Abstract
Objective: To test the influence of functional cerebral reorganization in amyotrophic lateral sclerosis (ALS) on disease progression. Methods: Nineteen predominantly right‐handed ALS patients and 21 controls underwent clinical evaluation, functional Magnetic Resonance Imaging (fMRI), and diffusion tensor imaging. Patients were clinically re‐evaluated 1 year later and followed until death. For fMRI, subjects executed and imagined a simple hand‐motor task. Between‐group comparisons were performed, and correlations were searched with motor deficit arm Medical Research Council (MRC) score, disease progression ALS Functional Rating Scale (ALSFRS), and survival time. Results: By the MRC score, the hand strength was lowered by 12% in the ALS group predominating on the right side in accordance with an abnormal fractional anisotropy (FA) limited to the left corticospinal tract (37.3% reduction vs. controls P < 0.01). Compared to controls, patients displayed overactivations in the controlateral parietal (P < 0.004) and somatosensory (P < 0.004) cortex and in the ipsilateral parietal (P < 0.01) and somatosensory (P < 0.01) cortex to right‐hand movement. Movement imagination gave similar results while no difference occurred with left‐hand tasks. Stepwise regression analysis corrected for multiple comparisons showed that controlateral parietal activity was inversely correlated with disease progression (R 2 = 0.43, P = 0.001) and ipsilateral somatosensory activations with the severity of the right‐arm deficit (R 2 = 0.48, P = 0.001). Conclusions: Cortical Blood Oxygen Level Dependent (BOLD) signal changes occur in the brain of ALS patients during a simple hand‐motor task when the motor deficit is still moderate. It is correlated with the rate of disease progression suggesting that brain functional rearrangement in ALS may have prognostic implications. Hum Brain Mapp 34:2391–2401, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: fMRI, plasticity, prognosis, controlateral activation, DTI
INTRODUCTION
Neuroplasticity refers to structural and functional brain reorganizations that occur during normal aging or cerebral lesions and reflects the adaptative power of the brain to compensate functional loss [Caramia et al., 1996; Pascual‐Leone et al., 1996]. In amyotrophic lateral sclerosis (ALS) which is a consequence of a progressive degeneration of brain and spinal cord motor neurons, functional imaging studies have shown modulations of cortical and subcortical activity during the completion of motor and cognitive tasks, suggesting a reorganization of the motor system with recruitment of extramotor [Abrahams et al., 1996; Abrahams et al., 1996; Konrad et al., 1996; Konrad et al., 1996; Schoenfeld et al., 1996; Tessitore et al., 1996] and motor‐related areas [Luppino et al., 1996]. This activity is modulated with disease progression but whether it increases or decreases is controversial [Lulé et al., 1996; Mohammadi et al., 1996].
However, the dynamics of functional brain rearrangements in ALS is still poorly understood and whether this could have a prognostic value on disease progression and survival is unknown. While progression rates in ALS are usually linear with a median survival of 3 years, a great interindividual heterogeneity exists. A few clinical indicators have been associated with longer survival but predicting the rate of clinical decline in individual patients remains delicate [Magnus et al., 1996]. Here, we performed a study in ALS patients and were able to show that early brain cortical modifications correlated with the severity of motor deficit and the disease progression.
MATERIALS AND METHODS
Subjects
Nineteen patients with a definite diagnosis of ALS according to the revised El Escorial criteria [Brooks et al., 1996] and receiving riluzole were compared to 21 healthy controls. The initial ALS functional rating scale (ALSFRS) was >35/40 corresponding to an early stage of the disease. None of the subjects had any past medical history of cerebrovascular disease, neoplasia, hypertension, diabetes, alcoholism, or psychiatric illness. They were not depressed (Montgomery‐Asberg depression rating scale (MADRS) score <20/60), or demented (minimental state examination (MMSE) score >27/30) and had normal neuropsychological evaluation of frontal lobe functions on the Tower of London, Stroop, and verbal fluency tests (see Supporting Information Table 1). Handedness was tested using the Edinburgh handedness inventory (EHI) [Oldfield et al., 1996]. Before inclusion, written informed consent was obtained from all subjects according to the Declaration of Helsinki, and the study was approved by the regional Research Ethics Committee.
Table 1.
Characteristics of the two groups at inclusion
| ALS (n =19) mean (SD) | Controls (n =21) mean (SD) | P | |
|---|---|---|---|
| Age (years) | 63.8 (8.7) | 60.3 (8.0) | 0.20 |
| Sex ratio (% male) | 1.4 (57.8) | 1.1 (52.3) | 0.31 |
| Handedness EHI (% right handed) | 91.0 (24.0) | 93.5 (19.5) | 0.77 |
| MADRS/60 | 8.4 (5.1) | 2.6 (3.4) | 0.0003 |
| MMSE/30 | 29.1 (1.3) | 29.6 (0.7) | 0.20 |
| Clinical onset | 9LL‐7UL‐3B | n.a. | ‐ |
| Disease duration (months) | 18.2 (9.6) | n.a. | ‐ |
| ALSFRS/40 | 35.3 (2.7) | n.a. | ‐ |
| Norris limbs functional score/63 | 56 (5.9) | n.a. | ‐ |
| Norris bulbar functional score/39 | 37.4 (3.3) | n.a. | ‐ |
| MRC total limb and neck score/150 | 139.1 (11.3) | 150 (0) | <0.0001 |
| Total upper limb score/70 | 65.9 (4.2) | 70 (0) | 0.0002 |
| Total lower limb score/70 | 63.1 (11.4) | 70 (0) | 0.0006 |
| Neck score/10 | 10 (0) | 10 (0) | >0.99 |
MADRS, Montgomery‐Asberg Depression Rating Scale; MMSE, Mini‐Mental State Examination; n.a, not applicable; LL, lower limb; UL, upper limb; B, bulbar.
Study Design
We conducted a cross sectional fMRI/DTI study with a clinical longitudinal follow‐up. At inclusion, the patients and the controls underwent a full clinical evaluation, recording of ALSFRS, MRC scores, Norris limbs and bulbar scales [Brooks et al., 1996], and an MR examination, including fMRI and DTI. Then patients underwent a quarterly evaluation during which MRC scores and ALSFRS were recorded. After 1 year of follow‐up, the rate of disease progression was calculated as: (ALSFRS score at inclusion – ALSFRS score at last follow‐up)/(time in months from inclusion to last follow‐up). Survival time after inclusion was noted up to death or tracheotomy or after a 5‐year follow‐up corresponding to the end of the study. Clinical evaluations were performed blind of the MRI results.
Image Acquisition
The MR examination comprised sequences including 3D‐T1 anatomical, BOLD fMRI, and diffusion acquisitions on a 1.5 T Siemens Symphony scanner using an eight‐channel head coil.
Each fMRI session allowed to record 171 volumes using axial single‐shot gradient‐echo echo‐planar sequence (TR/TE: 2,880/66 ms, flip angle: 90°, acquisition matrix: 64 × 64, FoV: 256 mm, 28 contiguous 4‐mm slices).
Diffusion imaging was performed using a single‐shot spin‐echo echo‐planar sequence with two b values (0 and 1,000 s/mm2) along each of 12 gradient axes. One b = 0 image was acquired per dataset. The following parameters were used: TR/TE 6,000/89 ms, flip angle 90°, one acquisition, matrix 64 × 64, and field of view 240 × 240 mm2. Forty‐four contiguous 3.5‐mm slices were then acquired twice.
Experimental Design and fMRI Paradigm
The subjects performed two visually paced simple motor tasks. They had to execute or to imagine the action of opening and closing the hand with an autogenerated rhythm alternating with a rest condition. The two conditions were performed in a random sequence of 30‐s blocks. During the rest condition, the subjects were asked to stay still and to stare at a white cross. Each volunteer performed one motor session with the right hand and another with the left hand. Just before scanning, the subjects were trained to the experimental tasks in a dummy environment until the task was clearly understood and correctly performed. During scanning, the subjects were observed to ensure that the tasks were performed and to confirm the absence of movement during mental imagery. At the end of the imagination sequence, the patients were asked whether they have executed the task.
DATA ANALYSIS
Clinical Data
The nonparametric Mann–Whitney test was used for between‐group analyses. A paired t‐test was conducted to compare patients' clinical characteristics at initial and final evaluation.
fMRI Data
Preprocessing and statistical analysis were performed using SPM2 software
Preprocessing
All scans of each individual were realigned to the first image of each session to correct for movement artefacts. The data from all the subjects had translation corrections <3 mm and rotation corrections <3°. A mean functional volume was constructed for each subject from the realigned images. Functional images were normalized to a template brain image created by the Montreal Neurological Institute (MNI). The mean functional image was used to determine the parameters for the spatial normalization process of the EPI images. The parameters estimated from this normalization process were then applied to each of the functional images. The resulting voxel size in standard stereotaxic coordinates was 4 × 4 × 4 mm3. The normalized images were smoothed with a Gaussian kernel of 8‐mm full‐width half‐maximum.
Statistics
We searched for significantly activated voxels displaying the effects of interest using a two‐level random‐effect analysis. In a first level (fixed effects), we performed a single‐subject analysis. The task‐related neural activities for each condition were modeled with a canonical hemodynamic response function. Data were high‐pass filtered, with a cut off period set to 128 s. Serial correlations were corrected using an autoregressive model. Individual contrast images reflecting the contrasts of interest (activation > rest or rest > activation) for each subject were then obtained and entered into a second level (random effects) analyses. In this second level, within‐group (one sample t‐test) and between‐group (ANOVA) comparisons were carried out with the total MRC score as covariate to smooth the effect of the between‐patients variance in the global motor deficit. Statistical parametric maps were first thresholded at P < 0.001 at the voxel level then thresholded at P < 0.05 corrected for multiple comparisons at the cluster level. Anatomical labeling was performed by projecting the results on the mean anatomical group images and with the help of the automated anatomical labeling and anatomy toolboxes [Eickhoff et al., 1996; Tzourio‐Mazoyer et al., 1996].
Correlations Between Cortical Activations and Clinical Data
We searched for correlations between modifications of brain activity in the ALS group comparatively to controls and different clinical variables estimating the patient deficits or illness progression. We used the SPSS software to perform a stepwise multivariate regression analysis to select the clinical variable that independently explained BOLD signal changes in patients. The BOLD signal changes in regions with modified activity comparatively to controls were the dependent variable, and age, disease progression rate at last follow‐up, survival time since inclusion, and the lateralization of the motor deficit in the upper limbs (RUL/UL) were independent variables. The significant threshold was set to P < 0.05 after Bonferroni correction for multiple comparisons. For each task of both hands, first peak coordinates of significant clusters (P < 0.05) from the between group analyses were used as center of spherical regions of interest (ROIs). For each ROI, the activity estimates (β values) were extracted on individual contrast images and averaged for all the voxels within each of the regions. Then correlation analyses were performed using these activity estimates. To assess the lateralization of motor deficit, we created a specific ratio to take into account the possibility that brain modulation might depend on the lateralization of the motor deficit in the upper limbs. This ratio was calculated as (right or left upper limb MRC score)/(total upper limb MRC score) and was designated as the RUL/UL ratio for the right side and the LUL/UL ratio for the left side; the lower the ratio, the greater the contribution of that side to the whole deficit of the upper limbs.
DTI Data Processing
The DTI datasets were processed using DtiStudio (http://www.DtiStudio.org, Department of Radiology, Johns Hopkins University). Any potential small bulk motion or Eddy‐current distortion was removed, the b‐matrix modified after motion correction, and the images were realigned by affine transformation using Automated Image Registration [Woods et al., 1996]. The diffusion tensors were calculated for each voxel using multivariant linear fitting. After computation of eigenvalues and eigenvectors, fractional Anisotropy (FA) maps were obtained [Pierpaoli et al., 1996]. We used SPM2 and in‐house software written in Matlab to normalize images to the International Consortium for Brain Mapping (ICBM) standard template using a two‐step procedure. We first created a customized template of our FA maps based on the brains of all 40 participants. The first normalization step was performed using a 12‐parameter affine transformation and b=0 images. These transformation parameters were applied to each individual FA map, which were then averaged and smoothed (with a Gaussian kernel of 8‐mm full‐width half‐maximum) to create the customized FA template. In the second normalization step, a 12‐parameter affine transformation was used to match the original FA images of each individual to the FA template and was refined using 16 nonlinear iterations, medium regularization and a 25‐mm cut off. Each normalized image was finally resampled at 3 × 3 × 3 mm and smoothed with an 8‐mm full‐width half‐maximum kernel. The resulting FA maps of controls and ALS were compared using both a whole brain voxel‐based analysis (VBA) and a ROI study.
VBA
FA group differences were investigated using analysis of covariance in each voxel of the brain using the general linear formulation of SPM2. Age and gender were included as covariates in the model. Statistical parametric maps were thresholded at P < 0.05 corrected at the cluster level. MRIcro software was used for figure captions [Rorden et al., 1996].
ROI study
Six elliptical ROIs were manually drawn along the CST using the b = 0 and FA maps using DTIStudio/RoiEditor and standardized guidelines based on location and size. They included the cerebral peduncle, the internal capsule, and the stem of the corona radiata bilaterally. Mean and standard deviation were computed for each ROI and every subject. An ANOVA analysis with age and gender as covariates was computed for each ROI to investigate potential group differences.
RESULTS
Subject Characteristics
The characteristics of the patients and controls are summarized in Table 1. The MRC subscores of the hand were 17.75 ± 2.5/20 for the right hand and 18.88 ± 1.1/20 for the left hand indicating that patients had a minor hand deficit at first evaluation. As the RUL/UL ratio (0.48, SD 0.02) was significantly lower than the LUL/UL ratio (0.52, SD 0.02) (P = 0.009), the severity of motor deficit was greater on the right side. There was a significant correlation between the MRC score in the right upper limb and the RUL/UL ratio (Pearson 0.898, P < 0.001), but not for the left side (Pearson −0.361, P = 0.13). Thus, for a given patient, the lower the RUL/UL ratio, the greater the deficit in the right upper limb (Fig. 1).
Figure 1.
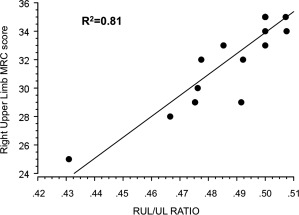
Correlation between the MRC score in the right upper limb and the RUL/UL ratio. RUL/UL ratio: right upper limb MRC score/total upper limb MRC score.
After the initial assessment, patients were regularly followed. One patient committed suicide, three went out of the study because of an important fatigue, and 15 patients were clinically reinvestigated 12 months later (mean 11.8; SD 1.1). As expected, there was a significant worsening of their neurological status (Table 2). The RUL/UL ratio was still significantly lower than the LUL/UL ratio (P = 0.04). The 15 patients except one died or underwent tracheotomy (one case) during the follow‐up (mean (SD) survival time after inclusion: 32.3 (8.6) varying from 13.2 to 48.5 months). The last patient was still alive at the end of the study after 64 months.
Table 2.
Clinical data of patients at inclusion (n = 19) and after 12 months of follow‐up (n= 15)
| ALS | Initial assessment mean (SD) | Follow‐up mean (SD) | P |
|---|---|---|---|
| Time since initial assessment (months) | – | 11.8 (1.1) | – |
| ALSFRS /40) | 34.4 (3.1) | 29.3 (2.5) | 0.0001 |
| Norris limbs functional score (/63) | 52.9 (7.1) | 44.1 (10.3) | 0.01 |
| MRC total limb and neck score (/150) | 138.3 (8.1) | 121.6 (14.5) | 0.005 |
| RUL/UL ratio | 0.48 (0.03) | 0.48 (0.04) | 0.567 |
| Disease progression rate | ‐ | 0.4 (0.2) | – |
*P < 0.05; **P < 0.005.
fMRI
Group activation maps (Fig. 2)
Figure 2.
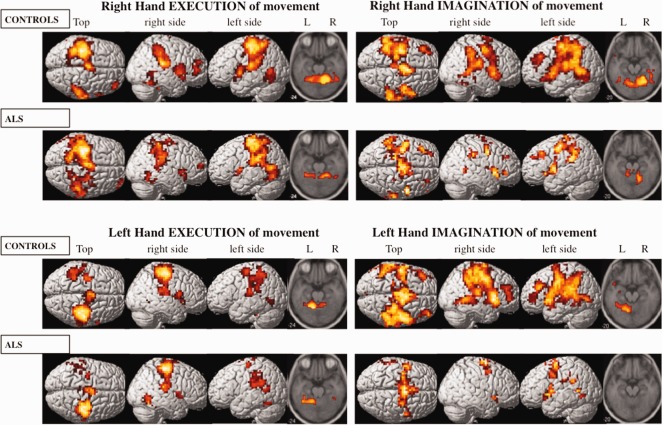
fMRI group activation maps during right‐hand (top two rows) and left‐hand (bottom two rows) tasks in controls and ALS groups. Execution and imagination of movements are in left and right panels, respectively. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Right‐hand tasks
During execution of right‐hand movement, the control group displayed activations in the controlateral primary motor cortex (BA 4), mid‐cingulate gyrus (BA 32, 24) and basal ganglia (thalamus and putamen), the ipsilateral frontopolar region (BA 10, 46) and the bilateral SMA (BA 6), primary somatosensory cortex (BA 3, 1, 2), parietal cortex (BA 40), temporal inferior cortex (BA 37), temporo‐operculo‐insular region (BA 22, 45), and cerebellum. The ALS group showed the same activations, except for the lateral premotor cortex (BA 6) and basal ganglia, which were activated bilaterally.
During imagination of right‐hand movement, the control group displayed bilateral activations in the SMA (BA 6), lateral premotor (BA 6), prefrontal dorsolateral (BA 9), motor (BA 4), somatosensory (BA 3, 1, 2), parietal (BA 40), mid‐cingulate gyrus (BA 24, 32), temporo‐operculo‐insular (BA 22, 45), superior and medial temporal (BA 21, 22) cortex, putamen, thalamus, and cerebellum. The ALS group displayed, to a lesser extent, the same bilateral activations as the controls except for no activation in the basal ganglia and a bilateral activation of the precuneus (BA 7).
Left‐hand tasks
The brain activity pattern for execution of left‐hand movements was very similar to the execution of right‐hand movements in patients and controls. However, in the control group, the frontopolar activation was absent and, in both groups, temporo‐operculo‐insular activation was only controlateral to movement.
During imagination of left‐hand movement, the control group showed the same bilateral activations as for the right hand but with additional activations in the contralateral precuneus and bilateral frontopolar cortex. The ALS group displayed less activity, restricted to the bilateral SMA, lateral premotor cortex, mid cingulate gyrus, temporo‐operculo‐insular area, and the ipsilateral parietal cortex.
Between group comparisons
Right‐hand tasks
During right‐hand‐side tasks, significant higher brain activity was seen in the ALS patients than in the control group (ALS > controls) but no decrease of activation was elicited in the ALS group.
In the ALS group, execution of right‐hand movement induced stronger BOLD signal changes than in controls in the controlateral motor, the somatosensory, and the parietal cortices (including precuneus) as well as in the ipsilateral somatosensory and parietal cortices.
During imagination of right‐hand movement, the ALS patients showed increased activity compared to controls in the left motor and somatosensory cortex (Fig. 3 and Table 3).
Figure 3.
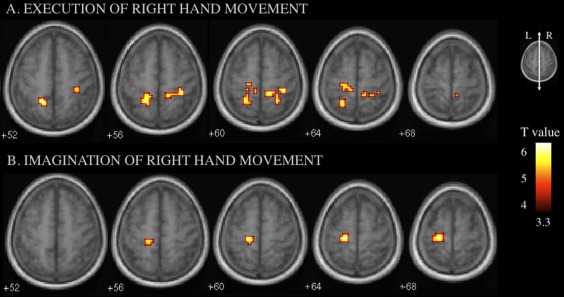
fMRI between‐group comparisons (ALS > controls). The corresponding regions and coordinates are shown in Table 3. A: During execution of right‐hand movement, increased activity in the left motor cortex and bilateral somatosensory and parietal cortex was observed in the ALS patients. B: During imagination of right‐hand movement, patients showed additional activations in the left motor and somatosensory cortex compared to controls. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 3.
Brain areas showing higher activity in ALS compared to controls during execution or imagination of movement of the right hand
| Side of hemisphere activation | Brain region (BA) | MNI coordinates x, y, and z (mm) | P Cluster | Cluster size (voxels) | T |
|---|---|---|---|---|---|
| Execution of right‐hand movement | |||||
| Left | Superior parietal lobule (5) | −16, −52, 52 | 0.004 | 49 | 5.28 |
| Somatosensory (3, 1, 2) | −12, −32, 64 | 0.004 | 49 | 4.02 | |
| Motor (4) | −20, −28, 64 | 0.004 | 49 | 3.81 | |
| Right | Somatosensory (3, 1, 2) | 36, −36, 60 | 0.010 | 41 | 5.04 |
| Parietal (5) | 24, −52, 60 | 0.010 | 41 | 4.24 | |
| Imagination of right‐hand movement | |||||
| Left | Somatosensory (3, 1, 2) | −16, −32, 60 | 0.017 | 35 | 5.52 |
| Motor (4) | −20, −28, 64 | 0.017 | 35 | 5.98 | |
BA, brodman area; P cluster, corrected probability of nondifference at the cluster level; T, value of peak voxel.
Left‐hand tasks
We did not find any significant difference between the two groups for the left‐hand‐side tasks.
Correlations Between Brain Activity and Clinical Data in ALS Patients
Activity from execution of right‐hand movement
By stepwise regression analysis, age was an independent variable of ipsilateral parietal activity (Standardised Coefficient SC= 0.67, R 2 = 0.41, P = 0.001) and the RUL/UL ratio of ipsilateral somatosensory activations (right somatosensory cortex: SC= ‐0.61, R 2 = 0.48, P = 0.001). In addition, disease progression rate at 1 year was an independent variable of controlateral parietal areas activity (left superior parietal lobule: SC= −0.66, R 2 = 0.39, P = 0.001; left precuneus: SC= −0.69, R 2 = 0.43, P = 0.001) (corrected for multiple comparison) (Fig. 4).
Figure 4.
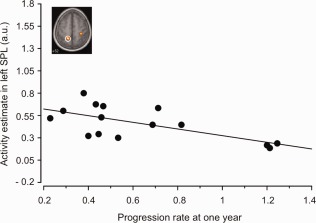
Brain activity differences between ALS and controls during movement of the right hand and linear correlation between the superior parietal lobule (SPL) activations and the disease progression at 1 year. a.u., arbitrary unit. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Activity from imagination of right‐hand movement
We did not find any significant correlation between activity from imagination of right‐hand movement and clinical data.
DTI
VBA
Group comparison revealed a decreased FA in the CST of ALS patients compared to the controls in a region of the left corona radiata projecting to the precentral gyrus (Fig. 5).
Figure 5.
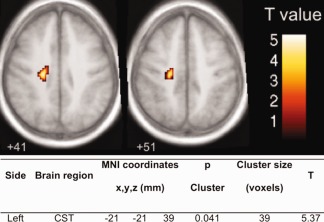
Whole brain voxel‐based group comparison of the FA scores of ALS patients and controls. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
ROI analysis
The ALS patients showed a decreased FA in both cerebral peduncles compared to the controls (right peduncle: controls 0.20, ALS 0.13, P < 0.01; left peduncle: controls 0.19, ALS 0.12, P < 0.01) with a decrease of 34.2% in the right cerebral peduncles and 37.3% in the left one.
DISCUSSION
Using a simple motor task in fMRI, our study demonstrated (1) that increased cortical BOLD signal changes occurred in specific regions of the brain of ALS patients when their motor deficit was still moderate, and that this early signal changes correlated with (2) the lateralization of the motor deficit or hand predominance, and more importantly, (3) with the rate of disease progression at 1 year, suggesting that modulations of cerebral activity in ALS may have functional implications.
The population of ALS patients included in this work was different from those in previous fMRI studies. First, the age (mean: 63.8 years) was more representative of sporadic ALS [Annegers et al., 1996] than that in previous studies, which included younger patients (mean age: 44–55.1 years) [Konrad et al., 1996, 2004; Lulé et al., 1996; Stanton et al., 1996; Stanton et al., 1996; Tessitore et al., 1996]. This point is important, because age is an essential factor in brain activation [Mattay et al., 1996; Ward et al., 1996] and structural anatomy [Bennett et al., 1996; Yoon et al., 1996]. In addition, our patients had a shorter disease duration (mean: 18.6 months) at time of first assessment compared to 21.5–50 months in others studies [Konrad et al., 1996; Lulé et al., 1996; Stanton et al., 1996, 2004; Tessitore et al., 1996], making it possible to detect earlier changes. Moreover, the prolonged follow‐up (clinical re‐evaluation after 1 year and follow‐up until the end of the study after 64 months) allowed us to assess the predictive values of cortical reorganization in terms of disease progression. Compared to the matched control group, the ALS patients were mildly depressed on the MADRS score. However, we do not believe that this state of mood influenced our results. Indeed, the patients performed the task properly, producing homogeneous activations in the same way as controls. Moreover, the MADRS score was not correlated with any functional activity (data not shown). One subject in each group was left handed. Each of them showed an individual pattern of activity similar to that of other subjects so that handedness probably did not introduced a significant bias in this study.
The MRC subscores of the hand showed that the testing of the hand was normal at 88.75% for the right hand and 94.4% for the left one. Thus, as hand deficits were only minor, we thought that they were compatible with the realization of the task. The lack of control of performance during fMRI may be a drawback, but we think that the motor paradigm we used for fMRI was effective and not altered by the absence of objective monitoring of the force and frequency of movement, as the results matched the classical pattern of activations obtained with a simple motor task of the hand. Furthermore, the results of the within‐group analysis conformed to those already published.
Another important feature of our population, which was not planned, but proved to be important for the understanding of the modulation of brain activation, was that the burden of UL motor deficit predominated in the right side while patients were predominantly right handed. This result is in accordance with recent studies which showed a strong concordance for the side of onset and handedness in ALS patients with upper limb‐onset [Turner et al., 1996]. In keeping with this clinical observation, the VBA in our patients showed a significant lateralization of FA reduction in the left CST compared to controls and the ROI analysis confirmed this asymmetry. Our DTI results are in accordance with previous DTI studies which have demonstrated significant decreased of FA in the CST and the brain of patients with ALS [Agosta et al., 1996; Ciccarelli et al., 1996; Ellis et al., 1996; Hong et al., 1996; Sach et al., 1996; Sage et al., 1996, 2004; Toosy et al., 1996; Wang et al., 1996] which is thought to reflect upper motor neuron degeneration. Correlations of diffusion parameters with measures of disease duration or progression and disease severity have been established [Ciccarelli et al., 1996; Ellis et al., 1996; Sage et al., 1996; Wang et al., 1996] but so far, no correlations were elicited with the lateralization of the motor deficit.
Imagination and execution of movements share a common neural circuitry in normal subjects [Porro et al., 1996]. In ALS patients, motor imagery may be useful for exploring the functional motor network without the requirement for movement execution. In our study, during the imagination task, both controls and patients recruited motor networks overlapping with those activated during movement execution and the controlateral motor cortex was part of this network. In normal subjects, the involvement of the primary motor cortex (M1) in the mental performance of hand movements is controversial [Nair et al., 1996; Porro et al., 1996], but is strongly supported by studies using transcranial magnetic stimulation [Facchini et al., 1996], magnetoencephalography [Pfurtscheller et al., 1996], event‐related potentials [Romero et al., 1996], and high spatial resolution fMRI [Dechent et al., 1996]. M1 activation has also been reported in ALS patients, [Lulé et al., 1996; Stanton et al., 1996]. In addition to this motor‐shared network, we also found activity in the bilateral lateral premotor cortex, prefrontal dorsolateral cortex, and superior temporal cortex, as well as ipsilateral activation of motor and somatosensory cortex. The lateral premotor cortex is known to participate in the preparation of movement [Geyer et al., 1996], whereas the prefrontal dorsolateral cortex reactivates the representation of a specific motor action during working memory [Lacourse et al., 1996] and is highly implicated in autoinitiated motor movement [Lehéricy et al., 1996]. Moreover, the ALS group also displayed bilateral activation of the posteromedial portion of the parietal lobe and the precuneus. Recent studies suggest a central role for the precuneus in a wide spectrum of highly integrated tasks, including visuospatial imagery, episodic memory retrieval, and self‐processing operations, and that the anterior region of the precuneus is involved in self‐centered mental imagery strategies [Cavanna et al., 1996], which would fit with our results.
While the within‐group analyses were very similar in ALS patients and controls, the between‐groups comparison highlighted higher activity in ALS patients during execution and imagery of the right‐hand movement, but not during the left‐hand tasks. When executing the right‐hand movement, ALS patients presented an increased activation in the controlateral motor, somatosensory, and parietal cortex, as well as in the ipsilateral somatosensory and parietal cortex. During imagination of the right‐hand movement, ALS patients showed a higher activation in controlateral motor and somatosensory cortex. Different patterns of activation/deactivation have been reported in ALS, probably because of different task paradigms and population characteristics [Konrad et al., 1996; Lulé et al., 1996; Schoenfeld et al., 1996; Tessitore et al., 1996; Stanton et al., 1996, 2004]. However, heightened activity in controlateral areas involved in motor execution, sensation, and organization of motor representations have commonly been observed and interpreted as a compensation of the consequences of cell loss in the precentral regions during motor task realization. Usually, two‐thirds of pyramidal tract fibers originate from the precentral areas (BA 4, 6) and one‐third from the somatosensory and parietal areas (BA 3, 1, 2, 40). The loss of precentral neurons, shown by the decrease in FA in this area in our patients, might lead to overactivation of residual neurons from the pyramidal tract and explain the modified cortical activations. The loss of interneuron inhibition and the need for increasing sensory input to maintain motor performance were also hypothesized [Konrad et al., 1996; Schoenfeld et al., 1996; Stanton et al., 1996].
Ipsilateral activation was observed during right‐hand movement but not with movement imagination. Ipsilateral activation observed during execution of hand movement has been reported in ALS [Konrad et al., 1996; Schoenfeld et al., 1996] and is a well‐known phenomenon after acute brain injury, such as stroke [Caramia et al., 1996]. This ipsilateral overactivity was described in areas usually engaged in sensation of movement, but not in execution. This might be explained by reinforcement of movement execution from both sensory areas through transhemispheric connections. As these areas were not overactivated during movement imagination, we may conclude that ipsilateral activations need to be recruited for movement realization only. This assertion must however be modulated by the fact that the absence of significant task‐related signal does not necessarily mean that there was no activation but that the sensitivity of the method may not allow the detection of significant differences. This ipsilateral overactivity was negatively correlated with the RUL/UL ratio. Thus, it implies that patients with the highest relative right‐hand deficit showed the highest level of activity in the right ipsilateral cortex.
An important result was that the ALS patients did not show higher cerebral activity than the controls during left‐hand movements. A default of task execution is unlikely because the within‐group analysis showed a similar pattern of activity compared to the control group. The right‐side predominance of the upper limb deficit was the most likely explanation. As the patients also had simultaneous left‐hand involvement, the absence of overactivation in the controlateral cortex can be interpreted in four ways. First, to develop or to be detected by fMRI, plasticity needs to reach a certain threshold of neuronal loss in the motor cortex. Second, the available resources for brain plasticity are preferentially mobilized for the most affected side. Third, they are mostly attributed to the dominant side, as in our patients. Fourth, the sensitivity of fMRI was not sufficient to detect differences in signal changes.
The most important finding of our study that has not been reported previously in ALS was that development of brain reorganization in the controlateral hemisphere was negatively correlated with disease progression at 1 year. The underlying structural changes occurring at this stage and responsible for BOLD activity changes are of course unknown. They certainly result from neuronal loss or dysfunction but whether they imply mechanisms of plasticity can at present only be guessed. However, over activation of specific cortical areas involved in motor‐task realization or control is more consistent with plasticity than the only result of cell loss. Longitudinal fMRI studies have showed that these changes are transient and occur at the initial phase of the disease while later in the evolution BOLD activity disappears from these regions [Mohammadi et al., 1996]. Whatever the events occurring at the cellular level, patients who developed a high level of brain reorganization experienced a less severe course of their disease. Two hypotheses can be suggested to explain this correlation. First, patients with a slow spontaneous evolution of disease have more time and chance to develop brain rearrangements. Second, the fact that patients are able to develop brain reorganization allows the installation of compensatory mechanisms, which can slow down the course of the disease. Interestingly, the ipsilateral overactivations were not correlated with the disease progression, probably because they were likely to be only involved in the reinforcement of the executed movement, a reinforcement which, in turn, depended on the severity of the right‐hand deficit.
In conclusion, cerebral activations during a simple motor task, investigated by fMRI, may be considered as a biomarker of disease progression. But future longitudinal studies investigating larger cohorts of patients with repeated clinical assessment and fMRI measures are needed to confirm or not whether functional activity can be used as a marker of disease progression in ALS.
Supporting information
Supporting Information Table 1. Neuropsychological assessment of the two groups at inclusion.
ACKNOWLEDGMENTS
The authors are grateful for the contribution of the patients and their relatives. The authors thank the clinicians, Christophe Vial, Frédérique Brudon, and Emmanuel Broussolle, who referred patients to us.
REFERENCES
- Abrahams S, Goldstein L, Kew J, Brooks D, Lloyd C, Frith C, Leigh PN (1996): Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain 119:2105–2120. [DOI] [PubMed] [Google Scholar]
- Abrahams S, Goldstein L, Simmons A, Brammer M, Williams S, Giampietro V, Leigh PN (2004): Word retrieval in amyotrophic lateral sclerosis: A functional magnetic resonance imaging study. Brain 127:1507–1517. [DOI] [PubMed] [Google Scholar]
- Agosta F.Pagani E, Rocca M, Caputo D, Perini M, Salvi F, Prelle A, Filippi M (2007): Voxel‐based morphometry study of brain volumetry and diffusivity in amyotrophic lateral sclerosis patients with mild disability. Hum Brain Mapp 28:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annegers J, Appel S, Lee J, Perkins P (1991): Incidence and prevalence of amyotrophic lateral sclerosis in Harris County Texas 1985–1988. Arch Neurol 48:589–593. [DOI] [PubMed] [Google Scholar]
- Bennett I, Madden D, Vaidya C, Howard D, Howard J (2010): Age‐related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp 31:378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B (1994): El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci 124( Suppl):96–107. [DOI] [PubMed] [Google Scholar]
- Brooks B, Miller R, Swash M, Munsat T, World Federation of Neurology Research Group on Motor Neuron Diseases (2000): El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299. [DOI] [PubMed] [Google Scholar]
- Caramia M, Palmieri M, Giacomini P, Iani C, Dally L, Silvestrini M (2000): Ipsilateral activation of the unaffected motor cortex in patients with hemiparetic stroke. Clin Neurophysiol 111:1990–1996. [DOI] [PubMed] [Google Scholar]
- Cavanna A, Trimble M (2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129:564–583. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Behrens T, Johansen‐Berg H, Talbot K, Orrell R, Howard R, Nunes R, Miller D, Matthews P, Thompson A, Smith S (2009): Investigation of white matter pathology in ALS and PLS using tract‐based spatial statistics. Hum Brain Mapp 30:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechent P, Merboldt KD, Frahm J (2004): Is the human primary motor cortex involved in motor imagery? Brain Res Cogn Brain Res 19:138–144. [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Stephan K, Mohlberg H, Grefkes C, Fink G, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Ellis CM, Simmons A, Jones DK, Bland J, Dawson JM, Horsfield MA, Williams SC, Leigh PN (1999): Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 53:1051–1058. [DOI] [PubMed] [Google Scholar]
- Facchini S, Muellbacher W, Battaglia F, Boroojerdi B, Hallett M (2002): Focal enhancement of motor cortex excitability during motor imagery: A transcranial magnetic stimulation study. Acta Neurol Scand 105:146–151. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Zilles K (2000): Functional neuroanatomy of the primate isocortical motor system. Anat Embryol (Berl) 202:443–474. [DOI] [PubMed] [Google Scholar]
- Hong YH, Sung JJ, Kim SM, Park KS, Lee KW, Chang KH, Song IC (2008): Diffusion tensor tractography‐based analysis of the pyramidal tract in patients with amyotrophic lateral sclerosis. J Neuroimaging 18:282–287. [DOI] [PubMed] [Google Scholar]
- Konrad C, Henningsen H, Bremer J, Mock B, Deppe M, Buchinger C, Turski P, Knecht S, Brooks B (2002): Pattern of cortical reorganization in amyotrophic lateral sclerosis: A functional magnetic resonance imaging study. Exp Brain Res 143:51–56. [DOI] [PubMed] [Google Scholar]
- Konrad C, Jansen A, Henningsen H, Sommer J, Turski P, Brooks B, Knecht S (2006): Subcortical reorganization in amyotrophic lateral sclerosis. Exp Brain Res 172:361–369. [DOI] [PubMed] [Google Scholar]
- Lacourse M, Orr E, Cramer S, Cohen M (2005): Brain activation during execution and motor imagery of novel and skilled sequential hand movements. Neuroimage 27:505–519. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Bardinet E, Tremblay L, Van de Moortele PF, Pochon JB, Dormont D, Kim DS, Yelnik J, Ugurbil K (2006): Motor control in basal ganglia circuits using fMRI and brain atlas approaches. Cereb Cortex 16:149–161. [DOI] [PubMed] [Google Scholar]
- Lulé D, Diekmann V, Kassubek J, Kurt A, Birbaumer N, Ludolph A, Kraft E (2007): Cortical plasticity in amyotrophic lateral sclerosis: Motor imagery and function. Neurorehabil Neural Repair 21:518–526. [DOI] [PubMed] [Google Scholar]
- Luppino G, Rizzolatti G (2000): The organization of the frontal motor cortex. News Physiol Sci 15:219–224. [DOI] [PubMed] [Google Scholar]
- Magnus T, Beck M, Giess R, Puls I, Naumann M, Toyka KV (2002): Disease progression in amyotrophic lateral sclerosis: Predictors of survival. Muscle Nerve 25:709–714. [DOI] [PubMed] [Google Scholar]
- Mattay V, Fera F, Tessitore A, Hariri A, Das S, Callicott J, Weinberger DR (2002): Neurophysiological correlates of age‐related changes in human motor function. Neurology 58:630–635. [DOI] [PubMed] [Google Scholar]
- Mohammadi B, Kollewe K, Samii A, Dengler R, Münte TF (2011): Functional neuroimaging at different disease stages reveals distinct phases of neuroplastic changes in amyotrophic lateral sclerosis. Hum Brain Mapp 32:750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair D, Purcott K, Fuchs A, Steinberg F, Kelso J (2003): Cortical and cerebellar activity of the human brain during imagined and executed unimanual and bimanual action sequences: A functional MRI study. Cogn Brain Res 15:250–260. [DOI] [PubMed] [Google Scholar]
- Oldfield R (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A, Amedi A, Fregni F, Merabet L (2005): The plastic human brain cortex. Annu Rev Neurosci 28:377–401. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C (1997): Motor imagery activates primary sensorimotor area in humans. Neurosci Lett 239:65–68. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser P. (1996): Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 36:893–906. [DOI] [PubMed] [Google Scholar]
- Porro C, Cettolo V, Francescato M, Baraldi P (2000): Ipsilateral involvement of primary motor cortex during motor imagery. Eur J Neurosci 12:3059–3063. [DOI] [PubMed] [Google Scholar]
- Romero D, Lacourse M, Lawrence K, Schandler S, Cohen M (2000): Event‐related potentials as a function of movement parameter variations during motor imagery and isometric action. Behav Brain Res 117:83–96. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M (2000): Stereotaxic display of brain lesions. Behav Neurol 12:191–200. [DOI] [PubMed] [Google Scholar]
- Sach M, Winkler G, Glauche V, Liepert J, Heimbach B, Koch MA, Büchel C, Weiller C (2004): Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 127:340–350. [DOI] [PubMed] [Google Scholar]
- Sage CA, Peeters RR, Görner A, Robberecht W, Sunaert S (2007): Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis. Neuroimage 34:486–499. [DOI] [PubMed] [Google Scholar]
- Sage CA, Van Hecke W, Peeters R, Sijbers J, Robberecht W, Parizel P, Marchal G, Leemans A, Sunaert S (2009): Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis: Revisited. Hum Brain Mapp 30:3657–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld M, Tempelmann C, Gaul C, Kühnel G, Düzel E, Hopf JM, Feistner H, Zierz S, Heinze HJ, Vielhaber S (2005): Functional motor compensation in amyotrophic lateral sclerosis. J Neurol 252:944–952. [DOI] [PubMed] [Google Scholar]
- Stanton B, Williams V, Leigh P, Williams S, Blain C, Giampietro V, Simmons A (2007a): Cortical activation during motor imagery is reduced in Amyotrophic Lateral Sclerosis. Brain Res 1172:145–151. [DOI] [PubMed] [Google Scholar]
- Stanton B, Williams V, Leigh P, Williams S, Blain C, Jarosz J, Simmons A (2007b): Altered cortical activation during a motor task in ALS. Evidence for involvement of central pathways. J Neurol 254:1260–1267. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Esposito F, Monsurrò M, Graziano S, Panza D, Russo A, Migliaccio R, Conforti FL, Morrone R, Quattrone A, Di Salle F, Tedeschi G (2006): Subcortical motor plasticity in patients with sporadic ALS: An fMRI study. Brain Res Bull 69:489–494. [DOI] [PubMed] [Google Scholar]
- Toosy AT, Werring DJ, Orrell RW, Howard RS, King MD, Barker GJ, Miller DH, Thompson AJ (2003): Diffusion tensor imaging detects corticospinal tract involvement at multiple levels in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatr 74:1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MR, Wicks P, Brownstein CA, Massagli MP, Toronjo M, Talbot K, Al‐Chalabi A (2011): Concordance between site of onset and limb dominance in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 82:853–854. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Wang S, Poptani H, Bilello M, Wu X, Woo JH, Elman LB, McCluskey LF, Krejza J, Melhem ER (2006): Diffusion tensor imaging in amyotrophic lateral sclerosis: Volumetric analysis of the corticospinal tract. AJNR 27:1234–1238. [PMC free article] [PubMed] [Google Scholar]
- Ward N, Frackowiak R (2003): Age‐related changes in the neural correlates of motor performance. Brain 126:873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R, Grafton S, Holmes C, Cherry S, Mazziotta J (1998): Automated image registration: I. General methods and intrasubject intramodality validation. J Comput Assist Tomogr 22:139–152. [DOI] [PubMed] [Google Scholar]
- Yoon B, Shim YS, Lee KS, Shon YM, Yang DW (2008): Region‐specific changes of cerebral white matter during normal aging: A diffusion‐tensor analysis. Arch Gerontol Geriatr 47:129–138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table 1. Neuropsychological assessment of the two groups at inclusion.


