Abstract
In this study, we investigated central/supraspinal processes mediating cessation of a muscle fatiguing exercise. Fifteen male subjects performed 39 intermittent, isometric handgrip contractions (13 s on, 5–6 s off) with the dominant right hand while brain activation was assessed by means of functional magnetic resonance imaging (fMRI). An adaptive, partly stochastic protocol was designed such that in approximately 50% of the contraction trials the required force could not be held until the end of the trial (task failure trial). Trials performed in compliance with the force requirements (succeeded trial) were compared with task failure trials concerning neural activity during a small time window before task failure occurred. The data revealed significantly increased activation contralaterally in both the mid/anterior insular cortex and the thalamus during the investigated time window in the case of subsequent task failure. In accordance with other studies investigating sensations that alert the organism to urgent homeostatic imbalance such as air hunger, hunger for food, and pain, we assume that an increased thalamo‐insular activation in the context of a fatigue‐induced handgrip exercise could reflect increased homeostatic disturbance in the exercising muscle and may be of essential importance by mediating task failure to maintain the integrity of the organism. Hum Brain Mapp, 2011. © 2010 Wiley Periodicals, Inc.
Keywords: fMRI, isometric contraction, homeostasis
INTRODUCTION
Exercise‐induced muscle fatigue can be defined as a reversible decline in force‐ or power‐generating capacity of the neuromuscular system [Bigland‐Ritchie et al.,1983; Fitts and Holloszy,1976]. Muscle fatigue arises not only from peripheral processes within the working muscle but also from central mechanisms residing in the sensorimotor pathway of the central nervous system (CNS).
As one factor exerting influence on central fatigue, activity of small‐diameter (groups III and IV) muscle afferents has been considered [Gandevia,2001; Nybo and Secher,2004]. Responding to a range of noxious, mechanical and chemical changes, these afferents have been shown to increase their firing rate when metabolites in the fatigued muscle accumulate or when muscle pain was experimentally evoked [Kniffki et al.,1979; Li and Sinoway,2002; Mense,1977; Paintal,1960; Rotto and Kaufman,1988; Sinoway et al.,1993]. Activity of such fatigue‐sensitive small‐diameter afferents has been supposed to affect motor cortical cells [Gandevia et al.,1996; Martin et al.,2008; Taylor et al.,1996; Taylor et al.,2000]. However, it is debatable whether afferent influence on the motor cortex is exerted directly or via intermediate relay stations, and exact cortical mechanisms underlying central fatigue remain hypothetical.
Using functional magnetic resonance imaging (fMRI), several studies investigated the time course of brain activity during sustained or intermittent submaximal or maximal contractions, and revealed an increased activity of the sensorimotor cortex contralateral to the fatiguing muscles, corresponding to an increase in fatigue [Benwell et al.,2007; Liu et al.,2003; Post et al.,2009; van Duinen et al.,2007]. In a maximal 2‐min handgrip contraction, the number of activated voxels in the sensorimotor cortex gradually increased during the first minute of the contraction but then decreased to a level similar to that at the initial phase [Liu et al.,2002]. An early increase in the size of the activation cluster was thought to be due to additional neuron recruitment to compensate for force loss, whereas a decrease of cluster size at later stages of the contraction was supposed to result from inhibitory influence of group III and IV afferents acting possibly via brain structures such as the cingulate and insular cortices [Liu et al.,2002,2003]. Experimentally induced muscle pain has indeed been proven to increase neural activity within the anterior and mid cingulate cortex and widespread insular regions, along with other regions typically also activated by other forms of pain [Henderson et al.,2006; Kupers et al.,2004]. Notably, deviations of muscle pain processing from processing of surface pain seem to be most pronounced within primary sensory cortex and motor regions [Henderson et al.,2006].
The final moment of cessation during a fatiguing task (task failure) has been supposed to be determined by the CNS to ensure that homeostasis is maintained [Noakes et al.,2005]. This concept of a central governor [St Clair Gibson and Noakes,2004] has gained considerable value in the past few years and faces the originally hypothesized “catastrophe” model, which posits that a fatiguing exercise terminates when physiological and biochemical limits of the body are exceeded leading to a catastrophic failure of intracellular homeostasis [Edwards,1983]. Centrally governed changes in pace and termination of a fatiguing exercise have been assumed to occur as part of a regulated system [Noakes et al.,2005] and locomotor power output has been shown to be causally connected with afferent feedback from exercising muscles [Amann et al.,2009]. As an alternative hypothesis, changes in the concentration of specific brain neurotransmitters, e.g., serotonin [Blomstrand,2001; Davis,1995], dopamine [Bailey et al.,1993; Ziv et al.,1998], and acetylcholine [Conlay et al.,1992] cause fatigue. Hereby, it is not clear if precursors produced in the peripheral tissue and crossing the blood–brain barrier [Blomstrand et al.,1991; Guezennec et al.,1998] or an increased neural activity causes the changes in the concentration.
With increasing perception of discomfort produced by exhausting exercise, the conscious desire to override the control mechanism decreases [Noakes,2004], thus also accounting for determination of exercise cessation [Sgherza et al.,2002]. Neuroimaging investigations of sensations of discomfort alerting the organism to urgent homeostatic imbalance such as air hunger (urge to breath) revealed activations in the anterior cingulate as well as in the anterior insular cortex [Brannan et al.,2001; Evans et al.,2002; Liotti et al.,2001] and in the thalamus [Banzett et al.,2000; Evans et al.,2002]. In line, the same structures were also identified during hunger for food [Tataranni et al.,1999], and both the anterior cingulate cortex and the thalamus were shown to participate in sensations of thirst [Denton et al.,1999]. Taken together, in various homeostatic processes consistent activations were described within the anterior insular as well as within the anterior cingulate cortex and the thalamus, with the former two structures also being involved in integrating afferent input.
On the basis of the assumption that task failure is a process related to sensory feedback signaling homeostatic imbalance, we hypothesized that in the context of muscle fatigue a centrally governed system comprising sensorimotor regions, the anterior insular cortex and the anterior cingulate cortex is involved in the mediation of task failure by exerting influence on motor regions. This contrasts the view of loss of neural efficiency in motor regions as cause for task failure. Thus, an fMRI study was designed to allow for comparison of blood oxygen level–dependent (BOLD) signal during the final seconds before task failure with an equivalently defined time window in control trials without task failure.
METHODS
Subjects
Fifteen right‐handed (self‐report) male subjects [26.4 years (SD 4.5)] with no record of neurological or psychiatric illness volunteered to participate in the study. All subjects were familiar with endurance and/or strength training. Written informed consent was obtained from each participant. All procedures were approved by the Ethics Committee of the canton Zurich and performed according to the guidelines of the Helsinki Declaration.
Motor Task
Subjects performed a muscle fatiguing exercise of the right finger flexors during fMRI data acquisition of the brain. The examination protocol comprised 39 intermittent, submaximal, isometric handgrip contraction trials each lasting 13 s, and separated by a resting period of randomized 5–6 s. Before the experiment, individuals' maximal voluntary contraction (MVC) was assessed. Force requirement for the initial contraction was set at 70% MVC. Increased or reduced force was demanded with a probability of 80% after two succeeding trials in which subjects succeeded or failed, respectively, to maintain the target force. The amount of increase, or reduction, respectively, of target force, was 20%. Purpose of this adaptive, partly stochastic protocol was to induce task failure trials (see below) in approximately 50% of the contractions evenly distributed over all trials.
To minimize head movements throughout the scanner session, subjects' head was fixated with an adjustable vacuum cushion (Synmedic, Zurich, Switzerland).
For motivational reasons, the experiment was performed as a competition honoring the best three volunteers' performances with a prize money of CHF 300.‐, 200.‐, and 100.‐. The ranking based on (1) the MVC assessed before the experiment; (2) the duration of achieved target forces summed up throughout the 39 trials; and (3) the number of succeeded trials (see below). The participants were aware of these ranking conditions and the number of rivals.
Force Measurement
The applied forces were assessed with a custom made MRI‐compatible isometric handgrip dynamometer (Sensory‐Motor Systems Laboratory, ETH Zurich and University of Zurich, Switzerland). During the fMRI experiment, subjects were comfortably lying supine with the right upper arm positioned along the body and an elbow flexion of 90° thus not to enable the subjects to make any contact with the handgrip dynamometer except for the right hand they were pressing with. The handgrip dynamometer was grasped in a squeezing grip with the thumb opposite to the fingers. Force values were converted to a serial signal (RS232) and logged on a PC running Presentation® control software (Neurobehavioral Systems, Albany, NY) with a sampling frequency of 60 Hz. The PC was connected to a projector to allow for real‐time visual feedback of the applied force in relation to the required target force.
Visual Feedback
At the beginning of each trial, a rectangular dark‐green target field positioned above a red horizontal bar on a black background was projected onto a screen located in front of the scanner inside the darkened MRI room. A mirror fixed to the head coil directly above the subjects' eyes provided a clear, unobstructed sight of these projections while lying in the scanner. Subjects had to press the dynamometer handgrip to raise the red horizontal bar into the target field, and to hold this position as long as possible. As visual help, the color of the bar changed from red to lime green when the target field was reached. Furthermore, to inform the subjects about time progression, a yellow horizontal midline in the target field linearly prolonged from the centre and reached the rectangle's margins after 13 s. During the resting periods between the contractions, a white cross was presented in the middle of the black screen to prevent eye movements. After each set of 10 trials, columns with the height indicating the duration of maintained target forces were presented for each performed trial. Stimuli presentation and force response acquisition was controlled with Presentation® 11.0.
fMRI Data Collection
Data acquisition occurred on a Philips Achieva 3T whole body MR unit (Philips Medical Systems, Best, The Netherlands) equipped with an 8‐channel Philips SENSE head coil. Structural images of the whole brain were acquired by using a T1‐weighted three‐dimensional, spoiled, gradient echo pulse sequence [repetition time (RT) = 20 ms; echo time (TE) = 2.30 ms; flip angle = 20°; field of view (FOV) = 220 × 220 mm; matrix size = 224 × 224; voxel size = 0.98 × 0.72 × 1.22 mm, resliced to 0.98 × 0.98 × 1.5 mm, 180 slices]. Functional data of the whole brain comprised 315 scans per run and were obtained by using a sensitivity encoded (SENSE, R = 2.0) single‐shot echo planar imaging (EPI) technique (RT = 2500 ms; TE = 35 ms; flip angle = 78°; FOV = 220 × 220 mm; matrix size = 80 × 80; voxel size = 2.75 × 2.75 × 4 mm, resliced to 1.72 × 1.72 × 4 mm; 33 transverse slices acquired in interleaved order).
fMRI Data Analysis
fMRI data were preprocessed and analyzed using SPM5 (http://fil.ion.ucl.ac.uk/spm) running on MATLAB 7.7.0 (Mathworks, Natiek, MA). All images were realigned to the first volume to correct for movement artifacts, stereotactically normalized into standard brain space (EPI‐template provided by the Montreal Neurological Institute) and smoothed using a 6‐mm full‐width‐at‐half‐maximum (FWHM) Gaussian kernel.
To identify activated voxels, the General Linear Model (GLM) approach was used. At first‐level‐analysis, task failure trials and succeeded trials were modeled: Trials were defined as failed, if the target force was achieved for ≥3.5 s but <12.5 s; if the required force was performed for ≥12.5 s, trials were classified as succeeded. Trials in which the target force was sustained for <3.5 s were discarded from further analysis. From the shortest task failure trial performed by a subject, time windows W of a consistent length (≥3.5 s) were determined such that the endpoint of W matched the occurrence of task failure resulting in a window length of 4.76 ± 1.0 s (mean ± SD) over the subjects. To each task failure trial, we randomly assigned a succeeded trial with an equivalently defined initial‐ and endpoint of W (Fig. 1).
Figure 1.
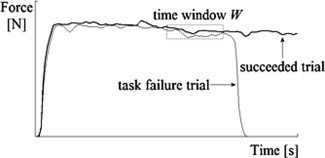
Illustration of a task failure trial and a succeeded trial laid on top of each other from a single subject. A time window W was defined such that the endpoint of W matched the occurrence of task failure. To each task failure trial, a succeeded trial with an equivalently defined initial‐ and endpoint of W was randomly assigned.
To investigate the contrast of interest, namely brain areas showing specific activity related to task failure, single‐subject estimates of the time‐windowed task failure trial conditions were compared with those obtained from the corresponding time window of the assigned succeeded trial conditions for each voxel and each subject [Friston et al.,1995] resulting in a set of voxel values yielding a statistical parametric map of the t statistic (SPM {t}) for the investigated contrast in each subject. Contrast images were further smoothed using an 8‐mm FWHM Gaussian kernel, leading to an overall smoothing of (62 + 82)0.5 = 10 mm FWHM, to reduce remaining interindividual variance and increase signal to noise ratio. To allow for population inferences, a second‐level analysis over the entire group was performed by comparing voxel activation values (beta weights) of the time‐windowed task failure trial and succeeded trial conditions using the smoothed contrast images obtained from all fifteen subjects. Reported are clusters that survived significance thresholding at P < 0.05, family wise corrected for multiple comparisons with a spatial extent threshold of k = 10 voxels.
RESULTS
Behavioral Data
On average, 17.1 ± 5.0 (mean ± SD) task failure trials, and 18.3 ± 2.0 succeeded trials were performed by the subjects. A two‐tailed paired t test revealed no significant difference in the number of task failure trials as well as succeeded trials for the first and second half of the experiment [t(15) = −1.8 and t(15) = −0.4, respectively; both P > 0.05]. The distribution of succeeded trials and task failure trials over the experimental session of 39 trials performed by the fifteen participants is shown in Figure 2 together with discarded trials. Group mean values of maximal forces performed in task failure trials were significantly higher than in succeeded trials [220.9 ± 29.0 N vs. 208.7 ± 33.0 N; t(15) = −4.0; P = 0.001]. Averaged maximal forces of the last five succeeded trials were significantly lower than those obtained in the first five succeeded trials [167.7 ± 35.3 N vs. 271.6 ± 46.4 N; t(15) = 8.8; P < 0.001].
Figure 2.
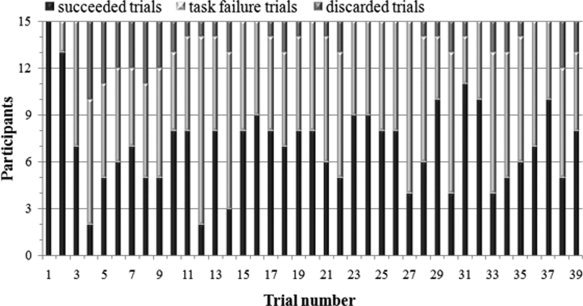
Distribution of succeeded trials and task failure trials over the experimental session of overall 39 trials. Trials in which the participants did not reach the target force for at least 3.5 s were discarded.
Imaging Data
Group results of the analysis relevant to test our hypothesis: time‐windowed task failure trial > succeeded trial. A significantly activated cluster contralaterally to the used hand in both the mid/anterior insular cortex and the thalamus is revealed, as shown in Figure 3 and Table I.
Figure 3.
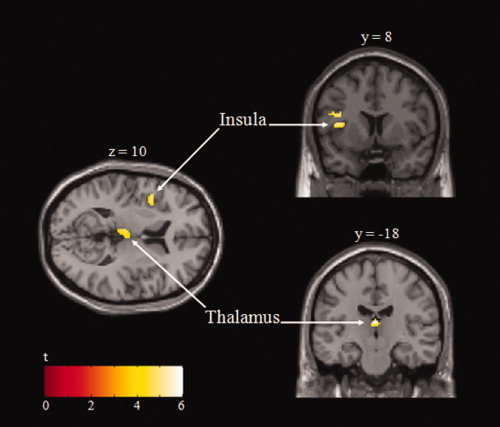
Statistical maps of significant brain activity associated with task failure superimposed onto transversal and coronal sections of the structural standard MNI template. Max t values for the activated clusters are indicated by the scale bar (thresholded at P < 0.05, corrected for multiple comparisons).
Table I.
Activation sites from group analysis of the task failure trial > succeeded trial contrast
| Anatomical description | Cluster size [n voxel] | MNI coordinates X·Y·Z [mm] | Max t value | ||
|---|---|---|---|---|---|
| Thalamus | 104 | 0 | −18 | 14 | 5.99 |
| Insular Cortex | 101 | −42 | 8 | 10 | 5.06 |
Cluster size, coordinates in MNI space, and max t values are shown for the local maxima in each cluster (P < 0.05, corrected for multiple comparisons, cluster extent threshold = 10 voxels).
For completeness, activations obtained from the contrast succeeded trial > baseline, and task failure trial > baseline are reported in Table II and Table III, respectively.
Table II.
Activation sites from group analysis of the succeeded trial > baseline contrast
| Anatomical description | Cluster size [n voxel] | MNI coordinates X·Y·Z [mm] | Max t value | ||
|---|---|---|---|---|---|
| Cerebellum, parahippocampal gyrus, hippocampus | 1572 | −6 | −32 | −16 | 8.64 |
| Parahippocampal gyrus, hippocampus | 246 | −8 | −30 | 10 | 8.54 |
| Subcallosal cortex, medial frontal cortex, basal ganglia | 249 | −2 | 12 | −12 | 6.08 |
| Inferior temporal gyrus | 146 | 54 | −50 | −20 | 5.70 |
| Parietal operculum cortex | 239 | 56 | 4 | 8 | 5.07 |
If an activated region spans several anatomically distinct areas, locations of further local maxima are given after the comma.
Cluster size, coordinates in MNI space, and max t values are shown for the local maxima in each cluster (P < 0.05, corrected for multiple comparisons, cluster extent threshold = 10 voxels).
Table III.
Activation sites from group analysis of the task failure trial > baseline contrast
| Anatomical description | Cluster size [n voxel] | MNI coordinates X·Y·Z [mm] | Max t value | ||
|---|---|---|---|---|---|
| STG/temporal pole, insular cortex, amygdala | 2574 | −52 | 4 | −16 | 7.49 |
| Temporal fusiform cortex, inferior temporal gyrus | 249 | −36 | −32 | −16 | 7.26 |
| Frontal orbital cortex, insular cortex | 662 | 34 | 30 | −14 | 6.78 |
| Cerebellum | 105 | 10 | −90 | −32 | 5.98 |
| Cerebellum | 66 | −22 | −88 | −32 | 5.60 |
| Temporal fusiform cortex, inferior temporal gyrus | 131 | 42 | −24 | −20 | 5.57 |
| Temporal pole/STG | 219 | 48 | 16 | −28 | 5.19 |
| Frontal orbital cortex | 145 | −18 | 20 | −26 | 5.05 |
| Parahippocampal gyrus | 69 | 16 | 4 | −30 | 5.00 |
If an activated region spans several anatomically distinct areas, locations of further local maxima are given after the comma.
Cluster size, coordinates in MNI space, and max t values are shown for the local maxima in each cluster (P < 0.05, corrected for multiple comparisons, cluster extent threshold = 10 voxels).
STG, Superior temporal gyrus.
DISCUSSION
In this study, central/supraspinal mechanisms mediating cessation of a fatiguing, intermittent handgrip exercise were investigated by using fMRI. As expected, few seconds before failure of a 13‐s isometric, submaximal contraction performed with the right hand, the BOLD response in the contralateral mid/anterior insular cortex was significantly increased. Additionally, the data revealed an increased hemodynamic signal in the contralateral thalamus.
Behavioral Results and Their Implications
The mean number of succeeded contraction trials and failed contraction trials, respectively, indicates that the protocol designed to induce task failure trials in approximately 50% of contractions was appropriate. Moreover, task failure trials were evenly distributed over the 39 performed trials thus no sequence effect was observed, which might have influenced fMRI data. Because group mean values of maximal forces performed in task failure trials were significantly higher than in succeeded trials, an alternative explanation for increased insular and thalamic activity during task failure trials may be their involvement in force coding. To assess this possibility an additional analysis was conducted to test for brain regions showing a positive correlation between the BOLD signal and the magnitude of the applied force including all trials. However, no increase in motor cortex activity was found for the more forcefully performed task failure trials. Furthermore, because differences between force levels, although significant, were much smaller than in studies designed to investigate force coding, we assume that the protocol used here to compare task failure with succeeded trials was not sensitive to effects of force coding. More important to note is the fact that in the insular cortex as well as in the thalamus the activity did not correlate with the applied force, neither in our results nor in other studies of force coding [Dai et al.,2001; Dettmers et al.,1995]. Therefore, increased activity in both the insular cortex and the thalamus revealed by contrasting time‐windowed task failure trials > succeeded trials cannot be attributed to higher force requirements in task failure trials.
fMRI Results
In the present study, a prominent cluster of increased neuronal activity was observed few seconds before cessation of a muscle fatiguing handgrip contraction in the mid/anterior insular cortex. This finding may be related to the long discussed notion that the insular cortex has a function in autonomic processes. In fact, the insular cortex has been shown to be involved in the control of cardiac function: by electrically stimulating the insular cortex, changes in both the heart rate and the blood pressure were elicited not only in monkeys [Kaada et al.,1949; King et al.,1999] but also in patients undergoing surgery for epilepsy [Oppenheimer et al.,1992]. Similar cardiovascular effects after patients' insular cortex stimulation were also reported by other researchers [Sander and Klingelhofer,1995; Svigelj et al.,1994] and next to respiratory inhibition several sensory experiences such as pain and smell was evoked [Penfield and Faulk,1955]. In an fMRI study of healthy humans, King et al., [1999] suggested that both the anterior and posterior insular cortex as well as the thalamus have some role in the control and regulation of autonomic conditions because these regions revealed higher neuronal activity during periods of significant increases in blood pressure and/or heart rate, induced by maximal inspiration, Valsalva's maneuver or isometric handgrip contractions. Due to technical limitations throughout our exercise of fatiguing handgrip contractions in the scanner, cardiovascular changes could not be assessed, so that we have to take into account that they could be related to insular and thalamic activation preceding task failure. However, in our task setting, no marked differences in heart rate and blood pressure are expected between task failure trials and succeeded trials, which were both performed near the individual performance limit. In line, another study of handgrip exercise found increased insular activity not associated with blood pressure elevation [Williamson et al.,2003], and it was supposed that a neural network including insular and cingulate cortex interpret sensory input in concert or independently to elicit appropriate autonomic adjustments to exercise [Jouanin et al.,2009; Williamson et al.,2003;2006]. In the context of processing both visceral and cutaneous pain, the anterior insular cortex has consistently been shown to play an important role [Baciu et al.,1999; Binkofski et al.,1998; Casey,1999; Derbyshire et al.,1997; Iadarola et al.,1998; for reviews see Apkarian et al., [2005] and Peyron et al., [2000]]. Similar to pain, air hunger and hunger for food are strong sensations to alert the organism of a potential physiological threat. As it was demonstrated in several neuroimaging experiments, the anterior insular cortex has been thought to participate in the evaluation of such distressing [Cannon,1935] internal stimuli [Banzett et al.,2000; Brannan et al.,2001; Evans et al.,2002; Liotti et al.,2001; Parsons et al.,2001; Peiffer et al.,2001; Tataranni et al.,1999] and was denoted as a nonspecific “alarm center” for physiological threat [Reiman,1997]. Increased activity of insular cortex immediately before task failure thus strengthens the view of this structure being involved in regulatory processes to keep up a homeostatic balance. The current study provides evidence for task failure, or more general motor fatigue, to rely on similar processes. With regard to lateralization of autonomic regulatory sites, handedness may play a role. King et al. [1999] revealed opposite lateralization of insular activity due to autonomic regulation in a single left‐handed subject compared to the right‐handed individuals. However, lateralization in the cingulate and insular cortex in studies of cold pressor application to the forehead, Valsalva maneuver and hyperoxia has been demonstrated to appear independently of handedness and inconsistent for the stimulus [Harper et al.,2000; Macey et al.,2007]. Therefore we do not attempt to interpret the fact that the current activity pattern was left lateralized (contralateral to the used hand) as an indication in favor of or against autonomic regulation of homeostasis playing a role in the current study.
Considerable evidence exist for a lamina I spino‐thalamic pathway processing homeostatic regulation in humans. Neurons originating in the lamina I of the superficial spinal dorsal horn are monosynaptically activated by small‐diameter afferents that provide information about physiological changes within various tissues of the body, e.g., evoked by pain (nociceptors), heat/cold (thermoreceptors), acidic pH (metaboreceptors), hypo‐osmolarity (osmoreceptors) [Craig et al.,2000]. In primates, lamina I neurons project to spinal autonomic and brainstem homeostatic integration sites including the caudal and rostral ventrolateral medulla, parabrachial nucleus, periaqueductal gray and catecholamine cell groups A1‐A2, A5‐A7. Furthermore, ascending in the lateral spinothalamic tract the lamina I neurons also project to two sides of the contralateral thalamus: the posterior part of the ventral medial nucleus (VMpo) and the ventral caudal part of the medial dorsal nucleus (MDvc). Such sensory projections in homeostasis have also been proposed to play an important role during prolonged cycling exercise [Rauch et al.,2005] and effects on autonomic cardiorespiratory adjustments to muscular work have been proven [Wilson et al.,2002]. Whereas VMpo has been assumed to project topographically to the mid/anterior insular cortex [Craig et al.,1994], MDvc is thought to have efferents to the anterior cingulate cortex, which has been associated with the affective motivational component of pain [Vogt,2005]. Although anatomic knowledge would suggest VMpo thalamic activity corresponding with insular activation, we rather estimate the location of the thalamic activation cluster found in our study as MDvc. Activation in the MDvc observed in the present study contralaterally to the used hand just before task failure thus supports the proposed role of the thalamus as a visceral relay‐point supervising the maintenance of essential homeostatic balances and allowing their regulation in accord with insular activity. Nevertheless, no significant influence on the anterior cingulate cortex could be revealed. Interpretation of such a null finding must remain cautious and with the current study, a separation of autonomic regulation and pain processing is difficult. However, activations in typical “pain matrix” regions such as anterior cingulate cortex, somatosensory cortex, or parietal opercular cortex that account for experience of pain were absent. The networks involved in homeostatic regulation of several functions and in pain processing seem to partly overlap, especially regarding anterior cingulate, insular and thalamic activity which underlines the view of pain as a homeostatic emotion [Craig,2003]. Interestingly, overlap with these systems also exists with the current data concerning mid/anterior insular cortex and thalamus, pointing to a common denominator of the involved processes.
From the viewpoint of a homeostatically regulated determination of an exhaustive exercise task to prevent body harm, the commonly used term “task failure” [Hunter et al.,2004; Maluf and Enoka,2005] is probably not appropriate. Because cessation of a fatiguing exercise might not reflect a failure but rather a biologically wise decision, we suggest an alternative denotation for future studies considering the aspect of body protection.
Similarly, the fact that data from this experiment indicate a brain system actively leading to a reduction in force production, contrasts the view that reduction of neural efficiency, e.g., due to neurotransmitter depletion, causes task cessation.
With the current study, however, it was not possible to demonstrate whether an increase in insular and thalamic activity merely reflects homeostatic disturbances in the muscle and/or indeed reduces neuronal output from the motor cortex. To assess an effective connectivity between these regions, a “dynamic causal modeling” (DCM) approach [Friston et al.,2003] could be applied in future studies. Because well discernable “key regions” on the first‐level analysis is an essential prerequisite to compute DCM, the current study design should be adjusted to additionally comprise activity in the motor cortex, e.g., by lengthening the time period between the contraction trials to provide more complete recovery and thus detection of a re‐increase of the BOLD signal in the motor cortex.
Because the fatiguing exercise was performed as a competition with attractive prize money to maximally motivate the subjects, the central mechanism of task failure proposed here is not likely to be influenced from areas involved in motivational processes, e.g., the prefrontal cortex. However, other brain areas “upstream” of the motor cortex still have to be considered when discussing the mechanisms of central fatigue [Gandevia et al.,1996].
Taken together, our data revealed significantly increased activity in both the contralateral mid/anterior insular cortex and the thalamus during the final few seconds before subjects had to cease a fatiguing isometric handgrip contraction (task failure), compared with an equivalently defined time window in control contractions not exhibiting task failure. In accordance with previous studies of autonomic regulation and homeostatic processes, we conclude that increased thalamo‐insular activation may be of essential relevance during a force demanding handgrip exercise by acting as a central instance mediating the termination of a muscle fatiguing task to protect the integrity of the organism.
REFERENCES
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA ( 2009): Opioid‐mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587: 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK ( 2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484. [DOI] [PubMed] [Google Scholar]
- Baciu MV, Bonaz BL, Papillon E, Bost RA, Le Bas JF, Fournet J, Segebarth CM ( 1999): Central processing of rectal pain: A functional MR imaging study. AJNR Am J Neuroradiol 20: 1920–1924. [PMC free article] [PubMed] [Google Scholar]
- Bailey SP, Davis JM, Ahlborn EN ( 1993): Neuroendocrine and substrate responses to altered brain 5‐HT activity during prolonged exercise to fatigue. J Appl Physiol 74: 3006–3012. [DOI] [PubMed] [Google Scholar]
- Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L ( 2000): Breathlessness in humans activates insular cortex. Neuroreport 11: 2117–2120. [DOI] [PubMed] [Google Scholar]
- Benwell NM, Mastaglia FL, Thickbroom GW ( 2007): Changes in the functional MR signal in motor and non‐motor areas during intermittent fatiguing hand exercise. Exp Brain Res 182: 93–97. [DOI] [PubMed] [Google Scholar]
- Bigland‐Ritchie B, Johansson R, Lippold OC, Woods JJ ( 1983): Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. J Neurophysiol 50: 313–324. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Schnitzler A, Enck P, Frieling T, Posse S, Seitz RJ, Freund HJ ( 1998): Somatic and limbic cortex activation in esophageal distention: A functional magnetic resonance imaging study. Ann Neurol 44: 811–815. [DOI] [PubMed] [Google Scholar]
- Blomstrand E ( 2001): Amino acids and central fatigue. Amino Acids 20: 25–34. [DOI] [PubMed] [Google Scholar]
- Blomstrand E, Hassmen P, Newsholme EA ( 1991): Effect of branched‐chain amino acid supplementation on mental performance. Acta Physiol Scand 143: 225–226. [DOI] [PubMed] [Google Scholar]
- Brannan S, Liotti M, Egan G, Shade R, Madden L, Robillard R, Abplanalp B, Stofer K, Denton D, Fox PT ( 2001): Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proc Natl Acad Sci USA 98: 2029–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB ( 1935): Stresses and strains of homeostasis. Am J Med Sci 189: 13–14. [Google Scholar]
- Casey KL ( 1999): Forebrain mechanisms of nociception and pain: Analysis through imaging. Proc Natl Acad Sci USA 96: 7668–7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlay LA, Sabounjian LA, Wurtman RJ ( 1992): Exercise and neuromodulators: Choline and acetylcholine in marathon runners. Int J Sports Med 13( Suppl 1): S141–142. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2003): A new view of pain as a homeostatic emotion. Trends Neurosci 26: 303–307. [DOI] [PubMed] [Google Scholar]
- Craig AD, Bushnell MC, Zhang ET, Blomqvist A ( 1994): A thalamic nucleus specific for pain and temperature sensation. Nature 372: 770–773. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM ( 2000): Thermosensory activation of insular cortex. Nat Neurosci 3: 184–190. [DOI] [PubMed] [Google Scholar]
- Dai TH, Liu JZ, Sahgal V, Brown RW, Yue GH ( 2001): Relationship between muscle output and functional MRI‐measured brain activation. Exp Brain Res 140: 290–300. [DOI] [PubMed] [Google Scholar]
- Davis JM ( 1995): Central and peripheral factors in fatigue. J Sports Sci 13: S49–53. [DOI] [PubMed] [Google Scholar]
- Denton D, Shade R, Zamarippa F, Egan G, Blair‐West J, McKinley M, Fox P ( 1999): Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proc Natl Acad Sci USA 96: 2532–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL ( 1997): Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain 73: 431–445. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS ( 1995): Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol 74: 802–815. [DOI] [PubMed] [Google Scholar]
- Edwards RH ( 1983): Biochemical bases for fatigue in exercise performance: Catastrophe theory in muscular fatigue In: Knuttgen HG, Vogel JA, Poortmans J, editors. Biochemistry of Exercise. Champaign, IL: Human Kinetics; pp 1–28. [Google Scholar]
- Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR ( 2002): BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol 88: 1500–1511. [DOI] [PubMed] [Google Scholar]
- Fitts RH, Holloszy JO ( 1976): Lactate and contractile force in frog muscle during development of fatigue and recovery. Am J Physiol 231: 430–433. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RS ( 1995): Characterizing evoked hemodynamics with fMRI. Neuroimage 2: 157–165. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W ( 2003): Dynamic causal modelling. Neuroimage 19: 1273–1302. [DOI] [PubMed] [Google Scholar]
- Gandevia SC ( 2001): Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL ( 1996): Supraspinal factors in human muscle fatigue: Evidence for suboptimal output from the motor cortex. J Physiol 490: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guezennec CY, Abdelmalki A, Serrurier B, Merino D, Bigard X, Berthelot M, Pierard C, Peres M ( 1998): Effects of prolonged exercise on brain ammonia and amino acids. Int J Sports Med 19: 323–327. [DOI] [PubMed] [Google Scholar]
- Harper RM, Bandler R, Spriggs D, Alger JR ( 2000): Lateralized and widespread brain activation during transient blood pressure elevation revealed by magnetic resonance imaging. J Comp Neurol 417, 195–204. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Bandler R, Gandevia SC, Macefield VG ( 2006): Distinct forebrain activity patterns during deep versus superficial pain. Pain 120: 286–296. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Shin IS, Enoka RM ( 2004): Men are more fatigable than strength‐matched women when performing intermittent submaximal contractions. J Appl Physiol 96, 2125–2132. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Berman KF, Zeffiro TA, Byas‐Smith MG, Gracely RH, Max MB, Bennett GJ ( 1998): Neural activation during acute capsaicin‐evoked pain and allodynia assessed with PET. Brain 121: 931–947. [DOI] [PubMed] [Google Scholar]
- Jouanin JC, Peres M, Ducorps A, Renault B ( 2009): A dynamic network involving M1‐S1, SII‐insular, medial insular, and cingulate cortices controls muscular activity during an isometric contraction reaction time task Hum Brain Mapp 30: 675–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaada BR, Pribram KH, Epstein JA ( 1949): Respiratory and vascular responses in monkeys from temporal pole, insula, orbital surface and cingulate gyrus: A preliminary report. J Neurophysiol 12: 347–356. [DOI] [PubMed] [Google Scholar]
- King AB, Menon RS, Hachinski V, Cechetto DF ( 1999): Human forebrain activation by visceral stimuli. J Comput Neurol 413: 572–582. [PubMed] [Google Scholar]
- Kniffki KD, Schomburg ED, Steffens H ( 1979): Synaptic responses of lumbar alpha‐motoneurones to chemical algesic stimulation of skeletal muscle in spinal cats. Brain Res 160: 549–552. [DOI] [PubMed] [Google Scholar]
- Kupers RC, Svensson P, Jensen TS ( 2004): Central representation of muscle pain and mechanical hyperesthesia in the orofacial region: A positron emission tomography study. Pain 108: 284–293. [DOI] [PubMed] [Google Scholar]
- Li J, Sinoway LI ( 2002): ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol 283: H2636–2643. [DOI] [PubMed] [Google Scholar]
- Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, Robillard R, Lancaster J, Zamarripa FE, Fox PT, Denton D ( 2001): Brain responses associated with consciousness of breathlessness (air hunger). Proc Natl Acad Sci USA 98: 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Dai TH, Sahgal V, Brown RW, Yue GH ( 2002): Nonlinear cortical modulation of muscle fatigue: A functional MRI study. Brain Res 957: 320–329. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Shan ZY, Zhang LD, Sahgal V, Brown RW, Yue GH ( 2003): Human brain activation during sustained and intermittent submaximal fatigue muscle contractions: An FMRI study. J Neurophysiol 90: 300–312. [DOI] [PubMed] [Google Scholar]
- Macey PM, Woo MA, Harper RM ( 2007): Hyperoxic brain effects are normalized by addition of CO2 . PLoS Med 4: e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maluf KS, Enoka RM ( 2005): Task failure during fatiguing contractions performed by humans. J Appl Physiol 99: 389–396. [DOI] [PubMed] [Google Scholar]
- Martin PG, Weerakkody N, Gandevia SC, Taylor JL ( 2008): Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol 586: 1277–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S ( 1977): Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol 267: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes TD ( 2004): Linear relationship between the perception of effort and the duration of constant load exercise that remains. J Appl Physiol 96: 1571–1572; author reply 1572–1573. [DOI] [PubMed] [Google Scholar]
- Noakes TD, St Clair Gibson A, Lambert EV ( 2005): From catastrophe to complexity: A novel model of integrative central neural regulation of effort and fatigue during exercise in humans: Summary and conclusions. Br J Sports Med 39: 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo L, Secher NH ( 2004): Cerebral perturbations provoked by prolonged exercise. Prog Neurobiol 72: 223–261. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC ( 1992): Cardiovascular effects of human insular cortex stimulation. Neurology 42: 1727–1732. [DOI] [PubMed] [Google Scholar]
- Paintal AS ( 1960): Functional analysis of group III afferent fibres of mammalian muscles. J Physiol 152: 250–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LM, Egan G, Liotti M, Brannan S, Denton D, Shade R, Robillard R, Madden L, Abplanalp B, Fox PT ( 2001): Neuroimaging evidence implicating cerebellum in the experience of hypercapnia and hunger for air. Proc Natl Acad Sci USA 98: 2041–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer C, Poline JB, Thivard L, Aubier M, Samson Y ( 2001): Neural substrates for the perception of acutely induced dyspnea. Am J Respir Crit Care Med 163: 951–957. [DOI] [PubMed] [Google Scholar]
- Penfield W, Faulk ME Jr ( 1955): The insula; further observations on its function. Brain 78: 445–470. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia‐Larrea L ( 2000): Functional imaging of brain responses to pain. A review and meta‐analysis. Neurophysiol Clin 30: 263–288. [DOI] [PubMed] [Google Scholar]
- Post M, Steens A, Renken R, Maurits NM, Zijdewind I ( 2009): Voluntary activation and cortical activity during a sustained maximal contraction: An fMRI study. Hum Brain Mapp 30: 1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch HG, St Clair Gibson A, Lambert EV, Noakes TD ( 2005): A signalling role for muscle glycogen in the regulation of pace during prolonged exercise. Br J Sports Med 39: 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM ( 1997): The application of positron emission tomography to the study of normal and pathologic emotions. J Clin Psychiatry 58( Suppl 16): 4–12. [PubMed] [Google Scholar]
- Rotto DM, Kaufman MP ( 1988): Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313. [DOI] [PubMed] [Google Scholar]
- Sander D, Klingelhofer J ( 1995): Changes of circadian blood pressure patterns and cardiovascular parameters indicate lateralization of sympathetic activation following hemispheric brain infarction. J Neurol 242: 313–318. [DOI] [PubMed] [Google Scholar]
- Sgherza AL, Axen K, Fain R, Hoffman RS, Dunbar CC, Haas F ( 2002): Effect of naloxone on perceived exertion and exercise capacity during maximal cycle ergometry. J Appl Physiol 93: 2023–2028. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Hill JM, Pickar JG, Kaufman MP ( 1993): Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol 69: 1053–1059. [DOI] [PubMed] [Google Scholar]
- St Clair Gibson A, Noakes TD ( 2004): Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. Br J Sports Med 38: 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svigelj V, Grad A, Tekavcic I, Kiauta T ( 1994): Cardiac arrhythmia associated with reversible damage to insula in a patients with subarachnoid hemorrhage. Stroke 25: 1053–1055. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E ( 1999): Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 96: 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM, Gandevia SC ( 1996): Changes in motor cortical excitability during human muscle fatigue. J Physiol 490: 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Petersen N, Butler JE, Gandevia SC ( 2000): Ischaemia after exercise does not reduce responses of human motoneurones to cortical or corticospinal tract stimulation. J Physiol 525: 793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duinen H, Renken R, Maurits N, Zijdewind I ( 2007): Effects of motor fatigue on human brain activity, an fMRI study. Neuroimage 35: 1438–449. [DOI] [PubMed] [Google Scholar]
- Vogt BA ( 2005): Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JW, Fadel PJ, Mitchell JH ( 2006): New insights into central cardiovascular control during exercise in humans: A central command update. Exp Physiol 91: 51–58. [DOI] [PubMed] [Google Scholar]
- Williamson JW, McColl R, Mathews D ( 2003): Evidence for central command activation of the human insular cortex during exercise. J Appl Physiol 94: 726–34. [DOI] [PubMed] [Google Scholar]
- Wilson LB, Andrew D, Craig AD ( 2002): Activation of spinobulbar lamina I neurons by static muscle contraction. J Neurophysiol 87: 1641–5. [DOI] [PubMed] [Google Scholar]
- Ziv I, Avraham M, Michaelov Y, Djaldetti R, Dressler R, Zoldan J, Melamed E ( 1998): Enhanced fatigue during motor performance in patients with Parkinson's disease. Neurology 51: 1583–6. [DOI] [PubMed] [Google Scholar]


