Abstract
The neural mechanisms behind active and passive touch are not yet fully understood. Using fMRI we investigated the brain correlates of these exploratory procedures using a roughness categorization task. Participants either actively explored a surface (active touch) or the surface was moved under the participant's stationary finger (passive touch). The stimuli consisted of three different grades of sandpaper which participants were required to categorize as either coarse, medium, or fine. Exploratory procedure did not affect performance although the coarse and fine surfaces were more easily categorized than the medium surface. An initial whole brain analysis revealed activation of sensory and cognitive areas, including post‐central gyrus and prefrontal cortical areas, in line with areas reported in previous studies. Our main analysis revealed greater activation during active than passive touch in the contralateral primary somatosensory region but no effect of stimulus roughness. In contrast, activation in the parietal operculum (OP) was significantly affected by stimulus roughness but not by exploration procedure. Active touch also elicited greater and more distributed brain activity compared with passive touch in areas outside the somatosensory region, possibly due to the motor component of the task. Our results reveal that different cortical areas may be involved in the processing of surface exploration and surface texture, with exploration procedures affecting activations in the primary somatosensory cortex and stimulus properties affecting relatively higher cortical areas within the somatosensory system. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: fMRI, passive touch, active touch, texture, roughness
INTRODUCTION
Since Gibson first documented the effect of active over passive touch on the perception of shapes [Gibson,1962] there has been a growing interest in investigating the relative efficiency of one type of tactile exploration over the other [for a review see Symmons et al.,2004]. Although our knowledge of the factors that affect behavioral performance during each type of tactile encoding has increased, it is still poorly understood how active or passive touch affects the processing of tactile information in the somatosensory cortices. Here we investigated how activations in areas within the somatosensory system typically involved in the perception of surface texture are affected by the manner in which the surface is explored.
In tactile texture perception roughness is one of the most important characteristics of a textured surface [Hollins et al.,1993] and it is evident that, at least for fine surfaces, motion plays an important role in extracting roughness information from textured surfaces. Information about the roughness of a surface can be encoded not only through active exploration of that surface but also by the surface rubbing passively against one's skin. Though it is intuitive that active touch (i.e., movement of the fingers over a surface) is the most natural way of exploring a surface, some studies have found that both active touch and dynamic passive touch (i.e., the surface moving under static fingers) elicit a similar perception of roughness [Heller,1989; Lamb,1983; Lederman,1981,1983; Verrillo et al.,1999], leading researchers to conclude that it is the relative motion of the surface against the finger that allows for relevant information related to surface roughness to be encoded, rather than the active exploration of the surface per se. The similar perceptual abilities observed between active and passive touch imply that tactile information is encoded independently of exploration. Indeed, Vega‐Bermudez et al. [1991] suggested that the same tactile inputs are generated in the two situations and processed in a similar way within the central nervous system.
Even though active touch does not change the perception of roughness relative to passive touch, it is nevertheless clear that different mechanisms are engaged during these two exploratory procedures. For example, passive touch involves only the activation of the cutaneous receptors of the skin whereas active touch implies the operation of the cutaneous, kinaesthetic, and proprioceptive senses. Furthermore studies involving electrophysiology have consistently reported suppression of afferent information to the primary somatosensory cortex during active movement, a phenomenon usually referred to as movement‐related gating of sensory transmission [e.g., Chapman and Ageranioti‐Belanger,1991; Chapman et al.,1987]. It is thought that the gating of sensory information in the presence of multiple sensory inputs is necessary for the efficient representation of stimulus properties and this gating has been reported to happen at the level of the lemniscal pathway [Coulter,1974]. In the somatosensory modality, motor gating was first reported with electroencephalography by Giblin [1964]. He observed that somatosensory evoked potentials (SEPs) elicited by electric stimulation of the median nerve at the wrist were diminished during voluntary movement. Later, in two separate studies, Coquery [1978] and Dyhre‐Poulsen [1978] reported that active movement could diminish the perception of tactile information in both humans and cats. The suppression of electrophysiological somatosensory activity due to movement has been widely reported using EEG [e.g., Abbruzzese et al.,1981; Cheron and Borenstein,1987; Cohen and Starr,1987; Giblin,1964; Huttunen and Homberg,1991; Rushton et al.,1981; Tapia et al.,1987] and more recently using magnetoencephalography [MEG; e.g., Kakigi et al.,1997; Kristeva‐Feige et al.,1996].
The suppression of sensory inputs during movement suggests that better behavioral performance would be associated with passive touch than active touch. However this is not the case since, according to Chapman [1994], active touch can allow for the more selective encoding of stimulus properties. For instance, by reducing the speed of movement at critical points along a surface this results in a more strategic and controlled way of encoding sensory information, and consequently optimizes the encoded information for the perceptual task [see Lederman and Klatzky,1987]. Passive dynamic exploration, on the other hand, may not allow for the optimal encoding of information, particularly if the surface is not homogenous, and such exploration may require extracting the critical information from irrelevant or nonoptimal information. On the other hand, behavioral differences between the two exploration procedures can often be reduced such as when the information available to explore is similarly constrained across the different tactile explorations or if a surface is uniform or homogenous in texture.
According to Hollins et al. [2001] and Hollins and Risner [2000] coarse and fine textures are mediated by different tactile receptors in the skin, a theory called the duplex theory of texture perception. Whereas coarse surfaces are perceived based on their spatial variation such as distance between the texture elements, and mediated by the slowly adapting Type I receptors [Blake et al.,1997; Connor and Johnson,1992; Connor et al.,1990; Hsiao et al.,1993; Lederman,1983; Yoshioka et al.,2001] the perception of fine surfaces is more based on temporal factors such as the vibrations elicited on the skin during exploration and mediated by the rapidly adapting receptors [Bensmaïa and Hollins,2003]. According to the duplex theory, the perception of a surface with element size <100 μm is impaired in the absence of movement [Hollins and Risner,2000].
In the present study we investigated brain activity elicited by active and passive dynamic touch while participants performed a roughness categorization task. Based on previous literature, we reasoned that activation in cortical areas typically involved in sensory processing of texture information would be affected by the nature of the exploration of the stimulus. For example, early work by Randolph and Semmes [1974] in monkeys showed that ablation of Brodmann area (BA) 3b in the primary somatosensory cortex impaired shape and texture discrimination, whereas ablation of BA1 impaired texture perception only. Differential activation in these areas has been further observed during discrimination of textured surfaces in both monkeys [Darian‐Smith et al.,1982; Phillips et al.,1988] and humans [Carey et al.,2008]. Another brain region reported to play an important role in roughness discrimination is the secondary somatosensory cortex (SII), contained within the parietal operculum subregion OP1 [Eickhoff et al.,2006a,b]. Unilateral and bilateral ablation of SII in monkeys has been shown to impair texture discrimination [Garcha and Ettlinger,1980; Ridley and Ettlinger,1976,1978]. Using fMRI, activation in the medial and lateral OP and in the posterior insula has been observed in humans during both haptic exploration of textured surfaces [Stilla and Sathian,2008] and tasks involving roughness estimation [Kitada et al.,2005]. Another set of studies using positron emission tomography (PET) have also reported increased activation in the lateral OP for roughness over length discrimination [Ledberg et al.,1995; O'Sullivan et al.,1994; Roland et al.,1998]. Taken together, these studies highlight the importance of the lateral OP and the posterior insula in the processing of the microgeometric properties of an object such as its surface texture, rather than its macrogeometry such as its shape‐related characteristics.
The present study was specifically designed not only to investigate activation differences between active and passive touch but also to determine the neural correlates of roughness perception during each exploratory procedure. To that end, participants performed a roughness categorization task in two different exploratory conditions: active touch (AT) and passive touch (PT). All parameters of the task were controlled across conditions except for the voluntary movement of the participant's finger during the AT condition. Although previous behavioral studies have reported no differences between active and dynamic passive touch in roughness discrimination [Heller,1989; Lamb,1983; Lederman,1981,1983; Verrillo et al.,1999], it remains probable that the neural mechanisms underlying each tactile procedure differ. For example, unlike passive touch, active touch involves an input from proprioception, however, it is not clear what effect this input has on the BOLD signal in the primary somatosensory regions in particular. Furthermore, we expected no difference between active and passive touch in activation levels in hierarchically higher regions of the somatosensory system involved in roughness perception, provided behavioral performance was equivalent across these conditions. The aim of our study therefore was to identify the brain network activated during each of these exploratory procedures and to provide insights into which cortical areas are most relevant when performing the roughness categorization task.
METHODS
Participants
Sixteen right‐hand dominant undergraduate and postgraduate students from Trinity College Dublin (seven males; mean age 23.6 years; age range 18–30) took part in this study for nominal pay. All reported normal or corrected‐to‐normal vision, no tactile impairments and no history of neurological or psychological disorders. The study was approved by the Ethics Committee of the School of Psychology in Trinity College Dublin, and accordingly, participants provided written informed consent once the task and the fMRI procedure were fully explained to them.
Stimuli and Apparatus
The stimuli consisted of three different grades of aluminium oxide sandpaper (coarse: P100; medium: P180 and fine: P320) and one flat smooth surface (paper). Our apparatus was adapted from that described by Kitada et al. [2005] and comprised a plain wooden disc to which a wooden dowel was attached through the centre, perpendicular to the disc surface, in order to rotate and provide stability to the device. Each stimulus surface was cut to fit one quarter of the wooden disc and was subsequently glued onto the surface of the disc (see Fig. 1A).
Figure 1.
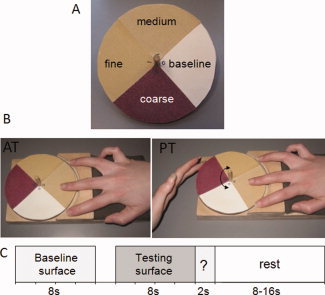
A schematic representation of the stimulus and experimental design. A. Stimuli: the disk contains three different sandpaper surfaces, fine (P320), medium (P180), and coarse (P100) and a flat baseline surface. B. Tactile conditions. During the active touch (AT) condition participants actively explored the surface with their right middle finger while the disk remained stationary whereas during the passive touch (PT) condition the experimenter rotated the disk while the participant's finger remained stationary against the surface of the stimulus. C. Experimental design. Each trial began with the presentation of the baseline surface for 8 s immediately followed by the testing surface for another 8 s. The participant was then required to make a button response categorizing the roughness of the testing surface as either “smooth,” “rough,” or “medium.”
Design and Procedure
The experiment was based on a within‐subjects design with two main factors: exploration procedure (active touch and passive touch) and surface roughness (fine, medium, and coarse). The two exploratory procedures were run in separate blocks.
Practice Session
Prior to scanning, all participants underwent a practice session to become trained on the active and passive exploratory procedures and to be exposed to all the testing stimuli. For the AT condition, we constrained the movement rate at which the participant's finger moved over the stimulus to a frequency of 0.7 Hz. This meant that during the 8‐s exploratory period participants moved their middle finger laterally 11 times (either from right to left or from left to right). Participants were instructed not to make any movement other than lateral movement and the experimenter monitored this movement until the participant performed it correctly. They practiced the lateral exploratory procedure several times against a smooth surface, i.e., not a testing surface, first with auditory cues to help them learn the movement rhythm and then without any auditory cue. Participants repeated this procedure in a self‐timed manner, but at least three times, until they reported having learned the exploratory procedure. The experimenter also observed that they had learned it without the auditory cues. For the passive PT condition participants were instructed to simply rest their fingertip against the surface while the disc was rotated underneath. Again, we constrained the rate at which the stimulus was moved under the participant's finger to 0.7 Hz and the rotation of the disk to 80° so it would be comparable with the finger movement range during the AT condition. Furthermore, participants were instructed to always use the same part of the fingertip and to maintain a comfortable and comparable level of force against the different surfaces during both exploratory conditions. This ensured that the same amount of tactile information was encoded across active and passive exploration conditions. In total, each participant was exposed to each of the testing surfaces twice. As soon as the participant was trained on these procedures they were then taken into the MRI scanner in order to conduct the main experiment.
fMRI Task
In the MR scanner, participants lay in a supine position with their right and left arms positioned alongside their body. Participants placed their middle finger of their right hand over the haptic apparatus and held a response box in their left hand. An experimenter was present in the scanner room to administer the stimuli to the participant. The participant was unable to view the apparatus or stimuli during the experiment. Participants performed a roughness categorization task under two different touch conditions: passive touch (PT) and active touch (AT). The middle finger of the participant's right hand lightly touched the stimulus disk and, depending on the testing condition, the disk was either moved by the experimenter under the participant's stationary finger (PT) or the participant moved their finger over the stimulus (AT). In the PT condition specifically, the experimenter systematically rotated the disk clockwise and anticlockwise. This movement was guided by auditory cues presented over headphones to the experimenter only (i.e., the participant did not receive these auditory instructions). During the AT condition the disk was kept still by the experimenter while the participant moved his/her middle finger across the surface as instructed during the training session (the participant did not receive any auditory instructions during the task) (Fig. 1B).
The participant's task was to explore a tactile surface and to categorize the surface as either coarse, medium, or fine. Throughout the course of the experiment, the participant was cued to either explore the stimulus or to stop exploring and respond via instructions presented visually. These visual instructions were projected onto a mirror placed in the head coil from a panel located behind the scanner. At the same time, the experimenter received the auditory instructions via headphones, indicating the exact timing at which to present the different stimuli and the nature of the stimulus exploration (i.e., AT or PT).
The entire experiment consisted of four 5.4‐min runs with two runs per exploratory condition and each run containing nine trials. The order of the tactile conditions was counterbalanced across participants. Each of the three testing surfaces was presented six times in each condition in a pseudorandom order with the constraint that the same testing surface was not presented in consecutive trials. A trial began with the presentation of the reference surface (i.e., smooth paper) for 8 s, after which the participant was instructed to lift their right middle finger from the apparatus. During the next 2 s the experimenter rotated the disk and placed the testing stimulus, i.e., one of the three sandpapers, underneath the participant's finger. The participant was then visually cued to either move their finger over the testing surface or to remain stationary whilst the experimenter moved the stimulus under the finger for 8 s. At the end of the exploration, the participant was cued to categorize the roughness of the surface by pressing one of three buttons on the response box indicating fine, medium, or coarse surface using their left index, middle, or ring fingers, respectively. A jitter rest period of between 8 and 16 s preceded the next trial (see Fig. 1C).
Data Acquisition
All scanning was conducted using a Philips Intera Achieva 3.0 Tesla MR system (Best, The Netherlands) equipped with a mirror that reflected the 1,280 × 1,024 display, projected on a panel placed behind the participant's head outside the magnet. The mirror was mounted on the head coil in the participant's line of vision. Scanning started with 31.5 s of standard scout images to adjust the head positioning, followed by a reference scan to resolve sensitivity variations.
High‐resolution anatomical images were acquired using an MPRAGE sequence (180 oblique‐axial slices, FOV 230 mm, thickness 0.9 mm, voxel size 0.9 × 0.9 × 0.9, total duration 5.43 min) to allow subsequent activation localization and spatial normalization.
Functional data of the entire brain were collected using a T2* weighted echo‐planar imaging sequence with the following parameters: 32 noncontiguous (10% gap) 3.5‐mm axial slices; TE = 35 ms, TR = 2,000 ms, FOV 224 mm, 64 × 64 mm2 matrix size in Fourier space. Each one of the four functional scans had duration of 5.4 min. The spontaneous changes in frequency were automatically corrected by the Intera Achieva by means of a dynamic stabilization (real time frequency adjustment) after each TR. Imaging used a parallel SENSitity Encoding (SENSE) approach [Pruessmann et al.,1999] with a reduction factor of 2.
Data Analysis
All analyses were conducted using Analysis of Functional Neuroimages software [AFNI, http://afni.nimh.nih.gov/afni; Cox,1996].
After image reconstruction, the time‐series data were then linearly detrended and motion‐corrected using 3D volume registrations (least‐squares alignment of three translational and three rotational parameters). An edge detection algorithm was used to remove activations from outside the brain area and functional and anatomical images were coregistered.
The analysis of functional data included data acquired during all trials, i.e., even the trials where participants categorized surface roughness incorrectly (see below for region‐of‐interest analysis involving data acquired to either all trials or to trials in which the participant correctly responded). For each participant a hemodynamic response model was generated for each one of the four surfaces based on the convolution of the corresponding time series with a gamma function [Cox,1996]. A regression analysis followed, comprising five task‐related regressors for each exploration condition (four regressors for the four different textures and one regressor for response) and the motion‐corrected time‐series files were accommodated for nuisance variance. The boxcar regression parameter values for each one of the time series were converted into percentage change scores and served as the block activation measures.
After the anatomical normalization, individual activation maps were warped onto the Talairach space [Talairach and Tournoux,1988] and spatially blurred with a 3‐mm isotropic rms Gaussian kernel filter. Prior to group analysis the activation map for the baseline surface was subtracted from each one of the testing surfaces in the respective tactile exploration conditions (i.e., passive baseline from the passive textures and active baseline from the active textures). This subtraction was conducted to eliminate the motor component of the activation present during the active condition and done in the passive condition for consistency of analysis.
Initial group analysis of the functional data was a whole brain analysis combining both exploratory procedures and the three different surfaces. This initial approach to the data was simply to establish all the brain regions activated by our task independently of the exploratory procedure used. To this end initial group activation maps for each of the three testing surfaces during active and passive exploration were determined with one‐sample t‐tests against the null hypothesis of no activation change. Significant voxels passed a voxelwise statistical threshold (t = 5.23, P ≤ 0.0001) and were required to be part of a larger 63 μL cluster of contiguous significant voxels. This cluster size was determined through a Monte Carlo simulation and resulted in less than a 5% probability of a cluster surviving due to chance. To conduct statistical comparisons between the two exploratory conditions and the three surfaces, the six distinct activation maps (three for each of the two exploratory condition) were combined in a single map which included the voxels that were significant in at least one of the six constituent maps. These maps are normally referred to as “OR‐map” (like the Boolean “OR” operator) and include the clusters that are significantly activated by at least one of the conditions.
The aim of our study was to look specifically at the activation differences between the AT and PT conditions during the roughness categorization task. To accomplish this we performed a voxelwise analysis using a three‐factor ANOVA with two fixed factors including tactile exploration (two levels: active and passive) and surface roughness (three levels: smooth, medium, and rough) and one random factor consisting of participants (16 levels). Unlike the previous approach, this voxelwise ANOVA reveals only the brain areas that show a main effect for tactile exploration or for surface roughness and levels out any brain area that is equally activated by the different conditions.
For this analysis voxels which passed a voxelwise statistical threshold set at P ≤ 0.005 and were part of a larger 284 μL cluster of contiguous significant voxels were included. This cluster size was determined through a Monte Carlo simulation and resulted in less than a 5% probability of significant activation within a cluster surviving due to chance.
Region of Interest Analysis
To explore further the role of the main somatosensory regions activated during the exploration procedures for the roughness task, a region of interest analysis (ROI) was conducted in the primary somatosensory cortex SI and in the parietal operculum region OP1 in the contralateral (left) hemisphere. To avoid the problem of nonorthogonal contrasts [see Kriegeskorte et al.,2009] both ROI coordinates were based on coordinates which have been previously reported in the fMRI literature. The Talairach coordinates for the centre of mass of the left SI region, i.e., the somatosensory region contralateral to the exploration hand, were (−41, −26, 55) [see Blankenburg et al.,2003]. For the OP1 region we determined the coordinates of the ROI to be (−51, −26, 25), based on the work by Eickhoff et al. [2006a,b]. Both ROIs consisted of 10‐mm radius spheres (which were drawn using the AFNI plugin) and for the SI ROI we repeated the analysis for a much smaller sphere of 1.5‐mm radius. The ROI analyses were performed on trials involving exploration of the coarse and fine surfaces to avoid effects due to performance differences across the conditions (performance was consistently better to the extreme surfaces than to the middle surface and there was no difference in performance to the extreme surfaces). For each one of these regions a 2 × 2 ANOVA (surface × tactile exploration) was performed. For these two ROIs two different analyses were performed: for the sensory‐based analysis all trials for each of the different surfaces were included irrespective of the participant's response (i.e., six trials per surface); for the perceptual‐based analysis only those trials in which participants correctly categorized the roughness of the surface were included (i.e., on average five correct trials for the fine and coarse surfaces).
RESULTS
Behavioral Results
The mean accuracy performance across participants for the roughness categorization task is presented in Figure 2. The accuracy scores for each surface was calculated as the percentage of the number of correct responses (i.e., categorizing the surface as coarse, medium, or fine) over the total number of presentations of that surface. The mean overall accuracy was 70.0%, which was well above chance level of 33.3%. A 2 × 3 ANOVA with tactile exploration (AT or PT) and surface roughness (coarse, medium, or fine) was conducted on the accuracy scores and revealed no effect of tactile exploration (F (1,15) = 0.42, n.s.) and a main effect of surface roughness (F (2,30) = 19.38, P < 0.001). There was no interaction between the two factors (F (2,30) = 0.94, n.s.). Bonferroni post‐hoc tests on the main effect of roughness revealed significantly lower accuracy for the medium grit surface (51.6%) than either the coarse (79.7%) or fine surfaces (78.6%; both P < 0.001). Furthermore, an analysis of the errors for the medium surface shows that this surface was systematically misidentified as the fine surface (97.2%), rather than the coarse surface (2.8%; P < 0.0001).
Figure 2.
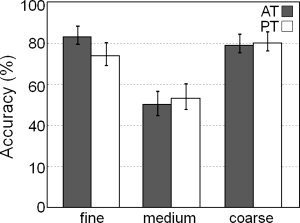
Plot showing the mean accuracy performance across each of the experimental conditions (error bars represent ±1 SEM).
fMRI Results
Our initial analysis looked at the brain areas activated by the categorization task for both exploratory conditions and for all three testing surfaces. The results revealed a wide network of brain regions, including sensory (e.g., left postcentral gyrus) and higher cognitive regions (e.g., left middle forntal gyrus, left insula, and cingulate cortex) activated by the task. The centers of mass of all the clusters across the whole brain are reported in Table I.
Table I.
Brain regions showing significant activity elicited during the roughness categorization task, for all three testing surfaces in both exploratory procedures
| Anatomical structure | BA | Centre of mass | Volume | ||
|---|---|---|---|---|---|
| x | y | z | μl | ||
| Frontal | |||||
| Mid Frontal G | 32/6 | −1 | 8 | 46 | 7630 |
| Mid Frontal G | 6 | −28 | 18 | 55 | 557 |
| Mid Frontal G | 9 | −46 | 18 | 36 | 122 |
| Mid Frontal G | 46 | −41 | 35 | 17 | 76 |
| Mid Frontal G | 9 | 44 | 26 | 29 | 623 |
| Mid Frontal G | 10 | 37 | 49 | 9 | 362 |
| Mid Frontal G | 6 | 24 | −11 | 48 | 225 |
| Precentral G | 6 | −57 | 0 | 31 | 719 |
| Precentral G | 6 | −15 | −18 | 68 | 323 |
| Precentral G | 6 | 57 | 2 | 28 | 293 |
| Cingulate G | 24 | 3 | 2 | 30 | 91 |
| Parietal | |||||
| Postcentral G | 40 | −46 | −31 | 39 | 11800 |
| Postcentral G | 40 | 56 | −22 | 21 | 1212 |
| Inf Parietal Lobe | 40 | 37 | −55 | 42 | 1056 |
| Inf Parietal Lobe | 40 | 51 | −29 | 44 | 64 |
| Temporal | |||||
| Mid Temporal G | 39 | −44 | −74 | 14 | 2968 |
| Mid Temporal G | 39 | −47 | −77 | 29 | 109 |
| Sup Temporal G | 41 | 51 | −31 | 17 | 64 |
| Occipital | |||||
| Fusiform G | 19 | 37 | −73 | −14 | 134 |
| Mid Occipital G | 39 | 38 | −77 | 13 | 306 |
| Culmen | −27 | −39 | −23 | 582 | |
| Culmen | −9 | −53 | 2 | 76 | |
| Precuneus | 31 | −11 | −59 | 20 | 364 |
| Precuneus | 19 | 33 | −71 | 40 | 124 |
| Insula and Subcortical | |||||
| Inf Frontal G | 47 | 38 | 18 | −4 | 1911 |
| Insula | 47/13 | −32 | 19 | 1 | 1545 |
| Insula | 13 | −40 | −6 | 4 | 559 |
| Insula | 13 | 39 | −5 | 2 | 72 |
| Caudate | 12 | 14 | 2 | 203 | |
| Lentiform Nucleus | 20 | 14 | −13 | 291 | |
| Lentiform Nucleus | 37 | −73 | −14 | 190 | |
| Parahippocampal G | 30 | −49 | 6 | 97 | |
Note: The coordinates are given within the framework of the standardized stereotaxic brain atlas of Talairach and Tournoux [1988]. Positive values for x, y, and z denote, respectively, locations to the right, anterior, and superior of the anterior commissure.
To look specifically at the brain activation differences between AT and PT a voxelwise 2 × 3 ANOVA was performed. In this analysis active touch (AT) was contrasted with passive touch (PT) for all three testing surfaces. Unlike the previous analysis, this approach levels out activity that is common to both exploratory conditions and reveals only the brain regions showing a significant differences between active and passive exploration or between the three different surface roughness (see Table II and Fig. 3). Table II reports the centre of mass for each of the clusters revealed by the critical comparisons in the 2 × 3 ANOVA (i.e. tactile exploration × surfaces) and the statistical significance of the comparisons. The overall results revealed significant activations in the left post‐central gyrus, bilateral insula, frontal gyrus, and bilateral cerebellum to both exploration conditions. Further analysis involving one‐sample t‐tests against baseline activation on these regions showed that only the post‐central gyrus cluster, corresponding to the primary somatosensory region (SI), showed significant activity above baseline for both AT and PT exploratory conditions. Furthermore, SI activation levels during the PT condition were roughly half of those observed during the AT condition (0.28% and 0.53% signal change, respectively; F (1,15) = 65.07; P < 0.0001). All the remaining clusters were active above baseline during the AT condition only (see Table II).
Table II.
Brain regions showing significant main effect for exploratory procedure resulting from the voxelwise ANOVA analysis
| Anatomical structure | Hemisphere | Centre of mass | Volume | F (1,15) | P | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | μl | |||||
| Postcentral gyrus | Left | −39 | −30 | 51 | 5303 | A>P | 65.07 | *** |
| Sup temporal lobule | Left | −36 | −65 | 45 | 388 | A>P | 29.43 | *** |
| Sup temporal gyrus | Right | 60 | −10 | 4 | 378 | A>P | 21.01 | *** |
| Insula | Left | −33 | −3 | 7 | 1797 | A>P | 74.54 | *** |
| Insula | Right | 32 | 19 | −1 | 709 | A>P | 38.24 | *** |
| Sup frontal gyrus | Left | −2 | 17 | 56 | 591 | A>P | 24.90 | *** |
| Inf frontal gyrus | Right | 46 | 40 | 0 | 291 | A>P | 29.18 | *** |
| Middle frontal gyrus | Left | −49 | 26 | 32 | 585 | A>P | 26.39 | *** |
| Middle frontal gyrus | Right | 34 | 13 | 31 | 326 | A>P | 38.16 | *** |
| Lentiform nucleus | Right | 26 | 0 | 1 | 533 | A>P | 34.98 | *** |
| Lingual gyrus | Left | −5 | −87 | −14 | 3434 | A>P | 38.52 | *** |
| Cerebellum | Right | 9 | −57 | −28 | 9319 | A>P | 41.73 | *** |
| Cerebellum | Left | −24 | −59 | −34 | 928 | A>P | 22.92 | *** |
Note: The coordinates are given within the framework of the standardized stereotaxic brain atlas of Talairach and Tournoux [1988]. Positive values for x, y, and z denote, respectively, locations to the right, anterior and superior of the anterior commissure. A = Active touch; P = Passive touch (***P < 0.001).
Figure 3.
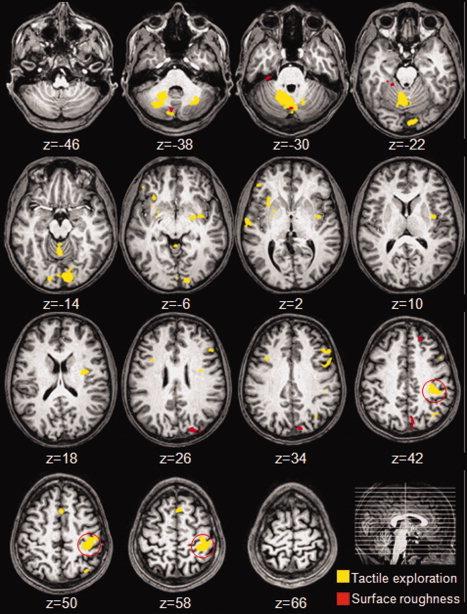
Axial views of the group‐averaged maps for the significant activations superimposed on the brain of an individual participant. The clusters in yellow correspond to regions showing a main effect for tactile exploration, and the clusters in red indicate a main effect for surface roughness. The red circle surrounds the activation cluster in the left primary somatosensory region which was the only cluster significantly activated for tactile explorations. For all contrasts the statistical threshold applied was P < 0.005 (uncorrected) and for the cluster a threshold of P < 0.05 was applied (corrected for multiple comparisons).
A further investigation of the activation cluster in SI was conducted using probabilistic cytoarchitectonic maps [Eickhoff et al.,2005]. This analysis allows us to infer the extent of cortical activation, i.e., whether the activation is focused on SI or if it extends outside the somatosensory regions. The results revealed that a large number of voxels in this cluster (26.2% out of 86.7%) overlapped with Brodmann area 1 (BA1) and a smaller number of voxels overlapped with areas BA2 (19%) and BA3b (13%). Furthermore, although the majority of the voxels in the cluster overlapped with SI, a minority overlapped with motor regions BA4a (8.9%), BA4p (8.0%) and BA6 (8.7%).
The centre of mass for each of the clusters showing a main effect for surface roughness, as revealed by the critical comparisons in the 2 × 3 ANOVA, are reported in Table III. These were the cerebellum (two different clusters), left precuneus and left superior temporal gyrus (see Fig. 3 and Table III). For the cluster in the cerebellum (coordinates 5, −69, −34), significant activity above baseline was observed for the AT condition only but not for PT condition (F (1,15) = 9.68; P < 0.01). Post‐hoc tests in this cluster revealed that the fine and medium surfaces elicited stronger activation compared with the coarse surface (P < 0.001 and P < 0.01, respectively). In all the remaining clusters we found effects of exploratory procedure (see Fig. 3) that were mainly based on differences in deactivations rather than activations for the medium and coarse surfaces only.
Table III.
Brain regions showing significant main effect for surface roughness resulting from the voxelwise ANOVA analysis
| Anatomical structure | Hemisphere | Centre of mass | Volume | F (1,15) | P | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | μl | |||||
| Cuneus | Left | −17 | −82 | 34 | 1071 | F<M<C | 15.71 | *** |
| Cerebellum | Right | 28 | −29 | −26 | 435 | F<M<C | 12.71 | *** |
| Cerebellum | Right | 5 | −69 | −34 | 370 | F=M>C | 12.72 | *** |
| Sup frontal gyrus | Left | −20 | 45 | 46 | 298 | F<M=C | 11.98 | *** |
Note: The coordinates are given within the framework of the standardized stereotaxic brain atlas of Talairach and Tournoux [1988]. Positive values for x, y, and z denote, respectively, locations to the right, anterior and superior of the anterior commissure. F = fine; M = medium, and C = coarse (***P < 0.001).
ROI Analysis
To explore further the role of the main somatosensory regions activated during the exploration procedures for the roughness task a region of interest analysis (ROI) was conducted on activations in the left primary somatosensory cortex and a region in the left parietal opercular, OP1, corresponding to the secondary somatosensory cortex [Eickhoff et al.,2006a,b]. Our ROI analyses were confined to a 2 × 2 ANOVA (as opposed to the 2 × 3 ANOVA previously conducted on the whole brain) as it was necessary to remove activations to the middle surface only since behavioral performance was worst to this condition. In contrast, behavioral performance did not differ across the remaining conditions, either across the fine and coarse surfaces nor across surface roughness (F (1,15) = 1.20, n.s. and F (1,15) = 0.03, n.s., respectively) and there was no interaction between the two factors (F (1,15) = 1.47, n.s.). We were therefore assured that effects observed with the ROI analyses were not due to differences in behavioral performance.
We first conducted a 2 × 2 ANOVA on the sensory data (i.e., brain activation levels across all trials, including those in which roughness was misclassified) with roughness (coarse or fine) and exploration (AT or PT) as factors. The data included all activations centered on the ROIs in the primary somatosensory cortex. This analysis revealed a main effect of tactile exploration (F (1,15) = 28.76, P < 0.0001) and no difference between the activation levels observed across the fine and the coarse surfaces (smooth: 0.42% rough: 0.44%, n.s.). There was no interaction between tactile exploration and surface roughness (F (1,15) = 0.01, n.s.; see Fig. 4A). Further analysis of the main effect of exploration revealed that activation levels during the PT condition were roughly half of those observed during the AT condition (0.32% vs. 0.54%, respectively, P < 0.0001).1
Figure 4.
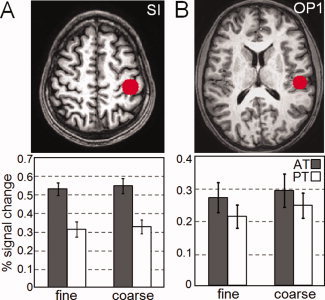
Plots showing the region of interest (ROI) analysis and associated activation differences. A. The ROI was defined as a 10‐mm radius sphere at the centre of mass of the cluster in the contrast of the AT against PT (Talaraich coordinates: x = −41, y = −26, z = 55). The bars indicate the cluster activation (mean ±1 SEM) for the fine and coarse surfaces during both active touch (dark grey) and passive touch (white) conditions. A main effect for tactile exploration (P < 0.0001) was found but no effect of surface roughness. B. The centre‐of‐mass of the ROI over the OP1 region was calculated based on the cytoarchitectonic maps done by Eickhoff et al. [2006a], (Talaraich coordinates: x = −51, y = −22, z = 20, 10‐mm radius). Activation in this region, which comprises the secondary somatosensory cortex, revealed a main effect between the fine and coarse surfaces (P < 0.05) but no difference between the AT and PT conditions.
A similar ANOVA based on brain activations in the OP1 region revealed the opposite effect. Here we found a main effect of surface roughness (F (1,15) = 4.89, P < 0.05) with the coarse surface associated with greater activation levels than the fine surface (coarse = 0.27%; fine = 0.24%; P < 0.05). However, we found no main effect for tactile exploration (AT: 0.28%, PT: 0.23%, n.s.) and no interaction between the two factors (F (1,15) = 0.22, n.s.; see Fig. 4B).
We then conducted an ROI analysis on the data which included activations during those trials to which the participant correctly responded. Analysis of the functional data on correct trials only revealed comparable results to the analysis conducted on all trials. Again, we found a main effect for tactile exploration to activations within the SI region (F (1,15) = 25.56, P < 0.0001) with significantly larger activation levels observed during the AT than PT conditions (0.54% and 0.33%, respectively, P < 0.0001). There was no main effect for surface roughness (F (1,15) < 0.84, n.s.) nor an interaction between the factors observed (F (1,15) < 1, n.s.). For the activations in the OP1, a main effect of surface roughness was observed (F (1,15) = 5.942, P < 0.05) with the coarse surface eliciting stronger activations in this region (0.28%) than the fine surface (0.23%; P < 0.05). As before, there was no main effect of tactile exploration (F (1,15) = 2.306, n.s.) nor an interaction between the factors (F (1,15) < 1, n.s.).
DISCUSSION
The aim of our study was to compare brain activity across active or passive tactile exploration of a stimulus during a roughness categorization task. While many behavioral studies have investigated the effect of exploration procedures on behavioral performance across a range of tasks, most neuroimaging studies on roughness perception have opted for one exploration procedure over the other in the task without evaluating the effect of neural activation elicited by each exploration type. The design of the present study allowed us to look at brain regions where activation patterns varied due to different tactile explorations and/or to the roughness of the stimuli. The results revealed differences in brain activity elicited by active and passive touch in the primary somatosensory region (SI), with stronger activation for active than passive touch. However, differences in surface roughness did not result in a detectable BOLD response difference in this region. In contrast, activation differences across the exploration procedures were no longer observed in the secondary somatosensory region (SII) although activation was related to the roughness of the surface textures (with greater activation for coarse than fine surfaces). In sum, we found that activation in early somatosensory cortex was more related to exploratory procedure than the texture properties of the surface whereas activation in later somatosensory areas was more related to stimulus properties, namely roughness, than exploration type. Thus, our results suggest a hierarchy of processing from how the information is encoded to the nature of the information encoded itself.
Overall, accuracy results for the roughness categorization task were not affected by the tactile exploration procedure since performance was comparable across both the AT and PT conditions. This lack of superiority of one exploratory procedure over the other is in line with many previous studies investigating roughness discrimination through AT and PT when similar information is encoded across exploration conditions [Heller,1989; Lamb,1983; Lederman,1981,1983]. These results also suggest that the relevant information used for roughness perception is invariant to the dynamic procedures with which it is acquired [e.g., Heller,1984; Lederman,1981,1983]. The testing surfaces used in this study were chosen to fall in the two distinct ranges of roughness perception [Hollins et al.,1993]. As such, our surfaces, with element sizes of roughly 162 μm (coarse), 82 μm (medium), and 47 μm (fine), were deliberately chosen to cross the roughness spectrum, such that the coarse surface would require an analysis of spatial variation and the medium and fine surfaces would rely on the motion information present in both exploratory conditions. Our results revealed no interaction between the type of exploration procedure employed (i.e., with or without finger motion) and the roughness of the surface, either on behavioral performance or on neural activations. It is possible, however, that fMRI is unable to reveal any subtle interactions that may exist between exploration type and stimulus roughness within regions of the somatosensory cortex. In any case, our behavioral results showed that categorization of the medium surface was more difficult than categorization of either the coarse or the fine surfaces: accuracy for the medium surface dropped to around 50% and it was systematically misclassified as the fine surface. This bias may be due to the particle sizes for the fine and medium surfaces being closer than the particle sizes for the medium and coarse surfaces and consequently more confusable as the fine surface. Moreover, according to the duplex theory, perception of the fine and medium surfaces are mediated by a different mechanism than the coarse surface, which may also have rendered these surfaces as perceptually more similar. Alternatively, the relatively poor performance for the medium surface might simply be due to a generally higher uncertainty associated with the middle option compared with the extreme surfaces of coarse and fine.
Our initial whole‐brain analysis of the functional data combining activations to both exploratory procedures and to all three surfaces, revealed a wide network of brain regions involved in the roughness categorization task. Specifically, greater activation was observed in the somatomotor region and in higher cognitive areas including the anterior cingulate cortex, the dorsolateral prefrontal cortex (BA9) and the fronto parietal region (BA10), all areas known to be involved in perceptual decision making [Heekeren et al.,2004,2006,2008; Pleger et al.,2006]. This map of cortical activations is similar to that observed in another roughness discrimination study by Kitada et al. [2005] during a roughness estimation task involving textured gratings. They observed activation in prefrontal regions only when participants had to make a judgment about the surface roughness, and not when participants simply felt the surfaces without making any judgments about their roughness. In our task we observed that the prefrontal activation was not different across AT and PT conditions, suggesting that the task complexity was equivalent across the two conditions.
Our main analysis compared activation patterns across the AT and PT conditions using the voxelwise ANOVA approach which levels out activation that is common to both conditions revealing only clusters of activation where there are significant differences between the two exploratory procedures. The main activation difference between the AT and PT conditions was observed in SI, the only area that was active to both exploratory conditions but with larger activation elicited by the AT than the PT condition. An in‐depth investigation of the cytoarchitecture within this activation cluster revealed that it overlaps mostly with somatosensory areas 1, 2, and 3b. These three regions have consistently been reported to include texture sensitive neurons in monkeys [Ageranioti‐Belanger and Chapman,1992; Chapman and Ageranioti‐Belanger,1991; Darian‐Smith et al.,1982; Tremblay et al.,1996], and lesions in area 1 and 3b typically impair texture discrimination [Randolph and Semmes,1974]. Activation in areas 1 and 3b have also previously been implicated in the processing of moving tactile stimuli presented to the passive participant. For example, in a PET study reported by Bodegard et al. [2000], hemodynamic activity in these subregions was observed when participants felt a rotating brush against their fingers while performing a speed recognition task. Area 2 is involved in later stages of somatosensory processing and contains a map of both cutaneous and deep receptors. Its role is to integrate tactile and proprioceptive information both in monkeys [Huffman and Krubitzer,2001; Kaas,1983] and in humans [Mima et al.,1996,1997]. In our experiment, the activity observed in area BA 2 during AT exploration might be influenced by a proprioceptive input to this area. In contrast to activation related to PT, where only cutaneous information is present, activation during the AT condition may be related to both cutaneous and proprioceptive inputs into the primary somatosensory area. As such, the possible increase in the number of activated neurons within a single functional area might result in the observation of a stronger activation level in that cortical area during the AT condition.
Another possible explanation for the difference of activity observed in SI across the exploration types may be due to the input from the motor cortex during the AT condition. It is well known from animal studies that there are reciprocal connections between the primary somatosensory and motor cortices [e.g., Jones et al.,1989; Joschko and Sanderson,1987; Kaas,2004] which allow the exchange of information between the two systems required during motor control [Aschersleben et al.,2001]. In our task the planning and control of the movement during the AT condition required an ongoing exchange of information between the motor and sensory cortices which may have contributed to the result of stronger activation in the primary somatosensory cortex during the AT compared with the PT condition. However, the nature of the input from the motor cortex to areas SI and SII in the human brain is, to our knowledge, unknown therefore it is difficult to evaluate the relative combination of proprioception and kinaesthetic information on activation in these areas during the AT exploration.
Activation differences between AT and PT were also observed in other parts of the brain: a large number of brain areas were recruited during the AT but not during the PT condition. Two of these regions were the cerebellum and the lentiform nucleus, one of the nuclei of the basal ganglia. It is known that the basal ganglia and cerebellum are part of a network involved in motor generation and coordination [for a review see Middleton and Strick,2000] and the basal ganglia‐cerebellar‐cortical network is crucial in both motor tuning and in the timing of movements [Ivry et al.,1988; Salman,2002; Taniwaki et al.,2003]. For example, Taniwaki et al. [2003] observed a strong correlation within this network for self‐initiated movements, but not for externally triggered movements. Similarly, bilateral activation of the cerebellum has been previously observed during memory‐timed finger movement, as opposed to visually cued movement [Kawashima et al.,2000]. In our study, participants were trained outside the scanner to make a highly controlled, self‐generated exploratory movement when exploring the stimulus during the AT condition. Once inside the scanner the timing of this learned movement had to be retrieved from memory. This memory retrieval may have affected activation in the cerebellum and may explain why activation was so extensive and bilateral in this region. Several other cortical areas are the target of cerebellar and basal ganglia outputs, including frontal areas, supplementary motor cortex, and parietal regions. We found that all of these regions showed increased activity during the AT condition, suggesting that these regions are involved in the motor component of the task rather than in the roughness categorization task itself.
Our results also show that the contralateral OP region was selectively activated by the roughness but not exploration conditions in that activation was greater to the coarse than the fine surface. The area OP1 in the parietal operculum, which corresponds to the human secondary somatosensory cortex (SII) [Eickhoff et al.,2006a,b] has been systematically implicated in roughness discrimination [e.g., Kitada et al.,2005; Ledberg et al.,1995; O'Sullivan et al.,1994; Roland et al.,1998; Stilla and Sathian,2008]. Our results confirm the involvement of OP1 in a roughness categorization task. The perception of fine surface textures in the tactile system is thought to rely on the vibration elicited by the relative movement of the surface against the skin [Bensmaïa and Hollins,2003; Hollins and Risner,2000]. In contrast, the perceptions of more coarse textures are thought to rely more on the spatial properties of the stimulus [e.g., Lederman,1983]. Several studies have reported activation in the SI and PO after passive vibratory stimulation [Burton et al.,1993; Coghill et al.,1994; Francis et al.,2000] but whereas activity in SI varies with the amplitude of the vibration [Nelson et al.,2004], activity in SII is modulated by the frequency of the vibration, a measure that is also strongly correlated with roughness [Francis et al.,2000; Harrington and Hunter Downs,2001]. We found a graded activation of the OP1 region which was related to the stimulus properties and to the perceived roughness. Our finding, together with the reports of OP1 activity elicited by the frequency of vibration, further emphasize the role of this region in roughness perception and the role of the relative movement in its perception.
With regards to motor gating effects, little is known about how such gating affects activation in the OP somatosensory area in the human brain. A recent MEG study by Wasaka et al. [2007] reported that activity in SI, elicited by electrical stimulation of the index fingers, is always suppressed by either ipsi‐ or contralateral thumb movement, while activation in area OP1 could be suppressed, enhanced or not affected. Compared with SI, the OP1 is considered to serve a higher level of cognitive function in tactile perception and is known to be modulated by attention [Hsiao et al.,1993]. Our results also point in this direction since activation in OP1 was related to roughness perception but was not related to the nature of the exploration procedure. Moreover, that activation differences were not found in higher levels of the somatosensory cortex across the AT and PT conditions is consistent with the idea that there was no difference in behavioral performance across these exploratory procedures.
CONCLUSIONS
We investigated the neural correlates of AT and PT in roughness perception and our findings suggest that differences between the two exploratory procedures are manifested in activation patterns in area SI, the primary area of somatosensory input, but not in a hierarchically higher somatosensory area (OP) where no difference between the two procedures was observed. The finding that area OP1 was equally activated by AT and PT exploration, may underlie the equivalent behavioral performance across these conditions. This somatosensory area is, however, selectively activated by differences in surface roughness both from the sensory properties of the stimulus itself but also from the perceived roughness of the surface. In contrast, activations in SI showed no detectable BOLD difference across the surface roughness types. Finally, we also found evidence that the basal ganglia‐cerebellar network, together with the cortical regions receiving its afferents, is selectively activated for the self‐initiated movement when textured surfaces are actively explored but not during passive stimulation of the surface.
Acknowledgements
The authors thank Dr. Erik O'Hanlon for assistance with data acquisition, the IITAC research project, the HEA PRTLI Cycle 3 program, the National Development Plan, and the Trinity Centre for High Performance Computing for the provision of computational facilities and support.
Footnotes
We repeated this analysis based on a smaller 1.5‐mm sphere ROI centered on the same coordinates which comprised voxels in the somatosensory region only. We observed a similar result of a main effect of tactile exploration only (F (1,15) = 14.45, P < 0.01) with activation levels during the AT condition almost being double of those observed during the PT condition (0.71% vs. 0.44%, respectively, P < 0.0001).
REFERENCES
- Abbruzzese G,Ratto S,Favale E,Abbruzzese M ( 1981): Proprioceptive modulation of somatosensory evoked potentials during active or passive finger movements in man. J Neurol Neurosurg Psychiatry 44: 942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ageranioti‐Belanger SA,Chapman CE ( 1992): Discharge properties of neurones in the hand area of primary somatosensory cortex in monkeys in relation to the performance of an active tactile discrimination task. II. Area 2 as compared to areas 3b and 1. Exp Brain Res 91: 207–228. [DOI] [PubMed] [Google Scholar]
- Aschersleben G,Gehrke J,Prinz W ( 2001): Tapping with peripheral nerve block: A role for tactile feedback in the timing of movements. Exp Brain Res 136: 331–339. [DOI] [PubMed] [Google Scholar]
- Bensmaïa S,Hollins M ( 2003): The vibrations of texture. Somatosens Mot Res 20: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT,Hsiao SS,Johnson KO ( 1997): Neural coding mechanisms in tactile pattern recognition: The relative contributions of slowly and rapidly adapting mechanoreceptors to perceived roughness. J Neurosci 17: 7480–7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenburg F,Ruben J,Meyer R,Schwiemann J,Villringer A ( 2003): Evidence for a rostral‐to‐caudal somatotopic organization in human primary somatosensory cortex with mirror‐reversal in areas 3b and 1. Cereb Cortex 13: 987–993. [DOI] [PubMed] [Google Scholar]
- Bodegard A,Geyer S,Naito E,Zilles K,Roland PE ( 2000): Somatosensory areas in man activated by moving stimuli: Cytoarchitectonic mapping and PET. Neuroreport 11: 187–191. [DOI] [PubMed] [Google Scholar]
- Burton H,Videen TO,Raichle ME ( 1993): Tactile‐vibration‐activated foci in insular and parietal‐opercular cortex studied with positron emission tomography: Mapping the second somatosensory area in humans. Somatosens Mot Res 10: 297–308. [DOI] [PubMed] [Google Scholar]
- Carey LM,Abbott DF,Egan GF,Donnan GA ( 2008): Reproducible activation in BA2, 1 and 3b associated with texture discrimination in healthy volunteers over time. Neuroimage 39: 40–51. [DOI] [PubMed] [Google Scholar]
- Chapman CE ( 1994): Active versus passive touch: Factors influencing the transmission of somatosensory signals to primary somatosensory cortex. Can J Physiol Pharmacol 72: 558–570. [DOI] [PubMed] [Google Scholar]
- Chapman CE,Ageranioti‐Belanger SA ( 1991): Discharge properties of neurones in the hand area of primary somatosensory cortex in monkeys in relation to the performance of an active tactile discrimination task. I. Areas 3b and 1. Exp Brain Res 87: 319–339. [DOI] [PubMed] [Google Scholar]
- Chapman CE,Bushnell MC,Miron D,Duncan GH,Lund JP ( 1987): Sensory perception during movement in man. Exp Brain Res 68: 516–524. [DOI] [PubMed] [Google Scholar]
- Cheron G,Borenstein S ( 1987): Specific gating of the early somatosensory evoked potentials during active movement. Electroencephalogr Clin Neurophysiol 67: 537–548. [DOI] [PubMed] [Google Scholar]
- Coghill RC,Talbot JD,Evans AC,Meyer E,Gjedde A,Bushnell MC,Duncan GH ( 1994): Distributed processing of pain and vibration by the human brain. J Neurosci 14: 4095–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LG,Starr A ( 1987): Localization, timing and specificity of gating of somatosensory evoked potentials during active movement in man. Brain 110 ( Part 2): 451–467. [DOI] [PubMed] [Google Scholar]
- Connor CE,Johnson KO ( 1992): Neural coding of tactile texture: Comparison of spatial and temporal mechanisms for roughness perception. J Neurosci 12: 3414–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CE,Hsiao SS,Phillips JR,Johnson KO ( 1990): Tactile roughness: Neural codes that account for psychophysical magnitude estimates. J Neurosci 10: 3823–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquery J ( 1978): Role of active movement in control of afferent input from skin in cat and man In: Gordon G, editor. Active Touch. Pergamon Press, Oxford: pp 161–169. [Google Scholar]
- Coulter JD ( 1974): Sensory transmission through lemniscal pathway during voluntary movement in the cat. J Neurophysiol 37: 831–845. [DOI] [PubMed] [Google Scholar]
- Cox RW ( 1996): AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Darian‐Smith I,Sugitani M,Heywood J,Karita K,Goodwin A ( 1982): Touching textured surfaces: Cells in somatosensory cortex respond both to finger movement and to surface features. Science 218: 906–909. [DOI] [PubMed] [Google Scholar]
- Dyhre‐Poulsen P ( 1978): Perception of tactile stimuli before ballistic and during tracking movements In: Gordon G, editor. Active Touch. Pergamon Press, Oxford: pp 171–176. [Google Scholar]
- Eickhoff SB,Stephan KE,Mohlberg H,Grefkes C,Fink GR,Amunts K,Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB,Amunts K,Mohlberg H,Zilles K ( 2006a): The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cerebral Cortex 16: 268–279. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB,Schleicher A,Zilles K,Amunts K ( 2006b): The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex 16: 254–267. [DOI] [PubMed] [Google Scholar]
- Francis ST,Kelly EF,Bowtell R,Dunseath WJ,Folger SE,McGlone F ( 2000): fMRI of the responses to vibratory stimulation of digit tips. Neuroimage 11: 188–202. [DOI] [PubMed] [Google Scholar]
- Garcha HS,Ettlinger G ( 1980): Tactile discrimination learning in the monkey: The effects of unilateral or bilateral removals of the second somatosensory cortex (area SII). Cortex 16: 397–412. [DOI] [PubMed] [Google Scholar]
- Giblin DR ( 1964): Somatosensory evoked potentials in healthy subjects and in patients with lesions of the nervous system. Ann NY Acad Sci 112: 93–142. [DOI] [PubMed] [Google Scholar]
- Gibson JJ ( 1962): Observations on active touch. Psychol Rev 69: 477–491. [DOI] [PubMed] [Google Scholar]
- Harrington GS,Hunter Downs J III ( 2001): FMRI mapping of the somatosensory cortex with vibratory stimuli. Is there a dependency on stimulus frequency? Brain Res 897: 188–192. [DOI] [PubMed] [Google Scholar]
- Heekeren HR,Marrett S,Bandettini PA,Ungerleider LG ( 2004): A general mechanism for perceptual decision‐making in the human brain. Nature 431: 859–862. [DOI] [PubMed] [Google Scholar]
- Heekeren HR,Marrett S,Ruff DA,Bandettini PA,Ungerleider LG ( 2006): Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proc Natl Acad Sci USA 103: 10023–10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heekeren HR,Marrett S,Ungerleider LG ( 2008): The neural systems that mediate human perceptual decision making. Nat Rev Neurosci 9: 467–479. [DOI] [PubMed] [Google Scholar]
- Heller MA ( 1984): Active and passive touch: The influence of exploration time on form recognition. J Gen Psychol 110: 243–249. [DOI] [PubMed] [Google Scholar]
- Heller MA ( 1989): Texture perception in sighted and blind observers. Percept Psychophys 45: 49–54. [DOI] [PubMed] [Google Scholar]
- Hollins M,Risner SR ( 2000): Evidence for the duplex theory of tactile texture perception. Percept Psychophys 62: 695–705. [DOI] [PubMed] [Google Scholar]
- Hollins M,Faldowski R,Rao S,Young F ( 1993): Perceptual dimensions of tactile surface texture: A multidimensional scaling analysis. Percept Psychophys 54: 697–705. [DOI] [PubMed] [Google Scholar]
- Hollins M,Bensmaia SJ,Washburn S ( 2001): Vibrotactile adaptation impairs discrimination of fine, but not coarse, textures. Somatosens Mot Res 18: 253–262. [DOI] [PubMed] [Google Scholar]
- Hsiao SS,O'Shaughnessy DM,Johnson KO ( 1993): Effects of selective attention on spatial form processing in monkey primary and secondary somatosensory cortex. J Neurophysiol 70: 444–447. [DOI] [PubMed] [Google Scholar]
- Huffman KJ,Krubitzer L ( 2001): Area 3a: Topographic organization and cortical connections in marmoset monkeys. Cereb Cortex 11: 849–867. [DOI] [PubMed] [Google Scholar]
- Huttunen J,Homberg V ( 1991): Modification of cortical somatosensory evoked potentials during tactile exploration and simple active and passive movements. Electroencephalogr Clin Neurophysiol 81: 216–223. [DOI] [PubMed] [Google Scholar]
- Ivry RB,Keele SW,Diener HC ( 1988): Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp Brain Res 73: 167–180. [DOI] [PubMed] [Google Scholar]
- Jones SJ,Halonen JP,Shawkat F ( 1989): Centrifugal and centripetal mechanisms involved in the “gating” of cortical SEPs during movement. Electroencephalogr Clin Neurophysiol 74: 36–45. [DOI] [PubMed] [Google Scholar]
- Joschko MA,Sanderson KJ ( 1987): Cortico‐cortical connections of the motor cortex in the brushtailed possum (Trichosurus vulpecula). J Anat 150: 31–42. [PMC free article] [PubMed] [Google Scholar]
- Kaas JH ( 1983): What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol Rev 63: 206–231. [DOI] [PubMed] [Google Scholar]
- Kaas JH ( 2004): Evolution of somatosensory and motor cortex in primates. Anat Rec A Discov Mol Cell Evol Biol 281: 1148–1156. [DOI] [PubMed] [Google Scholar]
- Kakigi R,Shimojo M,Hoshiyama M,Koyama S,Watanabe S,Naka D,Suzuki H,Nakamura A ( 1997): Effects of movement and movement imagery on somatosensory evoked magnetic fields following posterior tibial nerve stimulation. Brain Res Cogn Brain Res 5: 241–253. [DOI] [PubMed] [Google Scholar]
- Kawashima R,Okuda J,Umetsu A,Sugiura M,Inoue K,Suzuki K,Tabuchi M,Tsukiura T,Narayan SL,Nagasaka T, et al. ( 2000): Human cerebellum plays an important role in memory‐timed finger movement: An fMRI study. J Neurophysiol 83: 1079–1087. [DOI] [PubMed] [Google Scholar]
- Kitada R,Hashimoto T,Kochiyama T,Kito T,Okada T,Matsumura M,Lederman SJ,Sadato N ( 2005): Tactile estimation of the roughness of gratings yields a graded response in the human brain: An fMRI study. Neuroimage 25: 90–100. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N,Simmons WK,Bellgowan PS,Baker CI ( 2009): Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci 12: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristeva‐Feige R,Rossi S,Pizzella V,Lopez L,Erne SN,Edrich J,Rossini PM ( 1996): A neuromagnetic study of movement‐related somatosensory gating in the human brain. Exp Brain Res 107: 504–514. [DOI] [PubMed] [Google Scholar]
- Lamb GD ( 1983): Tactile discrimination of textured surfaces: Psychophysical performance measurements in humans. J Physiol 338: 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledberg A,O'Sullivan BT,Kinomura S,Roland PE ( 1995): Somatosensory activations of the parietal operculum of man. A PET study. Eur J Neurosci 7: 1934–1941. [DOI] [PubMed] [Google Scholar]
- Lederman SJ ( 1981): The perception of surface roughness by active and passive touch. Bull Psychon Soc 18: 253–255. [Google Scholar]
- Lederman SJ ( 1983): Tactual roughness perception: Spatial and temporal determinants. Can J Psychol 37: 498–511. [Google Scholar]
- Lederman SJ,Klatzky RL ( 1987): Hand movements: A window into haptic object recognition. Cognit Psychol 19: 342–368. [DOI] [PubMed] [Google Scholar]
- Middleton FA,Strick PL ( 2000): Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Brain Res Rev 31: 236–250. [DOI] [PubMed] [Google Scholar]
- Mima T,Terada K,Maekawa M,Nagamine T,Ikeda A,Shibasaki H ( 1996): Somatosensory evoked potentials following proprioceptive stimulation of finger in man. Exp Brain Res 111: 233–245. [DOI] [PubMed] [Google Scholar]
- Mima T,Ikeda A,Terada K,Yazawa S,Mikuni N,Kunieda T,Taki W,Kimura J,Shibasaki H ( 1997): Modality‐specific organization for cutaneous and proprioceptive sense in human primary sensory cortex studied by chronic epicortical recording. Electroencephalogr Clin Neurophysiol 104: 103–107. [DOI] [PubMed] [Google Scholar]
- Nelson AJ,Staines WR,Graham SJ,McIlroy WE ( 2004): Activation in SI and SII: The influence of vibrotactile amplitude during passive and task‐relevant stimulation. Brain Res Cogn Brain Res 19: 174–184. [DOI] [PubMed] [Google Scholar]
- O'Sullivan BT,Roland PE,Kawashima R ( 1994): A PET study of somatosensory discrimination in man: Microgeometry versus macrogeometry. Eur J Neurosci 6: 137–148. [DOI] [PubMed] [Google Scholar]
- Phillips JR,Johnson KO,Hsiao SS ( 1988): Spatial pattern representation and transformation in monkey somatosensory cortex. Proc Natl Acad Sci USA 85: 1317–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger B,Ruff CC,Blankenburg F,Bestmann S,Wiech K,Stephan KE,Capilla A,Friston KJ,Dolan RJ ( 2006): Neural coding of tactile decisions in the human prefrontal cortex. J Neurosci 26: 12596–12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessmann KP,Weiger M,Scheidegger MB,Boesiger P ( 1999): SENSE: Sensitivity encoding for fast MRI. Magn Reson Med 42: 952–962. [PubMed] [Google Scholar]
- Randolph M,Semmes J ( 1974): Behavioral consequences of selective subtotal ablations in the postcentral gyrus of Macaca mulatta . Brain Res 70: 55–70. [DOI] [PubMed] [Google Scholar]
- Ridley RM,Ettlinger G ( 1976): Impaired tactile learning and retention after removals of the second somatic sensory projection cortex (SII) in the monkey. Brain Res 109: 656–660. [DOI] [PubMed] [Google Scholar]
- Ridley RM,Ettlinger G ( 1978): Further evidence of impaired tactile learning after removals of the second somatic sensory projection cortex (SII) in the monkey. Exp Brain Res 31: 475–488. [DOI] [PubMed] [Google Scholar]
- Roland PE,O'Sullivan B,Kawashima R ( 1998): Shape and roughness activate different somatosensory areas in the human brain. Proc Natl Acad Sci USA 95: 3295–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton DN,Rothwell JC,Craggs MD ( 1981): Gating of somatosensory evoked potentials during different kinds of movement in man. Brain 104: 465–491. [DOI] [PubMed] [Google Scholar]
- Salman MS ( 2002): The cerebellum: It's about time! But timing is not everything—New insights into the role of the cerebellum in timing motor and cognitive tasks. J Child Neurol 17: 1–9. [DOI] [PubMed] [Google Scholar]
- Stilla R,Sathian K ( 2008): Selective visuo‐haptic processing of shape and texture. Hum Brain Mapp 29: 1123–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symmons M,Richardson B,Wuillemin D ( 2004): Active versus passive touch: Superiority depends more on the task than the mode In: Ballesteros S, Heller M, editors. Touch, Blindness and Neuroscience. Madrid: Universidad Nacional de Educacion a Distancia; pp 179–185. [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain. New York: Thieme. [Google Scholar]
- Taniwaki T,Okayama A,Yoshiura T,Nakamura Y,Goto Y,Kira J,Tobimatsu S ( 2003): Reappraisal of the motor role of basal ganglia: A functional magnetic resonance image study. J Neurosci 23: 3432–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia MC,Cohen LG,Starr A ( 1987): Selectivity of attenuation (i.e., gating) of somatosensory potentials during voluntary movement in humans. Electroencephalogr Clin Neurophysiol 68: 226–230. [DOI] [PubMed] [Google Scholar]
- Tremblay F,Ageranioti‐Belanger SA,Chapman CE ( 1996): Cortical mechanisms underlying tactile discrimination in the monkey. I. Role of primary somatosensory cortex in passive texture discrimination. J Neurophysiol 76: 3382–3403. [DOI] [PubMed] [Google Scholar]
- Vega‐Bermudez F,Johnson KO,Hsiao SS ( 1991): Human tactile pattern recognition: Active versus passive touch, velocity effects, and patterns of confusion. J Neurophysiol 65: 531–546. [DOI] [PubMed] [Google Scholar]
- Verrillo RT,Bolanowski SJ,McGlone FP ( 1999): Subjective magnitude of tactile roughness. Somatosens Mot Res 16: 352–360. [DOI] [PubMed] [Google Scholar]
- Wasaka T,Kida T,Nakata H,Akatsuka K,Kakigi R ( 2007): Characteristics of sensori‐motor interaction in the primary and secondary somatosensory cortices in humans: A magnetoencephalography study. Neuroscience 149: 446–456. [DOI] [PubMed] [Google Scholar]
- Yoshioka T,Gibb B,Dorsch AK,Hsiao SS,Johnson KO ( 2001): Neural coding mechanisms underlying perceived roughness of finely textured surfaces. J Neurosci 21: 6905–6916. [DOI] [PMC free article] [PubMed] [Google Scholar]


