study. J Neurosci 24: 7524–7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller MA ( 1984): Active and Passive touch: The influence of exploration time on form recognition. J Gen Psychol 110: 243–249. [DOI] [PubMed] [Google Scholar]
- Hlushchuk Y, Hari R ( 2006): Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J Neurosci 26: 5819–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura Y ( 1998): Hierarchical somatosensory processing. Curr Opin Neurobiol 8: 522–528. [DOI] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Servos P, Menon RS, Goodale MA ( 2002): Haptic study of three‐dimensional objects activates extrastriate visual areas. Neuropsychologia 40: 1706–1714. [DOI] [PubMed] [Google Scholar]
- Jones EG ( 1986): Connectivity of the primate sensory‐motor cortex In: Jones EG, Peters A, editors. Cerebral Cortex, Sensory‐Motor Areas and Aspects of Cortical Connectivity, Vol. 5 New York: Plenum Press; pp 113–215. [Google Scholar]
- Katz D ( 1989): The World Touch. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Kitada R, Hashimoto T, Kochiyama T, Kito T, Okada T, Matsumura M, Lederman SJ, Sadato N ( 2005): Tactile estimation of the roughness of gratings yields a graded response in the human brain: An fMRI study. NeuroImage 25: 90–100. [DOI] [PubMed] [Google Scholar]
- Kitada R, Kito T, Saito DN, Kochiyama T, Matsumura M, Sadato N, Lederman SJ ( 2006): Multisensory activation of the intraparietal area when classifying grating orientation: A functional magnetic resonance imaging study. J Neurosci 26: 7491–7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzky RL, Lederman SJ, Metzger VA ( 1985): Identifying objects by touch: An “expert system.” Percept Psychophys 37: 299–302. [DOI] [PubMed] [Google Scholar]
- Kostopoulos P, Albanese MC, Petrides M ( 2007): Ventrolateral prefrontal cortex and tactile memory disambiguation in the human brain. PNAS 104: 10223–10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima T, Sadato N, Yazawa S, Hanakawa T, Fukuyama H, Yonekura Y, Shibasaki H ( 1999): Brain structures related to active and passive finger movement in man. Brain 22: 1989–1997. [DOI] [PubMed] [Google Scholar]
- Miquée A, Xerri C, Rainville C, Anton JL, Nazarian B, Roth M, Zennou‐Azogui Y ( 2008): Neuronal substrates of haptic shape encoding and matching: A functional magnetic resonance imaging study. Neuroscience 152: 29–39. [DOI] [PubMed] [Google Scholar]
- Newmana SD, Klatzky RL, Lederman SJ, Just MA ( 2005): Imagining material versus geometric properties of objects: An fMRI study. Cognit Brain Res 23: 235–246. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Peltier S, Stilla R, Mariola E, LaConte S, Hu X, Sathian K ( 2007): Activity and effective connectivity of parietal and occipital cortical regions during haptic shape perception. Neuropsychologia 45: 476–483. [DOI] [PubMed] [Google Scholar]
- Pleger B, Ruff CC, Blankenburg F, Bestmann S, Wiech K, Stephan KE, Capilla A, Friston KJ, Dolan RJ ( 2006): Neural coding of tactile decisions in the human prefrontal cortex. J Neurosci 26: 12596–12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CL, Shoham S, Halgren E ( 2004): Neural substrates of tactile object recognition: An fMRI study. Hum Brain Mapp 21: 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CL, Klatzky RL, Halgren E ( 2005): What vs. where in touch: An fMRI study. NeuroImage 25: 718–726. [DOI] [PubMed] [Google Scholar]
- Roland PE, O'sillivan B, Kawashima R ( 1998): Shape and roughness activate different somatosensory areas in the human brain. PNAS 95: 3295–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Brody CD, Hernández A, Lemus L ( 1999): Neuronal correlates of parametric working memory in the prefrontal cortex. Nature 399: 470–473. [DOI] [PubMed] [Google Scholar]
- Saito DN, Okada T, Morita Y, Yonekura Y, Sadato N ( 2003): Tactile‐visual cross‐modal shape matching: A functional MRI study. Cognit Brain Res 17: 14–25. [DOI] [PubMed] [Google Scholar]
- Stilla R, Deshpande G, LaConte S, Hu X, Sathian K ( 2007): Posteromedial parietal cortical activity and inputs predict tactile spatial acuity. J Neurosci 27: 11091–11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE, Stanford TR ( 2008): Multisensory intergration current issues from the perspective of the single neuron. Nat Rev Neurosci 9: 255–266. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Ingeholm JE, Beauchamp MS, Bikle PC, Ungerleider LG ( 2005): Tactile form and location processing in the human brain. PNAS 102: 12601–12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrillo RI, Bolanowski SJ, McGlone F ( 2003): Intra‐ and interactive touch on the face. Somatosens Motor Res 20: 3–11. [DOI] [PubMed] [Google Scholar]
- Weiller C, Juptner M, Fellows S, Rijntjes M, Leonhardt G, Kiebel S ( 1996): Brain representation of active and passive movements. NeuroImage 4: 105–110. [DOI] [PubMed] [Google Scholar]
- Wu J, Yang J, Ogasa T ( 2010): Raised Angle Discrimination under passive hand movement. Perception 39: 993–1006. [DOI] [PubMed] [Google Scholar]
- Yang J, Wu J ( 2010): Development and evaluation of a multi‐model tactile pattern delivery device for fMRI study. J Inform 13: 1823–1832. [Google Scholar]
- Zhang M, Mariola E, Stilla R, Stoesz M, Mao H, Hu X, Sathian K ( 2005): Tactile discrimination of grating orientation: fMRI activation patterns. Hum Brain Mapp 25: 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]


