Abstract
Motor recovery after stroke requires continuous interaction of motor and somatosensory systems. Integration of somatosensory feedback with motor programs is needed for the automatic adjustment of the speed, range, and strength of the movement. We recorded somatosensory evoked fields (SEFs) to tactile finger stimulation with whole‐scalp magnetoencephalography in 23 acute stroke patients at 1 week, 1 month, and 3 months after stroke to investigate how deficits in the somatosensory cortical network affect motor recovery. SEFs were generated in the contralateral primary somatosensory cortex (SI) and in the bilateral parietal opercula (PO) in controls and patients. In the patients, SI amplitude or latency did not correlate with any of the functional outcome measures used. In contrast, the contralateral PO (cPO) amplitude to the affected hand stimuli correlated significantly with hand function in the acute phase and during recovery; the weaker the PO activation, the clumsier the hand was. At 1 and 3 months, enhancement of the cPO activation paralleled the improvement of the hand function. Whole‐scalp magnetoencephalography measurements revealed that dysfunction of somatosensory cortical areas distant from the ischemic lesion may affect the motor recovery. Activation strength of the PO paralleled motor recovery after stroke, suggesting that the PO area is an important hub in mediating modulatory afferent input to motor cortex. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: stroke, recovery, magnetoencephalography, secondary somatosensory cortex, somatosensory evoked fields, parietal operculum
INTRODUCTION
Hand dexterity demands continuous inflow of somatosensory input to motor system. Deficits in the motor or somatosensory circuits or impaired interaction between these two systems may all result in hand dysfunction. For example, monkey studies have indicated that section of the dorsal columns and the sensory cortex may abolish purposeful hand movements [Asanuma and Arissian,1984]. Accordingly, a patient with severe peripheral sensory neuropathy and with intact motor circuits was unable to use his hands in everyday tasks, as the strength and range of the movements were not automatically corrected due to insufficient afferent input [Rothwell et al.,1982].
In ischemic stroke patients, motor recovery is associated with plastic changes of the motor cortex [Calautti et al.,2001; Ward et al.,2003a]. Changes in somatosensory activation have been less extensively studied, but previous reports have shown that interhemispheric differences in the locations and latencies of the primary somatosensory (SI) cortex activation are associated with functional recovery of stroke patients [Gallien et al.,2003; Rossini et al.,1998; Tecchio et al.,2006].
In healthy subjects, somatosensory stimulation activates, in addition to the SI cortex, the bilateral secondary somatosensory cortex (SII) in the parietal opercula (PO) [Forss et al.,1994; Hari et al.,1984b; Kakigi et al.,2000; Lin et al.,2000]. These higher‐order somatosensory areas converge input from the two hands, participate in sensorimotor integration, and are associated with tactile discriminative learning and retention [Burton,1986; Ridley and Ettlinger,1976]. Therefore, it is likely that ischemic lesions altering the activation of the higher‐order somatosensory areas affect hand motor recovery. So far, this has not been studied by whole‐head magnetoencephalography (MEG) recordings, allowing simultaneous survey of the secondary somatosensory cortices in both hemispheres. Although studies in monkeys and in humans have revealed at least two somatotopically arranged areas in the PO sharing a common boundary, SII cortex, and the parietal ventral area (PV), [Disbrow et al.,2000; Krubitzer et al.,1995], the patterns of activation within SII and PV have been shown to be variable even across healthy subjects [Disbrow et al.,2000]. In the present study, brain edema in the acute phase may impair the separation of SII activation from possible PV activation, and therefore we call the long‐latency activation arising from the Sylvian fissure as PO responses.
We applied whole‐scalp MEG to investigate the effect of lesions in the somatosensory cortical network on motor recovery after stroke. We recorded somatosensory evoked fields (SEFs) to tactile finger stimulation in 23 acute stroke patients and correlated the measured brain activity with clinical tests of the patients in three subsequent follow‐up measurements. MEG suits stroke studies well as it detects directly the neuronal activation instead of hemodynamic changes [Rossini et al.,2003], and because damaged tissue has minimal effects on the distribution of the magnetic fields [Huang et al.,1990].
MATERIALS AND METHODS
Patients and Clinical Testing
SEFs were recorded from 23 patients suffering from a first‐ever ischemic stroke in the territory of the middle cerebral artery (11 females, 12 males, mean age 64 years, range, 42–84 years; all right‐handed), affecting motor function of the upper extremity, and from 18 healthy control subjects (11 females, 7 males, mean age 55 years). A neurologist of the research team recruited the patients within 3 days after stroke from the Department of Neurology, Helsinki University Central Hospital. The study protocol was accepted by the local Ethics Committee. All patients and control subjects gave their written informed consent before the measurements. Exclusion criteria were multiple strokes, other neurological diseases, previous neurosurgical operations or severe head traumas, severe psychiatric disorder, unstable cardiovascular condition, and poor general condition. From the 23 patients, five were excluded after the first measurement. In three of them, MRI revealed silent brain infarcts, and one patient developed a second stroke after the first measurement. One patient was excluded because of technical problems. Thus, 18 patients (9 females, 9 males, aged 65 ± 3 years, all right‐handed) participated in follow‐up measurements. One patient refused the last measurement because of his claustrophobia.
MEG measurements and clinical testing of the patients were performed 1–7 days (T0), 3–4 weeks (T1), and 3 months (T2) after the stroke. MRI was acquired at T0 and T1 with a 3‐T Philips™ system. National Institutes of Health Stroke Scale (NIHSS), modified Rankin Scale (mRS), and Barthel Index (BI) were performed by a neurologist at T0, T1, and T2. The upper limb functions were tested by physio‐ or ergotherapists using the Action Research Arm Test (ARAT) and 9‐hole pegboard test (PEG). ARAT contains four subcategories (grasp, grip, pinch, and gross motor function of upper limb), and high‐ARAT scores indicate good arm function. In PEG, a patient has to take out and put back nine pegs in holes as fast as possible with one hand. In our study, 120‐s maximum time was given if the patient could not complete the test faster or could not perform the test at all.
MEG Recordings
During MEG measurement, the patient was sitting or lying down while tactile stimuli (duration 141 ms and peak 50 ms) were delivered to the fingertips with balloon diaphragms driven by compressed air. Tactile stimuli are natural stimuli that activate only the tactile receptors in the fingertips. Furthermore, they are quick to apply in acutely ill patients and do not require active contribution of the patient. Sensory feedback from the movement elicited by mixed nerve stimulation above the motor threshold is also avoided by tactile stimulation. The stimuli were delivered alternately to left and right index fingers (D2), with a 3‐s interstimulus interval. SEFs were recorded with a 306‐channel helmet‐shaped neuromagnetometer (Elekta Neuromag®, Helsinki, Finland). The exact position of the head with respect to the sensors was found by measuring the magnetic signals produced by currents led into four indicator coils placed at known sites on the scalp. The locations of the coils with respect to anatomical landmarks on the head were determined with a 3‐D digitizer to allow alignment of the MEG and MRI coordinate systems. The signals were bandpass‐filtered through 0.03–308 Hz and digitized at 941 Hz. The 700‐ms analysis period for evoked responses included a 100‐ms prestimulus baseline; about 60 epochs were averaged for each condition. Responses coinciding with amplitudes exceeding 150 μV in the simultaneously recorded electro‐oculogram were automatically rejected from the analysis.
Data Analysis
If visual inspection of the data revealed artifacts, we applied the signal space separation (SSS) method [Taulu et al., 2005] or its temporal extension spatiotemporal signal space separation (tSSS) [Taulu and Simola,2006], implemented in Maxfilter™ software, to suppress artifacts caused by nearby sources. SSS method, based on sensor geometry and Maxwell's equations, separates brain‐related and external interference signals arising inside the measurement room, for example, from the stimulators or magnetized electrodes [Taulu et al., 2005]. However, if significant artifact sources exist very close to the sensors (such as magnetic dental materials or other magnetic particles on the head), the SSS method alone cannot separate these sources. Artifacts from these nearby sources can be extracted by a simple statistical analysis in the time domain and projected out. This tSSS method thus recognizes and removes both external interference and the artifacts produced by nearby sources. Testing with artificial current dipoles and interference sources and clinical data from patients have demonstrated that the method removes the artifacts without altering the field patterns of the brain signals [Taulu and Simola,2006].
If one of three measurements of a patient contained artifacts, SSS or tSSS was applied to all of them to ensure that the data were equally preprocessed within subjects.
To identify the SEF sources, equivalent current dipoles, best‐describing local source currents at the peak of the responses, were found one by one by a least‐squares search using a subset of channels (usually 16–18) over the response area. After identifying the single dipoles, the analysis was extended to the whole signal duration, and all channels were taken into account in computing a time‐varying multidipole model.
Statistical Analyses
Repeated‐measures ANOVA with within‐subject factors time (T0, T1, and T2) and hemisphere (unaffected, UH; affected, AH) were used to analyze the differences in hand‐function tests and SEF amplitudes. Two‐tailed t‐test was used for pairwise comparisons. Spearman's correlation coefficient was calculated to test correlations between MEG parameters and clinical tests, and, for multiple comparisons, Bonferroni's correction was used. Because the ARAT scores tended to cluster at T1 and T2, chi‐square test was calculated to ensure that the correlation was not driven by outliers.
RESULTS
Clinical Testing
Patient characteristics are summarized in Table I. Five patients had received thrombolysis therapy. Five patients with right hemisphere lesions showed signs of neglect in the acute phase; four of them had ischemic lesions in the parietal lobe extending to the PO and insular area. In the MRIs at T1, the ischemic lesion extended into the SI hand area in one patient. In five others, large lesions comprised the superior parts of the parietal operculum, including the estimated location of the SII cortex. In 12 patients, ischemic lesion did not extend to the SI or SII cortices.
Table I.
Clinical characteristics of the patients
| PAT | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | M | M | M | M | F | F | F | M | M | F | F | F | M | F | M | F | F | M |
| Age | 68 | 44 | 61 | 60 | 68 | 72 | 46 | 69 | 75 | 85 | 69 | 72 | 62 | 74 | 78 | 73 | 48 | 61 |
| AH | R | L | R | R | L | L | R | L | L | R | L | R | L | R | L | L | L | R |
| Site | S | CS | CS | C | S | C | CS | CS | C | C | S | CS | CS | S | S | S | S | S |
| Size | 7 | 34 | 106 | 0.1 | 1 | 0.3 | 70 | 48 | 0.4 | 1 | 3 | 24 | 5 | 5 | 10 | 3 | 1 | 4 |
| Neglect | + | − | + | − | − | − | + | − | − | − | − | + | − | − | − | − | − | + |
| Sensory threshold | N | D | D | D | D | D | D | D | D | D | N | D | D | D | D | D | N | D |
AH, affected hemisphere; C, cortical; CS, cortico‐subcortical; S, subcortical. Size, lesion volume in cm3. Sensory threshold; N, normal; D, decreased.
Hand‐function tests revealed significantly worse performance of the affected than healthy hand at T0 (35.2 vs. 55.6, P < 0.005 for ARAT and 84 s vs. 33 s, P < 0.001 for PEG). A significant main effect for the factors time and hemisphere was found for both ARAT and PEG results. The ad hoc comparison revealed that ARAT and PEG scores were significantly improved from T0 to T1 and T2 (P < 0.005 for both tests, Table II). This improvement was steepest during the first month (see Fig. 1). Healthy hand performance was slightly but significantly lower at T0 than at T2 (P < 0.005 for ARAT and P < 0.001 for PEG). NIHSS, mRS, and BI indicated significant improvement from T0 to T1 (P < 0.001) and further recovery at T2 (P < 0.05).
Table II.
Clinical test results of the patients
| ARAT a | PEG (s) a | ARAT hh | PEG (s) hh | NIHSS | BI | mRS | |
|---|---|---|---|---|---|---|---|
| T0 | 35.2 ± 5 | 84 ± 9 | 55.6 ± 0 | 33 ± 2 | 4.1 ± 1 | 63 ± 7 | 3.4 ± 0.3 |
| T1 | 46.2 ± 5 | 59 ± 10 | 56.9 ± 0 | 27 ± 1 | 2.0 ± 1 | 92 ± 4 | 2.1 ± 0.2 |
| T2 | 48.2 ± 4 | 52 ± 9 | 57.0 ± 0 | 26 ± 1 | 1.5 ± 0 | 96 ± 3 | 1.8 ± 0.2 |
T0, 1–7 days; T1, 1 month; T2, 3 months after stoke; a, affected hand; hh, healthy hand s, seconds.
Figure 1.
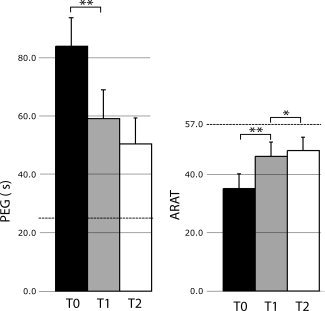
Mean (±SEM) PEG times (s) and ARAT scores of the affected hand at T0, T1, and T2. Dotted lines indicate the mean value for the healthy hand (*P < 0.05, **P < 0.005, two‐tailed t‐test).
Somatosensory Evoked Fields
In controls, tactile stimulation elicited responses in the contralateral SI at 58 ± 1 ms and in the bilateral SII at 109 ± 6 ms and 118 ± 4 ms (for contra‐ and ipsilateral SII, respectively), in line with previous reports [Hari et al.,1984a; Kakigi et al.,2000]. SEF amplitudes and latencies to right and left hand stimuli did not differ.
Figure 2 illustrates alterations of SEFs in one patient during recovery. SI responses were found in 15 patients and contralateral PO (cPO) responses in nine patients to affected hand stimuli at T0.
Figure 2.
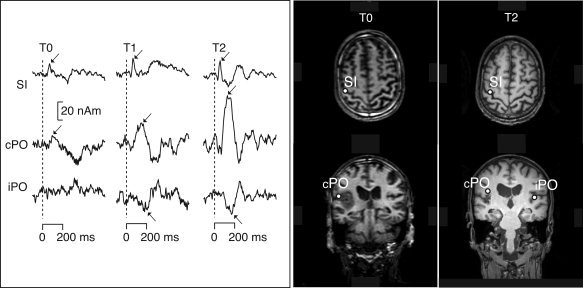
Left source waveforms of the Patient 8 at T0, T1, and T2. The peak responses with dipolar field patterns are marked with an arrow. Right source locations of SEFs to affected hand stimuli in the same patient at T0 and T2.
Figure 3 illustrates mean ± SEM amplitudes of the SI and contra‐ and ipsilateral PO responses of controls and patients. In patients, ANOVA showed a significant main effect for the factor hemisphere; the amplitude of the SI response was significantly smaller to affected than to healthy hand stimulation at T0 and T1 (P < 0.01 and P < 0.005, respectively, Table III). The amplitude of the SI responses to healthy hand stimulation tended to be stronger than in controls (Fig. 3).
Figure 3.
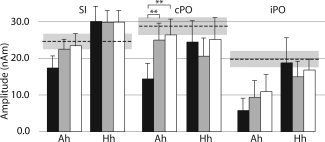
Mean (±SEM) amplitudes (nAm) of SI, cPO, and iPO sources of the patients to affected (Ah) and healthy hand (Hh) stimulation. The mean ± SEM amplitudes of the controls (right and left pooled) are shown with dashed and gray horizontal lines (**P < 0.005, two‐tailed t‐test).
Table III.
Mean (±SEM) amplitudes and latencies of the SEFs in patients and in healthy controls
| Amplitude (nAm) | Latency (ms) | |||||
|---|---|---|---|---|---|---|
| Patients ah | Patients hh | Controls r + l | Patients ah | Patients hh | Controls r + l | |
| SI | ||||||
| T0 | 17 ± 3 | 30 ± 4 | 25 ± 2 | 60 ± 2 | 60 ± 1 | 58 ± 1 |
| T1 | 22 ± 3 | 30 ± 3 | 60 ± 2 | 59 ± 2 | ||
| T2 | 23 ± 3 | 30 ± 3 | 61 ± 2 | 59 ± 2 | ||
| cPO | ||||||
| T0 | 14 ± 4 | 25 ± 6 | 29 ± 2 | 109 ± 11 | 113 ± 9 | 109 ± 6 |
| T1 | 25 ± 5 | 21 ± 5 | 101 ± 5 | 110 ± 6 | ||
| T2 | 26.± 4 | 25 ± 6 | 101 ± 6 | 101 ± 4 | ||
| iPO | ||||||
| T0 | 19 ± 7 | 6 ± 3 | 20 ± 2 | 119 ± 14 | 107 ± 28 | 118 ± 4 |
| T1 | 15 ± 4 | 9 ± 4 | 122 ± 10 | 147 ± 15 | ||
| T2 | 17 ± 3 | 11 ± 5 | 126 ± 8 | 113 ± 13 | ||
SI, primary somatosensory cortex; cPO and iPO, contra‐ and ipsilateral parietal operculum; ah, affected hand; hh, healthy hand; r, right hand; l, left hand. For controls, SEF amplitudes and latencies to right and left hand stimulation are pooled.
Amplitude of the cPO responses to the affected hand stimulation was weaker in patients than in controls (P < 0.01). ANOVA indicated a significant interaction between time and hemisphere for cPO amplitude; ad hoc comparison revealed that cPO amplitude to affected hand stimuli was significantly enhanced at T1 and T2 compared to T0 (P < 0.005).
Correlation with Clinical Recovery
Lesion size correlated significantly with NIHSS and mRS in all three measurements (r = 0.7, P < 0.005 and r = 0.5, P < 0.05 at T2); the larger the lesion, the higher the patient's NIHSS and mRS scores were. In contrast, NIHSS, mRS, or BI scores did not correlate with hand‐function tests. Neither ARAT nor PEG results correlated with the lesion size.
Amplitudes or latencies of the SI responses from the affected hemisphere did not correlate with any of the clinical test results. In contrast, amplitudes of the cPO responses to affected hand stimuli correlated significantly with the hand‐function tests in all three measurements (Fig. 4; P < 0.001 and P < 0.01 at T0 for ARAT and PEG, respectively, and P < 0.05 at T1 and T2 for both tests). At T0, weak PO activation was associated with low‐ARAT scores and long PEG times, whereas at T1 and T2, the cPO amplitudes increased in parallel with improving hand‐function tests. In contrast to PEG results, ARAT scores tended to cluster at T1 and T2 indicating that ARAT may not be sensitive enough to find subtle differences in hand dexterity as the patients have almost fully recovered. However, chi‐square test confirmed that the relationship between high‐ARAT scores and strong cPO amplitudes was significant at T1 and T2 [X2(1, N = 26) = 4.74, P = 0.029; X2(1, N = 17) = 4.16, P = 0,041, respectively]. Amplitude of the cPO response to affected hand stimulation at 1 month (T1) correlated significantly with the hand‐function tests at 3 months (P < 0.05 for ARAT and PEG at T2, respectively), and at acute phase (T0) the correlation between the cPO amplitude and hand‐function tests approached significance (P < 0.06).
Figure 4.
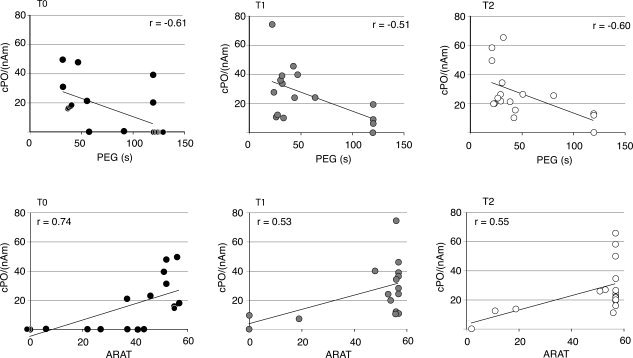
Correlation of cPO amplitude (nAm) with PEG times (above) and ARAT scores (below) of the affected hand.
For healthy hand stimulation, SEF amplitudes or latencies did not correlate with PEG or ARAT results of the affected or the healthy hand. However, at T0 correlation between cPO amplitude to healthy hand stimulation and healthy hand ARAT approached significance (r = −0.5, P < 0.06) but in a different direction than in the affected hemisphere; larger cPO responses in the intact hemisphere indicated worse healthy hand performance in ARAT.
DISCUSSION
The amplitude of the early SI response has been shown to correlate with tactile discrimination ability [Knecht et al.,1996; Wikstrom et al.,2000], whereas association of SI activation strength with other functional outcome measures has been more variable [Gallien et al.,2003; Tecchio et al.,2006]. In the present study, no correlation between SI strength and motor recovery was observed at acute phase or during recovery. Instead, the results showed, for the first time, that the activation of cPO in the affected hemisphere correlates with hand function (tested with ARAT and PEG) both at the acute phase and during recovery. Strength of the PO activation did not correlate with tests that measure stroke‐induced deficits in overall neurological function (NIHSS, BI, mRS), implicating that PO activation is associated with hand sensorimotor functions rather than the overall outcome from stroke.
Somatosensory input is crucial for accurate motor functions, but it is not well understood how afferent input affects the motor cortex. It has been suggested that somatosensory input mediates its effect on the motor system by modulating the excitability of the motor cortex [Favorov et al.,1988]. Animal and human studies have shown that intracortical inhibition affects the reorganization of the motor cortex after injury [Chen et al.,1998; Jacobs and Donoghue,1991]. In healthy subjects, peripheral stimulation may reduce intracortical inhibition, as estimated by transcranial magnetic stimulation (TMS), indicating that afferent input is capable of altering activity in cortical inhibitory circuits and is highly relevant for mechanisms involved in cortical reorganization [Ridding and Rothwell,1999].
The afferent input may reach the motor cortex via several ascending pathways. In addition to direct thalamic connections to motor cortex [Asanuma et al.,1979], areas 3a and 2 of the SI are strongly connected to motor cortex area 4. Projections between area 3b, the major cutaneous zone in SI, and the area 4 are weak or nonexisting [Jones et al.,1978; Stepniewska et al.,1993]. Instead, several studies have reported strong connections from SII to area 4 [Jones and Wise,1977; Mori et al.,1989; Stepniewska et al.,1993], providing a cortical loop for somatosensory input via SII to the motor cortex [Burton,1986]. Indeed, microstimulation of the monkey SII cortex has shown to produce movements contralateral to the side of stimulation [Mori et al.,1985], suggesting that SII might be important in mediating the somatosensory input to the motor cortex.
Although the functional role of SII is not fully understood, prior studies have shown a close interaction of SII activation and motor functions. Navigated TMS of the SII region facilitated motor performance in healthy subjects [Raij et al.,2008] and a motor task modulated strongly the SII activation [Lin et al.,2000]. In addition, some patient studies have found an association between SII activation and hand‐motor functions. Unverricht–Lundborg‐type progressive myoclonus epilepsy patients with absent SII activation had more severe motor symptoms, such as clumsiness, than the patients with spared SII activity [Forss et al.,2001]. Furthermore, an increase in activity of SII, premotor cortex, and cerebellum correlated with increased grip strength ratio after a 2‐week home‐based rehabilitation therapy in chronic stroke patients [Johansen‐Berg et al.,2002]. Taken together, prior monkey and human studies indicate that the SII cortex participates in sensorimotor integration, particularly in tasks involving multiple functionally related body parts like digits of the hand [Disbrow et al.,2000; Krubitzer et al.,1995]. Thus, it is possible that deficient PO activation, observed in our acute stroke patients, reflects impaired flow of sensory feedback information to motor cortex, leading to impaired sensorimotor integration.
A direct ischemic lesion in PO explained lack of activation in five patients, but in other patients, the unresponsiveness of PO was probably due to disrupted thalamocortical and corticocortical connections. SII may receive input from the ipsilateral thalamic nuclei, the SI, and the contralateral SII via commissural connections [Friedman and Jones,1980; Jones and Powell,1970], but the functional organization of SI and SII cortices vary among species [Pons et al.,1992; Zhang et al.,1996]. Our prior study with incompletely recovered chronic stroke patients suggested that in humans SI and SII cortices are sequentially activated within one hemisphere, whereas SII ipsilateral to the stimulation may receive direct input from the thalamus [Forss et al.,1999]. The observed increase of PO activation during recovery might thus be associated with increased input from the recovering SI rather than with enhanced sensorimotor integration. However, the SI amplitudes did not correlate with cPO amplitudes, indicating that the strength of cPO activation does not solely depend on SI activity. This is in line with a recent fMRI study revealing independent behavior of SI and PO responses after thalamic stroke [Taskin et al.,2006]. Moreover, SI amplitudes did not correlate with improving hand‐motor function, further supporting the role of PO region in sensorimotor integration.
Patients with incomplete recovery have enhanced activation of the intact hemisphere [Forss et al.,1999; Ward et al.,2003b], which may have an unfavorable effect on recovery by abnormally inhibiting the lesioned hemisphere [Floel et al.,2008; Hummel and Cohen,2006]. In the present study, the responses of the intact hemisphere were not abnormally enlarged, possibly because of good recovery of our patients. However, larger cPO responses in the intact hemisphere were related to worse performance of the healthy hand at T0, suggesting that excitability changes of the intact hemisphere also affect the healthy hand performance.
In monkeys, selective attention to tactile stimuli affects more SII than SI activity [Meftahel et al.,2002]. In accordance, SII activation of healthy subjects is modified by attention and vigilance changes [Mauguiere et al.,1997]. Thus, lowered vigilance or deficits in directional attention may have affected PO responses in the acute phase. However, the cPO responses to healthy hand stimuli at T0 did not differ from those of the healthy controls. Furthermore, only 5 of 18 patients had unilateral neglect and behavior of their cPO responses did not differ from patients having left‐sided lesions and no attentional deficits. Therefore, it is likely that attentional factors cannot alone explain the observed changes in the PO activation.
Correlation of cPO amplitude of the affected hemisphere at 1 month with affected hand performance at 3 months suggests that the strength of activation in PO could be useful in predicting hand motor recovery already in the subacute phase. However, because of the relatively small number of patients, such conclusion cannot be definitely drawn. Future studies with larger patient groups are needed to evaluate the predictive value of cPO activation in motor recovery.
In conclusion, the present results show that even remote somatosensory lesions may affect motor recovery after stroke. Activation in PO paralleled motor recovery suggesting that PO area is important in mediating modulatory afferent input to motor cortex utilized for sensorimotor integration.
Acknowledgements
We report no conflicts of interest. We thank Mr Jari Kainulainen and Ms Suvi Heikkilä for their valuable help in the recordings, Dr Lauri Nummenmaa for expert help in statistical analysis, and the ergo‐ and physiotherapists of the Department of Neurology, HUCH for their skilful clinical testing of the patients.
REFERENCES
- Asanuma H, Arissian K ( 1984): Experiments on functional role of peripheral input to motor cortex during voluntary movements in the monkey. J Neurophysiol 52: 212–227. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Larsen KD, Zarzecki P ( 1979): Peripheral input pathways projecting to the motor cortex in the cat. Brain Res 172: 197–208. [DOI] [PubMed] [Google Scholar]
- Burton H ( 1986): Second somatosensory cortex and related areas In: Jones EG, Peters A, editor. New York: Plenum; pp 31–98. [Google Scholar]
- Calautti C, Leroy F, Guincestre JY, Baron JC ( 2001): Dynamics of motor network overactivation after striatocapsular stroke: A longitudinal PET study using a fixed‐performance paradigm. Stroke 32: 2534–2542. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Yaseen Z, Hallett M, Cohen LG ( 1998): Mechanisms of cortical reorganization in lower‐limb amputees. J Neurosci 18: 3443–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disbrow E, Roberts T, Krubitzer L ( 2000): Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: Evidence for SII and PV. J Comp Neurol 418: 1–21. [DOI] [PubMed] [Google Scholar]
- Favorov O, Sakamoto T, Asanuma H ( 1988): Functional role of corticoperipheral loop circuits during voluntary movements in the monkey: A preferential bias theory. J Neurosci 8: 3266–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A, Hummel F, Duque J, Knecht S, Cohen LG ( 2008): Influence of somatosensory input on interhemispheric interactions in patients with chronic stroke. Neurorehabil Neural Repair 22: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forss N, Hari R, Salmelin R, Ahonen A, Hamalainen M, Kajola M, Knuutila J, Simola J ( 1994): Activation of the human posterior parietal cortex by median nerve stimulation. Exp Brain Res 99: 309–315. [DOI] [PubMed] [Google Scholar]
- Forss N, Hietanen M, Salonen O, Hari R. ( 1999): Modified activation of somatosensory cortical network in patients with right‐hemisphere stroke. Brain 122 ( Pt 10): 1889–1899. [DOI] [PubMed] [Google Scholar]
- Forss N, Silen T, Karjalainen T ( 2001): Lack of activation of human secondary somatosensory cortex in Unverricht‐Lundborg type of progressive myoclonus epilepsy. Ann Neurol 49–90–97. [PubMed] [Google Scholar]
- Friedman DP, Jones EG ( 1980): Focal projection of electrophysiologiaclly defined groupings of thalamic cells on the monkey somatic sensory cortex. Brain Res 191: 249–252. [DOI] [PubMed] [Google Scholar]
- Gallien P, Aghulon C, Durufle A, Petrilli S, de Crouy AC, Carsin M, Toulouse P ( 2003): Magnetoencephalography in stroke: A 1‐year follow‐up study. Eur J Neurol 10: 373–382. [DOI] [PubMed] [Google Scholar]
- Hari R, Reinikainen K, Kaukoranta E, Hamalainen M, Ilmoniemi R, Penttinen A, Salminen J, Teszner D ( 1984a): Somatosensory evoked cerebral magnetic fields from SI and SII in man. Electroencephalogr Clin Neurophysiol 57: 254–263. [DOI] [PubMed] [Google Scholar]
- Hari R, Reinikainen K, Kaukoranta E, Hamalainen M, Ilmoniemi R, Penttinen A, Salminen J, Teszner D ( 1984b): Somatosensory evoked cerebral magnetic fields from SI and SII in man. Electroencephalogr Clin Neurophysiol 57: 254–263. [DOI] [PubMed] [Google Scholar]
- Huang JC, Nicholson C, Okada YC ( 1990): Distortion of magnetic evoked fields and surface potentials by conductivity differences at boundaries in brain tissue. Biophys J 57: 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG ( 2006): Non‐invasive brain stimulation: A new strategy to improve neurorehabilitation after stroke? Lancet Neurol 5: 708–712. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP ( 1991): Reshaping the cortical motor map by unmasking latent intracortical connections. Science 251: 944–947. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM ( 2002): Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain 125( Pt 12): 2731–2742. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Hendry SH ( 1978): Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. J Comp Neurol 181: 291–347. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TP ( 1970): An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain 93: 793–820. [DOI] [PubMed] [Google Scholar]
- Jones EG, Wise SP ( 1977): Size, laminar and columnar distribution of efferent cells in the sensory‐motor cortex of monkeys. J Comp Neurol 175: 391–438. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Hoshiyama M, Shimojo M, Naka D, Yamasaki H, Watanabe S, Xiang J, Maeda K, Lam K, Itomi K, Nakamura A. ( 2000): The somatosensory evoked magnetic fields. Prog Neurobiol 61: 495–523. [DOI] [PubMed] [Google Scholar]
- Knecht S, Kunesch E, Schnitzler A ( 1996): Parallel and serial processing of haptic information in man: Effects of parietal lesions on sensorimotor hand function. Neuropsychologia 34: 669–687. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Clarey J, Tweedale R, Elston G, Calford M ( 1995): A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. J Neurosci 15( 5, Pt 2): 3821–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YY, Simoes C, Forss N, Hari R ( 2000): Differential effects of muscle contraction from various body parts on neuromagnetic somatosensory responses. Neuroimage 11: 334–340. [DOI] [PubMed] [Google Scholar]
- Mauguiere F, Merlet I, Forss N, Vanni S, Jousmaki V, Adeleine P, Hari R ( 1997): Activation of a distributed somatosensory cortical network in the human brain. A dipole modelling study of magnetic fields evoked by median nerve stimulation, Part 1: Location and activation timing of SEF sources. Electroencephalogr Clin Neurophysiol 104–281–289. [DOI] [PubMed] [Google Scholar]
- Meftahel M, Shenasa J, Chapman CE ( 2002): Effects of a cross‐modal manipulation of attention on somatosensory cortical neuronal responses to tactile stimuli in the monkey. J Neurophysiol 88: 3133–3149. [DOI] [PubMed] [Google Scholar]
- Mori A, Babb RS, Waters RS, Asanuma H ( 1985): Motor effects produced by stimulation of secondary somatosensory (SII) cortex in the monkey. Exp Brain Res 58: 440–442. [DOI] [PubMed] [Google Scholar]
- Mori A, Waters RS, Asanuma H ( 1989): Physiological properties and patterns of projection in the cortico‐cortical connections from the second somatosensory cortex to the motor cortex, area 4 gamma, in the cat. Brain Res 504: 206–210. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Mishkin M ( 1992): Serial and parallel processing of tactual information in somatosensory cortex of rhesus monkeys. J Neurophysiol 68: 518–527. [DOI] [PubMed] [Google Scholar]
- Raij T, Karhu J, Kicić D, Lioumis P, Julkunen P, Lin FH, Ahveninen J, Ilmoniemi RJ, Mäkelä JP, Hämäläinen M, Rosen BR, Belliveau JW ( 2008): Parallel input makes the brain run faster. Neuroimage 40: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC ( 1999): Afferent input and cortical organisation: A study with magnetic stimulation. Exp Brain Res 126: 536–544. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Ettlinger G ( 1976): Impaired tactile learning and retention after removals of the second somatic sensory projection cortex (SII) in the monkey. Brain Res 109: 656–660. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Calautti C, Pauri F, Baron JC ( 2003): Post‐stroke plastic reorganisation in the adult brain. Lancet Neurol 2: 493–502. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Caltagirone C, Castriota‐Scanderbeg A, Cicinelli P, Del Gratta C, Demartin M, Pizzella V, Traversa R, Romani GL ( 1998): Hand motor cortical area reorganization in stroke: A study with fMRI, MEG and TCS maps. Neuroreport 9: 2141–2146. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Day BL, Obeso JA, Thomas PK, Marsden CD ( 1982): Manual motor performance in a deafferented man. Brain 105 ( Pt 3): 515–442. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH ( 1993): Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (M1) of owl monkeys. J Comp Neurol 330: 238–271. [DOI] [PubMed] [Google Scholar]
- Taskin B, Jungehulsing GJ, Ruben J, Brunecker P, Krause T, Blankenburg F, Villringer A ( 2006): Preserved responsiveness of secondary somatosensory cortex in patients with thalamic stroke. Cereb Cortex 16: 1431–1439. [DOI] [PubMed] [Google Scholar]
- Taulu S, Kajola M, Simola J ( 2004): Suppression of interference and artifacts by the Signal Space Separation Method. Brain Topogr 16: 269–275. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J ( 2006): Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51: 1759–1768. [DOI] [PubMed] [Google Scholar]
- Tecchio F, Zappasodi F, Tombini M, Oliviero A, Pasqualetti P, Vernieri F, Ercolani M, Pizzella V, Rossini PM ( 2006): Brain plasticity in recovery from stroke: An MEG assessment. Neuroimage 32: 1326–1334. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS ( 2003a): Neural correlates of motor recovery after stroke: A longitudinal fMRI study. Brain 126( Pt 11): 2476–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS ( 2003b): Neural correlates of outcome after stroke: A cross‐sectional fMRI study. Brain 126( Pt 6): 1430–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom H, Roine RO, Aronen HJ, Salonen O, Sinkkonen J, Ilmoniemi RJ, Huttunen J ( 2000): Specific changes in somatosensory evoked magnetic fields during recovery from sensorimotor stroke. Ann Neurol 47: 353–360. [PubMed] [Google Scholar]
- Zhang HQ, Murray GM, Turman AB, Mackie PD, Coleman GT, Rowe MJ ( 1996): Parallel processing in cerebral cortex of the marmoset monkey: Effect of reversible SI inactivation on tactile responses in SII. J Neurophysiol 76: 3633–3655. [DOI] [PubMed] [Google Scholar]


