Abstract
A repetition–suppression functional magnetic resonance imaging paradigm was used to explore the neuroanatomical substrates of processing two types of acoustic variation—speaker and accent—during spoken sentence comprehension. Recordings were made for two speakers and two accents: Standard Dutch and a novel accent of Dutch. Each speaker produced sentences in both accents. Participants listened to two sentences presented in quick succession while their haemodynamic responses were recorded in an MR scanner. The first sentence was spoken in Standard Dutch; the second was spoken by the same or a different speaker and produced in Standard Dutch or in the artificial accent. This design made it possible to identify neural responses to a switch in speaker and accent independently. A switch in accent was associated with activations in predominantly left‐lateralized areas including posterior temporal regions, including superior temporal gyrus, planum temporale (PT), and supramarginal gyrus, as well as in frontal regions, including left pars opercularis of the inferior frontal gyrus (IFG). A switch in speaker recruited a predominantly right‐lateralized network, including middle frontal gyrus and prenuneus. It is concluded that posterior temporal areas, including PT, and frontal areas, including IFG, are involved in processing accent variation in spoken sentence comprehension. Hum Brain Mapp, 2012. © 2010 Wiley Periodicals, Inc.
Keywords: repetition–suppression, speech, speaker, auditory cortex
INTRODUCTION
The human speech comprehension system seems to effortlessly extract the linguistic message from the acoustic signal. This is a remarkable feat, given the variability inherent to this signal, for instance, as a result from speaker differences [Peterson and Barney,1952]. These differences are not only anatomical/physiological in nature, but also emerge from social factors such as a speaker's geographical background and socioeconomic status. These social factors result in different spoken varieties of the standard language, which can be exemplified by phonetic and phonological variation in the sounds of a language [Wells,1982]. For instance, the Dutch word bed (bed) is pronounced with the vowel/ε/in the western part of the Netherlands, but with a vowel close to/a/as in bad (bath) in the south‐eastern part [Adank et al.,2007]. Listeners are continuously confronted with ambiguities in speech that they have to resolve perceptually (or, normalize) to extract the linguistic message [Nearey,1989]. This disambiguating process requires cognitive effort; reflected in longer response times for comprehension of sentences spoken with an unfamiliar regional or foreign accent compared to listeners' native accent [Adank et al.,2009; Floccia et al.,2006; Rogers et al.,2004; Van Wijngaarden,2001].
Behaviorally, listeners process accented speech by shifting their phonetic boundaries to match those of the speaker, when confronted with a speaker whose speech displays accent or specific idiosyncrasies [Evans and Iverson,2003; Norris et al.,2003]. It has finally been suggested that the adaptation process involves pattern matching mechanisms [Hillenbrand and Houde,2003; Nearey,1997] that are based on statistical learning [Nearey and Assmann,2007].
The neural bases underlying processing of accent‐related variation are largely unknown. It has been hypothesized that the planum temporale (PT) is involved in processing complex spectrotemporal variation in speech [Griffiths and Warren,2002; Warren et al.,2005]. PT is a large region in the temporal lobe, posterior to Heschl's gyrus in the superior temporal gyrus (STG), and represents the auditory association cortex. PT is involved in elementary acoustic pattern perception [Binder et al.,2000; Giraud et al.,2000; Hall et al.,2002; Penhune et al.,1998], spatial processing [Warren et al.,2005] auditory scene analysis [Bregman,1990], musical perception [Zatorre et al.,1994], and, more specifically, speech perception, [Binder et al.,1996; Giraud and Price,2001; Shtyrov et al.,2000]. PT is hypothesized to be involved in continuous updating of incoming traces required for phonological working memory and speech production [Binder et al.,2000]. Griffiths and Warren [2002] propose a functional model for the processing in PT of spectrotemporally complex sounds that change over time. PT continuously analyses these incoming signals and compares them with those previously experienced using pattern matching. Griffiths and Warren furthermore suggest that PT is associated with “…constructing a transient representation of the spectrotemporal structures embodied in spoken words, regardless of whether these are heard or retrieved from lexical memory (i.e., a phonological template.)”.
The present study aimed to provide insights into the neural locus of processing accent and speaker variation using functional magnetic resonance imaging (fMRI). We investigated whether PT is involved in disambiguation processes required for understanding accented speech using a repetition–suppression fMRI design. Repetition suppression is based on the finding that the repeated presentation of a stimulus induces a decrease in brain activity. This decrease can be detected using fMRI [Grill‐Spector and Malach,2001; Grill‐Spector et al.,1999]. This technique can be used to identify brain areas involved in processing specific stimulus characteristics. By varying the property that is repeated, the neural bases involved in processing that specific property are uncovered. For example, repetition–suppression paradigms have been used to locate the neural substrates of speaker processing [Belin and Zatorre,2003], spoken syllables [Zevin and McCandliss,2005], spoken words [Orfanidou et al.,2006], and spoken sentences [Dehaene‐Lambertz et al.,2006].
In the experiment, listeners heard two sentences presented in quick succession. The first sentence was spoken in Standard Dutch; the second sentence was spoken by the same or a different speaker in Standard Dutch or in a novel accent of Dutch. This design allowed us to identify neural responses to a switch in speaker, in accent, or both. Recordings were made for a male and a female speaker of Dutch to maximize the amount of variation related to anatomical/physiological differences between speakers. Accent and speaker were implemented in a factorial design with both factors crossed, allowing us to determine the neural bases associated with processing both variation types independently. Phonological/phonetic variation was introduced into the speech signal by creating an artificial, nonexisting, accent. Using a nonexisting accent has two advantages: first, speaker and accent were not confounded as both factors were manipulated independently. Second, the use of a novel accent ensures that all listeners are equally unfamiliar with the accent. This is necessary as familiarity with an accent affects language comprehension: processing slow when listeners are unfamiliar with the accent [Floccia et al.,2006], especially in noisy conditions [Adank et al.,2009].
MATERIALS AND METHODS
Participants
Twenty participants (14F and 6M, mean 21.2 years; range, 18–26 years) took part in the study, although the data from two (2F) were subsequently excluded due to (i) to excessive head movement (>3 mm) and (ii) an unexpected brain anomaly. The remaining 18 participants were right‐handed, native monolingual speakers of Dutch, with no history of oral or written language impairment, or neurological or psychiatric disease. All gave written informed consent and were paid for their participation. The study was approved by the local ethics committee.
Experiment and Design
The present repetition–suppression fMRI experiment used a miniblock design, with continuous scanning. The choice of continuous rather than sparse sampling was based on a trade‐off between the ability to reliably detect suppression in the blood oxygen level‐dependent (BOLD) signal and the length of the experiment. Continuous sampling results in both acoustic masking of the auditory sentences [Shah et al.,1999] and contamination of the BOLD signal response in auditory regions [Bandettini et al.,1998; Hall et al.,1999; Talavage et al.,1999]. The former, however, was not a problem as a relatively quiet acquisition sequence (∼80 dB) coupled with sound attenuating headphones (∼30 dB attenuation) ensured that the sentences were easily heard. Indeed, all participants confirmed their ability to hear and understand the sentences during a familiarization session in which only sentences in Standard Dutch (not included in the main experiment) were presented. Contamination of the BOLD signal was potentially more problematic, because scanner noise elevates BOLD responses in auditory areas [Gaab et al.,2006; Hall et al.,1999], and these effects need not be identical across regions [Tamer et al.,2009; Zaehle et al.,2007]. In the current experiment, however, we were specifically interested in relative reductions in BOLD signal. As a result, elevated BOLD responses may not be problematic; only responses driven to saturation levels by the scanner noise would reduce sensitivity, and previous studies have clearly shown that typical EPI sequences reduce, but do not eliminate, the dynamic range of the BOLD response [Gaab et al.,2006; Zaehle et al.,2007]. Moreover, to avoid scanner‐noise contamination and ensure an adequate sampling of the evoked hemodynamic response function requires silent periods between volume acquisitions lasting between 16 and 32 s [Eden et al.,1999; Edmister et al.,1999; Hall et al.,1999; Hickok et al.,1997; Tamer et al.,2009]. A sparse design would therefore result in the experiment lasting up to twice as long as using a continuous design, which was deemed likely to reduce participants' ability to attend to the sentences. Consequently, we chose to use a continuous sampling paradigm.
Listeners were presented with two sentences in quick succession in four conditions as in Table I. The first sentence was always spoken in Standard Dutch, followed by the same sentence spoken by the same speaker in the same accent (condition SS, same speaker, and same accent), spoken by a different speaker in the same accent (DS, different speaker, same accent, representing a switch of speaker), by the same speaker in a different accent (DS, same speaker, different accent, representing a switch of accent), or finally by a different speaker in a different accent (DSDA, different speaker, different accent, representing a switch of speaker and accent). Thirty‐two sentences were presented per condition in eight miniblocks of four stimuli. Participants were required to listen to the sentences and to pay close attention. There was no additional task.
Table I.
Experimental conditions: speaker and accent of the second sentence in the design
| Name | Speaker | Accent |
|---|---|---|
| SS | Same speaker | Same accent |
| DS | Different speaker | Same accent |
| DA | Same speaker | Different accent |
| DSDA | Different speaker | Different accent |
The first sentence was always spoken in Standard Dutch.
Stimulus Materials
The total stimulus set consisted of 256 sentences. The sentences were taken from the speech reception threshold corpus or SRT [Plomp and Mimpen,1979]. This corpus has been widely used for assessing intelligibility of different types of stimuli, for example, for speech in noise [Zekveld et al.,2006] or foreign‐accented speech [van Wijngaarden et al.,2002]. The SRT consists of 130 sentences designed to resemble short samples of conversational speech. All consist of maximally eight or nine syllables and do not include words longer than three syllables. Two versions of 128 of the SRT‐sentences were recorded in Standard Dutch and in the novel accent. The novel accent was designed to merely sound different from Standard Dutch and was not intended to replicate an existing accent.
The novel accent, also used in [Adank and Janse,2010], was created by instructing the speaker to read sentences with an adapted orthography. The orthography was systematically altered to achieve the following changes in all 15 Dutch vowels: the switching of all tense‐lax vowel pairs (e.g.,/e:/was pronounced as/ε/and vice versa),/u/(not having a lax counterpart in Dutch) was pronounced as /Y/, and all diphthongal vowels were realized as monophthongal vowels (e.g.,/εi/was pronounced as/e:/). All changes are listed in Table II, and all sentences are listed in Appendix I. Only vowels bearing primary or secondary stress were included in the conversion of the orthography. An example of a sentence in Standard Dutch and a converted version is given below, including a broad phonetic transcription using the International Phonetic Alphabet [IPA,1999]:
Table II.
Intended vowel conversions for obtaining the novel accent
| Orthography | Phonetic (IPA) |
|---|---|
| a → aa | /a/ → /a:/ |
| aa → a | /a:/ → /a/ |
| e → ee | /ε/ → /e:/ |
| ee → e | /e:/ → /ε/ |
| i → ie | /I/ → /i:/ |
| ie → i | /i:/ → /I/ |
| o → oo | / / → /o:/ / → /o:/ |
| oo →o | /o:/ → / / / |
| uu → u | /y:/ → /Y/ |
| u → uu | /Y/ → /y:/ |
| oe → u | /u/ → /Y/ |
| eu →u | /ø/ → /Y/ |
| au → oe | /ou/ → /u/ |
| ei →ee | /εi/ → /e:/ |
| ui → uu | /œy/ → /y:/ |
The left column shows the altered orthography in Standard Dutch, and the right column shows the intended change in pronunciation of the vowel in broad phonetic transcription, using the International Phonetic Alphabet (IPA,1999).
Standard Dutch: “De bal vloog over de schutting” 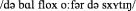 [The ball flew over the fence]
[The ball flew over the fence]
After conversion: “De baal flog offer de schuuttieng” 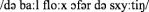
These sentences were recorded in both accents by a female and a male speaker of Dutch. The recordings were made in a sound‐attenuated booth. Sentences were presented on the screen of a desktop computer. The speakers were instructed to read the sentences aloud as a declarative statement and with primary sentence stress on the first noun, as to keep the intonation pattern relatively constant across all sentences. First, two tokens were recorded of each Standard Dutch version followed by one token of the artificial accent version. Every sentence in the artificial accent was repeated until it was pronounced as instructed and sounded as fluent as the Standard Dutch sentences. The speakers were monitored from sentence to sentence during recording by the first author (a trained phonetician). After recording, the sentences were checked by the first author, and all sentences with mistakes were re‐recorded, using the same procedure. Finally, 14 additional sentences were recorded in Standard Dutch for the control task in the fMRI experiment. All sentences were recorded to hard disk directly via an Imix DSP chip plugged into the USB port of an Apple Macbook.
Next, all sentences were saved into separate sound files with begin and end trimmed at zero crossings and resampled at 16 kHz. Subsequently, the speech rate differences across all six tokens of a specific sentence (two Standard Dutch tokens and one artificial accent token, for two speakers) were equalized, so that every token for a given sentence had the same length. This ensured that both sentences in each repetition–suppression stimulus pair were equally long. First, for each of the 128 sentences (four experimental conditions × 32 sentences), the average duration across all six tokens for that sentence was calculated. Second, each token was digitally shortened or lengthened to fit the average length for the sentence, using PSOLA [Moulines and Charpentier,1990], as implemented in the Praat software package, version 4.501 [Boersma and Weenink,2003]. Second, every sentence was peak‐normalized at 99% of its maximum amplitude and then saved at 70 dB (SPL).
Procedure
The participants listened to the stimuli and were instructed to pay close attention and told that they would be tested after the experiment. A single trial (see Fig. 1) began with a tone signal of 200 ms, followed by a pause of 300 ms, the presentation of the first sentence of the pair (always in Standard Dutch), a pause of 300 ms, and the second sentence of the pair. The interstimulus‐interval was effectively jittered by adding a waiting period that was randomly varied between 4,000 and 6,000 ms to the offset of the second sentence. The average sentence duration was 2,495 ms (range, 2,074–3,064 ms).
Figure 1.

Timeline of the presentation of a single sentence pair.
To improve statistical power, trials occurred in short blocks of four sentences of one experimental condition, followed by a silent baseline trial (duration randomly varied from 4,000 to 6,000 ms). The identity of the speaker did not vary across the first sentences of a pair in a miniblock. Every unique sentence was presented only once in the experiment, and all were presented in a semirandomized order and counterbalanced across conditions, so that the 128 sentences were presented in all four conditions across participants. The presentation of the 128 sentence trials and the 32 silent trials lasted 23 min. Before the main experiment, listeners were presented with six sentences (not included in the main experiment) spoken by the same speaker as in the main experiment to ensure that the sentences were audible over the scanner noise and presented at a comfortable loudness level (adapted for each individual participant).
Participants were informed that the sentences were presented in pairs and that the first sentence of a pair was always spoken in Standard Dutch and that the second one often varied in speaker, accent, or both. They were also informed that the sentence itself did not vary, that is, that they would be presented with two tokens of the same sentence within a stimulus pair. Presenting participants with a sentence in Standard Dutch ensured that they would be able to understand the linguistic message and second that they would not have to make additional cognitive efforts to understand the linguistic content of the second sentence in a pair.
After the main experiment, participants heard 28 single sentences in Standard Dutch. Half of these sentences had not been presented before (these sentences were not part of the SRT corpus, but had been constructed to resemble the SRT sentences as much as possible)—and the other half had been presented in the main experiment. After the main experiment had finished, participants responded through a button‐press with their right index finger when they had heard the sentence in the main experiment. Stimulus presentation was performed using Presentation (Neurobehavioral Systems, Albany, CA), running on a Pentium 4 with 2 GB RAM, and a 2.8 GHz processor.
Functional MRI Data Acquisition
Whole‐brain imaging was performed at the Donders Centre for Brain, Cognition, and Behaviour, Centre for Cognitive Neuroimaging, at a 3T MR scanner (Magnetom Trio, Siemens Medical Systems, Erlangen, Germany). The sentences were presented over electrostatic headphones (MRConFon, Magdeburg, Germany) during continuous scanner acquisition (GE‐EPI, repetition time = 2,282 ms; echo time = 35 ms; 32 axial slices; slice thickness = 3 mm; voxel size = 3.5 × 3.5 × 3.5 mm; field of view = 224 mm; flip angle = 70°)—in other words, over the noise of the scanner. All participants confirmed their ability to hear and understand the sentences during a short practice session when the scanner was on. All functional images were acquired in a single run. Listeners watched a fixation cross that was presented on a screen and viewed through a mirror attached to the head coil.
After the acquisition of functional images, a high‐resolution structural scan was acquired (T1‐weighted MP‐RAGE, 192 slices, repetition time = 2,282 ms; echo time = 3.93 ms; field of view = 256 mm, slice thickness = 1 mm). Total scanning time was 40 min.
Analyses
The neuroimaging data were preprocessed and analyzed using SPM5 (Wellcome Imaging Department, University College London, London, UK). The first two volumes of every functional run from each participant were excluded from the analysis to minimize T1‐saturation effects. Next, the image time series were spatially realigned using a least‐squares approach that estimates six rigid‐body transformation parameters [Friston et al.,1995] by minimizing head movements between each image and the reference image, that is, the first image in the time series. Next, the time series for each voxel was temporally realigned to acquisition of the middle slice. Subsequently, images were normalized onto a custom Montreal Neurological Institute (MNI)‐aligned EPI template (based on 28 male brains acquired on the Siemens Trio at the Donders Institute for Brain, Cognition and Behaviour, Centre for Neuroimaging) using both linear and nonlinear transformations and resampled at an isotropic voxel size of 2 mm. All participants' functional images were smoothed using an 8‐mm FWHM Gaussian filter. Each participant's structural image was spatially co‐registered to the mean of the functional images [Ashburner and Friston,1997] and spatially normalized with the same transformational matrix applied to the functional images. A high‐pass filter was applied with a 0.0078 Hz (128 s) cut‐off to remove low‐frequency components from the data, such as scanner drifts.
The fMRI time series were analyzed within the context of the General Linear Model using an event‐related approach. Repetition suppression was operationally defined as the difference between stimulus pairs for each of the four condition (SS, DS, DA, and DSDA), following Noppeney and Penny's [2006] categorical approach for analyzing repetition–suppression designs (see also [Chee,2009; Chee and Tan,2007; Henson et al.,2004]). The rationale behind this approach is as follows: as the first sentence in each stimulus pair was always spoken in Standard Dutch, and all 128 sentences were randomized and counterbalanced across in all four conditions across all participants, it may be assumed that the first sentences did not differ systematically across conditions. Activation differences between conditions could therefore only be caused by the different patterns of neural suppression after presentation of the second sentence per condition, that is, be due to an interaction between an overall suppression effect and the speaker or accent variation present in the second sentences.
Four events of interest were identified and entered into a subject‐specific General Linear Model, consisting of the 32 stimulus pairs per condition (SS, DS, DA, and DSDA). All onsets within these events were modeled with a length equaling the duration of the both sentences presented and started at the onset of the first sentence in a stimulus pair. Parameter estimates were calculated for each voxel, and contrast maps were constructed for each participant. Finally, the statistical model also considered six separate covariates describing the head‐related movements (as estimated by the spatial realignment procedure).
Linear‐weighted contrasts were used to specify four contrasts. The conditions SS, DS, DA, and DSDA were analyzed in an 2 × 2 factorial design with accent and speaker as factors. A switch of accent occurred in DA and DSDA, a switch of speaker in DS and DSDA, while SS was associated with neither a switch of accent or speaker. We determined main effects of each factor and the interaction term. A main effect of processing a switch of accent was assessed by (DA + DSDA) − (SS + DS), a main effect of processing a switch of speaker was assessed by (DS + DA) − (SS + DA), and the interaction term by (SS + DSDA) − (DS + DA).
The statistical thresholding of the second‐level activation maps associated with these three contrasts was an uncorrected threshold of P < 0.001 in combination with a minimal cluster extent of 80 voxels. This yields a whole‐brain alpha of P < 0.05, determined using a Monte‐Carlo Simulation with 1,000 iterations, using a function implemented in Matlab [Slotnick et al.,2003].
RESULTS
Behavioral Results
For each participant, the proportion of correct responses was calculated for the after‐task. A response was correct whenever the participant had pressed the button and the sentence had been present in the main experiment, or whenever the participant had not pressed the button and the sentence had not been present in the main experiment. Participants correctly detected whether a sentence was present (or not) on average for 79.2% (SD 10.1%; range, 60.7–96.4%) of the sentences, which are significantly higher than chance level (50%), t(17) = 12.142, P < 0.05. All individual participants' scores were significantly higher than chance level (P < 0.05). Given that all participants could judge whether a sentence had been present in the main experiment above chance level, it seems plausible that participants paid attention to the sentences played in the scanner in the main experiment.
Accent
We assessed which cortical regions showed an effect when a switch of accent was present, versus no switch, (DA + DSDA) − (SS + DS). The coordinates of the peak voxels for these effects are listed in Table III and displayed in Figure 2. The peak of the relative increase in BOLD signal for a switch of accent was located in posterior STG bilaterally extending to the ventral supramarginal gyrus (SMG) and left PT. The activation appears more widespread on the left. A second cluster is found in left inferior frontal gyrus (IFG), in pars opercularis (POp), extending into pars triangularis.
Table III.
Activation for peaks separated by at least 8 mm for the contrasts (DA + DSDA) − (SS + DS) (switch of accent only), (DS + DSDA) − (SS + DA) (switch of speaker only)
| Structure | Hemisphere | X | Y | z | T‐value | Z‐value |
|---|---|---|---|---|---|---|
| (DA + DSDA) − (SS + DS) | ||||||
| Posterior STG/SMG | Left | −54 | 40 | 4 | 5.91 | 5.29 |
| Posterior STG/PT | Left | −60 | −34 | 8 | 4.76 | 4.41 |
| Posterior MTG | Left | −60 | −26 | −4 | 4.10 | 3.87 |
| Posterior STG/SMG | Right | 60 | −32 | 2 | 6.10 | 5.43 |
| POp/PG | Left | −50 | 12 | 24 | 4.04 | 3.81 |
| POp/FOC | Left | −46 | 16 | 12 | 3.97 | 3.75 |
| POp/PTr | Left | −46 | 16 | 12 | 3.50 | 3.35 |
| Posterior STG/MTG/SMG | Right | 54 | −26 | −2 | 5.07 | 4.65 |
| Anterior STG/TP/MTG | Right | 54 | 4 | −16 | 4.87 | 4.49 |
| Central opercular cortex | Right | 38 | 18 | 26 | 4.36 | 4.08 |
| Right | ||||||
| (DS + DSDA) − (SS + DA) | ||||||
| LOC/OP | Left | −26 | −92 | 32 | 4.77 | 4.41 |
| LOC | Left | −26 | −76 | 24 | 4.12 | 3.88 |
| LOC | Right | 14 | −66 | 66 | 5.47 | 4.96 |
| Precuneus/LOC/SPL | Right | 12 | −58 | 60 | 4.32 | 4.05 |
| Precuneus | Right | 6 | −52 | 50 | 4.06 | 3.83 |
| MFG | Right | 46 | 24 | 46 | 5.02 | 4.67 |
| MFG/FP | Right | 40 | 36 | 42 | 3.72 | 4.54 |
Coordinates in MNI standard space. FOC, frontal opercular cortex; FP, frontal pole; LOC, lateral occipital cortex; MFG, middle frontal gyrus; MTG, middle temporal gyrus; PG, precentral gyrus; POp, pars opercularis; PT, planum temporale; PTr, pars triangularis; SMG, supramarginal gyrus; SPL, superior parietal lobule; TP, temporal pole.
Figure 2.
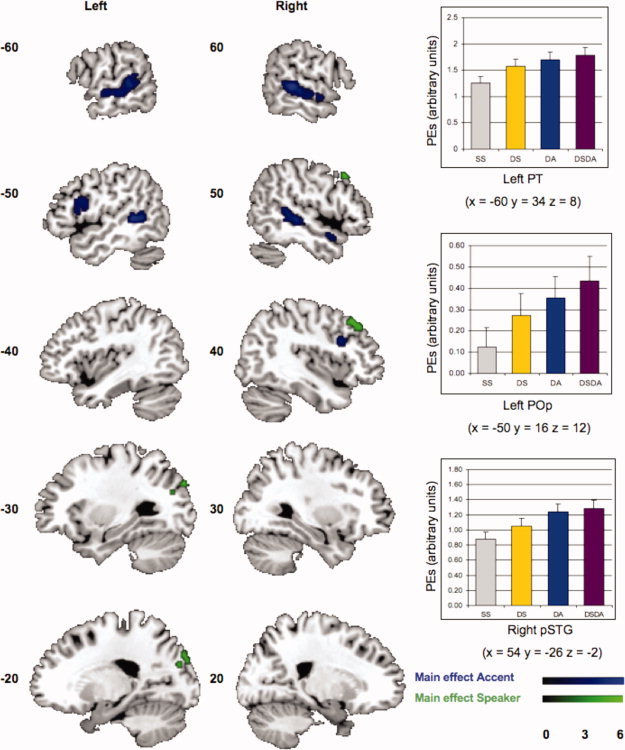
Main effect for a switch of accent only (DA + DSDA) − (SS + DS) in blue, a switch of speaker (DS + DSDA) − (SS + DA) in green. Depiction of the brain activation in arbitrary units for the peak voxels in left planum temporale (PT), right superior temporal gyrus (pSRG), and left pars opercularis (POp). The different conditions are colour coded: yellow (SS), orange (DS), blue (DA), and purple (DSDA). The parameter estimates were based on a 10‐mm sphere around the peak activation and extracted using the MarsBaR toolbox within SPM5 [Brett et al., 2002].
We ensured that the activations in left posterior STG/PT for the contrasts (DA + DSDA) − (SS + DS) were located in PT using the probability map in Westbury et al. [1999]. The group peak activation results in Figure 2 for (DA + DSDA) − (SS + DS) in left posterior STG/PT (−60, −34, 8) is inside the 25–45% probability area, after conversion from MNI to Talairach coordinates [Talairach and Tournoux,1988], which was necessary to use the Westbury et al. [1999] probability map.
Speaker
We assessed which cortical regions showed an effect when a switch of speaker was present, versus no switch, (DS + DSDA) − (SS + DA) (cf. Table III and Fig. 2). Peaks for a relative increase in BOLD for a switch of speaker were located in lateral occipital cortex bilaterally, right precuneus, and right middle frontal gyrus extending into frontal pole. Activations appeared to be more right‐lateralized. Finally, no activated clusters were found at the selected significance level for the interaction term (SS + DSDA) − (DS + DA).
DISCUSSION
The present study aimed to establish the neural bases of processing variation in spoken sentences related to speaker and accent in general and to investigate the role of PT in processing accent variation. Specifically, it was hypothesized that BOLD‐activity in PT would vary as a result of an increase in accent‐related phonetic/phonological variation in the speech signal.
Accent
Several areas in the temporal lobes, including anterior and posterior STG, PT. and SMG, and in the frontal lobes, including POp, showed a relative increase whenever there was a switch of accent present: (DA + DSDA) − (SS + DS).
Bilateral STG has been associated with different cognitive functions. Left pSTG (including PT) is generally regarded as part of a pathway for processing comprehensible speech [Davis and Johnsrude,2003; Poldrack et al.,2001], and it has been suggested that it serves as an interface between the perception and long‐term representation in mental lexicon of familiar words [Wise et al.,2001] and is implicated in resolving semantic ambiguities in spoken sentence comprehension [Rodd et al.,2005].
Earlier studies demonstrate that (left) POp [Blumstein et al.,2005; Burton,2001; Burton et al.,2000; Golestani et al.,2002; Myers,2007; Wilson and Iacoboni,2006; Zatorre et al.,1996] as well as posterior STG/PT [Callan et al.,2004; Warren et al.,2005; Wilson and Iacoboni,2006] is associated with phonetic‐analytic listening to speech sounds or syllables. In addition, POp has previously been associated with disambiguation tasks at a syntactic processing level [Fiebach et al., 2004] and has been named as a key structure in models for processing phonetic/phonological variation in speech comprehension [Callan et al.,2004; Skipper et al., 2006]. Furthermore, left POp has been associated with implicit phonemic analysis processes in speech comprehension [Burton et al.,2000], and it may be expected that processing accented speech may rely in part on increased (low‐level) auditory analysis of the speech signal.
We also found activations in left SMG for a switch in accent. SMG shows sensitivity to phonological changes in speech [Dehaene‐Lambertz et al.,2005]. It has been suggested [Obleser and Eisner,2009] that SMG has access to abstract (i.e., normalised) phonological units. Activations in SMG in speech perception tasks are thus in many cases interpreted as reflecting involvement in phonological working memory (e.g., [Jacquemot et al.,2003].
Speaker
We found several areas that showed a relative increase when a switch of accent was present for the contrast (DA + DSDA) − (SS + DS). These activations included a relative increase in areas in lateral occipital cortex bilaterally, right precuneus, and right middle frontal gyrus extending into frontal pole. These results are generally in line with earlier studies investigating neural activation related to the speaker's voice. Stevens [2004] and Belin et al. [2000] found increases in BOLD activation in MFG for processing voice variation versus nonvocal stimuli. Belin et al. [2000] and Kriegstein and Giraud [2004] both report activations in the precuneus for processing voices.
Nevertheless, most studies on processing speaker variation report more extensive activations in predominantly right‐lateralized temporal areas. Studies on processing speaker‐related information show a wide variety in the neural locus of this process; some report activation in an area close to the left temporal pole in left anterior STS [Belin and Zatorre,2003], while others report that perceptual normalization for the speaker occurs in the superior/middle temporal gyri bilaterally and the superior parietal lobule bilaterally [Wong et al.,2004]. Furthermore, a recent repetition–suppression study on spoken sentence processing also does not report whole‐brain effects for switching between speakers [Dehaene‐Lambertz et al.,2006]. Dehaene‐Lambertz et al. [2006] report a small normalization effect for speaker differences in left STG, after applying a more sensitive analysis. It seems plausible that the differences between our results and the aforementioned studies arise both from differences in task. Moreover, previous studies did not explicitly control for regional accent differences between speakers. The present study shows predominantly activations outside the temporal regions when accent differences between speakers are accounted for. Therefore, it could be the case that accent differences between speakers used in previous studies could have affected results, especially in temporal areas.
Speaker and Accent Normalization
The question arises whether phonological and phonetic variation in speech is processed in the same way as speech stimuli that have been distorted or degraded, for instance, by presenting sentences at a lower signal‐to‐noise ratio [Zekveld et al.,2006] or by noise‐vocoding [Obleser et al.,2007]. Relatively few studies investigate the specific effect of different types of distortion of the speech signal and identified areas that are involved more when the intelligibility decreases. Only one study addresses this question in‐depth [Davis and Johnsrude,2003]. Davis and Johnsruhe evaluated the effect of three types of distortions (speech in noise, noise‐vocoded speech, and segmented speech) on speech processing. They found that left STG and left IFG became more active for distorted versus intelligible speech. However, activity in left posterior STG varied dependent on the type of distortion, while left anterior STG's responses were form‐independent (i.e., showed elevated activation independent from the type of distortion used). It is at this point not feasible to determine whether accented speech and distorted speech are processed differently in left posterior STG, as Davis and Johnsruhe did not include accented speech and our study did not include distorted speech.
CONCLUSION
We conclude that bilateral posterior STG (including PT) and POp are involved in processing various types of distortions in the speech signal. However, further study is required to establish whether these areas differentiate between various types of speech‐intrinsic (such as accent, speech rate, or clarity of speech) and speech‐extrinsic variation (such as added noise).
Finally, our results provide further evidence for the hypothesis that PT is associated with processing accent and speaker variation during spoken language comprehension and thus support the theory [Griffiths and Warren,2002; Warren et al.,2005] that PT serves as a computational hub for processing spectrotemporal variation in auditory perception.
Acknowledgements
We thank Paul Gaalman for technical assistance, Esther Aarts, for lending her voice, and Joseph T. Devlin and Penny Lewis for useful comments.
Table AI.
Sentences from the SRT corpus before and after conversion
| Sentence no. | Standard Dutch | Novel accent of Dutch |
|---|---|---|
| 1 | De bal vloog over de schutting | De baal vlog offer de schuuttieng |
| 2 | Morgen wil ik maar één liter melk | Moorgen wiel iek mar èn litter meelk |
| 3 | Deze kerk moet gesloopt worden | Desse keerk mut geslopt woorden |
| 4 | De spoortrein was al gauw kapot | De sportreen waas aal goew kaappoot |
| 5 | De nieuwe fiets is gestolen | De niwwe fits ies gestollen |
| 6 | Zijn manier van werken ligt mij niet | Zeen mannir vaan weerken liegt mee nit |
| 7 | Het slot van de voordeur is kapot | Het sloot vaan de vordur ies kaappoot |
| 8 | Dat hotel heeft een slechte naam | Daat hotteel heft 'n sleechte nam |
| 9 | De jongen werd stevig aangepakt | De joongen weerd steffig angepaakt |
| 10 | Het natte hout sist in het vuur | Het naatte hoet siest ien het vur |
| 11 | Zijn fantasie kent geen grenzen | Zeen faantassih keent gèn greenzen |
| 12 | De aardappels liggen in de schuur | De ardaappels liegen ien de schur |
| 13 | Alle prijzen waren verhoogd | Aalle preezen warren verhogt |
| 14 | Zijn leeftijd ligt boven de dertig | Zeen lèfteed liegt boffen de deertieg |
| 15 | Het dak moet nodig hersteld worden | Het daak mut noddieg heersteeld woorden |
| 16 | De kachel is nog steeds niet aan | De kaachel ies noog stèds nit an |
| 17 | Van de viool is een snaar kapot | Vaan de vij‐jol ies 'n snar kaappoot |
| 18 | De tuinman heeft het gras gemaaid | De tuunmaan heft het graas gemajt |
| 19 | De appels aan de boom zijn rijp | De aappels an de bom zeen reep |
| 20 | Voor het eerst was er nieuwe haring | Vor het erst waas eer niwwe harrieng |
| 21 | Het loket bleef lang gesloten | Het lokkeet blef laang geslotten |
| 22 | Er werd een diepe kuil gegraven | Eer weerd 'n dippe koel gegraffen |
| 23 | Zijn gezicht heeft een rode kleur | Zeen geziecht hèft 'n rodde klur |
| 24 | Het begon vroeg donker te worden | Het beggoon vrug doonker te woorden |
| 25 | Het gras was helemaal verdroogd | Het graas waas hèllemal verdrogt |
| 26 | Spoedig kwam er een einde aan | Spuddieg kwaam eer 'n eende an |
| 27 | Ieder half uur komt hier een bus langs | Idder haalf ur koomt hir 'n buus laangs |
| 28 | De bel van de voordeur is kapot | De beel vaan de vordur ies kaappoot |
| 29 | De wind waait vandaag uit het westen | De wiend wajt vaandag uut het weesten |
| 30 | De slang bewoog zich door het gras | De slaang bewog ziech dor het graas |
| 31 | De kamer rook naar sigaren | De kammer rok nar siggarren |
| 32 | De appel had een zure smaak | De aappel haad 'n zurre smak |
| 33 | De trein kwam met een schok tot stilstand | De treen kwaam meet 'n schook toot stielstaand |
| 34 | De koeien werden juist gemolken | De kujjen weerden juust gemoolken |
| 35 | Het duurt niet langer dan een minuut | Het durt nit laanger daan 'n minnut |
| 36 | De grijze lucht voorspelt regen | De greeze luucht vorspeelt règgen |
| 37 | Hij kon de hamer nergens vinden | Hee koon de hammer neergens vienden |
| 38 | Deze berg is nog niet beklommen | Desse beerg ies noog nit bekloommen |
| 39 | De bel van mijn fiets is kapot | De beel vaan meen fits ies kaappoot |
| 40 | De auto heeft een lekke band | De oetoh hèft 'n leekke baand |
| 41 | Het moeilijke werk bleef liggen | Het muj‐leekke weerk blef lieggen |
| 42 | Het vliegtuig vertrekt over een uur | Het vligtuug vertreekt offer 'n ur |
| 43 | De jongens vechten de hele dag | De joongens veechten de hèlle daag |
| 44 | De schoenen moeten verzoold worden | De schunnen mutten verzold woorden |
| 45 | In de krant staat vandaag niet veel nieuws | Ien de kraant stat vaandag nit vèl niws |
| 46 | Door de neus ademen is beter | Dor de nus addemmen ies better |
| 47 | Het kind was niet in staat te spreken | Het kiend waas nit ien stat te sprekken |
| 48 | De witte zwaan dook onder water | De wiette zwan dok oonder watter |
| 49 | Hij nam het pak onder zijn arm | Hee naam het paak oonder zeen aarm |
| 50 | Gelukkig sloeg de motor niet af | Geluukkieg slug de mottor nit aaf |
| 51 | De leraar gaf hem een laag cijfer | De lèrrar gaaf heem 'n lag seeffer |
| 52 | Het huis brandde tot de grond toe af | Het huus braande toot de groond tuh aaf |
| 53 | De foto is mooi ingelijst | De fotto ies moi iengeleest |
| 54 | Mijn broer gaat elke dag fietsen | Meen brur gat eelke daag fitsen |
| 55 | Een kopje koffie zal goed smaken | Een koopje kooffih zaal gud smakken |
| 56 | De schrijver van dit boek is dood | De schreeffer vaan diet buk ies dot |
| 57 | Zij heeft haar proefwerk slecht gemaakt | Zee heft har prufweerk sleecht gemakt |
| 58 | De sigaar ligt in de asbak | De siggar liegt ien de aasbaak |
| 59 | De appelboom stond in volle bloei | De aappelbom stoond ien voolle bluj |
| 60 | Er wordt in dit land geen rijst verbouwd | Eer woordt ien diet laand gèn reest verbuwd |
| 61 | Hij kan er nu eenmaal niets aan doen | Hee kaan eer nuh ènmal nits an dun |
| 62 | De kleren waren niet gewassen | De klerren warren nit gewaassen |
| 63 | Het gedicht werd voorgelezen | Het gediecht weerd vorgelèssen |
| 64 | Haar gezicht was zwart van het vuil | Har geziecht waas zwaart vaan het vuul |
| 65 | De letters stonden op hun kop | De leetters stoonden oop huun koop |
| 66 | De groene appels waren erg zuur | De grunne aappels warren eerg zur |
| 67 | In het gebouw waren vier liften | Ien het geboew warren vir lieften |
| 68 | Lopen is gezonder dan fietsen | Loppen ies gezoonder daan fitsen |
| 69 | Het lawaai maakte hem wakker | Het lawwai makte heem waakker |
| 70 | Mijn buurman heeft een auto gekocht | Meen burmaan heft 'n oetoh gekoocht |
| 71 | Als het flink vriest kunnen we schaatsen | Aals het flienk frist kuunnen we schatsen |
| 72 | De kast was een meter verschoven | De kaast waas 'n metter verschoffen |
| 73 | Oude meubels zijn zeer in trek | Oede mubbels zeen zèr ien treek |
| 74 | De portier ging met vakantie | De poortir gieng meet vaakkaantih |
| 75 | De lantaarn gaf niet veel licht meer | De laantarn gaaf nit vèl liecht mer |
| 76 | Door zijn snelheid vloog hij uit de bocht | Door zeen sneelheed vlog hee uut de boocht |
| 77 | Het is hier nog steeds veel te koud | Het ies hir noog steds vèl te koed |
| 78 | De oude man was kaal geworden | De oede maan waas kal gewoorden |
| 79 | De bomen waren helemaal kaal | De bommen warren hèllemal llemal kal |
| 80 | Rijden onder invloed is strafbaar | Reedden oonder ienvlud ies straafbar |
| 81 | Onze bank geeft vijf procent rente | Oonze baank geft veef prosseent reente |
| 82 | Het verslag in de krant is kort | Het verslaag ien de kraant ies koort |
| 83 | In de vijver zwemmen veel vissen | Ien de veeffer zweemmen vel viessen |
| 84 | Honden mogen niet in het gebouw | Hoonden moggen nit ien het geboew |
| 85 | Een flinke borrel zal mij goed doen | Een flienke boorrel zaal mee gud dun |
| 86 | Gisteren waaide het nog harder | Giesteren wajde het noog haarder |
| 87 | Het meisje stond lang te wachten | Het meesje stoond laang te waachten |
| 88 | De volgende dag kwam hij ook niet | De voolgende daag kwaam hee ok nit |
| 89 | Het geschreeuw is duidelijk hoorbaar | Het geschrew ies duudeleek horbar |
| 90 | Eindelijk kwam de trein op gang | Eendeleek kwaam de treen oop gaang |
| 91 | De grote stad trok hem wel aan | De grotte staad trook heem weel an |
| 92 | De bus is vandaag niet op tijd | De buus ies vaandag nit oop teed |
| 93 | Onze dochter speelt goed blokfluit | Oonze doochter spèlt gud blookfluut |
| 94 | Ook in de zomer is het hier koel | Ok ien de zommer ies het hir kul |
| 95 | Zij moesten vier uur hard werken | Zee musten vir ur haard weerken |
| 96 | Niemand kan de Fransman verstaan | Nimmaand kaan de Fraansmaan verstan |
| 97 | Eiken balken zijn erg kostbaar | Eeken baalken zeen eerg koostbar |
| 98 | Het aantal was moeilijk te schatten | Het antaal waas muujleek te schaatten |
| 99 | Er waaide een stevig briesje | Er waj‐de 'n stèffieg brisje |
| 100 | De vis sprong een eind uit het water | De vies sproong 'n eend uut het watter |
| 101 | Iedereen genoot van het uitzicht | Idderèn genot vaan het uutziecht |
| 102 | Het regent al de hele dag | Het règgent aal de hèlle daag |
| 103 | Het tempo was voor hem veel te hoog | Het teempoh waas vor heem vèl te hog |
| 104 | In juni zijn de dagen het langst | Ien junnih zeen de daggen het laangst |
| 105 | De bakkers bezorgen vandaag niet | De baakkers bezoorgen vaandaag nit |
| 106 | Het licht in de gang brandt nog steeds | Het liecht ien de gaang braandt noog steds |
| 107 | De wagen reed snel de berg af | De waggen red sneel de beerg aaf |
| 108 | Lawaai maakt je op den duur doof | Lawai makt je oop deen dur dof |
| 109 | In de kerk wordt mooi orgel gespeeld | Ien de keerk woordt moi oorgel gespèld |
| 110 | De schaatsen zijn in het vet gezet | De schatsen zeen ien het veet gezeet |
| 111 | Toch lijkt me dat een goed voorstel | Tooch leekt mee daat 'n gud vorsteel |
| 112 | Hij probeerde het nog een keer | Hee probbèrde het noog 'n kèr |
| 113 | De zak zat vol oude rommel | De zaak zaat vool oede roommel |
| 114 | Zij werd misselijk van het rijden | Zee weerd miesselleek vaan het reedden |
| 115 | Door zijn haast maakte hij veel fouten | Dor zeen hast makte hee vèl foeten |
| 116 | De nieuwe zaak is pas geopend | De niwwe zak ies paas ge‐oppend |
| 117 | Dat is voor hem een bittere pil | Daat ies vor heem 'n biettere piel |
| 118 | Op het gras mag men niet lopen | Oop het graas maag meen nit loppen |
| 119 | Steile trappen zijn gevaarlijk | Steelle traappen zeen gevarleek |
| 120 | De zon gaat in het westen onder | De zoon gat ien het weesten oonder |
| 121 | De hond blafte de hele nacht | De hoond blaafte de hèlle naacht |
| 122 | De kat van de buren is weg | De kaat vaan de burren ies weeg |
| 123 | De trein vertrekt over twee uur | De treen vertreekt offer twe ur |
| 124 | Het was heel stil in de duinen | Het waas hel stiel ien de duunnen |
| 125 | Hij rookte zijn sigaret op | Hee rokte zeen siggarreet oop |
| 126 | De rivier trad buiten haar oevers | De riffir traad buutten har uffers |
| 127 | De jongen ging er gauw vandoor | De joongen gieng eer goew vaandor |
| 128 | Moeizaam klom de man naar boven | Mujzam kloom de maan nar boffen |
| 129 | De biefstuk is vandaag erg mals | De bifstuuk ies vaandaag eerg maals |
| 130 | De kat likt het schoteltje leeg | De kaat liekt het schotteltje lèg |
Sentences 1–128 were presented in the fMRI experiment.
REFERENCES
- Adank P, Janse E ( 2010): Comprehension of a novel accent by younger and older listeners. Psychol Aging 25: 736–740. [DOI] [PubMed] [Google Scholar]
- Adank P, van Hout R, Van de Velde H ( 2007): An acoustic description of the vowels of Northern and Southern Standard Dutch II: Regional varieties. J Acoust Soc Am 121: 1130–1141. [DOI] [PubMed] [Google Scholar]
- Adank P, Evans BG, Stuart‐Smith J, Scott SK ( 2009): Familiarity with a regional accent facilitates comprehension of that accent in noise. J Exp Psychol: Hum Percept Perform 35: 520–529. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K ( 1997): Multimodal image coregistration and partitioning—A unified framework. NeuroImage 6: 209–217. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, van Kylen J, Birn RM, Hyde JS ( 1998): Functional MRI of brain activation induced by scanner acoustic noise. Magn Reson Med 39: 410–416. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ ( 2003): Adaptation to speaker's voice in right anterior temporal lobe. NeuroReport 14: 2105–2109. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B ( 2000): Voice‐selective areas in human auditory cortex. Nature 403: 309–312. [DOI] [PubMed] [Google Scholar]
- Binder J, Frost J, Hammeke T, Rao S, Cox R ( 1996): Function of the left planum temporale in auditory and linguistic processing. Brain 119: 1229–1247. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, Possing ET ( 2000): Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex 10: 512–528. [DOI] [PubMed] [Google Scholar]
- Blumstein SE, Myers EB, Rissman J ( 2005): The perception of voice onset time: An fMRI investigation of phonetic category structure. J Cogn Neurosci 17: 1353–1366. [DOI] [PubMed] [Google Scholar]
- Boersma P, Weenink D ( 2003): Praat: Doing Phonetics by Computer. Retrieved August 11, 2008, from http://www.fon.hum.uva.nl/praat [Google Scholar]
- Bregman AS ( 1990): Auditory Scene Analysis. Cambridge: MIT Press. [Google Scholar]
- Burton MW ( 2001): The role of inferior frontal cortex in phonological processing. Cogn Sci 25: 695–709. [Google Scholar]
- Burton MW, Small SL, Blumstein SE ( 2000): The role of segmentation in phonological processing: An fMRI investigation. J Cogn Neurosci 12: 679–690. [DOI] [PubMed] [Google Scholar]
- Callan DE, Jones JA, Callan AM, Akahane‐Yamada R ( 2004): Phonetic perceptual identification by native‐ and second‐language speakers differentially activates brain regions involved with acoustic phonetic processing and those involved with articulatory‐auditory/or‐ osensory internal models. NeuroImage 22: 1182–1194. [DOI] [PubMed] [Google Scholar]
- Chee MWL ( 2009): fMR‐adaptation and the bilingual brain. Brain Lang 109: 75–79. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Tan CC ( 2007): Inter‐relationships between attention, activation, fMR adaptation and long‐term memory. NeuroImage 37: 1487–1495. [DOI] [PubMed] [Google Scholar]
- Davis MH, Johnsrude IS ( 2003): Hierarchical processing in spoken language comprehension. J Neurosci 23: 3423–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene‐Lambertz G, Pallier C, Serniclaes W, Sprenger‐Charolles L, Jobert A, Dehaene S ( 2005): Neural correlates of switching from auditory to speech perception. Neuroimage 24: 21–33. [DOI] [PubMed] [Google Scholar]
- Dehaene‐Lambertz G, Dehaene S, Anton J, Campagne A, Ciuciu P, Dehaene GP, Denghien I, Jobert A, LeBihan D, Sigman M, Pallier C, Poline J ( 2006): Functional segregation of cortical language areas by sentence repetition. Human Brain Mapp 27: 360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden GF, Joseph JE, Brown HE, Brown CP, Zeffiro TA ( 1999): Utilizing hemodynamic delay and dispersion to detect fMRI signal change without auditory interference: The behavior interleaved gradients technique. Magn Reson Med 41: 13–20. [DOI] [PubMed] [Google Scholar]
- Edmister WB, Talavage TM, Ledden TJ, Weisskoff RM ( 1999): Improved auditory cortex imaging using clustered volume acquisitions. Human Brain Mapp 7: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BG, Iverson P ( 2003): Vowel normalization for accent: An investigation of best exemplar locations in northern and southern British English sentences. J Acoust Soc Am 115: 352–361. [DOI] [PubMed] [Google Scholar]
- Floccia C, Goslin J, Girard F, Konopczynski G ( 2006): Does a regional accent perturb speech processing? J Exp Psychol: Hum Percept Perform 32: 1276–1293. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J‐B, Heather JD, Frackowiak RSJ ( 1995): Spatial registration and normalization of images. Human Brain Mapp 2: 165–189. [Google Scholar]
- Gaab N, Gabrieli JD, Glover GH ( 2006): Assessing the influence of scanner background noise on auditory processing. An fMRI study comparing three experimental designs with varying degrees of scanner noise. Human Brain Mapp 28: 703–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Price CJ ( 2001): The constraints functional neuroimaging places on classical models of auditory word processing. J Cogn Neurosci 13: 754–765. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Lorenz C, Ashburner J, Wable J, Johnsrude I, Frackowiak R, Kleinschmidt A ( 2000): Representation of the temporal envelope of sounds in the human brain. J Neurophysiol 84: 1588–1598. [DOI] [PubMed] [Google Scholar]
- Golestani N, Paus T, Zatorre RJ ( 2002): Anatomical correlates of learning novel speech sounds. Neuron 35: 997–1010. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Warren JD ( 2002): The planum temporale as a computational hub. Trends Neurosci 25: 348–353. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K, Malach R ( 2001): fMR‐adaptation: A tool for studying the functional properties of human cortical neurons. Acta Psychol 107: 293–321. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R ( 1999): Differential processing of objects under various viewing conditions in the human lateral occipital cortex. Neuron 24: 187–203. [DOI] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliot MR, Gurney EM, Bowtell RW ( 1999): “Sparse” temporal sampling in auditory fMRI. Human Brain Mapp 7: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, Johnsrude IS, Haggard MP, Palmer AR, Akeroyd MA, Summerfield AQ ( 2002): Spectral and temporal processing in human auditory cortex. Cereb Cortex 12: 140–149. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rylands A, Ross E, Vuilleumeir P, Rugg MD ( 2004): The effect of repetition lag on electrophysiological and haemodynamic correlates of visual object priming. NeuroImage 21: 1674–1689. [DOI] [PubMed] [Google Scholar]
- Hickok G, Love T, Swinney D, Wong EC, Buxton RB ( 1997): Functional MR imaging during auditory word perception: A single trial presentation paradigm. Brain Lang 58: 197–201. [DOI] [PubMed] [Google Scholar]
- Hillenbrand JM, Houde RA ( 2003): A narrow band pattern‐matching model of vowel perception. J Acoust Soc Am 113: 1044–1055. [DOI] [PubMed] [Google Scholar]
- IPA ( 1999): Handbook of the International Phonetic Association: A Guide to the Use of the International Phonetic Alphabet. Cambridge: Cambridge University Press. [Google Scholar]
- Jacquemot C, Pallier C, LeBihan D, Dehaene S, Dupoux E ( 2003): Phonological grammar shapes the auditory cortex: A functional magnetic resonance imaging study. J Neurosci 23: 9541–9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein K, Giraud A ( 2004): Distinct functional substrates along the right superior temporal sulcus for the processing of voices. NeuroImage 22: 948–955. [DOI] [PubMed] [Google Scholar]
- Moulines E, Charpentier F ( 1990): Pitch‐synchronous waveform processing techniques for text‐to‐speech synthesis using diphones. Speech Commun 9: 453–467. [Google Scholar]
- Myers EB ( 2007): Dissociable effects of phonetic competition and category typicality in a phonetic categorization task: An fMRI investigation. Neuropsychologia 45: 1463–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nearey TM ( 1989): Static, dynamic, and relational properties in speech perception. J Acoust Soc Am 85: 2088–2113. [DOI] [PubMed] [Google Scholar]
- Nearey TM ( 1997): Speech perception as pattern recognition. J Acoust Soc Am 101: 3241–3254. [DOI] [PubMed] [Google Scholar]
- Nearey TM, Assmann PF ( 2007): Probabilistic ‘sliding‐template’ models for indirect vowel normalization In: Solé MJ, Beddor PS, Ohala M, editors. Experimental Approaches to Phonology. Oxford: Oxford University Press; pp 246–269. [Google Scholar]
- Noppeney U, Penny W ( 2006): Two approaches to repetition suppression. Human Brain Mapp 27: 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris D, McQueen JM, Cutler A ( 2003): Perceptual learning in speech. Cogn Psychol 47: 204–238. [DOI] [PubMed] [Google Scholar]
- Obleser J, Eisner F ( 2009): Pre‐lexical abstraction of speech in the auditory cortex. Trends Cogn Sci 13: 14–19. [DOI] [PubMed] [Google Scholar]
- Obleser J, Wise RJS, Dresner MA, Scott SK ( 2007): Functional integration across brain regions improves speech perception under adverse listening conditions. J Neurosci 27: 2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfanidou E, Marslen‐Wilson WD, Davis MH ( 2006): Neural response suppression predicts repetition priming of spoken words and pseudowords. J Cogn Neurosci 18: 1237–1252. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Zatorre RJ, Evans AE ( 1998): Cerebellar contributions to motor timing: A PET study of auditory and visual rhythm reproduction. J Cogn Neurosci 10: 752–765. [DOI] [PubMed] [Google Scholar]
- Peterson GE, Barney HL ( 1952): Control methods used in a study of the vowels. J Acoust Soc Am 24: 175–184. [Google Scholar]
- Plomp R, Mimpen AM ( 1979): Improving the reliability of testing the speech reception threshold for sentences in quiet for sentences. Audiology 18: 42–53. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich M, Gabrieli JDE ( 2001): Relations between the neural bases of dynamic auditory processing and phonological processing: Evidence from fMRI. J Cogn Neurosci 13: 687–697. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Davis MH, Johnsrude IS ( 2005): The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cereb Cortex 15: 1261–1269. [DOI] [PubMed] [Google Scholar]
- Rogers CL, Dalby J, Nishi K ( 2004): Effects of noise and proficiency level on intelligibility of Chinese‐accented English. Lang Speech 47: 139–154. [DOI] [PubMed] [Google Scholar]
- Shah NJ, Jäncke L, Grosse‐Ruyken ML, Muller‐Gartner HW ( 1999): Influence of acoustic masking noise in fMRI of the auditory cortex during phonetic discrimination. J Magn Reson Imag 9: 19–25. [DOI] [PubMed] [Google Scholar]
- Shtyrov Y, Kujala T, Palva S, Ilmoniemi RJ, Naatanen R ( 2000): Discrimination of speech and of complex nonspeech sounds of different temporal structure in the left and right cerebral hemispheres. Neuroimage 12: 657–663. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart JJ ( 2003): Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cogn Brain Res 17: 75–82. [DOI] [PubMed] [Google Scholar]
- Stevens AA ( 2004): Dissociating the neural bases for voices, tones, and words. Cogn Brain Res 18: 162–171. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme. [Google Scholar]
- Talavage TM, Edmister WB, Ledden TJ, Weisskoff RM ( 1999): Quantitative assessment of auditory cortex responses induced by imager acoustic noise. Human Brain Mapp 7: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamer G, Talavage T, Wen‐Ming L ( 2009): Characterizing response to acoustic imaging noise for auditory event‐ related fMRI. IEEE Trans Biomed Eng 56: 1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wijngaarden SJ ( 2001): Intelligibility of native and non‐native Dutch speech. Speech Commun 35: 103–113. [Google Scholar]
- van Wijngaarden SJ, Steeneken HJ, Houtgast T ( 2002): Quantifying the intelligibility of speech in noise for non‐native talkers. J Acoust Soc Am 112: 3004–3013. [DOI] [PubMed] [Google Scholar]
- Warren JE, Wise RJS, Warren JD ( 2005): Sounds do‐able: Auditory–motor transformations and the posterior temporal plane. Trends Neurosci 28: 636–643. [DOI] [PubMed] [Google Scholar]
- Wells JC ( 1982): Accents of English. Three Volumes and Cassette. Cambridge: Cambridge University Press. [Google Scholar]
- Westbury CF, Zatorre RJ, Evans AC ( 1999): Quantifying variability in the planum temporale: A probability map. Cereb Cortex 9: 392–405. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Iacoboni M ( 2006): Neural responses to non‐native phonemes varying in producibility: Evidence for the sensorimotor nature of speech perception. NeuroImage 33: 316–325. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA ( 2001): Separate neural subsystems within ‘Wernicke's area’. Brain 124: 83–95. [DOI] [PubMed] [Google Scholar]
- Wong PCM, Nusbaum HC, Small SL ( 2004): Neural bases of talker Normalization. J Cogn Neurosci 16: 1173–1184. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Schmidt CF, Meyer M, Baumann S, Baltes C, Boesiger P, Jancke L ( 2007): Comparison of “silent” clustered and sparse temporal fMRI acquisitions in tonal and speech perception tasks. NeuroImage 37: 1195–1204. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E ( 1994): Neural mechanisms underlying melodic perception and memory for pitch. J Neurosci 14: 1908–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Meyer E, Gjedde A, Evans AC ( 1996): PET studies of phonetic processing of speech: Review, replication, and reanalysis. Cereb Cortex 6: 21–30. [DOI] [PubMed] [Google Scholar]
- Zekveld AA, Heslenfeld DJ, Festen JM, Schoonhoven R ( 2006): Top–down and bottom–up processes in speech comprehension. NeuroImage 32: 1826–1836. [DOI] [PubMed] [Google Scholar]
- Zevin JD, McCandliss BD ( 2005): Dishabituation of the BOLD response to speech sounds. Behav Brain Funct 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]


