Abstract
Previous studies have indicated that increasing working memory (WM) load can affect the attentional selection of signals originating from one object/location. Here we assessed whether WM load affects also the selection of multiple objects/locations (divided attention). Participants monitored either two object‐categories (vs. one category; object‐based divided attention) or two locations (vs. one location; space‐based divided attention) while maintaining in WM either a variable number of objects (object‐based WM load) or locations (space‐based WM load). Behavioural results showed that WM load affected attentional performance irrespective of divided or focused attention. However, fMRI results showed that the activity associated with object‐based divided attention increased linearly with increasing object‐based WM load in the left and right intraparietal sulcus (IPS); while, in the same areas, activity associated with space‐based divided attention was not affected by any type of WM load. These findings support the hypothesis that WM contributes to the maintenance of resource‐demanding attentional sets in a domain‐specific manner. Moreover, the dissociable impact of WM load on performance and brain activity suggests that increased IPS activation reflects a recruitment of additional, domain‐specific processing resources that enable dual‐task performance under conditions of high WM load and high attentional demand. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: working memory, load, focused, divided, spatial attention, intraparietal sulcus, fMRI
INTRODUCTION
A growing body of literature has started to investigate the relationship between working memory (WM) and selective attention, which were traditionally studied as separate processes. The interplay between these two cognitive functions can be addressed by asking whether attention influences the operation of the WM system, but also whether the engagement of WM resources modulates attentional selection. On the first point, extensive research indicates that attention can “bias” the likelihood of sensory information to access WM [e.g., Botta et al., 2010; Schmidt et al., 2002; see Awh et al., 2006, for a review], consistent with the view that attention selects relevant information to be processed in WM, which is a limited‐capacity system [e.g., Cowan, 2005, 2010].
By contrast, less is known about the role of WM for the allocation and control of attentional resources. A few previous studies suggested that increasing WM load results in a reduced capability to filter out task‐irrelevant information [e.g., Lavie, 2000, 2004; see also Lavie, 2005, for a review]. For instance, during visual search, attentional capture by task‐irrelevant salient stimuli increases when participants are performing a concurrent high‐load WM task [Lavie and De Fockert, 2005]. Neuroimaging studies indicated a possible neural substrate for this effect, showing that distractor‐related activation in visual cortex increases under high‐load WM conditions [De Fockert et al., 2001]. Lavie et al. proposed that high WM load interferes with executive control, reducing the brain's capability to maintain stimulus‐processing priorities. As a consequence, task‐irrelevant low‐priority distractors would interfere more with the processing of task‐relevant stimuli [e.g., Lavie, 2000]. This highlights one possible contribution of WM for successful selective attention.
However, attention does not only operate by selecting a single object or location at the time, but can also be deployed to monitor simultaneously multiple objects and/or locations (divided attention). The monitoring of multiple objects/locations typically results in a decrement of processing efficacy, as documented both behaviorally and neurophysiologically [Castiello and Umiltà, 1992; Eriksen and St. James, 1986; McMains and Somers, 2004, 2005; Müller et al., 2003a, b]. The nature of these costs is linked to increased demands of top‐down control signals from high‐level control areas (e.g., frontal eye fields or the parietal cortex) to lower‐level sensory areas during divided attention [McMains and Somers, 2004; see also Tong, 2004], and to limited processing capacity of the high‐level control systems [see also Driver, 2001; Nebel et al., 2005].
With regards to the second aspect, it is hypothesized that the costs of dividing attention may arise because dividing attention requires maintaining multiple target representations in WM [e.g., Kastner et al., 1999; Luck et al., 1997; see also Fagioli and Macaluso, 2009]. Accordingly, WM would play a direct role in divided attention control, with the two systems utilizing a common pool of processing resources. Indirect evidence supporting this view come from neuroimaging studies showing that associative areas in the fronto‐parietal cortex engage both during divided attention and WM tasks. For instance, Fagioli and Macaluso [ 2009] found that dividing attention between multiple object–categories or multiple locations activates a fronto‐parietal (FP) attention network, including the prefrontal cortex (PFC) bilaterally, the middle and dorsal premotor cortex (comprising the frontal eye‐fields, FEF), and the intraparietal sulcus (IPS). The same areas activate consistently in WM studies during the maintenance phase of WM tasks [Courtney et al., 1998; Jha and McCarthy, 2000; Leung et al., 2004; Linden et al., 2003; Munk et al., 2002; Petit et al., 1998; Rowe et al., 2000; Todd and Marois, 2004; Xu, 2007; Xu and Chun, 2006; Zarahn et al., 1999], which is the main focus of our current investigation. Nonetheless, the mere colocalization of activation for attention and WM is insufficient to infer the existence of a common neural substrate. In fact, different populations of WM‐specific and attention‐specific neurons can generate overlapping activation in fMRI experiments. Hence, here we utilized a dual task‐procedure requiring subjects to perform non‐spatial (i.e., object‐based) or spatial divided attention tasks, while—at the same time—maintaining a variable number of objects or locations in WM. This enabled us to directly assess whether changes of WM requirements (high vs. low WM load) affect activity associated with divided attention, which would imply that these two cognitive functions utilize a common set of processing resources.
An additional issue that needs to be considered is the specific type of information to be selected (attention) and maintained (WM). Extensive investigation in the WM domain highlighted a segregation between areas preferentially processing object‐related information in the most ventral part of PFC vs. space‐related information in the most dorsal part of PFC, plus the parietal cortex [e.g., Munk et al., 2002; see also Courtney et al., 1996; McCarthy et al., 1996; Rissman et al., 2008; Tresch et al., 1993; Ventre‐Dominey et al., 2005]. Studies on selective attention also revealed some difference between non‐spatial and spatial attention activating extrastriate occipital regions versus parietal areas (i.e., the IPS and the precuneus), respectively [Slagter et al., 2007; see also Fink et al., 1997]. This distinction may parallel the classical segregation of ‘what’ (i.e., occipito‐temporal) and “where” (i.e., occipito‐parietal) pathways for the processing of visual information [see Goodale and Milner, 1992; Underleider and Mishkin, 1982]. Other studies have reported differential activation between the IPS and the right superior parietal cortex for nonspatial and spatial attention, respectively [e.g., Coull and Frith, 1998].
However, it should also be noted that these distinctions are often relative rather than absolute. For example, activation of the PFC, FEF, and IPS during the maintenance period of spatial WM tasks [Courtney et al., 1998; Leung et al., 2004; Petit et al., 1998; Rowe et al., 2000; Zarahn et al., 1999] was found also in nonspatial WM tasks [e.g., Jha and McCarthy, 2000; Linden et al., 2003]. Similarly, attentional control has been shown to activate the PFC, FEF, and IPS irrespective of the type of the to‐be‐attended material [i.e., object‐ and space‐based; Corbetta et al., 2005; Fink et al., 1997; Slagter et al., 2005; see also Arrington et al., 2000]. Finally, as noted above, also in the field of divided attention, the PFC, FEF, and IPS have been found to activate during both monitoring of multiple object–categories and attending to multiple spatial locations [Fagioli and Macaluso, 2009]. In this context, the maintenance of multiple target representations may recruit same or different substrates, depending on whether the task‐relevant dimension concerns the number or objects/features (monitor two vs. one object‐category) or the number of locations (divided vs. focused spatial attention).
Accordingly, the aim of our current study was to investigate the contribution of WM for the control of divided attention, and to test whether any interplay between these two systems depends on the type of information (spatial vs. nonspatial) that the subject is attending to and has to maintain in WM. The divided attention tasks involved either monitoring two object‐categories (vs. one object‐category, as a control condition) or attending two locations [vs. one location; cf. Fagioli and Macaluso, 2009]. Subjects performed the object‐based and the space‐based attention tasks while they maintained in WM either a variable number of objects (object‐based WM load) or locations (space‐based WM load). Our analyses assessed whether increasing the amount of object‐based or space‐based information held in WM modulates activity within the FP network associated with object‐based divided attention and space‐based divided attention, indexed using the difference score “divided attention conditions minus focused attention conditions.”
We expected that if WM and divided attention utilize a common, limited‐capacity pool of processing resources, increasing WM load would interfere with the divided attention tasks, leading to changes of performance and/or brain activation associated with the attention tasks. Moreover, if attention and WM make use of specific object‐based vs. space‐based resources [e.g., Fagioli and Macaluso, 2009; Leung et al., 2004; Linden et al., 2003], we would expect different patterns of interaction depending on the specific attentional and WM requirements: e.g., increasing object‐based WM load may modulate differentially attention‐related activation depending on whether the divided attention task involves monitoring multiple object–categories or attending to multiple spatial locations.
METHODS AND MATERIALS
Participants
Thirteen right‐handed volunteers took part in the study that included two fMRI sessions, one for the object‐based WM experiment and one for the space‐based WM experiments. The two sessions were carried out on separate days, with the order of the WM tasks counterbalanced across participants. All participants were in good health, free of psychotropic or vasoactive medication, with no past history of psychiatric or neurological disease. All had normal or corrected‐to‐normal (with contact lenses) visual acuity. Two participants were excluded from statistical analysis because of within‐fMRI‐run head‐movements larger than 2 mm or 2°, leaving 11 participants (four males, mean age: 26.6 years, range: 20–32 years). After having received an explanation of the procedures, all participants gave their written consent. The study was approved by the independent Ethics Committee of the Santa Lucia Foundation (Scientific Institute for Research Hospitalization and Health Care).
Paradigm
In two separate fMRI sessions, the participants performed either an object‐based or a space‐based WM task, while engaging in specific object‐based or space‐based divided attention tasks (see below). This enabled us to manipulate concurrently WM and attentional requirements and to investigate possible interactions between these two cognitive processes. The two WM tasks were (see also Fig. 1):
-
1
Object‐based WM task: Decide whether the item presented in the memory test display had been presented (50% of trials) or not (50% of trials) in the memory sample display.
-
2
Space‐based WM task: Decide whether the location cued in the memory test display had contained (50% of trials) or not (50% of trials) an item in the memory sample display.
Figure 1.
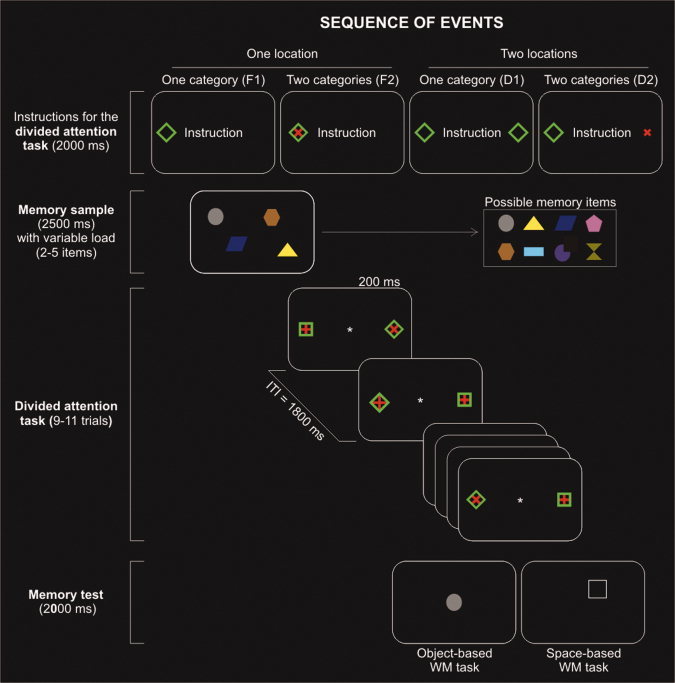
Schematic diagram showing an example of the sequence of events. Each block began with a visual display providing target‐instruction about the upcoming selective attention task. A memory sample consisting of 2–5 items was then presented and followed by 9–11 attention trials, including presentation of two independent visual streams on each side. Depending on the instruction, the participants monitored one or two of the four visual streams, responding to target stimuli in the relevant stream/s while ignoring all other stimuli. Finally, a memory test display required the participants to decide whether a given item was included in the memory sample (object‐based WM task) or whether the location cued by the white box contained an item in the memory sample (spatial‐based WM task), as instructed at the start of the experiment.
In each of the two sessions, there were four fMRI‐runs that differed according to the number of the to‐be‐remembered items presented in the memory samples (from 2 to 5; WM load).
While holding spatial or object information in WM, participants were presented with sequences of attentional trials. Each trial included presentation of four shapes flashed simultaneously in the left and right hemifields (one red and one green on each side; see Fig. 1 and below for details) and participants were asked to perform one of the four attention tasks. The tasks were generated by crossing factorially the number of attended object–categories (two vs. one: divided object‐based attention) and the number of attended locations (two vs. one: divided space‐based attention). Accordingly, the four attention tasks were:
-
a
Divided both object‐based and space‐based attention: Attend to one object‐category in one hemifield, and the other object‐category in the opposite hemifield (D2); e.g., “attend green shapes on the left side and red shapes on the right side.”
-
b
Divided object‐based attention only: Attend to both object–categories in the same hemifield (F2/L or F2/R, for focused spatial attention to the left or right hemifield); e.g., “attend green shapes and red shapes on the left side.”
-
c
Divided space‐based attention only: Attend to one single object–category, but monitoring both hemifields at the same time (D1); e.g., “attend green shapes both on the left and right side.”
-
d
Focused attention both in object‐based and space‐based domains: Attend to one object–category in one hemifield (F1/L or F1/R); e.g., “attend only green shapes presented on the left side.”
Therefore, the basic design was 2 × 4 × 2 × 2 factorial design, with the independent factors: (I) the type of WM task (object‐based or space‐based; between sessions); (II) the number of to‐be‐remembered items, i.e., the WM load (2, 3, 4, or 5; between fMRI‐runs); (III) the number of attended locations (one or two; blocked); (IV) the number of attended object–categories (one or two; blocked). Our analyses tested whether object‐based or space‐based WM load modulated brain activation associated with object‐based divided attention (i.e., main effect of monitor “two vs. one” categories) or space‐based divided attention (i.e., main effects of attend to “two vs. one” locations, see also Supporting Information for additional tests regarding higher‐order interactions). Accordingly, here we assessed whether increasing the amount of a specific type of information held in WM affects activity in brain regions involved in object‐based or space‐based divided attention.
Stimuli and Procedure
Participants lay in the scanner in a dimly‐lit environment and viewed the back‐projected visual display via a mirror system. Each block of trials began with the presentation for 2,000 ms of an instruction display, which informed the participant about the relevant object–category/s and location/s for the attention task. The instruction display consisted of a text string “instruction,” plus one or two shapes indicating the relevant position and object–category (see Fig 1, top panel). In other words, the display showed the target stimuli (defined by position, shape, and orientation) that participants should detect and respond to. All stimuli in task‐irrelevant streams had to be ignored, including shapes of a currently relevant category and orientation presented in an unattended position (e.g., a green diamond presented in the right hemifield, when participants had to monitor green shapes on the left and red shapes on the right). After the offset of the instruction display, a memory sample was presented for 2,500 ms (WM encoding). The memory sample included from two to five items depending on the current level of load (L2‐5). Items of different shape were presented in semi‐randomized positions selected from 28 possible locations on the screen, with the constraint of having the same number of items in each hemifield. In the L3 and L5 conditions, one item was presented along the middle vertical axis. The items were randomly chosen from a pool of eight different shapes each filled with a different color (see Fig. 1, inset on the right). In the WM encoding phase, subjects were allowed to freely move their eyes.
After the offset of the memory sample display, a consecutive series of attention trials was presented [see also Fagioli and Macaluso, 2009, who used the same attention tasks]. Briefly, on each attention trial, four shapes were presented simultaneously on the screen: two on the left and two on the right of the central fixation point. There was always a red and a green shape on each side, one above the other (see Fig. 1). Each shape could be presented in one of two orientations: i.e., a red “+” or a red “×”; and a green square or green diamond. On each trial, the orientation of the four shapes changed independently and unpredictably. According to instructions, the participant monitored one or two of these streams (see “Paradigm” above) and responded with a right‐hand key‐press whenever a target shape was presented in a task‐relevant stream (e.g., a green diamond on the left; see condition: “one location/one category” in Fig. 1, top panel). The series of attention trials comprised from 9 to 11 trials, in order to make the participants uncertain as regard the start of the memory test. The frequency of target shapes in attended stream/s was adjusted so that there were five targets when the sequence included 10 trials; 4 or 5 targets when the sequence included 9 trials; and 5 or 6 targets when the sequence included 11 trials. In each trial, the attentional display was presented for 200 ms, followed by a fix interstimulus interval (ITI) of 1,800 ms. During the entire block of attention trials, participants were asked to maintain central gaze, while covertly monitoring the peripheral stream/s.
At the end of the sequence of attention trials, a memory test display was presented for 2,000 ms. In the object‐based WM task, an item was presented in the centre of the display and the participant was asked to press either a key with the index or middle finger of the right hand to indicate whether the item was included or not in the memory sample. In the space‐based WM task, a white box was presented at one location and the participant was asked to indicate whether the memory sample contained an item at that location. Irrespective of the WM task, in half of trials the target item was the same, or presented in the same location, as the memory sample, while in the remain half of trails it was not. For each WM session/task (object‐ or space‐based) all participants underwent four fMRI scanning runs (lasting ∼9.5 min each), one for each level of WM load. Every fMRI run comprised 24 attention‐blocks, repeating each attention task four times (F1/L, F1/R, F2/L, F2/R, D1, D2). Over the entire experimental session, each participant was presented with 96 WM trials (24 repetitions for each WM load condition) and 960 attention trials (160 repetitions for each attention condition).
Together with the four dual‐task fMRI runs, each session included also a WM‐only localizer run to identify areas involved in the WM tasks, but now without any concurrent attention task. In this run the participant performed only either the object‐ or the space‐based WM task (according to the current WM session). The sequence and the timing of stimuli were the same as in the dual‐task runs, with the exception that the instruction display for the attention task was not presented, i.e., each block of trials started with the memory sample display. During the presentation of 9–11 “attention trials,” the participants were simply instructed to wait for the memory test, while holding the items in WM and maintaining central fixation. This run lasted for ∼10 min and included 28‐WM trials, equally divided among the four different levels of WM load (here with load randomized between trials). Before the beginning of the fMRI session (outside the scanner room), the participants underwent a WM‐only block (28 WM trials) and a dual‐task block (24 WM trials, with the corresponding 240 attention trials) to familiarize with the tasks.
Gaze‐Position Recording
The gaze‐position was recorded during fMRI using an ASL eye‐tracking system, adapted for use in the scanner (Applied Science Laboratories, Bedford, MA; Model 504, sampling rate 60 Hz). Eye‐position traces were examined for each attention trial in a temporal window of 400 ms, starting 200 ms before the onset of the visual display containing the four attention streams and lasting for all its duration (200 ms). Failures to maintain fixation were identified as changes in horizontal eye‐position greater than ±2° of visual angle and modelled as a separate event‐type in the fMRI analyses. Overall, the participants made few eye‐movements both in the object‐based (3%) and in space‐based (5%) sessions.
Magnetic Resonance Imaging
A Siemens Allegra (Siemens Medical Systems, Erlangen, Germany) operating at 3T and equipped for echo‐planar imaging (EPI) acquired functional magnetic resonance (MR) images. A quadrature volume head coil was used for radio frequency transmission and reception. Head movement was minimized by mild restraint and cushioning. Thirty‐two slices of functional MR images were acquired using blood oxygenation level‐dependent imaging (3 × 3 mm2, 2.5‐mm thick, 50% distance factor, repetition time = 2.08 s, time echo = 30 ms), covering the entirety of the cortex.
fMRI Data Analysis
We used SPM5 (Wellcome Department of Cognitive Neurology) implemented in MATLAB 7.1 (The MathWorks, Natick, MA) for data preprocessing and statistical analyses. For all participants, we acquired 1,402 fMRI volumes in each fMRI session (290 in the WM‐only localizer run, and 1,112 in the four dual‐task runs). After having discarded the first 4 vol. of each run, all images were corrected for head movements. Slice‐acquisition delays were corrected using the middle slice as reference. All images were normalized to the standard SPM5 EPI template, resampled to 2 mm isotropic voxel size, and spatially smoothed using an isotropic Gaussian kernel of 8 mm FWHM. Time series at each voxel for each participant were high‐pass filtered at 220 s and pre‐whitened by means of autoregressive model AR(1).
Our fMRI analyses aimed testing for: (1) encoding, maintenance, and retrieval‐related activations1, and any load modulation thereof, in the WM‐only localizer runs (object‐ and space‐based WM); (2) the effect of increasing object‐based WM load on the activity associated with monitoring two vs. one category (object‐based divided attention task), and on the activity associated with attending to two vs. one location (space‐based divided attention task); (3) the effect of increasing space‐based WM load on activity associated with the object‐based and with the space‐based divided attention tasks. In the Supporting Information we also report additional analyses regarding the effect of WM load on areas showing an interaction between monitoring two vs. one category and attending to two vs. one location.
For all analyses, statistical inference was based on a random effects approach [Penny and Holmes, 2004], which comprised two steps: first‐level analyses estimating contrasts of interest for each subject, followed by second‐level analyses for statistical inference at the group‐level [with non‐sphericity correction; Friston et al., 2002]. For each subject we run four separate first‐level analyses. Two models concerned the WM‐localizer runs: one for object‐based WM and one for space‐based WM. The other two models concerned the dual‐task runs: one model to estimate attention‐related effects under different levels of object‐based WM, and one for attentional effects under different levels of space‐based WM.
The first‐level multiple regression models for WM‐only localizer runs included separate predictors for the 3 WM phases (encoding, maintenance and retrieval) and the four levels of WM load (2, 3, 4, or 5 to‐be‐remembered objects or locations). The predictor for the encoding phase was time‐locked to the presentation of the memory sample; the predictor for retrieval phase was time‐locked to the presentation of the memory test (in both cases, delta functions convolved with the SPM5 hemodynamic response function, HRF). The maintenance phase was modeled as a variable duration block between the memory sample and the memory test, convolved with the HRF. The parameters of head movements were also included in the multiple regression models as covariates of no interest. Linear contrasts were used to determine responses for the 12 effects of interest (three phases × four loads). These underwent the second‐level analyses, comprising three separate analyses of variance: one for each WM phase (encoding, maintenance and retrieval). Each group‐level ANOVA included eight conditions modeling the four levels of load, for the object‐based and space‐based WM tasks in the same analysis.
The first‐level models of the dual‐task fMRI runs included delta functions time‐locked to the onsets of each attention trial divided according to the four main attention conditions (F1, F2,—collapsing L/R conditions—and D1, D2) and the four levels of WM load (L2‐5), convolved with the HRF. Two separate models assessed the effects of divided attention with concurrent object‐based or space‐based WM tasks, which were acquired in the same subjects but on different days. The onsets of instruction‐displays, memory samples and memory tests were also included in the multiple regression models as covariates of no interest, along with the parameters of head movements. Linear contrasts were used to determine the difference scores associated with object‐based divided attention (F2 + D2) − (F1 + D1), and space‐based divided attention (D1 + D2) − (F1 + F2), separately for each level of WM load. Accordingly, for each regression model (object‐ and space‐based WM) this resulted in 8 contrast images corresponding to the two effects of divided attention (object‐ and space‐based) measured under the four levels of the WM tasks (L2‐5). Two separate ANOVAs were then used to assess the effect of WM load on the attention‐related activations. One model included all contrast images concerning the difference scores associated with object‐based divided attention: i.e., four images for (F2 + D2) − (F1 + D1) at the four levels of object‐based WM task, plus four images of the same attention contrast at the four levels of space‐based WM task. The second model concerned the difference scores associated with space‐based divided attention, thus including four contrast images for (D1 + D2) − (F1 + F2) at the four levels of object‐based WM task, plus the corresponding four contrasts for four levels of the space‐based WM task.
The critical comparisons of interest concerned the effect of increasing object‐based or space‐based WM load on activity associated with the monitoring two vs. one category or attending to two vs. one location. First, we highlighted linear changes of activity across WM load conditions (L2‐5) either in the object‐based, in the space‐based, or in both of the WM tasks (i.e., there was no averaging across the two WM tasks) during the maintenance phase of the WM‐only localizer (F‐contrast, p‐FDR‐corrected = 0.05 at voxel level, considering the whole brain as the volume of interest). As an additional constraint, we considered only voxels showing an overall activation across object/space tasks and loads (T‐contrast, p‐unc. = 0.05), ensuring that we selected only regions activated during the maintenance phase. This procedure identified two regions: the left and right intraparietal sulcus (IPS, see Fig. 3A and Table I) that also overlapped with the fronto‐parietal network activated by the divided attention tasks [cf. Fagioli and Macaluso, 2009; see also Fig. 4, central panel]. For these regions we constructed spherical ROIs centred on the two maxima of the F‐map (ROI diameter = 8 mm, matching the FWHM of the smoothing filter). MarsBar 0.41 (“MARSeille Boîte À Région d'Intérêt” SPM toolbox) was used to average activity within each ROI and to test for specific interactions between attention and WM in the dual‐task experiment. Within each ROI we tested for the effect of increasing object‐based WM load on the activity associated with object‐based and space‐based divided attention, and for the effect of increasing space‐based WM load on activity associated with the same two divided attention effects.
Figure 3.
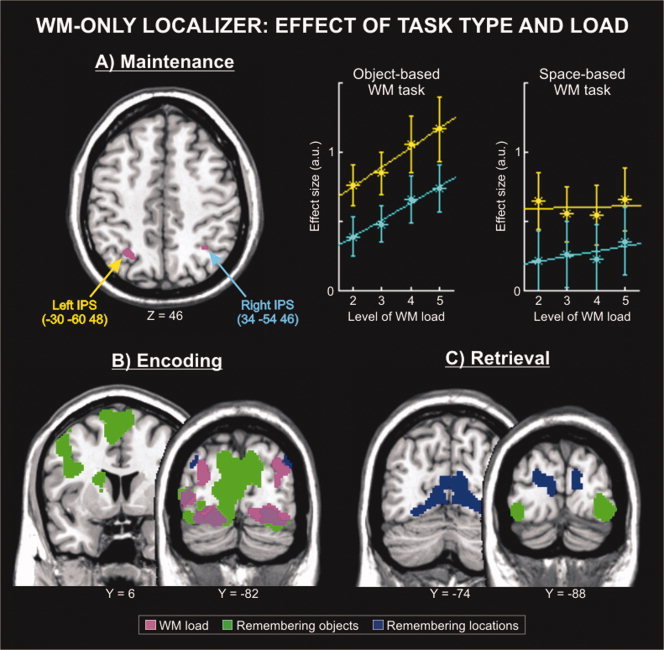
Brain areas activated by object‐ and space‐based tasks during the WM‐only localizer. (A) Maintenance phase: Transversal sections highlighting regions that showed a linear change of activation with changing WM load (F‐contrast). The corresponding signal plots report the estimated activity for the left and right intraparietal sulcus (IPS), both showing a linear increase of activation with increasing load during the object‐based WM task. The level of activity is expressed in arbitrary units (a.u., ±90% confidence interval). (B) Encoding phase: Coronal sections showing regions activated by remembering objects (vs. location, in green) or remembering locations (vs. objects, in blue), averaging across load conditions. (C) Retrieval phase: Coronal sections showing regions activated by remembering objects (vs. location, in green) or remembering locations (vs. objects, in blue), averaging across load conditions. All activation maps are displayed at a threshold of p‐FDR‐corr. = 0.05.
Table I.
WM‐only localiser: coordinates, Z‐values, and P‐FDR‐corrected for the main effects of remembering objects vs. locations, locations vs. objects, and for the modulatory effect of WM load (F‐contrast testing for linear changes during object‐based or space‐based WM tasks)
| WM‐load | Objects > locations | Locations > objects | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P‐corr | Z‐value | x y z | P‐corr | Z‐value | x y z | P‐corr | Z‐value | x y z | |
| Maintenance | |||||||||
| Left IPS | 0.027 | 3.57 | −30 −60 48 | ||||||
| Right IPS | 0.033 | 3.37 | 34 −54 46 | ||||||
| Right preG | 0.029 | 3.50 | 60 2 34 | ||||||
| Left PT | 0.038 | 3.22 | −66 −14 10 | ||||||
| Encoding | |||||||||
| Left IOG | <0.001 | 5.96 | −20 −84 −14 | ||||||
| Right IOG | <0.001 | 6.19 | 28 −80 −12 | ||||||
| Left MOG | <0.001 | 5.91 | −28 −88 18 | <0.001 | 4.98 | −46 −82 4 | |||
| Right MOG | <0.001 | 4.85 | 38 −84 26 | <0.001 | 4.70 | 34 −90 0 | |||
| Left cun | <0.001 | 5.53 | −12 −76 8 | ||||||
| Right cun | 0.001 | 4.47 | 14 −76 26 | ||||||
| Left linG | 0.001 | 4.57 | −10 −70 −4 | ||||||
| Right linG | 0.001 | 4.44 | 14 −72 −4 | ||||||
| Left cinG | 0.005 | 3.60 | −6 24 38 | ||||||
| Right cinG | 0.008 | 3.42 | 6 20 40 | ||||||
| Left MFG | <0.001 | 4.65 | −48 6 44 | ||||||
| Left angG | 0.197 | 3.82 | −36 −82 32 | ||||||
| Right angG | 0.179 | 4.16 | 42 −78 34 | ||||||
| Retrieval | |||||||||
| Left IOG | <0.001 | 6.87 | −28 −96 −8 | ||||||
| Right IOG | <0.001 | 6.67 | 32 −94 −8 | ||||||
| Left MOG | <0.001 | 4.69 | −16 −88 18 | ||||||
| Right MOG | 0.004 | 4.02 | 12 −90 22 | ||||||
| Left linG | <0.001 | 5.23 | −10 −78 −6 | ||||||
| Right linG | <0.001 | 6.00 | 22 −74 −6 | ||||||
Note: IPS: intraparietal sulsus; preG: precentral gyrus; PT: planum temporale; IOG: inferior occipital gyrus; MOG: middle occipital gyrus; cun: cuneus; linG: lingual gyrus; cinG: cingulated gyrus; MFG: middle frontal gyrus; angG: angular gyrus.
All effects were tested separately in the three WM phases.
Figure 4.
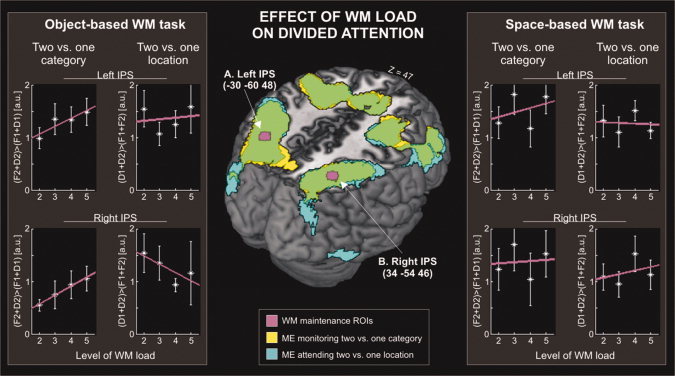
Modulation of divided attention by WM load. Central panel: Transversal sections through a 3D rendering of the canonical MNI template showing the activation associated with the two main effects of dividing attention: monitoring two vs. one object categories (in yellow), and attending to two vs. one locations (in cyan). The 3D renderings also show the localization of two ROIs selected on the basis of the WM‐only localizer (i.e., areas showing an effect of WM‐load, see Fig. 3). Left and right panels: For each ROI, the signal plots show the activation associated with dividing object‐based attention (monitoring two vs. one object–categories) and dividing space‐based attention (attending to two vs. one location) as a function of WM load (L2‐5). Panels on the left shows the effect of changing object‐based WM load; panels on the right show the effect of changing space‐based WM load. Signal plots on the left side show that activity in left and right IPS linearly increased as a function of the increased object‐based WM load, selectively when participants monitored two vs. one stimulus–categories (i.e., the object‐based divided attention task). In all plots, the level of activity is expressed in arbitrary units (a.u., ±90% confidence interval).
RESULTS
Behavioral Data
The behavioral results are summarized in Figure 2. We conducted analyses on error rates and reaction times (RT) for the WM and attention tasks. Trials in which participants responded erroneously to the WM memory (22%) or to the divided attention task (12%) were excluded from the analysis of the RT data.
Figure 2.
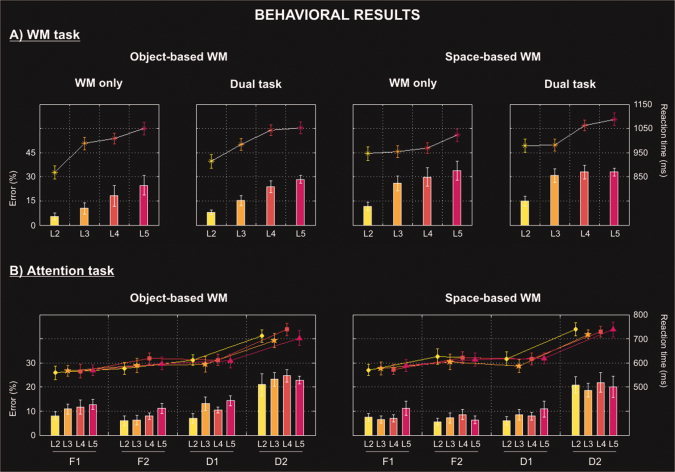
Behavioral data (errors and reaction times, RTs) for (A) the two WM‐tasks (object‐/space‐based); and (B) the four main selective attention tasks (F1, D1, F2, D2). The error bars represent the standard error of the means.
First, a three‐way within‐participants ANOVA with the factors of “WM task” (object‐ or space‐based), “WM load” (L2‐5), and “number of tasks” (WM‐only or dual‐task) was performed on the WM data (see Fig. 2A). The analysis of the RTs revealed a main effect of WM load [F(3, 30) = 8.8, P < 0.001], which was confirmed by the error rates analysis [F(3, 30) = 27.9, P < 0.001]. Error rates also differed between WM tasks [F(1, 10) = 9.6, P = 0.011], with less errors in the object‐based (17%) than in the space‐based WM task (27%). No other main effect or interaction was found either in the RTs (all Fs < 2.0; all Ps > 0.189) or error rate data (all Fs < 3.1; all Ps > 0.111).
Additionally, we specifically tested whether the WM behavioral data changed linearly with load (analogous to our fMRI analyses; see below). For the object‐based WM task we found a linear decrease of behavioral performance with increasing WM load, both in the WM‐only task (accuracy: P = 0.030; RTs: P = 0.015) and in the dual‐task condition (accuracy: P < 0.001; RTs: P = 0.005). Similar effects were found also in the space‐based WM task. Performance decreased with increasing load both in the WM‐only task (accuracy: P = 0.009, RTs: P = 0.091) and in the dual‐task condition (accuracy: P < 0.001; RTs: P = 0.015).
Next, we assessed the behavioral performance associated with the attention tasks. A four‐way within‐participants ANOVA included the following factors: “WM task” (object‐ or space‐based), “WM load” (L2‐5), “number of monitored categories” (one or two), and “number of attended locations” (one or two; see Fig. 2B). Both RTs and error rates revealed significant main effects of the number of attended locations (RTs: [F(1, 10) = 168.2, P < 0.001], and errors: [F(1, 10) = 44.0, P < 0.001]), a main effect of the number of monitored categories (RTs: [F(1, 10) = 218.2, P < 0.001], and errors: [F(1, 10) = 16.9, P = 0.002]), and a significant interaction between these two factors (RTs: [F(1, 10) = 36.2, P < 0.001], and errors: [F(1, 10) = 59.1, P < 0.001]). Thus, the discrimination performance in the attention task was more difficult when participants had to monitor two categories at two different locations (D2: 722 ms and 22%) compared with the other attention conditions (F1: 571 ms and 9%; F2: 606 ms and 7%; and D1: 608 ms and 10%).
The analyses of the error rates revealed a main effect of WM load [F(3, 30) = 3.7, P = 0.022], with participants making fewer errors in the attention task when they were asked to maintain in WM two (6%) as compared to four (8%; P = 0.018) or five (9%; P = 0.034) objects or locations. The type of WM task also affected the overall accuracy in the attention task, with more discrimination errors when participants had to maintain object‐based (13%) vs. space‐based (11%) information in WM (main effect of WM task: F(1, 10) = 8.2, P = 0.017]). These analyses did not reveal any other significant main effect or interaction (RT: all Fs <1.8; all Ps > 0.177; and error: all Fs <3.1; all Ps > 0.111).
Overall, our behavioral analyses did not reveal any significant interaction between WM load (L2‐5; object‐ or space‐based) and divided‐attention performance (F1, F2, D1, D2): i.e., the difference scores comparing object‐ or space‐based “divided minus focused attention” were similar across WM load conditions. This was true both for the RTs and the error rates. However, it must be noted that there was a significant main effect of WM load irrespective of attention conditions (see Fig. 2B), indicating that WM load affected focused and divided attention to a similar degree. This suggests either that WM and divided attention utilize independent processing resources or that additional mechanisms were recruited to attain comparable levels of attentional performance irrespective of WM load. This was addressed with the following fMRI analyses.
fMRI Data
WM‐only localizer
For each WM phase (encoding, maintenance and retrieval) we tested for the main effects of object‐based and space‐based WM tasks. Moreover, we used F‐contrasts to test for linear changes of activity across load conditions (L2‐5) either in object‐based or in space‐based WM task. Figure 3 shows the anatomical location of the areas activated for these comparisons, separately in the three WM phases (see also Table I).
Maintenance phase
Our analyses of the WM‐only localizer focused on the maintenance phase, as this was the trial phase cooccurring with the attention tasks in the main experiment (i.e., the dual‐task). During the maintenance phase, we did not find any significant main effect of object‐ or space‐based WM tasks. However, several brain regions showed a significant effect of WM load (see Fig. 3A and Table I). These included the left and right IPS, consistently with previous studies [e.g., Jha and McCarthy, 2000; Leung et al., 2004; Linden et al., 2003; Todd and Marois, 2004; Xu and Chun, 2006; see also Magen et al., 2009]. The signal plots for these areas showed that activity increased with an increasing amount of information held in WM, and that this effect was more pronounced for the object‐based than for space‐based WM task (see the signal plots in Fig. 3A). The same analysis also highlighted an effect of load in the left planum temporale and the right precentral gyrus. Activation of temporal and precentral areas during WM maintenance areas has been reported before [e.g., Habeck et al., 2005; Piekema et al., 2006; see, for a review, Wager and Smith, 2003], but these regions are not part of the fronto‐parietal network involved in divided attention tasks [Fagioli and Macaluso, 2009; Santangelo et al., 2010; see also Fig. 4]. Accordingly, the analyses of the dual‐task experiment were restricted to the left and right IPS (see below).
Encoding and retrieval phases
For completeness, we tested for the effect of WM task (object‐ and space‐based) and WM load also in the encoding and retrieval phases. Briefly, encoding objects vs. locations activated the medial and lateral occipital cortices, plus frontal regions in the left middle frontal gyrus and the bilateral cingulate gyrus (see Fig. 3B, activations displayed in green, and Table I). The reverse contrast, encoding locations vs. objects, activated the left and right angular gyri (Fig. 3B, in blue). An effect of WM load was found in the occipital cortex (Fig. 3B, in violet), where activity increased with increasing load in both object‐ and space‐based WM tasks; but note that, at encoding, the high load conditions included the presentation of more items than the low load conditions. In the retrieval phase (see Fig. 3C and Table I), we found greater activation for the retrieval of object‐ vs. space‐based information in inferior‐lateral occipital areas (activations displayed in green). By contrast, retrieval of the items' location recruited more medial regions (in blue). In the retrieval phase we did not find any significant effect of load.
Overlap between spatial and nonspatial divided attention, and WM maintenance in the fronto‐parietal network
In the main experiment (dual‐task), we first highlighted the main effects of monitoring two vs. one category (F2 + D2 > F1 + D1) and attending to two vs. one location (D1 + D2 > F1 + F2), averaging across WM load. These two main effects of non‐spatial and spatial divided attention recruited largely overlapping areas in the fronto‐parietal cortex (see Fig. 4, central panels). This FP network included the IPS and extensive activation in the frontal lobe, comprising the superior and middle frontal gyri, and the anterior supplementary motor area; but also ventral regions including the inferior frontal gyri plus the insula (see Table II). Moreover, these areas showed an interaction between object‐ and space‐based divided attention, with maximal activation when subjects monitored two different object–categories in opposite hemifields [see Supporting Information, and cf. Fagioli and Macaluso, 2009].
Table II.
Coordinates, Z‐values, and p‐FDR‐corrected for areas activated by the main effect (ME) of monitoring multiple categories and attending to multiple locations
| ME of monitoring two vs. one category (F2 + D2) > (F1 + D1) | ME of attending to two vs. one location (D1 + D2) > (F1 + F2) | ||||||
|---|---|---|---|---|---|---|---|
| Hem | P‐corr | Z‐value | x y z | P‐corr | Z‐value | x y z | |
| IPS | L | <0.001 | >8 | −32 −54 48 | <0.001 | 7.57 | −28 −56 46 |
| R | <0.001 | 6.55 | 36 ‐54 52 | <0.001 | 7.02 | 36 −52 50 | |
| IOG | L | 0.020 | 4.42 | −48 −64 −12 | =0.001 | 5.60 | −46 −64 12 |
| R | n.s. | 3.32 | 52 −60 −12 | =0.007 | 4.67 | 52 −60 −16 | |
| SFG/FEF | L | <0.001 | 7.75 | −28 2 52 | <0.001 | >8 | −28 2 52 |
| R | <0.001 | 7.61 | 30 14 52 | <0.001 | 7.36 | 28 4 54 | |
| MFG | L | <0.001 | 7.75 | −28 2 52 | <0.001 | >8 | −28 2 52 |
| R | <0.001 | 7.61 | 30 14 52 | <0.001 | 7.03 | 32 10 56 | |
| IFG | L | <0.001 | 7.63 | −50 28 28 | <0.001 | 7.08 | −50 28 28 |
| R | 0.005 | 6.95 | 46 34 22 | <0.001 | 5.76 | 48 32 20 | |
| Insula | L | 0.006 | 4.75 | −30 22 6 | =0.023 | 4.36 | −32 20 4 |
| R | 0.002 | 5.00 | 32 22 2 | =0.002 | 5.00 | 32 24 0 | |
| AntSMA | L | <0.001 | 5.39 | −6 14 50 | =0.001 | 5.05 | −2 16 50 |
| R | 0.001 | 5.23 | 2 18 50 | =0.001 | 5.28 | 6 18 50 | |
Note: IPS: intraparietal sulcus; IOG: inferior occipital gyrus; SFG/FEF: superior frontal girus/frontal eye fields; MFG: middle frontal gyrus; IFG: inferior frontal gyrus; AntSMA: anterior supplementary motor area.
The 3D‐renderings in Figure 4 also highlight the two regions showing an effect of load during the maintenance phase of the WM‐only localizer (left and right IPS; in violet) that overlapped with the fronto‐parietal network activated by the two main effects of divided attention. Accordingly, our data confirm that the same regions can activate for WM and attention [e.g., Kincade et al., 2005; Linden et al., 2003; Munk et al., 2002; Serences and Yantis, 2007), here extending this observation to conditions of object‐ and space‐based divided attention. The following analyses aimed to clarify whether this colocalization involves independent WM‐specific and attention‐specific responses (separate resources), or rather there is a common underlying neural substrate that contributes both to WM maintenance and to the control of divided attention (shared resources).
Dual‐task conditions: The effect of increasing WM load on attention‐related activations
The main analysis of this experiment investigated whether increasing the load of a WM task (object‐ or space‐based) affects attention‐related activations associated with dividing attention between multiple categories or between multiple locations. We considered two ROIs that showed an effect of load during the maintenance phase of the WM‐only localizer and that were located within the fronto‐parietal network engaged by the divided attention tasks (i.e., the left and right IPS, see above and Fig. 4).
First, we investigated the effect of increasing load of the object‐based WM task on the activity associated with the monitoring two vs. one category (i.e., object‐based divided attention, irrespective of the number of attended locations). This revealed a positive modulation both in the left and the right IPS (linear increase: t = 2.10; P = 0.019 and t = 2.48; P = 0.008, respectively; with both modulations passing Bonferroni correction for multiple comparisons: α‐value = 0.025). Figure 4 (left‐most plots) shows the effect of object‐based WM load on the activity associated with the monitoring of multiple categories: In both ROIs attention‐related activation increased linearly with increasing object‐based WM load.
This modulatory effect of the object‐based WM was selective for the object‐based divided attention task. Increasing object‐based WM load did not significantly affect the activation associated with the space‐based divided attention task (i.e., the difference score related to attending to two vs. one location; linear increase: t = 0.32; P = 0.374; and t = −1.35; P = 0.909, for the left and right IPS, respectively). If any, space‐based attentional effects in the right IPS tended to decrease with increasing object‐based WM load. This inverse relationship can be seen in Figure 4 (left second‐most plot on the bottom), where the main effect of attending to multiple locations is plotted separately for the four levels of load of the object‐based WM task.
Next, we turned to the space‐based WM task testing whether also changes of load in this task affected attention‐related activation in the IPS. None of the linear contrasts revealed a significant effect of space‐based WM load on the attention‐related activations. T‐values ranged between ‐1.01 and ‐0.17 (all P‐values > 0.566) for the object‐based divided attention conditions (monitoring two vs. one category); and between ‐0.11 and 0.57 (all P‐values >0.283) for the space‐based divided attention conditions (attending to two vs. one location; see Fig. 4, plots on the right).
DISCUSSION
The present study assessed the contribution of WM to the control of divided attention and examined the interplay between these two systems, specifically in relation to the type of information involved (object‐based vs. spatial‐based). Using a dual‐task we manipulated both WM maintenance and divided attention, indexed by the difference between “divided attention conditions” and “focused attention conditions”. We found increased activity in the IPS with increasing WM load, specifically for conditions involved with monitoring multiple object–categories while holding several objects in WM. This suggests that WM and divided attention may utilize a common, limited‐capacity pool of processing resources in the IPS, which mediates the storage of target/object‐related information for on‐line processing and short‐term memory maintenance.
WM Maintenance of Object‐Based and Space‐Based Information
In the WM‐only localizer task, we separated transient activation at the encoding and retrieval phases of the trial, versus sustained activation during the retention (or maintenance) interval. During the maintenance phase, we found activation in the left and right IPS, irrespective of whether participants were asked to maintain object‐ or space‐based information. Traditionally, the posterior parietal cortex (together with the prefrontal cortex) is thought to play a key role in WM tasks [e.g., Naghavi and Nyberg, 2005], whereas the prefrontal cortex is involved in control and manipulation processes of WM. For example, activity in the prefrontal cortex has been shown to vary according to retrieval demands of WM tasks [e.g., Champod and Petrides, 2007; Owen et al., 1996; Petrides, 2000]. By contrast, posterior parietal cortex is associated with successful storing and maintenance of information [see Zimmer, 2008, for a review]. The current finding of IPS activation irrespective of the type of information (object‐ and space‐based) is in line with previous studies, showing increased activation in the parietal cortex during retention of both spatial and nonspatial information [e.g., Belger et al., 1998; Coull and Frith, 1998; Majerus et al., 2007; see also Leung et al., 2004; Linden et al., 2003; Munk et al., 2002; Todd and Marois, 2004; Xu, 2007; Xu and Chun, 2006, specifically testing for maintenance‐related activations].
Although the posterior parietal cortex was activated during maintenance of both object‐ and space‐based information, the effect of WM load modulated the IPS activity differentially with the type of material held in WM. The bilateral IPS was sensitive only to object‐based WM load (see the signal plot in Fig. 3), but not with space‐based WM. This finding was surprising, because our behavioral data showed that both object‐ and space‐based WM load affected performance (WM‐only and dual‐task conditions, cf. Fig. 1A; though note that performance leveled off at around L3 in the space‐based WM task, see also discussion below). Several previous imaging studies reported modulation of IPS activity irrespective of spatial vs. nonspatial material [e.g., Magen et al., 2009; Todd and Marois, 2004; Xu and Chun, 2006; see also Belger et al., 1998; Coull and Frith, 1998; Majerus et al., 2007, who did not separate the different trial phases]. One interpretation of material‐independent effects of load in IPS is that participants shift attention between the different objects held in memory during maintenance. Within this framework, IPS activation during non‐spatial WM task would reflect implicit spatial components of the task, i.e., greater shift demands when subjects had to maintain many objects in memory [see, e.g., Magen et al., 2009; Pollmann and von Cramon, 2000; see also Harrison et al., 2010].
More recently, several studies isolated the effect of increasing object‐based load, while holding the number of task‐relevant locations constant [e.g., Harrison et al., 2010; Xu, 2007; Xu and Chun, 2006]. Using specific sequences of to‐be‐remembered stimuli (changing locations vs. changing colors), Harrison et al. [ 2010] emphasized the task‐relevance of either stimulus location or stimulus identity. Their fMRI analyses indicated that the activity in IPS was related to the number of locations successfully held in WM, but not with objects/colors. However, their experimental protocol did not separate brain activity associated with the different phases of a WM trial (encoding, maintenance, and retrieval) and, therefore, it remains inconclusive of whether the primacy of “location information” is maintenance‐specific. In fact, spatial shifting at encoding, when the stimuli are physically present in the visual display would be also consistent with these results. With a different approach, Xu and Chun [ 2006] manipulated the complexity of the objects to‐be‐held in memory and revealed that the superior IPS represents the number of object's features held in WM. Control experiments further confirmed that the IPS maintains object‐based signals irrespective of the number of locations. Information regarding the number of locations was represented in the inferior IPS instead. Thus, in contrast with traditional views about the segregation of “what” and “where” pathways in dorsal and ventral processing streams [Ungerleider and Mishkin, 1982], Xu and Chun's results indicate that parietal cortex contributes also to object processing. The later proposal by Xu and Chun [ 2009] that the superior IPS contains visual representations of objects [i.e., “object‐files”; see Kahneman et al., 1992] for objects' identification and visual short‐term memory is also consistent with our current findings of object‐selective load effects in IPS [at coordinates corresponding to the superior IPS reported by Xu and Chun, 2006].
Colocalization of WM and Divided Attention in Fronto‐Parietal Cortex
In our main experiment (dual‐task procedure), we tested for the overall effect of monitoring two vs. one object‐category (effect of object‐based divided attention) and the effect of attending to two vs. one location/hemifield (space‐based divided attention), irrespective of WM load. This revealed that both attention tasks activated an extensive network including the dorsal fronto‐parietal network, plus more ventral premotor/prefrontal regions (see Fig. 4, central panel). Within these regions we found an interaction between object‐ and spatial‐based divided attention, with maximal activation when subjects monitored two different object–categories in opposite hemifields (see Supporting Information). These results replicate Fagioli and Macaluso [ 2009; see also Santangelo et al., 2010, for a multisensory version of the same task], indicating interactions between object‐ and space‐based divided attention at the neuronal level, rather than mere interdigitated populations of neurons specialized for one or the other task [cf. Beauchamp, 2005, for related arguments about the interpretation of interaction effects in fMRI].
We show that there was an overlap between the divided attention network and regions activated during the maintenance phase of the WM task in the parietal cortex (i.e., the left and right IPS; see Fig. 4, central panel). These findings highlighted a common substrate for WM and attention [LaBar et al., 1999; Silk et al., 2010; see also Naghavi and Nyberg, 2005, for a meta‐analysis], showing for the first time that WM and divided attention share common areas in the parietal cortex. Overlapping activations between WM and attention is often interpreted as entailing the shifting of spatial attention between memory of locations during WM maintenance [Magen et al., 2009; Majerus et al., 2007; Pollmann and von Cramon, 2000; see also Lepsien and Nobre, 2006, for related studies on shifting attention between “internal stimulus representations”]. The current findings thus suggest that the overlap might not be solely due to spatial shifting processes (see also discussion on attention–WM interplay, below), given that the object‐based task did not require any spatial shift (participants had to focus on a single location) and that the spatial shifting demand was also minimal in the spatial‐based task (the stimuli were presented for 200 ms only, an arguably insufficient time to identify the stimulus—target/nontarget—on one side, disengage attention from that side, shift to the opposite hemifield, reengage attention there and, finally, identify/judge the second task‐relevant stimulus). Accordingly, our data suggest that the overlap (and interaction, see below) between WM and attention is not merely related to attention shifting during maintenance of information in WM [cf. Harrison et al., 2010; Pollmann and von Cramon, 2000].
Functional Interplay Between WM and Divided Attention
As noted above, the mere colocalization of activation for WM maintenance and divided attentional control may be insufficient to infer the existence of a common pool of cognitive resources [e.g., interdigitated populations of “WM neurons” and “attention neurons”; see Beauchamp, 2005]. Here we tackled this issue by introducing a dual‐task procedure that required subjects to perform object‐ or space‐based divided attention tasks, while—at the same time—maintaining a variable number of objects or locations in WM. With this procedure we assessed whether changes of WM requirements (high vs. low WM load) affect activity associated with the divided attention tasks.
Behaviorally, we found an impact of WM load and WM task (object‐ vs. space‐based) on attentional performance, but no specific interaction between load and attention conditions (i.e., F1, F2, D1, D2), indicating that maintaining objects or locations information in WM affected focused and divided attention to a similar degree. One possible explanation for this finding is that WM and divided attention utilize independent processing resources. Alternatively, additional resources may become available to perform the divided attention tasks, when WM is engaged in a high load primary task.
Our fMRI results are in line with the second hypothesis. We found that in the left and right IPS the activity associated with monitoring two vs. one object–category increased linearly with increasing object‐based WM load. This demonstrates that IPS does not simply contain independent pools of neural resources for the WM and divided attention. Rather, the specific interaction between the two tasks indicates that both cognitive functions engage a set of common resources. We suggest that the increased activity in IPS when subjects monitored two object–categories while maintaining several objects in WM reflects the augmented processing demands on this common system.
By contrast, WM load did not affect activity associated with dividing attention between locations (see signal plots in Fig. 4), which is a limitation of the present study. One might argue that the absence of any interplay between space‐based divided attention and space‐ or object‐based WM load could relate to the possibility that there are independent resources for attentional monitoring available for the left and right hemifields [cf. Alvarez and Cavanagh, 2005, who showed that monitoring two objects was not more difficult than monitoring one object, as long as the two objects were presented in opposite hemifields]. Consequently, space‐based divided attention might be less affected by any types of WM load as compared to object‐based divided attention. To further investigate this hypothesis, we performed a three‐way ANOVA on the data derived from the dual‐task, now using only one‐category attentional conditions (i.e., F1 or D1). Specifically, the factors included in this analysis were: “WM task” (object‐ or space‐based), “WM load” (L2‐5), and “number of attended locations” (i.e., F1 or D1 attentional conditions). On the error data, this analysis revealed a main effect of WM load [F(1, 10) = 6.3, P = 0.030] and a main effect of WM task [F(1, 10) = 7.3, P = 0.022], in line with our main analysis. However, this analysis did not reveal neither a main effect of the number of attended locations (i.e., F1 vs. D1) nor any interaction of this factor with the others (all Fs <1; all Ps > 0.540), thus replicating Alvarez and Cavanagh's [ 2005] main findings. However, while Alvarez and Cavanagh reported only the accuracy data, here we collected and analyzed also RT data. Actually, the same analysis performed on the RT data revealed a main effect of the number of attended locations [F(1, 10) = 24.1, P < 0.001], indicating larger processing time needed to attend to two locations (i.e., D1; M = 608 ms) than one location (i.e., F1; 571 ms), even when a single object‐category was task‐relevant. No other significant effects were found in this additional analysis: all Fs <1.6; all Ps > 0.216. These results extend Alvarez and Cavanagh's findings indicating that attentional monitoring in opposite hemifields involves a limited pool of resources, when the attention task requires speeded responses. This is in line with traditional models of divided attention that have highlighted a decrement of processing efficacy when monitoring multiple (as compared to single) objects/locations [e.g., Castiello and Umiltà, 1992; Eriksen and St. James, 1986; McMains and Somers, 2004, 2005; Müller et al., 2003a, b].
Although further investigation is needed to clarify to what extent attentional resources are independently available to each hemifield/hemisphere, the current results seem to rule out the possibility that space‐based divided attention is less affected by WM load because there are (entirely) independent processing resources for the two hemifields/hemispheres. An alternative explanation for the absence of any interplay between space‐based divided attention and WM load is that any putative “space‐based specific WM buffer” reached capacity limits in our dual‐task condition already at a relatively low level of WM load, i.e., L3 (see error rates for the space‐based WM task, rightmost panel in Fig. 2A). Although this account necessitates additional evidence, the specificity of the object‐based WM task on the object‐based divided attention task demonstrates that the interplay between the WM and attention does not merely relates to overall dual‐task difficulty. Rather, our results highlight a domain‐specific contribution of WM for the control of divided attention. We suggest that IPS provides specific resources for the maintenance of object‐based target information, rather than some general, all‐purposes short‐term storage system [see also Cusack et al., 2010].
Object‐Based Working Memory and Attention in Posterior Parietal Cortex
The posterior parietal cortex is associated with spatial attention [Corbetta et al., 2000; Kelley et al., 2008; Molenberghs et al., 2007; Vandenberghe et al., 2005; Yantis et al., 2002], as well as with the storage of spatial information in working memory [Leung et al., 2004; Munk et al., 2002; Xu and Chun, 2006; see also Zimmer, 2008, for a review]. Indeed, many authors suggested that the engagement of posterior/superior parietal regions during non‐spatial (i.e., object‐based) WM tasks actually reflects implicit spatial attentional functions associated with the rehearsal of material held in WM [e.g., attention shifting; Magen et al., 2009; Pollmann and von Cramon, 2000; see also Harrison et al., 2010].
Manipulating both attention and WM within the same paradigm, Silk et al. [ 2010] asked their participants to perform a visual search task during the maintenance phase of a spatial WM task. Both WM and visual search loads were modulated parametrically. The imaging results revealed a significant interaction between attention and WM in the IPS. Specifically, there was a reduction of the activation associated with the high load WM task at the highest search/attention loads. This pattern was qualitatively different from that observed in the present study. Here we found maximal IPS activity when the dual‐task required at the same time greater WM and attentional resources (i.e., divided object‐based attention, while maintaining multiple objects in WM; see signal plots in Fig. 4). One substantial difference between Silk et al.'s experiment and our present study concerns the role of spatial attention. The search task in Silk et al. required continuous spatial shifts of gaze/attention, while our attention tasks did not (see also section above). The pattern of behavioral data also substantially differed between these two studies: Silk et al. found that the spatial WM task interfered with the search task; while here we did not find any load‐dependent effect of WM specifically on the divided attention tasks. We suggest that spatial information (and attention shifting) dominated both WM and attention tasks in Silk et al.'s study [see also Cavanagh and Alvarez, 2005, for related interactions between multi‐objects memory and attention, using moving stimuli to involve shifts of spatial attention]; while in the current study object‐based information was the most relevant dimension.
We propose that the activation of IPS relates to the active maintenance of target priorities, including the storage of multiple objects/features, which characterized both high load object‐based WM task and the object‐based divided attention task. Specifically, the object‐based divided attention task required our participants to maintain two objects in WM in order to perform the attention task (e.g., a green‐diamond and a red “x”). By contrast, the space‐based divided attention task required monitoring the same features‐defined object at two different locations (green diamond on both sides). Thus, in the space‐based divided attention task, any feature‐based visual short‐term memory system had to store only two target‐elements [one color and one orientation; cf. also Xu and Chun, 2009]; by contrast during the object‐based divided attention task this system had to maintain four independent target‐elements (two colors and two orientations). Accordingly, the object‐specific effect of WM on object‐based divided attention would fit with the hypothesis that the IPS can store objects' information irrespective of location [Cusack et al., 2010; Xu, 2007; but see also Shafritz et al., 2002]. In our dual‐task settings, this object‐specific storage system would be used concurrently for the retention of memory samples‐identity (WM task) and online perception/identification of the feature‐defined attentional targets [see also Mitchell and Cusack, 2008, who showed IPS load effects in multi‐objects perceptual tasks].
CONCLUSION
Our findings extend previous evidence of an interplay between WM and attention [LaBar et al., 1999; Silk et al., 2010; see also Naghavi and Nyberg, 2005] in the domain of divided attention control. We demonstrated that object/space‐based WM maintenance and object/space‐based divided attention recruited overlapping regions in the left and right IPS. Common activation of these areas does not merely reflect colocalization of independent pools of neural resources recruited by either WM or divided attention. Instead, both processes engaged these areas in an interactive manner. Specifically, the activity associated with monitoring of two vs. one object–categories increased linearly with increasing object‐based WM load; while, in the same areas, activity associated with dividing attention between locations decreased with increasing object‐based WM load. This indicates a domain‐specific contribution of WM for the control of divided attention, with the amount of object‐related information stored in WM modulating the involvement of the IPS during monitoring of multiple object‐categories. We propose that WM and divided attention utilize a common, limited‐capacity pool of processing resources in the IPS, which mediates the storage of target/object‐related information for on‐line processing and short‐term memory maintenance.
Supporting information
Additional Supporting Information may be found in the online version of this article
Supporting Information
Acknowledgements
The authors thank Dr. S.C. Kwok for language revision of the manuscript.
The issue of separating multiple phases occurring within a single trial (here encoding, maintenance and retrieval) has been discussed in several previous papers (e.g., Postle et al., 2000; Ollinger et al., 2001; Ruge et al., 2009; see also Curtis and D'Esposito, 2003). Here we used long and variable maintenance phases (range: 16‐20 sec), so as to reduce the co‐variation/correlation between the model's predictors associated with the three trial phases. Indeed, the correlation between the encoding and the maintenance predictors ranged between −0.070 and 0.172 in the WM‐only experiment; and between −0.196 and −0.157 in the dual‐task experiment. This allowed us to separate brain activity associated with the different trial phases in an efficient manner.
The issue of separating multiple phases occurring within a single trial (here encoding, maintenance and retrieval) has been discussed in several previous papers [e.g., Ollinger et al., 2001; Postle et al., 2000; Ruge et al., 2009; see also Curtis and D'Esposito, 2003]. Here we used long and variable maintenance phases (range: 16–20 s), so as to reduce the covariation/correlation between the model's predictors associated with the three trial phases. Indeed, the correlation between the encoding and the maintenance predictors ranged between ‐0.070 and 0.172 in the WM‐only experiment; and between ‐0.196 and ‐0.157 in the dual‐task experiment. This allowed us to separate brain activity associated with the different trial phases in an efficient manner
REFERENCES
- Alvarez GA, Cavanagh P ( 2005): Independent resources for attentional tracking in the left and right visual hemifields. Psychol Sci 16: 637–643. [DOI] [PubMed] [Google Scholar]
- Arrington CM, Carr TH, Mayer AR, Rao SM ( 2000): Neural mechanisms of visual attention: Object‐based selection of a region in space. J Cogn Neurosci 12: 106–117. [DOI] [PubMed] [Google Scholar]
- Awn E, Vogel EK, Oh S‐H ( 2006): Interactions between attention and working memory. Neuroscience 139: 201–208. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS ( 2005): Statistical criteria in fMRI studies of multisensory integration. Neuroinformatics 3: 93–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belger A, Puce A, Krystal JH, Gore JC, Goldman‐Rakic P, McCarthy G ( 1998): Dissociation of mnemonic and perceptual processes during spatial and nonspatial working memory using fMRI. Hum Brain Mapp 6: 14–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta F, Santangelo V, Raffone A, Olivetti Belardinelli M, Lupianez J ( 2010): Exogenous and endogenous spatial attention effects on visuo‐spatial working memory. Q J Exp Psychol 27: 1–13. [DOI] [PubMed] [Google Scholar]
- Castiello U, Umiltà C ( 1992): Splitting focal attention. J Exp Psychol Hum Percept Perform 18: 837–848. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Alvarez GA ( 2005): Tracking multiple targets with multifocal attention. Trends Cogn Sci 9: 349–354. [DOI] [PubMed] [Google Scholar]
- Champod AS, Petrides M ( 2007): Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proc Natl Acad Sci USA 104: 14837–14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL ( 2000): Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3: 292–297. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Tansy AP, Stanley CM, Astafiev SV, Snyder AZ, Shulman GL ( 2005): A functional MRI study of preparatory signals for spatial location and objects. Neuropsychologia 43: 2041–2056. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD ( 1998): Differential activation of right superior parietal cortex and intraparietal sulcus by spatial and nonspatial attention. Neuroimage 8: 176–187. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV ( 1996): Object and spatial visual working memory activate separate neural systems in human cortex. Cereb Cortex 6: 39–49. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV ( 1998): An area specialized for spatial working memory in human frontal cortex. Science 279: 1347–1351. [DOI] [PubMed] [Google Scholar]
- Cowan N ( 2005): Working Memory Capacity. New York: Psychology Press. [Google Scholar]
- Cowan N ( 2010): The magical mystery four: How is working memory capacity limited, and why? Curr Dir Psychol Sci 19: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M ( 2003): Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7: 415–423. [DOI] [PubMed] [Google Scholar]
- Cusack R, Mitchell DJ, Duncan J ( 2010): Discrete object representation, attention switching, and task difficulty in the parietal lobe. J Cogn Neurosci 22: 32–47. [DOI] [PubMed] [Google Scholar]
- De Fockert JW, Rees G, Frith CD, Lavie N ( 2001): The role of working memory in visual selective attention. Science 291: 1803–1806. [DOI] [PubMed] [Google Scholar]
- Driver J ( 2001): A selective review of selective attention research from the past century. Br J Psychol 92: 53–78. [PubMed] [Google Scholar]
- Eriksen CW, St. James JD ( 1986): Visual attention within and around the field of focal attention: A zoom lens model. Percept Psychophys 40: 225–240. [DOI] [PubMed] [Google Scholar]
- Fagioli S, Macaluso E ( 2009): Attending to multiple visual streams: Interactions between spatial and non‐spatial selection. J Cogn Neurosci 21: 1628–1641. [DOI] [PubMed] [Google Scholar]
- Fink GR, Dolan RJ, Halligan PW, Marshall JC, Frith CD ( 1997): Space‐based and object‐based visual attention: Shared and specific neural domains. Brain 120: 2013–2028. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J ( 2002): Classical and Bayesian inference in neuroimaging: Applications. Neuroimage 16: 484–512. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD ( 1992): Separate visual pathways for perception and action. Trends Neurosci 15: 20–25. [DOI] [PubMed] [Google Scholar]
- Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, Stern Y. ( 2005): An event‐related fMRI study of the neural networks underlying the encoding, maintenance, and retrieval phase in a delayed‐match‐to‐sample task. Cogn Brain Res 23: 207–220. [DOI] [PubMed] [Google Scholar]
- Harrison A, Jolicoeur P, Marois R ( 2010): “What” and “where” in the intraparietal sulcus: An FMRI study of object identity and location in visual short‐term memory. Cereb Cortex 20: 2478–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AP, McCarthy G ( 2000): The influence of memory load upon delay‐interval activity in a working‐memory task: An event‐related functional MRI study. J Cogn Neurosci 12: 90–105. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Treisman A, Gibbs BJ ( 1992): The reviewing of object files: Object‐specific integration of information. Cogn Psychol 24: 175–219. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk M, De Weerd P, Desimone R., Ungerleider L ( 1999): Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22: 751–761. [DOI] [PubMed] [Google Scholar]
- Kelley TA, Serences JT, Giesbrecht B, Yantis S ( 2008): Cortical mechanisms for shifting and holding visuospatial attention. Cereb Cortex 18: 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M ( 2005): An event‐related functional magnetic resonance imaging study of voluntary and stimulus‐driven orienting of attention. J Neurosci 25: 4593–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M ( 1999): Neuroanatomic overlap of working memory and spatial attention networks: A functional MRI comparison within subjects. Neuroimage 10: 695–704. [DOI] [PubMed] [Google Scholar]
- Lavie N ( 2000): Selective attention and cognitive control: Dissociating attentional functions through different types of load In: Monsell S, Driver J, editors. Attention and Performance, Vol. 18 Cambridge, MA: MIT Press; pp 175–194. [Google Scholar]
- Lavie N ( 2005): Distracted and confused?: Selective attention under load. Trends Cogn Sci 9: 75–82. [DOI] [PubMed] [Google Scholar]
- Lavie N, De Fockert J ( 2005): The role of working memory in attentional capture. Psychon Bull Rev 12: 669–674. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E ( 2004): Load theory of selective attention and cognitive control. J Exp Psychol Gen 133: 339–354. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Nobre AC ( 2006): Cognitive control of attention in the human brain: Insights from orienting attention to mental representations. Brain Res 1105: 20–31. [DOI] [PubMed] [Google Scholar]
- Leung HC, Seelig D, Gore JC ( 2004): The effect of memory load on cortical activity in the spatial working memory circuit. Cogn Affect Behav Neurosci 4: 553–563. [DOI] [PubMed] [Google Scholar]
- Linden DE, Bittner RA, Muckli L, Waltz JA, Kriegeskorte N, Goebel R, Singer W, Munk MH. ( 2003): Cortical capacity constraints for visual working memory: Dissociation of fMRI load effects in a fronto‐parietal network. Neuroimage 20: 1518–1530. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R ( 1997): Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol 77: 24–42. [DOI] [PubMed] [Google Scholar]
- Magen H, Emmanouil TA, McMains SA, Kastner S, Treisman A ( 2009): Attentional demands predict short‐term memory load response in posterior parietal cortex. Neuropsychologia 47: 1790–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus S, Bastin C, Poncelet M, Van der Linden M, Salmon E, Collette F, Maquet P. ( 2007): Short‐term memory and the left intraparietal sulcus: Focus of attention? Further evidence from a face short‐term memory paradigm. Neuroimage 35: 353–367. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman‐Rakic P ( 1996): Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb Cortex 6: 600–611. [DOI] [PubMed] [Google Scholar]
- McMains SA, Somers DC ( 2004): Multiple spotlights of attentional selection in human visual cortex. Neuron 42: 677–686. [DOI] [PubMed] [Google Scholar]
- McMains SA, Somers DC ( 2005): Processing efficiency of divided spatial attention mechanisms in human visual cortex. J Neurosci 25: 9444–9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DJ, Cusack R ( 2008): Flexible, capacity‐limited activity of posterior parietal cortex in perceptual as well as visual short‐term memory tasks. Cereb Cortex 18: 1788–1798. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Mesulam MM, Peeters R, Vandenberghe RRC ( 2007): Remapping attentional priorities: Differential contribution of superior parietal lobule and intraparietal sulcus. Cereb Cortex 17: 2703–2712. [DOI] [PubMed] [Google Scholar]
- Müller MM, Malinowski P, Gruber T, Hillyard SA ( 2003): Sustained division of the attentional spotlight. Nature 424: 309–312. [DOI] [PubMed] [Google Scholar]
- Müller NG, Bartelt OA, Donner TH, Villringer A, Brandt SA ( 2003): A physiological correlate of the “zoom lens” of visual attention. J Neurosci 23: 3561–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk MH, Linden DE, Muckli L, Lanfermann H, Zanella FE, Singer W, Goebel R. ( 2002): Distributed cortical systems in visual short‐term memory revealed by event‐related functional magnetic resonance imaging. Cereb Cortex 12: 866–876. [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L ( 2005): Common fronto‐parietal activity in attention, memory, and consciousness: Shared demands on integration? Conscious Cogn 14: 390–425. [DOI] [PubMed] [Google Scholar]
- Nebel K, Wiese H, Stude P, de Greiff A, Diener HC, Keidel M ( 2005): On the neural basis of focused and divided attention. Cogn Brain Res 25: 760–776. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL ( 2001): Separating processes within a trial in event‐related functional MRI. Neuroimage 13: 218–229. [DOI] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M ( 1996): Evidence for a two‐stage model of spatial working memory processing within the lateral frontal cortex: A positron emission tomography study. Cereb Cortex 6: 31–38. [DOI] [PubMed] [Google Scholar]
- Penny WD, Holmes AP ( 2004): Random effects analysis In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan R, Price CJ, Zeki S, Ashburner J, Penny WD. editors. Human Brain Function. New York: Academic Press; pp 843–850. [Google Scholar]
- Petit L, Courtney SM, Ungerleider LG, Haxby JV ( 1998): Sustained activity in the medial wall during working memory delays. J Neurosci 18: 9429–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M ( 2000): Dissociable roles of mid‐dorsolateral prefrontal and anterior inferotemporal cortex in visual working memory. J Neurosci 20: 7496–7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekema C, Kessels RP, Mars RB, Petersson KM, Fernández G ( 2006): The right hippocampus participates in short‐term memory maintenance of object‐location associations. Neuroimage 33374–33382. [DOI] [PubMed] [Google Scholar]
- Pollmann S, von Cramon DY ( 2000): Object working memory and visuospatial processing: Functional neuroanatomy analyzed by event‐related fMRI. Exp Brain Res 133: 12–22. [DOI] [PubMed] [Google Scholar]
- Postle BR, Zarahn E, D'Esposito M ( 2000): Using event‐related fMRI to assess delay‐period activity during performance of spatial and nonspatial working memory tasks. Brain Res Brain Res Protoc 5: 57–66. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M ( 2008): Dynamic adjustments in prefrontal, hippocalmpal, and inferior temporal interactions with increasing visual working memory load. Cereb Cortex 18: 1618–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE ( 2000): The prefrontal cortex: Response selection or maintenance within working memory? Science 288: 1656–1660. [DOI] [PubMed] [Google Scholar]
- Ruge H, Goschke T, Braver TS ( 2009): Separating event‐related BOLD components within trials: The partial‐trial design revisited. Neuroimage 47: 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo V, Fagioli S, Macaluso E ( 2010): The costs of monitoring simultaneously two sensory modalities decrease when dividing attention in space. Neuroimage 49: 2717–2727. [DOI] [PubMed] [Google Scholar]
- Schmidt BK, Vogel EK, Woodman GF, Luck SJ ( 2002): Voluntary and automatic attentional control of visual working memory. Percept Psychophys 64: 754–763. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S ( 2007): Spatially selective representations of voluntary and stimulus‐driven attentional priority in human occipital, parietal, and frontal cortex. Cereb Cortex 17: 284–293. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Gore JC, Marois R ( 2002): The role of the parietal cortex in visual feature binding. Proc Natl Acad Sci USA 99: 10917–10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk TJ, Bellgrove MA, Wrafter P, Mattingley JB, Cunnington R ( 2010): Spatial working memory and spatial attention rely on common neural processes in the intraparietal sulcus. Neuroimage 53: 718–724. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Giesbrecht B, Kok A, Weissman DH, Kenemans JL, Woldorff MG, Mangun GR. ( 2007): fMRI evidence for both generalized and specialized components of attentional control. Brain Res 1177: 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JJ, Marois R ( 2004): Capacity limit of visual short‐term memory in human posterior parietal cortex. Nature 428: 751–754. [DOI] [PubMed] [Google Scholar]
- Tong F ( 2004): Splitting the spotlight of visual attention. Neuron 42: 524–526. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Sinnamon HM, Seamon JG ( 1993): Double dissociation of spatial and object visual memory: Evidence from selective interference in intact human subjects. Neuropsychologia 31: 211–219. [DOI] [PubMed] [Google Scholar]
- Underleider LG, Mishkin M ( 1982): Two cortical visual systems In: Ingle MA, Goodale MI, Masfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; pp 549–586. [Google Scholar]
- Vandenberghe R, Geeraerts S, Molenberghs P, Lafosse C, Vandenbulcke M, Peeters K, Peeters R, Van Hecke P, Orban GA. ( 2005): Attentional responses to unattended stimuli in human parietal cortex. Brain 128: 2843–2857. [DOI] [PubMed] [Google Scholar]
- Ventre‐Dominey J, Bailly A, Lavenne F, Lebars D, Mollion H, Costes N, Dominey PF. ( 2005): Double dissociation in neural correlates of visual working memory: A PET study. Cognit Brain Res 25: 747–759. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE ( 2003): Neuroimaging studies of working memory: A meta‐analysis. Cogn Affect Behav Neurosci 3: 255–274. [DOI] [PubMed] [Google Scholar]
- Xu Y ( 2007): The role of the superior intraparietal sulcus in supporting visual short‐term memory for multifeature objects. J Neurosci 27: 11676–11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Chun MM ( 2006): Dissociable neural mechanisms supporting visual short‐term memory for objects. Nature 440: 91–95. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chun MM ( 2009): Selecting and perceiving multiple visual objects. Trends Cogn Sci 13: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. ( 2002): Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci 5: 995–1002. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre GK, D'Esposito M ( 1999): Temporal isolation of the neural correlates of spatial mnemonic processing with fMRI. Brain Res Cogn Brain Res 7: 255–268. [DOI] [PubMed] [Google Scholar]
- Zimmer HD ( 2008): Visual and spatial working memory: From boxes to networks. Neurosci Biobehav Rev 32: 1373–1395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article
Supporting Information


