Abstract
We aimed to investigate the effect of hand effector and handedness on the cerebral lateralization of pantomiming learned movements. Fourteen right‐handed and 14 left‐handed volunteers performed unimanual and bimanual tool‐use pantomimes with their dominant or nondominant hand during fMRI. A left hemispheric lateralization was observed in the right‐ and left‐handed group regardless of which hand(s) performed the task. Asymmetry was most marked in the dorsolateral prefrontal cortex (DLPFC), premotor cortex (PMC), and superior and inferior parietal lobules (SPL and IPL). Unimanual pantomimes did not reveal any significant differences in asymmetric cerebral activation patterns between left‐ and right‐handers. Bimanual pantomimes showed increased left premotor and posterior parietal activation in left‐ and right‐handers. Lateralization indices (LI) of the 10% most active voxels in DLPFC, PMC, SPL, and IPL were calculated for each individual in a contrast that compared all tool versus all control conditions. Left‐handers showed a significantly reduced overall LI compared with right‐handers. This was mainly due to diminished asymmetry in the IPL and SPL. We conclude that the recollection and pantomiming of learned gestures recruits a similar left lateralized activation pattern in right and left‐handed individuals. Handedness only influences the strength (not the side) of the lateralization, with left‐handers showing a reduced degree of asymmetry that is most readily observed over the posterior parietal region. Together with similar findings in language and visual processing, these results point to a lesser hemispheric specialization in left‐handers that may be considered in the cost/benefit assessment to explain the disproportionate handedness polymorphism in humans. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: tool use, pantomiming, cerebral lateralization, transitive gestures, hemispheric specialization, unimanual gestures, bimanual gestures, handedness, lateralization, functional asymmetry
INTRODUCTION
Learned movements such as tool use and symbolic gestures are pivotal in daily life. A common test to evaluate the integrity of a person's repertoire of learned gestures is tool‐use pantomiming [Bartolo et al., 2008; van Heugten et al., 1999]. In tool‐use pantomime, an instrumental grasp and movement is mimicked with an imaginary object. Although these pantomimes are derived from actual tool use, they are merely communicative gestures that symbolize the tool and its action [Goldenberg et al., 2007]. Therefore, the kinematic properties of pantomimed and actual tool use are not identical [Laimgruber et al., 2005]. Nevertheless, the pantomiming of tool use still has considerable clinical and scientific relevance, and in behavioral neurology and clinical neuropsychology, it is considered to be a sensitive test for apraxia, a disorder of learned movement resulting from left brain damage [Bartolo et al., 2008; Goldenberg et al., 2003; Liepmann, 1920]. Neuroimaging studies have used pantomiming tasks for several purposes, among others to determine its neural correlates [Choi et al., 2001; Rumiati et al., 2004], or to evaluate the neural background of error patterns in apraxia [Ohgami et al., 2004]. They have also proven to be useful to reveal differences between executed and imagined or planned pantomimes [Fridman et al., 2006; Imazu et al., 2007; Johnson‐Frey et al., 2005; Moll et al., 2000], to compare actual tool use with pantomimed tool use [Hermsdorfer et al., 2007; Imazu et al., 2007], and to explore the neural correlates of mechanical problem solving [Vingerhoets et al., 2010a]. These studies have certainly advanced our insight into the cognitive neuroanatomy of pantomiming tool use. In line with lesion studies in apraxic patients, they have confirmed a consistent left hemispheric dominance of the activated regions in right‐handed volunteers [Buxbaum et al., 2005; Goldenberg et al., 2007; Haaland et al., 2000]. What remains largely unexplored; however, are the factors that influence the lateralization of cerebral activity during the pantomiming of tool use. In this fMRI study, we aim to investigate three such factors: bimanual performance, hand (in)dependence, and handedness.
Unimanual Versus Bimanual Pantomimes
In daily life, the skilled manipulation of objects frequently requires the use of both hands, for example when eating with knife and fork, threading a needle, or sharpening a pencil. Although in most cases the preferred hand plays a dominant role in the interaction, the nonpreferred hand is crucial for holding and positioning the additional manipulandum in space. This everyday bimanual tool use is clearly not reflected in the tasks that are commonly used in pantomime research. For clinical use, bimanual tasks are not well suited, as many apraxic patients also suffer from a contralesional hemiplegia that makes the quality of a bimanual performance difficult to assess. In cognitive neuroscience, too, bimanual pantomiming is rarely used and its neural correlates therefore still remain unexplored. In unimanual tool pantomiming, the resulting left hemispheric activation pattern is taken to reflect a lateralized network that contains the functional knowledge about objects and how to use them. As apraxia after left hemispheric damage is usually expressed in both hands (as revealed by apraxics without hemiplegia), this left hemispheric network is assumed to control learned movements for both sides of the body. Bimanual tool pantomiming provides an interesting test for the strength of this lateralization, as it can be argued that the different functional roles of both hands may require increased right hemispheric input in order to functionally guide the nondominant hand. In this study, we will compare the neural correlates of unimanual versus bimanual pantomimes of tool use in a paradigm that controls for nontool‐related motor behavior and the visual complexity of the stimuli.
Hand (In)Dependency
If a left lateralized network guides the learned tool‐related gestures of both hands, then this network should be activated regardless of which hand is used to carry out the task. This idea was tested in three neuroimaging studies that compared right and left hand pantomimes of tool use in right‐handed volunteers [Choi et al., 2001; Johnson‐Frey et al., 2005; Moll et al., 2000]. These studies indeed confirmed strong left hemispheric lateralization regardless of which hand was used, although pantomimes with the preferred hand did produce an additional activation in the left temporal and subcortical regions [Choi et al., 2001] and the left frontal cortex [Johnson‐Frey et al., 2005]. Notwithstanding the paucity of scientific data, the role of the effector on the lateralization of tool‐use‐related cerebral activation is of clinical importance and should be documented in more detail. Although epidemiological data are scarce, in more than 85% of the cases apraxia is the direct result from left hemispheric damage [Zwinkels et al., 2004], and the majority of these patients also develop aphasia and right‐sided hemiplegia. As a result, the assessment of apraxia in these patients is often based on their performance with the nonpreferred hand. Only when tool‐use‐related cerebral activation is independent of effector side, will apraxia testing with the nondominant hand genuinely reflect the severity of the functional deficit and, therefore, accurately predict the need for rehabilitation. Here, we will compare performances with the dominant and nondominant hand for unimanual and bimanual pantomimes of tool use.
Handedness
Development and activation of the primary motor cortex show a mirror‐opposite pattern in right‐ and left‐handers [Dassonville et al., 1997; Hammond, 2002; Kim et al., 1993]. An analogous shift in dominance for praxis to the right hemisphere might be expected in left‐handed persons, at least if one reasoned that hand dominance and skillful learned control are intricately linked. Several clinical reports argue in favor of such an obligatory link and mention left‐handed patients with apraxia after right hemispheric damage [Ochipa et al., 1989; Poeck and Kerschen, 1971; Poeck and Lehmkuhl, 1980]. Other case studies, however, observe apraxia following right brain damage in right‐handers [Basso et al., 1985; Marchetti and Della Sala, 1997; Rapcsak et al., 1987; Raymer et al., 1999] and refute the alleged association between handedness and learned motor skills.
To further our understanding of the relation between hand preference and the cerebral representation of pantomimed tool use, we will compare unimanual and bimanual pantomimes in matched groups of left‐ and right‐handed volunteers. Pantomimes with their dominant and nondominant hands will be controlled for basic motor action in order to focus on regions that are specifically associated with the use of familiar tools. Besides a comparison of the resulting statistical activation patterns, we will calculate lateralization indices of multiple regions of interest to evaluate the strength of the asymmetries in left‐ and right‐handers.
MATERIALS AND METHODS
Participants
Twenty‐eight healthy volunteers (age range 19–26 years, mean age 21.5 years, 11 women and 17 men) from the student population at Ghent University entered the study after approval from the local ethics committee. All the participants signed written informed consent and none of them had a history of neurological or psychiatric disease. Fourteen (six women and eight men) were right‐handed as determined by the Edinburgh Handedness Inventory [Oldfield, 1971]: M = 97.1%, SD = 6.1%, and 14 (five women and nine men) were left‐handed: M = −95.0%, SD = 7.6%. The left‐ and right‐handed groups revealed neither significant differences in age and strength of handedness, nor in male/female ratio.
Stimuli
To keep the visual load equal between conditions, each stimulus of the pantomime paradigm adhered to the same pictorial format. Every stimulus presented two objects, one on the right and one on the left, and three lines, one below each object and one between the objects. The objects were shown in color, and two of the lines were always black and one was always red. In the tool conditions the depicted objects were familiar tools. In 20 slides, the line under the right tool object was red and these slides were mirrored over the vertical axis to obtain 20 similar slides in which the line under the left object was red. In 20 other slides, the line between the two tool objects was red. In the control conditions, designed to control for transitive pantomime movements in general (that is, movement unrelated to tools), the objects were eggs. Eggs are familiar objects that are easy to manipulate, but are not associated with tool‐like qualities. In each control slide, only one of the eggs was aligned vertically. Six slides were constructed for each control condition (six with a red line under the [vertical] right egg, six with a red line under the [vertical] left egg, six with a red line between the eggs and with the vertical egg on the right, and six with a red line between the eggs and with the vertical egg on the left). Some examples of the stimuli are depicted in Figure 1, and a list of the familiar tools used in the unimanual and bimanual conditions can be found in the Appendix.
Figure 1.
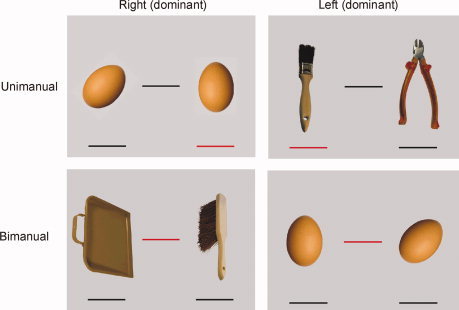
Some examples of tool and control stimuli. The red line indicates whether the pantomime is bimanual (between objects) or unimanual (under one object). The position of the object determines with which hand the action is performed (or execution dominance in bimanual trials) (left object/left hand, right object/right hand). In bimanual control trials, the side of the vertically aligned egg determines the hand that makes the dominant movement. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Procedure
Tool pantomime paradigm
Prior to scanning, the volunteers completed a prescan MRI‐safety questionnaire and the Edinburgh Handedness Inventory. They were instructed that the position of the red line indicated the type of movement to be carried out. If the red line was under one of the objects, a unimanual response was expected, and if the line between the objects was marked red, then a bimanual movement had to be performed. During unimanual pantomimes, the nonactive hand should remain still on the scanner table alongside the body. The participants were also informed that the position of the object indicated with which hand the movement had to be made. If the red line was under the right object, the pantomime had to be executed with the right arm and hand; if it was under the left object, the left arm and hand should be used. If the red line was between objects, the position of the objects still dictated how the bimanual movement was to be performed. The object on the left had to be pantomimed with the left hand, and the one on the right with the right hand. We felt it necessary to switch hands in bimanual conditions as well, because in most bimanual tasks, like pencil sharpening or threading a needle, one hand is clearly dominant over the other, and left‐ and right‐handers would perform these tasks differently. In unimanual control conditions, the volunteers were instructed to pantomime a rotating movement with the wrist while they imagined holding the egg with their fingers. In bimanual control conditions, they were asked to pantomime holding one egg in a central position, while rotating with the other egg around it. In these bimanual conditions, the vertically depicted egg signaled with which hand the dominant movement had to be made. Together, these instructions gave rise to eight different conditions: (1) unimanual right tool pantomime (UniToolRight), (2) unimanual left tool pantomime (UniToolLeft), (3) bimanual right dominant tool pantomime (BiToolRight), (4) bimanual left dominant tool pantomime (BiToolLeft), (5) unimanual right control pantomime (UniControlRight), (6) unimanual left control pantomime (UniControlLeft), (7) bimanual right dominant control pantomime (BiControlRight), and (8) bimanual left control pantomime (BiControlLeft). Participants were presented with several examples of the stimuli until it was ascertained that they understood every instruction.
The volunteers were positioned head first and supine in the magnet. Their left and right arms were positioned comfortably alongside the body on the scanner table. A nylon ribbon was tightened over the chest and arms at the elbows, thus limiting movements of the upper arms. Participants were reminded of the fact that MR‐imaging is very sensitive to movement and were therefore instructed to restrict their head movements and lie as still as possible. Their heads were gently fixed in place with foam cushions. The procedure also required the subjects to perform the pantomimes rather calmly, using only their underarms, wrists, and hands. Stimulus presentation was controlled by a commercially available experiment generator (Presentation, Neurobehavioral Systems Inc., Albany CA) that was digitally synchronized with the MRI‐scanner. The stimuli were back projected on a screen at the back of the magnet bore, and were viewed via a mirror that was attached to the head coil. The paradigm was arranged as a conventional block design with eight conditions, each condition consisting of eight blocks. A block lasted 21 s and contained six stimuli of the same type that were each projected for 3,500 ms. The total experiment thus took 22.4 min. To avoid consecutive presentation of two blocks with the same type of stimuli, blocks were ordered semi‐randomly and the stimuli were distributed at random over their conditions' blocks.
The performance of the participants was monitored continuously by one of the coauthors. All participants were able to do the required pantomimes during their fMRI session. In the postscan session, they all completed a postscan MRI safety questionnaire, and were debriefed.
Saccadic eye movement paradigm
As the bimanual stimulus conditions may give rise to more eye movements than in unimanual conditions, a localizer task was used to define the frontal and parietal eye fields. A yellow dot jumped erratically to different positions on a black screen with a frequency of 2 Hz (i.e., saccade condition), or sometimes remained fixed in the middle of the screen (i.e., rest condition). The number of saccadic eye movements to each quadrant (and within the quadrants of each quadrant) was equated, with a maximal amplitude of 17.2° in the horizontal dimension and 12.1° in the vertical one. The size of the yellow dot was 0.4°. The saccade paradigm was arranged as a blocked design in which volunteers alternated performing the oculomotor task and the control task for 15 s each. Each block was repeated six times, resulting in a paradigm of 3 min.
Scanning Procedure
Scanning was performed at 3.0 T on a Siemens Trio MRI scanner (Siemens Medical Systems, Erlangen, Germany) equipped with echo planar imaging (EPI) capabilities. We used an 8‐channel PA head coil for radio frequency transmission and signal reception. After automatic shimming of the magnetic field on each participant, a 3‐D high‐resolution T 1 anatomical image of the whole brain in the sagittal plane was acquired for coregistration with the functional images (3D MPRAGE, 176 slices, slice thickness = 0.9, in‐plane resolution = 0.9 × 0.9 mm2, TR = 2,530 ms, TE = 2.58). Next, 545 functional EPI images in the axial plane were obtained for the tool pantomime paradigm and 70 for the saccadic localizer. They had the following parameters: TR = 2.5 s, TE = 33 ms; flip angle = 90°, 33 slices, slice thickness = 2.5 mm, slice gap = 1.25 mm, FOV = 192 mm and matrix = 64 × 64, resulting in a resolution of 3 × 3 × 2.5 mm3.
Image Analysis
Data analysis was achieved by means of Brain Voyager QX for preprocessing and statistical inference [Goebel et al., 2006]. Functional data of each paradigm were subjected to a standard sequence of preprocessing steps comprising slice scan time correction by means of sinc interpolation, 3‐D motion correction by spatial alignment to the first volume also by means of sinc interpolation, and temporal filtering using linear trend removal and high pass filtering for low‐frequency drifts of three or fewer cycles. For the volume‐based analysis, spatial smoothing with a Gaussian filter (FWHM = 8 mm) was applied. The anatomical data for each subject were resampled to 1‐mm resolution, and transformed into Talairach standard space using sinc interpolation. The functional data for each subject were coregistered with the subject's 3‐D anatomical dataset and also converted into Talairach space.
For each subject's paradigm, a protocol file was derived that represented the onset and duration of each block for the different conditions. Factorial design matrices were automatically defined from the created protocols. The BOLD response in each condition was modeled by convolving these neural functions with a canonical hemodynamic response function (gamma) to form covariates in a General Linear Model (GLM). After the GLM had been fitted and the effects of temporal serial correlation allowed for (by means of AR(1) modeling, see [Bullmore et al., 1996]), group (random effects procedure) t‐maps were generated to evaluate the effects of tool pantomiming under different conditions. The details of the specific whole‐brain contrasts are described in the respective parts of the results section below. For all analyses of the pantomime paradigm, we used a threshold of P < 0.05 corrected for multiple comparisons using false discovery rate (FDR) correction [Genovese et al., 2002]. For the saccadic localizer, alpha was set to < 0.001 (FDR‐corrected).
Lateralization Indices
Based on the functional data, four regions of interest (ROI) were selected that showed robust lateralization during tool‐use pantomiming: dorsolateral prefrontal cortex (DLPFC, with peak activation in Brodmann Area [BA] 46), premotor cortex (PMC, with peak activity in ventral BA 6), superior parietal lobule (SPL, with peak activation over BA 7), and inferior parietal lobule (IPL, with peak activity in BA 40)(see bounding boxes in Figures 2E and 3E). ROIs were drawn over the four areas using a combination of the functional activation of the entire group (N = 28) based on the all tools conditions > all control conditions contrast (with alpha <0.05, Family Wise Error‐correction), bounded by a priori defined anatomical ROIs that were drawn on a group‐averaged 3‐D anatomical image of the scanned group using the brain atlases of Mai et al. [ 2008] and Talairach and Tournoux [ 1988]. The DLPFC‐ROI measured 15.316 voxels, the PMC‐ROI encompassed 19.050 voxels, the SPL‐ROI counted 23.784 voxels, and the IPL‐ROI consisted of 13.573 voxels. We then mirrored these ROIs to obtain symmetrical ROIs over the left and right hemisphere for each of the four locations. The ROIs were projected over each participant's normalized functional data set to demarcate the activated voxels. To take interindividual variability in cerebral activation into account, the activation threshold was individually adjusted to expose the 10% most active voxels of the total (left and right) ROI volume for each participant [Jansen et al., 2006]. We then counted the number of active voxels in the left and right hemisphere separately for each ROI and calculated the lateralization index (LI) with the formula [(R − L)/(R + L)] × 100, resulting in values that range between +100 (complete right hemispheric lateralization) and −100 (complete left hemispheric lateralization).
Figure 2.
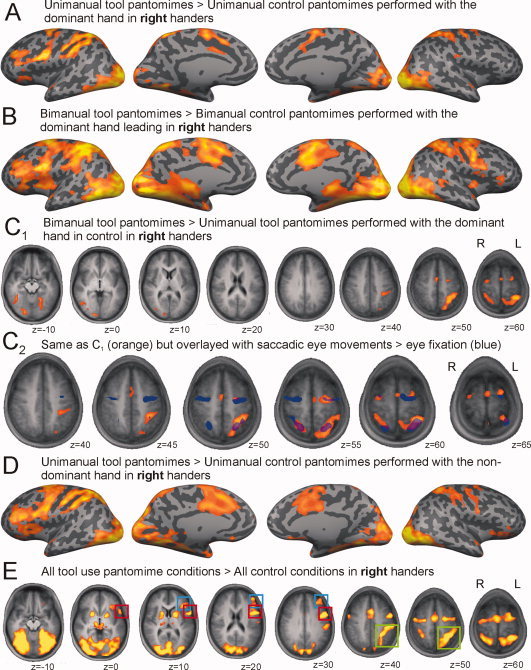
Right‐handed group. Whole‐brain activation during (A) unimanual right hand pantomimes and (B) bimanual pantomimes with the right hand leading projected over an inflated cortical surface mesh. C1: Depicts the contrast between bimanual versus unimanual tool use pantomimes with the right hand (leading). C2: The same contrast is detailed over the superior part of the brain and overlayed with the activation of a saccadic localizer task (in blue) at alpha (FDR) < 0.001. D: Whole‐brain activation during unimanual left hand pantomimes projected on an inflated cortical mesh. E: Describes the contrast between all tool pantomimes versus all control conditions. Colored boxes are drawn over lateralized regions of activation (red, premotor cortex; blue, dorsolateral prefrontal cortex; green, posterior parietal cortex). All pantomime activation maps at alpha (FDR) < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
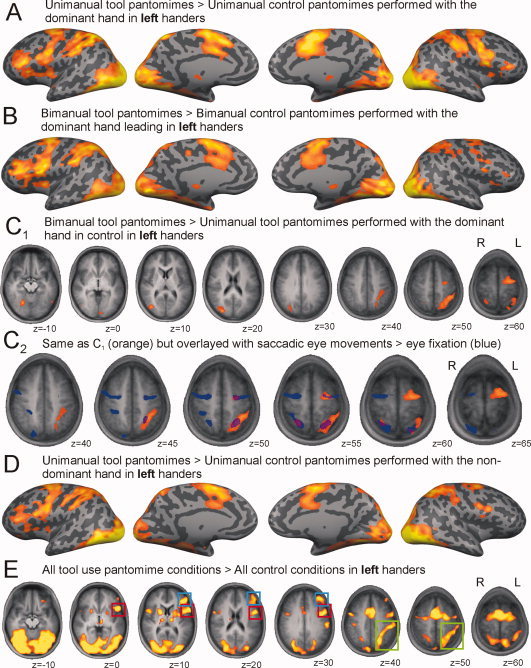
Left‐handed group. Whole‐brain activation during (A) unimanual left hand pantomimes and (B) bimanual pantomimes with the left hand leading projected over an inflated cortical surface mesh. C1: The contrast between bimanual versus unimanual tool use pantomimes with the left hand (leading). C2: The same contrast is detailed over the superior part of the brain and overlayed with the activation of a saccadic localizer task (in blue) at alpha (FDR) < 0.001. D: Depicts whole‐brain activation during unimanual right hand pantomimes projected on an inflated cortical mesh. E: Describes the contrast between all tool pantomimes versus all control conditions. Colored boxes are drawn over lateralized regions of activation (red, premotor cortex; blue, dorsolateral prefrontal cortex; green, posterior parietal cortex). All pantomime activation maps at alpha (FDR) < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
RESULTS
Activation Patterns
Unimanual versus bimanual pantomimes
In right‐handed volunteers, unimanual tool pantomimes that are performed with the dominant upper limb elicit neural activation in precentral (Left [L] = Right [R]), ventral premotor (L ≫ R), dorsolateral prefrontal (L ≫ R), posterior parietal (L > R), inferior temporal (L = R), and occipital (L = R) regions, over and above brain areas that are involved in the execution of simple unimanual motor gestures (Fig. 2A: UniToolRight > UniControlRight). Bimanual tool pantomimes with the dominant hand leading, show a similar and more robust pattern of activation with activation also spreading to the medial temporal region (Fig. 2B: BiToolRight > BiControlRight). When bimanual and unimanual tool pantomiming are compared directly—in each case correcting for single differences between their respective control tasks: (BiToolRight > UniToolRight) ∩ (BiToolRight > BiControlRight) ∩ (UniToolRight > UniControlRight)—it becomes clear that bimanual tool pantomimes result in additional activation in premotor/precentral regions (L > R), the posterior parietal cortex (L ≫ R), and temporo‐occipital regions (R > L) (Fig. 2C1 and upper part of Table I). As the bimanual condition may evoke additional stimulus‐related eye movements, we describe the results of a saccadic localizer in the lower part of Table I and Figure 2C2. The localizer detects symmetric bilateral activation in the frontal and parietal eye fields that partly overlaps with the additional activation elicited by bimanual tool pantomimes.
Table I.
A: Additional brain activation of right‐handers during bimanual compared with unimanual pantomiming of tool use, alpha (FDR) > 0.05; (B) cerebral activation during eye movements compared with eye fixation, alpha (FDR) > 0.001
| Brain region | BA | Talairach coordinates (Left) | Cluster size | t max | Talairach coordinates (Right) | Cluster size | t max | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | ||||||
| A: (BiToolRight > UniToolRight) ∩ (BiToolRight > BiControlRight) ∩ (UniToolRight > UniControlRight) | |||||||||||
| Frontal cluster | |||||||||||
| Superior/middle frontal gyrus | 6 | −22 | −5 | 60 | 1,301 | 5.69 | 30 | −10 | 61 | 652 | 5.31 |
| Medial frontal gyrus | 6 | −1 | −5 | 63 | 5.60 | ||||||
| Parietal clusters | |||||||||||
| Precuneus | 7 | −19 | −64 | 46 | 1,149 | 6.00 | |||||
| Intraparietal sulcus | 7/40 | −27 | −50 | 53 | 11,598 | 7.71 | 29 | −47 | 8 | 2,315 | 5.63 |
| Temporal and occipital clusters | |||||||||||
| Occipital gyri | 19 | 16 | −85 | −5 | 2,987 | 6.66 | |||||
| Fusiform gyrus | 37 | −22 | −55 | −13 | 2,948 | 6.15 | 31 | −54 | −17 | 1,574 | 5.60 |
| Middle temporal gyrus | 37 | 41 | −62 | −2 | 4,003 | 5.98 | |||||
| B: Saccadic eye movements > eye fixation | |||||||||||
| Frontal clusters | |||||||||||
| Precentral gyrus | 4 | −26 | −9 | 53 | 6,600 | 15.17 | 34 | −7 | 52 | 3,002 | 17.63 |
| Parietal cluster | |||||||||||
| Superior parietal lobule | 7 | −28 | −53 | 56 | 6,013 | 21.42 | 24 | −54 | 54 | 4,046 | 23.18 |
| Temporo‐occipital cluster | |||||||||||
| Bilateral visual areas | 18 | −2 | −78 | 1 | 43,262 | 32.48 | 3 | −77 | 6 | 38,872 | 31.48 |
Hand (in)dependency
When unimanual tool pantomimes are performed with the nondominant hand of right‐handed participants (Fig. 2D: UniToolLeft > UniControlLeft), largely the same activation pattern appears as during execution with the dominant hand (compare with Fig. 2A). A direct comparison of control‐corrected unimanual nondominant versus unimanual dominant tool pantomiming—(UniToolLeft > UniToolRight) ∩ (UniToolLeft > UniControlLeft) ∩ (UniToolRight > UniControlRight)—uncovers no surviving clusters in right‐handers. A similar result was obtained when we contrasted bimanual pantomimes with the nondominant hand leading versus bimanual pantomimes with the dominant hand in control. The opposite contrasts (preferred hand > nonpreferred hand) also remained empty.
Tool pantomiming regardless of hand effector
We contrasted all tool pantomime conditions against all control conditions, thus making abstraction of the hand(s) performing the task and maximizing the use of the obtained data set (Fig. 2E). In right‐handers, tool pantomiming versus control evokes neural activation in premotor (L ≫ R), dorsolateral prefrontal (L ≫ R), medial frontal (L = R), posterior parietal (L ≫ R), and temporo‐occipital (L = R) regions.
Effect of handedness
In Figure 3, we depicted exactly the same contrasts for the left‐handed volunteers as we did in Figure 2 for the right‐handers. Unimanual tool pantomimes performed with the dominant upper limb of left‐handed volunteers evokes neural activation in precentral (L = R), premotor (L = R), dorsolateral prefrontal (L ≫ R), posterior parietal (L ≫ R), inferior temporal (L = R), and occipital (L = R) regions, over and above brain areas involved in the execution of simple unimanual motor gestures (Fig. 3A: UniToolLeft > UniControlLeft). In bimanual tool pantomimes, the asymmetry is more prominent (Fig. 3B: BiToolLeft > BiControlLeft). Activated regions for the bimanual > unimanual contrast in the left‐handed volunteers are described in Figure 3C1 and the upper part of Table II: (BiToolLeft > UniToolLeft) ∩ (BiToolLeft > BiControlLeft) ∩ (UniToolLeft > UniControlLeft). In left‐handers, bimanual tool pantomimes elicit additional activation in premotor/precentral regions (L > R), posterior parietal cortex (L > R), and temporo‐occipital regions (R > L). In the same left‐handed participants, the saccadic localizer brought about symmetric bilateral activation in the frontal and parietal eye fields that partly overlaps with the additional activation elicited by bimanual tool pantomimes. Tool pantomime performance with the nondominant hand alone—compared with unimanual control—exhibits asymmetric activation similar to unimanual tool pantomiming with the dominant hand (Fig. 3D: UniToolRight > UniControlRight, compare with Fig. 3B). Analogous to right‐handers, left‐handers show no surviving clusters in the similar control‐corrected contrasts between unimanual and bimanual pantomimes with the nondominant hand (in control) versus unimanual bimanual pantomimes with the dominant hand in control, or vice versa.
Table II.
A: Additional brain activation of left‐handers during bimanual compared with unimanual pantomiming of tool use, alpha (FDR) > 0.05; (B) cerebral activation during eye movements compared with eye fixation, alpha (FDR) > 0.001
| Brain region | BA | Talairach coordinates (Left) | Cluster size | t max | Talairach coordinates (Right) | Cluster size | t max | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | ||||||
| A: (BiToolLeft > UniToolLeft) ∩ (BiToolLeft > BiControlLeft) ∩ (UniToolLeft > UniControlLeft) | |||||||||||
| Frontal cluster | |||||||||||
| Superior/middle frontal gyrus | 6 | −23 | −8 | 61 | 2,274 | 5.96 | |||||
| Parietal clusters idem | |||||||||||
| Intraparietal sulcus | 7/40 | −31 | −51 | 50 | 3,561 | 6.02 | 24 | −54 | 57 | 168 | 5.37 |
| Temporal and occipital clusters | |||||||||||
| Occipital gyri | 19 | 29 | −78 | 23 | 830 | 6.22 | |||||
| 39 | 45 | −72 | 4 | 333 | 5.86 | ||||||
| Cuneus | 17 | −8 | −90 | 4 | 140 | 5.49 | |||||
| Fusiform gyrus | 19 | 22 | 61 | −8 | 554 | 7.07 | |||||
| B: Saccadic eye movements > eye fixation | |||||||||||
| Frontal cluster | |||||||||||
| Precentral gyrus | 4 | −33 | −9 | 49 | 1,524 | 10.82 | 38 | −6 | 50 | 3,412 | 11.60 |
| Parietal clusters | |||||||||||
| Superior parietal lobule | 7 | −23 | −57 | 54 | 1,982 | 11.92 | 21 | −58 | 57 | 1,399 | 10.68 |
| Inferior parietal lobule | 40 | 33 | −36 | 46 | 682 | 9.26 | |||||
| Temporo‐occipital cluster | |||||||||||
| Bilateral visual areas | 18 | −10 | −72 | −1 | 30,106 | 23.37 | 9 | −78 | −1 | 36,301 | 23.37 |
In left‐handers, the all‐tool‐conditions versus all‐control‐conditions contrast displays an almost identical pattern of left lateralized premotor, dorsolateral prefrontal, and posterior parietal activation than that of the right‐handed participants (Fig. 3E, and compare with Fig. 2E). Figure 4A shows the frontal and parietal activation patterns of each individual participant of the right‐ and left‐handed group.
Figure 4.
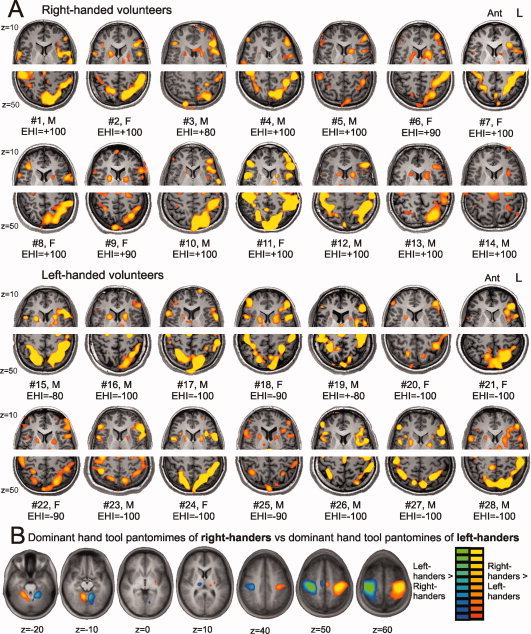
Individual activation patterns and the effect of motor execution. A: Individual activation patterns of right‐ and left‐handed volunteers over the inferior/middle frontal (z = 10) and posterior parietal (z = 50) region. EHI: Edinburgh Handedness Inventory‐score. B: Contrast of the dominant hand tool conditions between right‐ and left‐handers (without control‐correction for motor execution) showing an asymmetric and opposite activation pattern of primary, supplementary, and subcortical motor regions. Activation map at alpha (FDR) < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
An ANOVA was performed on the statistical activation maps with condition as within‐subjects factor and handedness as between‐subjects factor, to test the effect of handedness on the contrasts used thus far: unimanual tool dominant > unimanual control dominant, bimanual tool dominant > bimanual control dominant, bimanual > unimanual, unimanual nondominant > unimanual dominant, and all tools > all control conditions. In comparison with left‐handers, right‐handers show additional activation in a region of 856 voxels with a maximum t‐value of 5.36 over the right precentral gyrus (BA 4, x = 33, y = −23, z = 53) during bimanual compared with unimanual pantomiming. All other contrasts revealed no significant differences in cerebral activation between left‐ and right‐handers. Finally, we directly compared the dominant hand conditions between left‐ and right‐handers without control correction to evaluate the impact of motor execution. The result of the latter comparison is depicted in Figure 4B. Left‐ and right‐handed volunteers reveal a clear‐cut asymmetric and opposite activation pattern over the contralateral primary motor cortex (BA 4), supplementary motor area (medial BA 6), and deep grey nuclei (thalamus and putamen), and over the ipsilateral cerebellum.
Lateralization indices
A summary of the lateralization indices is provided in Table III. A multivariate analysis of variance with the lateralization indices of the four ROIs as within‐subjects factor, and handedness as between‐subjects factor was calculated. A main effect of ROI was found, F[3,24] = 4.64, P = 0.01, indicating that the LIs of different regions show differences in lateralization strength. A main effect of handedness was also obtained, F[1,26] = 8.93, P < 0.01, and unveiled a significantly reduced lateralization in left‐handers. Finally, a ROI × handedness interaction effect was observed, F[3,24] = 3.75, P < 0.05, suggesting that handedness is associated with region specific differences in LI. Post hoc analyses revealed that the reduced lateralization of left‐handed participants is nonsignificant in premotor cortex, shows a trend toward significance in the dorsolateralprefrontal region (P‐value around 0.1), and demonstrates a significant difference in lateralization strength over the inferior parietal area (P < 0.05) and the superior parietal lobule (P < 0.001). In fact, three left handed participants (#18, #19, and #27) showed slight to moderate right hemispheric lateralization in the parietal ROIs (Fig. 4A).
Table III.
Lateralization indices of left‐and right‐handers over the selected ROIs: M (SD)
| Region of interest | Total group (N = 28) | Right‐handed group (n = 14) | Left‐handed group (n = 14) | F‐value | P‐value |
|---|---|---|---|---|---|
| Dorsolateral prefrontal cortex | −80.2 (24.8) | −87.3 (20.8) | −73.0 (27.0) | 2.45 | 0.13 |
| Premotor cortex | −68.8 (37.6) | −74.2 (28.2) | −63.5 (45.5) | 0.55 | 0.47 |
| Inferior parietal lobule | −73.6 (44.3) | −92.6 (11.6) | −54.6 (56.2) | 6.15 | 0.02 |
| Superior parietal lobule | −53.8 (42.7) | −80.4 (31.7) | −27.1 (42.2) | 17.71 | <0.001 |
Finally Pearson correlations (two‐tailed) were calculated between the cerebral LIs of the four ROIs. A significant correlation between the LIs of the premotor and both parietal ROIs was observed (IPL: r = 0.62, P < 0.001; SPL: r = 0.42, P < 0.03). The DLPFC‐LI was significantly associated with the LI of the inferior parietal lobule only (r = 0.49, P < 0.01). Finally, the LIs of both posterior parietal ROIs were significantly correlated (r = 0.71, P < 0.001).
DISCUSSION
Standard unilateral tool pantomiming with the dominant hand in a group of right‐handed volunteers uncovers the typical praxis system activated during the recollection and production of tool use skills [Lewis, 2006]. This network involves the inferior and superior parietal lobules, dorsal and ventral premotor cortex, middle frontal cortex (dorsolateral prefrontal cortex), and the temporo‐occipital area. Apart from the latter region, the former foci reveal a clear left hemispheric lateralization. The putative functions of these regions have been inferred from neuroimaging and neuropsychological research. In short, the superior parietal lobule is thought to combine multimodal sensory inputs to form a common coordinate system that allows on‐line interaction with external objects [Andersen, 1997; Binkofski et al., 1999; Culham et al., 1998; Molenberghs et al., 2007; Tunik et al., 2008; Wolpert et al., 1998]. The inferior parietal lobule is involved with stored knowledge for the skillful manipulation of familiar tools, including hand position and learned gestures, and appears to be implicated in the discrimination of action intention [Buxbaum et al., 2003, 2006, 2007; Vingerhoets, 2008; Vingerhoets et al., 2009, 2010b]. The dorsal premotor cortex is associated with the timing and sequencing of motor commands [Abe et al., 2007; Bortoletto and Cunnington, 2010; Grafton et al., 1998; Nakai et al., 2003], whereas the ventral premotor cortex is involved in the planning and preparation of arm and hand movements [Binkofski et al., 1999; Johnson‐Frey et al., 2003; Rizzolatti et al., 2002]. The middle frontal region, also described as the dorsolateral prefrontal cortex, is particularly left lateralized during tool planning and pantomiming tasks [Grezes et al., 2003; Johnson‐Frey et al., 2005]. Speculation regarding its function range from interpretation of observed prehensile action to motor syntax [Johnson‐Frey et al., 2003; Thoroughman and Shadmehr, 2000]. Bilateral temporo‐occipital activation, in particular the fusiform cortex, is frequently reported in paradigms involving (unfamiliar) tool observation and naming, and appears responsible for the visual processing of the features and contours of tool objects [Creem‐Regehr and Lee, 2005; Vingerhoets, 2008]. We will now turn to a discussion of the impact of hand‐effector and handedness on the activation of the praxis system.
Bimanual Versus Unimanual Pantomimes
In bimanual tool pantomiming, when the nondominant hand joins the action while manipulating a second tool object, we observed increased left lateralized premotor and posterior parietal activation of the classical “neural tool network” described above. Rather than being asymmetrical in favor of the contralateral hemisphere to “steer” the joining nondominant hand, the additional parietal activation is located along the lateral and medial banks of the left intraparietal sulcus. Interestingly, the right posterior parietal activation that survives this contrast is confined to the superior parietal lobule in right‐ and left‐handers (best observed in Figures 2C2 and 3C2). It has been posited that this region is part of the dorso‐dorsal stream, which is a rostral section of the dorsal visual pathway and is believed to serve as an object‐independent stream responsible for the on‐line control of action [Buxbaum and Kalenine, 2010; Rizzolatti and Matelli, 2003; Tanne‐Gariepy et al., 2002]. In line with the functional segregation of the dorso‐dorsal and ventro‐dorsal pathways, we speculate that the increased bilateral activation of the superior parietal lobule during bimanual pantomimes merely reflects the increased difficulty to control complex coordinated movements involving both upper limbs. The increased activation in the left inferior parietal lobule, on the other hand, is the result of the additional recruitment of the ventro‐dorsal pathway that provides additional conceptual input needed to guide the functional manipulation of multiple objects [Buxbaum and Kalenine, 2010; Rizzolatti and Matelli, 2003; Tanne‐Gariepy et al., 2002; Vingerhoets et al., 2009].
Coordinates of the frontal and parietal eye fields (FEF and PEF respectively) elicited by the saccadic localizer task are very similar to the coordinates that were described in previous research on eye movements [Berman et al., 1999]. Maximal PEF activation is typically located near the middle of the intraparietal sulcus, whereas peak activity for the pantomime task is typically located in the anterior and caudal parts of the intraparietal sulcus. The partial overlap suggests that some of the regions that are activated in the pantomime contrast may indeed be related to increased eye movements in the bimanual condition. But clearly, eye movements do not account for all of the remaining activation.
Hand (In)Dependency
In agreement with previous research, our results showed strong left hemispheric lateralization during tool‐use pantomimes in right‐handers regardless of which hand was used [Choi et al., 2001; Johnson‐Frey et al., 2005; Moll et al., 2000]. This leftward asymmetry was very similar in the left‐handed group, and indeed no significant group differences for this contrast were revealed when both groups were compared in a between‐subjects ANOVA (see further). In contrast with previous literature, we observed no additional activation during actions that were conducted with the preferred hand versus the non‐preferred hand or vice versa.
Since no additional activation in the inferior parietal lobules was registered during pantomiming with the nondominant hand, it appears that this region, which is strongly linked with action semantics and conceptual input, is equally active regardless of hand effector. It is therefore likely that apraxia testing with the nonpreferred hand can provide an adequate estimation of the functional deficit expressed by damage to praxis representations.
Effect of Handedness
Between‐group comparison in an ANOVA model did not unveil any significant differences in the activation patterns in right‐ and left‐handers for the contrasts described earlier (with the exception of an increased right precentral activation during a bimanual task in right‐handers). This is a new and noteworthy finding, as there has been a lot of speculation regarding the effect of handedness on the cerebral organization of learned movements. One theory claimed that the left hemispheric dominance for learned movement can be explained by its direct control of the more skilful right hand, and that this dominance would therefore be mirror‐organized in left‐handers [Geschwind and Galaburda, 1985; Goldenberg, 2003; Liepmann, 1920]. Alternatively, manual control and praxis could be seen as separate mechanisms, but that both mechanisms would benefit from sharing the same hemisphere, because such an organization would eliminate the need for interhemispheric transfer [Frey, 2008]. Frey already pointed out that these two hypotheses assume a clear association between handedness and praxis skills, but that case studies of left‐handers with apraxia following left hemispheric lesions and right‐handers with apraxia after right brain damage do not support this assumption [Basso et al., 1985; Hecaen et al., 1981; Kimura and Archibal, 1974; Marchetti and Della Sala, 1997; Rapcsak et al., 1987; Raymer et al., 1999].
If, on the other hand, handedness and praxis are unrelated, and praxis shows left hemispheric preference, then most people, left‐handers included, should present a left hemispheric dominance for praxis [Frey, 2008]. Arguments in favor of this view come from two studies. In the first, 90 epilepsy patients underwent a presurgical intracarotid amobarbital procedure (IAP or Wada‐test), during which each cerebral hemisphere was temporarily inactivated in order to assess language and praxis dominance [Meador et al., 1999]. The ability to pantomime tool use actions appeared closely associated with language dominance, and this relation was irrespective of hand preference. In the second, behavioral study, tool‐use pantomimes were compared in one left‐handed and one right‐handed callosotomy patient. The results of this study revealed right hand (left hemisphere) advantages in both patients, suggesting that the left hemispheres of both right‐ and left‐handed split‐brain patients were specialized for representing acquired tool‐use skills [Frey et al., 2005].
To the best of our knowledge, the present fMRI study provides the first account of motion‐controlled cerebral activation during different effector conditions of tool pantomiming in left‐ and right‐handed participants. The findings of this study are most supportive to the view that handedness and praxis representations are unrelated, seeing that two groups of opposite handedness, but matched for the strength of their handedness, showed only marginal differences in cerebral activation patterns during tool use pantomiming. Recalling and performing pantomimes of learned gestures induces robust left lateralized activity in dorsolateral prefrontal, premotor, and posterior parietal regions compared to control movements. This cerebral activation pattern is observed in right‐ and left‐handed participants, can be demonstrated on the individual level, and reveals no major statistical differences in activation maps between both groups.
In contrast with the similarity in activation pattern, left‐ and right‐handers seem to differ in the degree (not the side) of lateralization with left‐handers showing less asymmetry over the inferior parietal lobule and especially over the superior parietal lobule. Reduced hemispheric specialization in left‐handed individuals has been reported for functions favoring the left hemisphere such as language [Knecht et al., 2000a, b] and for functions favoring the right hemisphere such as face‐selective and body‐selective visual processing [Willems et al., 2010]. This study adds the left hemispheric dominance for praxis to this list.
Hand preference has been attributed to a complex interplay of genetic, anatomical, hormonal, developmental, and cultural factors [Annett, 1973; Corballis, 2009; Geschwind and Levitsky, 1968; McManus and Bryden, 1991; Thatcher et al., 1987]. The observation that left handedness occurs at a low frequency in the human population (between 5% and 25.9% [Raymond and Pontier, 2004]) suggests that left handedness could have an evolutionary cost [Llaurens et al., 2009; Schaafsma et al., 2009]. It remains to be determined whether reduced hemispheric specialization in left‐handers may be relevant for the assessment of fitness differences between left‐ and right‐handers and thus play a role in the evolutionary explanation for the persistence of the handedness polymorphism in humans. Reduced hemispheric specialization may provide an advantage in tasks requiring bihemispheric control such as intermanual coordination, and left‐handers have indeed shown superior performances in such tasks [Gorynia and Egenter, 2000; Judge and Stirling, 2003]. Less pronounced hemispheric specialization is also associated with less accentuated functional deficits following unilateral brain damage [Heiss et al., 2003]. On the other hand, lateralization of cognitive functions is viewed as an efficient evolutionary solution for the maximal exploitation of the limited amount of available cortical space [Levy, 1988], or might be due to functional incompatibility between the logical demands of basic cognitive functions [Vallortigara et al., 1999]. In this case, reduced lateralization could have a cost. These observations suggest that the cost/benefit assessment of hemispheric specialization may be a complex puzzle, with fitness depending on the type of function and the amount of lateralization [Badzakova‐Trajkov et al., 2010]. Future research should investigate the relation between the degree of cerebral lateralization of a given cognitive function and the behavioral proficiency on tasks that depend on that function [Corballis, 2009].
Interestingly, the correlations between the LI‐indices of some ROIs seem to suggest that the degree of lateralization is reproduced in anatomically connected, but topographically remote cortical areas. This reoccurrence of lateralization strengths within individuals performing the same task can be taken to suggest a functional relation based on hemispheric specificity. If, for example, the DLPF‐region is involved with action understanding and motor syntax (combinations of motor primitives)[Johnson‐Frey et al., 2003; Thoroughman and Shadmehr, 2000], it is not illogical to find a significant correlation with the LI of the inferior parietal area believed to play a role in knowledge on the skillful manipulation of familiar tools, rather than with the superior parietal ROI associated with the on‐line control of object interactions. Also, the LI of the premotor ROI linked with the planning of arm and hand movements appears significantly associated with the LIs of the posterior parietal ROIs, but is unrelated to the LI of the adjacent DLPF cortex. Our data suggest the exciting possibility that functional connectivity may also be reflected in the degree of lateralization during task performance, and that the LI of one brain region might be predicted based on the LIs of related areas. Finer‐grained lateralization studies in larger groups of participants showing a wide variety in the amount of individual lateralization are necessary to corroborate these findings.
Limitations of the Study
If the hemispheric lateralization of praxis is linked to language dominance as suggested by the Meador et al. study [Meador et al., 1999; Xu et al., 2009], then a study that compares praxis dominance in left‐ and right‐handers should control for language lateralization, because left‐ and right‐handers exhibit a different variability in language dominance [Badzakova‐Trajkov et al., 2010; Knecht et al., 2000a, b]. However, language dominance was not assessed in the present study and therefore we cannot rule out the possibility that there may be an imbalance in language lateralization between both groups. Nevertheless, this imbalance, if present, did not give rise to any significant differences in praxis lateralization between left‐ and right‐handers. Additional research is necessary to evaluate the strength of the relation between cerebral asymmetry for language and praxis skills.
Because we selected rather extreme left‐handers to match the right‐handers on strength of handedness, we can only conclude that strongly left‐handed individuals show less lateralized posterior parietal activation. It remains to be determined if this trend can be reproduced in a larger sample including people with less extreme left‐handedness scores.
Tools Used in the Unimanual Conditions
Clothes pin, coffee spoon, comb, computer mouse, cup, eraser, filling‐knife, fountain pen, garden shears, hair brush, house key, ice‐cream scoop, office stamp, paintbrush (painting), paintbrush (wall), pincers, salt shaker, sponge, wire brush, wire cutters.
Tools Used in the Bimanual Conditions
Badminton racket and shuttle, ballpoint pen and measuring rule, beer bottle and bottle opener, can and can opener, cork screw and wine bottle, dustpan and brush, fountain pen and notebook, hammer and chisel, knife and fork, lemon and lemon squeezer, nut and nut cracker, oyster and oyster knife, paper and scissors, pencil and pencil sharpener, saw and miter box, screw and screwdriver, tennis racket and ball, thread and needle, tooth paste and tooth brush, whisk and bowl.
REFERENCES
- Abe M, Hanakawa T, Takayama Y, Kuroki C, Ogawa S, Fukuyama H ( 2007): Functional coupling of human prefrontal and premotor areas during cognitive manipulation. J Neurosci 27: 3429–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RA ( 1997): Multimodal integration for the representation of space in the posterior parietal cortex. Philos Trans R Soc Lon Ser B Biol Sci 352: 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett M ( 1973): Handedness in families. Ann Hum Genet 37: 93–105. [DOI] [PubMed] [Google Scholar]
- Badzakova‐Trajkov G, Haberling IS, Roberts RP, Corballis MC ( 2010): Cerebral asymmetries: Complementary and independent processes. Plos One 5: e9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolo A, Cubelli R, Della Sala S ( 2008): Cognitive approach to the assessment of limb apraxia. Clin Neuropsychol 22: 27–45. [DOI] [PubMed] [Google Scholar]
- Basso A, Capitani E, Laiacona M, Zanobio ME ( 1985): Crossed Aphasia—One or more syndromes. Cortex 21: 25–45. [DOI] [PubMed] [Google Scholar]
- Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, Thulborn KR, Sweeney JA ( 1999): Cortical networks subserving pursuit and saccadic eye movements in humans: An FMRI study. Hum Brain Mapp 8: 209–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund HJ ( 1999): A fronto‐parietal circuit for object manipulation in man: evidence from an fMRI‐study. Eur J Neurosci 11: 3276–3286. [DOI] [PubMed] [Google Scholar]
- Bortoletto M, Cunnington R ( 2010): Motor timing and motor sequencing contribute differently to the preparation for voluntary movement. Neuroimage 49: 3338–3348. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Brammer M, Williams SCR, Rabehesketh S, Janot N, David A, Mellers J, Howard R, Sham P ( 1996): Statistical methods of estimation and inference for functional MR image analysis. Magn Reson Med 35: 261–277. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kalenine S ( 2010): Action knowledge, visuomotor activation, and embodiment in the two action systems. Ann NY Acad Sci 1191: 201–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LJ, Sirigu A, Schwartz MF, Klatzky R ( 2003): Cognitive representations of hand posture in ideomotor apraxia. Neuropsychologia 41: 1091–1113. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Menon R ( 2005): On beyond mirror neurons: Internal representations subserving imitation and recognition of skilled object‐related actions in humans. Cogn Brain Res 25: 226–239. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Tang K, Detre JA ( 2006): Neural substrates of knowledge of hand postures for object grasping and functional object use: Evidence from fMRI. Brain Res 1117: 175–185. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle K, Grossman M, Coslett HB ( 2007): Left inferior parietal representations for skilled hand‐object interactions: Evidence from stroke and corticobasal degeneration. Cortex 43: 411–423. [DOI] [PubMed] [Google Scholar]
- Choi SH, Na DL, Kang E, Lee KM, Lee SW, Na DG ( 2001): Functional magnetic resonance imaging during pantomiming tool‐use gestures. Exp Brain Res 139: 311–317. [DOI] [PubMed] [Google Scholar]
- Corballis MC ( 2009): The evolution and genetics of cerebral asymmetry. Philos Trans R Soc B Biol Sci 364: 867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creem‐Regehr SH, Lee JN ( 2005): Neural representations of graspable objects: Are tools special? Cogn Brain Res 22: 457–469. [DOI] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RBH ( 1998): Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol 80: 2657–2670. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Zhu XH, Ugurbil K, Kim SG, Ashe J ( 1997): Functional activation in motor cortex reflects the direction and the degree of handedness. Proc Natl Acad Sci USA 94: 14015–14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey SH ( 2008): Tool use, communicative gesture and cerebral asymmetries in the modern human brain. Philos Trans R Soc B Biol Sci 363: 1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey SH, Funnell MG, Gerry VE, Gazzaniga MS ( 2005): A dissociation between the representation of tool‐use skills and hand dominance: Insights from left‐ and right‐handed callosotomy patients. J Cogn Neurosci 17: 262–272. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Immisch I, Hanakawa T, Bohlhalter S, Waldvogel D, Kansaku K, Wheaton L, Wu T, Hallett M ( 2006): The role of the dorsal stream for gesture production. Neuroimage 29: 417–428. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W ( 1968): Human brain—Left‐right asymmetries in temporal speech region. Science 161: 186–187. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM ( 1985): Cerebral lateralization—Biological mechanisms, associations, and pathology. III. A hypothesis and a program for research. Arch Neurol 42: 634–654. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E ( 2006): Analysis of functional image analysis contest (FIAC) data with BrainVoyager QX: From single‐subject to cortically aligned group general linear model analysis and self‐organizing group independent component analysis. Hum Brain Mapp 27: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G ( 2003): Apraxia and beyond: Life and work of Hugo Liepmann. Cortex 39: 509–524. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Hartmann K, Schlott I ( 2003): Defective pantomime of object use in left brain damage: Apraxia or asymbolia? Neuropsychologia 41: 1565–1573. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Hermsdorfer J, Glindemann R, Rorden C, Karnath HO ( 2007): Pantomime of tool use depends on integrity of left inferior frontal cortex. Cereb Cortex 17: 2769–2776. [DOI] [PubMed] [Google Scholar]
- Gorynia I, Egenter D ( 2000): Intermanual coordination in relation to handedness, familial sinistrality and lateral preferences. Cortex 36: 1–18. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fagg AH, Arbib MA ( 1998): Dorsal premotor cortex and conditional movement selection: A PET functional mapping study. J Neurophysiol 79: 1092–1097. [DOI] [PubMed] [Google Scholar]
- Grezes J, Tucker M, Armony J, Ellis R, Passingham RE ( 2003): Objects automatically potentiate action: An fMRI study of implicit processing. Eur J Neurosci 17: 2735–2740. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight RT ( 2000): Neural representations of skilled movement. Brain 123: 2306–2313. [DOI] [PubMed] [Google Scholar]
- Hammond G ( 2002): Correlates of human handedness in primary motor cortex: A review and hypothesis. Neurosci Biobehav Rev 26: 285–292. [DOI] [PubMed] [Google Scholar]
- Hecaen H, Deagostini M, Monzonmontes A ( 1981): Cerebral organization in left‐handers. Brain Lang 12: 261–284. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Thiel A, Winhuisen L, Muhlberger B, Kessler J, Herholz K ( 2003): Functional imaging in the assessment of capability for recovery after stroke. J Rehabil Med 35: 27–33. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Terlinden G, Muhlau M, Goldenberg G, Wohlschlager AM ( 2007): Neural representations of pantomimed and actual tool use: Evidence from an event‐related fMRI study. Neuroimage 36: T109–T118. [DOI] [PubMed] [Google Scholar]
- Imazu S, Sugio T, Tanaka S, Inui T ( 2007): Differences between actual and imagined usage of chopsticks: An fMRI study. Cortex 43: 301–307. [DOI] [PubMed] [Google Scholar]
- Jansen A, Menke R, Sommer J, Forster AF, Bruchmann S, Hempleman J, Weber B, Knecht S ( 2006): The assessment of hemispheric lateralization in functional MRI—Robustness and reproducibility. Neuroimage 33: 204–217. [DOI] [PubMed] [Google Scholar]
- Johnson‐Frey SH, Maloof FR, Newman‐Norlund R, Farrer C, Inati S, Grafton ST ( 2003): Actions or hand‐object interactions? Human inferior frontal cortex and action observation. Neuron 39: 1053–1058. [DOI] [PubMed] [Google Scholar]
- Johnson‐Frey SH, Newman‐Norlund R, Grafton ST ( 2005): A distributed left hemisphere network active during planning of everyday tool use skills. Cereb Cortex 15: 681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge J, Stirling J ( 2003): Fine motor skill performance in left‐ and right‐handers: Evidence of an advantage for left‐handers. Laterality 8: 297–306. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Ugurbil K, Georgopoulos AP ( 1993): Functional magnetic‐resonance‐imaging of motor cortex—Hemispheric‐asymmetry and handedness. Science 261: 615–617. [DOI] [PubMed] [Google Scholar]
- Kimura D, Archibal Y ( 1974): Motor functions of left hemisphere. Brain 97: 337–350. [DOI] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Drager B, Bobe L, Lohmann H, Ringelstein EB, Henningsen H ( 2000a): Language lateralization in healthy right‐handers. Brain 123: 74–81. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein EB, Henningsen H ( 2000b): Handedness and hemispheric language dominance in healthy humans. Brain 123: 2512–2518. [DOI] [PubMed] [Google Scholar]
- Laimgruber K, Goldenberg G, Hermsdorfer J ( 2005): Manual and hemispheric asymmetries in the execution of actual and pantomimed prehension. Neuropsychologia 43: 682–692. [DOI] [PubMed] [Google Scholar]
- Levy J ( 1988): The evolution of human cerebral asymmetry In: Jerison HJ, Jerison I, editors. Intelligence and Evolutionary Biology. Berlin: Springer Verlag; 157–173. [Google Scholar]
- Lewis JW ( 2006): Cortical networks related to human use of tools. Neuroscientist 12: 211–231. [DOI] [PubMed] [Google Scholar]
- Liepmann H ( 1920): Apraxie In: Brugsch H, editor. Ergebnisse der gesamten Medizin. Wien Berlin: Urban & Schwarzenberg; 516–543. [Google Scholar]
- Liepmann H ( 1920): Apraxie. Ergeb Ges Med 516–540. [Google Scholar]
- Llaurens V, Raymond M, Faurie C ( 2009): Why are some people left‐handed? An evolutionary perspective. Philos Trans R Soc B Biol Sci 364: 881–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T ( 2008): Atlas of the Human Brain, 3 ed Amsterdam: Elsevier. [Google Scholar]
- Marchetti C, Della Sala S ( 1997): On crossed apraxia. Description of a right‐handed apraxic patient with right supplementary motor area damage. Cortex 33: 341–354. [DOI] [PubMed] [Google Scholar]
- McManus IC, Bryden MP ( 1991): Geschwind theory of cerebral lateralization—Developing a formal, causal model. Psychol Bull 110: 237–253. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Loring DW, Lee K, Hughes M, Lee G, Nichols M, Heilman KM ( 1999): Cerebral lateralization—Relationship of language and ideomotor praxis. Neurology 53: 2028–2031. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Mesulam MM, Peeters R, Vandenberghe RRC ( 2007): Remapping attentional priorities: Differential contribution of superior parietal lobule and intraparietal sulcus. Cereb Cortex 17: 2703–2712. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira‐Souza R, Passman LJ, Cunha FC, Souza‐Lima F, Andreiuolo PA ( 2000): Functional MRI correlates of real and imagined tool‐use pantomimes. Neurology 54: 1331–1336. [DOI] [PubMed] [Google Scholar]
- Nakai T, Kato C, Glover GH, Toma K, Moriya T, Matsuo K ( 2003): A functional magnetic resonance imaging study of internal modulation of an external visual cue for motor execution. Brain Res 968: 238–247. [DOI] [PubMed] [Google Scholar]
- Ochipa C, Rothi LJG, Heilman KM ( 1989): Ideational apraxia—A deficit in tool selection and use. Ann Neurol 25: 190–193. [DOI] [PubMed] [Google Scholar]
- Ohgami Y, Matsuo K, Uchida N, Nakai T ( 2004): An fMRI study of tool‐use gestures: Body part as object and pantomime. Neuroreport 15: 1903–1906. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Poeck K, Kerschen M ( 1971): Ideomotor apraxia following right‐sided cerebral lesion in a left‐handed subject. Neuropsychologia 9: 359. [DOI] [PubMed] [Google Scholar]
- Poeck K, Lehmkuhl G ( 1980): Ideatory apraxia in a left‐handed patient with right‐sided brain lesion. Cortex 16: 273–284. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Rothi LJG, Heilman KM ( 1987): Apraxia in a patient with atypical cerebral‐dominance. Brain Cogn 6: 450–463. [DOI] [PubMed] [Google Scholar]
- Raymer AM, Merians AS, Adair JC, Schwartz RL, Williamson DJG, Rothi LJG, Poizner H, Heilman KM ( 1999): Crossed apraxia: Implications for handedness. Cortex 35: 183–199. [DOI] [PubMed] [Google Scholar]
- Raymond M, Pontier D ( 2004): Is there geographical variation in human handedness? Laterality 9: 35–51. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M ( 2003): Two different streams form the dorsal visual system: Anatomy and functions. Exp Brain Res 153: 146–157. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V ( 2002): Motor and cognitive functions of the ventral premotor cortex. Curr Opin Neurobiol 12: 149–154. [DOI] [PubMed] [Google Scholar]
- Rumiati RI, Weiss PH, Shallice T, Ottoboni G, Noth J, Zilles K, Fink GR ( 2004): Neural basis of pantomiming the use of visually presented objects. Neuroimage 21: 1224–1231. [DOI] [PubMed] [Google Scholar]
- Schaafsma SM, Riedstra BJ, Pfannkuche KA, Bouma A, Groothuis TGG ( 2009): Epigenesis of behavioural lateralization in humans and other animals. Philos Trans R Soc B Biol Sci 364: 915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐Planar Sereotaxic Atlas of the Human Brain. Stuttgart: G. Thieme. [Google Scholar]
- Tanne‐Gariepy J, Rouiller EM, Boussaoud D ( 2002): Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: Evidence for largely segregated visuomotor pathways. Exp Brain Res 145: 91–103. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Walker RA, Giudice S ( 1987): Human cerebral hemispheres develop at different rates and ages. Science 236: 1110–1113. [DOI] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R ( 2000): Learning of action through adaptive combination of motor primitives. Nature 407: 742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E, Ortigue S, Adamovich SV, Grafton ST ( 2008): Differential recruitment of anterior intraparietal sulcus and superior parietal lobule during visually guided grasping revealed by electrical neuroimaging. J Neurosci 28: 13615–13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallortigara G, Rogers LJ, Bisazza A ( 1999): Possible evolutionary origins of cognitive brain lateralization. Brain Res Rev 30: 164–175. [DOI] [PubMed] [Google Scholar]
- van Heugten CM, Dekker J, Deelman BG, Stehmann‐Saris FC, Kinebanian A ( 1999): A diagnostic test for apraxia in stroke patients: Internal consistency and diagnostic value. Clinical Neuropsychologist 13: 182–192. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G ( 2008): Knowing about tools: Neural correlates of tool familiarity and experience. Neuroimage 40: 1380–1391. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, Acke F, Vandemaele P, Achten E ( 2009): Tool responsive regions in the posterior parietal cortex: Effect of differences in motor goal and target object during imagined transitive movements. Neuroimage 47: 1832–1843. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, Vandekerckhove E, Honoré P, Vandemaele P, Achten E ( 2011): Neural correlates of pantomiming familiar and unfamiliar tools: Action semantics versus mechanical problem solving? Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets G, Honoré P, Vandekerckhove E, Nys J, Vandemaele P, Achten E ( 2010): Multifocal intraparietal activation during discrimination of action intention in observed tool grasping. Neuroscience 169: 1158–1167. [DOI] [PubMed] [Google Scholar]
- Willems RM, Peelen MV, Hagoort P ( 2010): Cerebral lateralization of face‐selective and body‐selective visual areas depends on handedness. Cereb Cortex 20: 1719–1725. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Goodbody SJ, Husain M ( 1998): Maintaining internal representations the role of the human superior parietal lobe. Nat Neurosci 1: 529–533. [DOI] [PubMed] [Google Scholar]
- Xu J, Gannon PJ, Emmorey K, Smith JF, Braun AR ( 2009): Symbolic gestures and spoken language are processed by a common neural system. Proc Natl Acad Sci USA 106: 20664–20669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwinkels A, Geusgens C, van de Sande P, van Heugten C ( 2004): Assessment of apraxia: Inter‐rater reliability of a new apraxia test, association between apraxia and other cognitive deficits and prevalence of apraxia in a rehabilitation setting. Clin Rehabil 18: 819–827. [DOI] [PubMed] [Google Scholar]


