Abstract
We aimed to identify the brain areas involved in verbal and visual memory processing in normal controls and patients with unilateral mesial temporal lobe epilepsy (MTLE) associated with unilateral hippocampal sclerosis (HS) by means of functional magnetic resonance imaging (fMRI). The sample comprised nine normal controls, eight patients with right MTLE, and nine patients with left MTLE. All subjects underwent fMRI with verbal and visual memory paradigms, consisting of encoding and immediate recall of 17 abstract words and 17 abstract drawings. A complex network including parietal, temporal, and frontal cortices seems to be involved in verbal memory encoding and retrieval in normal controls. Although similar areas of activation were identified in both patient groups, the extension of such activations was larger in the left‐HS group. Patients with left HS also tended to exhibit more bilateral or right lateralized encoding related activations. This finding suggests a functional reorganization of verbal memory processing areas in these patients due to the failure of left MTL system. As regards visual memory encoding and retrieval, our findings support the hypothesis of a more diffuse and bilateral representation of this cognitive function in the brain. Compared to normal controls, encoding in the left‐HS group recruited more widespread cortical areas, which were even more widespread in the right‐HS group probably to compensate for their right mesial temporal dysfunction. In contrast, the right‐HS group exhibited fewer activated areas during immediate recall than the other two groups, probably related to their greater difficulty in dealing with visual memory content. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: cognitive functions, neuropsychological evaluation, hippocampal atrophy, functional reorganization
INTRODUCTION
Neuropsychological evaluation of patients with refractory mesial temporal lobe epilepsy (MTLE) associated with hippocampal atrophy (HA) and other MRI signs of hippocampal sclerosis (HS) usually reveal a mild‐to‐moderate memory deficit [Engel et al., 1997; French et al., 1993]. According to the classic material‐specific model of memory, lesions in the hippocampus of the left temporal lobe (dominant for language) may implicate in verbal memory deficits [Meyer and Yates, 1955; Milner, 1972; Novelly et al., 1984] and those of the right temporal lobe, in visual memory deficits [Jones‐Gotman, 1987; Kimura, 1963; Milner et al., 1962]. However, more recent studies in epilepsy surgery have shown that the relationship between lateralized hippocampal pathology and memory dysfunction is more evident in left MTLE than in right MTLE [Alessio et al., 2004a, b; Hermann et al., 1997; Jones‐Gotman, 1996; Lencz et al., 1992; Saling et al., 1993; Trenerry et al., 1995]. Evidences have suggested that the most important predictor factor for postoperative memory decline is the functional capacity of the ipsilateral mesial temporal lobe (MTL; hippocampal adequacy model) rather than the functional reserve of the contralateral MTL (hippocampal reserve model) to sustain memory performance [Powell et al., 2007; Rabin et al., 2004]. However, MRI findings associated with neuropsychological data are not always sufficient to determine the risk of language and/or memory impairment following mesial temporal resection. Application of more invasive procedures, such as intracranial monitoring with depth electrodes (SEEG) and intracarotid sodium amytal test (IAT or Wada test), may be considered necessary [Dinner, 1991; Golby et al., 2002; Lineweaver et al., 2006; Rausch and Langfitt, 1991].
Even though considered the gold standard, the IAT has a series of limitations, including its invasiveness, poor spatial resolution, insufficient time for detailed evaluation of language and memory functions and limited ability to distinguish verbal versus visual memory deficits [Dade and Jones‐Gotman, 1997; Gotman et al., 1992; Loring et al., 1990; Lukban et al., 1994; Rausch, 2002]. Because of these limitations of IAT, some researchers have aimed to explore alternative methods, such as fMRI [Aldenkamp et al., 2003; Golby et al., 2002; Rausch, 2002].
Although the role of fMRI in the lateralization of hemispheric dominance for language is relatively well‐established, such an application has so far not been proved reliable in the lateralization of verbal and visual memories [Jokeit et al., 2001]. fMRI evaluation of memory is more difficult than fMRI evaluation of other cognitive functions due to some neuropsychological and technical issues [Alessio et al., 2004b; Detre et al., 1998; Jones‐Gotman, 1996; Powell et al., 2004, 2005 Strange, 2002; Richardson et al., 2003].
Keeping all the above factors in mind, the purposes of this study were: (1) to identify the brain areas involved in verbal and visual memory processing in normal controls and patients with unilateral MTLE associated with ipsilateral HS, by means of fMRI; and (2) to assess the sensitivity and potential clinical role of memory tests applied during fMRI experiments in lateralizing and localizing verbal and visual memory functions, during two distinct stages of memory processing, namely: encoding and retrieval.
PATIENTS AND METHODS
Ascertainment of Subjects
In this study we included patients with diagnosis of refractory MTLE followed at our epilepsy clinic. We also included a control group, which were submitted to the same protocols. All individuals signed a written consent, approved by the ethics committee of UNICAMP Medical School.
The clinical criterion was a history of simple partial and/or complex partial seizures with characteristics of MTL origin, such as: rising epigastric sensation, fear, experiential phenomena, and autonomic signs and symptoms. As a complement of this clinical criterion, no suggestion of any other partial epilepsy syndrome could be present. The EEG criterion was the presence of interictal epileptiform discharges over mid‐inferomesial temporal regions and no clear‐cut epileptiform abnormalities elsewhere.
The selected 26 individuals were divided into three groups, as follows: control group, composed of nine normal controls with no history of epilepsy or any other neurological and/or psychiatric pathology, and at ages and education level similar to the patient groups; right‐HS group, composed of eight patients who fulfilled clinical‐EEG criteria for right MTLE and who had right HA and other signs of unilateral right HS on MRI; and finally, left‐HS group, composed of nine patients who also had clinical and EEG diagnosis of left MTLE and had left HA and other signs of unilateral left HS on MRI. All patients had normal hippocampal volumes contralateral to the EEG lateralization, determined by MRI volumetric analysis according to a previously defined protocol [Bonilha et al., 2004]. Other MRI signs of HS, including hyperintense T2 signal were evaluated clinically by one of the authors with experience in neuroimaging investigation in epilepsies (F.C.).
Neuropsychological Evaluation
Both patient groups were submitted to an extensive neuropsychological evaluation, which included: (1) vocabulary and block design subtests of the Wechsler adult intelligence scale‐revised (WAIS‐R) to estimate IQ; (2) the Edinburgh handedness inventory and dichotic listening test to determine hemispheric dominance for language and, by inference, to lateralize verbal and visual memories; (3) the logical memory and verbal paired associates of the Wechsler memory scale‐revised (WMS‐R) to investigate verbal memory; and (4) the figural memory, visual reproduction, and visual paired associates of the WMS‐R to investigate visual memory. To control other cognitive functions that could somehow influence memory tasks, they were also submitted to tests for language (verbal fluency test and Boston naming test/BNT), and attention [Strub and Black Vigilance Test; Fromm‐Auch and Yeudall, 1983; Kaplan et al., 1983; Oldfield, 1971; Spreen and Strauss, 1998; Strub and Black, 1993; Wechsler, 1981, 1987]. These tests were adapted for our population by means of few stimuli substitutions. However, the procedures were kept the same as the originals. The results of each test were compared with results for normal controls matched by age and educational level. We did not use the same fMRI control group for neuropsychological data.
Verbal and Visual Memory Tests During fMRI
Verbal memory evaluation during fMRI consisted of encoding and immediate recall of 17 abstract and emotionally neutral words [the equivalent Portuguese translations for HONOR, OPINION, PROBLEM, DUTY, INTEREST, PATIENCE, SOUL, LAW, OPTION, HAZARD, SYMPATHY, PRIDE, DECISION, CRITERIA, SYSTEM, LIFE, METHOD, as proposed by Jones‐Gotman et al., 1997] presented in four blocks alternating with a non‐word string (ARLTIP) presented in five blocks. Likewise, visual memory evaluation consisted of encoding and immediate recall of 17 abstract drawings presented in four blocks alternating with a fixation point (“+”) presented in five blocks [Damasceno et al., 2005; Jones‐Gotman et al., 1997; see Fig. 1].
Figure 1.
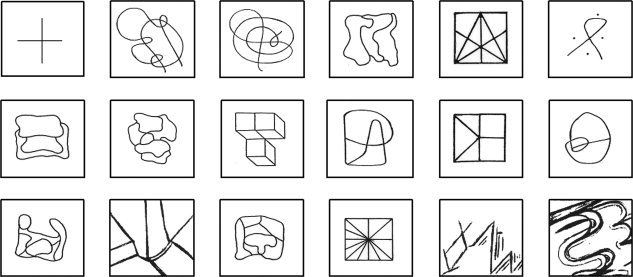
The cross and the 17 abstract drawings.
Individuals were instructed to focus their attention on the non‐word/fixation point for 34 s during the baseline condition, and to try to memorize the 17 words/drawings in 34 s during the experimental condition. At the end of this encoding period, they rested for 120 s and, soon afterward, they attempted to recall silently all words/drawings during 60 s (see Fig. 2). After this immediate recall period, the individuals were requested to try to recognize, by pressing a push‐button, the 17 memorized words/drawings among another 17 new abstract words/drawings.
Figure 2.
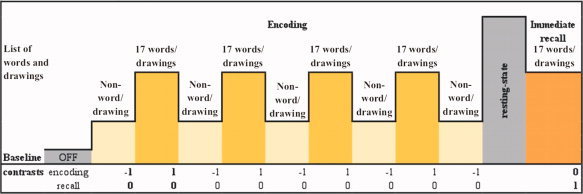
Verbal and visual memory paradigms. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
fMRI Acquisition and Data Analysis
Before getting into the MR scanner, all subjects received complete instructions about the verbal and visual memory tests they were supposed to perform inside the machine.
Images were acquired in a bottom/up interleaved mode, using a 2T Elscint Prestige (Haifa, Israel) MR scanner with an EPI protocol (TR = 2 s, TE = 45 ms, voxel size = 3 × 3 × 6 mm3). Two hundred and ninety cerebral volumes with 20 slices each were obtained in the run.
The functional images acquired were then (1) reconstructed and temporally reorganized, (2) transformed from DICOM‐2D into ANALYSE‐3D format by using the MRIcro software, and finally (3) slice timed, realigned, normalized (MNI standard template), smoothed (6 mm/FWHM) and analyzed by using the SPM2 software package (http://www.fil.ion.ucl.ac.uk/spm/). The imaging preprocessing was carried out individually for each subject and run, while the data analysis was performed putting together all subjects of the same group (control group, right‐HS group and left‐HS group).
We attempted to maximize the signal‐to‐noise ratio before acquiring the EPI images by following a semi‐automatic shimming procedure to homogenize the magnetic field strength in the brain region to the sub‐ppm level. In order to reduce the chance for ghost artifacts, we have made use of an algorithm proposed by Buonocore and Gao [ 1997] during the image reconstruction stage.
For data analysis, the following parameters were adopted: gama function with window length of 32 and order 1, whereas model interaction, parametric modulation, other regressors, removal of global effects, high‐pass filter and correction for serial correlations were left aside. Three T‐test contrasts were created in order to identify cerebral areas related to verbal memory encoding (word blocks—minus—nonword blocks) and immediate recall (words recall). The same procedure was adopted for visual memory encoding and immediate recall (see Fig. 2). In other words, the first two contrasts designed were word blocks and nonword blocks and the third one, immediate recall. In order to identify the cerebral areas activated during encoding stage, we assigned the value “1” to the word blocks and the value “‐1” to the nonword blocks, while no value was assigned to the baseline condition (OFF). We also assigned the value “0” to the immediate recall block because in doing so it was left aside of this analysis. Then, the program performed not only the subtraction of word blocks—minus—nonword blocks but also compared this result to the baseline condition result. Soon afterwards, to identify the cerebral regions activated during immediate recall stage, we assigned the value “1” to the immediate recall block and no value to the baseline condition. Once again, the value “0” was assigned to the word and non‐word blocks, which were left aside of this second analysis.
Finally, for the results visualization a threshold of P < 0.001, uncorrected for multiple comparisons, and clusters of 125/0 voxels were used for the encoding/retrieval phase.
RESULTS
The three groups had similar age and educational level (Table I). The two patient groups were similar in age of seizure onset, duration of epilepsy, seizure frequency, and number of antiepileptic drugs used, as well as in the results of the Edinburgh handedness inventory, dichotic listening test, Boston naming test, verbal fluency test, Strub&Black vigilance test, visual memory tests, and fMRI recognition tests. The only difference between them was that patients with left HS had lower IQ (F = 9.656; P = 0.008) and worse performance on tests of general memory (F = 15.387; P = 0.002), verbal memory (F = 14.510; P = 0.002), and delayed recall (F = 6.345; P = 0.025) than patients with right HS (Table II).
Table I.
Demografic and fMRI data of subjects
| Subjects | Age (years) | Education (years) | fMRI verbal memory (correct answers) | fMRI visual memory (correct answers) | Edinburgh handedness inventory |
|---|---|---|---|---|---|
| Controls | |||||
| 1 | 38 | 4 | 7 | 13 | right |
| 2 | 23 | 16 | 17 | 14 | right |
| 3 | 19 | 11 | 12 | 8 | right |
| 4 | 29 | 15 | 10 | 8 | right |
| 5 | 31 | 11 | 10 | 16 | right |
| 6 | 50 | 7 | 12 | 11 | right |
| 7 | 40 | 15 | 17 | 17 | right |
| 8 | 35 | 5 | 12 | 8 | right |
| 9 | 31 | 11 | 16 | 12 | right |
| Right‐HS group | |||||
| 1 | 34 | 11 | 12 | 12 | right |
| 2 | 32 | 11 | 5 | NA | right |
| 3 | 43 | 11 | 13 | 11 | right |
| 4 | 49 | 11 | 14 | 14 | left |
| 5 | 50 | 16 | 14 | 15 | right |
| 6 | 41 | 13 | 15 | 11 | right |
| 7 | 38 | 8 | 12 | 13 | right |
| 8 | 35 | 8 | 17 | 16 | right |
| Left‐HS group | |||||
| 1 | 48 | 15 | 15 | 14 | right |
| 2 | 42 | 4 | 7 | 11 | right |
| 3 | 24 | 11 | 14 | 11 | right |
| 4 | 34 | 11 | 8 | 13 | right |
| 5 | 47 | 8 | 11 | 13 | left |
| 6 | 33 | 11 | 8 | 9 | right |
| 7 | 42 | 7 | 0 | NA | right |
| 8 | 20 | 4 | 15 | 14 | Right |
| 9 | 31 | 5 | 11 | 12 | Right |
| F and | 1.5 | 1.574 | 1.402 | 0.508 | 0.536 |
| P values | 0.244 | 0.229 | 0.266 | 0.609 | 0.592 |
NA, not available.
Table II.
MRI, clinical, and neuropsychological data of patients
| Patients | MRI | Side of TL spikes on EEG | Age at seizure onset (years) | Duration of epilepsy (years) | Seizure frequency (P/month) | AEDs | Dichotic listening test | WAIS‐R estimated IQ | Boston naming test (z score) | Verbal fluency test (z score) | Vigilance test (errors) | WMS‐R general memory (z score) | WMS‐R verbal memory (z score) | WMS‐R visual memory (z score) | WMS‐R delayed recall (z score) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right‐HS group | |||||||||||||||
| 1 | RHA | right | 4 | 30 | 20 | CBZ; CLN | left | 100 | 0.12 | −0.63 | 0 | 1.23 | 1.53 | ‐0,05 | 1,95 |
| 2 | RHA | right | 8 | 24 | 12 | CBZ; CLB | NA | 97 | −4.8 | −0.63 | 0 | 0.4 | 0.68 | ‐0,37 | ‐0,24 |
| 3 | RHA | right | 7 | 36 | 2 | PNT; CLB | left | 86 | −3.39 | −0.19 | 0 | −0.55 | −0.54 | ‐0,76 | ‐1,02 |
| 4 | RHA | right | 0 | 49 | 14 | CBZ; CLB | left | 100 | −2.13 | −0.85 | 1 | 0.46 | −0.02 | 1,68 | ‐0,45 |
| 5 | RHA | right | 19 | 31 | 2 | LMT; CLB | left | 115 | −0.11 | 0.02 | 0 | 2.38 | 2.96 | 0,46 | 3,16 |
| 6 | RHA | right | 6 | 34 | 12 | CBZ; CLB | left | 100 | 1.32 | 0.46 | 1 | 0.9 | 0.77 | 0,63 | 0,48 |
| 7 | RHA | right | 4 | 34 | 30 | CBZ | left | 92 | −1.11 | NA | 0 | −0.04 | 0.07 | ‐0,11 | ‐1 |
| 8 | RHA | right | 4 | 31 | 4 | CBZ; CLB | left | 97 | −0.58 | −0.85 | 0 | 1.29 | 1.07 | 1,04 | 1,88 |
| Left‐HS group | |||||||||||||||
| 1 | LHA | left | 1 | 47 | 2 | OXC; CLB | NA | 94 | −1.26 | −0.85 | 0 | −0.76 | −1.19 | 0.78 | −0.77 |
| 2 | LHA | left | 20 | 22 | 40 | CBZ; CLB | left | 80 | −501 | −1.02 | 1 | −0.23 | −0.22 | −0.24 | −1.3 |
| 3 | LHA | left | 2 | 22 | 6 | CBZ; TPM | left | 88 | −4.49 | −0.85 | 0 | −1.25 | −1.12 | −0.5 | −0.24 |
| 4 | LHA | left | 4 | 29 | 1 | CBZ; PNT; CLB | left | 94 | −2.69 | −0.19 | 0 | −0.83 | −1.55 | 0.59 | −0.65 |
| 5 | LHA | left | 3 | 44 | 5 | CBZ; CLB | left | 89 | −1.27 | 0.46 | 0 | −0.04 | −0.22 | 0.27 | −0.03 |
| 6 | LHA | left | 7 | 26 | 2 | LMT; TPM; CLB | left | 89 | −1 | 1.34 | 0 | −0.04 | −0.09 | 0.01 | −0.59 |
| 7 | LHA | left | 2 | 40 | 4 | CBZ; CLB | left | 80 | −7.97 | −1.64 | 0 | −1.83 | −2.3 | 0.01 | −1.73 |
| 8 | LHA | left | 2 | 18 | 11 | CBZ | left | NA | NA | NA | NA | NA | NA | NA | NA |
| 9 | LHA | left | 4 | 27 | 4 | CBZ | left | 86 | −4.45 | NA | 0 | −1.51 | −1.12 | −1.53 | −3.15 |
| F and | 0.287 | 0.482 | 0.461 | 0.204 | 9.656 | 3.855 | 0.001 | 0.368 | 15.387 | 14.51 | 1.061 | 6.345 | |||
| P values | 0.600 | 0.498 | 0.508 | 0.658 | 0.008 | 0.07 | 0.979 | 0.554 | 0.002 | 0.002 | 0.32 | 0.025 |
TL, temporal lobe; RHA, right hippocampal atrophy; LHA, left hippocampal atrophy; AEDs, antiepileptic drugs; CBZ, carbamazepine; CLB, clobazam; PNT, phenytoin; LMT, lamotrigine; TPM, topiramate; CLN, clonazepam; NA, not available.
As already mentioned, three contrasts were designed during the SPM2 analysis aiming to visualize the results for encoding [‐1 1 0] and immediate recall [0 0 1] stages. The differences between cerebral areas activated during encoding and immediate recall of verbal versus visual memories are shown in Table III.
Table III.
Comparison of verbal and visual memories activation areas
| Activation areas | Encoding of verbal memory | Immediate recall of verbal memory | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control group | Right‐HS group | Left‐HS group | Control group | Right‐HS group | Left‐HS group | |||||||||||||
| R | L | B | R | L | B | R | L | B | R | L | B | R | L | B | R | L | B | |
| Superior temporal cortex | * | * | * | * | * | |||||||||||||
| Infero‐medial temporal cortex | * | * | ||||||||||||||||
| Middle frontal cortex | * | * | * | |||||||||||||||
| Ventro‐lateral frontal cortex | * | * | * | * | ||||||||||||||
| Parietal cortex | * | * | * | * | * | * | ||||||||||||
| Occipital cortex | * | * | * | * | * | * | ||||||||||||
| Encoding of visual memory | Immediate recall of visual memory | |||||||||||||||||
| Inferior temporal cortex | * | * | * | * | ||||||||||||||
| Mid‐temporal cortex | * | * | ||||||||||||||||
| Hippocampal cortex | * | |||||||||||||||||
| Superior and inferior frontal cortex | * | * | * | |||||||||||||||
| Prefrontal cortex | * | * | * | * | * | |||||||||||||
| Parietal cortex | * | * | * | * | * | * | ||||||||||||
| Occipital cortex | * | * | * | * | * | * | ||||||||||||
| Cerebellum | * | * | * | |||||||||||||||
R, right; L, left; B, bilateral; * presence of activation.
Verbal Memory Test
The data analysis of the encoding stage revealed activations of: (1) bilateral occipital cortices in the three groups; (2) right parietal cortex in control group, and bilateral parietal cortices in right‐HS and left‐HS groups; (3) left superior temporal cortex in control group, bilateral superior temporal cortices in right‐HS group, and right superior temporal cortex in left‐HS group; (4) bilateral middle frontal cortices in control and left‐HS groups, and left middle frontal cortex in right‐HS group (see Fig. 3); and (5) bilateral>left ventro‐lateral frontal cortices in right‐HS group and bilateral>right ventro‐lateral frontal cortices in left‐HS group, while there was no such activation in control group (see Fig. 4).
Figure 3.
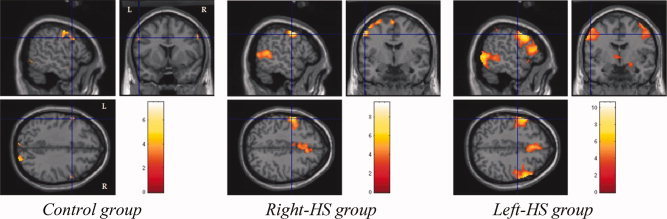
Encoding activations of bilateral middle frontal cortices in control and Left‐HS groups, and left middle frontal cortex in Right‐HS group.
Figure 4.
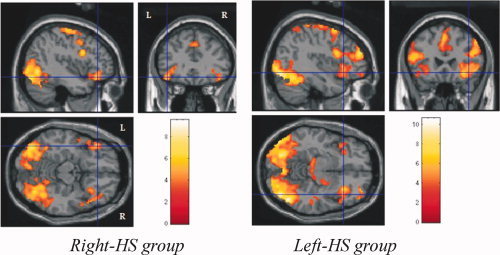
Encoding activations of bilateral > left ventro‐lateral frontal cortices in Right‐HS group and bilateral > right ventro‐lateral frontal cortices in Left‐HS group.
The data analysis of immediate recall showed activations of: (1) left occipital cortex in control and right‐HS groups, and bilateral occipital cortices in left‐HS group; (2) left parietal cortex in the three groups; (3) right superior temporal cortex in control and left‐HS groups, whereas no such activation was detected in right‐HS group; (4) bilateral>right infero‐medial temporal cortices in control group and right infero‐medial temporal cortex in right‐HS group, and absence of such activation in left‐HS group (see Fig. 5); (5) right ventro‐lateral frontal cortex in right‐HS and left‐HS groups, while no such activation was identified in control group (see Fig. 6); and (6) right prefrontal cortex in control and right‐HS groups, and bilateral prefrontal cortices in left‐HS group.
Figure 5.
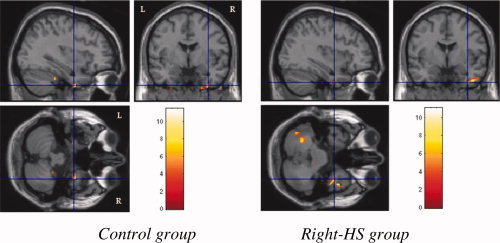
Immediate recall activations of bilateral > right infero‐medial temporal cortices in control group and right infero‐medial temporal cortex in Right HS group.
Figure 6.
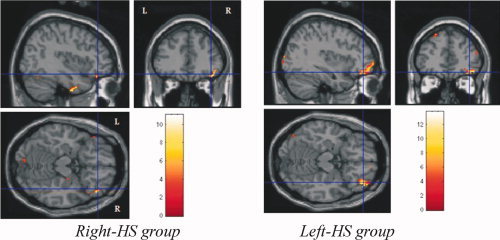
Immediate recall activations of right ventro lateral frontal cortex in Right HS and left HS groups.
Visual Memory Test
The data analysis of the encoding stage revealed activations of: (1) bilateral occipital and parietal cortices in the three groups, (2) right frontal cortex in control group, and bilateral superior and inferior frontal and prefrontal cortices in right‐HS and left‐HS groups (see Fig. 7); and (3) bilateral inferior temporal cortices in right‐HS group (see Fig. 8), while there was no such activation in control and left‐HS groups.
Figure 7.
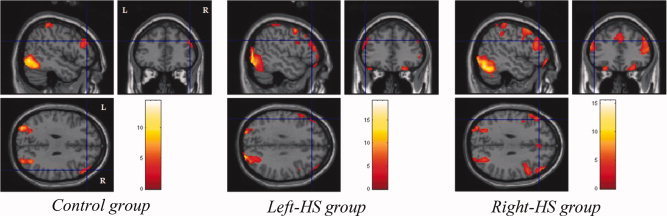
Encoding activations of right frontal cortex in control group, and bilateral superior and inferior frontal and prefrontal cortices in Right HS and left HS groups.
Figure 8.
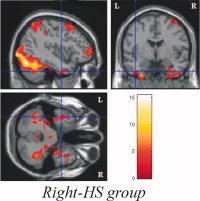
Encoding activations of bilateral inferior temporal cortices in Right HS group.
The data analysis of immediate recall showed activations of: (1) bilateral cerebellum in the three groups; (2) right occipital cortex in control group, and left occipital cortex in left‐HS and right‐HS groups; (3) bilateral parietal cortices in control group, and left parietal cortices in left‐HS and right‐HS groups; (4) right prefrontal cortex in control group and bilateral prefrontal cortex in left‐HS group (see Fig. 9), whereas no such activation was detected in right‐HS; (5) bilateral inferior temporal cortices in control group, and right inferior temporal cortex in left‐HS and right‐HS groups; (6) right mid‐temporal cortex in control and left‐HS groups, while there was no such activation in right‐HS; and (7) bilateral hippocampal cortices in control group (see Fig. 10).
Figure 9.
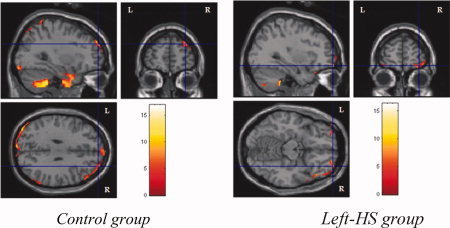
Immediate recall activations of right prefrontal cortex in control group and bilateral prefrontal cortices left HS group.
Figure 10.
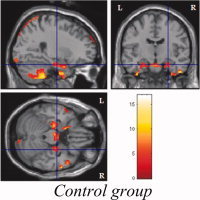
Immediate recall activations of bilateral hippocampal cortices in control group.
DISCUSSION
As already well‐established in the literature, neuropsychological evaluation of patients with refractory MTLE associated with HA usually reveals a mild‐to‐moderate memory deficit [Engel et al., 1997; French et al., 1993]. According to our previous studies [Alessio et al., 2004a, b], patients with refractory MTLE have more memory deficits than those with drug‐responsive MTLE, regardless of the presence and degree of HA on MRI. On the other hand, individuals with HA on MRI exhibit more memory impairment than individuals with normal MRI, regardless of the presence and frequency of seizures. However, the interaction of refractory seizures and HA is related to the worst memory performance [Alessio et al., 2004a, b].
Verbal Memory
A matter of less agreement in the literature concerns the classic material‐specific model of memory. More recent studies in epilepsy surgery have shown that the relationship between lateralized hippocampal pathology and memory dysfunction is more evident in left MTLE for verbal memory deficits than in right MTLE for visual memory [Alessio et al., 2004a, b]. However, it is not well established what kind of verbal memory process (encoding, consolidation, or retrieval) is related to which cortical brain regions in these patients, and this issue is particularly important when selecting candidates for resection of mesial temporal lobe structures.
Hence, one of the purposes of this study was to identify and compare the cerebral areas involved in verbal memory processing in normal controls and in patients with refractory MTLE by means of fMRI. More specifically, we tried to localize and lateralize verbal memory function, during two distinct stages of memory processing: encoding and retrieval.
The first important result of this study is the fact that the two patient groups were similar as regards several variables which can influence verbal memory performance, such as age of seizure onset, duration of epilepsy, seizure frequency, and number of antiepileptic drugs used, as well as vigilance, language and visual memory functions. Nevertheless, patients with left HS had worse performance on verbal memory, general memory and delayed recall than patients with right HS. Moreover, they also had lower IQ, which may have contributed to their inferior memory performance.
In addition, although the three groups were similar in age, educational level, handedness, and hemispheric dominance for language, they exhibited different patterns of activations not only in the encoding stage, but also in the retrieval stage of verbal memory processing.
With regard to the encoding stage, patients with left HS showed more widespread areas of activations than patients with right HS, and even more than normal controls. Although this pattern had been observed in the occipital (Brodmann areas [BA]: 17/18), parietal (BA: 7) and temporal regions (BA: 21/22), it seemed to be mostly remarkable in the middle and ventro‐lateral frontal regions (BA: 6/8 and 44/45, respectively). These findings are in accordance with previous fMRI studies that have demonstrated a larger involvement of frontal cerebral areas in verbal memory processing in patients with left MTLE [Dupont et al., 2000; Golby et al., 2001].
Patients with left HS also tended to exhibit more bilateral or right lateralized encoding related activations (Figs. 3 and 4). This functional reorganization and, consequently, cortical reallocation of verbal memory encoding in more widespread bilateral fronto‐parietal, and right temporal areas in patients with left MTLE may indicate a compensatory strategy for the dysfunction of left MTL system [Dupont et al., 2000]. A less evident functional reorganization of verbal memory encoding was also detected in the bilateral parietal, bilateral>left ventro‐lateral frontal, left middle frontal, and bilateral superior temporal cortices in patients with right HS, as compared to normal controls. We could also hypothesize that this functional rearrangement can be secondary to the failure of right MTL system.
Another interpretation of our regional activation data during verbal encoding is that the block designed visual presentation of the abstracts words could also activate the semantic working memory processing of these words, whose meanings (concepts) are accessed almost automatically. Indeed, a “semantic working memory system” responsible for retrieving, maintaining, monitoring and manipulating semantic representations and composed particularly by the left anterior‐inferior prefrontal cortex and the polar region of the left temporal lobe, has been proposed by Gabrieli et al. [ 1998], Wagner [ 1999], and Martin and Chao [ 2001]. Additional evidence for the relevance of the left lateral prefrontal cortex for semantic processing of abstract words has been yielded afterward by other authors [Fiebach and Friederici, 2004; Goldberg et al., 2007]. In an event‐related fMRI study of normal readers Fiebach and Friederici [ 2004] found that different semantic classes of nouns are processed in distinct cortical regions within the left hemisphere, with concrete nouns activating preferentially the left basal temporal cortex, and abstract nouns the left inferior frontal cortex. In patients with left MTLE and HS, on the contrary, similar studies have shown a shift of activations to homologous regions in the right hemisphere during the processing of abstract words as compared to concrete words and non‐words [Edwards et al., 2005; Koylu et al., 2006]. Our group of left MTLE and HS patients showed similar shifted distribution of activations: right temporal, right or bilateral inferolateral frontal, bilateral middle frontal and bilateral parietal. Thus, this widespread contralateral and ipsilateral cortical activation may be interpreted as resulting not only from the episodic encoding but also from the semantic working memory processing of the words presented.
With respect to the retrieval stage, patients with MTLE associated with left HS continued to show more widespread areas of activations than patients with right HS and normal controls. Once more, this pattern was more evident in the frontal region. However, it is important to emphasize that the activation areas observed in the retrieval stage were smaller than those detected in the encoding stage in the three groups.
In the retrieval period, as opposed to the encoding stage, not only patients with left HS, but also patients with right HS and normal controls tended to exhibit more bilateral and right‐sided activations (Figs. 5 and 6).
This prevalence of right hemisphere activations during the retrieval stage is in agreement with the hemispheric encoding/retrieval asymmetry (HERA) model, which predicts prefrontal activation during encoding of new information mainly in the left hemisphere, whereas retrieval of previously learned information is accompanied by increased activity in right prefrontal areas [Dupont et al., 2000; Kapur et al., 1994; Kennepohl et al., 2007; Powell et al., 2004; Tulving et al., 1994]. Although it concerns only to the material‐independent lateralization of certain memory tasks in frontal lobes, some authors have recommended that it be extended to temporal lobe regions [Kennepohl et al., 2007]. During retrieval, our group of left MTLE patients showed bilateral prefrontal activation with right frontal ventrolateral predominance, which indicates a reduction of the functional hemispheric asymmetry in this task, similarly to that observed with normal aging (the so called HAROLD, Hemispheric Asymmetry Reduction in Older adults), [Cabeza, 2002]. In patients with left MTLE, the increased (bilateral) prefrontal activation not only may be compensatory, but also may represent a decrease in the level of functional differentiation of the task‐relevant neural systems, as proposed by Chen et al. [ 2002] for the HAROLD model. It remains unexplained why our group of patients with right MTLE presented ipsilateral activation (right prefrontal, frontal ventrolateral, and temporal inferomedial), thus preserving the prevalence of right hemisphere activations during retrieval. It is possible that their long epilepsy duration with early development of right HS has led to intrahemispheric reorganization of cognitive functions.
Visual Memory
Our previous studies did not show a significant correlation between the degrees of right HA and visual memory deficits in patients with right MTLE [Alessio et al., 2004a, b, 2006; Bonilha et al., 2007]. Two hypotheses have been raised to explain this lack of correlation: (1) the visual memory may have a more diffuse and bilateral representation in the brain [Helmstaedter and Kurthen, 2001; Jones‐Gotman, 1996], and/or (2) the visual memory tests employed may not be robust enough to identify nondominant hippocampal dysfunction [Jones‐Gotman, 1996]. We believe in a combination of these two hypotheses, because: (1) we found visual memory deficits in MTLE patients with bilateral HA and rarely in those with right unilateral HA, and (2) we observed that some patients made use of verbal strategies in order to memorize a visual content [Alessio et al., 2004a].
Hence, another purpose of this study was to identify and compare the cerebral areas involved in visual memory processing in normal controls and patients with refractory MTLE by means of fMRI.
With regard to the encoding stage, patients with right MTLE showed more widespread and bilateral areas of activations than patients with left MTLE, and even more than normal controls. Moreover, while normal controls tended to exhibit asymmetrical (bilateral>right) activated regions, patients with right HS tended to show more symmetrical and bilateral activated regions probably to compensate for the failure of their right MTL system (Figs. 7 and 8).
These findings are in agreement with the hypothesis of a more diffuse and bilateral representation of visual memory in the brain. Not only MTL structures, but also temporoparietal and prefrontal areas seem to be involved with the functional neuroanatomy of visual memory, as follows: (1) posterior parietal cortex provides a bridge from perception to recognition, it is related to attention and spatial awareness, and it takes part in spatial memory; and (2) dorsolateral prefrontal cortex is associated with attention, working memory and executive functions that are also critical for memory processes [Burgess et al., 2001; Desgranges et al., 1998].
However, in contrast to some authors [Detre et al., 1998; Stern et al., 1996; Szaflarski et al., 2004] who have found bilateral‐symmetrical hippocampal activation in normal controls and bilateral‐asymmetrical hippocampal activation in MTLE patients, we did not find hippocampal activations in the three groups during encoding stage of a visual content. This discrepancy could be explained by (1) differences in the fMRI task design; and/or (2) lack of MTL activation in our study owing to those technical limitations mentioned before, such as geometric distortions, signal loss artifacts, and partial volume effects [Figueiredo et al., 2008; Powell et al., 2004, 2005, 2007]. For different reasons some other functional studies have also failed to demonstrate hippocampal activation either during encoding or retrieval stages [Cabeza and Nyberg, 2000; Schacter and Wagner, 1999].
With respect to the retrieval stage, the activation patterns found in the three groups were exactly the opposite of those detected in the encoding stage. Compared to patients with right MTLE, left MTLE patients retrieval produced more diffuse activation, which was even more diffuse in normal controls (Figs. 9 and 10). It is important to note that in the retrieval stage we were able to detect bilateral symmetrical hippocampal activation in the control group [Jokeit et al., 2001]. Again, not only MTL structures but also temporal, parietal and prefrontal areas seem to be involved in visual memory processing [Burgess et al., 2001; Engelsen et al., 2006; Fletcher, 1995; Ungerleider et al., 1998].
In addition, patients with right‐HS tended to exhibit more left‐sided activated areas during retrieval as opposed to patients with left‐HS and normal controls, which tended to show more bilateral and right‐sided activations. This prevalence of right hemisphere activations during the retrieval stage in normal controls is in agreement with the hemispheric encoding/retrieval asymmetry (HERA) model. Patients with right MTLE, on the contrary, tended to show more left‐sided activated areas probably to compensate for the failure of their right MTL system.
Limitations
As already mentioned, fMRI evaluation of memory is more difficult than fMRI evaluation of other cognitive functions, due to some neuropsychological and technical issues [Powell et al., 2004, 2005]. As regards the neuropsychological aspects, we polarized as much as possible the verbal versus the nonverbal (visual) memory contents, by using a list of 17 abstract and emotionally neutral words as opposed to a series of 17 abstract drawings. In spite of this, some of the drawings may have led the patients to use verbal strategies in order to memorize the visual content. In addition, the drawings may have led to a different pattern of activation because they were more complex than the fixation point (“+”) of the baseline condition. In addition, the technical aspects could not be so well controlled, because (1) the fMRI acquisitions were made using EPI sequence, which is particularly susceptible to geometric distortions and signal loss, and (2) the BOLD response is naturally lower in the hippocampal regions. All these problems may have contributed to the relative lack of MTL activations, and their solution requires paradigms with even less verbalizable visual‐spatial test material and more complex stimulus than a fixation point, as well as fMRI equipments with higher spatial resolution and greater sensitivity.
However, we believe that the restrictive and rigorous statistical analysis that we performed in the present study make our findings quite reliable.
CONCLUSION
A complex network including parietal, temporal and frontal cortices seems to be involved in verbal memory encoding and retrieval in normal controls. The extension of such activations was larger in patients with right and left HS, more so in patients with left HS. Whereas normal controls and patients with right HS tended to exhibit more left‐sided activated areas, patients with left HS tended to show more bilateral and right‐sided activated regions in the encoding stage. These findings can indicate either a dysfunction or a functional reorganization of verbal memory processing in other cerebral regions, particularly in frontal lobes. In contrast, the three groups presented more right‐sided activated areas in the retrieval stage, which is in agreement with the HERA model.
As regards visual memory encoding and retrieval, our findings support the hypothesis of a more diffuse and bilateral representation of this cognitive function in the brain. Compared to normal controls, encoding of visual material in patients with left HS recruited more widespread cortical areas, which were even more widespread in patients with right HS probably to compensate for their right mesial temporal dysfunction. On the other hand, when compared to the right MTLE group, the drawing retrieval task produced more diffuse activation in the left MTLE group and even more diffuse activation in the control group (including also both hippocampi). In spite of their effort in memorizing (encoding) the 17 drawings, patients with right MTLE exhibited fewer activated areas during immediate recall probably related to their greater difficulty in dealing with visual memory content.
REFERENCES
- Aldenkamp AP, Boon PA, Deblaere K, Achten E, Backes WH, Boon P, Hofman P, Troost J, Vandemaele P, Vermeulen J, Vonck K, Wilmink J ( 2003): Usefulness of language and memory testing during intracarotid amobarbital testing: Observations from an fMRI study. Acta Neurol Scand 108: 147–152. [DOI] [PubMed] [Google Scholar]
- Alessio A, Kobayashi E, Damasceno BP, Lopes‐Cendes I, Cendes F ( 2004a): Evidence of memory impairment in asymptomatic individuals with hippocampal atrophy. Epilepsy Behav 5: 981–987. [DOI] [PubMed] [Google Scholar]
- Alessio A, Damasceno BP, Camargo CHP, Kobayashi E, Guerreiro CAM, Cendes F ( 2004b): Differences in memory performance and other clinical characteristics in patients with mesial temporal lobe epilepsy with and without hippocampal atrophy. Epilepsy Behav 5: 22–27. [DOI] [PubMed] [Google Scholar]
- Alessio A, Bonilha L, Rorden C, Kobayashi E, Li ML, Damasceno BP, Cendes F ( 2006): Memory and language impairments and their relationship to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy Behav 8: 593–600. [DOI] [PubMed] [Google Scholar]
- Baxendale SA ( 1997): The role of the hippocampus in recognition memory. Neuropsychologia 35: 591–598. [DOI] [PubMed] [Google Scholar]
- Baxendale SA, Thompson P, Harkness W, Duncan J ( 2006): Predicting memory decline following epilepsy surgery: A multivariate approach. Epilepsia 47: 1887–1894. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Kobayashi E, Cendes F, Li LM ( 2004): Protocol for volumetric segmentation of medial temporal structures using high resolution 3D MRI. Hum Brain Mapp 22: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Alessio A, Rorden C, Baylis G, Damasceno BP, Li ML, Cendes F ( 2007): Extrahippocampal gray matter atrophy and memory impairment in patients with medial temporal lobe epilepsy. Hum Brain Mapp 28: 1376–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD ( 2001): Prefrontal regions involved in keeping information in and out of mind. Brain 124: 2074–2086. [DOI] [PubMed] [Google Scholar]
- Buonocore MH, Gao L ( 1997): Ghost artifact reduction for echo planar imaging using image phase correction. Magn Reson Med 38: 89–100. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O'Keefe J ( 2001): A tempoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage 14: 439–453. [DOI] [PubMed] [Google Scholar]
- Cabeza R ( 2002): Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging 17: 85–100. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L ( 2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Chen J, Myerson J, Hale S ( 2002): Age‐related dedifferentiation of visuospatial abilities. Neuropsychologia 40: 20–50. [DOI] [PubMed] [Google Scholar]
- Dade LA, Jones‐Gotman M ( 1997): Sodium amobarbital memory tests: What do they predict? Brain Cogn 33: 189–209. [DOI] [PubMed] [Google Scholar]
- Damasceno A, Alessio A, Damasceno BP, Li LM, Cendes F ( 2005): Spatial and emotional memory in patients with temporal lobe epilepsy. Neurology 64 ( Suppl 1): A359–A360. [Google Scholar]
- Desgranges B, Baron JC, Eustache F ( 1998): The functional neuroanatomy of episodic memory: The role of the frontal lobes, the hippocampal formation and other areas. Neuroimage 8: 198–213. [DOI] [PubMed] [Google Scholar]
- Detre JA, Maccotta L, King D, Alsop DC, Glosser G, D'Esposito M, Zarahn E, Aguirre GK, French JA ( 1998): Functional MRI lateralization of memory in temporal lobe epilepsy. Neurology 50: 926–932. [DOI] [PubMed] [Google Scholar]
- Dinner DS ( 1991): Intracarotid amobarbital test to define language lateralization In: Luders H, editor. Epilepsy Surgery. New York: Raven Press; pp 503–506. [Google Scholar]
- Dupont S, Van de Moortele PF, Samson S, Hasboun D, Poline JB, Adam C, Lehéricy S, Le Bihan D, Samson Y, Baulac M ( 2000): Episodic memory in left temporal lobe epilepsy: A functional MRI study. Brain 123: 1722–1732. [DOI] [PubMed] [Google Scholar]
- Edwards J, Bass A, Goodyear B, Federico P ( 2005): An fMRI study of semantic reorganization in temporal lobe epilepsy. J Neurol Sci 238 ( Suppl 1): S122. [Google Scholar]
- Engel J, Willimson PD, Wieser HG ( 1997): Mesial temporal lobe epilepsy In: Engel J, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. Philadelphia: Lippincott‐Raven Publishers; pp 2417–2426. [Google Scholar]
- Engelsen BA, Gramstad A, Thomsen T, Beneventi H, Ersland L, Smievoll AI, Lundervold A, Hugdahl K ( 2006): Frontoparietal activation during delayed visuospatial recall in patients with epilepsy due to hippocampal sclerosis. Epilepsy Behav 8: 565–574. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD ( 2004): Processing concrete words: fMRI evidence against a specific right‐hemisphere involvement. Neuropsychologia 42: 62–70. [DOI] [PubMed] [Google Scholar]
- Figueiredo P, Santana I, Teixeira J, Cunha C, Machado E, Sales F, Almeida E, Castelo‐Branco M ( 2008): Adaptative visual memory reorganization in the right medial temporal lobe epilepsy. Epilepsia 49: 1395–1408. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ ( 1995): The mind's eye—Precuneus activation in memory‐related imagery. Neuroimage 2: 195–200. [DOI] [PubMed] [Google Scholar]
- French JA, Willimson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD ( 1993): Characteristics of temporal lobe epilepsy: I results of history and physical examination. Ann Neurol 34: 774–780. [DOI] [PubMed] [Google Scholar]
- Fromm‐Auch D, Yeudall LT ( 1983): Normative data for the Halstead‐Reitan neuropsychological tests. J Clin Neuropsychol 5: 221–238. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Poldrack RA, Desmond JE ( 1998): The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA 95: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleissner U, Helmstaedter C, Schramm J, Elger CE ( 2004): Memory outcome after selective amygdalohippocampectomy in patients with temporal lobe epilepsy: One‐year follow up. Epilepsia 45: 960–962. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Brewer JB, Spencer D, Desmond JE, Aron AP, Gabrieli JD ( 2001): Material‐specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain 124: 1841–1854. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Illes J, Chen D, Desmond JE, Gabrieli JDE ( 2002): Memory lateralization in medial temporal lobe epilepsy assessed by functional MRI. Epilepsia 43: 855–863. [DOI] [PubMed] [Google Scholar]
- Goldberg RF, Perfetti CA, Fiez JA, Schneider W ( 2007): Selective retrieval of abstract semantic knowledge in left prefrontal cortex. J Neurosci 27: 3790–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J, Bouwer MS, Jones‐Gotman M ( 1992): Intracranial EEG study of brain structures affected by internal carotid injection of sodium amobarbital. Neurology 42: 2136–2143. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Kurthen M ( 2001): Memory and epilepsy: Characteristics, course, and influence of drugs and surgery. Curr Opin Neurol 14: 211–216. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Connell B, Barr WB, Wyler AR ( 1995): The utility of the Warrington recognition memory test for temporal lobe epilepsy: Pre and postoperative results. J Epilepsy 8: 139–145. [Google Scholar]
- Hermann BP, Seidenberg M, Schoenfeld J, Davies K ( 1997): Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol 54: 369–376. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Okujava M, Woermann FG ( 2001): Memory fMRI lateralizes temporal lobe epilepsy. Neurology 57: 1786–1793. [DOI] [PubMed] [Google Scholar]
- Jones‐Gotman M ( 1987): Commentary: Psychological evaluation: Testing hippocampal function In: Engel JR, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; pp 68–76. [Google Scholar]
- Jones‐Gotman M ( 1996): Psychological evaluation for epilepsy surgery In: Shorvon S, Dreifuss F, Fish D, Thomas D, editors. The Treatment of Epilepsy. Oxford: Blackwell Science; pp 621–630. [Google Scholar]
- Jones‐Gotman M, Zatorre RJ, Olivier A, Andermann F, Cendes F, Staunton H, McMackin D, Siegel A, Wieser HG ( 1997): Learning and retention of words and designs following excision from medial or lateral temporal‐lobe structures. Neuropsychologia 35: 963–973. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintaub S ( 1983): The Boston Naming Test. Philadelphia: Lea & Febiger. [Google Scholar]
- Kapur S, Craik FI, Tulving E, Wilson AA, Houle S, Brown GM ( 1994): Neuroanatomical correlates of encoding in episodic memory: Levels of processing effect. Proc Natl Acad Sci USA 91: 2008–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennepohl S, Sziklas V, Garver KE, Wagner DD, Jones‐Gotman M ( 2007): Memory and the temporal lobe: Hemispheric specialization reconsidered. Neuroimage 36: 969–978. [DOI] [PubMed] [Google Scholar]
- Kimura D ( 1963): Right temporal lobe damage. Perception of unfamiliar stimuli after damage. Arch Neurol 8: 264–271. [DOI] [PubMed] [Google Scholar]
- Koylu B, Trinka E, Ischebeck A, Visani P, Trieb T, Kremser C, Bartha L, Schocke M, Benke T ( 2006): Neural correlates of verbal semantic memory in patients with temporal lobe epilepsy. Epilepsy Res 72: 178–191. [DOI] [PubMed] [Google Scholar]
- Lencz T, McCarthy G, Bronen RA, Scott TM, Inserni JA, Sass KJ, Novelly RA, Kim JH, Spencer DD ( 1992): Quantitative magnetic resonance imaging in temporal lobe epilepsy: Relationship to neuropathology and neuropsychological function. Ann Neurol 31: 629–637. [DOI] [PubMed] [Google Scholar]
- Lineweaver TT, Morris HH, Naugle RI, Najm IM, Diehl B, Bingaman W ( 2006): Evaluating the contributions of state‐of‐the‐art assessment techniques to predicting memory outcome after unilateral anterior temporal lobectomy. Epilepsia 47: 1895–1903. [DOI] [PubMed] [Google Scholar]
- Loring DW, Lee GP, Meador KJ, Flanigin HF, Smith JR, Figueroa RE, Martin, RC ( 1990): The intracarotid amobarbital procedure as a predictor of memory failure following unilateral temporal lobectomy. Neurology 40: 605–610. [DOI] [PubMed] [Google Scholar]
- Lukban A, Dean G, Lisbona R, Dubeau F, McMackin D, Evans AC ( 1994): Anatomical localization of seizure foci using registered SPECT/MRI brain volumes. Neurology 44: 385.8145903 [Google Scholar]
- Martin A, Chao LL ( 2001): Semantic memory and the brain: Structure and processes. Curr Opin Neurobiol 11: 194–201. [DOI] [PubMed] [Google Scholar]
- Meyer V, Yates AJ ( 1955): Intellectual changes following temporal lobectomy for psychomotor epilepsy. J Neurol Neurosurg Psychiatry 18: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B ( 1972): Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg 19: 421–446. [DOI] [PubMed] [Google Scholar]
- Milner B, Branch C, Rasmussen T ( 1962): Study of short‐term memory after intracarotid injection of sodium amytal. Trans Am Neurol Assoc 87: 224–226. [Google Scholar]
- Novelly R, Augustine EA, Mattson RH, Glaser GH, Willimson PD, Spencer DD, Spencer SS ( 1984): Selective memory improvement and impairment in temporal lobectomy for epilepsy. Ann Neurol 15: 64–67. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Powell HW, Koepp MJ, Richardson MP, Symms MR, Thompson PJ, Duncan JS ( 2004): The application of functional MRI of memory in temporal lobe epilepsy: A clinical review. Epilepsia 45: 855–863. [DOI] [PubMed] [Google Scholar]
- Powell HW, Koepp MJ, Symms MR, Boulby PA, Salek‐Haddadi A, Thompson PJ, Duncan JS, Richardson MP ( 2005): Material‐specific lateralization of memory encoding in the medial temporal lobe: blocked versus event‐related design. Neuroimage 27: 231–239. [DOI] [PubMed] [Google Scholar]
- Powell HW, Richardson MP, Symms MR, Boulby PA, Thompson PJ, Duncan JS, Koepp MJ ( 2007): Reorganization of verbal and nonverbal memory in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsia 48: 1512–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin ML, Narayan VM, Kimberg DY, Casasanto DJ, Glosser G, Tracy JI, French JA, Sperling MR, Detre JA ( 2004): Functional MRI predicts post‐surgical memory following temporal lobectomy. Brain 127: 2286–2298. [DOI] [PubMed] [Google Scholar]
- Rausch R ( 2002): Epilepsy surgery within the temporal lobe and its short‐term and long‐term effects on memory. Curr Opin Neurol 15: 185–189. [DOI] [PubMed] [Google Scholar]
- Rausch R, Langfitt JT ( 1991): Memory evaluation during the intracarotid sodium amobarbital procedure In: Luders H, editor. Epilepsy Surgery. New York: Raven Press; pp 507–514. [Google Scholar]
- Richardson MP, Strange BA, Duncan JS, Dolan RJ ( 2003): Preserved verbal memory function in left medial temporal pathology involves reorganization of function to right medial temporal lobe. Neuroimage 20 ( Suppl 1): S112–S119. [DOI] [PubMed] [Google Scholar]
- Saling MM, Berkovic SF, O'Shea MF, Kalnins RM, Darby DG, Bladin PF ( 1993): Lateralization of verbal memory and unilateral hippocampal sclerosis. Evidence of task specific effects. J Clin Exp Neuropsychol 15: 608–618. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD ( 1999): Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 9: 7–24. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E ( 1998): Language tests In: Spreen O, Strauss E, editors. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. New York: Oxford University Press; pp 423–480. [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR ( 1996): The hippocampal formation participates in novel picture encoding: Evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA 93: 8660–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ ( 2002): Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. J Neurosci 22: 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strub RL, Black FW ( 1993): Mental Status Examination in Neurology. Philadelphia: FA Davis. [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Dunn RS, Privitera MD ( 2004): High‐resolution functional MRI at 3T in health and epilepsy subjects: Hippocampal activation with picture encoding task. Epilepsy Behav 5: 244–252. [DOI] [PubMed] [Google Scholar]
- Trenerry MR, Jack CR Jr, Ivnik RJ, Sharbrough FW, Cascino GD, Hirschorn KA, Marsh WR, Kelly PJ, Meyer FB ( 1993): MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology 43: 1800–1805. [DOI] [PubMed] [Google Scholar]
- Trenerry MR, Westerveld M, Meador KJ ( 1995): MRI hippocampal volume and neuropsychology in epilepsy surgery. Magn Reson Imaging 13: 1125–1132. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S ( 1994): Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proc Natl Acad Sci USA 91: 2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV ( 1998): A neural system for human visual working memory. Proc Natl Acad Sci USA 95: 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD ( 1999): Working memory contributions to human learning and remembering. Neuron 22: 19–22. [DOI] [PubMed] [Google Scholar]
- Wechsler D ( 1981): Wechsler Adult Intelligence Scale‐Revised. New York: Pshychological Corp. [Google Scholar]
- Wechsler D ( 1987): Wechsler Memory Scale‐Revised: Manual. San Diego: Psychological Corp/Harcourt Brace Jovanovich. [Google Scholar]


